Abstract
With the discovery of STIM1 and Orai1 and gating of both TRPC and Orai1 channels by STIM1, a central question is the role of each of the channels in the native store-operated Ca2+ influx (SOCs). Here, we used a strategy of knockdown of Orai1 and of TRPC1 alone and in combination and rescue by small interfering RNA-protected mutants (sm) of smOrai1 and smTRPC1 to demonstrate that in human embryonic kidney (HEK) cells, rescue of SOCs required co-transfection of low levels of both smOrai1 and smTRPC1. The pore mutant Orai1(E106Q) failed to rescue the SOCs in the presence or absence of TRPC1 and, surprisingly, the pore mutant TRPC1(F562A) failed to rescue the SOCs in the presence or absence of Orai1. TRPC1 is gated by electrostatic interaction between TRPC1(D639D,D640D) with STIM1(K684K, K685K). Strikingly, the channel-dead TRPC1(D639K,D640K) that can be rescued only by the STIM1(K684E,K685E) mutant could restore SOCs only when expressed with Orai1 and STIM1(K684E,K685E). Accordingly, we found a mutual requirement of Orai1 and TRPC1 for their interaction with the native STIM1 in HEK cells. By contrast, SOC and the CRAC current in Jurkat cells were inhibited by knockdown of Orai1 but were not influenced by knockdown on TRPC1 or TRPC3. These findings define the molecular makeup of the native SOCs in HEK cells and the role of a STIM1-Orai1-TRPC1 complex in SOC activity.
Ca2+ influx across the plasma membrane mediates many of the cellular functions of Ca2+, and several diseases, such as pancreatitis (1) and severe combined immune deficiency syndrome (2), are the result of aberrant Ca2+ influx. In non-excitable cells, Ca2+ influx is mediated by store-operated Ca2+ channels (SOCs)4 (3). The molecular identity of the SOCs and their regulation by Ca2+ stored in the endoplasmic reticulum (ER) have been revealed over the last few years with the discoveries of STIM1 (4, 5) and the Orai channels (2, 6, 7).
STIM1 is the ER Ca2+ content sensor. It has an N-terminal EF hand, which resides in the ER lumen, as well as cytoplasmic C-terminal Ezrin-Radixin-Moesin (ERM), serine/proline, and lysine-rich domains (8, 9). Ca2+ release from the ER results in the assembly of STIM1 into puncta at ER/plasma membrane interfaces. The STIM1 puncta subsequently interact with and activate the SOCs (9–11).
The two Ca2+ influx channel types that are regulated by STIM1 and function as SOCs are the TRPC (12–17) and Orai (2, 6, 7) channels. Deletion of TRPC1 and TRPC4 in mice (18, 19) and silencing of several TRPC channels (TRPCs) by siRNA (9, 20, 21) showed that TRPCs contribute to receptor-stimulated and store-mediated Ca2+ influx in various cell types. More recently, we showed that all TRPCs, except TRPC7, are gated by STIM1 (13) and that this regulation requires the lysine-rich domain at the C terminus of STIM1 (12, 22). Furthermore, native STIM1 is sufficient to fully activate the ectopically expressed TRPCs (12, 13). The Orai family consists of Orai1, Orai2, and Orai3 (2, 6, 7). Orai1 mediates the CRAC current, and co-expression of the Orais with STIM1 is obligatory for the Orais to function as channels (23–26). In fact, expression of Orai1 alone results in inhibition of the native Ca2+ influx (27, 28), possibly as a result of scavenging the native STIM1 (14).
The expression and function of the Orais, TRPCs, and STIM1 in the same cells raise the question of the contribution of each channel to the native SOCs. Several recent studies suggest that Orai1 and TRPC channels may interact with and contribute to Ca2+ influx by SOCs. In the human salivary gland cell line HSG, knockdown (KD) of Orai1 alone reduced SOCs-mediated Ca2+ influx by more than 90%, and KD of TRPC1 alone reduced Ca2+ influx by more than 50% (29). Co-expression of Orai1, TRPC1, and STIM1 in HEK cells resulted in a larger SOC-mediated Ca2+ influx than expression of the individual channels with STIM1 (30). Accordingly, a complex of Orai1-TRPC1-STIM1 could be co-immunoprecipitated from HEK cells transfected with Orai1, STIM1, and TRPC1 (29). KD of Orai1 and expression of Orai1 channel mutants reduced the current measured in cells transfected with TRPC1 and STIM1 and treated with thapsigargin (30). In the rat mast cell line RBL-2H3, KD of Orai1 reduced Ca2+ and Sr2+ influx by about 70%, and KD of TRPC5 reduced the influx by more than 50% (31). Further studies reported that transfection of low levels of Orai1 in HEK cells stably transfected with TRPC3 or TRPC6 converted the TRPC channels from store-independent to store-operated channels (15) and increased the CRAC current (14). Although these findings suggest that ectopically expressed Orai1 and TRPC channels interact, their contribution to the native SOCs and whether the channel function of Orai1 and of TRPC channels is required for the native SOC are not known. Furthermore, the properties of the SOCs are cell-specific. For example, blood-born cells, like T, B, and mast cells, have a large CRAC current, whereas the CRAC current is small or undetectable in most other non-excitable cells (3). The cell-specific behavior of Orai1 and TRPC channels is not well understood.
Here, we used a strategy of KD of Orai1 and TRPCs and rescue with low levels of channel-functional and channel-dead transcripts and expression of STIM1 and TRPC1 mutants that specifically isolate the function of TRPCs to show that the channel function of both TRPCs and Orai1 is required for the native SOCs and that the requirement for TRPCs and Orai1 for the native SOCs is cell-specific. These findings have implications for how receptor stimulation regulates Ca2+ signaling and the role of TRPC channels in Ca2+ signaling.
MATERIALS AND METHODS
Reagents, Cell Culture, and Transfection—Anti-FLAG, anti-HA and anti-STIM1 antibodies were from Sigma, Covance and BDBiosciences, respectively. Fura-2-AM and cyclopiazonic acid were purchased from Tef Labs and Alomone Labs, respectively. All the siRNA and primers used in the present study were obtained from Integrated DNA Technologies. The TRPC1 siRNA sequence used for most experiments is 5′-cca ccu gua aga aga uaa uga cug u-3′,3′-cgg gug gac auu cuu cua uua cug aca-5′, and the Orai1 siRNA sequence used for most experiments is 5′-ugg aac ugu cgg uca guc uua ugg cua-3′,3′-acc uug aca gcc agu cag aau acc g-5′. The efficiency of the siRNAs was determined by RT-PCR because the available antibodies that detect the expressed proteins (Sigma and Alomone Labs) were not sufficiently sensitive to detect the native channels. The efficiency of the siRNAs was further verified by showing their ability to prevent expression of the transfected channels (see Figs. 1 and 2 below).
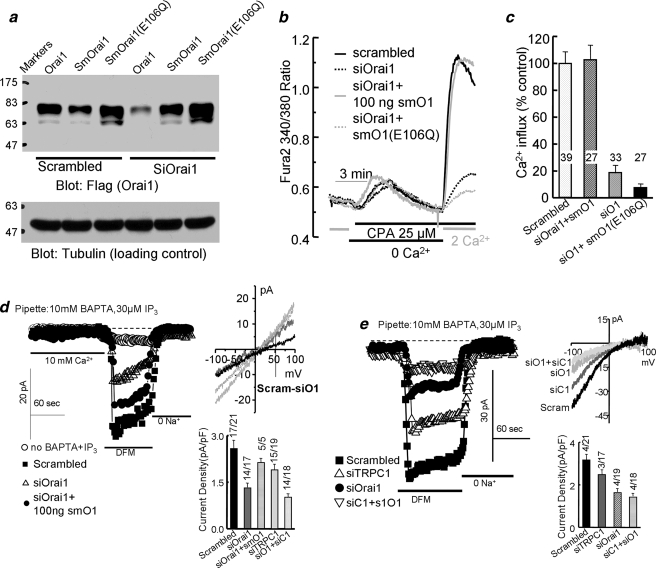
FIGURE 1.
The channel function of Orai1 is required for the native SOCs. a shows analysis of the protein level of FLAG-mCherry-tagged Orai1, Orai1 with silent mutation (smOrai1), and the mutant Orai1(E106Q) with silent mutation (smOrai1(E106Q)) in cells treated with scrambled or with Orai1 siRNA (siOrai1) and transfected with 100 ng of cDNA of the respective constructs. Tubulin levels are given as loading and specificity controls. b shows measurement of SOC activity in cells treated with scrambled siRNA (black) or siOrai1 (all other conditions) for 48 h and transfected with 100 ng/dish of vector (dashed black trace), mCherry-tagged smOrai1 (gray) or smOrai1(E106Q) (dashed gray trace). After an additional 24 h, the cells were used to measure SOC activity. Stores were depleted by perfusion with 25 μm cyclopiazonic acid (CPA) in Ca2+-free medium containing 1 mm EGTA for 7.5 min, and Ca2+ influx was measured using medium containing 2 mm Ca2+. c shows the mean ± S.E. of the indicated number of cells from 4 experiments. d shows the store-independent current recorded with pipette solution containing 70 nm Ca2+ buffered with 5 mm EGTA (○). The store-dependent current was recorded with pipette solution containing 140 mm Cs+, 10 mm BAPTA, and 30 μm inositol 1,4,5-trisphosphate (IP3). Bath solution was the same for all conditions and contained 10 mm Ca2+ and then was divalent-free medium supplemented with 5 mm EGTA. Cells were treated with scrambled Orai1 (▪) or siOrai1 (▵) and transfected with 100 ng of smOrai1 (•). Current recording started about 20 s after break-in. The I/V plots are from the peak current of each condition. The black trace is the Orai1-dependent current obtained by subtracting the current recorded in cells treated with siOrai1 from the current recorded in cells treated with scrambled siRNA. The columns show the mean ± S.E. of the indicated number of experiments. e shows example traces, I/V plots, and the mean ± S.E. of the CRAC-like current found in 4/21 cells treated with scrambled siRNA, 3/17 cells treated with siTRPC1, 4/19 cells treated with siOrai1, and 4/18 cells treated with siTRPC1 + siOrai1.
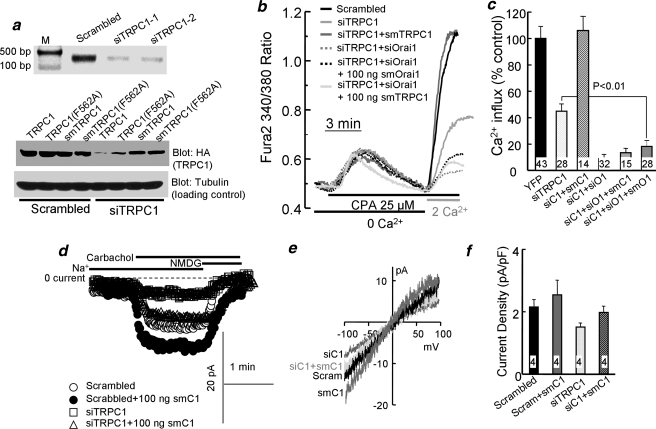
FIGURE 2.
TRPC1 is required for the native SOCs. The upper blot of a shows TRPC1 RT-PCR of HEK cells treated with siTRPC1. The lower blots show analysis of protein levels of HA-tagged TRPC1, TRPC1(F562A), TRPC1 with silent mutation (smTRPC1), and smTRPC1(F562A) in cells treated with scrambled or with TRPC1 siRNA (siTRPC1) and transfected with 100 ng of cDNA of the respective constructs. Tubulin levels are given as loading and specificity controls. b shows measurement of SOC activity in cells treated with scrambled siRNA (black), siTRPC1 (light gray), and transfected with smTRPC1 (gray) or treated with siTRPC1 + siOrai1 for 48 h (all other conditions) and transfected with enhanced green fluorescent protein and 100 ng/dish of vector (dashed gray trace), 100 ng of smOrai1 (dashed black trace), or 100 ng of smTRPC1 (light gray trace). After 24 h, cells were used to measure SOC activity by store depletion in Ca2+-free media and the readdition of 2 mm Ca2+ to the medium, as in Fig. 1. CPA, cyclopiazonic acid. c shows the mean ± S.E. of the indicated number of cells from at least 4 experiments. YFP, yellow fluorescent protein. d shows the current recorded with pipette solution containing 140 mm CsCl, 5 mm EGTA, and 1.5 mm CaCl2 to clamp free Ca2+ at 70 nm and bath solution containing 140 mm Na+ or NMDG+ and 0.5 mm EGTA. Cells were treated with scrambled TRPC1 (○, •) or siTRPC1 (□, ▵) and transfected with 100 ng of smTRPC1 (•, ▵). e shows the corresponding I/V at peak current of each condition. f shows the mean ± S.E. of 4 of the experiments.
HEK293 and Jurkat cells were maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich) and RPMI 1640 (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Invitrogen) and incubated in 5% CO2. The day before transfection, cells were seeded at 80% confluence in 35-mm dishes. Cells were transfected using either Lipofectamine 2000 (Invitrogen) or FuGENE 6 (Roche Applied Science) following the manufacturers' protocols. Briefly, siRNA and Lipofectamine or FuGENE were diluted at the indicated amount in Opti-MEM and then incubated for 20 min before treating the cell. The transfected cells were replated onto a cover glass the following day and incubated for an additional 24 h. Then the cells were transfected with the indicated combination of cDNAs and Ca2+ influx, or current was measured 24 h after transfection.
Cloning and Mutagenesis—HA-TRPC1 (32) and Myc-STIM1 (12), which were used as a template in mutagenesis, were previously described. Full-length Orai1 was generated by PCR using Orai1-pCMV-SPORT6 as template. A cherry-red tag was inserted into the p3xFLAG-CMV-7.1 (Sigma)vector at the HindIII cleavage site. To obtain the cherry-tagged Orai1 construct, Orai1 was subcloned into the p3xFLAG-CMV-7.1 vector using NotI(5′) and SalI(3′). All the point or silent mutations (sm) were generated by PCR mutagenesis. Silent mutation of TRPC1, TRPC1(F562A), and TRPC1(D639K,D640K) was performed by exchanging the nucleotide sequence 943aag aag948 with 943aaa aaa948. Silent mutation of Orai1 and Orai1 E106Q was performed by exchanging the 796act gac801 with 796aca gat801.
[Ca2+]i Measurement—Cells were loaded with Fura-2 at 5 μm final concentration and incubated for 50 min at 37 °C. Cells attached to cover glass that formed the bottom of a perfusion chamber were perfused with prewarmed (37 °C) bath solution containing (in mm): 140 mm NaCl, 5 mm KCl, 1 mm MgCl2,10 mm HEPES, 1 mm CaCl2, 10 mm glucose, adjusted to 310 mosm, pH 7.4). Chemicals were diluted in standard or Ca2+-free bath solution that contained 1 mm EGTA. [Ca2+]i was measured at the 340- and 380-nm excitation wavelengths. The emitted fluorescence was collected by digital camera (Roper Scientific) at 510 nm and analyzed using the Metafluor software. Results are presented as the ratio of 340/380.
Western Blot—Whole cell extracts were prepared in 1% Triton X-100 buffer containing protease inhibitor (1% Triton X-100, 10 mm sodium pyrophosphate, 50 mm NaF at pH 7.4, and 1 mm NaVO3). The cell lysates were sonicated and centrifuged at 12,000 rpm for 10 min to remove insoluble material. Proteins (10–50 μg) were separated on 10% SDS-PAGE and probed with anti-HA (1:1000) or anti-FLAG (1:3000) antibodies. The extracts were also used for immunoprecipitation of STIM1 using 1 μg/100 μl.
Current Measurement—Current in HEK or Jurkat cells treated with siOrai1 and expressing smOrai1 was measured using pipette solution containing (in mm) 140 cesium aspartate, 6 MgCl2, 10 BAPTA, and 10 HEPES (pH 7.2 with CsOH) and using bath solution containing 130 NaCl, 5 KCl, 10 CaCl2,1 MgCl2, and 10 HEPES (pH 7.4 with NaOH). The divalent-free bath solution contained (in mm) 150 NaCl, 10 EDTA, and 10 HEPES (pH 7.4 with NaOH). The current was recorded by application of 400-ms rapid alterations of membrane potential from –100 to +100 mV from a holding potential of 0 mV at 5-s intervals. The current recorded at –100 mV was used to plot the traces and to calculate current density as pA/pF.
Cation current was measured in HEK cells treated with siTRPC1 and transfected with smTRPC1 as described previously (32). In brief, the pipette solution contained (in mm) 140 CsCl, 2 MgCl2, 1 ATP, 5 EGTA, 1.5 CaCl2 (free Ca2+ clamped at 70 nm), and 10 HEPES at pH 7.2 with CsOH, to eliminate K+ current and prevent inhibition of the channel by high cytoplasmic Ca2+. The bath solution contained (in mm) 140 NaCl or 140 NMDG-Cl, 5 KCl, 0.5 EGTA, and 10 HEPES at pH 7.4 with NaOH or NMDG-OH). The current was recorded by the application of 400 ms –100 to +100 mV rapid alterations of membrane potentials from a holding potential of 0 mV at 5-s intervals. The current recorded at –100 mV was used to plot the traces and calculate current density as pA/pF.
Statistics—Results are expressed as means ± S.E. from the number of indicated cells obtained in at least 3–4 independent experiments. The statistical significances of differences between groups were determined by analysis of variance.
RESULTS AND DISCUSSION
The Channel Function of Orai1 Is Required for SOC-mediated Ca2+ Influx in HEK Cells—To understand the role of TRPC and Orai1 channels in native SOC function, we used a complementary strategy and compared the effect of KD of TRPC1 or Orai1 on SOC and the CRAC current and on the channel activity required to restore SOCs activity. The efficiency of the siRNAs was tested by RT-PCR (see “Materials and Methods”) and verified by reduction in Ca2+ influx and expression of recombinant channels (see below). To study the role of Orai1 channel activity in SOC, we introduced silent mtations to generate siRNA-protected Orai1 (smOrai1) and siRNA-protected channel-dead Orai1(E106Q) (smOrai1(E106Q)) (26) using Orai1 tagged with FLAG and mCherry. This construct retained full activity when expressed with STIM1 (not shown). Fig. 1a shows that the smOrai1 and smOrai1(E106Q) are indeed protected against the Orai1 siRNA used in the present study.
Fig. 1, b and c, show that KD of Orai1 in HEK cells reduced SOC activity by about 85%. Transfecting HEK cells treated with siOrai1 with 100 ng of smOrai1 fully restored SOC activity. Transfection with 50 ng was less effective than transfection with 100 ng of smOrai1, and transfection with 150 or 250 ng of smOrai1 was again less effective than transfection with 100 ng of smOrai1, probably due to inhibition of native SOC by high levels of Orai1 (27, 28). Interestingly, transfecting the cells with different concentrations of the channel-dead smOrai1(E106Q) mutant failed to restore SOC, indicating that the channel function of Orai1 is essential for the native SOC in HEK cells.
To further study the role of Orai1 in SOCs, we measured the store-dependent current in HEK cells treated with siOrai1 and transfected with smOrai1. The store-independent leak current was measured in cells with filled stores dialyzed with a solution containing 70 nm Ca2+ (Fig. 1d, open circles). The store-dependent current was measured in cells with depleted stores dialyzed with 10 mm BAPTA and 30 μm inositol 1,4,5-trisphosphate. In Fig. 1d, the first portion of the traces shows that the HEK cell line used in the present study has no discernable Icrac-mediated Ca2+ current. In divalent-free medium, Orai1 (24, 25, 28) and the native Icrac (3) mediate a large transient inwardly rectifying monovalent cation current. Searching for a similar current in our HEK cells revealed such a current with very low frequency (Fig. 1e). In cells treated with scrambled siRNA, a CRAC-like current with strongly inward rectifying I/V was observed in 4/21 cells. This current was reduced by KD of TRPC1 in the 3/17 cells, KD of Orai1 in the 4/19 cells, or KD of both TRPC1 + Orai1 in the 4/18 cells, in which CRAC-like current was detectable. Orai1 likely mediates the CRAC-like current, although this current was reduced by KD of TRPC1, suggesting that the function of the native Orai1 is influenced by TRPC1.
Most of the cells did not show CRAC-like current. Rather, store depletion and incubation in divalent-free medium resulted in a small current with a close to linear I/V (Fig. 1d). Nevertheless, similar to the findings with the CRAC-like current, KD of TRPC1 partially reduced the current, and KD of Orai1 and of Orai1 + TRPC1 reduced the current more than KD of TRPC1. Notably, the portion of the current inhibited by KD of Orai1 could be partially restored by expression of 100 ng of smOrai1. The current associated with the SOCs is considered as the current remaining after subtracting the current remaining after treatment with siOrai1 from the current in control cells. This resulted in a small current with shallow inward rectification and a reversal potential of about 10 mV. This is different from the properties of CRAC channels in T and RBL cells (33).
The analysis in Fig. 1 suggests that in the minority of our HEK cells, the native SOCs current is carried mostly by Orai1 and that it is modulated or enhanced by TRPC1. However, in most of our HEK cells, although the SOC current is activated by store depletion and is reduced by KD of Orai1, the properties of the current are different from those of the CRAC current mediated by Orai1 alone. This current with a reversal potential of about 10 mV may be mediated by Orai1 that is modified by TRPC channels, or it may be mediated by TRPC channels but requires activation by Orai1.
TRPC1 Is Involved in SOC-mediated Ca2+ Influx in HEK Cells—To further examine the requirement for native SOC in HEK cells, we determined the effect of KD of TRPC1 on Ca2+ influx and on the ability of low levels of Orai1 to restore SOC. In Fig. 2a, the RT-PCR blot shows that the two siTRPC1s examined reduced TRPC1 mRNA by about 80%. Most experiments below were performed with siTRPC1-2, although in selective experiments, we verified similar effects with siTRPC1-1 to minimize off-target effects. In Fig. 2a, the lower blots show that treating HEK cells with siTRPC1 reduced expression of transfected TRPC1 and of the channel dead TRPC1(F562A) and that the silent mutations significantly protected smTRPC1 and smTRPC1(F562A). Fig. 2, b and c, confirm previous reports in several cell types that KD (20, 21, 29, 30) or knock-out (18) of TRPC1 reduce SOC-mediated Ca2+ influx by about 60%. SOC is fully restored by expression of only 100 ng of smTRPC1 in cells treated with siTRPC1. The current associated with KD and expression of TRPC1 is shown in Fig. 2, d–f. Control HEK cells showed a small receptor-activated current with a nearly linear I/V that slightly increased by expression of 100 ng of TRPC1. The conditions used to record the receptor-activated current favor detection of current by TRPC channels (see “Materials and Methods”) and are not suitable to detect the CRAC current (3). Treating the cell with siTRPC1 tended to reduce this current, which was restored by 100 ng of smTRPC1. However, due to the small current size, the changes in current size did not reach statistical significance. Nevertheless, the results in Fig. 2, d–f, indicate that expression of 100 ng of smTRPC1 is not associated with induction of a large current but rather results in current comparable with that measured in control cells. Hence, rescue and lack of rescue of SOCs under the different conditions described below are not the results of generation of large TRPC1-mediated current.
The combined KD of TRPC1 and Orai1 reduced SOC-mediated Ca2+ influx by about 90%. Note that inhibition of SOC-mediated Ca2+ influx by Orai1 (85%) and TRPC1 (60%) are not additive. Moreover, a notable finding in Fig. 2, b and c, is that expression of smOrai1 cannot restore SOC activity to cells treated with siOrai1 and siTRPC1. Similarly, expression of smTRPC1 failed to restore SOCs in cells treated with siTRPC1 and siOrai1. These findings suggest that both channels, Orai1 and TRPC1, are required for SOC activity in HEK cells. This is also suggested by the inability of smOrai1 to partially restore SOC activity to cells treated with siOrai1 + siTRPC1 to the extent found in cells treated with siTRPC1 (Fig. 2c, columns 2 and 6). It is possible that the smOrai1 was too low to rescue the SOCs or that reassembly of the SOCs requires both TRPC1 and Orai1.
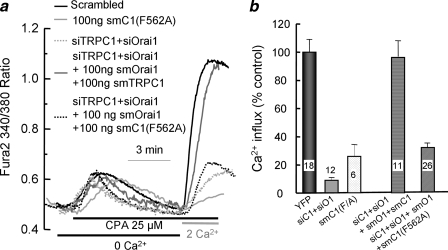
The Channel Function of TRPC1 Is Required to Restore SOC Activity—To further characterize the role of TRPC1 in SOC activity, we compared the ability of siRNA-protected TRPC1 and the channel dead TRPC1(F562A) mutant (34) to restore SOC activity in cells treated with siOrai1 and siTRPC1. Fig. 3 shows that expression of low levels of TRPC1(F562A) alone inhibited the SOC by about 70%, providing the first evidence that the channel function of TRPC1 is required for the native SOC. Furthermore, co-transfection of low levels of smTRPC1 together with smOrai1 completely restored SOC in cells treated with siTRPC1 and siOrai1. However, the channel-dead smTRPC1(F562A) fail to restore SOC when co-expressed with smOrai1.
FIGURE 3.
The channel function of TRPC1 is required for the native SOCs. In a, cells transfected with TRPC1(F562A) (light gray trace) for 24 h were used to measure SOCs-mediated Ca2+ influx as in Fig. 1. Cells were also treated with scrambled siRNA (black) or siTRPC1 + siOrai1 (dashed light gray and gray traces) for 48 h and were then transfected with 100 ng of smOrai1 + smTRPC1 (gray trace) or smOrai1 + smTRPC1(F562A) (dashed black trace), and 24 h later, they were used to measure SOCs, as in Fig. 1. CPA, cyclopiazonic acid. b shows the mean ± S.E. of the indicated number of cells from 3 experiments. YFP, yellow fluorescent protein.
In a recent study (22), we showed that STIM1 gates TRPC1 by electrostatic interaction between STIM1(K684K,K685K) and TRPC1-(D639D,D640D). Charge swapping experiments showed that TRPC1(D639K,D640K) is not active and STIM1(K684E,K685E) acts as dominant negative to inhibit channel activity of wild-type TRPCs. However, STIM1-(K684E,K685E) fully restores channel function to TRPC1-(D639K,D640K). Importantly, all STIM1 Lys-684 and Lys-685 mutants normally activate Orai1 (22). To develop further evidence for the role of the channel function of TRPC1 in native SOCs, we tested how the TRPC1(D639K,D640K) and STIM1(K684E,K685E) mutants affect native SOCs.
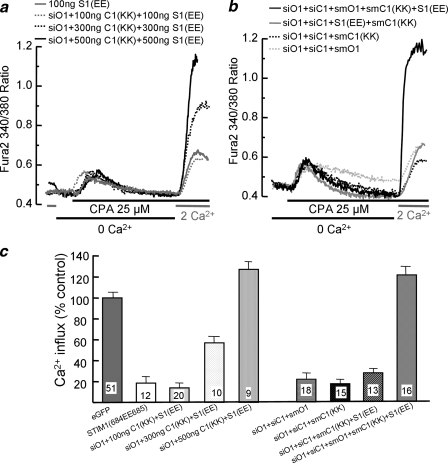
First, we asked whether STIM1(K684E,K685E), which inhibits TRPCs but fully activates Orai1 (22), affects native SOC activity. Fig. 4a and the summary in Fig. 4c show that STIM1(K684E,K685E) strongly inhibits the native SOC, further indicating that TRPCs do participate in native SOCs. Next, we asked whether TRPC1 can function as SOC in the absence of Orai1. This question was tested by expressing increasing amounts of TRPC1(D639K,D640K) and STIM1(K684E,K685E) in cells treated with siOrai1. Fig. 4a and the summary in Fig. 4c show that at the low level of 100 ng, TRPC1(D639K,D640K) and STIM1(K684E,K685E) show minimal SOC activity. Interestingly, 300 and 500 ng of TRPC1-(D639K,D640K) and STIM1(K684E, K685E) showed, and increased SOC activity, and 500 ng (Fig. 4) and 1 μg (not shown) showed SOC activity that was comparable or higher than the native SOCs. Because (a) the SOCs activity of TRPC1(D639K, D640K) + STIM1(K684E,K685E) is observed in siOrai1-treated cells, (b) STIM1(K684E,K685E) inhibits the native TRPCs, and (c) the only channel that can be activated by STIM1-(K684E,K685E) is TRPC1(D639K, D640K), these findings provide strong evidence that TRPC1 can be activated by passive store depletion and, thus, functions as SOC.
FIGURE 4.
The SOC function of TRPC1 and Orai1 are required for the native SOCs. In a, untreated cells were transfected with 100 ng of STIM1(K684E,K685E) alone (gray trace), or cells treated with siOrai1 (all remaining) were transfected with the combination of the mutants TRPC1(D639K,D640K) + STIM1(K684E,K685E) at 100 ng (dashed gray trace), 300 ng (dashed black trace), or 500 ng (black trace). After 24 h, the transfected cells were used to measure SOC activity. CPA, cyclopiazonic acid. In b, all cells were treated with siOrai1 and siTRPC1 for 48 h and then transfected with 100 ng each of smOrai1 (dashed gray trace), smTRPC1(D639K,D640K) (dashed black trace), smTRPC1(D639K,D640K) + STIM1(K684E,K685E) (gray trace), or smOrai1 + smTRPC1(D639K,D640K) + STIM1(K684E,K685E) (black trace). SOC activity was measured 24 h after transfection as in Fig. 1. c shows the mean ± S.E. of the indicated number of cells from 4 experiments. eGFP, enhanced green fluorescent protein.
The next question we addressed is whether the combination of low levels of channel-dead TRPC1-(D639K,D640K) and of STIM1-(K684E,K685E), which acts as dominant negative on wild-type TRPCs but selectively activates TRPC1-(D639K,D640K), can combine with Orai1 to restore SOC in cells treated with siOrai1 and siTRPC1. Fig. 4b and the summary in Fig. 4c show that 100 ng each of smOrai1, smTRPC1-(D639K,D640K), or smTRPC1-(D639K,D640K) + STIM1(K684E, K685E) could not restore SOCs activity. Notably, the combination of 100 ng of smOrai1 + smTRPC1-(D639K,D640K) + STIM1(K684E, K685E) fully restored the SOCs. Because the only functional TRPC channel available to interact with Orai1 is TRPC1-(D639K,D640K), which must be activated by STIM1-(K684E,K685E), we consider these findings as our strongest evidence that the channel function of both Orai1 and TRPC1 is required for the native SOC in HEK cells and possibly other cells that do not show strong Icrac current.
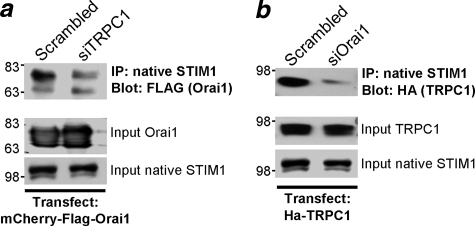
Mutual Requirement of Orai1 and TRPC1 for Interaction with STIM1—Previous work with transfected proteins at high levels suggested that Orai1, STIM1, and TRPC1 are present in the same complex (29). We asked whether the two channels influence the interaction of each other with the native STIM1 when expressed at low levels, closer to the level of the native channels. The cells were transfected with 100 ng of Orai1 or TRPC1. Fig. 5a shows that treating cells with siTRPC1 significantly reduces the interaction of Orai1 with the native STIM1, and Fig. 5b shows that treating cells with siOrai1 reduces the interaction of TRPC1 with the native STIM1. Hence, the interaction of Orai1 and TRPC1 with STIM1 is stabilized and/or enhanced by the presence of both channels in the complex.
FIGURE 5.
Mutual dependence of Orai1 and TRPC1 for interaction with STIM1. HEK cells treated with scrambled siRNA (a and b) and siTRPC1 (a) or siOrai1 (b) were transfected with 100 ng of mCherry-FLAG-Orai1 (a) or HA-TRPC1 (b) and were used to immunoprecipitate the native STIM1 and blot for co-immunoprecipitation (IP) of Orai1 (a, with anti-FFLAG) or TRPC1 (b, anti-HA). Also shown are the inputs for Orai1, TRPC1, and the native STIM1. Similar results were obtained in 3 experiments.
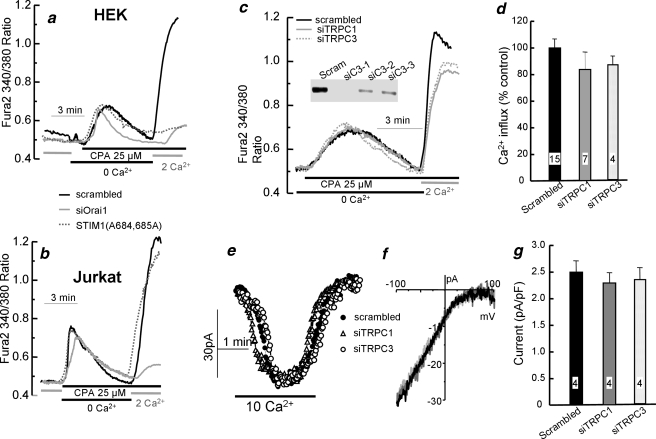
TRPC1 and TRPC3 Are Not Required for SOC Activity and Icrac Current in Jurkat Cells—The findings in Figs. 1, 2, 3, 4, 5 suggest that Ca2+ influx by the native SOCs is mediated by the associated function of Orai1 and TRPC channels. A question raised by these findings is whether this is also the case in cells in which a large Orai1-mediated CRAC current can be measured. To address this question, we examined the role of TRPC channels in Ca2+ influx and CRAC current in the model system of Jurkat cells. A tool to differentiate the contribution of Orai1 and TRPC channels to the native SOCs is mutants in STIM1 Lys-684 and Lys-685 that inhibit TRPCs but not Orai1, such as STIM1(K684E,K685E) and STIM1(K684A,K685A). In control experiments, we verified that KD of Orai1 (Fig. 6, a and b) inhibited SOC activity in the HEK and Jurkat cell lines used in the present work. Fig. 6a shows that STIM1(K684A,K685A) nearly abolished SOC-mediated Ca2+ influx in HEK cells. Significantly, STIM1(K684A,K685A) does not inhibit SOC activity in Jurkat cells (Fig. 6b). The same differential effects were observed with STIM1(K684E,K685E) (not shown).
FIGURE 6.
TRPC1 and TRPC3 are not required for Icrac and SOCs in Jurkat cells. In a and b, HEK (a) and Jurkat cells (b) were treated with scrambled (black traces) or Orai1 siRNA (gray traces) for 72 h or transfected with STIM1(K684A,K685A) for 24 h (dashed gray traces) and used to measure SOC activity. CPA, cyclopiazonic acid. The inset in c shows TRPC3 RT-PCR of HEK cells treated with siTRPC3. In c–g, Jurkat cells were treated with scrambled siRNA (Scram) or siTRPC1 or siTRPC3 for 72 h (c–g) before measurement of SOC activity (c and d) or the CRAC current (e–g). f shows representative I/V plots, and d and g show the mean ± S.E. of the indicated number of experiments.
Previous work showed that Jurkat cells express mainly TRPC1 and TRPC3, and a Jurkat cell line with reduced SOC and CRAC current was reported to be deficient in TRPC3 (35). Therefore, we tested the effect of KD of TRPC1 and TRPC3 on SOC activity and CRAC current in Jurkat cells. Fig. 2a shows the efficiency of the siTRPC1, and in Fig. 6c, the inset shows the efficiency of the siTRPC3 to down-regulate the level of TRPC3 mRNA. All the experiments below were performed with siTRPC3 number 1. Fig. 6, c–f, show that KD of TRPC1 or TRPC3 in Jurkat cells had no measurable effect on SOC activity (Fig. 6, c and d) or the CRAC current (Fig. 6, e and f). Similar findings were reported very recently in an endothelial cell line with a large CRAC current in which knockdown of TRPC1 and TRPC4 had no effect on CRAC current and Ca2+ influx that was inhibited by knockdown of STIM1 and Orai1 (36). Hence, the combined findings in Fig. 6 suggest that although TRPC channels and their interaction with STIM1 are essential for SOC activity in HEK cells, such an interaction is not required for activation of SOC activity and CRAC current in Jurkat cells, and that Orai1 can function independently of TRPCs to mediate CRAC current and SOC activity in Jurkat cells. However, this appears to be cell-specific and/or a function of the expression level of Orai1.
Conclusions—The present work shows that TRPC1 can function as a bona fide SOC and that the channel function of TRPC1 and Orai1 is required for native SOCs. Several studies showed that disruption of TRPC1 expression reduces SOC activity (reviewed in Ref. 37), and we reported that TRPC1 is regulated by the ER Ca2+ sensor STIM1 (12, 13). However, direct demonstration that TRPC1 can be activated by passive store depletion and that this function is independent of any other Ca2+ influx channel was lacking. Here, we used expression of TRPC1(D639K,D640K) and STIM1(K684E,K685E) in cells treated with siOrai1 to provide direct evidence for the function of TRPC1 as SOC. In siOrai1-treated cells that express STIM1(K684E,K685E), the only functional channel is TRPC1-(D639K,D640K) because STIM1(K684E,K685E) inhibits the activity of the native wild-type TRPCs and specifically activates TRPC1(D639K,D640K) (see Ref. 22 for details). At high expression levels, the combination of TRPC1(D639K,D640K) + STIM1(K684E,K685E) resulted in Ca2+ influx that can be readily activated by passive store depletion.
The present work examines the role of Orai1 and TRPC channels and STIM1 in native SOC activity in the two models of HEK and Jurkat cells. KD by siRNA and rescue by expression of low levels of Orai1 and TRPC1 or their channel-dead mutants lead us to conclude that Orai1 and STIM1 are required for SOC activity in both cell types but that TRPC channel function is required in SOC activity only in HEK cells. How can two channels that can function independently as SOCs when expressed alone at high levels be required for native SOCs? It is clear that the channels cooperate with each other. Hence, the Ca2+ influx mediated by 100 ng of TRPC1 or Orai1 alone is nearly undetectable, yet the combination of 100 ng of TRPC1 + Orai1 generates a large Ca2+ influx, comparable with the native SOC. The requirement of functional channels to observe the rescue of the SOC would suggest that TRPC1 and Orai1 may activate each other. Moreover, the pores of the two channels appear to communicate and sense the function of each other. This can be due to activation of the channels by the ions flowing through each channel or that only the open channel conformations can positively affect each other.
When present in the same complex, TRPC1 and Orai1 may stabilize the SOC complex by the interaction of the STIM1 ERM domain with TRPC1 (12) and of STIM1 with the C terminus of Orai1 (38). TRPC1 may also aid in targeting the SOC complex to particular plasma membrane microdomains. Two recent studies reported that the TRPC1-STIM1 complex translocates to caveolae upon store depletion (16, 17) to facilitate the function of TRPC1 as SOC. This implies that the interaction of STIM1 with TRPC channels in HEK cells is required for the interaction of STIM1 with Orai1 and vice versa, whereas in Jurkat cells, STIM1 can interact with Orai1 independent of TRPC channels. The mutual effect of Orai1 and TRPC1 on their co-immunoprecipitation with the native STIM1 is consistent with this possibility.
Comparing the roles of STIM1 and TRPC1 in HEK and Jurkat cells reveals cell-specific SOC complexes. SOC and CRAC current in Jurkat cells are not inhibited by STIM1 mutants that inhibit SOCs in HEK cells and are not affected by KD of TRPC1 or TRPC3. These findings suggest that SOC channels can be assembled as Orai1-STIM1, TRPCs-STIM1, or Orai1-STIM1-TRPCs complexes depending on the cell type and probably the Ca2+ signaling complex. Hence, the present findings provide a potential molecular mechanism to explain the cell-specific properties of SOCs found in a large number of previous studies (reviewed in Refs. 3 and 37). This form of differential assembly of SOC complexes can aid in generating cell-specific Ca2+ influx mechanisms in cellular microdomains (39) to regulate specific cell functions (40).
This work was supported, in whole or in part, by National Institutes of Health Grant Grants DE12309 and DK38938 and by the National Institute on Drug Abuse (Grants DA00266 and DA10309) and the National Institute of Mental Health (Grant MH068830) (to P. F. W.).
Footnotes
The abbreviations used are: SOCs, store-operated channels; STIM1, stromal interaction molecule 1; TRPC, transient receptor potential canonical; smTRPC1, TRPC1 with silent mutation; KD, knockdown; smOrai1, Orai1 with silent mutation; siRNA, small interfering RNA; HEK, human embryonic kidney; ER, endoplasmic reticulum; RT-PCR, reverse transcription-PCR; HA, hemagglutinin; BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; NMDG, N-methyl-d-glucamine; CRAC, calcium release-activated calcium; pF, picofarads.
References
- 1.Petersen, O. H., Sutton, R., and Criddle, D. N. (2006) Cell Calcium 40 593–600 [DOI] [PubMed] [Google Scholar]
- 2.Feske, S., Gwack, Y., Prakriya, M., Srikanth, S., Puppel, S. H., Tanasa, B., Hogan, P. G., Lewis, R. S., Daly, M., and Rao, A. (2006) Nature 441 179–185 [DOI] [PubMed] [Google Scholar]
- 3.Parekh, A. B., and Putney, J. W., Jr. (2005) Physiol. Rev. 85 757–810 [DOI] [PubMed] [Google Scholar]
- 4.Roos, J., DiGregorio, P. J., Yeromin, A. V., Ohlsen, K., Lioudyno, M., Zhang, S., Safrina, O., Kozak, J. A., Wagner, S. L., Cahalan, M. D., Velicelebi, G., and Stauderman, K. A. (2005) J. Cell Biol. 169 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou, J., Kim, M. L., Heo, W. D., Jones, J. T., Myers, J. W., Ferrell, J. E., Jr., and Meyer, T. (2005) Curr. Biol. 15 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vig, M., Peinelt, C., Beck, A., Koomoa, D. L., Rabah, D., Koblan-Huberson, M., Kraft, S., Turner, H., Fleig, A., Penner, R., and Kinet, J. P. (2006) Science 312 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, S. L., Yeromin, A. V., Zhang, X. H., Yu, Y., Safrina, O., Penna, A., Roos, J., Stauderman, K. A., and Cahalan, M. D. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 9357–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziadek, M. A., and Johnstone, L. S. (2007) Cell Calcium 42 123–132 [DOI] [PubMed] [Google Scholar]
- 9.Worley, P. F., Zeng, W., Huang, G. N., Yuan, J. P., Kim, J. Y., Lee, M. G., and Muallem, S. (2007) Cell Calcium 42 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahalan, M. D., Zhang, S. L., Yeromin, A. V., Ohlsen, K., Roos, J., and Stauderman, K. A. (2007) Cell Calcium 42 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu, M. M., Luik, R. M., and Lewis, R. S. (2007) Cell Calcium 42 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, G. N., Zeng, W., Kim, J. Y., Yuan, J. P., Han, L., Muallem, S., and Worley, P. F. (2006) Nat. Cell Biol. 8 1003–1010 [DOI] [PubMed] [Google Scholar]
- 13.Yuan, J. P., Zeng, W., Huang, G. N., Worley, P. F., and Muallem, S. (2007) Nat Cell Biol. 9 636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao, Y., Erxleben, C., Abramowitz, J., Flockerzi, V., Zhu, M. X., Armstrong, D. L., and Birnbaumer, L. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 2895–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao, Y., Erxleben, C., Yildirim, E., Abramowitz, J., Armstrong, D. L., and Birnbaumer, L. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 4682–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alicia, S., Angelica, Z., Carlos, S., Alfonso, S., and Vaca, L. (2008) Cell Calcium 44 479–491 [DOI] [PubMed] [Google Scholar]
- 17.Pani, B., Ong, H. L., Liu, X., Rauser, K., Ambudkar, I. S., and Singh, B. B. (2008) J. Biol. Chem. 283 17333–17340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, X., Cheng, K. T., Bandyopadhyay, B. C., Pani, B., Dietrich, A., Paria, B. C., Swaim, W. D., Beech, D., Yildrim, E., Singh, B. B., Birnbaumer, L., and Ambudkar, I. S. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 17542–17547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freichel, M., Suh, S. H., Pfeifer, A., Schweig, U., Trost, C., Weissgerber, P., Biel, M., Philipp, S., Freise, D., Droogmans, G., Hofmann, F., Flockerzi, V., and Nilius, B. (2001) Nat. Cell Biol. 3 121–127 [DOI] [PubMed] [Google Scholar]
- 20.Zagranichnaya, T. K., Wu, X., and Villereal, M. L. (2005) J. Biol. Chem. 280 29559–29569 [DOI] [PubMed] [Google Scholar]
- 21.Li, J., Sukumar, P., Milligan, C. J., Kumar, B., Ma, Z. Y., Munsch, C. M., Jiang, L. H., Porter, K. E., and Beech, D. J. (2008) Circ. Res. 103 e97–e104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng, W., Yuan, J. P., Kim, M. S., Choi, Y. J., Huang, G. N., Worley, P. F., and Muallem, S. (2008) Mol. Cell 32 439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakriya, M., Feske, S., Gwack, Y., Srikanth, S., Rao, A., and Hogan, P. G. (2006) Nature 443 230–233 [DOI] [PubMed] [Google Scholar]
- 24.Peinelt, C., Vig, M., Koomoa, D. L., Beck, A., Nadler, M. J., Koblan-Huberson, M., Lis, A., Fleig, A., Penner, R., and Kinet, J. P. (2006) Nat. Cell Biol. 8 771–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeromin, A. V., Zhang, S. L., Jiang, W., Yu, Y., Safrina, O., and Cahalan, M. D. (2006) Nature 443 226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vig, M., Beck, A., Billingsley, J. M., Lis, A., Parvez, S., Peinelt, C., Koomoa, D. L., Soboloff, J., Gill, D. L., Fleig, A., Kinet, J. P., and Penner, R. (2006) Curr. Biol. 16 2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soboloff, J., Spassova, M. A., Tang, X. D., Hewavitharana, T., Xu, W., and Gill, D. L. (2006) J. Biol. Chem. 281 20661–20665 [DOI] [PubMed] [Google Scholar]
- 28.Mercer, J. C., Dehaven, W. I., Smyth, J. T., Wedel, B., Boyles, R. R., Bird, G. S., and Putney, J. W., Jr. (2006) J. Biol. Chem. 281 24979–24990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong, H. L., Cheng, K. T., Liu, X., Bandyopadhyay, B. C., Paria, B. C., Soboloff, J., Pani, B., Gwack, Y., Srikanth, S., Singh, B. B., Gill, D. L., and Ambudkar, I. S. (2007) J. Biol. Chem. 282 9105–9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng, K. T., Liu, X., Ong, H. L., and Ambudkar, I. S. (2008) J. Biol. Chem. 283 12935–12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, H. T., Peng, Z., Hiragun, T., Iwaki, S., Gilfillan, A. M., and Beaven, M. A. (2008) J. Immunol. 180 2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan, J. P., Kiselyov, K., Shin, D. M., Chen, J., Shcheynikov, N., Kang, S. H., Dehoff, M. H., Schwarz, M. K., Seeburg, P. H., Muallem, S., and Worley, P. F. (2003) Cell 114 777–789 [DOI] [PubMed] [Google Scholar]
- 33.Bakowski, D., and Parekh, A. B. (2002) Cell Calcium 32 379–391 [DOI] [PubMed] [Google Scholar]
- 34.Kim, S. J., Kim, Y. S., Yuan, J. P., Petralia, R. S., Worley, P. F., and Linden, D. J. (2003) Nature 426 285–291 [DOI] [PubMed] [Google Scholar]
- 35.Philipp, S., Strauss, B., Hirnet, D., Wissenbach, U., Mery, L., Flockerzi, V., and Hoth, M. (2003) J. Biol. Chem. 278 26629–26638 [DOI] [PubMed] [Google Scholar]
- 36.Abdullaev, I. F., Bisaillon, J. M., Potier, M., Gonzalez, J. C., Motiani, R. K., and Trebak, M. (2008) Circ. Res. 103 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambudkar, I. S., Ong, H. L., Liu, X., Bandyopadhyay, B., and Cheng, K. T. (2007) Cell Calcium 42 213–223 [DOI] [PubMed] [Google Scholar]
- 38.Muik, M., Frischauf, I., Derler, I., Fahrner, M., Bergsmann, J., Eder, P., Schindl, R., Hesch, C., Polzinger, B., Fritsch, R., Kahr, H., Madl, J., Gruber, H., Groschner, K., and Romanin, C. (2008) J. Biol. Chem. 283 8014–8022 [DOI] [PubMed] [Google Scholar]
- 39.Chang, W. C., Di Capite, J., Singaravelu, K., Nelson, C., Halse, V., and Parekh, A. B. (2008) J. Biol. Chem. 283 4622–4631 [DOI] [PubMed] [Google Scholar]
- 40.Chang, W. C., and Parekh, A. B. (2004) J. Biol. Chem. 279 29994–29999 [DOI] [PubMed] [Google Scholar]