Abstract
Micron scale latex beads are well established as highly biocompatible reagents. Imbibing two fluorescent dyes into the interior of the beads enables the creation of a family of combinatorially colored labels. Previous use of such beads, in flow cytometry for example, has focused on beads of ~5μm diameter. We show here that 280 nm combinatorially labeled particles can be used to create ELISA-style assays in 200 μm scale virtual wells, using digital microscopy as the readout. The utility of this technique is illustrated by profiling the secreted cytokine footprints of peripheral blood mononuclear cells in a multiparametric version of the popular Elispot assay. Doing so reveals noncanonical classes of T lymphocytes. We further show that the secreting cell type can be concurrently identified by surface staining with a cell type specific antibody conjugated to the same multiplexed beads.
Keywords: cytokines, lymphocytes, multiplex, high content assay
Introduction
Latex (polystyrene) microspheres have been used for decades as assay reagents (Bangs, 1996). Originally used in agglutination reactions, microspheres are now more often used as reporters of molecule-molecule or molecule-surface binding interactions. In a typical binding assay, microspheres provide a high surface area in a small volume of liquid. With appropriate labeling, microsphere binding can be easily detected and localized.
In the past decade, several groups have described multiplexed assays based on combinatorially colored particles, a concept first described over twenty years ago (Fulwyler, 1985). Examples of single particles incorporating two or more distinguishable labels include fluorescently dyed beads (Fulton et al., 1997), beads incorporating quantum dots (Han et al., 2001), and beads incorporating cleavable labels readable by gas chromatography (Ohlmeyer et al., 1993). In some assays using these beads, analyte is mixed with an assortment of differently labeled beads, the surface of each bead analogous to the surface of a well in a polystyrene microplate. The combinatorial labeling is analogous to naming the well by row and column designators. The signal in the assay is typically generated by direct or competitive binding of a labeled probe to the bead surface.
One widely used version of this assay format is the multiplexed cytokine assay developed by Luminex and marketed by BioRad and others (de Jager et al., 2003). The advantage of this multiplexed assay is that multiple cytokines can be measured in a single sample concurrently, providing insight into the complex multifactorial regulation of the immune system. Since individual beads are small, very little cytokine is required to generate a signal per bead, but the overall assay sensitivity is limited by the requirement for a minimal quantity of fluid (and beads). In practice, the assay is used to measure pooled cytokines secreted by >10,000 cells, thus averaging the overall cellular activity in the sample.
Although useful in many contexts, a pooled assay format is not suitable for addressing questions relating to presence or absence of differing, sometimes rare, cell types within a larger population. For example, quantifying antigenic stimulation of T lymphocytes, under conditions when very few cells are in fact activated to the point of secreting cytokine, cannot be performed by a pooled assay and is typically accomplished using an Elispot assay. In this format, secreted cytokine is captured by antibodies linked to the surface upon which the cells are resting, with the captured cytokine visualized by an ELISA style assay (Czerkinsky et al., 1988). Spots at roughly cellular resolution are thereby generated, which can be counted by digital microscopy (Lehmann, 2005).
In this report, we demonstrate the feasibility of integrating the multiplexed bead concept into the Elispot format, enabling multifactorial definition of cell phenotype at single cell resolution (CellSpot™). We further show that cell surface markers in a mixed population of cytokine secreting cells can be labeled concurrently with the secreted protein footprints.
Methods
Combinatorially colored beads
Aldehyde modified polystyrene beads (280 nm diameter) (Interfacial Dynamics Corporation, Eugene, OR) were allowed to swell at 12.5 mg/mL in 5 mM citrate (pH3), 33% ethanol. Varying amounts of Nile red dye (Sigma) and Coumarin 6 (C6) dye (Sigma) at 1 mg/mL dissolved in 80% ethanol, 20% chloroform were added to the bead solution and incubated in a 65°C water bath for 15 minutes, followed by entrapment of the dye in the bead interior by three additions of pre-warmed water (33% of reaction volume) into the beads/dye mix at 15 minute intervals. The beads were subsequently removed from the solvent, washed, and suspended in buffer and stored at 4°C. Each bead type is defined by the ratio of Nile red and C6.
Protein conjugation to beads
A mixture of antibody and BSA (Sigma) (at 1:4.5 molar ratio) was conjugated to the dyed, aldehyde modified beads via reductive amination in 50mM Borate buffer (pH8.5). A stable secondary amine was formed by reduction with 3mg/mL of sodium cyanoborohydride at room temperature for 2 hours followed by blocking with 1% of BSA. After washing, the conjugated beads were stored in 50mM Borate buffer (pH8.5) with 1% of BSA.
Multiplexed assay conditions
Capture antibodies
R&D Systems: IFNγ (MAB2852), IL2 (AF202NA), IL4 (AF204NA), IL5 (MAB405), IL6(AF206NA); Pierce: IL10 (M010)
Detection antibodies
R&D Systems: IL2 (AF202NA), IL4 (AF204NA), IL6(AF206NA); Pierce: IFNγ (M701), IL10 (M011), IL5 (M551E)
100 μl of capture antibody (singly or in combination) was coated onto wells of a 96 well plate (Greiner, 96-well μClear) overnight at 1 μg/mL in 100 mM carbonate buffer (pH 9.6), then blocked with 3% of BSA. For calibration, the plates were incubated with a dilution series of cytokine(s) in PBS/0.2% Tween for 1 hour followed by washing with PBS/0.2% Tween. 100 μL of detection solution (0.1M citrate buffer pH 6/1% BSA/0.2% Tween) containing the beads conjugated with the detection antibodies was added into each well for an additional 16 hours in the dark. Beads of this size remain in suspension throughout this incubation period without agitation. The unbound beads were washed off with PBS/0.2% Tween, and the bound beads were fixed briefly with 4% paraformaldehyde. The plates were subsequently washed with water, and finally ethanol, and allowed to dry.
Because bound beads are more easily displaced by washing than conventionally labeled detection antibodies, plate washing was accomplished using a platewasher that has a low aspiration setting (Biotek Elx405 Select CW) with 50 μL residual buffer left in the wells after each wash to prevent the aspirator tip from approaching the surface.
The number of bound beads in this variation of the ELISA format was determined by automated counting of five random fields under 67x magnification using a modified Zeiss Discovery-12 fluorescent microscope. Fluorescence signal from both Nile red and C6 was collected in each field, and the ratio of the two colors in each bead was used to identify the bead type.
CellSpot™ assay format
Normal human PBMCs were obtained from AllCells (Emeryville, CA). For CellSpot assays, 105 PBMC, Th1 or Th2 selected cells were suspended in activation medium with phorbol myristate acetate (PMA) at 50 ng/mL and Ionomycin at 1μg/mL or control medium, and plated on cytokine-capturing 96-well plates. Cells were plated in a series of 2- or 3-fold dilutions to ensure a suitable cell density for imaging. The plates were covered and placed in a 37°C CO2 incubator for 16–20 hours (overnight). During this time, secreted cytokines were captured by the coated antibodies in a small area around the cell, the CellSpot. After cytokine capture, the plates were washed with water/0.2% Tween to lyse the cells, then with PBS/0.2% Tween. The large volume and rapid removal of the lysis buffer prevented released cytokine from the cells from binding to the surface. As noted below, in some experiments, the cells were fixed in place rather than lysed and removed, with no change in the secreted cytokine assay. Quantification of captured cytokines in CellSpots was performed as in the multiplexed ELISA assay described above, except that beads were counted in an area of 200 μm diameter around each cell.
The digital microscopy platform was based on a Stemi SV 11 dissecting microscope from Kramer Scientific (Valley Cottage, NY). Automation of the stage, of filter changing, and of objective lens changing were all developed at Trellis. A survey at low magnification (4x) allowed CellSpots to be detected and the position recorded. After switching to high magnification (40x), the individual beads at each location were imaged in two color channels.
Labeling of cell surfaces
For most of the experiments, cells were removed prior to staining the secreted footprints. We have subsequently determined that this is not necessary. Cytokine producing cells were fixed on the plate for 30 minutes by gently adding paraformaldehyde into culture media (4% final concentration). After washing off the paraformaldehyde, combinatorially colored beads conjugated with cell typing antibody, e.g. anti-CD8 (T cells), anti-CD19 (B cells), anti-CD14 (macrophages) (all from BD Bioscience), were added to the wells along with the cytokine detection beads for overnight binding at 4°C and processed as above.
Commercial Elispot reading service
Elispot assays were carried out according to the manufacturer (R&D Systems) suggested protocol with minor modification. Briefly, the capturing antibodies were coated onto wells of a 96 well plate (Greiner, 96-well μClear) overnight at 1 μg/mL in 100 mM carbonate buffer (pH 9.6). After blocking with 3% BSA for 2 hours, PMA/I or unstimulated cells were seeded into the wells and the plate was incubated for 16 hours. After washing away cells and unbound substances, a biotinylated polyclonal antibody specific for the target cytokine was added to the wells, followed by adding alkaline-phosphatase-conjugated streptavidin. A blue-black colored precipitate appeared as spots at the site of cytokine localization after adding substrate solution (BCIP/NBT). The number of spots on such Elispot plates was determined by a commercial service: Cellular Technology, Ltd. (Cleveland Ohio). Input cell counts were assessed by hemocytometer counting.
Selection for Th1 and Th2 phenotypes
Naïve CD4+ CD45RA+ T cells were selected from PBMCs using a magnetic T-cell isolation kit (Miltenyi Biotec, Auburn, CA) through a negative selection process, following the manufacturer’s recommended protocols. The cells were divided into two aliquots and cultured on plates coated with anti-CD3/anti-CD28 (BD Bioscience) at 1.5 μg/cm2 in either: a) IMDM supplemented with 10% FBS containing 10 ng/mL of IL-2, 1 ng/mL IL-12, and 10 μg/mL of a neutralizing antibody to IL-4 (R&D Systems) to direct the cells towards a Th1 phenotype, or b) 10 ng/mL of IL-2, 20 ng/mL of IL-4 and neutralizing antibodies to IL-12 (10 μg/mL) and IFNγ (10 μg/mL) (R&D Systems) to direct the cells towards a Th2 phenotype. The selected cells were cultured for two weeks in the selection conditions before assays.
Results
Multiplexed beads
The principal challenge in preparing multiplexed visualization beads is to obtain sets of beads that can be reliably distinguished from each other. Latex microspheres can be purchased in many different sizes, manufactured at high uniformity (cv of ~1%). Beads are dyed by swelling in organic solvent, with beads imbibing both solvent and dissolved fluorescent dyes. The beads are then transferred to aqueous media, trapping the hydrophobic dyes in the hydrophobic interior. Such beads suffer negligible photobleaching (< 5%) on the time scale of the assays described here. Photobleaching is believed to be due to bond breakage by hydroxyl radicals formed by UV illumination of water. Apparently, the sequestered dye is shielded from this insult. Aldehyde modification of the beads allows conjugation of proteins via Schiff base formation. Subsequent reductive amination results in a stable covalent amine bond that does not change the charge on the conjugated protein.
Others have described combinatorially colored beads, but typically in the size range of 5 μm (Fulton et al., 1997). For use in a multiplexed Elispot format, we have found that 280 nm is an optimal bead size. With smaller beads, the image of two nearby beads is not readily distinguishable from the image of one bead. With larger beads, fewer beads can be bound per CellSpot, thus limiting the complexity and dynamic range of the assay. A further advantage of beads at this size scale is that they remain in suspension without settling. When we tried agitation by various means during the assay, the result was that the beads accumulated at the sides of the well as compared to the center. Accordingly, no agitation was used for any of the results reported here. In this respect, the beads are well behaved tags, comparable to other tags larger and brighter than single fluorescent dyes, e.g. phycobiliproteins.
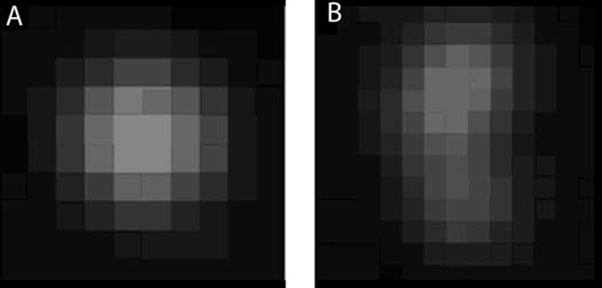
Since 280 nm is just below the resolution of the light microscope at visible wavelengths, the actual image of a bead is blurred by the optics, then pixilated by the solid state digital CCD camera used for detection. A typical bead image is shown in Figure 1(a), an image captured using a 10x, 0.45 N.A. objective lens with 4x zoom through an air interface. Figure 1(b) shows the image of two beads in close proximity. The first step in the automated image analysis is “blob” finding, a standard image processing technique that identifies adjacent pixels above a preset intensity threshold. The radial symmetry and uinformly declining intensity of the blob from center to periphery is then used to identify true beads from dirt or two beads in close proximity. An integrated intensity of the bead is then computed, with higher weighting given to the central pixels. The same beads are typically imaged twice, e.g. in red and green data channels, using an automated filter changer on the microscope. Chromatic aberration causes a systematic offset of the images by several pixels, and therefore the image processing software includes a compensatory alignment routine. A further source of noise arises from the fact that the plastic plate is not perfectly flat at micron scale, and thus some beads are slightly out of focus.
Figure 1.
Bead image analysis. The image of a 280 nm bead is expanded by the optics, requiring image processing to distinguish single beads (A) from aggregates (B). Alignment of images from two color channels is followed by determination of intensity ratio, using a pixel weighting function based on standard single bead calibrations.
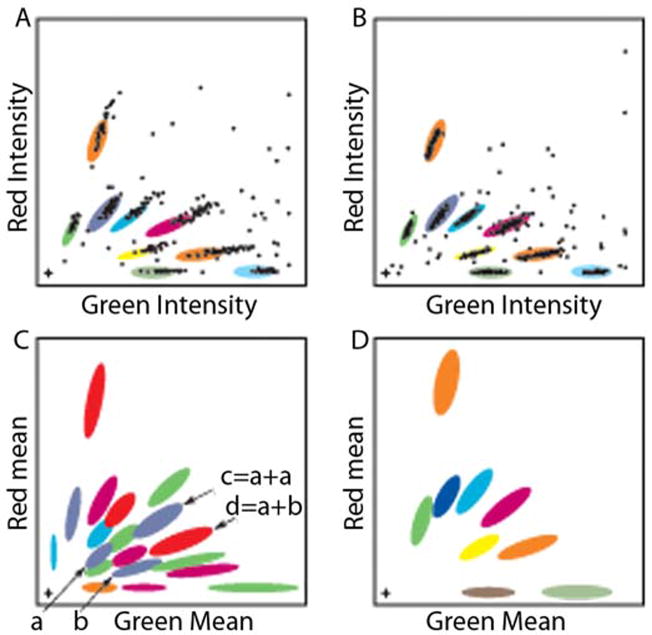
The red and green intensities for each bead are plotted in Figure 2 for a collection of beads dyed at varying ratios of the two fluorescent dyes. Initially, the resulting plot was classified into bead types using a fixed gate for each bead type (panel a), with the xy dimensions of each gate reflecting the manufacturing noise (primarily attributable to inhomogeneity in bead dyeing). In practice, lamp intensity variation proved to be an additional significant source of noise, causing beads to fall outside these fixed gates. Accordingly, a dynamic gating algorithm was implemented. Calibration beads are now included into one well per plate, which has proven sufficient to adjust the gates in a manner that results in ~85% of beads being correctly classified, as shown in Figure 2(b). Further optimization of the bead set was undertaken to eliminate residual misclassification caused by two bead images summing to a bead profile that is itself a legitimate bead type. Figure 2(c,d) illustrates this selection criterion. The same principles illustrated in Figure 2 for the red/green pairing can also be applied to other pairwise combinations of these fluors with fluors emitting in the far red and blue ends of the visible spectrum, and are applicable to any suite of combinatorially colored particles (readout by flow cytometry, for example).
Figure 2.
Bead classification algorithm and selection of working set. Distinguishable bead types are made by incorporating two fluors into the interior, at varying ratios. Due primarily to lamp intensity variations, a fixed gate classifier has a high error rate (A), but incorporation of an adaptive gating algorithm allows >85% of beads to be correctly classified (B), with the ambiguous beads excluded from further consideration. A useful working set of bead types minimizes artifacts arising from overlapping images of beads smaller than the wavelength of light. Although beads <280 nm are more subject to artifacts, larger beads occupy more space which limits the information that can be derived from single cells. Eighteen batches of multihued beads were prepared (C) from which a working set of nine was chosen (D) to avoid artifacts arising from overlap of bead images.
Beads are well behaved tags
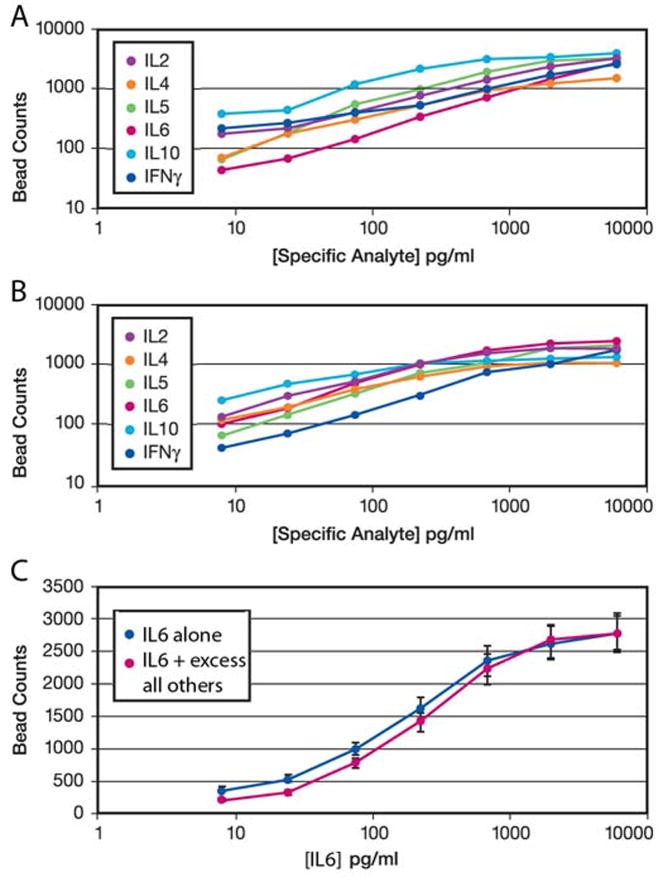
ELISA style assays using enzyme catalysis of a substrate gained popularity because they are quite sensitive. Use of fluorescent tags simplified immunoassays by converting a non-linear kinetic assay into a linear fixed endpoint assay, but with loss in sensitivity. The multiplexing beads are much brighter than a single fluor, increasing the sensitivity of the assay. Figure 3(a) shows a dose/response to analyte in this format for 6 different cytokines, each in a separate well with the detection antibody conjugated to a different bead type. The coefficient of variation (variance divided by the mean) is less than 5% for all the assays in Figure 3. One of the antibodies was also conjugated to 8 different beads, with no significant difference in assay results (data not shown). The dynamic range and absolute sensitivity are quite similar to a standard ELISA. Figure 3(b) shows that the presence of capture and detection antibodies for the other 5 cytokines in the panel does not affect the assay for one particular cytokine. Finally, Figure 3(c) shows that the dose/response curve for one cytokine is the same when measured alone or in the presence of excess amounts of all the other cytokines. To achieve these results, a suite of capture and detection antibodies is needed that has low cross-reactivity in all combinations. For the set used here, all cross-reactivities were < 0.5%. Varying the input bead concentration for each cytokine allows similar bead counts to be achieved even for cytokines that differ substantially in absolute amount. Trial and error across a representative cell sample was used to adjust the bead concentrations so as to achieve similar dynamic range for each cytokine.
Figure 3.
ELISA-style sandwich assays can be performed using number of beads per fixed area as the readout. Panel (A) shows six calibration assays for six cytokines. Panel (B) shows similar dose/response curves for each single cytokine even when all six sandwich reagent pairs are in the same well. Input bead concentrations have been adjusted to reflect the differing absolute amounts of different cytokines. Panel (C) shows that the dose/response to IL6 (blue) under the conditions of panel (B) is unaffected by presence of all the other cytokines in saturating excess (red). For all these measurements, the coefficient of variation (variance divided by mean) is <5%.
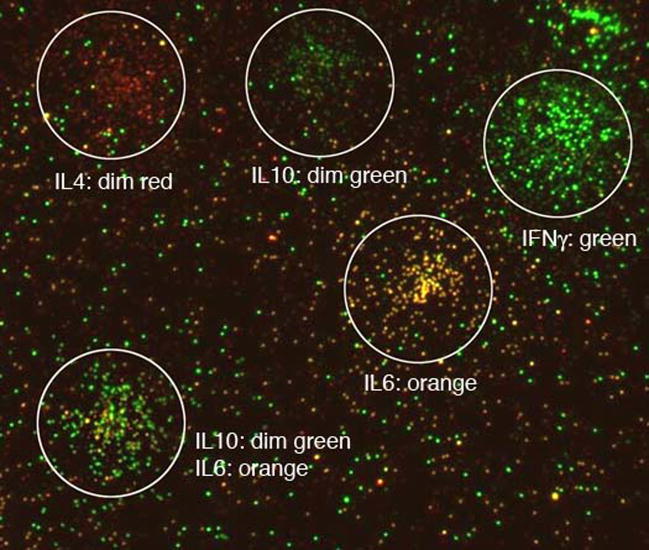
When cytokine is deposited by settled cells rather than a solution, the cell footprint can be analyzed. Figure 4 is a representative view of cell footprints from peripheral blood monocytes (PBMCs) illustrating the typical background level of beads between cell footprints and the higher density of beads in the vicinity of a cell. Different cells secrete different cytokines, with each detectable independently. With input bead concentrations appropriately adjusted, background levels were similar for all cytokines. Adjusting density of capture antibody for each cytokine is also useful for achieving uniform bead counts, but this approach is less linear and thus less convenient for efficient assay calibration.
Figure 4.
A representative montage of footprints of T lymphocytes with relatively simple phenotypes. After scanning the plate at low magnification (4x) to find the discrete footprints, high magnification (40x) images of those identified regions are captured (white circles). For illustration, images from the red and green filter channels have been overlaid. Each bead is classified, and the number of each type tabulated for each cell’s footprint. The automated process includes low to high magnification imaging, multiple color filter imaging, and data reduction to yield cell phenotype. Color code: pure red to pure green in the following order: IL2, IL4, IL5, IL6, IL10 and IFNγ.
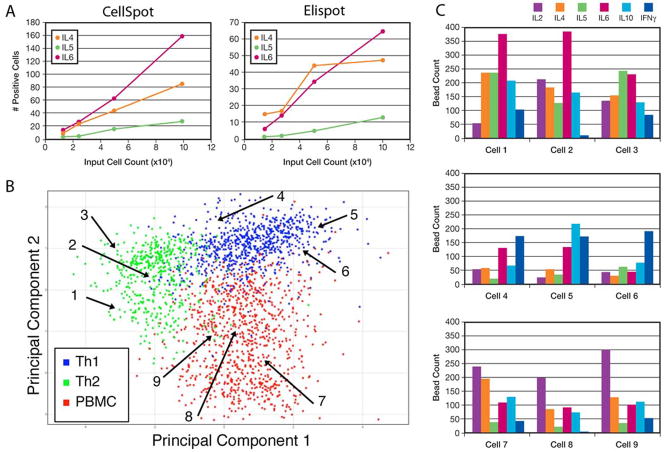
Detection of cell footprints by enzyme linked detection is a well established tool of cellular immunology, called Elispot. The assay is typically employed to count the number of cells responding to a particular stimulation condition by secreting an indicator cytokine, typically IFNγ or IL-2. Figure 5(a) shows that the multiplexed bead based assay (ie concurrent detection of multiple cytokines) gave comparable frequency results to data generated by a commercial Elispot service. In fact, the bead based assay is about 2-fold more sensitive (i.e. it detects footprints too faint for standard Elispot detection), and somewhat more linear across increasing number of input cells plated. For the comparison shown, three cytokines were chosen that gave similar frequency of spots. A higher dilution of the input cells was needed for IFNγ, for example (not shown).
Figure 5.
Use of multiple cytokine profiling to classify lymphocytes. Elispot is a standard assay for determining the fraction of cells secreting a particular cytokine, typically IFNγ. The multiplexed CellSpot analog shows good correlation with Elispot as conducted by a commercial service (A), with somewhat higher sensitivity and linearity across different cell plating densities. The three cytokines illustrated were chosen because they have similar absolute frequencies of producing cells, allowing easy graphing across cell dilutions. Each cell’s footprint following multiplexed analysis is a 6 parameter profile, which can be treated as a point in a 6-dimensional space. In (B), the data are plotted following projection down onto the most informative two dimensional plane (Principal Components Analysis). The profiles for 1,000 normal human PBMCs are shown in red. Profiles are shown in the same graphical representation for cells from subpopulations grown in a cytokine milieu that selects for either Th1 (blue) or Th2 (green) phenotype. Numbered arrows mark the cells whose detailed profiles are shown in panel (C).
Analyzing PBMC diversity at single cell level
Based on pilot assays, the midpoint of the assay was adjusted for each cytokine to match the average concentration in a normal human PBMC sample. This was accomplished by reducing the number of detector beads in the mix for abundant cytokines compared to rare cytokines. The same dose/response curve is preserved at the lower bead density, but with fewer beads per unit area (data not shown). This adjustment provides a more informative multiplexed readout.
To explore the utility of the multiplexed Elispot assay, control cell populations were established that show different phenotypic characteristics. CD4+, CD45RA+ naïve T cells were selected from PBMCs using magnetic beads, and the cells were divided into two aliquots. Cells were then cultured on plates coated with T cell stimulatory antibodies anti-CD3 and anti-CD28 in medium containing different cytokines for the two aliquots. A series of monoplex Elispot analyses showed that the Th1 cytokine exposed cells express IL-2 and IFNγ, and no discernable IL-4, IL-5, or IL-6, the expected profile. Cells from the Th2 cytokine exposed cells showed increased IL-4, IL-5, and IL-6, but still showed considerable IL-2 and IFNγ, so these are not canonical Th2 cells. Nevertheless, they still show a pattern distinct from the Th1 cells.
What cannot be determined from the monoplex Elispot assays (or from culture supernatant assays) is the cytokine profile of individual cells. Specifically, it is not clear from the Elispot results if the “Th2” cells are a combination of Th1 and Th2 cells or a combination of these and other cell types. Nor is it clear if all cells that express IL-2 also express IFNγ. To address these questions, the Th1 and Th2 cultured cells were analyzed in a multiplex CellSpot assay as described above, and compared to the parental human PBMC population from which they were derived.
Each footprint accommodates ~3,000 beads, with a background noise level <20 beads in that area, providing a dynamic range of >10-fold variation for each of 6 bead types. By adjusting the number of detection beads and/or the concentration of capture antibody, the midpoint of the dynamic range for each cytokine can be adjusted to match different absolute levels, thus allowing more informative readouts of experimental samples. In Figure 5(b), the profiles of the cells that were directed towards a Th1 phenotype are colored blue, and those of the Th2 biased cells are colored green. For this plot, the 6-dimensional data (level of each of the six cytokines for each cell) was projected onto the principal components of the distribution, a commonly used technique of multivariate statistical analysis (Jolliffe, 2002). The Th1 cells are more tightly clustered, indicating that the individual profiles of all cells in these cultures are more similar than for the Th2 population. Some of the Th2 biased cells coincide with the Th1 cells, but the other cells from this population show a range of phenotypes. Most of these cells are clearly distinguishable from Th1 cells, but do not cluster tightly. Rather, they form a continuum of phenotypes, a result that could not be obtained by 1-plex Elispot analysis. When the parent population of PBMCs was analyzed and plotted in the same manner (in red), a continuum of types beyond the simple Th1/Th2 typology was found. Figure 5(c) shows the detailed cytokine profile for three individual cells from each type. The map positions of these cells in panel (b) is marked by numbered arrows.
Assigning footprint to originating cell type
The combinatorially colored beads incorporate two fluorescent dyes, whose ratio defines the bead type. For quantifying each cytokine in a CellSpot, conditions were optimized for reading each bead as a single object. A typical footprint is 200 μm in diameter, accommodating ~3,000 beads. The producing cell is only about 10 μm in diameter. Following fixation of the cells, cell surface markers on the PBMCs can be stained, leading to a very bright signal from the densely crowded (unresolved) beads captured on the cell itself. The fixation procedure is accomplished after the cytokine footprints have been captured, and the procedure does not change the assay readout of the cytokines. The image of the cell is readily distinguishable from the footprint beads (Figure 6). Multiple cell types are thereby identifiable in this manner: T cells (CD8), B cells (CD19), and macrophages (CD14). For the illustrated cells, the predominant cytokines are: CD8 (IFNγ, with some IL2), CD19 (IL4, IL5), CD14 (IFNγ). The net effect of this additional capability is increased throughput when characterizing mixed cell populations. More importantly, it minimizes sample handling losses and perturbations attendant upon preparing each cell type as a pure population for analysis. In principle, this capability can be extended to additional cell types for which single identifying antigenic markers are available.
Figure 6.
Cell surface staining of a secreting cell allows cell type to be defined concurrently with assay of the secreted cytokine profile. Illustrated are a T lymphocyte stained with anti-CD8 antibody conjugated to a green bead (left), a B lymphocyte stained with anti-CD19 antibody conjugated to a red bead (middle), and a macrophage stained with anti-CD14 antibody conjugated to a yellow bead – i.e. 50:50 ratio of red and green fluors (right). The cell is the large, bright object in the center of the field of small beads which mark cytokine types secreted by the cell. Cytokine color code as in Figure 4.
Discussion
Regulation of cytokine expression in T cells is accomplished via parallel networks for each gene individually, accompanied by cross-regulation, not via a single master switch for a battery of cytokines (Callard et al., 1999). That is, canonical classifications such as Th1/Th2 are the result of several independent regulatory paths that operate to yield a uniform phenotype in healthy tissue. In diseased or damaged tissue, however, the abnormal cellular environment disrupts normal regulation, selecting for abnormal subpopulations of cells.
At the level of end stage disease, when cytokine profiles are grossly aberrant, skewed phenotypes are apparent at the level of bulk serum (Banchereau et al., 2004), yet the bulk cell population is likely to consist of a mixture of normal and abnormal cells. One plausible mechanism underlying the progressive nature of T cell mediated disorders, such as rheumatoid arthritis, is that an initial small set of deviant cells contributes, through cytokine release or cellular damage, to making the environment even more abnormal, thereby selecting for even more deviant phenotypes. Understanding progressive disease processes and learning how to treat them will benefit from profiling of populations of single cells and following the expansion of rare deviant cells into aberrant subpopulations.
A wide variety of cells secrete cytokines, including fibroblasts (Smith et al., 1997). Understanding the tissue level extracellular milieu is an emerging research arena, with microenvironments implicated in the normal growth and function of stem cells, for example. By various estimates, ~5,000 human genes are thought to code for secreted proteins (Klee et al., 2004). The function of most of these proteins is obscure but it is likely that at least some of them regulate other tissues in a manner analogous to the regulation of lymphocytes by cytokines. The multiplexed CellSpot assay described here offers a novel tool for elucidating the role of these proteins in tissue level regulation.
More immediately, a promising application for functional characterization of single cells is to monitor in vivo drug activity with a readout that is faster and easier to obtain than a clinical endpoint. A key barrier to lowering the cost of drug development is lack of functionally predictive biomarkers. Flow cytometry is one useful approach to this goal (Krutzik et al., 2004), although this technology is limited to abundant non-adherent cells. The CellSpot assay described here can be performed on biopsy quantities of tissue, while further allowing the labeling of cells by cell type, distinguishing therapeutic effects on abnormal cells from toxic effects on normal cells. Further, by characterizing cytokines actually secreted, the assay method avoids any potential artifacts associated with staining for intracellular cytokine stores, as is typically needed for flow cytometry assays. Future directions for improving the assay include expanding the number of cytokines monitored concurrently, and improving throughput by adding autofocusing and plate stacker capabilities to the instrument.
Acknowledgments
For help on this project, we thank our colleagues Carol Cain, Donna Chen, Neal DeChene, Stote Ellsworth, Beverly Freeman, Carlos Lorenzana, John Pease, Jim Quarato, David Rocke, Jason Tseng, Eric Walters, Jiangzhang Zhang.
This work was supported in part by SBIR grant # 2R44AI56878-02A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–50. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- Bangs LB. Diagnostic Applications of Microspheres. In: Knapp JZ, editor. Liquid- and Surface-borne Particle Measurement Handbook. Marcel Dekker, Inc.; New York: 1996. pp. 687–708. [Google Scholar]
- Callard R, George AJ, Stark J. Cytokines, chaos, and complexity. Immunity. 1999;11:507–13. doi: 10.1016/s1074-7613(00)80125-9. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C, Andersson G, Ekre HP, Nilsson LA, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10:133–9. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton RJ, McDade RL, Smith PL, Kienker LJ, Kettman JR., Jr Advanced multiplexed analysis with the FlowMetrix system. Clin Chem. 1997;43:1749–1756. [PubMed] [Google Scholar]
- Fulwyler MJ. USPTO . Apparatus for distinguishing multiple subpopulations of cells. 1985. [Google Scholar]
- Han M, Gao X, Su JZ, Nie S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol. 2001;19:631–5. doi: 10.1038/90228. [DOI] [PubMed] [Google Scholar]
- Jolliffe I. Principal Component Analysis. Springer; 2002. [Google Scholar]
- Klee EW, Carlson DF, Fahrenkrug SC, Ekker SC, Ellis LB. Identifying secretomes in people, pufferfish and pigs. Nucleic Acids Res. 2004;32:1414–21. doi: 10.1093/nar/gkh286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzik PO, Irish JM, Nolan GP, Perez OD. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin Immunol. 2004;110:206–21. doi: 10.1016/j.clim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Lehmann PV. Image analysis and data management of ELISPOT assay results. Methods Mol Biol. 2005;302:117–32. doi: 10.1385/1-59259-903-6:117. [DOI] [PubMed] [Google Scholar]
- Ohlmeyer MH, Swanson RN, Dillard LW, Reader JC, Asouline G, Kobayashi R, Wigler M, Still WC. Complex synthetic chemical libraries indexed with molecular tags. Proc Natl Acad Sci U S A. 1993;90:10922–6. doi: 10.1073/pnas.90.23.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–22. [PMC free article] [PubMed] [Google Scholar]