Abstract
Mutations in ATP8B1 cause severe inherited liver disease. The disease is characterized by impaired biliary bile salt excretion (cholestasis), but the mechanism whereby impaired ATP8B1 function results in cholestasis is poorly understood. ATP8B1 is a type 4 P-type ATPase and is a flippase for phosphatidylserine. Atp8b1-deficient mice display a dramatic increase in the biliary extraction of cholesterol from the canalicular (apical) membrane of the hepatocyte. Here we studied the hypothesis that disproportionate cholesterol extraction from the canalicular membrane impairs the activity of the bile salt transporter, ABCB11, and as a consequence causes cholestasis. Using single pass liver perfusions, we show that not only ABCB11-mediated transport but also Abcc2-mediated transport were reduced at least 4-fold in Atp8b1 deficiency. We show that canalicular membranes of cholestatic Atp8b1-deficient mice have a dramatically reduced cholesterol to phospholipid ratio, i.e. 0.75 ± 0.24 versus 2.03 ± 0.71 for wild type. In vitro depletion of cholesterol from mouse liver plasma membranes using methyl-β-cyclodextrin demonstrated a near linear relation between cholesterol content of the membranes and ATP-dependent taurocholate transport. Abcc2-mediated transport activity was not affected up to 30% of membrane cholesterol depletion but declined to negligible levels at 70% of membrane cholesterol depletion. These effects were reversible as cholesterol repletion of the liver membranes completely restored Abcb11- and Abcc2-mediated transport. Our data demonstrate that membrane cholesterol content is a critical determinant of ABCB11/ABCC2 transport activity, provide an explanation for the etiology of ATP8B1 disease, and suggest a novel mechanism protecting the canalicular membrane against luminal bile salt overload.
ATP8B1 deficiency causes progressive familial intrahepatic cholestasis type 1 (PFIC1)3 and benign recurrent intrahepatic cholestasis type 1 (BRIC1) (reviewed in Ref. 1). PFIC1 patients usually present at a young age with typical cholestatic symptoms; serum bile salt, bilirubin, and transaminase levels are elevated, but serum GGT levels are low. Liver histology reveals bridging fibrosis but no bile duct proliferation. Electron microscopic analysis shows that canalicular bile has a coarsely granular appearance as opposed to the amorphous or finely filamentous bile observed in other forms of cholestasis. PFIC1 patients can also develop extrahepatic symptoms, including watery diarrhea and pancreatitis. Orthotopic liver transplantation relieves the cholestasis; however, the extrahepatic phenotypes remain. BRIC1 is characterized by periodic bouts of cholestasis, the onset and resolution of which are caused by unknown factors, and leaves no liver damage. Recently it was shown that mutations in ATP8B1 also are associated with intrahepatic cholestasis of pregnancy (2, 3). The gene involved in these diseases, ATP8B1, encodes a member of the P4 subfamily of the P-type ATPase superfamily (4, 5). P4 ATPases are thought to function as phospholipid flippases, i.e. activities that translocate phospholipids from the exoplasmic to the cytoplasmic leaflet of membrane bilayers (6, 7). We have recently shown that ATP8B1 is a flippase for phosphatidylserine, a phospholipid that is exclusively confined to the cytoplasmic leaflet of the membrane bilayer (8). Absolute requirements for plasma membrane localization and activity of ATP8B1 were co-expression and the physical interaction of ATP8B1 with CDC50A, a potential β-subunit for ATP8B1. ATP8B1 is expressed in many tissues, including the liver, pancreas, and small intestine, where it localizes to the apical membrane of epithelial cells, including the canalicular membrane of hepatocytes (9). The mechanism underlying the cholestasis in patients with ATP8B1 deficiency is not completely understood. Interruption of the enterohepatic circulation of bile salts in PFIC1/BRIC1 patients results in a remarkable normalization of serum bile salt levels and restored hepatobiliary output of chenodeoxycholic acid (10, 11). This indicates that ATP8B1 deficiency affects the activity of the major bile salt transporter, ABCB11, in the canalicular membrane of the hepatocyte and that bile salt depletion improves the activity. We have studied the pathophysiological mechanisms in the mouse model for PFIC1, the Atp8b1G308V/G308V mutant mouse (further referred to as Atp8b1-deficient mouse) (12–15). We have previously shown that the hepatocyte canalicular membrane in Atp8b1-deficient mice is more sensitive to the detergent action of hydrophobic bile salts. This phenotype is underscored by a dramatic increase in the bile salt-dependent biliary excretion of cholesterol and canalicular ectoenzymes (13, 15). The enhanced biliary cholesterol output in Atp8b1-deficient mice was independent of the cholesterol transporter Abcg5/g8, because Atp8b1-deficient mice with inactivated Abcg5/g8 also displayed enhanced cholesterol output (15). Furthermore, in the isolated perfused liver, we showed that Atp8b1 deficiency leads to strongly impaired biliary output of taurodeoxycholate without affected Abcb11 expression and localization. We have hypothesized that ATP8B1 deficiency leads to loss of the normal phospholipid asymmetry of the canalicular membrane. As a result the canalicular membrane becomes more sensitive to extraction of cholesterol by hydrophobic bile salts, which impairs the activity of ABCB11 and, as a consequence, causes cholestasis.
In this study we have analyzed the effect of membrane cholesterol modulation on the activity of ABCB11. We show that canalicular membranes of cholestatic Atp8b1-deficient mice have a dramatically reduced cholesterol to phospholipid ratio. Furthermore, by selective extraction of membrane cholesterol using methyl-β-cyclodextrin, we show that ABCB11 activity is critically dependent on membrane cholesterol content.
EXPERIMENTAL PROCEDURES
Mice—Atp8b1G308V/G308V mutant (Atp8b1-deficient) mice are knock-in mice for a glycine-to-valine substitution at amino acid 308 leading to near absence of the protein (12). Atp8b1-deficient and wild-type mice (C57BL/6JOlaHsd) were housed in an animal facility on a 12-h light-dark cycle and were fed standard rodent chow. One week before experiments, animals were fed a purified semi-synthetic (20% casein) diet (K4068.02, Arie Blok, Woerden, The Netherlands) with or without 0.5% (w/w) cholic acid. In all experiments age-matched (3–6 months) male mice were anesthetized by intraperitoneal injection of Hypnorm (11.76 mg/kg fluanisone, 0.37 mg/kg fentanyl citrate) and 5.88 mg/kg diazepam. All animal experiments were approved by the Institutional Animal Care and Use Committee (IUCAC) of the Academic Medical Center.
Isolated, Single Pass Liver Perfusion—Mice were anesthetized, and gallbladder, portal vein, and superior vena cava were cannulated. The liver (left in situ) was placed in a 37 °C climate chamber and perfused in orthograde direction (3 ml/min) with carbogene (95% O2, 5% CO2)-gassed Krebs/bicarbonate buffer (120 mm NaCl, 24 mm NaHCO3, 1.2 mm KH2PO4, 1.2 mm MgSO4, 1.3 mm CaCl2, 0.9 mg/ml glucose). Taurodeoxycholate (TDC), containing trace amounts of [3H]TDC, was dissolved in Krebs buffer and continuously perfused at a 50 nmol/min·100 g rate for 90 min. Bile samples were taken every 5 min. At the end of the experiment, livers were weighed, and part was dissolved in 100% soluene (Packard Instrument Co.). Radioactivity was determined by liquid scintillation counting. The ABCC2 substrate 5′-carboxyfluorescein was perfused as a bolus at an 80 nmol/min·100 g rate for 5 min followed by 75 min in Krebs buffer. Bile samples were taken every 10 min, and 5′-carboxyfluorescein fluorescence was measured using a Novostar analyzer (BMG Labtech), with excitation 485 nm/emission 540 nm.
Isolation of Mouse Liver Plasma Membrane—Mouse liver mixed and canalicular plasma membranes were isolated as described (16). Briefly, livers were homogenized in 1 mm NaHCO3, pH 7.4, hypotonic buffer in a Dounce homogenizer (loose pestle, five strokes). After centrifugation of the homogenate (15 min, 1500 × g), the crude membrane pellet was diluted with 5.5 volumes of 56% sucrose. The homogenized crude membranes were layered over 56% sucrose and below a 44/36.5/8% sucrose gradient and centrifuged in a Beckman ultracentrifuge (3 h, 28,000 rpm in an SW28 rotor (141,245 × g) or 2 h in an SW41 rotor (134,314 × g)). Mixed liver membranes were collected from the 44/36% interface, washed, resuspended in isolation buffer (250 mm sucrose, 20 mm Hepes/Tris, pH 7.4), and revesiculated by passage of the suspension through a 27-gauge needle (30 times). Membrane vesicles were stored in liquid nitrogen. For canalicular membrane isolations, mixed liver membranes were homogenized in a Dounce homogenizer (tight pestle, 60 strokes). Homogenized mixed liver membranes were layered over a 29.7/34/38% sucrose gradient and centrifuged in a Beckman ultracentrifuge (40,000 rpm, 3 h in an SW41 rotor (274,111 × g)). Canalicular membranes were collected from the 31/29.7% interface and washed in isolation buffer. Canalicular membranes were collected at 50,000 rpm, 45 min in a Ti70 rotor (257,091 × g).
Cholesterol Depletion and Repletion of Mouse Liver Plasma Membranes—Cholesterol was depleted by incubation of membrane vesicles with 0.5–4 mm methyl-β-cyclodextrin (300 μg of protein/2 ml of isolation buffer) in isolation buffer for 1 h at room temperature. Subsequently, membranes were centrifuged for 10 min at 20,000 × g, washed in isolation buffer, and centrifuged again. For cholesterol repletion, membrane pellets were incubated for an additional hour at room temperature with methyl-β-cyclodextrin-cholesterol inclusion complex (3:1). The resulting membrane pellets were resuspended in isolation buffer to a final protein concentration of 1 μg/μl. Vesicle preparations were revesiculated by passage through a 27-gauge needle. Cholesterol-inclusion complex was prepared by adding 0.01 volume of a 0.1 m cholesterol stock (in CHCl3/CH3OH (1:2)) to a pre-warmed (80 °C) 3 mm methyl-β-cyclodextrin (in isolation buffer) solution. The solution was extensively vortexed, sonicated (three times for 10 s at 50%), and passed through a 0.45-μm filter. Cholesterol and choline-containing phospholipids in the vesicle preparation were measured as described above. Protein content was measured using the bicinchoninic acid method.
Membrane Vesicle Transport Assay—Uptake of [3H]taurocholate (TC) and dinitrophenyl-[glycine-2-[3H]glutathione (DNP-[3H]GS) was measured using the rapid filtration technique as described (17). Briefly, membrane vesicles (30 μg of protein) were incubated for 3 min (at 37 °C) in reaction mixture containing 250 mm sucrose and 20 mm Hepes/Tris, pH 7.4, 8 mm MgCl2, 20 mm creatine phosphate, 0.8 mg of creatine kinase per ml/1 μCi of [3H]TC per ml, with or without 4 mm ATP. Reactions were stopped, and after rapid filtration, filters were washed four times with 3 ml of stop buffer (250 mm sucrose and 20 mm Hepes/Tris, pH 7.4). Filters were placed in liquid scintillation fluid, and radioactivity was measured in a liquid scintillation counter.
Recombinant Protein Expression in Sf21 Cells—Recombinant human ABCB11 and ABCC2 were expressed in Sf21 cells using the baculovirus expression system as described previously (17). Baculovirus for ABCB11 was kindly provided by Dr. R. J. Thompson. Protein was produced either in suspension (3–5 × 108 cells per spinner) or in monolayer cultures (2 × 107 per flask). Cells were grown at 27 °C in Grace's insect medium supplemented with 10% fetal bovine serum, 1% glutamine, 1% penicillin/streptomycin and were incubated for 1 h with viral stocks at a multiplicity of infection of 10 and harvested 3 days post-infection. Membranes were isolated as described previously (17). Cholesterol depletion and repletion of Sf21 membranes were described above.
Bile Salts, Phospholipids, and Cholesterol Assays—Serum bile salt levels were determined using Diazyme total bile acids kit (Diazyme Laboratories, Poway, CA). Choline-containing phospholipids and cholesterol were determined enzymatically as described (13). All measurements were done on a Novostar analyzer (BMG Labtech GmbH, Offenburg, Germany).
Statistical Analyses—Statistical analyses were performed using Student's t test; a p value of less than 0.05 was considered significant. All data were expressed as means ± S.D.
RESULTS
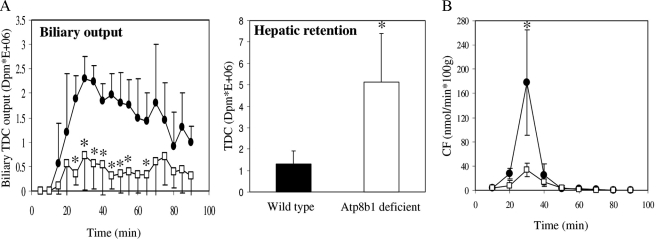
Atp8b1-deficient Mice Display Reduced Biliary Output of Organic Anions and Bile Salts—Our present studies are performed with Atp8b1-deficient mice on a C57Bl/6 background. We studied this background because in our hands the cholestatic phenotype caused by Atp8b1 deficiency is strongest in these animals (15). Single pass perfusion of Atp8b1-deficient livers with radiolabeled taurodeoxycholate showed a 4-fold reduced hepatobiliary output of label and a concomitant 4-fold enhanced hepatic retention of label compared with wild types (Fig. 1A). We subsequently investigated whether Atp8b1 deficiency only affects bile salt transport or also other prominent transport activities in the canalicular membrane. To this end we studied Abcc2-mediated organic anion excretion in Atp8b1-deficient mice using the Abcc2 substrate, 5′-carboxyfluorescein (5′-CF). To measure 5′-CF excretion, livers were perfused with a bolus of 5′-CF. Using Abcc2–/– mice, we first demonstrated that this organic anion is exclusively excreted into bile via Abcc2 (data not shown). In wild-type mice, hepatobiliary excretion of 5′-CF peaked at t = 30 min but was at least 4-fold reduced in Atp8b1-deficient mice (Fig. 1A). These data demonstrate that Atp8b1 deficiency not only impairs Abcb11-mediated bile salt transport but also Abcc2-mediated organic anion transport.
FIGURE 1.
Atp8b1 deficiency impairs biliary Abcb11-mediated TDC and Abcc2-mediated 5′-CF output in the isolated, single pass perfused liver. A, isolated livers were perfused with radiolabeled TDC, and radioactivity in bile samples and livers was determined. B, isolated livers were perfused at t = 10 min with a bolus 5′-CF for 5 min. Bile samples were analyzed for 5′-CF fluorescence intensity. •, wild-type mice; □, Atp8b1-deficient mice. Results are expressed as means ± S.D. of four experiments for both genotypes; *, p < 0.05.
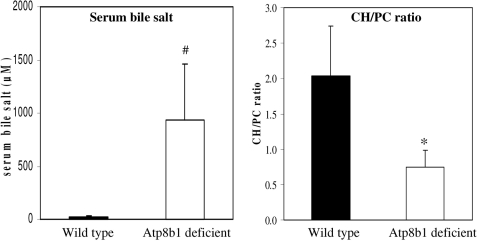
Canalicular Membranes of Atp8b1 Mutant Mice Have a Reduced Cholesterol to Phospholipid Ratio—Atp8b1-deficient mice displayed enhanced Abcg5/g8-independent biliary cholesterol output when challenged with taurocholate (13, 15), which suggests enhanced extraction of cholesterol from the canalicular membrane by bile salts. Thus, we hypothesized that Atp8b1-deficient canalicular membranes have reduced amounts of cholesterol compared with wild type. To study this, we isolated canalicular and basolateral membranes from wild-type and Atp8b1-deficient mice fed a 0.5% cholate-supplemented diet for 1 week, and we measured membrane cholesterol and choline-containing phospholipid content (Fig. 2). Atp8b1-deficient mice were cholestatic as judged from a dramatic increase in serum bile salt levels (∼50-fold). The cholesterol to choline-containing phospholipid ratio (CH/PC) of canalicular membranes of Atp8b1-deficient mice was 0.75 ± 0.24 compared with a ratio of 2.03 ± 0.71 for canalicular membranes from wild-type mice. Strikingly, the cholesterol depletion was not confined to the canalicular membrane but was also found in the basolateral membrane fraction. This is not surprising as lipids can freely pass the tight junctions via the inner leaflet of the plasma membrane (18). These data show that challenging Atp8b1-deficient mice with a cholate-supplemented diet strongly depletes the cholesterol content of the plasma membrane. These observations allow the hypothesis that cholestasis may be caused by impaired transporter function in a canalicular membrane that has a reduced cholesterol content.
FIGURE 2.
Canalicular membrane of Atp8b1-deficient mice has a reduced cholesterol to choline-containing phospholipid ratio. Mice were fed a 0.5% cholic acid-supplemented diet for 1 week. Canalicular membranes were isolated, and serum bile salt levels and membrane cholesterol and choline-containing phospholipid content were determined as described under “Experimental Procedures.” Results are expressed as means ± S.D. of five mice per genotype. Black bars, wild-type; white bars, Atp8b1-deficient. Statistical analyses were performed by a Student's t test. *, p < 0.005; #, p < 0.02.
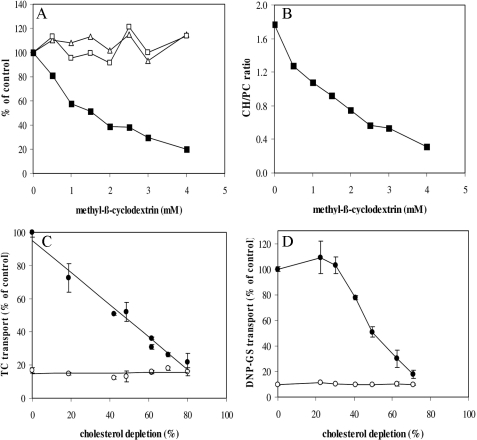
Cholesterol Depletion from Mouse Liver Plasma Membranes Reduces Abcb11- and Abcc2-mediated Transport—To test the hypothesis distilled from the experiments above (Figs. 1 and 2), we investigated the effect of modulation of membrane cholesterol content on Abcb11 and Abcc2 transport activities. To study this, we selectively extracted cholesterol from mouse liver plasma membranes with methyl-β-cyclodextrin (MβCD) and measured the uptake of the Abcb11- and Abcc2 model substrates TC and DNP-GS, respectively, by these vesicles. Cholesterol depletion did not interfere with plasma membrane integrity as it did not result in extraction of any protein or phosphatidylcholine (PC) (Fig. 3A). Consequently, the CH/PC ratio rapidly decreased upon treatment with increasing concentrations of MβCD (Fig. 3B). There was a striking, near-linear relation between cholesterol content of the membranes and ATP-dependent TC transport, with a complete abrogation of transport at 80% of cholesterol depletion (Fig. 3C). Abcc2-mediated transport was less sensitive to cholesterol depletion; DNP-GS uptake was not affected up to 30% of membrane cholesterol depletion. However, from that point on the ATP-dependent transport activity rapidly declined to negligible levels at 70% of membrane cholesterol depletion (Fig. 3D).
FIGURE 3.
Cholesterol depletion in mouse liver plasma membranes reduces Abcb11- and Abcc2-dependent transport. mLPMs were incubated with different concentrations of MβCD and cholesterol (▪), protein (Δ), and choline-containing phospholipid (□) content was measured (A), and cholesterol to phospholipid ratios were plotted (B). C, effect of cholesterol depletion on Abcb11-mediated TC uptake in mouse liver plasma membranes. A near linear relation between cholesterol content of the membranes and ATP-dependent TC transport is observed, with a complete abrogation of transport at 80% of cholesterol depletion. D, effect of cholesterol depletion on Abcc2-mediated DNP-GS uptake in mouse liver plasma membranes. Uptake of TC and DNP-GS was measured after 3 min at 37 °C. • indicates transport in the presence of ATP; ○ indicates transport in the absence of ATP. The results are shown for a representative experiment and are expressed as percentage of control (means ± S.D. (n = 3)).
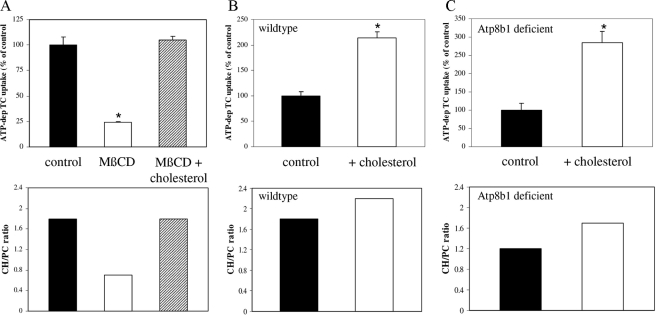
Cholesterol Addition Stimulates Abcb11- and Abcc2-mediated Transport—Next, we studied whether the inactivation of Abcb11- and Abcc2-mediated transport by membrane cholesterol depletion was reversible. Maximal membrane cholesterol depletion with 4 mm MβCD resulted in a decreased CH/PC ratio (0.7 versus 1.8 in cholesterol depleted versus control or repleted membranes) and a concomitant maximal inactivation of Abcb11-mediated TC transport (Fig. 4A, white bar). Subsequent repletion of these depleted membranes with cholesterol-saturated MβCD fully restored TC transport (Fig. 4A, hatched bar). We also studied whether we could stimulate Abcb11- and Abcc2-mediated transport to higher than endogenous levels by supplementation of membranes with cholesterol. Addition of cholesterol to wild-type membranes resulted in an enhanced CH/PC ratio (1.8 versus 2.2 in control versus cholesterol-repleted membranes) and a concomitant 100% increase in Abcb11-mediated TC uptake (Fig. 4B). Similarly, cholesterol repletion of Atp8b1-deficient membranes also enhanced the CH/PC ratio (1.2 versus 1.7 in control versus cholesterol-repleted membranes) and, as expected, resulted in an even stronger stimulation of Abcb11 activity by 250% (Fig. 4C). A similar cholesterol dependence of transport activity was observed for Abcc2 (not shown). These data indicate that membrane cholesterol content and/or the cholesterol to PC ratio is a critical determinant of the transport activity of Abcb11 and Abcc2.
FIGURE 4.
Modulation of mouse liver plasma membrane cholesterol content affects Abcb11- and Abcc2 transport activity. A, effect of cholesterol depletion (white bar) and depletion followed by repletion (hatched bar) on Abcb11-mediated TC uptake in mouse liver plasma membranes. Membranes were depleted from cholesterol with 4 mm MβCD and were repleted with cholesterol by incubation with MβCD-cholesterol (3:1) inclusion complex as described under “Experimental Procedures.” Cholesterol repletion following cholesterol depletion fully restored TC transport in these membranes. B and C, effect of cholesterol supplementation of liver plasma membranes from wild-type (B) and Atp8b1-deficient (C) mice on Abcb11-mediated TC transport. Membranes were incubated with MβCD-cholesterol (3:1) inclusion complex and membrane cholesterol, and choline-containing phospholipid and TC transport were measured. Cholesterol over choline-containing phospholipid (CH/PC) ratios are depicted. Results are plotted as percentage of control and are of a representative experiment. Data expressed as means ± S.D. (n = 3). Statistical analyses were performed by a Student's t test. *, p < 0.001.
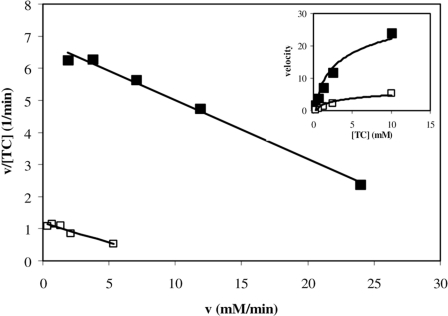
Kinetics of Abcb11-mediated TC Uptake in Liver Plasma Membranes upon Cholesterol Depletion—Next, we determined the kinetics of Abcb11-mediated TC uptake after depletion of cholesterol from liver plasma membranes (Fig. 5). Cholesterol was depleted from liver plasma membranes with 3 mm MβCD, and the initial uptake of different concentrations of TC was measured in control- and MβCD-treated membranes. TC uptake by both control and cholesterol-depleted membranes was saturable and obeyed Michaelis-Menten kinetics (Fig. 5, inset). When expressed in an Eadie-Scatchard plot, the data indicate that the Km value is largely unaffected (the slope of both lines are the same and equal 1/Km), indicating that cholesterol depletion does not affect the affinity of TC for the transporter. Because the y axis corresponds to Vmax/Km values, this experiment demonstrates that membrane cholesterol depletion reduces the Vmax, TC.
FIGURE 5.
Eadie-Scatchard plot displaying the kinetics of TC uptake by liver plasma membranes. Control membranes (▪) and 3 mm MβCD-treated membranes (□) were assayed for initial uptake of different concentrations of TC. TC uptake by both membrane preparations was saturable and obeyed Michaelis-Menten kinetics (see inset). Because the slopes of both lines are the same and equal 1/Km, the Km value is unaffected. The plot shows that membrane cholesterol depletion reduces the Vmax of TC uptake.
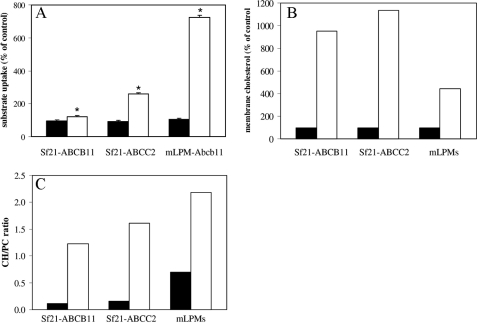
Effect of Cholesterol Repletion on ABCB11 and ABCC2 Activity in Sf21 Membranes—To study the relation between membrane cholesterol content and transporter activity in more detail, we expressed human ABCB11 and ABCC2 in Sf21 insect cells. Compared with mammalian cells, insect cells contain very little membrane cholesterol (19, 20), which makes these cells very suitable to study the relation between membrane cholesterol and protein activity. Indeed, the cholesterol content of Sf21 membranes (cells were grown in 10% fetal calf serum) is ∼6-fold lower compared with mouse liver plasma membranes (Table 1). Consequently, the CH/PC ratio of Sf21 membranes was 0.13 ± 0.02, which is in the same range as described for Sf9 cells (19, 20), and is 13-fold lower compared with liver plasma membranes (1.7 ± 0.2). Sf21 membranes expressing ABCB11 or ABCC2 protein and mouse liver plasma membranes were incubated with 3 mm MβCD or MβCD-cholesterol (3:1) inclusion complex. Subsequently, uptake of TC or DNP-GS by these membranes was measured (Fig. 6). As expected, MβCD treatment did not affect Sf21 membrane cholesterol and choline-containing phospholipid content nor did it affect ABCB11- and ABCC2-mediated transport activity (not shown). Cholesterol repletion of Sf21-ABCB11, Sf21-ABCC2, and liver plasma membranes resulted in 10-, 11-, and 4-fold increase in membrane cholesterol compared with cholesterol-depleted membranes, respectively (Fig. 6B). Despite the 10-fold increase in membrane cholesterol of Sf21-ABCB11 membranes, uptake of TC was only 1.3-fold enhanced (Fig. 6A), which contrasts the 7-fold increase in Abcb11-mediated TC uptake in liver plasma membranes. Uptake of DNP-GS by Sf21-ABCC2 membranes was ∼3-fold enhanced (and comparable with an ∼4-fold increase in liver plasma membranes (data not shown)). The cholesterol to PC ratios of the cholesterol-repleted membranes were 1.2, 1.6, and 2.2 for the Sf21-ABCB11, Sf21-ABCC2, and liver plasma membranes, respectively (Fig. 6C). These data show that also in Sf21 membranes the activity of ABCB11 and ABCC2 can be increased by addition of cholesterol to the membrane. The marginal increase in ABCB11-mediated transport may be explained by the difference in membrane composition/biophysical properties between Sf21 cells and liver plasma membranes. Altogether, our data indicate that the activity of the ABC transporters ABCB11 and ABCC2 in the canalicular membrane is very sensitive to modulation of membrane cholesterol content, albeit to a different extent.
TABLE 1.
Cholesterol and choline-containing phospholipid content of Sf21 and liver plasma membranes were measured and corrected for protein
Results are expressed as means ± S.D.
| Cholesterol | Phospholipid | Cholesterol/phospholipid ratio | |
|---|---|---|---|
| μmol/mg protein | μmol/mg protein | ||
| Mouse liver membranes | 0.47 ± 0.13 | 0.27 ± 0.05 | 1.7 ± 0.2 |
| Sf21 membranes | 0.08 ± 0.01 | 0.58 ± 0.05 | 0.13 ± 0.02 |
a p < 0.001 (n = 4 different vesicle preparations).
FIGURE 6.
Cholesterol repletion of Sf21 membranes stimulates ABCB11- and ABCC2-mediated transport. Sf21-ABCB11, Sf21-ABCC2, and mixed liver plasma membranes (mLPM) were incubated in 3 mm MβCD to deplete membrane cholesterol. One-half of each membrane preparation was repleted with cholesterol by incubation with MβCD-cholesterol-inclusion complex. Subsequently, TC (for ABCB11) and DNP-GS uptake (for ABCC2) (A) and membrane cholesterol content (B) were measured. C, cholesterol-to-phospholipid ratios (CH/PC) are shown. MβCD treatment did not change Sf21 membrane cholesterol and choline-containing phospholipid content nor did it affect ABCB11- and ABCC2-mediated transport activity. Black bars, cholesterol depletion; white bars, cholesterol repletion following cholesterol depletion. ATP-dependent TC uptake in depleted membranes was 0.91 ± 0.05 and 0.72 ± 0.04 pmol/min·mg protein for Sf21-ABCB11 and mixed liver plasma membranes, respectively. ATP-dependent DNP-GS uptake in depleted membranes was 98.2 ± 8.7 and 23.1 ± 0.8 fmol/min·mg protein for Sf21-ABCC2 and mLPM, respectively. Results are plotted as percentage of control (3 mm MβCD-treated) and are of a representative experiment. Data expressed as means ± S.D. (n = 3). Statistical analyses were performed by a Student's t test; *, p < 0.005.
DISCUSSION
Here we show that the activity of the canalicular transport proteins Abcb11 and Abcc2 is strongly dependent on canalicular membrane cholesterol content. To withstand the high millimolar concentrations of bile salts in the canalicular lumen, the hepatocyte canalicular membrane is protected by two different mechanisms (reviewed in Ref. 21). First, biliary excretion of bile salts is coupled to biliary output of phosphatidylcholine, a process mediated by ABCB4. PC is translocated from the cytoplasmic to the exoplasmic leaflet where it is readily extracted by bile salt micelles to form mixed micelles (22, 23). This process is essential to protect the canalicular and cholangiocyte apical membrane from the detergent action of bile salts. Second, the exoplasmic leaflet of the canalicular membrane is a rigid membrane that is enriched in (glyco)sphingolipids and cholesterol (24–26). Sphingolipid-cholesterolrich membrane domains or `rafts' are in a liquid-ordered (Lo) phase and are highly resistant toward detergents such as bile salts (21, 27). Based on our previous work, we have hypothesized that ATP8B1 activity is crucial in maintaining this Lo phase by flipping excess phosphatidylserine from the exoplasmic to the cytoplasmic leaflet of the canalicular membrane, thereby increasing the relative amount of sphingolipids (8, 13, 15). ATP8B1 deficiency dissipates normal phospholipid asymmetry of the canalicular membrane and causes a shift from an Lo to a more liquid-disordered phase. As a result the canalicular membrane becomes sensitive to extraction of cholesterol by hydrophobic bile salts. We hypothesized that enhanced cholesterol extraction results in reduced canalicular membrane cholesterol content and that this subsequently impairs ABCB11 activity (Fig. 3). Our present data support this hypothesis.
First, we show that the cholesterol to phospholipid ratio of the canalicular membrane in cholestatic Atp8b1-deficient mice was dramatically reduced (Fig. 2). This observation indicates that in cholate-fed Atp8b1-deficient mice cholesterol is extracted from the canalicular membrane, which impairs Abcb11 (and Abcc2) activity. A similar observation has been described for Abcg8–/– mice, which have impaired hepatobiliary excretion of cholesterol (28). Taurodeoxycholate infusion in these mice was associated with a 50% reduction in the cholesterol to phospholipid ratio of the canalicular membrane. Under this condition these mice also became cholestatic (28). Whereas in Atp8b1-deficient mice membrane cholesterol content is reduced because of extraction by hydrophobic bile salts, membrane cholesterol content in Abcg8–/– mice is reduced because of impaired cholesterol translocation to the exoplasmic leaflet. However, in both mouse models a cholesterol to phospholipid ratio of the canalicular membrane is associated with impaired Abcb11 activity.
Second, our in vitro experiments show that Abcb11 activity is very sensitive to modulation of membrane cholesterol content (Fig. 3). Extraction of only 10% of membrane cholesterol resulted in a reduction of Abcb11 activity of 10–20% and maximal inhibition of transport at 80% of cholesterol depletion, a process that was fully reversible. Abcc2 activity, on the other hand, was less sensitive to changes in membrane cholesterol content; depletion of membrane cholesterol up to 30% did not affect Abcc2 activity, but further cholesterol depletion also completely abrogated this activity.
It is not clear how cholesterol depletion affects these transport activities, but disturbance of the lipid shell of these transport proteins and/or changes in membrane fluidity may well impair protein structure and activity (for review see Ref. 29). We find that cholesterol depletion from liver plasma membranes does not affect the affinity of TC for Abcb11 but rather impairs the velocity of TC transport (Fig. 5). Similar findings have been reported for the multidrug transport protein ABCG2 expressed in Sf9 insect cells (30). Cholesterol repletion of ABCG2-expressing insect cell membranes stimulated ABCG2-mediated transport activity, with little effect on the substrate affinity for the protein. In another study, cholesterol repletion (10-fold increase in membrane cholesterol) stimulated the formation of the catalytic intermediate of ABCG2, and methotrexate transport was 15-fold enhanced (31). Our repletion experiments in ABCB11-expressing Sf21 insect cell membranes (which contain negligible amounts of cholesterol) show that, despite an ∼10-fold increase in membrane cholesterol content, transport activity was only marginally enhanced (1.3-fold). ABCC2 activity, on the other hand, was 3-fold enhanced with a concomitant 11-fold increase in membrane cholesterol content. In relation to this, it has recently been reported that modulation of membrane cholesterol content of ABCB1 and ABCC1-expressing Sf9 membranes also showed a negligible effects on these transport activities (31). Apparently, additional parameters are important for optimal activity of these type of proteins in insect cell membranes. An important possibility is the phospholipid composition of insect cell membranes, which contain much higher amounts of phosphatidylethanolamine and less (if any) sphingomyelin (19). In our own lipid analysis of Sf21 membranes (data not shown), we also observed a much higher phosphatidylethanolamine content (∼35% of total phospholipid in Sf21 compared with ∼17% in canalicular membranes).
Our study shows that canalicular membrane cholesterol content is an important determinant of ABCB11/ABCC2 activity. These data provide an explanation for the etiology of PFIC1/BRIC1 (see Fig. 7 for a model); intrahepatic cholestasis in PFIC1 is caused by an impaired aminophospholipid flippase activity that results in canalicular membrane phospholipid randomization; this renders the canalicular membrane more sensitive to extraction of cholesterol by bile salts, which reduces the cholesterol over phospholipid ratio and thereby impairs ABCB11 activity. The latter process causes cholestasis. These findings are not only relevant for PFIC1, they also suggest a novel mechanism protecting the canalicular membrane against luminal bile salt overload. When luminal bile salt levels become too high and excessively extract cholesterol from the membrane, ABCB11 activity falls. This lessens canalicular bile salt excretion, and thus prevents further membrane damage.
FIGURE 7.
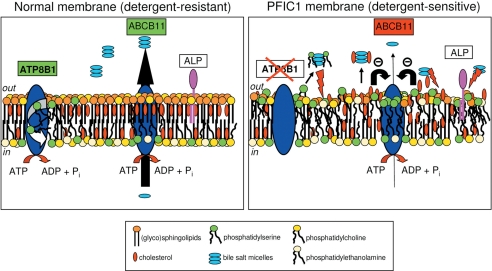
Hypothetical model that explains how ATP8B1 deficiency affects canalicular membrane composition and activity of ABCB11 (and ABCC2). The figure displays a schematic representation of a healthy (left) and an ATP8B1-deficient (right) canalicular membrane in the process of bile formation. The canalicular membrane is a highly rigid (glyco)sphingolipid/cholesterol-rich membrane, which is mostly in the liquid-ordered (Lo) state. This composition allows the membrane to resist high concentrations of luminal bile salts (up to 20 mm in the mouse). ATP8B1 is important for maintaining this Lo state by reducing the content of phosphatidylserine in the luminal leaflet, which increases the relative (glyco)sphingolipid content. In ATP8B1-deficient membranes, normal phospholipid asymmetry is lost, i.e. phosphatidylserine is exposed in the luminal leaflet, which results in disturbance of the Lo state. As a result, the canalicular membrane becomes more sensitive to extraction of lipids, including cholesterol and phosphatidylserine, and canalicular ectoenzymes (among others, alkaline phosphatase) by hydrophobic bile salts. The reduced cholesterol content of the membrane impairs the activity of ABCB11 (and ABCC2) and, as a consequence, causes cholestasis. Abbreviations: ATP8B1, phosphatidylserine flippase; ABCB11, the major bile salt transporter; ALP, alkaline phosphatase.
Footnotes
The abbreviations used are: PFIC1, progressive familial intrahepatic cholestasis type 1; BRIC1, benign recurrent intrahepatic cholestasis type 1; CH/PC, cholesterol to choline-containing phospholipid ratio; TC, taurocholate; DNP-GS, dinitrophenyl[glycine-2-]glutathione; TDC, taurodeoxycholate; 5′-CF, 5′-carboxyfluorescein; MβCD, methyl-β-cyclodextrin.
References
- 1.van Mil, S. W., Houwen, R. H., and Klomp, L. W. (2005) J. Med. Genet. 42 449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullenbach, R., Bennett, A., Tetlow, N., Patel, N., Hamilton, G., Cheng, F., Chambers, J., Howard, R., Taylor-Robinson, S. D., and Williamson, C. (2005) Gut 54 829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Painter, J. N., Savander, M., Ropponen, A., Nupponen, N., Riikonen, S., Ylikorkala, O., Lehesjoki, A. E., and Aittomaki, K. (2005) Eur. J. Hum. Genet. 13 435–439 [DOI] [PubMed] [Google Scholar]
- 4.Halleck, M. S., Lawler, J. F., Jr., Blackshaw, S., Gao, L., Nagarajan, P., Hacker, C., Pyle, S., Newman, J. T., Nakanishi, Y., Ando, H., Weinstock, D., Williamson, P., and Schlegel, R. A. (1999) Physiol. Genomics 1 139–150 [DOI] [PubMed] [Google Scholar]
- 5.Paulusma, C. C., and Oude Elferink, R. P. (2005) Biochim. Biophys. Acta 1741 11–24 [DOI] [PubMed] [Google Scholar]
- 6.Devaux, P. F., Lopez-Montero, I., and Bryde, S. (2006) Chem. Phys. Lipids 141 119–132 [DOI] [PubMed] [Google Scholar]
- 7.Pomorski, T., Holthuis, J. C., Herrmann, A., and van Meer, G. (2004) J. Cell Sci. 117 805–813 [DOI] [PubMed] [Google Scholar]
- 8.Paulusma, C. C., Folmer, D. E., Ho-Mok, K. S., de Waart, D. R., Hilarius, P. M., Verhoeven, A. J., and Oude Elferink, R. P. (2008) Hepatology 47 268–278 [DOI] [PubMed] [Google Scholar]
- 9.van Mil, S. W., van Oort, M. M., van den Berg, I., Berger, R., Houwen, R. H., and Klomp, L. W. (2004) Pediatr. Res. 56 981–987 [DOI] [PubMed] [Google Scholar]
- 10.Kurbegov, A. C., Setchell, K. D., Haas, J. E., Mierau, G. W., Narkewicz, M., Bancroft, J. D., Karrer, F., and Sokol, R. J. (2003) Gastroenterology 125 1227–1234 [DOI] [PubMed] [Google Scholar]
- 11.Stapelbroek, J. M., van Erpecum, K. J., Klomp, L. W., Venneman, N. G., Schwartz, T. P., van Berge Henegouwen, G. P., Devlin, J., van Nieuwkerk, C. M., Knisely, A. S., and Houwen, R. H. (2006) Hepatology 43 51–53 [DOI] [PubMed] [Google Scholar]
- 12.Pawlikowska, L., Groen, A., Eppens, E. F., Kunne, C., Ottenhoff, R., Looije, N., Knisely, A. S., Killeen, N. P., Bull, L. N., Elferink, R. P., and Freimer, N. B. (2004) Hum. Mol. Genet. 13 881–892 [DOI] [PubMed] [Google Scholar]
- 13.Paulusma, C. C., Groen, A., Kunne, C., Ho-Mok, K. S., Spijkerboer, A. L., de Waart, R., Hoek, F. J., Vreeling, H., Hoeben, K. A., van Marle, J., Pawlikowska, L., Bull, L. N., Hofmann, A. F., Knisely, A. S., and Oude Elferink, R. P. (2006) Hepatology 44 195–204 [DOI] [PubMed] [Google Scholar]
- 14.Groen, A., Kunne, C., Paulusma, C. C., Kramer, W., Agellon, L. B., Bull, L. N., and Oude Elferink, R. P. (2007) J. Hepatol. 47 114–122 [DOI] [PubMed] [Google Scholar]
- 15.Groen, A., Kunne, C., Jongsma, G., van den Oever, K., Mok, K. S., Petruzzelli, M., Vrins, C. L., Bull, L., Paulusma, C. C., and Oude Elferink, R. P. (2008) Gastroenterology 134 2091–2100 [DOI] [PubMed] [Google Scholar]
- 16.Meier, P. J., Sztul, E. S., Reuben, A., and Boyer, J. L. (1984) J. Cell Biol. 98 991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Waart, D. R., Paulusma, C. C., Kunne, C., and Oude Elferink, R. P. (2006) Liver Int. 26 362–368 [DOI] [PubMed] [Google Scholar]
- 18.van Meer, G., and Simons, K. (1986) EMBO J. 5 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marheineke, K., Grunewald, S., Christie, W., and Reilander, H. (1998) FEBS Lett. 441 49–52 [DOI] [PubMed] [Google Scholar]
- 20.Gimpl, G., Klein, U., Reilander, H., and Fahrenholz, F. (1995) Biochemistry 34 13794–13801 [DOI] [PubMed] [Google Scholar]
- 21.Oude Elferink, R. P. J., Paulusma, C. C., and Groen, A. K. (2006) Gastroenterology 130 908–925 [DOI] [PubMed] [Google Scholar]
- 22.Oude Elferink, R. P., and Groen, A. K. (2000) Semin. Liver Dis. 20 293–305 [DOI] [PubMed] [Google Scholar]
- 23.Trauner, M., Fickert, P., and Wagner, M. (2007) Semin. Liver Dis. 27 77–98 [DOI] [PubMed] [Google Scholar]
- 24.Mazzone, A., Tietz, P., Jefferson, J., Pagano, R., and LaRusso, N. F. (2006) Hepatology 43 287–296 [DOI] [PubMed] [Google Scholar]
- 25.Nibbering, C. P., and Carey, M. C. (1999) J. Membr. Biol. 167 165–171 [DOI] [PubMed] [Google Scholar]
- 26.Amigo, L., Mendoza, H., Zanlungo, S., Miquel, J. F., Rigotti, A., Gonzalez, S., and Nervi, F. (1999) J. Lipid Res. 40 533–542 [PubMed] [Google Scholar]
- 27.de Almeida, R. F., Fedorov, A., and Prieto, M. (2003) Biophys. J. 85 2406–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosters, A., Kunne, C., Looije, N., Patel, S. B., Oude Elferink, R. P., and Groen, A. K. (2006) J. Lipid Res. 47 1959–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, A. G. (2004) Biochim. Biophys. Acta 1666 62–87 [DOI] [PubMed] [Google Scholar]
- 30.Pal, A., Mehn, D., Molnar, E., Gedey, S., Meszaros, P., Nagy, T., Glavinas, H., Janaky, T., von Richter, O., Bathori, G., Szente, L., and Krajcsi, P. (2007) J. Pharmacol. Exp. Ther. 321 1085–1094 [DOI] [PubMed] [Google Scholar]
- 31.Telbisz, A., Muller, M., Ozvegy-Laczka, C., Homolya, L., Szente, L., Varadi, A., and Sarkadi, B. (2007) Biochim. Biophys. Acta 1768 2698–2713 [DOI] [PubMed] [Google Scholar]