Abstract
Common features of the extracellular carbohydrate-active virulence factors involved in host-pathogen interactions are their large sizes and modular complexities. This has made them recalcitrant to structural analysis, and therefore our understanding of the significance of modularity in these important proteins is lagging. Clostridium perfringens is a prevalent human pathogen that harbors a wide array of large, extracellular carbohydrate-active enzymes and is an excellent and relevant model system to approach this problem. Here we describe the complete structure of C. perfringens GH84C (NagJ), a 1001-amino acid multimodular homolog of the C. perfringens μ-toxin, which was determined using a combination of small angle x-ray scattering and x-ray crystallography. The resulting structure reveals unprecedented insight into how catalysis, carbohydrate-specific adherence, and the formation of molecular complexes with other enzymes via an ultra-tight protein-protein interaction are spatially coordinated in an enzyme involved in a host-pathogen interaction.
Microbial and viral invaders of the human body often exploit host glycans to aid in adherence and then must contend with the protective and structural sugar layers to enable invasion and further spread of the infection. Some of the more spectacular bacterial infections, such as the severe myonecrotic infections caused by Streptococcus pyogenes and Clostridium perfringens, involve extensive tissue destruction (1–4). The tissue destruction and bacterial spread appears to be aided by a variety of carbohydrate-active enzymes, which break down the polysaccharides of the extracellular matrix or potentiate the activity of other cytolytic toxins (5–8). A large number of other bacterial pathogens also feature carbohydrate-active enzymes as important virulence factors that figure in a variety of roles related to degrading and modifying host glycans (see for example Refs. 9–11).
A common feature of these microbial carbohydrate-active enzymes involved in processing eukaryotic glycans is their structural complexity; such proteins are frequently very large (over 1000 amino acids) and can comprise numerous modules and domains (12–16). C. perfringens, a myonecrotic (“flesh-eating”) bacterial pathogen and a leading cause of food-borne gastrointestinal illness, deploys numerous protein exo-toxins that work together to increase the pathogen's virulence (3, 17). Among its exo-toxin proteins are a considerable battery of large extracellular carbohydrate-active enzymes (supplemental Fig. S1), including the hyaluronidase μ-toxin, which destroys the polysaccharide hyaluronan, and the large sialidases (NanJ and NanI), which remove terminal sialic acid sugars and enhance the lethal cytolytic phospholipase activity of the α-toxin (5, 6, 18). The individual modules of such carbohydrate-active enzymes perform a variety of functions, which are most commonly catalysis, protein-carbohydrate interactions, or protein-protein interactions, and these contribute to the overall function and efficiency of the protein. However, because of their large size and flexibility, properties that make them recalcitrant to structural analyses, and their multifunctional natures, such large proteins have required a reductionist approach to their study whereby individual modules are heterologously produced for thorough structural and functional analysis. This has provided considerable insight into the individual functions of the modules. However, how the functions of the modules are spatially coordinated to cooperatively influence the overall activity of the full-length enzymes, knowledge that would have considerable biological relevance, is largely unknown as the complete structures of large carbohydrate-active bacterial virulence factors, for the most part, resist determination.
The considerable number of large and multimodular carbohydrate-active enzymes produced by C. perfringens makes this organism an excellent and relevant model system for the study of complex carbohydrate-active enzymes involved in bacterial pathogenesis. GH84C (NagJ) from C. perfringens is a homolog of the μ-toxin that comprises 1001 amino acids and four distinct modules: an N-terminal catalytic module followed by a family 32 carbohydrate-binding module (CBM32),5 a cohesin module (Coh), and a C-terminal fibronectin type III module (FN3) (Fig. 1). The structural and biochemical properties of the isolated catalytic module, CBM32, and Coh have been described separately (13–15, 19). These informative studies have revealed that the catalytic module is similar to human O-GlcNAcase and functions as an exo-β-d-N-acetylglucosaminidase, whereas the CBM32 preferentially recognizes the non-reducing terminus of N-acetyllactosamine (β-d-galactosyl-1,4-β-d-N-acetylglucosamine) bearing carbohydrate receptors (15, 19). The Coh module is perhaps the most unique component, because it functions to recognize and bind ultra-tightly to dockerin modules (Doc), such as that present at the C terminus of the μ-toxin, and plays a role in forming higher order complexes with other large C. perfringens exo-toxins, all of which are thought to contribute to the virulence of this bacterium (13). However, the overall three-dimensional structure and therefore spatial distribution of the cognate modules of GH84C remain unknown, as do the structure and function of the FN3 module. This information would provide considerable insight into the structural coordination of modular functions in this protein and provide a powerful model for understanding modularity in the carbohydrate-active enzymes of C. perfringens as well as carbohydrate-active virulence factors in other bacterial pathogens, which also frequently display modular complexity.
FIGURE 1.
Schematic of the modular structure of GH84C. At the N terminus is a Gram-positive secretion signal peptide (indicated by SP). This is followed by the family 84 catalytic module, a family 32 carbohydrate binding module (CBM), a cohesin module (Coh; previously called X82 module). At the C terminus of the enzyme is a fibronectin type III module (FN3). The amino acid numbers denoting the module boundaries are indicated.
Intact GH84C proved intractable to heterologous production and in vitro analysis. However, a less conventional approach that combined x-ray crystallography and small-angle x-ray scattering (SAXS) was applied in a “dissect-and-build” approach to generate a solution structure model of the complete enzyme. The reconstructed architecture of GH84C in complex with a Doc-containing fragment of the μ-toxin reveals unprecedented insight into how the recognition of substrate by the catalytic module, interaction of a CBM with a carbohydrate receptor, and recruitment of other carbohydrate-active enzymes through a high affinity protein-protein interaction are spatially coordinated in an enzyme involved in a host-pathogen interaction. Furthermore, the methodology employed here should be broadly applicable to a range of large and flexible multimodular proteins.
EXPERIMENTAL PROCEDURES
Materials—Unless otherwise stated, chemicals, carbohydrates, glycoproteins, and polysaccharides were purchased from Sigma.
Cloning, Protein Production, and Purification—The DNA fragments encoding the modules and combinations thereof from GH84C were amplified by PCR from C. perfringens genomic DNA (ATCC 13124) and, unless previously cloned, cloned into pET 28a(+) using described methods and the primers listed in supplemental Tables S1 and S2 (15, 20, 21). With the exception of Coh-FN3 and FIVAR-Doc, all constructs encoded polypeptides comprising a hexa-histidine tag fused to the desired module(s) by an N-terminal thrombin protease cleavage site. Coh-FN3 and FIVAR-Doc had C-terminal, non-cleavable hexa-histidine tags.
GH84C catalytic module and FIVAR-Doc were produced, purified by immobilized metal affinity chromatography, buffer-exchanged, and concentrated using previously described methods (13, 20, 22). This process was followed by further purification by size exclusion chromatography using a Sephacryl S-200 size exclusion column (Amersham Biosciences). The CBM32-Coh·FIVAR-Doc complex was prepared by mixing the two polypeptides with the FIVAR-Doc in ∼1.5-fold molar excess. The complex was purified using a Sephacryl S-200 size exclusion column. Protein concentrations were determined using UV absorbance at 280 nm and calculated extinction coefficients (23).
X-ray Crystallography—Crystallization and data collection procedures have been described previously for GH84C catalytic module (20). The hexahistidine tag was removed from the GH84C-CBM32 construct by incubation with thrombin protease for 16 h. The mixture was separated on a Sephacryl S-200 size exclusion column in 20 mm Tris-HCl, pH 8.0. All protein constructs were concentrated to 10–25 mg/ml for crystallization trials. Crystallization was performed using the hanging drop vapor diffusion method. GH84C-CBM32 was crystallized at 18 °C in 0.1 m Tris-HCl, pH 7.5, 0.1 m MgCl2, 15% (w/v) polyethylene glycol 4000. These crystals were cryoprotected in the crystallization solution supplemented with 20% ethylene glycol (v/v). The Coh-FN3 modular pair was crystallized in 0.1 m sodium acetate, pH 3.5, 4% (w/v) polyethylene glycol 8000. These crystals were cryoprotected in 20% (w/v) polyethylene glycol 400 with mother liquor. Diffraction data for GH84C-CBM32 was collected with a Rigaku R-AXIS IV++ area detector coupled to an MM-002 x-ray generator with Osmic “blue” optics and an Oxford Cryostream 700 at 113 K. Diffraction data for Coh-FN3 were collected at 100 K on beamline X6A at the National Synchrotron Light Source (Brookhaven National Laboratories). All data were processed with Crystal Clear/d*trek (24). Data collection statistics are given in Table 1.
TABLE 1.
X-ray crystal diffraction data collection and refinement statistics
| GH84C-CBM | GH84C catalytic | Coh-FN3 | |
|---|---|---|---|
| Data collection | |||
| Space group | P21 | I212121 | C2 |
| Cell dimensions | |||
| a, b, c (Å) | 46.85, 69.54, 136.78 | 130.39, 150.05, 155.43 | 148.25, 48.64, 36.90 |
| α, β, γ (°) | 90.00, 95.78, 90.00 | 90.00, 90.00, 90.00 | 90.00, 96.19, 90.00 |
| Resolution (Å) | 20.00-3.30 (3.39-3.30)a | 20.00-2.10 (2.15-2.10) | 20.00-1.80 (1.86-1.80) |
| Rmerge | 0.114 (0.287) | 0.103 (0.397) | 0.030 (0.170) |
| I/σI | 4.4 (2.2) | 12.4 (4.8) | 30.0 (8.0) |
| Completeness (%) | 92.8 (94.7) | 99.9 (100.0) | 95.7 (77.9) |
| Redundancy | 2.5 (2.5) | 16.1 (15.7) | 7.1 (6.1) |
| Refinement | |||
| No. reflections | 31,497 | 1,384,042 | 166,316 |
| Unique reflectionsb | 12,420 | 85,965 | 23,338 |
| Rwork/Rfree | 0.325/0.370 | 0.198/0.255 | 0.224/0.294 |
| No. atoms | |||
| Protein | 5,677 | 4,644 (A); 4,632 (B) | 1750 |
| Ligands | 3 (Ca) | 4 (Ca); 3 (Na); 25 (CAC) | 4 (ACT) |
| Water | 28 | 1,195 | 318 |
| B-factors (Å2) | |||
| Protein | 52.8 | 29.3 (A); 32.1 (B) | 28.3 |
| Ligands | 71.8 (Ca) | 44.3 (Ca); 22.0 (Na); 71.7 (CAC) | 30.2 (ACT) |
| Water | 27.5 | 34.3 | 38.7 |
| r.m.s.d. | |||
| Bond lengths (Å) | 0.006 | 0.010 | 0.018 |
| Bond angles (°) | 0.934 | 1.389 | 1.732 |
| Ramachandran statistics (%) | |||
| Favored | 84.1 | 90.4 | 91.6 |
| Allowed | 15.5 | 9.7 | 8.4 |
| Disallowed | 0.5 | 0.0 | 0.0 |
Values in parentheses are for the highest resolution shells.
All F > 2σF.
The structure of the GH84C catalytic module was solved by molecular replacement using PHASER (25) to find the positions of two GH84C molecules in the asymmetric unit. The preliminary coordinates of the family 84 glycoside hydrolase from Bacteroides thetaiotaomicron, kindly provided by Dr. Gideon Davies prior to deposition (PDB accession code 2J47) (26), was used as a search model. Three iterations of manual model correction using COOT (27), refinement with REFMAC (28), and solvent flattening with 2-fold non-crystallographic symmetry averaging using DM (29) were required to obtain a model of roughly 60% completeness with partially built side chains. Phases from this process were then sufficient for ARP/ wARP (30) to build a virtually complete model with docked side chains, which was then manually completed using COOT and refinement with REFMAC. Waters were added using the ARP/wARP option in REFMAC.
The GH84C-CBM32 modular pair structure was solved by molecular replacement by first running MOLREP (31) using the GH84C catalytic module as a search model to find the single molecule in the asymmetric unit. MOLREP was run a second time to place the CBM32 model (PDB accession 2J7M) in the asymmetric unit. Successive rounds of model correction were done using COOT and refinement with REFMAC. A limited number of waters was added manually. Due to substantial disorder in portions of this structure rather than use a bulk B-factor, we opted to refine individual B-factors.
The structure of the Coh-FN3 modular pair was determined by molecular replacement using PHASER and the coordinates of the isolated Coh module (PDB accession 2O4E) as a search model to find the single molecule of Coh-FN3 in the asymmetric unit. Although the molecular replacement solution contained only ∼50% of the asymmetric unit contents, the initial phases from restrained refinement with REFMAC were of sufficient quality for ARP/wARP to build a complete model, including the FN3 module, with docked side chains. Model correction was done manually using COOT and refinement with REFMAC. Waters were added using the ARP/wARP option in REFMAC.
In all cases, 5% of the reflections were flagged as “free” to monitor refinement procedures and judge model quality (32). Model validation was performed with SFCHECK (33) and PROCHECK (34). All model statistics are shown in Table 1. The coordinates and structure factors for the GH84C catalytic module, GH84C-CBM32, and Coh-FN3 have been deposited with the pdb codes of 2v5c, 2v5d, and 2w1n, respectively.
SAXS—SAXS data were collected at the X33 beamline of the European Molecular Biology Laboratory (Deutsches Electronen Synchrotron, Hamburg) using an MAR345 image plate detector or a Pilatus 500K detector. A 4.2 mg/ml solution of bovine serum albumin was measured as a reference and for calibration. The scattering patterns were measured with an exposure time of 2 min at 288 K. The wavelength was 1.5 Å. The sample-to-detector distance was set at 2.4 m, leading to scattering vectors q (defined as q = 4π /λ sinθ, where 2θ is the scattering angle) ranging from 0.06 Å–1 to 0.5 Å–1. The concentration of the protein samples ranged from 0.92 mg/ml to 13.48 mg/ml, depending on the protein, and each protein was measured at three to five concentrations. Background scattering was measured after each protein sample using the buffer solution and subsequently subtracted from the protein scattering patterns after proper normalization and correction from detector response.
The radii of gyration (Rg) were derived from the Guinier approximation: I(q) = I(0) exp(–q2R2g/3), where I(q) is the scattered intensity and I(0) is the forward scattered intensity (35). The radius of gyration and I(0) are inferred from the slope and the intercept, respectively, of the linear fit of ln[I(q)] versus q2 in the q-range q·Rg < 1.12. At low angles, the scattered intensities were very well approximated by the Guinier law, and revealed some repulsive interparticle interactions at high concentrations. All scattering curves were indicative of monomeric states of the molecules in solution. The distance distribution function P(r) was calculated on the merged curve by the Fourier inversion of the scattering intensity I(q) using GNOM (36) and GIFT (37).
The low resolution shapes of the protein constructs were determined ab initio from the scattering curve using the program GASBOR (38). Several independent fits were run with no symmetry restriction, and the stability of the solution was checked. These solutions were subsequently compared with the program DAMAVER (39), which computes the normalized spatial discrepancy value for the various obtained shapes (40). In all cases, calculations led to highly similar forms with normalized spatial discrepancy values ranging between 0.8 and 1.3. The atomic crystallographic structures of the individual modules were positioned in the envelopes using PyMOL. For each structural model obtained the theoretical SAXS profile, the Rg, and the corresponding fit to the experimental data were calculated using the program CRYSOL (41). The goodness of fit for all atomic models, as well as the low resolution models, with the experimental data were determined using the discrepancy χ, defined according to Konarev et al. (42). SAXS data are summarized in Table 2, and experimental SAXS curves and their fits are shown in supplemental Figs. S2 and S3.
TABLE 2.
Structural parameters of GH84C constructs obtained by SAXS
|
Construct (module
boundariesa)
|
Molecular
massb
|
Number of amino
acidsb
|
Rg
|
Dmax
|
Ab initio modeling
|
|
|---|---|---|---|---|---|---|
| dx (GASB) | Normalized spatial discrepancy | |||||
| kDa | Å | Å | ||||
| GH84C-CBM32-Coh (31-909) | 100 | 902 | 37 ± 1 | 116 ± 3 | 1.0 | 1.3 |
| GH84C-CBM32 (31-767) | 82.3 | 760 | 35 ± 6 | 103 ± 6 | 2.3 | 1.3 |
| CBM32-Coh (625-909) | 32.4 | 307 | 26 ± 3 | 85 ± 3 | 1.7 | 0.9 |
| CBM32-Coh·FIVAR-Doc (625-909/NAc) | 47.9 | 447 | 31 ± 3 | 95 ± 3 | 1.4 | 1.2 |
| Coh-FN3 (765-1001) | 27.0 | 246 | 37 ± 6 | 118 ± 6 | NDd | ND |
| Bovine serum albumin | 66.3 | 582 | 31 ± 1 | 90 ± 2 | ND | ND |
Construct boundaries are shown relative to the amino acid numbering in the intact GH84C protein.
Values are calculated including the N- or C-terminal six-histidine tags.
NA, not applicable.
ND, not determined.
RESULTS
Structure of the GH84C Catalytic Module—To determine the overall architecture of the enzyme we dissected GH84C into a series of modular combinations. These constructs were successfully overproduced in Escherichia coli and purified in sufficient quantities for structural analysis by x-ray crystallography and SAXS (see “Experimental Procedures” for details).
The catalytic module construct of GH84C comprising the residues 41–624 of the full protein sequence was crystallized, and its structure was determined by x-ray crystal diffraction to 2.1 Å (Table 1 and Fig. 2A). This fragment of GH84C comprises three domains that are not distinguishable through amino acid-based sequence comparisons. The N-terminal domain (amino acids 41–177) is a mixed β-sheet structure consisting of six β-strands sandwiched between three α-helices; two helices on one face of the β-sheet and one helix on the other. A central domain (amino acids 178–470) adopts a (β/α)8 barrel lacking the 7th helix, whereas the C-terminal domain (amino acids 471–624) is an elongated five-helix bundle (Fig. 2A). This is identical to the structure of a GH84C fragment from C. perfringens strain 13 reported by Rao et al. to 2.25 Å, and thus the additional structural and functional properties of this catalytic region have been discussed in detail previously (19).
FIGURE 2.
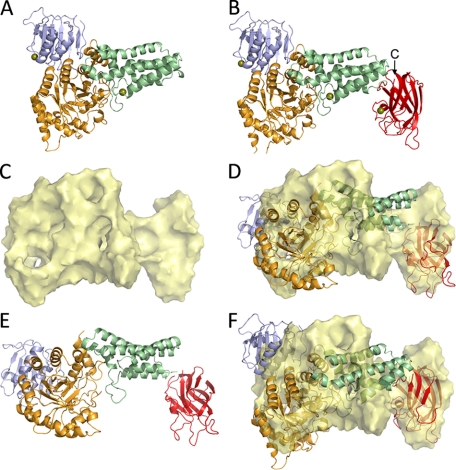
Structures of GH84C catalytic module and GH84C-CBM32 as determined using x-ray crystallography and SAXS. A and B show the crystal structures of GH84C catalytic module and GH84C-CBM32, respectively, in a ribbon representation. The arrow in B shows the C terminus of the CBM. C shows the GASBOR-generated SAXS envelope of GH84C-CBM32, whereas D shows the modules of GH84C-CBM32 manually fit into the SAXS envelope. E shows the model in D without the SAXS form. F shows the unmodified x-ray crystal structure, shown in B, fit into the SAXS-generated envelope. All of the structures are shown from identical orientations. The N-terminal domain is pictured in light blue, the catalytic TIM barrel is in orange, the helical bundle is in pale green, and the CBM in red.
Positioning the Carbohydrate-binding Module—A GH84C fragment comprising the catalytic module and the adjacent CBM32, herein called GH84C-CBM32, was also crystallized, and its structure was determined by x-ray crystallography to 3.3-Å resolution (Table 1 and Fig. 2B). Although these data were measured to comparatively low resolution the availability of the high resolution structures of the isolated catalytic module and CBM32, the latter previously determined at resolutions as high as 1.4 Å (15), made the placement of the modules by molecular replacement relatively facile and accurate. Despite the lack of excessively high mosaicity (<1) or anisotropy in the data, the refined R-factors for this structure remained relatively high at over 30%. This structure displayed substantial disorder in the N-terminal domain and portions of the (α/β)8 barrel. Indeed, the Wilson B-factor for this data were 62 Å2, which was roughly in keeping with the average refined B-factor of 52 Å2 for the protein; however, the B-factors for the N-terminal domain and portions of the (α/β)8 barrel approached 65–75 Å2 indicating their relative disorder. Thus, we primarily attributed the high R-factors to this disorder, which made it difficult to accurately model portions of the structure. This structure is important, however, not for the accurate appointment of individual atoms but for its disclosure of the relative placement of the protein's domains. The GH84C-CBM32 structure reveals the positioning of the CBM32 at the extremity of the rigid α-helical linker domain with the long axis of the CBM32 at roughly right angles to the axis of the α-helical bundle (Fig. 2B). The α-helical bundle and the CBM32 are separated by a short linker, which was clearly visible in electron density maps thus allowing unambiguous assignment of the CBM32 to the correct catalytic module in the crystal lattice. The C terminus of the CBM32 is positioned approximately at the tip of the α-helical bundle where, in the intact enzyme, the Coh module would immediately begin (Fig. 2B).
The solution conformation of the GH84C-CBM32 modular pair was also analyzed by SAXS (Table 2). The ab initio calculation of SAXS molecular envelopes for GH84C-CBM32 consistently yielded extended forms comprising a large globular region and a smaller region (Fig. 2C). An atomic model of GH84C-CBM32 based on the SAXS data was derived by two methods. In the first method, the coordinates of the composite modules were individually placed in the ab initio SAXS envelope with an attempt at minimizing bias toward the positioning of the modules in the crystal structure (Fig. 2, D and E). The crystal structure of the GH84C catalytic module was first placed in the SAXS envelope followed by rotation and translation of its domains to better fit the envelope. The coordinates of the CBM32 was then placed in the envelope in relation to the catalytic module. The relative positions of the N and C termini of contiguous domains were used as a constraint for placement. This produced a model with acceptable geometry in terms of the relative placement of the modules and gave a satisfactory χcrysol value of 2.0. Given the structural similarity of this model to the GH84C-CBM32 crystal structure, we also modeled the entire GH84C-CBM32 crystal structure into the ab initio SAXS envelope as a rigid body (Fig. 2F). The χcrysol value of the model generated in this manner was 2.1 and thus not substantially different from the approach where the modules were positioned individually. The similarity of the unbiased SAXS-derived model to the crystal structure and the goodness of the fit of the unmodified crystal structure to the SAXS data indicate that the GH84C-CBM32 crystal structure is indeed representative of the solution conformation of this polypeptide.
Orientation of the Cohesin Module—Modular constructs of GH84C larger than GH84C-CBM32 proved either recalcitrant to crystallization (GH84C-CBM32-Coh) or to heterologous production (full-length GH84C). Thus, we utilized a strictly SAXS-based approach to determine the structure of GH84C-CBM32-Coh and its complex with a fragment of the μ-toxin comprising its C-terminal Doc module and the single FIVAR module (found in various architectural regions) that precedes it, hereafter called FIVAR-Doc (see supplemental Fig. S1). These studies were initiated with the CBM32-Coh construct to provide a means of determining the relative orientations of the CBM32 and Coh modules. The maximum SAXS-derived dimension of this construct, as determined by the Dmax, was ∼85 Å (Table 2), which was somewhat smaller than the sum of the known maximum lengths of the isolated CBM32 (49 Å) and Coh (56 Å) modules determined from their crystal structures. The structure of this modular pair, determined by modeling the two modules into the ab initio SAXS envelope using the positions of the N and C termini as a constraint, supports an end-to-end arrangement with an angle of ∼115° between the two modules (Fig. 3A). The angling of the two modules creates a more compact structure, which explains the Dmax that is less than the sum of the end-to-end sizes of the modules. The relatively high χcrysol value of 2.9 for this model suggests some flexibility between these two modules that has not been accounted for in this single representative conformation. Consistent with this observation was the CBM32-Coh polypeptide's complete recalcitrance to crystallization, implying structural heterogeneity and/or flexibility that prevented packing in a crystalline lattice. Similar flexibility of bimodular carbohydrate-active enzymes has previously been observed for cellulosomal components (43). The Dmax for the CBM32-Coh·FIVAR-Doc complex was ∼95 Å and thus only slightly more extended than the CBM32-Coh structure (Table 2). An atomic model for this complex was generated by fitting the known high resolution x-ray crystal structure of the Coh·FIVAR-Doc complex (13) as a rigid body into the ab initio SAXS envelope followed by placement of the CBM32 module, again using the positions of the N and C termini as constraints (Fig. 3B). The modeled complex fit well into the envelope as judged by a χcrysol value of 1.2 and perhaps reflected a more structured relative arrangement of CBM32 and Coh modules induced by the binding of the FIVAR-Doc component.
FIGURE 3.
Structure of CBM32-Coh and CBM32-Coh·FIVAR-Doc and GH84C-CBM32-Coh as determined using SAXS. A shows, from left to right, the GASBOR-generated SAXS envelope for CBM32-Coh, the SAXS form with the structures of the CBM32 (red) and Coh (blue) modules fit into it, and the ribbon representation of the SAXS-derived model without the SAXS envelope. B shows the same for the CBM32-Coh module in complex with FIVAR-Doc from the μ-toxin (green denotes the FIVAR, pink the Doc, and yellow spheres the calcium atoms). C shows the crystal structure of GH84C-CBM32 that was overlapped with the GASBOR-generated SAXS envelope and model of CBM32-Coh, and D shows the crystal structure of GH84C-CBM32 that was overlapped with the GASBOR-generated SAXS envelope and model of the CBM32-Coh·FIVARDoc complex. E shows, from left to right, the GASBOR-generated SAXS envelope for GH84C-CBM32-Coh, the SAXS form with the structures of the catalytic module (purple, orange, and green), the CBM (red) and Coh (blue) modules fit into it, and the ribbon representation of the SAXS-derived model without the SAXS envelope.
An initial putative model of GH84C-CBM32-Coh was generated by overlaying the CBM32 of the CBM32-Coh modular pair and CBM32-Coh·FIVAR-Doc SAXS models with the CBM32 of the GH84C-CBM32 crystal structure; the orientations of Coh and Coh·FIVAR-Doc portions of the model were maintained relative to the CBM32 but placed to minimize clashes with the helical bundle of the GH84C catalytic module (Fig. 3, C and D). The two resulting models, with the FIVAR-Doc portion removed from the second model, agreed well with SAXS data collected on GH84C-CBM32-Coh with χcrysol values of 1.5 and 1.6, respectively. Indeed, the measured Dmax of ∼116 Å was consistent with the maximum dimensions of 124 for both of the two models (not considering the modeled FIVAR-Doc portions) (Table 2). To provide additional support for this modular arrangement the ab initio SAXS envelope for this polypeptide was also calculated using GASBOR. The GH84C catalytic module was modeled as a rigid body, whereas the CBM32 and Coh modules were rotated and translated relative to this component to best fit the envelope. The GH84C-CBM32 crystal structure was used as a guide for the placement of the CBM32, and, as above, the positions of the N and C termini of the component modules were used to constrain the potential orientations and spatial separation of the modules. The resulting model agreed very well with the scattering data and yielded a χcrysol of 1.2.
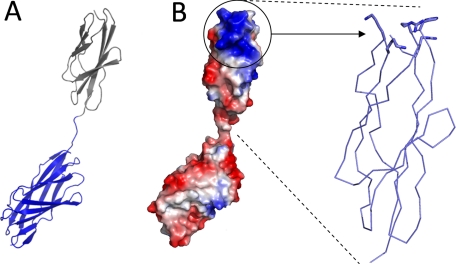
Generating a Complete Model of GH84C—The structural studies of the various GH84C modules were completed by analysis of the Coh-FN3 modular pair, whose crystal structure was determined at a resolution of 1.8 Å (Table 1 and Fig. 4A). The Coh module adopts a β-sandwich fold comprising a β-sheet of four anti-parallel β-strands packing against a β-sheet of five anti-parallel β-strands, identical to that previously described for the module in isolation (13, 14). The FN3 module also adopts a β-sandwich fold of three anti-parallel β-strands atop four anti-parallel β-strands, which is characteristic of this family of modules (44–46). Overall, the crystal structure reveals an elongated structure (109 Å long) with an extended eight-residue linker separating the two modules (Fig. 4A). SAXS analysis of this modular pair yielded a Dmax of ∼115 Å, consistent with the crystal structure and indicative of this polypeptide's tendency to adopt an extended structure (Table 2). However, standard ab initio determination of SAXS envelopes yielded inconsistent results, suggesting there may be considerable conformational flexibility between the modules, as one might expect from the inter-modular linker.
FIGURE 4.
Structural features of the Coh-FN3 modular pair. A shows a ribbon representation of the 1.8-Å crystal structure of Coh-FN3. The Coh module is depicted in blue, and FN3 is shown in black. B shows the surface representation of Coh-FN3 colored according to electrostatic potential (red is negative, and blue is positive). The basic patch of FN3 is circled and expanded to show a patch of basic residues.
Incorporation of the Coh-FN3 crystal structure into the crystal/SAXS-based structure of GH84C-CBM32-Coh enabled the generation of a complete composite model of GH84C (Fig. 5). The Coh module of the Coh-FN3 structure was overlapped with the Coh module of the GH84C-CBM32-Coh model with the lowest χcrysol value to provide an indication of the placement of the FN3 module. The Coh module of this model was then overlapped with the high resolution crystal structure of the Coh·FIVAR-Doc complex to incorporate the non-covalently associated FIVAR-Doc modules in the complete model. The resulting overall model clearly shows the splayed architecture of this complex (Fig. 5). However, this model represents only one potential conformation of the full-length enzyme. The low resolution of the SAXS analyses made it impossible to determine the absolute relative orientations of the CBM32 and Coh modules. Although the relative angles between the individual long axes of the modules could be approximated, their relative rotational orientations around these axes could not. Therefore, assuming the CBM32 is appropriately positioned based on the x-ray crystal structure of GH84C-CBM32, this leaves some uncertainty as to the exact relative positioning of the Doc-binding site on the Coh module. However, given the steric constraints from the closely neighboring CBM32, other potential orientations of the Coh module would likely have the Doc-binding site rotated away from the CBM32 (Fig. 5). Indeed, considering the complex structural architecture of this enzyme and the somewhat limited potential for inter-modular contacts, there is likely some capacity for movement between the modules, such as that suggested by the SAXS analysis of the CBM32-Coh structure. Inter-modular flexibility is likely to be particularly pronounced within the linker between the Coh and FN3. Thus, the FN3 in this enzyme likely samples a large number of spatial conformations relative to the remainder of the protein (Fig. 5).
FIGURE 5.
Composite structure of GH84C. The overall structure of GH84C in complex with the FIVAR-Doc module from the μ-toxin determined by the amalgamation of the SAXS data and crystallographic data is shown. Two Asp residues, 297, proposed to be involved in stabilizing the conformation of the acetamido group of the N-acetylglucosamine, and 298, proposed to be the catalytic acid, of GH84C are shown as sticks interacting with the competitive inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenyl carbamate, which was modeled on the basis of the C. perfringens GH84C complex determined by Rao et al. (19). The CBM32 module is shown interacting with its carbohydrate ligand, N-acetyllactosamine, which was modeled on the basis of the previously determined complex of the CBM with this sugar (15). Arrows near the FN3 module represents possible motion of this module due to the flexible linker region. The structure is also shown rotated by 90° around the horizontal axis running parallel to the page (right). The same structure is shown below in a solvent-accessible surface representation. The N-terminal domain is pictured in light blue, the catalytic TIM barrel is colored orange, the helical bundle is in pale green, the CBM32 is red, the cohesin module is blue, and the FN3 module is black. The FIVAR and Doc modules from the μ-toxin are shown in green and pink, respectively. Calcium atoms are shown in yellow.
DISCUSSION
The overall composite structure of GH84C is exceptionally revealing of how the known functions of the individual modules of GH84C are coordinated in three-dimensional space. The most immediate and striking observation is the relative positioning of the catalytic site and the binding site of the CBM32. GH84C is an exo-β-d-N-acetylglucosaminidase whose activity is likely directed toward terminal N-acetylglucosamine residues presented by host tissues (19). CBM32 binds carbohydrates bearing non-reducing terminal N-acetylgalactosamine disaccharide motifs but also binds other terminally galactosylated sugars (15). It is widely maintained that the CBMs present in carbohydrate-active enzymes target and hold it in proximity to substrate (47, 48). However, there are surprisingly few intact structures of carbohydrate-active enzymes, and no solution structures, that provide insight into how this adherence and catalysis might be coordinated. Our model of GH84C, which is based on both the crystal and solution conformations of the protein, reveals that the active site of the catalytic module and the carbohydrate-binding site of the CBM32, separated by ∼60 Å, are located on the same side of the protein and are oriented in the same direction (Fig. 5). This suggests an optimal arrangement to simultaneously interact with and hydrolyze terminal glycans presented on a surface, such as those clustered on the outer surface of a host cell or mucosal surface. Although such a coordinated attack of binding and catalysis has been implicated in CBM-containing carbohydrate-active enzymes, this suggestion has been mainly limited to enzymes that hydrolyze insoluble plant cell-wall polysaccharides and has not been directly supported by solution structure data. The available structures of multimodular carbohydrate-active enzymes involved in bacterial pathogenesis have been primarily restricted to the sialidases from Vibrio cholerae (VCNA) and, more recently, Streptococcus pneumoniae (NanB) (49, 50). These proteins both contain a β-propeller catalytic module, which is fused to two CBM40 modules in VCNA and one CBM40 in NanB. In both of these proteins, the sialic acid-binding site of the CBM is oriented in the same direction as the catalytic site, as in GH84C; however, the distance between catalytic sites and carbohydrate binding sites is only ∼25–30 Å. Although these multimodular enzymes lack the complexity typical of extracellular carbohydrate-active enzymes from bacterial pathogens, the arrangement of their catalytic sites and CBM carbohydrate-binding sites do support a model of coordinated catalysis and adherence.
One of the most fascinating and newly discovered features of C. perfringens enzymes is their capacity to interact with each other via an ultra-tight Coh-Doc interaction (13). The model of GH84C shows that the Doc-binding site on the Coh module is spatially removed from the carbohydrate-recognizing components of the enzyme thus allowing simultaneous substrate recognition and recruitment of other carbohydrate-active enzymes through the Coh-Doc interaction. Nevertheless, the structural placement of the Coh would permit another associated enzyme to lie in the same plane as the substrate-presenting surface thus allowing the second component to act on the carbohydrate-bearing surface as well. Indeed, three C. perfringens glycoside hydrolases, including the μ-toxin, are known to contain functional dockerin modules, and two of these also contain CBMs (13) (see supplemental Fig. S1). This latter observation raises the possibility of substantial avidity effects resulting from simultaneous binding to host glycans by two or more CBMs present in these enzyme complexes. The harmonized formation of such complexes would also harness the diversity of specificities displayed by C. perfringens enzymes to synergistically degrade host tissue complex glycans.
FN3 modules are found in numerous bacterial carbohydrate-active enzymes, yet little is known about their function(s). Indeed, the function of the FN3 module of GH84C remains enigmatic. In eukaryotic systems, FN3s are involved in a variety of molecular recognition processes such as cell adhesion, cell surface hormone receptors, and cytokine receptors by facilitating protein-protein interactions (51–53). The structural opposition of the FN3 from the active site of GH84C suggests that the FN3 module does not play a role in substrate recognition (Fig. 5). However, given the historical role of FN3s in protein-protein interactions and its spatial placement in the enzyme, it is conceivable that the GH84C FN3 module, like the Coh module, may mediate recruitment of other C. perfringens enzymes through an as yet unidentified interaction. Another plausible function is suggested by the unique surface charge of the FN3 module. The tip of the FN3 module that is most distal from the body of the enzyme has a distinct patch of basic residues, which is completely conserved among C. perfringens FN3 modules (not shown), leading to a cluster of positive charge at physiological pH (Fig. 4B). The charge of this patch is complementary to the negative charge of cell walls in Gram-positive bacteria, such as C. perfringens, which is imparted by the phosphodiester bonds of techoic acid moieties on the exterior of the bacterium (54, 55). Considering the spatial disposition of the FN3 and its associated basic patch, FN3s may function to bind the enzyme with the bacterial cell wall via an electrostatic interaction. In this scenario, the Doc-containing enzymes, which invariably lack FN3 modules or other obvious cell-wall attachment motifs, could also be tethered to the cell wall through their interaction with Coh-containing proteins, which always contain FN3 modules (supplemental Fig. S1).
The human body provides accommodation to a greater number of bacterial cells than it has of its own cells (56, 57). The majority of niches within the body that are occupied by these bacteria, such as the mucosal surfaces of gastrointestinal tract, the genitourinary tract, and the upper respiratory tract, display enormous carbohydrate content and diversity. Not surprisingly, commensal bacteria, which are often also opportunistic pathogens, as well as other dedicated pathogens, have developed extensive carbohydrate-degrading machineries to enable their persistence in the host and to promote infection. The enzymes comprising these carbohydrate-degrading systems are often large and complex and thus poorly understood. Here, we have provided a detailed look at the complete structure of such a complex enzyme from C. perfringens. As a model system, this structure provides a first glimpse at how carbohydrate adherence via a CBM, glycosidic bond hydrolysis by a catalytic module, complex formation through a protein-protein interaction, and potentially bacterial-cell wall attachment are coordinated spatially in one enzyme. Although the numerous bacterial carbohydrate-active virulence factors do not display an identical arrangement of modules, they do invariably contain catalytic modules, which are often associated with CBMs, and frequently numerous other modules of unknown functions. The investigation of GH84C presented here provides a paradigm to guide the analysis and interpretation of other important multimodular microbial enzymes.
Supplementary Material
Acknowledgments
We thank Prof. Gideon Davies for providing the coordinates of BtGH84 prior to their deposition and the staff members of beamline X6A at the National Synchrotron Light Source for beam time and assistance. We acknowledge A. Kikhney, A. Zozulya, and D. Svergun for the use of and assistance with the X33 SAXS beamline of the European Molecualr Biology Laboratory outstation, Deutsches Electronen Synchrotron, Hamburg.
This work was supported in part by a Canadian Institutes of Health Research (CIHR) operating grant (to A. B. B. and S. P. S.) and the CNRS (to M. C.). The atomic coordinates and structure factors (codes 2v5c, 2v5d, and 2win) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Tables S1 and S2.
Footnotes
The abbreviations used are: CBM32, family 32 carbohydrate-binding module; Coh, cohesin; FN3, fibronectin type III; Doc, dockerin; SAXS, small-angle x-ray scattering; FIVAR, found in various architectural regions module.
References
- 1.Bryant, A. E., Bayer, C. R., Chen, R. Y., Guth, P. H., Wallace, R. J., and Stevens, D. L. (2005) J. Infect. Dis. 192 1014–1022 [DOI] [PubMed] [Google Scholar]
- 2.Hynes, W. (2004) Front. Biosci. 9 3399–3433 [DOI] [PubMed] [Google Scholar]
- 3.Smedley, J. G., 3rd, Fisher, D. J., Sayeed, S., Chakrabarti, G., and McClane, B. A. (2004) Rev. Physiol. Biochem. Pharmacol. 152 183–204 [DOI] [PubMed] [Google Scholar]
- 4.Stevens, D. L., and Bryant, A. E. (2002) Clin. Infect. Dis. 35 S93–S100 [DOI] [PubMed] [Google Scholar]
- 5.Canard, B., Garnier, T., Saint-Joanis, B., and Cole, S. T. (1994) Mol. Gen. Genet. 243 215–224 [DOI] [PubMed] [Google Scholar]
- 6.Flores-Diaz, M., Alape-Giron, A., Clark, G., Catimel, B., Hirabayashi, Y., Nice, E., Gutierrez, J. M., Titball, R., and Thelestam, M. (2005) J. Biol. Chem. 280 26680–26689 [DOI] [PubMed] [Google Scholar]
- 7.Johnson, S., and Gerding, D. N. (1997) in The Clostridia Molecular Biology and Pathogenesis (Rood, J. I., McClane, B. A., Songer, J. G., and Titball, R. W., eds) pp. 117–140, Harcourt Brace & Company, London
- 8.Sheldon, W. L., Macauley, M. S., Taylor, E. J., Robinson, C. E., Charnock, S. J., Davies, G. J., Vocadlo, D. J., and Black, G. W. (2006) Biochem. J. 399 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figura, N. (1997) J. Clin. Gastroenterol. 25 S149–S163 [DOI] [PubMed] [Google Scholar]
- 10.Galen, J. E., Ketley, J. M., Fasano, A., Richardson, S. H., Wasserman, S. S., and Kaper, J. B. (1992) Infect. Immun. 60 406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelburne, S. A., Davenport, M. T., Keith, D. B., and Musser, J. M. (2008) Trends Microbiol. 16 318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott, D. W., Eirin-Lopez, J. M., and Boraston, A. B. (2008) Mol. Biol. Evol. 25 155–167 [DOI] [PubMed] [Google Scholar]
- 13.Adams, J. J., Gregg, K., Bayer, E. A., Boraston, A. B., and Smith, S. P. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 12194–12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chitayat, S., Gregg, K., Adams, J. J., Ficko-Blean, E., Bayer, E. A., Boraston, A. B., and Smith, S. P. (2008) J. Mol. Biol. 375 20–28 [DOI] [PubMed] [Google Scholar]
- 15.Ficko-Blean, E., and Boraston, A. B. (2006) J. Biol. Chem. 281 37748–37757 [DOI] [PubMed] [Google Scholar]
- 16.Thobhani, S., Ember, B., Siriwardena, A., and Boons, G. J. (2003) J. Am. Chem. Soc. 125 7154–7155 [DOI] [PubMed] [Google Scholar]
- 17.Rood, J. I., and Cole, S. T. (1991) Microbiol. Rev. 55 621–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boraston, A. B., Ficko-Blean, E., and Healey, M. (2007) Biochemistry 46 11352–11360 [DOI] [PubMed] [Google Scholar]
- 19.Rao, F. V., Dorfmueller, H. C., Villa, F., Allwood, M., Eggleston, I. M., and van Aalten, D. M. (2006) EMBO J. 25 1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ficko-Blean, E., and Boraston, A. B. (2005) Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 61 834–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ficko-Blean, E., Stubbs, K. A., Nemirovsky, O., Vocadlo, D. J., and Boraston, A. B. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 6560–6565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitayat, S., Adams, J. J., Furness, H. S., Bayer, E. A., and Smith, S. P. (2008) J. Mol. Biol. 381 1202–1212 [DOI] [PubMed] [Google Scholar]
- 23.Gasteiger, E., Gattiker, A., Hoogland, C., Ivanyi, I., Appel, R. D., and Bairoch, A. (2003) Nucleic Acids Res. 31 3784–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pflugrath, J. W. (1999) Acta Crystallogr. D Biol. Crystallogr. 55 1718–1725 [DOI] [PubMed] [Google Scholar]
- 25.Zwart, P. H., Afonine, P. V., Grosse-Kunstleve, R. W., Hung, L. W., Ioerger, T. R., McCoy, A. J., McKee, E., Moriarty, N. W., Read, R. J., Sacchettini, J. C., Sauter, N. K., Storoni, L. C., Terwilliger, T. C., and Adams, P. D. (2008) Methods Mol. Biol. 426 419–435 [DOI] [PubMed] [Google Scholar]
- 26.Dennis, R. J., Taylor, E. J., Macauley, M. S., Stubbs, K. A., Turkenburg, J. P., Hart, S. J., Black, G. N., Vocadlo, D. J., and Davies, G. J. (2006) Nat. Struct. Mol. Biol. 13 365–371 [DOI] [PubMed] [Google Scholar]
- 27.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60 2126–2132 [DOI] [PubMed] [Google Scholar]
- 28.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53 240–255 [DOI] [PubMed] [Google Scholar]
- 29.Cowtan, K. D., and Zhang, K. Y. (1999) Prog. Biophys. Mol. Biol. 72 245–270 [DOI] [PubMed] [Google Scholar]
- 30.Morris, R. J., Perrakis, A., and Lamzin, V. S. (2002) Acta Crystallogr. D Biol. Crystallogr. 58 968–975 [DOI] [PubMed] [Google Scholar]
- 31.Vagin, A., and Teplyakov, A. (1997) J. Appl. Crystallogr. 30 1022–1025 [Google Scholar]
- 32.Brunger, A. T. (1992) Nature 355 472–475 [DOI] [PubMed] [Google Scholar]
- 33.Vaguine, A. A., Richelle, J., and Wodak, S. J. (1999) Acta Crystallogr. D Biol. Crystallogr. 55 191–205 [DOI] [PubMed] [Google Scholar]
- 34.Roman, A., Laskowski, M. W. M., Moss, D. S., and Thornton, J. M. (1993) J. App. Crystallogr. 26 283–291 [Google Scholar]
- 35.Guinier, A. F., and Fournet, F. (1955) Small Angle Scattering of X-rays, Wiley Interscience, New York
- 36.Svergun, D. (1992) J. Appl. Crystallogr. 25 495–503 [Google Scholar]
- 37.Bergmann, A., Fritz, G., and Glatter, O. (2000) J. Appl. Crystallogr. 33 1212–1216 [Google Scholar]
- 38.Svergun, D. I., Petoukhov, M. V., and Koch, M. H. (2001) Biophys. J. 80 2946–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkov, V. V., and Svergon, D. I. (2003) J. Appl. Crystallogr. 36 860–864 [Google Scholar]
- 40.Putnam, C. D., Hammel, M., Hura, G. L., and Tainer, J. A. (2007) Q. Rev. Biophys. 40 191–285 [DOI] [PubMed] [Google Scholar]
- 41.Petoukhov, M. V., Eady, N. A., Brown, K. A., and Svergun, D. I. (2002) Biophys. J. 83 3113–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konarev, P. V., Volkov, V. V., Sokolova, A. V., Koch, M. H. J., and Svergun, D. I. (2003) J. Appl. Crystallogr. 36 1277–1282 [Google Scholar]
- 43.Hammel, M., Fierobe, H. P., Czjzek, M., Finet, S., and Receveur-Brechot, V. (2004) J. Biol. Chem. 279 55985–55994 [DOI] [PubMed] [Google Scholar]
- 44.Bisig, D., Weber, P., Vaughan, L., Winterhalter, K. H., and Piontek, K. (1999) Acta Crystallogr. D Biol. Crystallogr. 55 1069–1073 [DOI] [PubMed] [Google Scholar]
- 45.Leahy, D. J., Aukhil, I., and Erickson, H. P. (1996) Cell 84 155–164 [DOI] [PubMed] [Google Scholar]
- 46.Leahy, D. J., Erickson, H. P., Aukhil, I., Joshi, P., and Hendrickson, W. A. (1994) Proteins 19 48–54 [DOI] [PubMed] [Google Scholar]
- 47.Bolam, D. N., Ciruela, A., Mcqueen-Mason, S., Simpson, P., Williamson, M. P., Rixon, J. E., Boraston, A., Hazlewood, G. P., and Gilbert, H. J. (1998) Biochem. J. 331 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boraston, A. B., Bolam, D. N., Gilbert, H. J., and Davies, G. J. (2004) Biochem. J. 382 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gut, H., King, S. J., and Walsh, M. A. (2008) FEBS Lett. 582 3348–3352 [DOI] [PubMed] [Google Scholar]
- 50.Moustafa, I., Connaris, H., Taylor, M., Zaitsev, V., Wilson, J. C., Kiefel, M. J., von Itzstein, M., and Taylor, G. (2004) J. Biol. Chem. 279 40819–40826 [DOI] [PubMed] [Google Scholar]
- 51.Bencharit, S., Cui, C. B., Siddiqui, A., Howard-Williams, E. L., Sondek, J., Zuobi-Hasona, K., and Aukhil, I. (2007) J. Mol. Biol. 367 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen, S. M., Kohler, L. B., Li, S., Kiselyov, V., Christensen, C., Owczarek, S., Bock, E., and Berezin, V. (2008) J. Neurochem. 106 2030–2041 [DOI] [PubMed] [Google Scholar]
- 53.Yang, F., West, A. P., Jr., Allendorph, G. P., Choe, S., and Bjorkman, P. J. (2008) Biochemistry 47 4237–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myers, G. S., Rasko, D. A., Cheung, J. K., Ravel, J., Seshadri, R., DeBoy, R. T., Ren, Q., Varga, J., Awad, M. M., Brinkac, L. M., Daugherty, S. C., Haft, D. H., Dodson, R. J., Madupu, R., Nelson, W. C., Rosovitz, M. J., Sullivan, S. A., Khouri, H., Dimitrov, G. I., Watkins, K. L., Mulligan, S., Benton, J., Radune, D., Fisher, D. J., Atkins, H. S., Hiscox, T., Jost, B. H., Billington, S. J., Songer, J. G., McClane, B. A., Titball, R. W., Rood, J. I., Melville, S. B., and Paulsen, I. T. (2006) Genome Res. 16 1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weidenmaier, C., and Peschel, A. (2008) Nat. Rev. Microbiol. 6 276–287 [DOI] [PubMed] [Google Scholar]
- 56.Hooper, L. V., and Gordon, J. I. (2001) Science 292 1115–1118 [DOI] [PubMed] [Google Scholar]
- 57.Sears, C. L. (2005) Anaerobe 11 247–251 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.