Abstract
Hypoxia-inducible factor (HIF) accumulates when tumors grow under hypoxic conditions. The genesis of tumors, however, usually involves normoxic conditions. In this study, we were interested in examining the potential role of aryl hydrocarbon receptor nuclear translocator (ARNT)/HIF-1β in tumor growth under normoxic conditions, specifically when cells are treated with epidermal growth factor (EGF), which is known to affect the gene expression of tumor growth-related protein COX-2 (cyclooxygenase-2). The results showed that EGF receptor inhibitor, AG1478, abolished EGF-induced nuclear accumulation of ARNT as well as the expression of COX-2. ARNT small interfering RNA inhibited the promoter activity, mRNA level, and protein expression of COX-2 in cells treated with EGF. In contrast, CoCl2-induced HIF-1α exhibited no effect on COX-2 expression. EGF also stimulated the formation of the ARNT·c-Jun complex as well as the complex binding to the COX-2 promoter. ARNT small interfering RNAs blocked EGF-activated cell migration. Moreover, COX-2 and ARNT were cohorts present distinctively in clinical specimens of human cervical squamous cell carcinoma and were almost nondetectable in adjacent normal or noncancerous cervical tissues. Our results revealed that ARNT plays an important role in EGF-regulated COX-2 gene expression and may thus be related to either a cause or a consequence of tumorigenesis in cervical cancer.
The aryl hydrocarbon receptor nuclear translocator (ARNT)3 (also known as HIF-1β (hypoxia-inducible factor-1β)) is a member of the basic helix-loop-helix Per/aryl hydrocarbon receptor (AhR)/ARNT/Sim (bHLH-PAS) family of transcription factors and a general partner factor for bHLH-PAS proteins such as the AhR, HIF-1α (hypoxia-inducible factor-1α), and single-minded (SIM) proteins (1). Although the in vivo importance of ARNT homodimers remains unclear, each of the heterodimeric ARNT-containing complexes plays a critical function in pathways used to respond to environmental conditions. As in exposure to xenobiotic compounds, the AhR/ARNT heterodimer recognizes and promotes transcription from xenobiotic response elements found upstream of 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive genes (1). ARNT also cooperates with HIF-1α to form a heterodimer and regulates several genes involved in tumorigenesis under hypoxia (2). In addition, ARNT is essential for normal embryonic development (3). Loss of ARNT results in reduced tumor growth, decreased angiogenesis, and increased response to radiotherapy (4, 5). Inactivation of ARNT suppresses the development of liver hemangioma, polycythemia, and HIF-induced gene expression. Unlike ARNT, however, HIF-1α is insufficient to suppress the development of the Von Hippel-Lindau-associated polychthemia (6). These results demonstrate that the development of Von Hippel-Lindau-associated vascular tumors in the liver depends on functional ARNT but not in an HIF-1α/hypoxia-dependent manner.
Prostaglandin endoperoxide synthase, also known as cyclooxygenase, plays a regulatory role in the conversion of arachidonic acid to inflammatory prostaglandins (7). It is well documented that COX-2 expression contributes to increased antiapoptotic, proangiogenic, and metastatic potential in cancer cells, and COX-2 deregulation is correlated with tumor progression (8, 9). COX-2-derived PGE2 stimulates angiogenesis via HIF-1α, leading to the induction of vascular endothelial growth factor (10). Transcriptional up-regulation of the COX-2 gene by epidermal growth factor receptor (EGFR) and ErbB-2 has been found in cultured cancer cells (11–14). In addition to growth factors, COX-2 is induced under hypoxia by HIF-1α/ARNT in cells (15, 16).
Similar to COX-2, EGFR is highly expressed in a variety of human tumors and has been associated with poor prognosis and decreased survival (17). Activation of EGFR signaling pathways has many effects, including increased proliferation and angiogenesis and decreased apoptosis (18). Activation of EGFR signaling leads to increased mitogen-activated protein kinase activity, resulting in AP-1-mediated induction of COX-2 transcription (13, 14). COX-2-derived PGE2, however, can further activate EGFR signaling and thereby stimulate cell proliferation (19, 20). In preclinical studies, combining an inhibitor of COX-2 with an inhibitor of EGFR tyrosine kinase was more effective than either agent alone in suppressing tumor formation (21). In clinical studies, synchronous coexpression of EGFR and COX-2 has been demonstrated in carcinomas of the uterine cervix; the strong coexpression indicates a potential predictor of poor survival (22). So far, dual blockage of EGFR and COX-2 in head and neck cancer and in lung cancer has been investigated in clinical trials (23, 24).
Concerning the regulation of COX-2 gene expression, we recently found that N-terminal phosphorylation of c-Jun is not required for EGF-induced expression of COX-2 (25). Our results suggest a model in which c-Jun expression, induced by EGF, could recruit either c-Fos or other transcription factors to the promoter and regulate gene expression of COX-2. In order to identify factors involved in the regulation of EGF-induced COX-2 expression, factors bound to the CRE site of the COX-2 promoter were analyzed by a DNA affinity precipitation assay. The bound proteins were subsequently analyzed by two-dimensional SDS-PAGE. Proteins on the gel were identified by matrix-assisted laser desorption ionization time-of-flight. ARNT, which was one of the proteins bound to the probe, was clearly identified. In this study, we demonstrated for the first time that EGF-induced COX-2 expression was mediated by accumulation of ARNT in the nucleus, resulting in a complex formation of c-Jun·ARNT. Coexpression of COX-2 and ARNT was found clinically in human cervical cancer tissue. These results provide the new regulatory mechanism of HIF-1 factor involved in growth factor-induced gene expression under normoxia.
EXPERIMENTAL PROCEDURES
Cell Culture—The cell line of human squamous cell carcinoma (A431) was grown at 37 °C under 5% CO2 in 10-cm plastic dishes containing 10 ml of Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 units/ml penicillin. In this series of experiments, cells were treated with 50 ng/ml EGF (Pepro Technology, Rocky Hill, NJ) in culture medium supplemented with 10% fetal bovine serum, unless stated otherwise. The cell line of human cervical cancer, SiHa, and oral cavity squamous cell carcinoma, OEC-M1, were also grown in same manner but with different media, including Dulbecco's modified Eagle's medium and RPMI 1640, respectively, together with the supplement of 10% fetal bovine serum.
Immunofluorescence—Cells were seeded onto glass slides overnight and fixed with 4% paraformaldehyde (Sigma) in phosphate-buffered saline at 4 °C for 10 min. The cells were then rinsed with phosphate-buffered saline three times and permeabilized with 1% Triton X-100 for 7 min. Next, the cells were pretreated with 1% bovine serum albumin in phosphate-buffered saline at 25 °C for 60 min and incubated with rabbit anti-ARNT polyclonal antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a dilution of 1:100 for 1 h and treated with fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG polyclonal antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) at a dilution of 1:250 for 1 h. Finally, the cells were washed with phosphate-buffered saline, mounted in 90% glycerol containing 4,6-diamidino-2-phenylindole (Invitrogen), and examined by using a microscope (model DMI 4000 B; Leica).
Plasmid Construction—ARNT cDNA fragments were generated by PCR and were subcloned into pcDNA3.1myc/His vector (Invitrogen) at the BamHI and EcoRV sites. The forward and reverse primers were 5′-CGGGATCCATGGCGGCGACTACTG-3′ and 5′-CGGATATCTTCTGAAAAGGGGGGAAAC-3′, respectively. The vector sequence was confirmed by DNA sequencing.
Transfection of Cells with Plasmids and Luciferase Assay—Luciferase vectors bearing various lengths or mutants of the COX-2 gene promoter were used (14). Transient transfection of cells with plasmids was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions but with slight modifications. A431 cells were replated 36 h before transfection at a density of 3 × 105 cells in 2 ml of fresh culture medium in a 3.5-cm plastic dish. For use in transfection, 2 μl of Lipofectamine 2000 was incubated with 0.5 μg of pXC luciferase plasmid, ARNT siRNA, or scramble siRNA (Invitrogen) and plasmids, where indicated, such as Myc-ARNT in 1 ml of Opti-MEM medium for 30 min at room temperature. Cells were transfected by changing the medium with 1 ml of Opti-MEM medium containing the plasmids and Lipofectamine 2000 and then incubated at 37 °C in a humidified atmosphere of 5% CO2 for 24 h. Following the change of Opti-MEM medium to 2 ml of fresh culture medium, cells were incubated further for an additional 24 h unless stated otherwise. The luciferase activity in cell lysate was determined as described previously (26).
Reverse Transcription-PCR—Total RNA was isolated using the TRIzol RNA extraction kit (Invitrogen), and 5 μg of RNA was subjected to reverse transcription-PCR with SuperScript™II (Invitrogen). The COX-2-specific primers (sense, 5′-CCCACTTCAAGGGATTTT-3′; antisense, 5′-CCAGACCAAAGACCTCCT-3′), ARNT-specific primers (sense, 5′-TGGGTCCAGCCATTGCCTCT-3′; antisense, 5′-CGAGCCAGGGCACTACAGGT-3′), and glyceraldehyde-3-phosphate dehydrogenase primers were used. The PCR products were separated by 1% agarose gel electrophoresis and visualized with ethidium bromide staining.
Western Blotting—The cytoplasmic fractions and nuclear extracts of cells were prepared for Western blot analysis according to the method described (27). An analytical 10% SDS-PAGE was performed, and 30 μg of protein of each was analyzed, unless stated otherwise. For immunoblotting, proteins in the SDS gels were transferred to a polyvinylidene difluoride membrane by an electroblot apparatus. Antibodies against human HIF-1α (Upstate Biotechnology, Inc., Lake Placid, NY), c-Jun (Santa Cruz Biotechnology), COX-2 (Lab Vision Corp. (Fremont, CA) and Cayman (Ann Arbor, MI)), ARNT (Santa Cruz Biotechnology), and β-actin (Santa Cruz Biotechnology) were used as the primary antibodies. Mouse or rabbit IgG antibodies coupled to horseradish peroxidase were used as secondary antibodies. An enhanced chemiluminescence kit (Pierce) was used for detection.
Coimmunoprecipitation—A total of 200 μg of protein of nuclear extracts and lysate was incubated under gentle shaking at 4 °C overnight with a mixture of anti-c-Jun or anti-ARNT antibodies and protein A-agarose in 300 μl of immunoprecipitation buffer (20 mm HEPES, pH 7.9, 420 mm NaCl, 1.5 mm MgCl2, 25% glycerol (v/v), 0.5 mm phenylmethylsulfonyl fluoride, 1 mm orthovanadate, 2 μg/ml pepstatin A, and 2 μg/ml leupeptin). Beads were pelleted at 7500 × g for 2 min and washed three times with radioimmune precipitation buffer (50 mm Tris-HCl, pH 7.5, 1% IGEPAL CA-630 (v/v), 150 mm NaCl, and 0.5% sodium deoxycholate). Protein was removed from the beads by boiling in sample buffer (120 mm Tris-HCl, pH 6.8, 10% glycerol, 3% SDS, 20 mm dithiothreitol, and 0.4% bromphenol blue) for 5 min and subjected to SDS-PAGE on a 10% gel. Western blot analysis was carried out as described above.
DNA Affinity Precipitation Assay—Quantitation of the change of c-Jun and ARNT binding to the COX-2 promoter element was achieved by a DNA affinity precipitation assay according to the method previously described (28). In brief, 5′-biotinylated oligonucleotides and corresponding to the sense –67 to –42 bp and antisense strands of the COX-2 promoter element were annealed. The DNA affinity precipitation assay was performed by incubating 2 μg of biotinylated DNA probe with 200 μg of nuclear extract and 20 μl of streptavidin-agarose beads in phosphate-buffered saline at room temperature for 1 h with rotation. Beads were collected and washed three times with cold phosphate-buffered saline. The binding proteins were eluted by loading buffer and separated by SDS-PAGE, followed by Western blot analysis probed with specific antibodies.
Chromatin Immunoprecipitation Assay—A chromatin immunoprecipitation assay was performed as previously reported (29) with minor modifications. Briefly, A431 cells were treated with 1% formaldehyde for 15 min. The cross-linked chromatin was sonicated to 400–500 bp fragments. Lysates were precleaned with protein A beads and incubated overnight at 4 °C with antibodies specific to ARNT (Santa Cruz Biotechnology), c-Jun (Santa Cruz Biotechnology), or control rabbit IgG. Immune complex was precipitated with protein A beads preabsorbed with sonicated single-stranded DNA and bovine serum albumin. After reversal of cross-linking, levels of precipitated COX-2 promoter DNA were determined by PCR using oligonucleotides spanning the CRE binding sites (sense, 5′-CTGGGTTTCCGATTTTCTCA-3′; antisense, 5′-GAGTTCCTGGACGTGCTCCT-3′). The PCR products were separated by 1% agarose gel electrophoresis and visualized with ethidium bromide staining.
Transwell Migration and Wound Healing Assay—A431 cells were transfected with 50 nm ARNT siRNA or scramble oligonucleotides by lipofection. The cells were trypsinized, and 2 × 105 cells were added to the Boyden chambers (8-μm pore size; Millipore, Bedford, MA), and assay media with or without EGF was added to the culture plates. After EGF treatment for 15 h, the nonmotile cells at the top of the filter were removed, and the motile cells at the bottom of the filter were fixed with 70% ethanol and stained with a one-tenth dilution of Giemsa (Sigma). The number of migrating cells in each chamber was quantified by counting four fields. For wound healing assay, cells were transfected with 50 nm ARNT siRNA or scramble oligonucleotides by lipofection. After the cell layer reached confluence, a wound was made with a pipette tip, followed by extensive washing with serum-free medium to remove cell debris. Cells were then cultured as described under “Cell Culture,” treated with 50 ng/ml EGF and 10 μm PGE2, and allowed to migrate into the wound area for up to 60 h. The extent of wound closure was observed by using a phase-contrast microscope camera (model DMI 4000 B; Leica).
Surgical Specimens of Cervical Cancer—From January 1999 to December 2000, 100 consecutive patients with carcinoma of uterine cervix were scheduled for radical hysterectomy and pelvic lymphadenectomy at National Cheng Kung University Hospital, Taiwan. The clinical staging and histological classifications were based on the criteria of the International Federation of Gynecology and Obstetrics and the World Health Organization, respectively. Patients who had undergone conization of the cervix before radical hysterectomy and who had histopathology of squamous cell carcinoma were included in this study. For immunofluorescent stainings, sections of surgical specimens were stained with polyclonal antibodies against ARNT (Santa Cruz Biotechnology) and monoclonal antibody against COX-2 (Cayman), followed by exposure to Alexa 488-labeled or Alexa 594-labeled secondary antibodies (Molecular Probes, Inc., Eugene, OR) and by Hoechst 33258 (Sigma). At least five sections from each patient's specimen were analyzed for histology, ARNT, and COX-2 expression. Each section was examined blindly by two investigators trained in cervical pathology. ARNT and COX-2 staining were evaluated manually or by image analysis with the use of a CoolSnap-Pro color digital camera (Roper Scientific, Trenton, NJ) and Image-Pro Plus 4.1 software (Media Cybernetics, Silver Spring, MD) over 15–20 high power fields. The intensity and distribution of immunofluorescent stains of ARNT and COX-2 were graded using a scale of I–III in each specimen. At least 500 tumor cells were analyzed in each case, and the grade of stained tumor cells was recorded. For ARNT staining, tumors were considered grade III if more than 30% of the cells showed distinct strong nuclear stainings. Tumors were considered grade I of ARNT staining if fewer than 5% of the cells showed distinct nuclear stainings. For COX-2 staining, grade I indicates that COX-2 staining intensity and distribution are less than one-third of the tumor area. Grade II indicates that the staining intensity and distribution are greater than one-third but less than two-thirds of the tumor area. Grade III indicates that the staining intensity and distribution are greater than two-thirds of the tumor area.
RESULTS
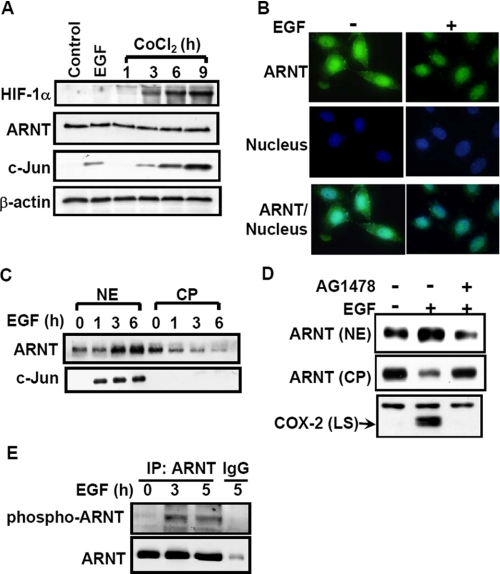
ARNT Translocates into Nucleus in EGF-treated A431 Cells—Since ARNT was clearly identified in the binding to COX-2 promoter (as shown in Fig. 4C), we then examined whether HIF-1, a HIF-1α/ARNT heterodimer, was involved in EGF-activated signaling pathways, resulting in the regulation of COX-2 expression. We first tested the effect of EGF on HIF-1α protein expression in A431 cells. As shown in Fig. 1A, HIF-1α was barely detectable in EGF-treated cells. On the other hand, a hypoxia-mimetic agent, CoCl2, could induce HIF-1α protein expression in a time-dependent manner. Both EGF and CoCl2 induced c-Jun expression but showed no effect on ARNT expression (Fig. 1A). Interestingly, although ARNT predominantly existed in the nucleus, EGF enhanced the accumulation of ARNT in the nucleus (Fig. 1, B and C); the accumulation of ARNT in the nucleus was abolished by the EGF receptor inhibitor AG1478 (Fig. 1D). Correlating with the decreased ARNT level caused by AG1478 in the nucleus, EGF-induced COX-2 expression was inhibited (Fig. 1D). In addition, EGF also stimulated the phosphorylation of ARNT (Fig. 1E). These results indicate that EGF induced the accumulation of ARNT in the nucleus but had no effect on the HIF-1α expression. The phospho-ARNT induced by EGF may account for the ARNT accumulation in the nucleus.
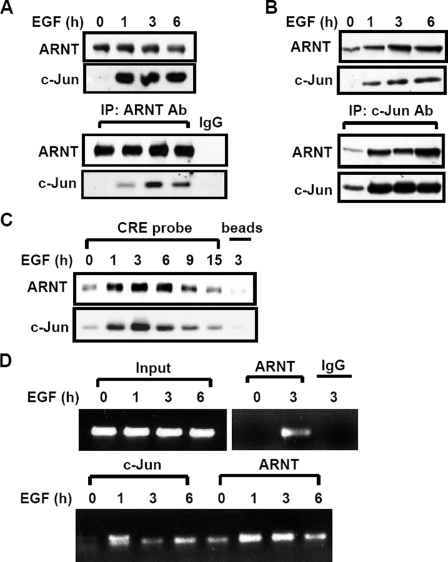
FIGURE 4.
EGF induces the complex formation and the binding of c-Jun·ARNT to the COX-2 gene promoter. A–C, cells were starved for 18 h in serum-free culture medium and then treated with EGF for various time periods (as indicated) in the culture medium without serum. Cellular lysates (A) and nuclear extracts (B) of cells were prepared and subjected to Western blot or immunoprecipitated (IP) with antibodies against c-Jun and ARNT bound to protein A-agarose. The proteins were subjected to SDS-PAGE and analyzed by Western blotting with anti-c-Jun and anti-ARNT antibodies. C, confluent cells were starved for 18 h in serum-free culture medium and then treated with EGF for various time periods (as indicated) in the culture medium without serum. Nuclear extracts were prepared, and DNA affinity precipitation assay was performed, as described under “Experimental Procedures.” Binding of c-Jun and ARNT proteins to CRE probes was analyzed by Western blot. The streptavidin-agarose beads were used to serve as a nonspecific binding control. D, cross-linked chromatin derived from EGF-treated cells was immunoprecipitated with c-Jun and ARNT antibodies and analyzed by PCR with specific primers for the region from –186 to +49 bp of the COX-2 promoter. Input, nonimmunoprecipitated cross-linked chromatin.
FIGURE 1.
Effect of EGF on HIF expression and translocation. A, cells were starved for 18 h in serum-free culture medium and then treated with 50 ng/ml EGF for 4 h or with 1 mm CoCl2 for various time periods (as indicated) in the culture medium without serum. Lysates of cells were prepared and subjected to SDS-PAGE and analyzed by Western blotting with antibodies against HIF-1α, ARNT, c-Jun, and β-actin. B, cells were treated with 50 ng/ml EGF for 4 h, fixed by using 4% paraformaldehyde, labeled with rabbit anti-ARNT antibodies, and then stained with secondary antibodies conjugated with fluorescein isothiocyanate. DNA was stained with 4,6-diamidino-2-phenylindole. Finally, the cells were examined using a microscope. C, cells were starved and then treated with 50 ng/ml EGF for various time periods (as indicated) in the culture medium without serum. Cytoplasmic fraction (CP) and nuclear extracts (NE) of cells were prepared in the same volume of buffer, and an aliquot of each fraction was subjected to Western blot analysis using antibodies against ARNT and c-Jun. D, cells were starved and then treated with 5 nm AG1478 for 30 min, followed by EGF treatment for 4 h. Nuclear extracts, cytoplasmic fraction, and lysates of cells (LS) were prepared and subjected to Western blot analysis using antibodies specific for ARNT and COX-2. E, cellular lysates of cells were prepared and subjected to immunoprecipitation (IP) with antibodies against ARNT bound to protein A-agarose. The proteins were subjected to SDS-PAGE and analyzed by Western blotting with anti-ARNT and anti-phosphothreonine antibodies.
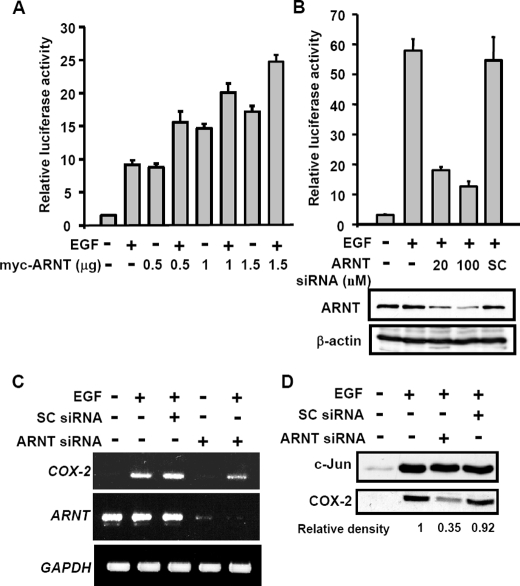
ARNT Regulates EGF-induced Cyclooxygenase-2 Gene Expression—To examine whether the increased level of ARNT in the nucleus plays a role in EGF-regulated COX-2 gene expression (14), we analyzed the effect of ARNT on the transcriptional activation by the reporter assay. As shown in Fig. 2A, overexpression of ARNT induced the promoter activity of COX-2. The ARNT-induced promoter activity was also enhanced by EGF. In order to confirm the functional role of endogenous ARNT in regulating the EGF-induced COX-2 expression, ARNT short interfering RNA (siRNA) oligonucleotide was transfected into A431 cells. The EGF-induced promoter activity, mRNA, and protein were all attenuated in those cells with decreased level of ARNT (Fig. 2, B and D). The inhibition of ARNT siRNA was further confirmed by using different sets of siRNA oligonucleotides (Fig. S1). In addition, the expression of EGF-induced c-Jun was not altered in ARNT knockdown cells (Fig. 2D). These results revealed that ARNT was involved in the regulation of EGF-induced COX-2 gene expression in normoxic conditions.
FIGURE 2.
Effect of ARNT on EGF-induced gene expression of COX-2. A, luciferase vector bearing the COX-2 gene promoter was constructed (pXC918) (25). Cells were transfected with pXC918 and ARNT expression vector by lipofection. Cells were treated with 50 ng/ml EGF and further cultured in fresh medium up to 6 h. The luciferase activities and protein concentrations were then determined and normalized. Values represent means ± S.E. of three determinations. B, cells were transfected with pXC918 and various amounts of ARNT siRNA oligonucleotides by lipofection. After EGF treatment for 6 h, the luciferase activities and protein concentrations were determined and normalized. Values represent means ± S.E. of three determinations. Expressions of ARNT and β-actin proteins were analyzed by Western blot analysis using anti-ARNT and anti-β-actin antibodies, respectively. C, cells were transfected with 50 nm ARNT siRNA or scramble oligonucleotides by lipofection. After EGF treatment for 6 h, total RNA was extracted for reverse transcription PCR with COX-2, ARNT, and glyceraldehyde-3-phosphate dehydrogenase primers. D, expressions of COX-2 and c-Jun proteins were analyzed by Western blot analysis using anti-COX-2 and anti-c-Jun antibodies, respectively. The relative density of COX-2 protein was quantified as indicated.
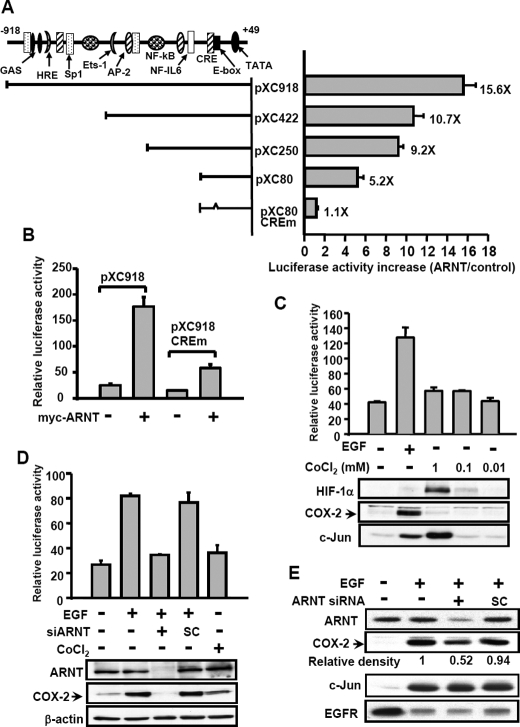
COX-2 Promoter Analysis for ARNT Responses—We then studied which binding site of the COX-2 promoter is responsible for the effect of ARNT on the induction of promoter activity. The 5′-deletion constructs of the COX-2 promoter were used. The results are summarized in Fig. 3A. A 30% decrease in the stimulatory response to ARNT transfection was observed in vectors bearing a promoter with a deletion from –918 to –422 bp (pXC918 to pXC422), indicating that a putative HRE site-containing region and a Sp1 site may play a partial role in the ARNT-induced promoter activity of COX-2. Compared with the stimulatory response of ARNT in pXC250 vector, a 50% decrease of the response was observed from pXC250 to pXC80. There was still about a 5-fold induction observed in vector pXC80. These results indicate that the promoter region, containing HRE, Sp1, NF-IL6, and CRE sites, etc., in the regions from –918 to –422 bp (pXC918 to pXC422), –250 to –80 bp (pXC250 to pXC80), and –80 to +49 bp (pXC80), respectively, may be responsive sites to the ARNT stimulation. Since the 5′-flanking region of the COX-2 gene ranging from –80 to –44 bp was required for the EGF response (14), we further focused on the effect of ARNT on this region. With the aid of site-directed mutagenesis at CRE (CREm), we found an almost complete elimination of the ARNT response in pXC80 CREm. To further confirm the requirement of the CRE element in the ARNT response, the pXC918 CREm or pXC918 was transfected with Myc-ARNT in cells. Although 35% promoter activity was still observed, ARNT response was significantly reduced in pXC918 CREm (Fig. 3B). Since the CRE element was essential for EGF-induced COX-2 expression, these results revealed that ARNT contributed to EGF-induced COX-2 expression through the CRE element. In addition to examining ARNT, we further checked whether HIF-1α played a role in regulating COX-2 gene expression. Although CoCl2 induced HIF-1α and c-Jun expression in cells, CoCl2 caused no significant effects on the promoter activity and protein expression of COX-2 (Fig. 3C). In order to check whether ARNT-regulated EGF-induced COX-2 expression was in a cell type-dependent manner, we also studied the effect of ARNT on EGF-induced COX-2 expression in squamous carcinoma of other origins. Similar to the results shown in Fig. 2B, ARNT siRNA inhibited EGF-induced expression of COX-2 in SiHa and OEC-M1 (Fig. 3, D and E). In addition, no significant effect of CoCl2 on COX-2 expression was observed in SiHa cells (Fig. 3D). These results suggest that ARNT, but not HIF-1α, was involved in the regulation of EGF-induced COX-2 gene expression in normoxic states.
FIGURE 3.
Analysis of ARNT-responsive regions in the 5′-flanking region of the COX-2 gene. A, luciferase vectors bearing various lengths of the COX-2 gene promoter were constructed as indicated. Site-directed mutagenesis of CRE consensus sequences in the 5′-flanking region ranging from –57 to –53 bp of the COX-2 gene was performed. The potential consensus sequences in the 5′-flanking region are indicated. Plasmid transfection was performed as described under “Experimental Procedures.” The luciferase activities and protein concentrations were then determined and normalized. The expression ratios of ARNT (1 μg)-treated cells to control cells are indicated. Results are expressed as means ± S.E. of three independent experiments in triplicate wells for each construct. B, cells were transfected with pXC918, pXC918 CREm, and ARNT expression vector by lipofection. The luciferase activities and protein concentrations were then determined and normalized. Values represent means ± S.E. of three determinations. C, cells were transfected with pXC918 by lipofection. After EGF or CoCl2 treatment for 6 h, the luciferase activities and protein concentrations were determined and normalized. Values represent means ± S.E. of three determinations. Expressions of HIF-1α, COX-2, and c-Jun proteins were analyzed by Western blot analysis using antibodies against HIF-1α, COX-2, and c-Jun, respectively. D, SiHa cells were transfected with pXC918 and ARNT siRNA oligonucleotides by lipofection. After EGF or CoCl2 treatment for 6 h, the luciferase activities and protein concentrations were determined and normalized. Values represent means ± S.E. of three determinations. Expressions of ARNT, COX-2, and β-actin proteins were analyzed by Western blot analysis using antibodies against ARNT, COX-2, and β-actin, respectively. E, OEC-M1 cells were transfected with ARNT siRNA oligonucleotides by lipofection. After EGF treatment for 6 h, the expression of ARNT, COX-2, c-Jun, EGFR, and β-actin was analyzed by Western blot analysis. The relative density of COX-2 protein was quantified as indicated.
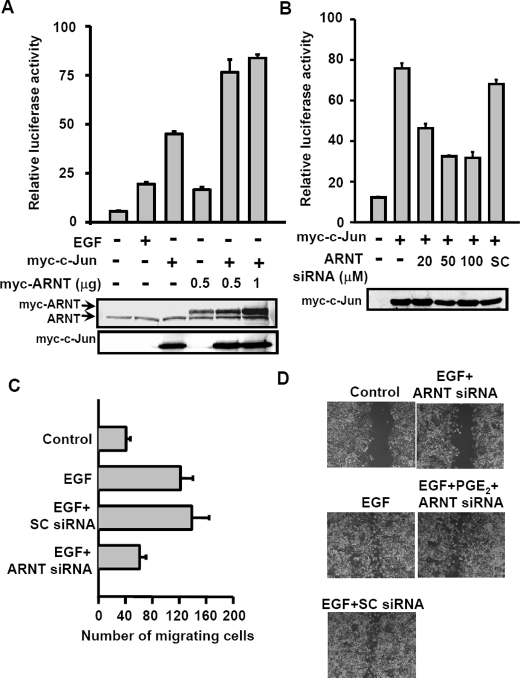
ARNT Cooperates with c-Jun in Regulating COX-2 Gene Transcription—We reported earlier that c-Jun is essential for EGF-induced COX-2 expression (14). From the results observed in Fig. 3A, we raised the hypothesis that the cooperation between c-Jun and ARNT was required in the regulation of EGF-induced COX-2 transcriptional activation. To address the possibility of interaction between c-Jun and ARNT in EGF-treated cells, immunoprecipitation assays were performed using anti-c-Jun or anti-ARNT antibodies. Indeed, by analyzing the samples prepared from lysates (Fig. 4A) and nucleus (Fig. 4B), we found that EGF dramatically enhanced the interaction between c-Jun and ARNT. In addition, EGF also enhanced the phosphorylation of ARNT (Fig. 1E), indicating that the post-translational modification of ARNT may be involved in the complex formation of c-Jun·ARNT. Since the CRE binding site was required for activation of COX-2 promoter by EGF, c-Jun, and ARNT, we then tested whether EGF-induced c-Jun·ARNT complex bound to the CRE site of the gene promoter. Indeed, both c-Jun and ARNT formed a complex and bound to the CRE element of the COX-2 promoter in EGF-treated cells, as revealed in a DNA affinity precipitation assay (Fig. 4C) and a chromatin immunoprecipitation assay (Fig. 4D). These results indicate that EGF induced the COX-2 gene expression by stimulating the binding of ARNT and c-Jun as a complex to the COX-2 promoter. Binding of ARNT·c-Jun complex to the COX-2 promoter suggests that ARNT and c-Jun cooperate to regulate the COX-2 gene expression. We next confirmed that the transactivation of the COX-2 promoter was enhanced by cooperation between c-Jun and ARNT in the reporter assays. The promoter activity was increased in EGF-treated, Myc-c-Jun- and Myc-ARNT-expressing cells (Fig. 5A). Although Myc-c-Jun had no significant effect on the expression of ARNT, the promoter activity was enhanced significantly more in Mycc-Jun- and Myc-ARNT-co-expressing cells than in individually Myc-c-Jun- or Myc-ARNT-expressing cells (Fig. 5A). Compared with 0.5 μg of Myc-ARNT-expressing cells, however, an excess of Myc-ARNT (1 μg) expression could not further induce higher promoter activity in Myc-c-Jun-coexpressing cells (Fig. 5A). Thus, the functional role of c-Jun and ARNT in the regulation of COX-2 expression prompted us to examine whether c-Jun-induced COX-2 expression in an ARNT-dependent manner. Notably, siRNA-mediated knockdown of ARNT significantly abolished the c-Jun-induced promoter activity of COX-2 (Fig. 5B). These results indicate that the cooperation between c-Jun and ARNT was essential for the COX-2 gene expression.
FIGURE 5.
Cooperation between ARNT and c-Jun in promoter activation of the COX-2 gene results in cell migration. A, cells were transfected with pXC-918, Myc-c-Jun, and Myc-ARNT expression vector by lipofection. Cells were treated with or without EGF for 6 h. The luciferase activities and protein concentrations were then determined and normalized. Values represent means ± S.E. of three determinations. Expressions of ARNT and Myc-c-Jun proteins were analyzed by Western blot analysis using anti-ARNT and anti-Myc antibodies, respectively. B, cells were transfected with pXC-918, Myc-c-Jun, and ARNT siRNA by lipofection. The luciferase activities and protein concentrations were then determined and normalized. Values represent means ± S.E. of three determinations. Expressions of Myc-c-Jun proteins were analyzed by Western blot analysis using anti-Myc antibodies. C, cells were transfected with 50 nm ARNT siRNA or scramble oligonucleotides by lipofection. Migration was assessed after 15 h in the presence or absence of EGF. The histogram displays the mean number of migrated cells obtained by counting four separate fields in three independent experiments. Error bars represent means ± S.D. D, cells were transfected with 50 nm ARNT siRNA or scramble oligonucleotides by lipofection. After a wound was made with a pipette tip, cells were treated with 50 ng/ml EGF and 10μm PGE2 for 60 h. The extent of wound closure was observed by using a phase-contrast microscope camera (model DMI 4000 B; Leica).
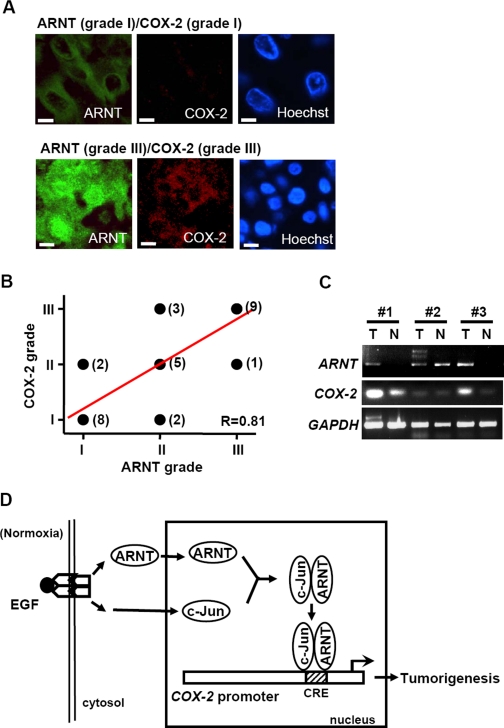
Expression Pattern of ARNT and COX-2 in Surgical Specimens of Cervical Carcinoma—Studies in cell culture systems have revealed that the ARNT signaling pathway increases COX-2 expression. To establish whether ARNT down-regulation affects the migratory behavior of EGF-treated cells, we examined cell motility in transwell and wound-healing assays. Down-regulation of ARNT with siRNA caused a dramatic inhibition of EGF-stimulated cell migration (Fig. 5C), suggesting that ARNT was required for EGF-regulated migration. We found that, consistent with PGE2 activation of the EGFR signaling pathway leading to increased inhibitors of DNA binding (Id)-1 transcription and cell invasion (30), the inhibition of ARNT siRNA in EGF-induced cell migration was rescued in cells treated with PGE2 (Fig. 5D). To evaluate the clinical relevance of these findings, we examined the expression pattern of ARNT and COX-2 in the surgical specimens (n = 30) of early stage cervical squamous cell carcinoma (stage Ib-IIa) by immunofluorescent staining. COX-2 expression in tumors was higher than in normal tissues. The higher COX-2 expression in tumors concurred with increased ARNT expressions (Fig. 6C). ARNT and COX-2 colocalized in the cervical cancer tissues (Fig. 6A). On the other hand, these two molecules were nearly undetectable by immunofluorescence microscopy in adjacent normal or noncancerous cervical tissues of all surgical specimens examined (n = 30). In addition, the expression levels of ARNT and COX-2 in the same surgical specimen showed a good linear correlation (R = 0.81), with stronger nuclear staining of ARNT indicating an up-regulation of COX-2 (Fig. 6B). These results suggest a likely involvement of ARNT in the COX-2 production in vivo.
FIGURE 6.
Expression patterns of ARNT and COX-2 in surgical specimens of cervical carcinoma. A, representative pictures of cervical cancer samples at different ARNT/COX-2 grades. The triple-immunofluorescent stain technique was used to identify ARNT (green), COX-2 (red), and nucleus (blue) in cervical cancer tissues. Scale bar, 5 μm. B, a linear correlation (R = 0.81) of ARNT and COX-2 expression in the same surgical specimen of cervical cancer tissues. The case number is indicated in parentheses beside each point. C, total RNA from tumor and normal tissue of different human cervical cancer patients (#1 to #3) was extracted for reverse transcription PCR with COX-2, ARNT, and glyceraldehyde-3-phosphate dehydrogenase primers. D, schematic pathway of COX-2 promoter regulation by EGF stimulation. Activation of EGFR signaling increases nuclear accumulation of ARNT under normoxic conditions. ARNT then interacts with transcriptional factor c-Jun and binds to the CRE site, which results in an increase of COX-2 expression and tumorigenesis.
DISCUSSION
Clinical and experimental data have indicated that increased COX-2 expression correlates closely with poor survival in various cancers. In this study, we showed that the COX-2 gene was transcriptionally activated in response to EGF through the binding of the ARNT·c-Jun complex to a functional CRE site within the COX-2 promoter and that this promoter could be activated even in normoxia by the overexpression of ARNT in squamous cell carcinoma cell lines. In addition, increased expression of COX-2 accompanied high amounts of ARNT in clinical samples of cervical cancer.
ARNT forms a complex with transcriptional factors and is essential for the normal function of HIF-1α, HIF-2α, and AhR. These heterodimeric complexes are required for cellular responses to hypoxia and environmental toxins (1). ARNT-containing dimers have been reported to regulate the expression of many genes via putative consensus binding sites in their promoters (including xenobiotic and hypoxic responsive genes). The consequence of the dysregulation of ARNT containing dimers is known to result in the formation of some diseases, including renal clear cell carcinoma (31). Although a putative HIF-1 binding site found in the COX-2 promoter is responsible for thioredoxin-1-induced gene expression (16), our data revealed no significant effects of hypoxia-mimetic agent-induced HIF-1α on the expression of COX-2. Alternatively, we found that ARNT interacted with c-Jun and bound to the CRE binding site to contribute the EGF-induced expression of COX-2. These results are consistent with reports showing that hypoxic induction of COX-2 seems to involve the cooperation of transcription factors Sp1, NF-κB, and the high mobility group protein I (Y) through their binding to the Sp1 and NF-κB sites of the COX-2 promoter (32, 33). Further experimental support would be needed, however, to establish that the putative HRE binding site is involved in the regulation of COX-2 expression.
ARNT has multiple potential partners, including the transcriptional factor c-Jun, as demonstrated in this study. Although the induction of c-Jun was critical for the EGF-induced COX-2 expression (14), our data showed that the increased c-Jun had no effect on the transcriptional activity without ARNT. This was further supported by an increase of c-Jun caused by hypoxic-mimetic agent which, however, cannot induce COX-2 expression. Induction of c-Jun mRNA expression and c-Jun phosphorylation by prolonged hypoxia are completely dependent on the presence of HIF-1α (34). Although the phosphorylation of c-Jun is increased in hypoxic conditions and the phosphorylation state of c-Jun is a primary determinant of the activity of c-Jun/AP1, c-Jun N-terminal phosphorylation was not required for EGF-induced expression of COX-2 (25). These data suggest that a cooperator is required in certain c-Jun-responsive genes. Our data show for the first time that c-Jun cooperates with ARNT in enhancing COX-2 gene expression in normoxic states.
It is well known that HIF-1α/ARNT dimer, a distinct common pathway induced by hypoxia, has a pivotal role in the development of various cancers. Since the hypoxic environment is frequently known in the tumor tissue, HIF-1α and -2α are activated and expressed in the nuclear site of the majority of solid tumors, including bladder, brain, breast, colon, ovarian, pancreatic, prostate, and renal carcinoma (35). Nevertheless, the consensus of expression and activity of HIF-1α is not always limited to hypoxic environment in recent studies and is regulated by signal pathways, including phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase (extracellular signal-regulated kinase) in normoxic condition (36–38). Unlike a previous study reporting that COX-2 is induced by HIF-1α in hypoxic condition (39), our study revealed that the expression of COX-2 was not related to the hypoxic-mimetic agent, CoCl2, or to the expression of HIF-1α. This suggests that, in normoxic conditions, tumor cells express COX-2 by the pathway that is related to ARNT but in a manner different from that of HIF-1α/ARNT dimer. Combination with HIF-1α, however, is not the only way for ARNT to promote cancer growth. For example, recent investigations in tumorigenesis in lung adenocarcinoma reveal that AHR-associated overexpression of CYPB1, which could increase intracellular oxidative stress and promote cell growth (40). In addition, the loss of cellular homostasis by the absence of AHR repressor, which is another member of the bHLH-PAS family that competes with AHR in the combination with ARNT, also resulted in tumorigenesis (41). In our study, EGF induced nuclear localization of ARNT, which, in combination with c-Jun, resulted in the transcription of COX-2 in the normoxic environment. The signal pathway reveals a novel function for ARNT in tumorigenesis when hypoxia is not present.
Another important implication for ARNT in this study lies in the correlation to COX-2, which is overexpressed in a variety of premalignant and malignant conditions (42). It is well known that increased expression of COX-2 is associated with poor survival in cervical cancer patients (43, 44). Based on the immunofluorescent study, patients with strong expression of ARNT in nucleus also had a high level of COX-2 expression (Fig. 6B). The expression of ARNT in the nucleus was also much higher in cancer tissues than in normal tissues examined by immunohistochemistry staining of oral cancer squamous cell carcinoma (Fig. S2). In addition, ARNT expression was much higher in cervical tumor than in normal tissues. Along with the previous consensus, these results showed that ARNT enhances the expression of COX-2 in tumor tissues and might result in dismal survival outcomes and represent another reliable indicator for prognosis for early to advanced stages of cervical cancers. In squamous cell carcinoma of the oral cavity, overexpression of COX-2 impacts on disease-free survival (45), and the expression of COX-2 was controlled by ARNT in OEC-M1 cells in our study. Along with other preliminary results that revealed the direct regulation of COX-2 by EGFR activation in cell lines, such as head and neck cancer (e.g. KB, UMSCC-1, and TU183 (data not shown)), it might be a reasonable rationale to target ARNT for treatment in these cancers.
Some impedance was observed in our study. In contrast to the clear association of EGFR, c-Jun, ARNT, and COX-2 in A431, SiHa, and OEC-M1, we could not demonstrate this pathway in lung cancer cell lines A549 and H157 (data not shown). In fact, a previous study has reported that there is no correlation between EGFR and COX-2 in clinical lung cancer tumor samples or cell lines (46). The difference in results might lie in the alternation of EGFR-downstream signals such as p38 mitogen-activated protein kinase and Sp1/Sp3 to induce COX-2 expression (47). In clinical trials, dual blockage of EGFR and COX-2 in lung cancer leads to disappointing outcomes, since this is not able to overcome the innate resistance of EGFR tyrosine kinase inhibitor (48, 49). As a consequence, the pathway for EGFR to the induction of COX-2 expression in different tumors is still a complicated network, and further investigation is needed.
In conclusion, we have identified a novel pathway that mediates COX-2 expression in EGFR signal-activated human tumor cells. Our results demonstrate that the activation of the EGFR signaling pathway leads to a nuclear accumulation of ARNT. We show that ARNT interacts with c-Jun, forming an ARNT·c-Jun complex that binds to the CRE site of the COX-2 promoter, resulting in the transcriptional activation of the COX-2 gene as well as tumorigenesis (Fig. 6D). Since the growth factor-induced signaling is common in tumors, it is possible that activation of ARNT may be required to regulate related gene expressions in normoxic tumor regions and execute the growth factor-mediated cell growth. Our study further indicates the importance of ARNT-mediated expression of COX-2 in cervical squamous cell carcinoma progression clinically. Based on the results, therapy exploiting ARNT may effectively prevent tumor progression.
Supplementary Material
Acknowledgments
We thank Dr. T. P. Su for critical review of the manuscript.
This work was supported in part by National Science Council of the Republic of China Grant NSC 95-2320-B-006-071-MY2 and by the National Cheng Kung University Project of Promoting Academic Excellence and Developing World-Class Research Centers.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: ARNT, aryl hydrocarbon receptor nuclear translocator; AhR, aryl hydrocarbon receptor; bHLH-PAS, basic helix-loop-helix Per/AhR/ARNT/Sim; EGF, epidermal growth factor; EGFR, EGF receptor; PGE2, prostaglandin E2; CRE, cAMP-response element; CREm, site-directed mutagenesis at CRE; siRNA, small interfering RNA.
References
- 1.Kewley, R. J., Whitelaw, M. L., and Chapman-Smith, A. (2004) Int. J. Biochem. Cell Biol. 36 189–204 [DOI] [PubMed] [Google Scholar]
- 2.Giaccia, A., Siim, B. G., and Johnson, R. S. (2003) Nat. Rev. Drug Discov. 2 803–811 [DOI] [PubMed] [Google Scholar]
- 3.Kozak, K. R., Abbott, B., and Hankinson, O. (1997) Dev. Biol. 191 297–305 [DOI] [PubMed] [Google Scholar]
- 4.Maltepe, E., Schmidt, J. V., Baunoch, D., Bradfield, C. A., and Simon, M. C. (1997) Nature 386 403–407 [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet, P., Dor, Y., Herbert, J-M., Fukumura, D., Brusselmans, K., Dewerchin, M., Neeman, M., Bono, F., Abramovitch, R., Maxwell, P., Koch, C. J., Ratcliffe, P., Moons, L., Jain, R. K., Collen, D., and Keshet, E. (1998) Nature 394 485–490 [DOI] [PubMed] [Google Scholar]
- 6.Rankin, E. B., Higgins, D. F., Walisser, J. A., Johnson, R. S., Bradfield, C. A., and Haase, V. H. (2005) Mol. Cell. Biol. 25 3163–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrasekharan, N. V., and Simmons, D. (2004) Genome Biol. 5 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turini, M. E., and DuBois, R. N. (2002) Annu. Rev. Med. 53 35–57 [DOI] [PubMed] [Google Scholar]
- 9.Gasparini, G., Longo, R., Sarmiento, R., and Morabito, A. (2003) Lancet Oncol. 4 605–615 [DOI] [PubMed] [Google Scholar]
- 10.Konturek, P. C., Kania, J., Burnat, G., Hahn, E. G., and Konturek, S. J. (2005) J. Physiol. Pharmacol. 56 57–73 [PubMed] [Google Scholar]
- 11.Kulkarni, S., Rader, J. S., Zhang, F., Liapis, H., Koki, A. T., Masferrer, J. L., Subbaramaiah, K., and Dannenberg, A. J. (2001) Clin. Cancer Res. 7 429–434 [PubMed] [Google Scholar]
- 12.Half, E., Tang, X. M., Gwyn, K., Sahin, A., Wathen, K., and Sinicrope, F. A. (2002) Cancer Res. 62 1676–1681 [PubMed] [Google Scholar]
- 13.Subbaramaiah, K., Norton, L., Gerald, W., and Dannenberg, A. J. (2002) J. Biol. Chem. 277 18649–18657 [DOI] [PubMed] [Google Scholar]
- 14.Chen, L-C., Chen, B-K., Chang, J-M., and Chang, W-C. (2004) Biochim. Biophys. Acta 1683 38–48 [DOI] [PubMed] [Google Scholar]
- 15.Schmedtje, JF, Jr., Ji, Y-S., Liu, W. L., DuBois, R. N., and Runge, M. S. (1997) J. Biol. Chem. 272 601–608 [DOI] [PubMed] [Google Scholar]
- 16.Csiki, I., Yanagisawa, K., Haruki, N., Nadaf, S., Morrow, J. D., Johnson, D. H., and Carbone, D. P. (2006) Cancer Res. 66 143–150 [DOI] [PubMed] [Google Scholar]
- 17.Salomon, D. S., Brandt, R., Ciardiello, F., and Normanno, N. (1995) Crit. Rev. Oncol. Hematol. 19 183–232 [DOI] [PubMed] [Google Scholar]
- 18.Yarden, Y., and Sliwkowski, M. X. (2001) Nat. Rev. Mol. Cell Biol. 2 127–137 [DOI] [PubMed] [Google Scholar]
- 19.Pai, R., Soreghan, B., Szabo, I. L., Pavelka, M., Baatar, D., and Tarnawski, A. S. (2002) Nat. Med. 8 289–293 [DOI] [PubMed] [Google Scholar]
- 20.Buchanan, F. G., Wang, D., Bargiacchi, F., and DuBois, R. N. (2003) J. Biol. Chem. 278 35451–35457 [DOI] [PubMed] [Google Scholar]
- 21.Torrance, C. J., Jackson, P. E., Montgomery E., Kinzler, K. W., Vogelstein, B., Wissner, A., Nunes, M., Frost, P., and Discafani, C. M. (2000) Nat. Med. 6 1024–1028 [DOI] [PubMed] [Google Scholar]
- 22.Kim, G. E., Kim, Y. B., Cho, N. H., Chung, H-C., Pyo, H. R., Lee, J. D., Park, T. J., Koom, W. S., Chun, M., and Suh, C. O. (2004) Clin. Cancer Res. 10 1366–1374 [DOI] [PubMed] [Google Scholar]
- 23.Wirth, L. J., Haddad, R. I., Lindeman, N. I., Zhao, X., Lee, J. C., Joshi, V. A., Norris, C. M., and Posner, M. R. (2005) J. Clin. Oncol. 23 6976–6981 [DOI] [PubMed] [Google Scholar]
- 24.Reckamp, K. L., Krysan, K., Morrow, J. D., Milne, G. L., Newman, R. A., Tucker, C., Elashoff, R. M., Dubinett, S. M., and Figlin, R. A. (2006) Clin. Cancer Res. 12 3381–3388 [DOI] [PubMed] [Google Scholar]
- 25.Chen, L. C., Chen, B. K., and Chang, W. C. (2005) Mol. Pharmacol. 67 2057–2069 [DOI] [PubMed] [Google Scholar]
- 26.Liu, Y. W., Arakawa, T., Yamamoto, S., and Chang, W. C. (1997) Biochem. J. 324 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews, N. C., and Faller, D. V. (1991) Nucleic Acids Res. 19 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu, Y., Saunders, M. A., Yeh, H., Deng, W-G., and Wu, K. K. (2002) J. Biol. Chem. 277 6923–6928 [DOI] [PubMed] [Google Scholar]
- 29.Saccani, S., Pantano, S., and Natoli, G. (2001) J. Exp. Med. 193 1351–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subbaramaiah, K., Benezra, R., Hudis, C., and Dannenberg, A. J. (2008) J. Biol. Chem. 283 33955–33968 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Semenza, G. L. (2003) Nat. Rev. Cancer 3 721–732 [DOI] [PubMed] [Google Scholar]
- 32.Ji, Y-S., Xu, Q., and Schmedtje, J. F., Jr. (1998) Circ. Res. 83 295–304 [DOI] [PubMed] [Google Scholar]
- 33.Xu, Q., Ji, Y-S., and Schmedtje, J. F., Jr. (2000) J. Biol. Chem. 275 24583–24589 [DOI] [PubMed] [Google Scholar]
- 34.Laderoute, K. R., Calaoagan, J. M., Gustafson-Brown C., Knapp, A. M., Li, G-C., Mendonca, H. L., Ryan, H. E., Wang, Z., and Johnson, R. S. (2002) Mol. Cell. Biol. 22 2515–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talks, K. L., Turley, H., Gatter, K. C., Maxwell, P. H., Pugh, W., Ratcliffen, P. J., and Harris, A. L. (2000) Am. J. Pathol. 157 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong, H., Chiles, K., Feldser, D., Laughner, E., Hanrahan, C., Georgescu, M-M., Simons, J. W., and Semenza, G. L. (2000) Cancer Res. 60 1541–1545 [PubMed] [Google Scholar]
- 37.Jiang, B-H., Jiang, G., Zheng, J. Z., Lu, Z., Hunter, T., and Vogt, P. K. (2001) Cell Growth & Differ. 12 363–369 [PubMed] [Google Scholar]
- 38.Dery, M-AC., Michaud, M. D., and Richard, D. E. (2005) Int. J. Biochem. Cell Biol. 37 535–540 [DOI] [PubMed] [Google Scholar]
- 39.Kaidi, A., Qualtrough, D., Williams, A. C., and Paraskeva, C. (2006) Cancer Res. 66 6683–6691 [DOI] [PubMed] [Google Scholar]
- 40.Chang, J. T., Chang, H., Chen, P-H., Lin, S-L., and Lin, P. (2007) Clin. Cancer Res. 13 38–45 [DOI] [PubMed] [Google Scholar]
- 41.Zudaire, E., Cuesta, N., Murty, V., Woodson, K., Adams, L., Gonzalez, N., Martinez, A., Narayan, G., Kirsch, I., Franklin, W., Hirsch, F., Birrer, M., and Cuttitta, F. (2008) J. Clin. Invest. 118 640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dannenberg, A. J., and Subbaramaiah, K. (2003) Cancer Cell 4 431–436 [DOI] [PubMed] [Google Scholar]
- 43.Ferrandina, G., Lauriola, L., Distefano, M. G., Zannoni, G. F., Gessi, M., Legge, F., Maggiano, N., Mancuso, S., Capelli, A., Scambia, G., and Ranelletti, F. O. (2002) J. Clin. Oncol. 20 973–981 [DOI] [PubMed] [Google Scholar]
- 44.Chen, H. HW, Su, W-C., Chou, C-Y., Guo, H-R., Ho, S-Y., Que, J., and Lee, W-Y (2005) Int. J. Radiat. Oncol. Biol. Phys. 63 1093–1100 [DOI] [PubMed] [Google Scholar]
- 45.Itoh, S., Matsui, K., Furuta, I., and Takano, Y. (2003) Oral Oncology 39 829–835 [DOI] [PubMed] [Google Scholar]
- 46.Richardson, C. M., Richardson, D., Swinson, D. E. B., Swain, W. A., Cox, G., and O'Byrne, K. J. (2005) Lung Cancer 48 47–57 [DOI] [PubMed] [Google Scholar]
- 47.Xu, K., and Shu, H-KG. (2007) Cancer Res. 67 6121–6129 [DOI] [PubMed] [Google Scholar]
- 48.Gadgeel, S. M., Ruckdeschel, J. C., Heath, E. I., Heilbrun, L. K., Venkatramanamoorthy, R. M., and Wozniak, A. (2007) J. Thorac. Oncol. 2 299–305 [DOI] [PubMed] [Google Scholar]
- 49.Agarwala, A., Fisher, W., Bruetman, D., McClean, M., Taber, D., Titzer, M., Juliar, B., Yu, M., Breen, T., Einhorn, L. H., and Hanna, N. (2008) J. Thorac. Oncol. 3 374–379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.