Abstract
The mammalian circadian system is orchestrated by a master pacemaker in the brain but many peripheral tissues also contain independent or quasi-independent circadian oscillators. The adaptive significance of clocks in these structures must lie, in large part, in the phase relationships between the constituent oscillators and their micro- and macro-environments. To examine the relationship between postnatal development, which is dependent on endogenous programs and maternal/environmental influences, and the phase of circadian oscillators, we assessed the circadian phase of pineal, liver, lung, adrenal, and thyroid tissues cultured from Period 1-luciferase (Per1-luc) rat pups of various postnatal ages. The liver, thyroid, and pineal were rhythmic at birth, but the phases of their Per1-luc expression rhythms shifted remarkably during development. To determine if the timing of the phase shift in each tissue could be the result of changing environmental conditions, we monitored the behavior of pups and their mothers. We found that the circadian phase of the liver shifted from the day to night around postnatal day (P) 22 as the pups nursed less during the light and instead ate solid food during the dark. Furthermore, the phase of Per1-luc expression in liver cultures from nursing neonates could be shifted experimentally from the day to the night by allowing pups access to the dam only during the dark. Peak Per1-luc expression also shifted from mid-day to early night in thyroid cultures at about P20, concurrent with the shift in eating times. The phase of Per1-luc expression in the pineal gland shifted from day to night coincident with its sympathetic innervation at around P5. Per1-luc expression was rhythmic in adrenal cultures and peaked around the time of lights-off throughout development, however, the amplitude of the rhythm increased at P25. Lung cultures were completely arrhythmic until P12 when the pups began to leave the nest. Taken together, our data suggest that the molecular machinery that generates circadian oscillations matures at different rates in different tissues and that the phase of at least some peripheral organs is malleable and may shift as the organ's function changes during development.
Keywords: development, circadian rhythms, suprachiasmatic nucleus, peripheral clocks, Period1, luciferase reporter, mammalian
Introduction
The hierarchial chrono-architecture of the mammalian circadian system is composed of multiple oscillators located in both central and peripheral tissues (Yamazaki et al., 2000; Davidson et al., 2003). A master pacemaker in the suprachiasmatic nuclei (SCN) of the hypothalamus orchestrates the rhythms of multiple peripheral clocks (Yoo et al., 2004). Under most conditions, light is the most important exogenous factor that entrains the circadian system and allows organisms to anticipate daily changes in the natural environment (Foster et al., 2007). Food availability is also a key environmental signal and food (or feeding) can entrain and decouple some (but not all) peripheral clocks from the control of the light-entrained SCN (Mistlberger, 1994; Stephan, 2002, Vujović et al., 2008). Using an in vitro reporter system to monitor circadian rhythms in tissues cultured from Period1-luciferase (Per1-luc) rats, in which the Per1 promoter drives the expression of luciferase, we have demonstrated that light and food signals entrain the peripheral as well as the central components of the circadian system (Yamazaki et al, 2000; Stokkan et al, 2001). While the rhythms of peripheral clocks are usually controlled by the SCN, they can also act independently of the master clock by adjusting their phase in response to exogenous signals such as rhythmic food availability (Damiola et al., 2000; Hara et al., 2001; Stokkan et al., 2001).
During postnatal development, the environment of rat pups changes drastically in a short period of time. Initially, neonates are confined to the nest and are fed by their mother. Later, the pups venture out of the nest and begin eating solid food. Concurrent with these changes in the environment, numerous internal developmental programs are progressing. While the SCN becomes rhythmic at embryonic day 19 to 20 (Reppert and Schwartz, 1984; Ohta et al., 2002), it is unknown whether peripheral clocks are rhythmic at birth (but see Ohta et al., 2008). Because both the endogenous and exogenous environments of perinatal rat pups undergo remarkable transformations, it is possible that circadian characteristics of peripheral oscillators may also change during development in order to maintain adaptive phase relationships with important aspects of the changing environment. To gain a better understanding of how the circadian system matures during development we examined rhythms of Per1-luc expression in postnatal peripheral oscillators.
Materials and Methods
Animals
Two different lines of Per1-luc transgenic rats [W(per1)1 and W(per1)2 (Yamazaki et al., 2000; referred to as L1 and L2, respectively)] were used in this study. All rats were raised and maintained in a 12:12 light/dark cycle (lights on at 5:00, lights off at 17:00 Eastern Standard Time). The day of birth was defined as postnatal day 0 (P0). Tissue cultures were prepared from L1 heterozygous and L2 homozygous pups at various days after birth (P0 to P90). Since no sex differences were identified, both male and female L1 and L2 rats were used. All procedures were approved by the Animal Care and Use Committee at the University of Virginia.
Tissue culture, bioluminescence recording and data analysis
Tissue culture, luminescence recordings and data analyses were performed as previously described (Yamazaki et al., 2002; Yamazaki and Takahashi, 2005; Yoshikawa et al., 20005). Tissues were harvested within the hour before lights-off. Tissues from L1 rats were cultured in Gibco DMEM [DMEM powder (#13000-021, Gibco), while Sigma DMEM powder (D-2902, Sigma) supplemented with 3.5 g of D-glucose (G7021 Sigma) was used for culturing L2 tissues (because Gibco discontinued the production of DMEM powder). Peak phase was determined from detrended (24 h moving average was subtracted from original data) and smoothed (3 h moving average) data as the highest point occurring between 12 h and 36 h in culture. Cultures were considered rhythmic when more than 2 circadian cycles of Per1-luc were expressed. Cultures with only one peak of Per1-luc were designated as arrhythmic.
Maternal Presence/Absence
Male homozygous L1 rats were crossed with wild type (WT) females. Mothers (WT) and their pups (L1 heterozygous) were maintained in a 12:12 light/dark cycle. Starting on postnatal day 5 (P5), maternal presence/absence (P/A) cycles were imposed on the pups. Individual litters were designated as control, light, or dark groups. Pups in the control group remained with their mother at all times (24 h per day). Litters in the light group were allowed access to the dam during the light phase (05:00–17:00) and were separated from their mother during the dark phase (17:00-05:00). To avoid dehydration or changes in body temperature while the litter of pups was separated from their mother, the litter was transferred from their mother’s cage to a glass dish, which was placed in a warm water bath (35°C). Litters in the dark group were allowed access to the dam during the dark phase and were placed in the water bath during the light phase. After either 2 or 7 full P/A cycles (on P7 or P12, respectively), three pups from each litter were randomly selected for tissue culture.
Monitoring nursing behavior and pups’ activity
A clear plastic rat cage (26 cm in width, 47 cm in length, 20 cm in height) was used to house each mother and her pups. An infrared video camera (2001BI, Spycameras4Less, Madisonville, LA) was connected to a computer with a capture card and video capture program (Microsoft VidCap). The program was set to capture one frame every minute, and the camera ran continuously, 24 hours a day from P2 until weaning on P21. Each day’s data were transferred to a CD and viewed at one frame-per-second (using Microsoft VidEdit, version 1.1). An observer (E.W.B.) watched the video and recorded the time when the mother was “on” or “off” the nest. The mother was considered “on” the nest when she was lying on or very close to the pups. She was considered “off” the nest when she had no physical contact with the pups or was lying away from the majority of the pups. Also recorded were the times each pup independently moved off the nest and when the pups began eating solid food. Data was recorded in 5 minute bins and plotted graphically using ActiView software (Mini Mitter Co. Inc, Bend, OR).
Taqman Analysis
Lungs from P3 (arrhythmic) and P30 (rhythmic) L1 heterozygous animals were harvested either at the time of lights on or lights off and were frozen immediately in liquid nitrogen. Total RNA was extracted with the Trizol reagent (Invitrogen). RT-Taqman PCR analysis (Taqman PCR 7900HT Sequence Detection System Applied Biosystems) was used to quantify mRNA levels. cDNA was synthesized using oligo dT primers (Applied Biosystems). A SYBR Green PCR Kit (Applied Biosystems) was used simultaneously to amplify synthesized cDNA and serial dilutions of genomic DNA (Promega) of known copy numbers to generate a standard curve. Each mRNA concentration was determined within the linear range of the standard curve. cDNA samples were normalized relative to the level of GAPDH (primers, Applied Biosystems). The primer pairs used were: Per1: 5’ AGCAGAGTGGAAGTTTTCAGCC 3’ and 5’ACCACTTCAGCAGCTTGTCAGC 3’. Per2: 5’ GGTGTGGCAGCTTTTGCTTC 3’ and 5’ CGGCACAGAAACGTACAGTGTG 3’. Per3: 5’ ACAGATGACGTTTTCCCAAAGC 3’ and 5’ TGTCTTCAGGTGACGGCTGAT 3’. Bmal1: 5’ TGGAGAAGGTGGCCCAAAGA 3’ and 5’ CCTATTTTTCCTGCTCCAGCTCT 3’. Clock: 5’ CATGCAGCATCTCAAAGACC 3’ and 5’ CCTTAGTTCTTCTTGCTGCCGA 3’. Cry1: 5’ TGTGTTCAGATCCCTTGGGACAA 3’ and 5’ ACTCTTCAAAGACCTTCATCCCTT 3’. Cry2: 5’ GGCCGAGGGCAAGACAG 3’ and 5’ AGCCCTCCTGCCTCAGTTG 3’. Timeless: 5’ GACCCTCAGTATCCGTACCTTTCA 3’ and 5’ TTGCGGTCTGTCAGCATCAT 3’. ck1ε: 5’ CGGTTCGATGATAAGCCTGAC 3’ and 5’ AGAAACCCTGCCGGTGAAA 3’. NPAS2: 5’ TCAGCATGTTCCAGACCATTAAA 3’ and 5’ GCTGCCACCGAATGTTGG 3’.
Results
Postnatal development of Per1-luc rhythm
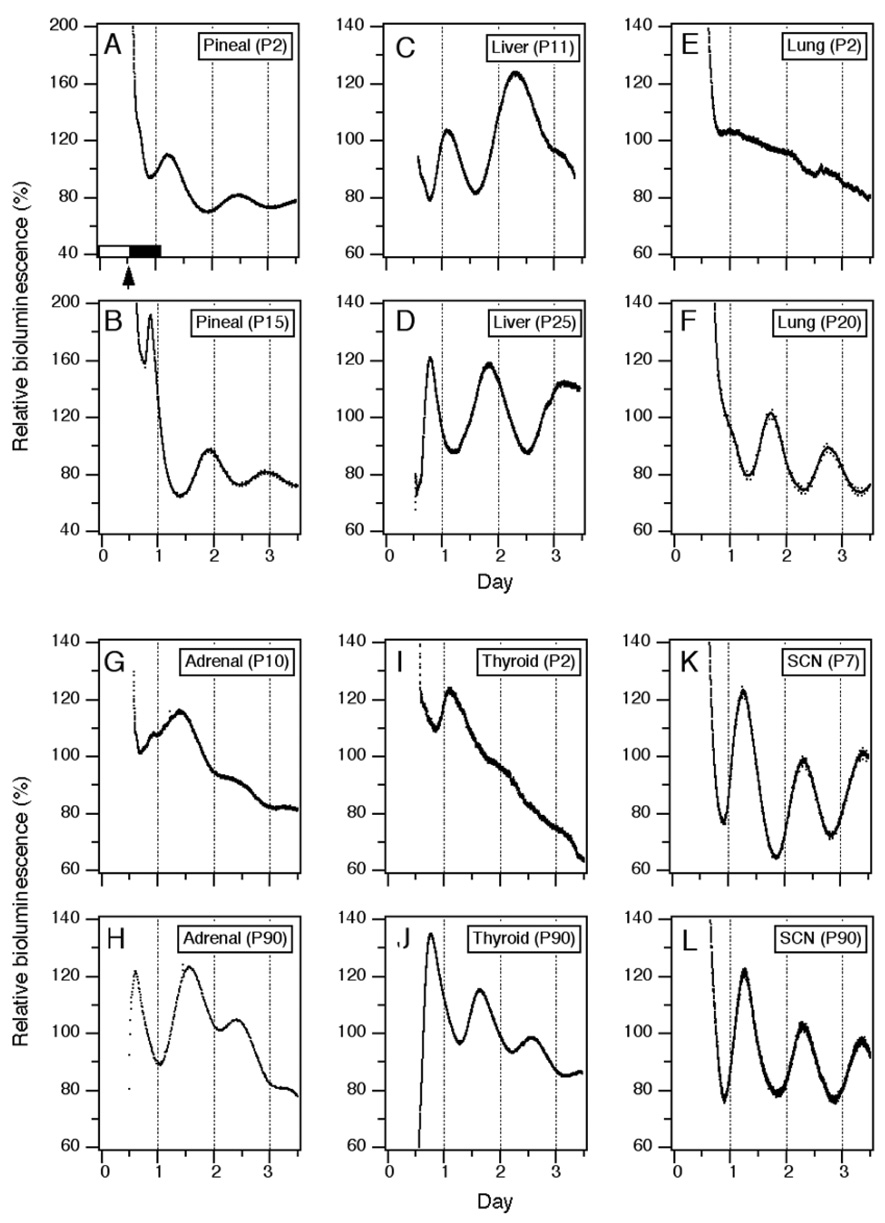
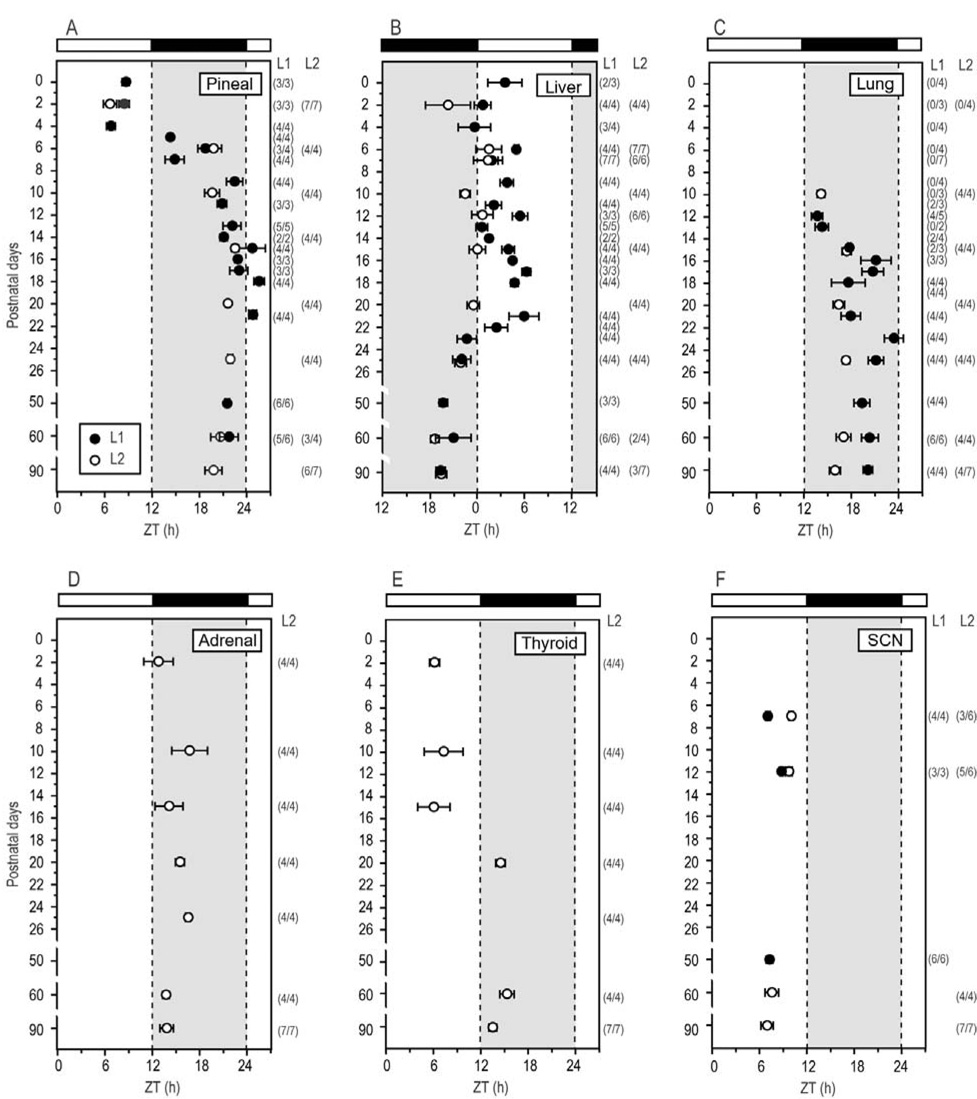
To understand how the circadian characteristics of central and peripheral clocks change postnatally, we analyzed Per1-luc expression in cultured SCN, pineal, liver, lung, adrenal, and thyroid tissues from pups of different ages. The rhythm of Per1-luc expression in pineal glands harvested from P0 pups was robust and peaked during the day (Fig. 1A, 2A). The phase of the Per1-luc rhythm remained stable until P4 and then it began to delay gradually each day. At P9, the phase stabilized in the late night (P9 to P90, Fig 1B and Fig 2A).
Figure 1. Representative bioluminescence rhythms in several tissues cultured at various postnatal days.
Within one hour before lights off (arrow, light/dark cycle indicated by white and black bars, respectively), static cultures of pineal (A, B), liver (C, D), lung (E, F), adrenal (G, H), thyroid (I, J) and SCN (K, L) were prepared from Per1-luc rat pups or adults maintained in 12:12 LD. Bioluminescence measurements (in counts per second) were collected at 10 minute intervals and are plotted for 3 days.
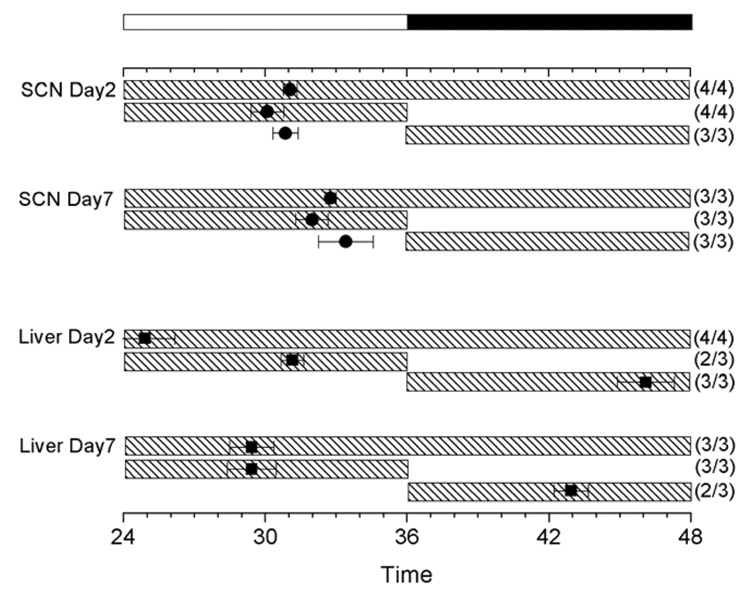
Figure 2. Phase changes in peripheral clocks during postnatal development.
Peaks of Per1-luc rhythms in pineal (A), liver (B), lung (C), adrenal (D), thyroid (E) and SCN (F) are plotted as a function of postnatal age. Black and white bars on the top indicates LD cycles in which the pups and their dams were held. The vertical axis indicates postnatal age at the time cultures were made. Average peak phase of rhythmic cultures (±SEM) from two transgenic lines of rats (L1, filled circle; L2, open circle) are plotted separately. Numbers in parentheses indicate rhythmic cultures per number of cultures tested. Note: none of lung tissue taken before P10 was rhythmic.
Per1-luc expression in liver cultures harvested from P0 to P21 pups peaked around early to mid-day (Fig. 1C and Fig 2B). Then, the phase began gradually advancing at P22 until it stabilized between P25 and P50 in the mid-night (Fig. 1D and Fig 2B).
At P2, Per1-luc expression in thyroid cultures peaked in mid-day (Fig. 1I and Fig 2E). This peak shifted to early night by P20 (Fig. 1J and Fig 2E).
In P2 adrenal cultures that contained both the cortex and medulla Per1-luc expression peaked in early night (Fig. 2D). Although no obvious change in peak phase was observed during the course of development, the amplitude of the rhythm was greater in cultures prepared after P25 than from P10 pups (see Figs. 1G and 1H).
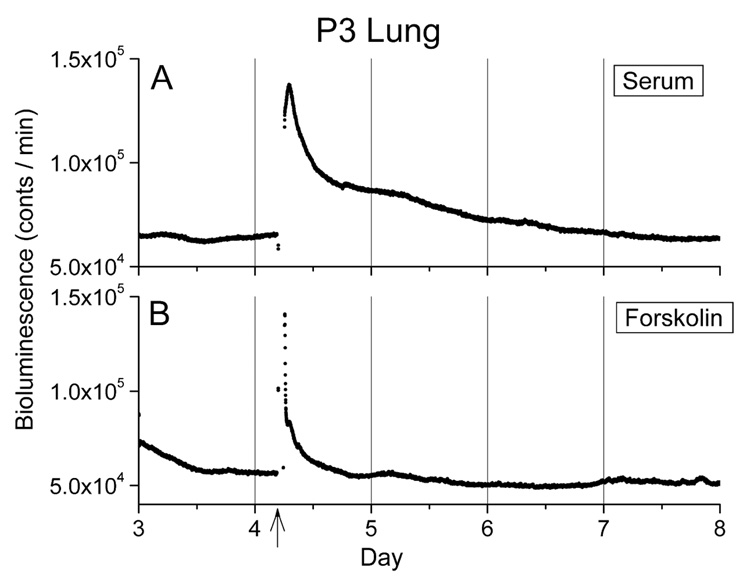
Lung tissue harvested from pups younger than P10 did not show rhythmic Per1-luc expression (Fig. 1E and Fig 2C). Rhythmic Per1-luc expression in lung cultures was first observed at P10 and peaked during the early night. The peak phase in the lung was slightly delayed as animals developed. One-hour stimulation with newborn calf serum or forskolin, which always reinitiates rhythmicity in damped adult tissues (Yamazaki et al., 2002), failed to induce rhythmic Per1-luc expression in arrhythmic cultures of lung taken before P10, although a transient increase in Per1-luc activity was observed (Figs. 3A and B). To determine whether the arrhythmicity of lung cultures from young rats could be caused by lack of expression of one or more circadian genes, we used RT-PCR to assess mRNA levels in the lung at P3 and P30. We found no significant differences between mRNA levels of Per1, Per2, Per3, Cry1, Cry2, Bmal1, Clock, NPAS2, Ck1ε and Tim mRNAs in lung tissue harvested from P3 and P30 rats, although differences in the levels of Per3, Bmal1 and Clock approached significance (TABLE 1).
Figure 3. Early postnatal lung was not capable of exhibiting Per1-luc rhythmicity in culture.
Neither 50 % new born calf serum (A; n=4) or 10 µM forskolin (B; n=4) stimulation could induce the rhythmicity in lung cultures made from P3 pups. Time of one hour stimulation (arrow) is indicated at the bottom of panel.
Table 1. Circadian gene expression in rhythmic and arrhythmic lungs.
mRNA levels of circadian clock related genes were compared between lung tissue at P3 (n=7) and P30 (n=4). Tissues harvested at ZT 0 and 12 were averaged and mean and SEM were plotted. No statistical differences (by t-test) were observed in the expression levels of Per1, Per2, Per3, Cry1, Cry2, Bmal1, Clock, NPAS2, Ck1ε and Tim.
Result of quantitative RT-PCR analysis.
| Postnatal day 3 | Adult | p value | |
|---|---|---|---|
| Per1 | 1.65 ± 0.09 | 1.83 ± 0.36 | 0.55 |
| Per2 | 2.38 ± 0.09 | 2.77 ± 0.37 | 0.22 |
| Per3 | 0.15 ± 0.02 | 0.23 ± 0.04 | 0.07 |
| Cry1 | 1.10 ± 0.17 | 1.25 ± 0.28 | 0.64 |
| Cry2 | 0.05 ± 0.01 | 0.07 ± 0.02 | 0.43 |
| Bmal1 | 1.01 ± 0.13 | 0.58 ± 0.16 | 0.06 |
| Clock | 0.15 ± 0.02 | 0.23 ± 0.04 | 0.07 |
| NPAS2 | 0.08 ± 0.02 | 0.09 ± 0.04 | 0.83 |
| Ck1ε | 1.05 ± 0.16 | 1.42 ± 0.22 | 0.20 |
| Tim | 0.90 ± 0.09 | 0.72 ± 0.02 | 0.19 |
Activity monitoring
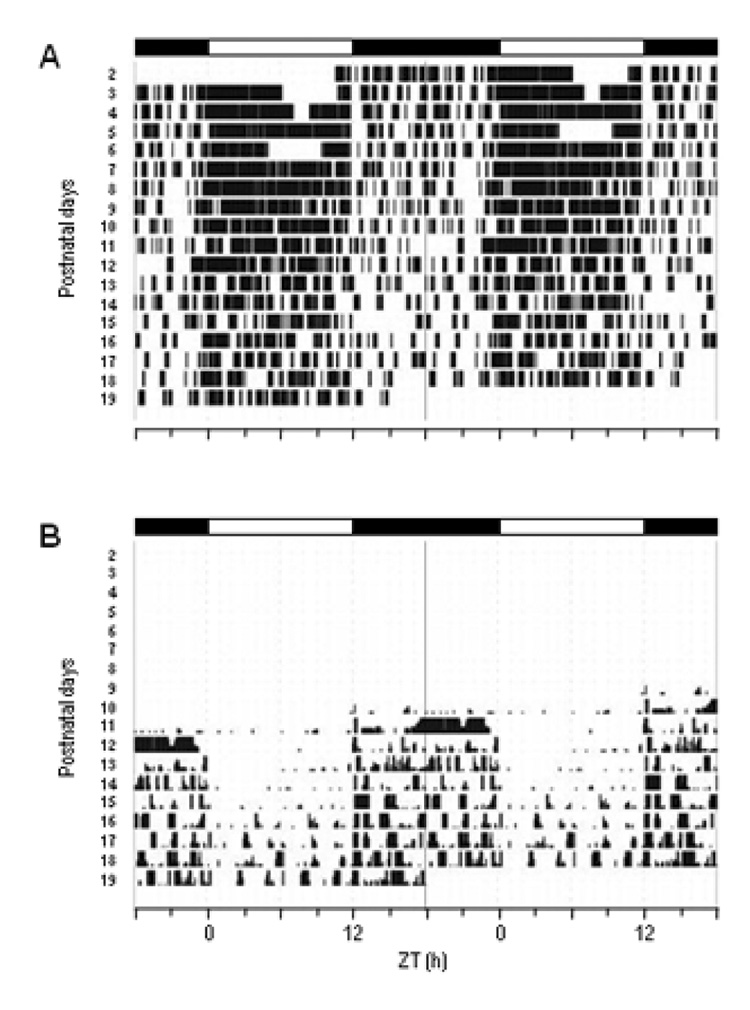
We hypothesized that shifts in the phase of Per1-luc expression rhythm might correlate with developmental milestones such as the time at which rat pups independently leave the nest or begin to eat solid food. To positively identify the times at which these events occurred, we recorded the behavior of each dam and her pups with an infra-red camera. The dam covered the nest for more of the day at early ages of the pups than at later ages (Fig. 4A). Therefore, the pups were likely nursed mostly during the day. This diurnal activity of nest covering by the dam gradually became less consolidated as the pups developed. Until P11, pups never spontaneously left the nest. Pups were first observed to leave the nest independently on P11, an activity that occurred primarily during the dark portion of the light cycle (Fig. 4B). These patterns were observed in 3 separate dam/pup groups. We first observed pups eating solid food around P15 (in two dam/pup groups, solid food was first eaten at P15; in the third dam/pup group, solid food was first eaten at P17). We observed that the pups ate during the dark part of the light/dark cycle which is consistent with a previous report (Levin and Stern, 1975).
Figure 4. Representative actograms of dam’s and pups' ";nest time. ".
Behavior of a mother and her pups were monitored by infra-red video camera. Events when the mother was “on” her nest are double plotted as black vertical bars (A). The number of pups that were out of the nest is indicated by the height of the black bars double plotted in (B). Black and white bars on the top of each panel indicate the LD cycles. Two other records show very similar activity patterns.
Effects of timing of nursing on Per1-luc rhythm
Activity monitoring indicated that pups were nursed mostly during the day, while weanlings and adults consumed solid food during the night. We hypothesized that the phase difference in Per1-luc rhythms of the liver cultures of nurslings and adults was caused by differences in the time of eating. To test this hypothesis, pups’ daily access to their dam was restricted to the 12 h of either the light or dark phase of the day [presence/absence cycles (P/A cycles)]. Exposure to either P/A cycle did not alter the phase of the Per1-luc rhythm in SCN cultures. In SCN cultures prepared from pups nursed only in the dark or only in the light, Per1-luc expression peaked during the day (Fig.5). In contrast to the SCN, the phase of Per1-luc expression rhythms in the liver cultures from the pups was significantly altered by changing time at which they had access to the dam (Fig. 5). Per1-luc expression in liver cultures from the control and light groups, in both of which pups were nursed during day, peaked during the day. In marked contrast, Per1-luc expression in liver harvested from the dark group, in which pups had access to the dam only in the dark, peaked during the night.
Figure 5. Effect of maternal presence/absence cycles on circadian rhythm phase in the SCN and liver.
The average times (±SEM) of peaks are plotted in Figure 2. The light cycle is plotted as a black and white bar at the top of the figure. Shaded bars indicate the time when pups were with their dams (top: control, mid: light group, bottom: dark group). Both SCN and liver were cultured after either 2 or 7 days of P/A cycles as indicated. No significant differences were found in SCN (by ANOVA). The phase of liver in the dark-fed group on Day 7 was significantly different from both the control and the light-fed group (ANOVA followed by Dunnett’s test, P<0.05).
Discussion
Our results indicate that peripheral clocks mature at different rates. Furthermore, the phases of the Per1-luc rhythms in some peripheral tissues change remarkably during postnatal development. The timing of this change varies by tissue, suggesting that each peripheral clock may respond differently to various neural, hormonal, physiological, and behavioral inputs. Under some conditions the timing of the culture procedure itself may influence the phase of rhythms recorded in the tissue explants (Yoshikawa et al., 2005). Although we cultured all tissues at the same times, it is possible that the changes in phase that we observed might reflect changes in sensitivity to the procedure. Ideally such phase changes could be confirmed by ex vivo experiments. Recently, Sládek et al. (2007) reported postnatal phase changes of circadian gene mRNA expression rhythms in Wistar rat liver. The phase of Per1 mRNA rhythms obtained by population sampling (ex vivo experiments) was similar to that which we measured using our in vitro real-time reporter assay. Our data also show that the time of maternal presence drastically affects the phase of the pup liver rhythm as measured by our in vitro assay. This supports the idea that the culture procedure as we have used it here has a minimal effect on the phase of tissue (at least in liver) explants.
In adult rats, melatonin is synthesized rhythmically by the pineal gland and peaks during the night. However, melatonin levels are not rhythmic immediately following birth (see review by Klein, Namboodiri and Auerbach, 1981). The day-to-night difference in pineal melatonin content becomes evident at P8 in Sprague-Dawley rats (Tamarkin et al., 1980) and at P11 in Wistar rats (Ribelayga et al., 1998). A small, but significant, nocturnal increase in the activity of arylalkylamine-N-acetyltransferase (AA-NAT), the rate-limiting enzyme for melatonin synthesis, is first identified at P4 and becomes prominent at P7 (Ellison, Weller and Klein, 1972). The expression of AA-NAT mRNA becomes rhythmic in the pineal gland of Wistar rats at P5 (Pfeffer and Stehle, 1998). The pineal gland is innervated by sympathetic neurons that originate in the superior cervical ganglion connected by a multisynaptic pathway to the SCN (see recent reviews, Klein 2007; Maronde and Stehle, 2007). Nocturnal release of norepinephrine from sympathetic terminals causes a rapid increase in AA-NAT mRNA expression. Hakanson and colleagues (1967) reported that sympathetic fibers are confined to the surface of the pineal in P0 to P4 rats. At P5 to P6, some fibers have entered the parenchyma of the pineal, suggesting that noradrenergic signals may be transmitted at this time. AA-NAT gene expression becomes rhythmic concurrent with sympathetic innervation of the pineal. Unlike the expression of AA-NAT mRNA, we found that Per1-luc expression in the pineal is rhythmic at birth, suggesting that the pineal clock is already running. AA-NAT expression may be arrhythmic at P0, because it is not yet coupled to the clock. Alternatively, AA-NAT may not be under the control of the pineal clock but rather may be driven rhythmically by the SCN through the sympathetic pathway. Since previous studies assessed AA-NAT mRNA expression at only two timepoints (one during the day and another in the night), it is possible that AA-NAT mRNA is, in fact, rhythmically expressed at birth, but its rhythmicity was not detected by this sampling protocol (Ellison et al., 1972; Pfeffer and Stehle, 1998). Detailed analysis of AA-NAT expression at birth will be necessary to determine if melatonin synthesis is controlled by the pineal clock at this early developmental stage.
The pineal clock is rhythmic at birth and the rhythm of Per1-luc expression peaks during the day. This is in contrast to adult rats, where pineal Per1-luc peaks during the night. Interestingly, we found that the phase of Per1-luc expression was gradually delayed from P5 to P7, concurrent with sympathetic innervation of the pineal, and the emergence of rhythmic AA-NAT mRNA expression, suggesting that the developmental program of the pineal clock is coordinated with the functional maturation of the pineal gland.
In previous studies on adult rodents, we and others have demonstrated that the phase of the liver clock entrains to the time of feeding (Damiola et al., 2000; Hara et al., 2001; Stokkan et al., 2001). In the current study, we found that the phase of Per1-luc expression in the liver changed from early in the day to late night at about P21. Since the phase shift occurred as pups were leaving the nest (where they were fed primarily during day) and beginning to eat solid food (which they eat at night), we hypothesized that feeding time controlled the phase of the liver clock in the presence of an unchanged light cycle. In support of this hypothesis, we were able to shift the phase of Per1-luc expression in livers harvested from P7 and P9 pups by limiting their access to the dam to either the day or the night (P/A cycle). We cannot exclude the possibility that stress associated with maternal separation and/or a small temperature change might also affect the phase of liver explants.
In contrast to the liver, the phase of the SCN was not affected by the P/A cycle. Our findings contrast with those of Ohta and colleagues (2002), who showed the SCN of enucleated pups entrains to the P/A cycle. However, at P5, when we exposed the pups to varying P/A cycles, the SCN is responsive to light (Weaver and Reppert, 1995). Therefore, the SCN of the pups in our experiment were likely entrained to the light/dark cycle even though they were exposed to the P/A cycle. This is a striking (though not unique) example of differential entrainment of different circadian oscillators in the same individual organism by hierarchically organized signals. It would be interesting to examine the effect of the P/A cycle on the phase of Per1-luc expression in the SCN of neonates before the retinohypothalamic tract becomes functional.
The peak phase of Per1-luc expression in both the liver and thyroid shifted from day to night at similar postnatal ages (P20) perhaps as a consequence of the change in feeding pattern. Since there is crosstalk between the hypothalamic-pituitary-thyroid (HPT) and hypothalamic-pituitary-adrenal (HPA) axis (Helmreich et al., 2005), we wondered whether the thyroid and adrenal clocks would shift phase at the same time in development. However, we found that the phase of the Per1-luc expression rhythm in the adrenal gland remained stable from P0 to P90, while the thyroid shifted between P15 and P20. This suggests that the rhythms of the adrenal and thyroid glands are separately controlled by the pituitary, or that factors apart from those of pituitary origin control these peripheral clocks. Interestingly, the amplitude of Per1-luc expression in cultured adrenals increased by P25 when daily rhythms of blood corticosterone are first observed in rats (Miyabo et al., 1980).
In contrast with the other tissues examined in this study, Per1-luc expression in lung tissue was arrhythmic until pups were 10 days old. Adding forskolin or serum to the medium induced a transient increase in Per1-luc expression, but neither initiated a rhythm in lungs from P3 pups. These data suggest that the lung is not capable of expressing an autonomous rhythm prior to P10 although other stimuli (e.g., glucocorticoid) might generate rhythmicity. Arrhythmicity in early postnatal lung cannot be attributed to deficient circadian gene expression since Per1, Per2, Per3, Cry1, Cry2, Bmal1, Clock, NPAS2, Ck1e and Tim mRNAs were expressed at comparable levels in arrhythmic P3 and rhythmic P30 lung tissue. However, we did not make any protein measurements. Interestingly, the lung becomes rhythmic at P10 and peaks during the night, concurrent with the increased activity of the pups as they leave the nest for the first time.
Our data indicate that the phase of Per1-luc rhythms in peripheral tissues may correspond with the physiology of the tissue and the behavior of the animal (e.g. liver rhythm peaks during feeding, lung develops a rhythm and peaks when pups are active outside of the nest). Perhaps the molecular apparatus that generates distinct circadian oscillations matures at different rates under the influence of tissue-specific signals. The coordination of these processes may allow animals to adapt to their environment as it changes throughout postnatal development.
Acknowledgements
We thank Julie Pendergast for critical reading this manuscript, Tom Breeden for computer programming, Hal Noakes for assistance of CCD imaging, Jay Hirsh for help with imaging. This work was supported in part by the NSF Center for Biological Timing and NIH grant MH56647 to M.M. T.Y. and R.N. were supported by fellowships from the Japan Society for the Promotion of Science for Young Scientists.
References
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp. 2003;253:110–121. [PubMed] [Google Scholar]
- Ellison N, Weller JL, Klein DC. Development of a circadian rhythm in the activity of pineal serotonin N-acetyltransferase. J Neurochem. 1972;19:1335–1341. doi: 10.1111/j.1471-4159.1972.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Foster RG, Hankins MW, Peirson SN. Light, photoreceptors, and circadian clocks. Methods Mol Biol. 2007;362:3–28. doi: 10.1007/978-1-59745-257-1_1. [DOI] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Hakanson R, Lombard des Gouttes MN, Owman C. Activities of tryptophan hydroxylase, dopa decarboxylase, and monoamine oxidase as correlated with the appearance of monoamines in developing rat pineal gland. Life Sci. 1967;6:2577–2585. doi: 10.1016/0024-3205(67)90107-5. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Parfitt DB, Lu XY, Akil H, Watson SJ. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005;81:183–192. doi: 10.1159/000087001. [DOI] [PubMed] [Google Scholar]
- Klein DC. Arylalkylamine N-acetyltransferase: "the Timezyme". J Biol Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- Klein DC, Namboodiri MA, Auerbach DA. The melatonin rhythm generating system: developmental aspects. Life Sci. 1981;28:1975–1986. doi: 10.1016/0024-3205(81)90644-5. [DOI] [PubMed] [Google Scholar]
- Levin R, Stern JM. Maternal influences on ontogeny of suckling and feeding rhythms in the rat. J Comp Physiol Psychol. 1975;89:711–721. doi: 10.1037/h0077038. [DOI] [PubMed] [Google Scholar]
- Maronde E, Stehle JH. The mammalian pineal gland: known facts, unknown facets. Trends Endocrinol Metab. 2007;18:142–149. doi: 10.1016/j.tem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Miyabo S, Yanagisawa KI, Ooya E, Hisada T, Kishida S. Ontogeny of circadian corticosterone rhythm in female rats: effects of periodic maternal deprivation and food restriction. Endocrinology. 1980;106:636–634. doi: 10.1210/endo-106-2-636. [DOI] [PubMed] [Google Scholar]
- Ohta H, Honma S, Abe H, Honma K. Effects of nursing mothers on rPer1 and rPer2 circadian expressions in the neonatal rat suprachiasmatic nuclei vary with developmental stage. Eur J Neurosci. 2002;15:1953–1960. doi: 10.1046/j.1460-9568.2002.02016.x. [DOI] [PubMed] [Google Scholar]
- Ohta H, Xu S, Moriya T, Iigo M, Watanabe T, Nakahata N, Chisaka H, Hanita T, Matsuda T, Ohura T, Kimura Y, Yaegashi N, Tsuchiya S, Tei H, Okamura K. Maternal feeding controls fetal biological clock. PLoS ONE. 2008;3(7):e2601. doi: 10.1371/journal.pone.0002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer M, Stehle JH. Ontogeny of a diurnal rhythm in arylalkylamine-N-acetyltransferase mRNA in rat pineal gland. Neurosci Lett. 1998;248:163–166. doi: 10.1016/s0304-3940(98)00356-5. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Gauer F, Pévet P, Simonneaux V. Ontogenesis of hydroxyindole-O-methyltransferase gene expression and activity in the rat pineal gland. Brain Res Dev Brain Res. 1998;110:235–239. doi: 10.1016/s0165-3806(98)00114-x. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Schwartz WJ. The suprachiasmatic nuclei of the fetal rat: characterization of a functional circadian clock using 14C-labeled deoxyglucose. J Neurosci. 1984;4:1677–1682. doi: 10.1523/JNEUROSCI.04-07-01677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sládek M, Jindráková Z, Bendová Z, Sumová A. Postnatal ontogenesis of the circadian clock within the rat liver. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1224–R1229. doi: 10.1152/ajpregu.00184.2006. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The "other" circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Tamarkin L, Reppert SM, Orloff DJ, Klein DC, Yellon SM, Goldman BD. Ontogeny of the pineal melatonin rhythm in the Syrian (Mesocricetus auratus) and Siverian (Phodopus sungorus) hamsters and in the rat. Endocrinology. 1980;107:1061–1064. doi: 10.1210/endo-107-4-1061. [DOI] [PubMed] [Google Scholar]
- Vujović N, Davidson AJ, Menaker M. Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am J Physiol: Regulatory Integrative Comp Physiol. 2008;295:355–360. doi: 10.1152/ajpregu.00498.2007. first published April 23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR, Reppert SM. Definition of the developmental transition from dopaminergic to photic regulation of c-fos gene expression in the rat suprachiasmatic nucleus. Mol Brain Res. 1995;33:136–148. doi: 10.1016/0169-328x(95)00117-b. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301. doi: 10.1016/S0076-6879(05)93012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Yamazaki S, Menaker M. Effects of preparation time on phase of cultured tissues reveal complexity of circadian organization. J Biol Rhythms. 2005;20:500–512. doi: 10.1177/0748730405280775. [DOI] [PMC free article] [PubMed] [Google Scholar]