Abstract
Cyclophilin D (CypD) is a mitochondrial immunophilin and a key positive regulator of the mitochondrial permeability transition (MPT). Several reports have shown that CypD is overexpressed in various tumors, where it has an anti-apoptotic effect. Because the MPT is a cell death-inducing phenomenon, we hypothesized that the anti-apoptotic effect of CypD is independent of the MPT but is due to its interaction with some key apoptosis regulator, such as Bcl2. Our data indicate that CypD indeed interacts with Bcl2 as confirmed with co-immunoprecipitation, pulldown, and mammalian two-hybrid assays. A cyclophilin D inhibitor, cyclosporine A, disrupts the CypD-Bcl2 interaction. CypD enhances the limiting effect of Bcl2 on the tBid-induced release of cytochrome c from mitochondria, which is not mediated via the MPT. Gain- and loss-of-function experiments confirm that CypD has a limiting effect on cytochrome c release from mitochondria and that such an effect of CypD is cyclosporine A- and Bcl2-dependent. On a cellular level, overexpression or knockdown of CypD respectively decreases or increases cytochrome c release from mitochondria and overall cell sensitivity to apoptosis progressing via the “intrinsic” pathway. Therefore, we here describe a novel function of CypD as a Bcl2 collaborator and an inhibitor of cytochrome c release from mitochondria independent of the MPT. This function of CypD may explain the anti-apoptotic effect of this protein observed in various cancer cells. The fact that some tumors overexpress CypD suggests that this may be an additional mechanism of suppression of apoptosis in cancer.
Apoptosis is a programmed mode of cell death and a universal phenomenon in multicellular eukaryotic organisms (1). Suppression of apoptosis is utilized by cancer cells to promote their survival (2). It is, therefore, important to pursue the mechanisms of suppression of apoptosis in cancer to define new therapeutic targets and develop new therapies. The anti-apoptotic effect and overexpression of cyclophilin D (CypD)2 in various tumors have recently been reported (3–5). The goal of this study was to elucidate the mechanism underlying the reported anti-apoptotic effect of CypD.
Cyclophilin D is a ppif gene product, a member of the immunophilin family of peptidyl-prolyl cis-trans isomerases, and a mitochondrial matrix protein that has a crucial role in protein folding (6). Therefore, the ability to interact with various proteins is an inherent feature of CypD. The most important interaction of CypD reported to date is with the components of the mitochondrial permeability transition (MPT) pore (7). The MPT can be induced in the presence of elevated calcium by a variety of signals (8). CypD has been shown to interact with the mitochondrial adenine nucleotide translocator (ANT), a putative MPT pore component (7, 9), and to promote its “open” conformation so that it can transport not only ADP and ATP but also solutes of up to 1.5 kDa (10). As a result, solutes and water equilibrate across the inner mitochondrial membrane, causing mitochondrial swelling, depolarization, and release of various intermembrane proteins including cytochrome c (CytC). Calcium strongly promotes binding of CypD to the ANT and is mandatory for MPT induction (7, 10). The immunosuppressor, cyclosporine A (CsA), binds CypD, prevents its interaction with the ANT, and is, therefore, an effective inhibitor of the MPT (11). Recently, Kokoszka et al. (12) have shown that mitochondria isolated from ANT knock-out mice remain sensitive to the MPT. However, various groups showed that mitochondria isolated from CypD knock-out mice completely lack the MPT (13–15). Therefore, CypD is a crucial regulator of the MPT, whereas the ANT is dispensable for this process. Lemasters and co-workers (16) have suggested that a role in MPT induction, like that of the ANT, may be played by other transporters and/or misfolded proteins.
In addition to its role in regulating the MPT, CypD has been shown to suppress apoptosis in various tumor cells via a yet unidentified mechanism (3–5). Because of the role of CypD in regulation of the MPT, the attempt has been made to relate the anti-apoptotic effect of CypD in cancer cells to the MPT (5). The MPT has been suggested as one of the mechanisms of release of CytC and other apoptogenic factors from mitochondria during apoptosis (17). In view of the pro-apoptotic effect of MPT opening, the anti-apoptotic effect of CypD, a key positive regulator of the MPT, seems controversial. Recently, the interaction between a member of the cyclophilin family of proteins, mitochondria-associated peptidyl-prolyl isomerase, FKBP38, and Bcl2, leading to modification of Bcl2 function, has been reported (18). We hypothesized that due to functional and structural similarity to FKBP38, CypD may exert a similar effect and interact with Bcl2. Such interaction between CypD and Bcl2, a major factor that regulates apoptosis, may also modify Bcl2 function and make it a more potent inhibitor of cell death. We here present evidence that CypD and Bcl2 indeed interact and cooperate in preventing CytC release from mitochondria, leading to inhibition of apoptosis.
EXPERIMENTAL PROCEDURES
Materials—Chemicals were from Sigma unless otherwise noted. Cell culture media and media components were from Invitrogen. Primary antibodies were from Epitomics (Bcl2, Bax), Mitosciences (CypD, CytC, ANT), Santa Cruz Biotechnology (mouse Ig), and Sigma (β-actin). CytC ELISA kit was from Mitosciences. Recombinant human tBid was from R&D Systems. Recombinant human Bcl2 fused to maltose-binding protein (MBP-Bcl2) was from Sigma. Recombinant human MBP-Bax was produced as described in detail in our previous work (19). HEK293T, Saos2, and HL60 cells were from ATCC.
Immunoprecipitation—Samples at 200 μg of protein per sample were precleared with protein G beads and then mixed with 2 μg of an α-Bcl2 antibody or control non-immune mouse Ig immobilized on protein G beads in an “immunoprecipitation buffer” (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1.5% Nonidet P-40) supplemented with protease inhibitors. The reactions were nutated overnight at 4 °C. Immunocomplexes were washed in an “immunoprecipitation wash buffer” (100 mm Tris-HCl, pH 7.5, 100 mm NaCl, 0.1% Triton X-100) and resuspended in 2× Laemmli buffer. The inputs were also mixed with 2× Laemmli buffer, and then the immunoprecipitation reactions and inputs were boiled for 10 min and spun down. The supernatants were subjected to immunoblotting as described below.
Immunoblotting—Samples were electrophoresed and then electroblotted onto polyvinylidene difluoride membranes (Bio-Rad). Blots were blocked in 5% solutions of nonfat dry milk in phosphate-buffered saline with Tween, probed with a primary antibody at 1 μg/ml and then probed with the corresponding horseradish peroxidase-conjugated secondary antibody at 0.2 μg/ml, developed using SuperSignal WestPico chemiluminescent substrate (Pierce), and photographed. Blots were then stripped in Re-Blot Plus stripping buffer (Chemicon) and either stained with Ponceau S (in case of isolated mitochondria) or reprobed with an α-β-actin antibody (in case of whole cell lysates or permeabilized cells) to verify equal loading.
Cyclophilin D Pulldown—Five μg of recombinant MBP-Bcl2 or MBP-Bax was immobilized on amylose resin (New England Biolabs) by mixing with 20 μl of resin and incubating for 30 min. The beads were washed twice with buffer B (25 mm Tris-HCl, pH 7.5, 200 mm NaCl, 1 mm dithiothreitol) and incubated for 1 h with either mitochondrial or whole cell protein extracts. Beads were pelleted, washed three times with buffer B, mixed with an equal volume of 2× Laemmli sample buffer, boiled for 10 min, and spun down. Supernatants were subjected to immunoblotting as described above.
Mammalian Two-hybrid Assay—The mammalian two-hybrid assay was performed using the Matchmaker kit (Clontech) according to the manufacturer's instructions. Briefly, human Bcl2 cDNA was cloned into the pM vector containing a DNA-binding domain (Fig. 1C, DNA-BD), and human CypD cDNA was cloned into the pVP16 vector containing the activation domain (Fig. 1C, AD). The pM-Bcl2 and pVP16-CypD constructs were co-transfected at different ratios into the HEK293T cells along with the Great EscApe secreted alkaline phosphatase (SEAP) reporter plasmid. In control experiments, the following combinations of vectors were co-transfected: (i) empty pM, empty VP16; (ii) pM-Bcl2, empty VP16; and (iii) empty pM, pVP16-CypD. The pM3-VP16 vector served as a positive control. At 48 h after transfections, media containing SEAP were collected, and SEAP was assayed using the chemiluminescent substrate in an Optocomp1 luminometer.
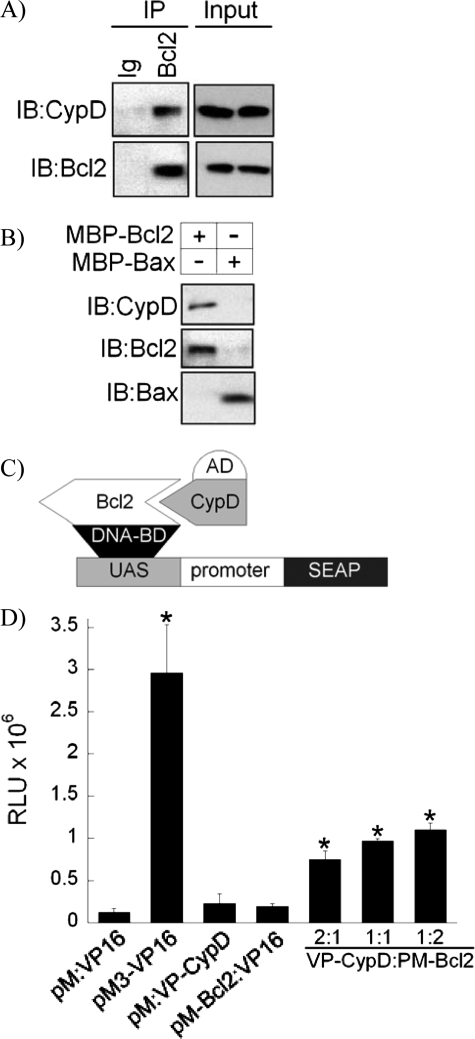
FIGURE 1.
Interaction of cyclophilin D with Bcl2. A, co-immunoprecipitation of CypD with Bcl2. Protein extracts from RLM were immunoprecipitated (IP) with an α-Bcl2 antibody or non-immune Ig, and immunoprecipitates and inputs were immunoprobed with an α-CypD antibody. Blots were then reprobed with an α-Bcl2 antibody. IB, immunoblot. B, pulldown of CypD by recombinant MBP-Bcl2. Protein extracts from RLM were incubated with MBP-Bcl2 or MBP-Bax immobilized on amylose resin, and CypD pulldown was assayed with immunoblotting with an α-CypD antibody. Blots were then reprobed with an α-Bcl2 or α-Bax antibody. Blots in A and B are representatives of four blots. C, a schematic diagram of the mammalian two-hybrid system to study interaction of CypD with Bcl2. AD, activation domain; BD, binding domain; UAS, upstream activator sequence. D, the mammalian two-hybrid assay. The assay performed as described in detail under “Experimental Procedures” shows a low SEAP activity in control reactions (pM:VP16, pM:VP-CypD, and pM-Bcl2:VP16) and high SEAP activity in the positive control (pM3-VP16). Co-transfection of VP-CypD and pM-Bcl2 constructs at the indicated ratios led to a significant increase in the SEAP signal when compared with control reactions. Data are means ± S.D. (n = 3). * indicates p < 0.05 when compared with control. RLU, relative light units.
Isolation of Mitochondria and Permeabilization of Cells—Mitochondria from rat liver or heart were prepared as described in detail in Refs. 20 and 21. Mitochondria from cells were isolated as described in Ref. 22. The functionality of isolated mitochondria was verified using oxygen consumption or calcium retention assays (20, 21). To prepare mitochondrial protein extracts, mitochondria were lysed in Golden lysis buffer for 30 min on ice, and the debris were spun down. Permeabilization of plasma membranes in HEK293T cells was performed using digitonin at 0.01% in KCl-based buffer (125 mm KCl, 2 mm K2HPO4, 10 mm K-HEPES (pH 7.4), 1 mm MgCl2, 5 mm potassium succinate, and 1 μm rotenone).
Loading of Isolated Mitochondria with Calcium—Isolated rat liver mitochondria (RLM) were incubated at 1 mg/ml in KCl-based buffer added with CaCl2 at various concentrations in a stirred cuvette. Calcium in the external medium was monitored with a calibrated selective electrode attached to a computer.
Cytochrome c Release Assay—Isolated mitochondria at 1 mg/ml or digitonin-permeabilized cells were incubated with recombinant human tBid at 1 μg/ml for 10 min at room temperature in KCl-based buffer. Samples were spun down, and supernatants, normalized for protein, were subjected to either immunoblotting for CytC or ELISA for CytC according to the manufacturer's instructions.
Cell Culture and Treatments—HEK293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. Stable transfections of HEK293T cells with pcDNA-CypD constructed as described previously in Ref. 22, of pIRES-Bcl2 constructed as described previously in Ref. 23, or of the empty vectors were performed using FuGENE HD (Roche Applied Science). Stable clones were selected by treating cells with G418 (Invitrogen) at 750 μg/ml for 2 weeks and maintained in G418 at 200 μg/ml. As a result, 293T-CypD, 293T-Bcl2, and 293T-EV stable cell lines were generated. To achieve knockdown of Bcl2 or CypD, cells were stably transfected with pKD-Bcl2 shRNA vector (Upstate Biotechnologies) or SureSilencing CypD shRNA vector (SABiosciences), respectively, and selected with 2 μm puromycin for 2 weeks. The controls were transfected with the corresponding empty vectors. To induce apoptosis, cells were treated with etoposide at 20 μm for 6 h or staurosporine at 2 μm for 6 h or TNF at 10 ng/ml for 24 h or were serum-starved for 48 h in the absence or presence of CsA at 1 μm. The optimal concentrations of the inducers and optimal treatment times were determined experimentally.
Nuclear Condensation Assay—Cells undergoing apoptosis and control cells were stained with the fluorescent nuclear probe Hoechst 33342 (Molecular Probes) at 1 μg/ml for 5 min. Nuclear morphology was assessed using a Zeiss Axiovert inverted fluorescence microscope, and the number of apoptotic nuclei showing condensed chromatin versus the total number of nuclei was counted.
Caspase-3 Activity Assay—Ten μg of cell lysates was mixed with the caspase-3-specific fluorogenic substrate, Ac-DEVD-amc (Calbiochem) at 20 μm in a total volume of 0.2 ml in 96-well plate and incubated for 30 min at 37 °C. The fluorescence at 440 nm (excitation at 380 nm) from the –amc tag cleaved by caspase-3 was measured in a Hitachi plate reader.
Statistical Analysis—Experiments were repeated 3–5 times. Mean values and standard deviations were calculated, and the statistical significance was determined using a Student's t test. Data with p < 0.05 were considered statistically significant.
RESULTS
Interaction of Cyclophilin D with Bcl2—As was shown recently by several groups, CypD is overexpressed in different types of cancer, where it has an anti-apoptotic effect (3–5). Because this anti-apoptotic effect of CypD in cancer is contradictory to its role in regulation of the MPT, which is a pro-death phenomenon, we hypothesized that this effect of CypD is independent of the MPT but is due to its interaction with some regulator of apoptosis. We, therefore, studied the interaction of CypD with a major anti-apoptotic factor, Bcl2. Fig. 1A shows that CypD co-immunoprecipitated with Bcl2 when protein extracts from isolated RLM were immunoprecipitated with an α-Bcl2 antibody and then immunoprobed with an α-CypD antibody. The co-immunoprecipitation of CypD with Bcl2 was specific because CypD did not co-immunoprecipitate with control non-immune Ig. Similar results were obtained with protein extracts from mitochondria isolated from rat hearts and human HEK293T, Saos2, and HL60 cells and with whole cell lysates from Saos2 or HEK293T cells (data not shown).
To further confirm the interaction of CypD with Bcl2, we performed a CypD pulldown assay by incubating mitochondrial protein extracts with recombinant MBP-Bcl2 fusion protein immobilized on amylose resin. Fig. 1B shows that CypD was pulled down by the MBP-Bcl2, indicating interaction between these proteins. To confirm the specificity of binding of CypD to Bcl2, we incubated mitochondrial protein extracts with a recombinant MBP-Bax fusion protein produced in our laboratory as described in Ref. 19 and immobilized the extracts on amylose resin. Fig. 1B shows that CypD was not pulled down by MBP-Bax and, therefore, the binding of CypD to Bcl2 is specific. Previous reports indicated that both CypD and Bcl2 can bind the ANT (7, 9, 24). To exclude the possibility that the observed interaction of CypD with Bcl2 is via the ANT, we probed the above immunoprecipitates and pulldowns with an α-ANT antibody. Our assay showed that the ANT was absent from our immunoprecipitates and pulldowns (data not shown), and, therefore, the interaction of CypD with Bcl2 is not via the ANT.
As a third line of evidence for the interaction of CypD with Bcl2, we designed a mammalian two-hybrid system that included a VP-CypD vector expressing CypD fused to an activation domain, a pM-Bcl2 vector expressing Bcl2 fused to a DNA-binding domain (Fig. 1C, DNA-BD), and a SEAP reporter vector as shown in a diagram in Fig. 1C. Co-transfection of VP-CypD with pM-Bcl2 at various ratios led to a significant induction of SEAP activity reaching 10-fold at a 1 to 2 ratio of VP-CypD to pM-Bcl2 when compared with a negative control (Fig. 1D). Transfection of VP-CypD with the empty pM vector or pM-Bcl2 with the empty VP vector did not induce any significant SEAP activity, confirming the specificity of interaction of CypD and Bcl2. Co-transfection of empty pM with VP16 was used as a negative control. Transfection of pM3-VP16 was used as a positive control as suggested by the manufacturer. Therefore, using various protein-protein binding assays including co-immunoprecipitation, pulldown, and the mammalian two-hybrid assay, we confirmed that CypD interacts with the anti-apoptotic mitochondrial protein, Bcl2.
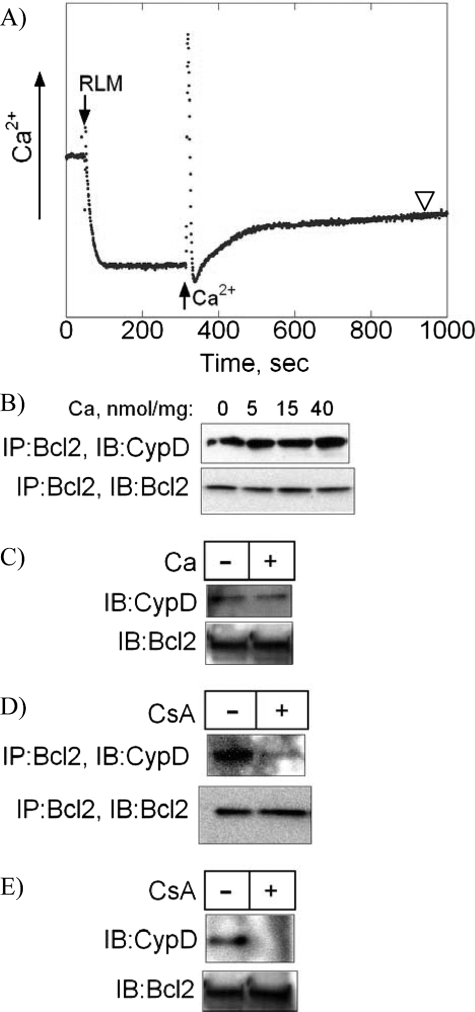
Effect of Calcium and Cyclosporine A on the Interaction of Cyclophilin D with Bcl2—The literature suggests that calcium and CsA counter-regulate the ability of CypD to interact with other mitochondrial proteins such as ANT (7–9). We, therefore, studied the effect of calcium and CsA on binding of CypD to Bcl2. CaCl2 was added to a suspension of isolated RLM at various concentrations, and the uptake of calcium into the mitochondria was monitored by measuring changes in calcium concentration in the medium with a calibrated calcium-sensitive electrode (Fig. 2A). After 10 min following calcium uptake, mitochondria were pelleted and lysed for immunoprecipitation. Fig. 2B shows that preincubation of isolated RLM with increasing concentrations of CaCl2 did not have any significant effect on the subsequent co-immunoprecipitation of CypD with Bcl2. It should be noted that to exclude the possibility of MPT induction by CaCl2, the concentration of CaCl2 used in our experiments did not exceed 40 nmol/mg of mitochondrial protein. As was determined experimentally (data not shown), more than 100 nmol of CaCl2/mg of mitochondrial protein was needed to induce the MPT in our RLM. To further confirm that calcium does not have an effect on binding of CypD to Bcl2, the pulldown of CypD from mitochondrial protein extracts using immobilized MBP-Bcl2 was performed in the absence or presence of CaCl2 at 500 μm. Fig. 2C shows that the pulldown of CypD by MBP-Bcl2 was similar in the absence or presence of CaCl2.
FIGURE 2.
The effect of calcium and cyclosporine A on the interaction of cyclophilin D with Bcl2. A, calcium uptake by isolated mitochondria. RLM at 1 mg/ml were resuspended in KCl-based media, and calcium in the media was monitored with calcium-selective electrode. CaCl2 at 0, 5, 15, or 40 nmol/mg of mitochondrial protein was added where indicated, and after 10 min (arrowhead), RLM were pelleted, lysed, and subjected to immunoprecipitation with an α-Bcl2 antibody. B, the effect of calcium on co-immunoprecipitation (IP) of CypD with Bcl2. Immunoprecipitates were immunoblotted (IB) with an α-CypD antibody. The blots were then reprobed with an α-Bcl2 antibody. C, the effect of calcium on CypD pulldown by MBP-Bcl2. Protein extracts from RLM were incubated with immobilized MBP-Bcl2 in the presence or absence of CaCl2 at 500 μm. The presence of CypD in the pulldowns was detected with immunoblotting using an α-CypD antibody. The blots were then reprobed with an α-Bcl2 antibody. D, the effect of CsA on co-immunoprecipitation of CypD with Bcl2. RLM were incubated with CsA at 1 μm for 10 min, lysed, immunoprecipitated with an α-Bcl2 antibody, and immunoprobed with an α-CypD antibody. The blots were then reprobed with an α-Bcl2 antibody. E, the effect of CsA on CypD pulldown by MBP-Bcl2. Protein extracts from RLM were incubated with immobilized MBP-Bcl2 in the presence or absence of CsA at 1 μm. The presence of CypD in the pulldowns was detected with immunoblotting using an α-CypD antibody. The blots were then reprobed with α-Bcl2 antibody. Blots in B–E are representatives of three blots.
CsA is known to bind CypD and inhibit its interactions with other proteins such as ANT (7). We, therefore, investigated the effect of CsA on the binding of CypD to Bcl2. Fig. 2D shows that in the presence of CsA at 1 μm, co-immunoprecipitation of CypD with Bcl2 was significantly reduced. In addition, CsA prevented CypD pulldown from mitochondrial protein extracts using immobilized MBP-Bcl2 (Fig. 2E). Together, our data indicate that although calcium does not have any significant effect on the interaction of CypD with Bcl2, CsA effectively inhibits this interaction.
Cyclophilin D Potentiates the Limiting Effect of Bcl2 on Cytochrome c Release from Mitochondria—Bcl2 is a major anti-apoptotic mitochondrial protein that functions by limiting the release of CytC from mitochondria during apoptosis (25). We hypothesized that binding of CypD may affect the ability of Bcl2 to inhibit CytC release from mitochondria. To test this hypothesis, we performed a series of experiments using recombinant human tBid, a potent inducer of CytC release from mitochondria, and isolated RLM. As shown in Fig. 3A (left blot), the supernatant from RLM incubated with tBid at 1 μg/mg of mitochondrial protein for 10 min contained significantly more CytC than the supernatant from control RLM as measured with immunoblotting. These data indicate that tBid induced the release of CytC from mitochondria. The CypD inhibitor, CsA, at 1 μm, which we have shown to prevent the CypD-Bcl2 interaction (Fig. 2), further increased the tBid-induced release of CytC from RLM. It should be noted that under these experimental conditions, tBid did not induce the MPT, as was determined by the absence of swelling of mitochondria (data not shown). The presence of calcium at 40 nmol/mg of mitochondrial protein did not significantly change the tBid-induced release of CytC from RLM into the supernatant (Fig. 3A, right blot). These data indicate that inhibition of CypD with CsA increases the tBid-induced release of CytC from mitochondria, which is not mediated via the MPT and not sensitive to calcium.
FIGURE 3.
The effect of cyclophilin D on cytochrome c release from mitochondria. A, the effect of CsA and calcium on tBid-induced release of CytC from RLM. RLM at 1 mg/ml were incubated with tBid at 1 μg/ml for 10 min in the absence or presence of CsA at 1 μm (left blot) or in the absence or presence of CaCl2 at 40 nmol/mg of mitochondrial protein (right blot) and spun down. CytC in the supernatants (SN) was detected with immunoblotting. The blots were then stained with Ponceau S to verify equal loading. The blots shown are representatives of four blots. B, the effect of gain- or loss-of-function of CypD and Bcl2 on CytC release. HEK293T cells, stably transfected with the indicated vectors and plated on 6-well plates, were permeabilized with digitonin and incubated with tBid at 1 μg/ml for 10 min in the absence or presence of CsA at 1 μm. To detect CytC released into supernatants, samples were normalized for total protein and subjected to ELISA assay. Ctrl, control. C, overexpression of Bcl2 or CypD or knockdown (KD) of CypD. Whole cell lysates from cells stably transfected with the indicated vectors were subjected to immunoblotting with an α-Bcl2 (left blot) or α-CypD (middle and right blots) antibody; the blots shown are representatives of three blots. D, knockdown of Bcl2. Total RNA and then cDNA were prepared from cells stably transfected with the pKD-Bcl2 shRNA or control puromycin (Puro) vector. Bcl2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were detected by real-time RT-PCR as described under “Experimental Procedures,” and Bcl2 was normalized to glyceraldehyde-3-phosphate dehydrogenase. Data are means ± S.D. (n = 3). * indicates p < 0.05 when compared with EV controls.
To further evaluate the effect of CypD on CytC release from mitochondria, we performed a series of gain- and loss-of-function experiments in HEK293T cells using the expression vectors for either CypD or Bcl2, constructed as described in detail in our previous works (22, 23), or shRNA vectors. Cells were stably transfected and then permeabilized with digitonin at 0.01%, a concentration sufficient to permeabilize the plasma membrane but not the outer mitochondrial membrane (22), to expose mitochondria to the external medium. The medium were similar to those used in the experiments with isolated mitochondria. These permeabilized cells were incubated with tBid for 10 min in the absence or presence of CsA. The supernatants were then analyzed for the presence of CytC using the CytC ELISA kit. Fig. 3B (column set EV) shows that the level of CytC in the supernatant from the empty vector-transfected cells (EV) incubated with tBid increased 4-fold when compared with untreated controls (Ctrl). CsA further increased the tBid-induced release of CytC from mitochondria in EV cells, leading to an 8-fold increase in the level of CytC in the supernatant when compared with untreated controls. This experiment demonstrates that tBid and CsA have a stimulating effect on CytC release in permeabilized cells similar to that observed in isolated RLM.
To inhibit the above effects, we stably overexpressed Bcl2 in HEK293T cells using the Bcl2 expression vector as confirmed with immunoblotting (Fig. 3C, left blot). Fig. 3B (column set Bcl2) shows that as was expected, Bcl2 overexpression abolished the tBid-induced release of CytC from mitochondria. Incubation of the Bcl2-expressing cells with the CypD inhibitor, CsA, restored the tBid-induced release of CytC from mitochondria and, therefore, reversed the inhibiting effect of Bcl2 on CytC release, suggesting that CypD plays a role in Bcl2-mediated inhibition of CytC release from mitochondria. To further confirm this finding, we knocked down 80% of the CypD in Bcl2-expressing cells using the shRNA technique as validated with immunoblotting shown in Fig. 3C (middle blot). Fig. 3B (column set Bcl2/KD CypD) shows that the specific knockdown of CypD reduced the limiting effect of Bcl2 on the tBid-induced release of CytC from mitochondria, leading to a more than 5-fold increase in CytC level in the supernatant. CsA further increased the CytC release from mitochondria in these cells; however, the effect was only marginal, probably because of the absence of the CsA target, CypD. These data indicate that CypD plays a role in Bcl2-mediated inhibition of CytC release from mitochondria.
To extend our study of the effect of CypD on CytC release, we stably overexpressed CypD in HEK293T cells using the CypD expression vector. As shown in Fig. 3B (column set CypD), overexpression of CypD, confirmed with immunoblotting (Fig. 3C, right blot), significantly reduced the tBid-induced release of CytC from mitochondria when compared with the EV controls. The effect was similar to that of Bcl2 overexpression. CsA again restored the tBid-induced release of CytC from mitochondria. To test whether the inhibitory effect of CypD on CytC release was dependent on Bcl2, we knocked down 80% of Bcl2 in CypD-overexpressing cells using the shRNA vector as validated with real-time RT-PCR (Fig. 3D). Fig. 3B (column set CypD/KD Bcl2) demonstrates that the specific knockdown of Bcl2 abolished the inhibitory effect of CypD on the tBid-induced release of CytC from mitochondria, leading to a 4-fold increase in the CytC level in the supernatant, which is similar to the level found in EV transfectants treated with tBid. CsA further increased the release of CytC from mitochondria in these cells. These results indicate that the effect of CypD on CytC release from mitochondria is dependent on Bcl2. Together, our data show that CypD and Bcl2 cooperate in limiting the release of CytC from mitochondria and that loss-of-function of either of these factors significantly diminishes the ability of the other factor to limit such a release.
Gain- and Loss-of-Function of Cyclophilin D Changes Cell Sensitivity to Apoptosis—The release of CytC from mitochondria is an important step in the “intrinsic,” receptor-independent apoptotic program (26), leading to activation of caspase-9 and then caspase-3. Therefore, the ability of CypD to limit such a release may influence the overall sensitivity of cells to undergo intrinsic apoptosis. To test this, we compared the sensitivity to apoptosis induced with etoposide, a compound known to trigger the CytC-dependent apoptotic pathway (23), in EV-transfected control cells and CypD-overexpressing cells. Cells were incubated with etoposide at 20 μm for 6 h and stained with Hoechst 33342 to detect condensed chromatin as a marker for apoptosis. Fig. 4 shows that on average, 56% of the EV-transfected control cells display apoptotic features after treatment with etoposide. Overexpression of CypD significantly reduced the number of cells undergoing apoptosis after treatment with etoposide to less than 30%. In the presence of CsA at 1 μm, the sensitivity of CypD-overexpressing cells to etoposide-induced apoptosis was restored (Fig. 4). Similar restoration of sensitivity to apoptosis was observed in CypD-overexpressing cells where knockdown of Bcl2 was performed. Therefore, these data indicate that CypD inhibits apoptosis induced with a compound known to trigger the CytC-dependent pathway. This anti-apoptotic effect of CypD can be reversed by CsA and is Bcl2-dependent, consistent with the data on CytC release from mitochondria shown in Fig. 3B.
FIGURE 4.
Overexpression of cyclophilin D inhibits apoptosis in a CsA- and Bcl2-dependent manner. HEK293T cells, stably transfected with the control empty vector or with the CypD expression vector, were treated with etoposide at 20 μm for 6 h in the absence or presence of CsA at 1 μm. A subset of cells was co-transfected with the pKD-Bcl2 shRNA vector to achieve knockdown (KD) of Bcl2 and also treated with etoposide. Apoptotic cells with condensed nuclei were detected using the Hoechst 33342 nuclear stain. Data are means ± S.D. (n = 3). * indicates p < 0.05 when compared with EV controls.
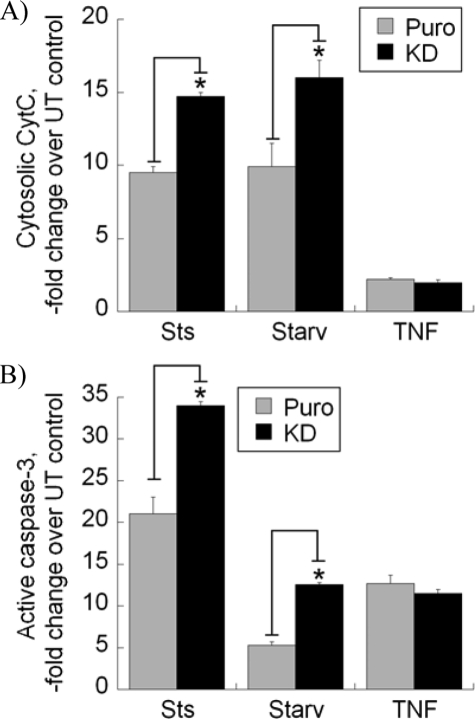
To further study the effect of CypD on cell sensitivity to apoptosis and to distinguish the effects on the intrinsic and “extrinsic” pathways, we induced apoptosis in cells that underwent stable shRNA-mediated knockdown of CypD using the inducers of apoptosis utilizing either the intrinsic pathway, such as staurosporine (Sts) or serum starvation (Fig. 5A, Starv), or the extrinsic, CytC-independent pathway, such as TNF (27). Fig. 5A shows that CypD knockdown significantly increased the cytosolic levels of CytC in cells treated with Sts or serum starvation when compared with EV-transfected controls (labeled Puro (for puromycin)). As was expected, the treatment with TNF did not induce any noticeable release of CytC into the cytosolic fraction, and the knockdown of CypD did not have any effect. As demonstrated in Fig. 5B, CypD knockdown also led to a significantly more pronounced activation of caspase-3 in Sts-treated or serum-starved cells but not in TNF-treated cells when compared with control transfectants. Therefore, loss of CypD function sensitized cells to the Sts- or serum starvation-induced intrinsic apoptosis, leading to the increase in CytC release from mitochondria and in caspase-3 activity. The above gain- and loss-of-function experiments demonstrate that manipulating CypD levels alters cell sensitivity to the intrinsic, CytC-dependent apoptosis but has no effect on TNF-induced extrinsic, CytC-independent cell death.
FIGURE 5.
Knockdown (KD) of cyclophilin D sensitizes cells to apoptosis induced with staurosporine or serum starvation. A, stable empty vector-(Puro (for puromycin)) or CypD shRNA-transfected cells were treated with Sts at 2 μm for 6 h or serum starvation (Starv) for 48 h or TNF at 10 ng/ml for 24 h. Cytosolic fractions or whole cell lysates were prepared and normalized for total protein. Cytosolic fractions were subjected to a CytC ELISA assay (A), and cell lysates were subjected to a caspase-3 activity assay (B), as described under “Experimental Procedures.” Data are means ± S.D. (n = 3). * indicates p < 0.05 when compared with puromycin (Puro) controls. UT, untransfected.
DISCUSSION
In this study, we report a novel interaction of CypD with the major anti-apoptotic protein and regulator of the CytC release from mitochondria, Bcl2. We confirmed our finding using several techniques including co-immunoprecipitation, pulldown, and mammalian two-hybrid assays. We have also found that the interaction of CypD with Bcl2 is important for limiting CytC release from mitochondria and inhibiting CytC-dependent apoptosis. As was confirmed with a gain- and loss-of-function approach, Bcl2 is significantly less effective as an inhibitor of CytC release from mitochondria and of ensuing apoptosis in the absence of CypD. On the other hand, the anti-apoptotic effect of CypD is abolished in the absence of Bcl2 and is, therefore, Bcl2-dependent. Thus, CypD and Bcl2 interact and cooperate in limiting CytC release from mitochondria and in inhibiting CytC-dependent apoptosis.
The CypD-Bcl2 interaction may play a role in cancer cells as an additional mechanism of suppression of apoptosis. It has been previously reported that various cancer cells, including but not limited to breast, ovary, and uterus cancer, overexpress CypD (3). We also found overexpression of CypD in osteosarcoma and leukemia cells.3 The previous work focused on elucidating the mechanism of the anti-apoptotic effect of CypD in cancer cells, pursuing the hypothesis that this effect of CypD is associated with its ability to regulate MPT pore opening (5). The controversy of the MPT-associated hypothesis is due to the fact that CypD is a key positive regulator of the MPT pore and that the MPT has a pronounced pro-death effect in both necrosis and apoptosis. Therefore, in the context of the MPT, CypD is not a pro-survival but a pro-death factor. In this study, we show that the anti-apoptotic effect of CypD is not related to its role as a regulator of the MPT but is due to its interaction with Bcl2. According to our data, CypD and Bcl2 cooperate in inhibiting MPT-independent CytC release from mitochondria. Such MPT-independent CytC release from mitochondria appears to be prevalent during various forms of apoptosis (17), whereas MPT-induced CytC release occurs primarily during necrosis (15). In addition, calcium, which is known to promote CypD binding to the ANT (7, 9), is mandatory for MPT opening (8). However, our data indicate that calcium does not have a significant effect on the CypD-Bcl2 interaction and on the resulting inhibition of CytC release. This is additional evidence that the MPT-independent mechanism is involved with the anti-apoptotic action of CypD. It should be noted that Shimisu et al. (28) have previously reported the possible interaction of the BH4 domain of Bcl2 with the putative component of the MPT pore, voltage-dependent anion channel, leading to inhibition of the MPT. Because interaction with CypD may change Bcl2 conformation, it may, therefore, change its ability to bind voltage-dependent anion channel and inhibit the MPT. This aspect was beyond the scope of our study and requires additional focused investigation.
Because Bcl2 is often considered to reside in the outer mitochondrial membrane and is, therefore, spatially separated from the mitochondrial matrix protein, CypD, we want to specifically address this issue. First, a careful evaluation of the literature leads to the conclusion that the data exist supporting both outer membrane (29–31) and inner membrane (32, 33) localization of Bcl2 in mitochondria. In fact, the most recent of these studies (33) utilized immunoelectron microscopy and the method of immunogold labeling developed by Tokuyasu that yields the best signal-to-noise ratio (34) and showed primarily inner membrane localization of Bcl2. It is, therefore, safest to conclude that Bcl2 can reside in both membranes. Second, Bcl2 has been found in the mitochondrial contact sites (17, 35) where the outer and inner mitochondrial membranes are in close contact and are virtually indistinguishable. In these regions, the membrane-associated domains of Bcl2, such as the C-terminal and the BH1 and BH2 domains (36), may span both membranes and be exposed to the matrix and, therefore, to CypD. Moreover, MPT complexes, including CypD, have also been localized to mitochondrial contact sites (17, 35), which increases the probability of CypD-Bcl2 interaction.
Our data suggest that targeting CypD and disrupting its interaction with Bcl2 may increase the sensitivity of cells to apoptosis. In this regard, it is important to discuss the effect of the CypD inhibitor, CsA. We have found that CsA effectively blocks the CypD-Bcl2 interaction. On a subcellular level, CsA increases CytC release from mitochondria. On a cellular level, CsA overcomes the anti-apoptotic effect of CypD overexpression and sensitizes cells to apoptosis. It remains to be determined whether CsA can be used in cancer treatments as an agent capable of sensitizing cancer cells to apoptosis. The literature suggests that in some cases, CsA has cancer protective effects, but there are also reports indicating the cancer-promoting effects of CsA, as reviewed by Weischer et al. (37). Further studies of this CsA effect in different cancer cells and in animal models of cancer are needed to fully elucidate its anti-tumor potential. In addition, CsA has effects not related to CypD, such as inhibition of calcineurin and of other cyclophilins. Such effects may mask or even reverse the sensitizing effect of CsA related to CypD. Moreover, it has recently been shown (38) that Bcl2 may interact with calcineurin and, therefore, the effect of CsA may be due to disruption of this Bcl2-calcineurin interaction and not the Bcl2-CypD interaction. The development of CsA derivatives, which are more specific for CypD, may help to overcome this issue. The development of new non-CsA derivative small peptides that will block the interaction of CypD with Bcl2 may also be a promising direction.
Overall, our study describes a novel mechanism of suppression of apoptosis via interaction and cooperation between CypD and Bcl2. Our data also provide an explanation for the previously reported phenomenon of overexpression and a pro-survival effect of CypD in various tumors. These findings open new perspectives in apoptosis and cancer research and may lead to development of new therapeutic avenues in the management of cancer. Targeting CypD may be an effective approach in selective sensitization of cancer cells to anti-cancer therapies.
Acknowledgments
We thank Dr. Paul Brookes and Dr. Karlene Gunter for fruitful discussions and the use of equipment.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 ES10041. This work was also supported by grants from the Wilmot Foundation and the Karen D'Amico Foundation.
Footnotes
The abbreviations used are: CypD, cyclophilin D; MPT, mitochondrial permeability transition; CsA, cyclosporine A; ANT, adenine nucleotide translocator; CytC, cytochrome c; ELISA, enzyme-linked immunosorbent assay; MBP, maltose-binding protein; SEAP, secreted alkaline phosphatase; RLM, rat liver mitochondria; shRNA, short hairpin RNA; TNF, tumor necrosis factor; Ac, acetyl; amc, 7-amino-4-methylcoumarin; Sts, staurosporine; EV, empty vector.
R. A. Eliseev, J. Malecki, T. Lester, Y. Zhang, J. Humpfrey, and T. E. Gunter, unpublished data.
References
- 1.Dragovich, T., Rudin, C. M., and Thompson, C. B. (1998) Oncogene 17 3207–3213 [DOI] [PubMed] [Google Scholar]
- 2.Reed, J. C. (1999) J. Clin. Oncol. 17 2941–2953 [DOI] [PubMed] [Google Scholar]
- 3.Schubert, A., and Grimm, S. (2004) Cancer Res. 64 85–93 [DOI] [PubMed] [Google Scholar]
- 4.Li, Y., Johnson, N., Capano, M., Edwards, M., and Crompton, M. (2004) Biochem. J. 383 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machida, K., Ohta, Y., and Osada, H. (2006) J. Biol. Chem. 281 14314–14320 [DOI] [PubMed] [Google Scholar]
- 6.Galat, A., and Metcalfe, S. M. (1995) Prog. Biophys. Mol. Biol. 63 67–118 [DOI] [PubMed] [Google Scholar]
- 7.Halestrap, A. P., Kerr, P. M., Javadov, S., and Woodfield, K. Y. (1998) Biochim. Biophys. Acta 1366 79–94 [DOI] [PubMed] [Google Scholar]
- 8.Gunter, T. E., and Pfeiffer, D. R. (1990) Am. J. Physiol. 258 C755–C786 [DOI] [PubMed] [Google Scholar]
- 9.Bernardi, P. (1999) Physiol. Rev. 79 1127–1155 [DOI] [PubMed] [Google Scholar]
- 10.Haworth, R. A., and Hunter, D. R. (1979) Arch. Biochem. Biophys. 195 460–467 [DOI] [PubMed] [Google Scholar]
- 11.Crompton, M., Ellinger, H., and Costi (1988) Biochem. J. 255 357–360 [PMC free article] [PubMed] [Google Scholar]
- 12.Kokoszka, J. E., Waymire, K. G., Levy, S. E., Sligh, J. E., Cai, J., Jones, D. P., MacGregor, G. R., and Wallace, D. C. (2004) Nature 427 461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baines, C. P., Kaiser, R. A., Purcell, N. H., Blair, N. S., Osinska, H., Hambleton, M. A., Brunskill, E. W., Sayen, M. R., Gottlieb, R. A., Dorn, G. W., II, Robbins, J., and Molkentin, J. D. (2005) Nature 434 658–662 [DOI] [PubMed] [Google Scholar]
- 14.Basso, E., Fante, L., Fowlkes, J., Petronilli, V., Forte, M., and Bernardi, P. (2005) J. Biol. Chem. 280 18558–18561 [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa, T., Shimizu, S., Watanabe, T., Yamaguchi, O., Otsu, K., Yamagata, H., Inohara, H., Kubo, T., and Tsujimoto, Y. (2005) Nature 434 652–658 [DOI] [PubMed] [Google Scholar]
- 16.Waldmeier, P. C., Zimmermann, K., Qian, T., Tintelnot-Blomley, M., and Lemasters, J. J. (2003) Curr. Med. Chem. 10 1485–1506 [DOI] [PubMed] [Google Scholar]
- 17.Forte, M., and Bernardi, P. (2006) Cell Death Differ. 13 1287–1290 [DOI] [PubMed] [Google Scholar]
- 18.Shirane M., and Nakayama K. I. (2003) Nat. Cell Biol. 5 28–37 [DOI] [PubMed] [Google Scholar]
- 19.Eliseev, R. A., Alexandrov, A., and Gunter, T. (2004) Protein Expression Purif. 35 206–209 [DOI] [PubMed] [Google Scholar]
- 20.Wingrove, D. E., and Gunter, T. E. (1986) J. Biol. Chem. 261 15159–15165 [PubMed] [Google Scholar]
- 21.Gunter, T. E., Miller, L. M., Gavin, C. E., Eliseev, R., Salter, J., Buntinas, L., Alexandrov, A., Hammond, S., and Gunter, K. K. (2004) J. Neurochem. 88 266–280 [DOI] [PubMed] [Google Scholar]
- 22.Eliseev, R. A., Filippov, G., Velos, J., VanWinkle, B., Goldman, A., Rosier, R. N., and Gunter, T. E. (2007) Neurobiol. Aging 28 1532–1542 [DOI] [PubMed] [Google Scholar]
- 23.Eliseev, R. A., Gunter, K. K., and Gunter, T. E. (2002) Mitochondrion (Kidlington) 1 361–370 [DOI] [PubMed] [Google Scholar]
- 24.Brenner, C., Cadiou, H., Vieira, H. L., Zamzami, N., Marzo, I., Xie, Z., Leber, B., Andrews, D., Duclohier, H., Reed, J. C., Kroemer, G. (2000) Oncogene 19 329–336 [DOI] [PubMed] [Google Scholar]
- 25.Adams, J. M., and Cory, S. (1998) Science 281 1322–1326 [DOI] [PubMed] [Google Scholar]
- 26.Kroemer, G., and Reed, J. C. (2000) Nat. Med. 6 513–519 [DOI] [PubMed] [Google Scholar]
- 27.Salvesen, G. S., and Dixit, V. M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 10964–10967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimisu, S., Konishi, A., Kodama, T., and Tsujimoto, Y. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 30100–30105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monaghan, P., Robertson, D., Amos, A. S., Dyer, M. S., Mason, D. Y., and Greaves, M. F. (1992) J. Histochem. Cytochem. 40 1819–1825 [DOI] [PubMed] [Google Scholar]
- 30.Akao, Y., Otsuki, Y., Kataoka, S., Ito, Y., and Tujimoto, Y. (1994) Cancer Res. 54 2468–2471 [PubMed] [Google Scholar]
- 31.Jong, D. D., Prins, F. A., Mason, D. Y., Reed, J. C., Ommen, G. B., and Kluin, P. M. (1994) Cancer Res. 54 256–260 [PubMed] [Google Scholar]
- 32.Hockenbery, D., Nunez, G., Milliman, C. L., Schreiber, R. D., and Korsmeyer, S. J. (1990) Nature 348 334–336 [DOI] [PubMed] [Google Scholar]
- 33.Motoyama, S., Kitamura, M., Saito, S., Minamiya, Y., Suzuki, H., Saito, R., Terada, K., Ogawa, J., and Inaba, H. (1998) Biochem. Biophys. Res. Commun. 49 628–636 [DOI] [PubMed] [Google Scholar]
- 34.Tokuyasu, K. T. (1989) Histochem. J. 21 163–171 [DOI] [PubMed] [Google Scholar]
- 35.Brdiczka, D. G., Zorov, D. B., and Sheu, S. S. (2006) Biochim. Biophys. Acta 1762 148–163 [DOI] [PubMed] [Google Scholar]
- 36.Peng, J., Tan, C., Roberts, J., Nikolaeva, O., Zhang, Z., Lapolla, S. M., Primorac, S., and Lin, J. (2006) J. Biol. Chem. 281 35802–35811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weischer, M., Rocken, M., and Berneburg, M. (2007) Exp. Dermatol. 16 385–393 [DOI] [PubMed] [Google Scholar]
- 38.Shibasaki, F., Kondo, E., Akagi, T., and McKeon, F. (1997) Nature 386 728–731 [DOI] [PubMed] [Google Scholar]