Abstract
GAT-1 is a sodium- and chloride-coupled γ-aminobutyric acid (GABA) transporter, which fulfills an essential role in the synaptic transmission by this neurotransmitter. Cysteine-399 is the major site of inhibition of GAT-1 by membrane-permeant sulfhydryl reagents. This cysteine residue was previously thought to reside on a cytoplasmic loop connecting transmembrane domains (TMs) 8 and 9. However, the crystal structure of LeuT, a bacterial homologue of the mammalian neurotransmitter:sodium symporters, revealed that the residue corresponding to Cys-399 is in fact located in the middle of TM 8. This residue is located to the cytoplasmic side of Asp-395 and Ser-396, whose side chains are thought to ligand one of the two cotransported sodium ions. To determine how the sulfhydryl reagents approach cysteine-399, a cysteine scan of all 35 residues of TM 8 was performed. Sulfhydryl reagents inhibited transport when a cysteine residue was present at either of the positions 399, 402, 406, and 410. SKF-89976A and other non-transportable analogues, which are expected to lock the transporter in a conformation facing the extracellular medium, protected against the sulfhydryl modification at positions 399, 402, and 406. Such a protection was not seen by GABA itself, which actually modestly potentiated the modification at positions 399 and 402. Our results point to an α-helical stripe on TM8 lining an aqueous access pathway from the cytoplasm into the binding pocket, which gets occluded in the conformation of the transporter where the binding pocket is exposed to the extracellular medium.
The sodium- and chloride-coupled GABA2 transporter GAT-1 (1, 2) plays a central role in the termination of the synaptic actions of this neurotransmitter. GAT-1 belongs to the NSS family of transporters (also called SLC6). Other members of the family include the transporters for serotonin, dopamine, glycine, and norepinephrine (for reviews, see Refs. 3 and 4). GAT-1 catalyzes the electrogenic cotransport of sodium, chloride, and GABA with a stoichiometry of 2:1:1, respectively (5–8). Although the precise order of events during a transport cycle is not yet established, it is clear that the uptake process is initiated by the binding of at least one sodium ion (6, 9, 10). In accordance with the principle of alternating access during transport (11), the cycle is expected to consist of transitions between outward and inward facing conformations. Moreover, by analogy with the high resolution crystal structures of the bacterial homologue LeuT (12) and those of transporters from other families, which are nevertheless structurally related (13, 14), conformations with the binding pocket occluded from both sides of the membrane are also likely to be involved in the cycle.
The LeuT structure appears to be an excellent model for the NSS neurotransmitter transporters (15–18). LeuT consists of 12 TMs with TMs 1–5 related to TMs 6–10 by a pseudo-two-fold axis in the membrane plane. TMs 1 and 6 have breaks in their helical structure approximately halfway across the membrane. These breaks expose main-chain carbonyl oxygen and nitrogen atoms for the binding of leucine and the two sodium ions, Na1 and Na2. The sodium ions in the binding pocket are both close to the substrate, which is in direct contact, through its carboxyl group, with Na1 (12). In contrast to NSS neurotransmitter transporters, LeuT and other bacterial homologues do not require chloride for transport (12, 19, 20), and indeed, no chloride was observed in the binding pocket of LeuT (12). Recently, the chloride binding site of the NSS neurotransmitter transporters was identified. This site is located near Na1, and it appears that the role of chloride is basically to compensate for the multiple positive charges provided by the sodium ions during the neurotransmitter translocation step (21, 22).
Wild-type GAT-1 transport activity is inhibited by the membrane-permeant sulfhydryl reagents MTSEA and NEM, which react with the thiolate anion. Previously, single substitutions of all endogenous cysteines revealed that Cys-399 is the major determinant of the sensitivity of GAT-1 to these two sulfhydryl reagents (23). According to the topology based on hydropathy plots of the sequence of the cloned transporter, Cys-399 was thought to be located on a cytoplasmic loop connecting TM 8 and TM 9 (2). However, the LeuT structure revealed that the residue corresponding to Cys-399 is located in the middle of the membrane close to the Na2 binding site (12). The approximate location of Cys-399 is indicated in the schematic topology (Fig. 1), which is based on the LeuT structure (12). Therefore, it is unclear whether the aqueous accessibility of Cys-399 to sulfhydryl reagents is from the extracellular or intracellular side of GAT-1. Because the transporter assumes outward and inward facing conformations during the transport cycle, the accessibility of Cys-399 should be influenced by transporter ligands predicted to favor either of these two conformations. Therefore, a cysteine scan of all 35 residues of TM 8 was performed. This was done in a construct where not only Cys-399 was eliminated but also the conserved Cys-74 located in the extracellular part of TM 1 (Fig. 1). This latter residue is the target for inhibition of transport by the impermeant sulfhydryl reagent (2-trimethylammonium) methanethiosulfonate (24, 25). We have tested the ability of NEM and MTSEA to affect [3H]GABA transport by the cysteine mutants under conditions favoring outward and inward facing conformations of the transporter. Our results are consistent with the participation of the intracellular part of TM 8 in an aqueous pathway leading from the binding pocket into the cytoplasm.
FIGURE 1.
Schematic NSS topology. This topology is adapted from the LeuT structure (12) such that it emphasizes the central position of the bundle consisting of TMs 1, 2, 6, and 7, proposed to play a central role in the transition between the outward and inward facing forms of the NSS transporters (32), and incorporates the feature that the two repeat units TMs 1–5 and TMs 6–10 are completely intertwined in the three-dimensional structure. Only the transmembrane domains are shown and numbered. The approximate positions of Cys-74 and Cys-399 in TMs 1 and 8 of GAT-1, respectively, are indicated by asterisks. The transmembrane domains are connected by extra- and intracellular loops, shown as thin lines. The membrane is marked by two thicker lines, with the extracellular (out) and intracellular side (in) indicated. The binding pocket, containing the binding sites for GABA, sodium, and chloride, is depicted as an ellipsoid. Amino and carboxyl termini are marked as N and C, respectively. TMs 3 and 8 pack onto each other in three-dimensional space.
EXPERIMENTAL PROCEDURES
Generation and Subcloning of Mutants—Mutations were made by site-directed mutagenesis of GAT-1, harboring the mutations C74A and C399A in the vector pBluescript SK– (Stratagene) using single-stranded uracil-containing DNA as described previously (26, 27). Briefly, the GAT-1-C74A/C399A containing plasmid was used to transform Escherichia coli CJ236 (dut-, ung-). From one of the transformants, single-stranded uracil-containing DNA was isolated upon growth in uridine-containing medium according to the standard protocol from Stratagene, using helper phage R408. This yields the sense strand, and consequently, mutagenic primers were designed to be antisense. The mutants were subcloned into GAT-1-C74A/C399A in the vector pBluescript SK–, using the unique restriction enzymes NheI and AgeI, and sequenced between these two restriction sites.
Cell Growth and Expression—HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 200 units/ml penicillin, 200 μg/ml streptomycin, and 2 mm glutamine. Infection with recombinant vaccinia/T7 virus vTF7-3 (28) and subsequent transfection with plasmid DNA, as well as GABA transport, were done as published previously (29). The expression vector was pBluescript SK–.
Inhibition Studies with Sulfhydryl Reagents—Prior to the transport assay, cells adhering to 24-well plates were washed twice with 1 ml of the transport medium containing 150 mm choline chloride instead of NaCl, except when the effect of chloride on reactivity was examined, in which case 150 mm sodium gluconate replaced NaCl. Each well was then incubated at room temperature with 200 μl of the preincubation medium (the different compositions and reagent concentrations are indicated in the figure legends) with the indicated concentrations of NEM (Sigma-Aldrich) or MTSEA (Anatrace). After 5 min, the medium was aspirated, and the cells were washed twice with the same solution without sulfhydryl reagents followed by [3H]GABA transport. The concentration of the sulfhydryl reagents chosen in the different experiments was optimized according to the experimental conditions of the mutants used. For instance, in Fig. 5, different concentrations were used for different mutants because some are more sensitive than others. Statistical evaluation of the inhibition of the different mutants by NEM or MTSEA utilized a one-way analysis of variance with a post-hoc Dunnett's multiple comparison test, where p < 0.05 was taken as significant. Results were plotted using normalized data for each mutant, where the untreated activity levels are normalized to 100%.
FIGURE 5.
Effect of the composition of the external medium on the inhibition of Cys-399, T406C and D410C by NEM. The three indicated cysteine mutants were transiently expressed in HeLa cells. Cells were preincubated 5 min with or without NEM in a 150 mm sodium chloride (NaCl)-, choline chloride (ChCl)-, or sodium gluconate (NaGluc)-containing solution. SKF-89976A (30 μm)(SKF) and GABA (1 mm) were added as indicated. After washing, [3H] GABA uptake activity was measured. Results represent the mean ± S.E. (error bars) of at least three experiments performed at least in triplicates and are given as a percentage of the uptake activity in samples preincubated in the same medium but without NEM. The concentrations of NEM used were 1, 0.3, and 0.25 mm for Cys-399, T406C, and D410C, respectively.
RESULTS
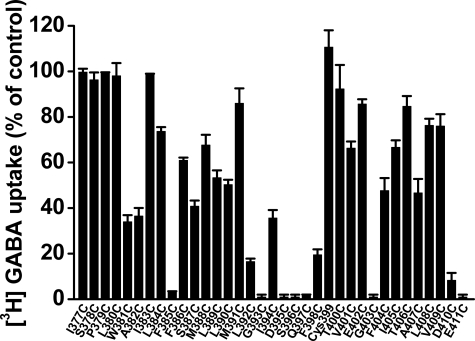
Cysteine-scanning Mutagenesis and Impact of NEM—GAT-1 residues 377–411 correspond to TM 8 as defined by the LeuT structure (12). Cysteines were introduced one at a time into each of these positions of GAT-1-C74A/C399A, a construct in which these two endogenous cysteines were replaced by alanine. In 5 out of the 35 cysteine mutants, no measurable [3H]GABA transport could be detected (Fig. 2). These included the mutants at positions 395 and 396, which correspond to LeuT positions, which coordinate Na2 (12), and were previously shown by us to be inactive in transport (15). The three other inactive mutants were G393C, G403C, and E411C. Moreover, two additional mutants, F385C and Q397C, had measurable yet very low activity, namely 3.1 ± 0.4 and 1.9 ± 0.2% of that of C74A/C399A, respectively (n = 3) (Fig. 2). The activity of the cysteine replacement mutant of the conserved Asp-410, which in the LeuT structure forms a charged pair with a conserved arginine at the cytoplasmic entrance of the transporter (12), was 8.3 ± 3.2% of C74A/C399A, which was sufficiently high to quantitatively study the effect of sulfhydryl reagents on transport activity. The activity of the other 27 mutants ranged from 16.5 to 100% of C74A/C399A (Fig. 2).
FIGURE 2.
Transport activity of TM8 cysteine mutants. GAT-1-C74A/C399A (control) and cysteine mutants of TM8 in the C74A/C399A background were transiently expressed in HeLa cells, and sodium-dependent [3H]GABA transport was measured at room temperature for 10 min, as described under “Experimental Procedures.” The data are given as the percentage of the activity of C74A/C399A and are the mean ± S.E. (error bars) of at least three separate experiments performed in triplicate.
Besides the mutant in which the cysteine at position 399 was restored (Cys-399; this construct bears the C74A mutation only), inhibition by the sulfhydryl reagent NEM (0.5 mm) was also observed with T406C and D410C (Fig. 3). Two other mutants, L392C and F398C, were also modified by NEM. However, instead of inhibition, the sulfhydryl reagent caused a stimulation of transport activity of these mutants (Fig. 3). When alanine instead of cysteine residues were introduced at positions 392, 398, 399, and 406, no effect of NEM on transport activity was observed (Fig. 4). This argues against the idea that the mutations caused the exposure of endogenous, previously inaccessible, cysteine residues. Rather, it appears that the introduced cysteines themselves reacted with the sulfhydryl reagent. In the case of position 410, it was impossible to assess the (lack of) effect of NEM on the alanine and serine mutants because these mutants were devoid of measurable transport activity (data not shown).
FIGURE 3.
Effect of NEM on Transport activity of TM 8 cysteine mutants. HeLa cells transiently expressing each of the indicated TM8 cysteine-mutants were preincubated for 5 min with transport solution containing 150 mm NaCl, with or without 0.5 mm NEM, as described under “Experimental Procedures” followed by washing and [3H]GABA transport. Results for each mutant are expressed as a percentage of its untreated control and represent the mean ± S.E. (error bars) of at least three experiments performed in triplicate or in quadruplicate. The means of the mutants were compared with those of C74A/C399A using a one-way analysis of variance with a post-hoc Dunnett's multiple comparison test (***, p < 0.001).
FIGURE 4.
Effect of sulfhydryl reagents on TM 8 alanine and cysteine mutants. The indicated cysteine (filled bars) and alanine (open bars) mutants were transiently expressed in HeLa cells, and the effect of preincubation with 0.5 mm NEM or of 0.75 mm MTSEA in the case of E402C on transport activity was determined as described in the legend for Fig. 3. Error bars indicate mean ± S.E.
Effect of GABA and SKF-89976A on Reactivity toward NEM—Positions 399, 406, and 410 are located on the intracellular part of TM8, and this suggests that the membrane-permeant NEM modifies Cys-399, T406C, and D410C from the cytoplasm. The non-transportable substrate analogue SKF-89976A is expected to “lock” GAT-1 in an outward facing conformation, thereby decreasing the aqueous accessibility from the intracellular side. Indeed, the inhibition of Cys-399 and T406C by NEM was significantly diminished in the presence of the non-transportable substrate analogue SKF-89976A (p < 0.001) (Fig. 5, A and B). A similar protection was observed with the non-transportable substrate analogue SKF-100330A, and the same was true for the analogue tiagabine, which was tested with T406C (data not shown). With Cys-399, this protection was dependent on the simultaneous presence of the co-substrates sodium and chloride (Fig. 5A). On the other hand, with T406C, a slight protection by SKF-89976A was seen even in the absence of chloride (replacement by gluconate) (p < 0.05) but not in the absence of sodium (replacement by choline) (Fig. 5B). The removal of chloride did not have much of an effect on the degree of inhibition by NEM (Fig. 5, A and B), but in the absence of sodium, a small potentiation of the inhibition was observed in Cys-399 (p < 0.05) (Fig. 5A). In contrast to SKF-89976A, GABA itself will be transported into the cell in the presence of external sodium and chloride, thereby increasing the proportion of inward facing transporters. In the presence of GABA, a small but significant potentiation of the inhibition by NEM was observed with Cys-399 (p < 0.05) (Fig. 5A), and this potentiation was only seen in the simultaneous presence of sodium and chloride (data not shown). On the other hand, GABA did not have a significant effect on the inhibition of T406C by NEM (Fig. 5B). In contrast to Cys-399 and T406C, none of the ligands had a significant effect on the inhibition of transport by NEM in D410C (Fig. 5C) or on its stimulation of transport activity in L392C (Fig. 6A) or F398C (Fig. 6B).
FIGURE 6.
Effect of the composition of the external medium on the inhibition of L392C and F398C. HeLa cells expressing mutants L392C (A) and F398C (B) were preincubated 5 min with NEM in media with the indicated compositions and processed as described in the legend for Fig. 5. Results represent the mean ± S.E. (error bars) of at least three experiments performed at least in triplicates and are given as a percentage of the uptake activity in samples that were not treated with NEM. The NEM concentration during preincubation was 0.5 mm. SKF, SKF-89976A; NaCl, sodium chloride; ChCl, choline chloride; NaGluc, sodium gluconate.
Impact of MTSEA—The pattern of reactivity of cysteine residues engineered into TM 8 to NEM suggests the presence of an α-helical stripe in contact with an aqueous access pathway. However, in this scenario, one would also expect reactivity of cysteine residues engineered at positions 402 and/or 403. The reactivity of G403C could not be tested because the mutant is devoid of transport activity (Fig. 2). E402C, which has transport activity similar to that of the C74A/C399A control (Fig. 2), was not inhibited by NEM (Fig. 3), but the activity of this mutant could be inhibited by 0.75 mm MTSEA (Fig. 7A). This was not the case for mutants with cysteine residues introduced at surrounding TM 8 positions (Fig. 7A). No significant inhibition by the same concentration of MTSEA was observed with E402A (Fig. 4), suggesting that the inhibition of E402C was due to the modification of the cysteine residue introduced at this position and not to a secondary effect caused by the mutation of the glutamate residue to a small neutral residue. Remarkably, the activity of T406C, although sensitive to NEM (Fig. 3), was not inhibited by MTSEA (Fig. 7A). Preincubation of T406C with MTSEA first followed by a second preincubation with NEM did not result in protection against inhibition by the latter (data not shown). This indicates that the lack of inhibition of transport of T406C by MTSEA is due to the inability of this reagent to modify the cysteine introduced at position 406. When this protocol was applied to cells expressing C74A/C399A, no inhibition of transport was observed, but when the first preincubation was with NEM and the second was with MTSEA, an inhibition of 50% or more was observed (data not shown). Therefore, we could not address the question whether the lack of inhibition of the activity of E402C by NEM was due to the inability of this sulfhydryl reagent to modify the introduced cysteine or that modification in fact took place yet was without functional consequences. MTSEA had only a minor effect on transport activity of a cluster of cysteine mutants from the extracellular half of TM 8; none of the mutants F386C, S387C, M388C, and L389C was inhibited by more than 20% (Fig. 7B).
FIGURE 7.
Effect of MTSEA on transport activity of TM8 cysteine mutants. HeLa cells transiently expressing cysteine replacement mutants at positions 400–409 (A) and 386–389 (B) were preincubated for 5 min with a 150 mm sodium chloride containing solution, with or without 0.75 mm MTSEA. Results for each mutant are expressed as a percentage of its untreated control and represent the mean ± S.E. (error bars) of at least three experiments performed at least in triplicates. The means of the mutants were compared with C74A/C399A using a one-way analysis of variance with a post-hoc Dunnett's multiple comparison test (***, p < 0.001; **, p < 0.01).
The degree of inhibition of E402C by MTSEA was dependent on the composition of the external medium (Fig. 8A) and was reminiscent of the characteristics of the inhibition of Cys-399 and T406C by NEM (Fig. 5, A and B). The non-transportable substrate analogue SKF-89976A also protected against the inhibition of E402C by MTSEA (p < 0.001) (Fig. 8A), and the same was true for SKF-100330A (data not shown). The protection of E402C by SKF-89976A was dependent on the simultaneous presence of sodium and chloride in the preincubation medium (Fig. 8A). The removal of sodium, but not that of chloride, resulted in an increased inhibition of the activity of E402C by MTSEA (p < 0.001) (Fig. 8A). As with Cys-399 (Fig. 5A), GABA caused a small but significant potentiation of the inhibition by MTSEA (p < 0.01) (Fig. 8A), and this effect was only observed in the presence of both sodium and chloride (data not shown).
FIGURE 8.
Effect of the composition of the external medium on the inhibition of E402C and S387C. HeLa cells expressing mutants E402C (A) and S387C (B) were preincubated 5 min with 0.75 mm MTSEA in media with the indicated compositions and processed as described in the legend for Fig. 5. Results represent the mean ± S.E. (error bars) of at least three experiments performed at least in triplicates and are given as a percentage of the uptake activity in samples that were not treated with MTSEA. SKF, SKF-89976A; NaCl, sodium chloride; ChCl, choline chloride; NaGluc, sodium gluconate.
In contrast to E402C, the slight sensitivity of S387C to MTSEA (p < 0.01) was not significantly influenced by the composition of the preincubation medium (Fig. 8B), and the same was true for NEM. In none of the mutants F386C, M388C, and L389C was a measurable inhibition by either MTSEA or NEM observed under any of the preincubation conditions described here (data not shown).
DISCUSSION
The results described in this study show that four cysteine residues introduced in positions 399, 402, 406, and 410, located on the intracellular half of TM8, react with membrane-permeant sulfhydryl reagents (Figs. 3 and 7A). This inhibition is apparently due to conformational restrictions, imposed by adding bulk and charge to the introduced cysteine residues. The sulfhydryl reagents employed here react with the thiolate anion, and therefore, their reactivity appears to reflect accessibility of the engineered cysteine residues from the aqueous medium. The reactivity of every third or fourth residue is consistent with the periodicity of an α-helix and suggests that only positions located on one face of the α-helix are accessible to the aqueous medium. This result is in harmony with information from the LeuT structure, where the secondary structure of all 12 TMs is α-helical (12). The reactivity of T406C with NEM (Fig. 3) but not with MTSEA (Fig. 7A) indicates that the accessibility at this position is also governed by geometric constraints, and the same may be true for position 402 (Figs. 3 and 7A). In accordance to our earlier observations (15), the cysteine mutants at positions 395 and 396, located one more turn of the α-helix in the extracellular direction, were devoid of [3H]GABA transport (Fig. 2), prohibiting the determination of the accessibility of sulfhydryl reagents to these positions. In LeuT, the positions corresponding to 395 and 396 of GAT-1 directly ligand Na2, one of the cotransported sodium ions (12). Indeed, in GAT-1 these two residues also appear to fulfill the same role (15), and in contrast to Na1, this site behaves as a low affinity sodium binding site where lithium can replace sodium (15). Therefore, our cysteine-scanning study indicates that the intracellular half of TM8 lines an aqueous accessibility pathway within the transporter, which leads from the cytoplasm to the binding pocket. Such a pathway is not seen in the LeuT structure, but this structure reflects an outward facing conformation of the transporter with the external gate closed (12). However, in accordance to the alternating access mechanism of transport proposed over 40 years ago (11), such a pathway should exist. Indeed, the non-transportable analogue SKF-89976A, expected to lock the transporter in an outward facing conformation, protects against the sulfhydryl modification (Figs. 5, A and B, and 8A), except for D410C (Fig. 5C). Position 410 is located at the extreme cytoplasmic end of TM 8 and is apparently exposed to the cytoplasm in all of the conformations, which GAT-1 assumes during transport.
In contrast to the effect of non-transportable analogues, GABA itself causes a mild potentiation of the inhibition of transport by the sulfhydryl reagents when the cysteine residues are (re)introduced at positions 399 (Fig. 5A) and 402 (Fig. 8A). Opposite effects on sulfhydryl reactivity by substrates and substrate analogues have been observed previously in other NSS transporters. When the cysteine residues were located in the proposed extracellular access pathway, reactivity was faster in the presence of a non-transportable substrate analogue and slower in the presence of a transportable substrate in the norepinephrine transporter NET (30). Conversely, several residues in the intracellular part of TM 5 of SERT reacted faster in the presence of substrate and slower in the presence of a non-transportable analogue and were proposed to line the intracellular pathway leading to the binding pocket (16). In GAT-1, GABA reduced the reactivity of cysteines located in the extracellular access pathway (25, 31). However, in several cases, the non-transportable analogue SKF100330A did not potentiate but protected (31), presumably by a physical blockade of the sulfhydryl reagent by the bulky blocker. Altogether, the present work and the previous studies suggest a concerted conformational change involving opposite accessibility changes in external and internal pathways.
Our results are in excellent agreement with a very recently published study on the serotonin transporter SERT (32). GAT-1 residues Cys-399, Glu-402, Thr-406, and Asp-410 correspond to Ala-441, Glu-444, Thr-448, and Asp-452 of SERT. Cysteine residues introduced at these positions of SERT reacted fastest with sulfhydryl reagents in membrane preparations (32), where both cytoplasmic and extracellular faces are accessible to ligands and MTS reagents (16). Moreover, this accessibility in SERT was also increased/decreased under conditions favoring the inward/outward facing conformation of the transporter, respectively (32). The excellent agreement with our study, where transport in intact cells was used, demonstrates that although a fraction of the applied sulfhydryl reagents may be destroyed by the internal reducing milieu of the cells, sufficient amounts are capable of reacting with the cysteine residues introduced at the cytoplasmic side of the transporter.
With two mutants, L392C and F398C, treatment with NEM led to stimulation rather than inhibition of activity (Fig. 3). It should be noted that in both these cases, the introduction of a cysteine led to a marked loss in activity (Fig. 2). This suggests that the adducts of the cysteine residues formed upon reaction with NEM are more conducive to executing the conformational changes required for transport than the free cysteines at these positions. The reactivity at both positions was not conformationally sensitive (Fig. 6), presumably because they are so close to the binding pocket that they are accessible from both sides of the membrane.
The cysteine residues introduced at positions 399 and 402 are less accessible in the presence of sodium than in its absence (Figs. 5A and 8A). It is likely that in the absence of sodium, the transporters are predominantly inward facing and that binding of sodium to the few outward facing transporters results in a shift in the equilibrium between the two conformations so as to cause a modest increase in the proportion of outward facing transporters. Indeed, the reactivity in the absence of sodium is similar to that in the presence of sodium and GABA (Figs. 5A and 8A), a condition expected to increase the proportion of inward facing transporters. The relatively limited protection by sodium could be due to an incomplete binding to all transporters at physiological concentrations because of a relatively low apparent affinity of GAT-1 for this cation (6, 9). On the other hand, it is also possible that the conformation of the transporter in the presence of sodium is distinct from that of the outward and inward facing forms. In this conformation, where the sodium could be in an occluded state (4), positions 399 and 402 could become somewhat accessible from the intracellular side. In such a conformation, the more intracellular position 406 could become even more exposed, and this could explain the more pronounced protection against sulfhydryl modification by SKF-89976A of T406C as well as the lack of the potentiation by GABA (Fig. 5B). The easiest way to explain the observations that the protection by sodium is dependent on the simultaneous presence of chloride (Figs. 5A and 8A) is the well known fact that chloride causes an increase of the apparent affinity of GAT-1 for sodium (6, 9). Except for T406C (Fig. 5B), the protective effect of the non-transportable analogue SKF-89976A is not only dependent on sodium but also on chloride (Figs. 5A and 8A). Apparently, sodium and chloride are required together for the binding of the blocker. It is possible that the apparent affinity of T406C for sodium is actually higher than in Cys-399 and E402C so that the blocker can bind already to some extent to T406C, even in the absence of chloride (Fig. 5B). Moreover, a small protective effect in the absence of chloride would be easier to detect with T406C, where the protection by the non-transportable substrate analogue is more pronounced than with Cys-399 and E402C (Figs. 5, A and B, and 8A).
The transition between the outward and inward facing forms of the NSS transporters was very recently proposed by Forrest et al. (32) to be due to a rocking motion of a bundle consisting of TMs 1, 2, 6, and 7 of the substrate-bound form of the transporter. This model of alternating access correctly predicted that the intracellular part of TM8 participated in the intracellular access pathway (32). Independent support for this role of TM8 comes from a structural study of a transporter from a different family, which nevertheless has the same fold as the NSS family (13). A different scenario leading to the substrate-induced conformational transition between outward and inward facing forms, namely the movement of TMs 3 and 8, has very recently been proposed by a different group (14). Whatever type of movement triggers this transition, the conformationally sensitive accessibility of engineered cysteine residues reported in this study indicates that in GAT-1, TM 8 also lines an aqueous access pathway from the cytoplasm to the binding pocket when the transporter becomes inward facing. Besides their importance in identification of the access pathways, accessibility studies of the type used here may also be used to experimentally probe novel transport mechanisms (33).
Acknowledgments
We thank Elia Zomot for helping with the preparation of the figures.
This work was supported by the Israel Science Foundation and the European Union Consortium European Drug Initiative on Channels and Transporters.
Footnotes
The abbreviations used are: GABA, γ-aminobutyric acid; TM, transmembrane domain; NSS, neurotransmitter:sodium symporter; MTSEA, 2-aminoethyl methanethiosulfonate; NEM, N-ethylmaleimide.
References
- 1.Radian, R., Bendahan, A., and Kanner, B. I. (1986) J. Biol. Chem. 261 15437–15441 [PubMed] [Google Scholar]
- 2.Guastella, J., Nelson, N., Nelson, H., Czyzyk, L., Keynan, S., Miedel, M. C., Davidson, N., Lester, H. A., and Kanner, B. I. (1990) Science 249 1303–1306 [DOI] [PubMed] [Google Scholar]
- 3.Nelson, (1998) J. Neurochem. 71 1785–1803 [DOI] [PubMed] [Google Scholar]
- 4.Kanner, B. I., and Zomot, E. (2008) Chem. Rev. 108 1654–1668 [DOI] [PubMed] [Google Scholar]
- 5.Lu, C. C., and Hilgemann, D. W. (1999) J. Gen. Physiol. 114 429–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mager, S., Naeve, J., Quick, M., Labarca, C., Davidson, N., and Lester, H. A. (1993) Neuron 10 177–188 [DOI] [PubMed] [Google Scholar]
- 7.Keynan, S., and Kanner, B. I. (1988) Biochemistry 27 12–17 [DOI] [PubMed] [Google Scholar]
- 8.Kavanaugh, M. P., Arriza, J. L., North, R. A., and Amara, S. G. (1992) J. Biol. Chem. 267 22007–22009 [PubMed] [Google Scholar]
- 9.Mager, S., Kleinberger-Doron, N., Keshet, G. I., Davidson, N., Kanner, B. I., and Lester, H. A. (1996) J. Neurosci. 16 5405–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilgemann, D. W., and Lu, C. C. (1999) J. Gen. Physiol. 114 459–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jardetzky, O. (1966) Nature 211 969–970 [DOI] [PubMed] [Google Scholar]
- 12.Yamashita, A., Singh, S. K., Kawate, T., Jin, Y., and Gouaux, E. (2005) Nature 437 215–223 [DOI] [PubMed] [Google Scholar]
- 13.Faham, S., Watanabe, A., Besserer, G. M., Cascio, D., Specht, A., Hirayama, B. A., Wright, E. M., and Abramson, J. (2008) Science 321 810–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weyand, S., Shimamura, T., Yajima, S., Suzuki, S., Mirza, O., Krusong, K., Carpenter, E. P., Rutherford, N. G., Hadden, J. M., O'Reilly, J., Ma, P., Saidijam, M., Patching, S. G., Hope, R. J., Norbertczak, H. T., Roach, P. C., Iwata, S., Henderson, P. J., and Cameron, A. D. (2008) Science 322 709–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou, Y., Zomot, E., and Kanner, B. I. (2006) J. Biol. Chem. 281 22092–22099 [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Y. W., and Rudnick, G. (2006) J. Biol. Chem. 281 36213–36220 [DOI] [PubMed] [Google Scholar]
- 17.Vandenberg, R. J., Shaddick, K., and Ju, P. (2007) J. Biol. Chem. 282 14447–14453 [DOI] [PubMed] [Google Scholar]
- 18.Dodd, J. R., and Christie, D. L. (2007) J. Biol. Chem. 282 15528–15533 [DOI] [PubMed] [Google Scholar]
- 19.Androutsellis-Theotokis, A., Goldberg, N. R., Ueda, K., Beppu, T., Beckman, M. L., Das, S., Javitch, J. A., and Rudnick, G. (2003) J. Biol. Chem. 278 12703–12709 [DOI] [PubMed] [Google Scholar]
- 20.Quick, M., Yano, H., Goldberg, N. R., Duan, L., Beuming, T., Shi, L., Weinstein, H., and Javitch, J. A. (2006) J. Biol. Chem. 281 26444–26454 [DOI] [PubMed] [Google Scholar]
- 21.Forrest, L. R., Tavoulari, S., Zhang, Y. W., Rudnick, G., and Honig, B. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 12761–12766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zomot, E., Bendahan, A., Quick, M., Zhao, Y., Javitch, J. A., and Kanner, B. I. (2007) Nature 449 726–730 [DOI] [PubMed] [Google Scholar]
- 23.Golovanevsky, V., and Kanner, B. I. (1999) J. Biol. Chem. 274 23020–23026 [DOI] [PubMed] [Google Scholar]
- 24.Bennett, E. R., and Kanner, B. I. (1997) J. Biol. Chem. 272 1203–1210 [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg, A., and Kanner, B. I. (2008) J. Biol. Chem. 283 14376–14383 [DOI] [PubMed] [Google Scholar]
- 26.Kunkel, T. A., Roberts, J. D., and Zakour, R. A. (1987) Methods Enzymol. 154 367–382 [DOI] [PubMed] [Google Scholar]
- 27.Kleinberger-Doron, N., and Kanner, B. I. (1994) J. Biol. Chem. 269 3063–3067 [PubMed] [Google Scholar]
- 28.Fuerst, T. R., Niles, E. G., Studier, F. W., and Moss, B. (1986) Proc. Natl. Acad. Sci. U. S. A. 83 8122–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keynan, S., Suh, Y. J., Kanner, B. I., and Rudnick, G. (1992) Biochemistry 31 1974–1979 [DOI] [PubMed] [Google Scholar]
- 30.Chen, J. G., and Rudnick, G. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou, Y., Bennett, E. R., and Kanner, B. I. (2004) J. Biol. Chem. 279 13800–13808 [DOI] [PubMed] [Google Scholar]
- 32.Forrest, L. R., Zhang, Y. W., Jacobs, M. T., Gesmonde, J., Xie, L., Honig, B. H., and Rudnick, G. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 10338–10343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi, L., Quick, M., Zhao, Y., Weinstein, H., and Javitch, J. A. (2008) Mol. Cell 30 667–677 [DOI] [PMC free article] [PubMed] [Google Scholar]