Abstract
The secreted immunoglobulin footprint of single hybridoma cells, containing ~10 fg of antibody purified in situ, has been probed for 9 properties concurrently by use of detection labels comprising 280 nm combinatorially colored fluorescent latex beads functionalized with proteins. Specificity of each individual hybridoma cell’s product has thereby been assessed in a primary screen. Varying the density of antigen on beads to modulate the avidity of the interaction between bead and secreted antibody footprint allowed rank ordering by affinity in the same primary screen. As more criteria were added to the selection process, the frequency of positive cells went down; in some cases, the favorable cell was present at <1/50,000. Recovery of the cell of interest was accomplished by plating the cells in a viscous medium on top of a membrane. After collecting the antibody footprint on a capture surface beneath the membrane, the immobilized cells were transferred to an incubator while the footprints were analyzed to locate the hybridoma cells of interest. The desired cells were then cloned by picking them from the corresponding locations on the membrane.
Keywords: Antibodies, hybridomas, specificity, multiplexed, single cell assay
Introduction
Hybridoma screening technology today is very similar to the technique first described in 1980 (de StGroth and Scheidegger, 1980). After fusion of immunized mouse spleen cells to an immortal myeloma cell line, and limited growth under conditions selecting for hybrids, the hybridoma library is plated out at a dilution yielding a small number of clones per well, trading off noise from multiple clones in a single well against cost of screening additional wells at true limiting dilution (a process described by Poisson statistics wherein about two thirds of the wells have no cells). Following growth of the clones, and accumulation of secreted immunoglobulin, supernatant is sampled for antigen binding in an ELISA format. Although a fusion may yield up to 10,000 independent clones, economic constraints often prohibit screening the entire library, particularly at sufficient over-sampling to assure identification of rare favorable cells. Furthermore, supernatant ELISA based methods require clones to produce sufficient antibody to be detected, a fact that skews primary screening away from low producing, slow growing clones which in fact may have desirable binding characteristics.
For strong immunogens, standard practice is often sufficient, as recently stimulated B cells are preferentially preserved in the hybridoma formation process (Schmidt et al., 2001). This can result in a high enough frequency for isolation of useful antibodies. Many immunogens do not generate a robust response, however, including conserved antigens, integral membrane proteins with small extracellular exposure, and peptides. Furthermore, quality criteria typically include affinity as well as specificity within a protein family or among different epitopes on the target protein. Even for immunogenic antigens, raising the antibody quality threshold to encompass all these parameters naturally leads to lowering the frequency of favorable clones.
One approach to overcoming the problem of low frequency antibodies is to increase the number of cells screened by shrinking the well size from the standard 96 well microplate format. For example, wells have been prepared via microlithography that are only large enough to accommodate one cell (Love et al., 2006). Fluid handling difficulties rise as the well size decreases, however, including differential evaporation between wells at different locations, and difficulty in achieving uniform washing. Assaying supernatant precisely is thus difficult in such formats. Without replicates, both false positive and false negative rates rise, reducing the value of the technique. Noise level issues also limit flow cytometry as a technique to extract favorable clones, particularly as the frequency of those clones drops (Gross et al., 1993). Although flow cytometry is in principle capable of considerable multiplexing, in practice, the absolute signal and the dynamic range (which impacts signal to noise) decline as multiplexing increases, further limiting utility.
A different approach to increasing the fraction of the immune repertoire surveyed is direct examination of primary B cells. In an illustrative example of this approach, antigen coated erythrocytes are lysed by locally high concentration of antibody around a lymphocyte secreting antibody specific for an antigen conjugated to the erythrocytes (a hemolytic plaque assay) (Babcook et al., 1996). This technique is poorly quantitative, and is limited by the inability to measure multiple parameters in order to define antibody quality.
Finally, it is possible to bypass animal immunization altogether and generate very large recombinant antibody libraries, often comprising 10 million or more independent antibody sequences. In general, the initial hope (Lerner et al., 1992) that this diversity source would eliminate the need for in vivo immunization has not been fulfilled. Such libraries are typically screened by phage display methods in a single parameter assay, relegating specificity screening to more laborious secondary screening (Hoogenboom, 2005). Natural immunization remains a particularly effective method for generating high quality antibodies, due in large part to the highly parallelized screening against all human antigens along with interative selection, that takes place in vivo (Or-Guil et al., 2007). Screening recombinant libraries in a similarly parallelized, iterative fashion is well beyond our current capability to reproduce in the laboratory
In spite of these limitations, monoclonal antibodies have become commonplace as research reagents over the past 25 years, and therapeutic antibodies have become the fastest growing segment of the pharmaceutical industry over the past 10 years (Reichert and Valge-Archer, 2007). The quality threshold for therapeutic agents is higher than for research reagents, requiring more exacting methods to meet therapeutic requirements. Thus, there is a need for reliable and efficient generation of antibodies meeting strict quality criteria, especially for poorly immunogenic antigens. Since specificity is one major aspect of quality, screening approaches based on a primary assay against a single antigen are not as likely to succeed as is a multiplexed primary screen.
The technology described here assays single cells using digital microscopy to read multiplexed probes bound to the secreted antibody footprint formed around individual cells. In effect, a microscopic virtual well is created in situ around each cell. The technique is readily useable for rapidly screening 100,000 to 1 million clones at a very high specificity threshold, enabling rapid examination of multiple libraries prepared by alternative immunization strategies. Modification of the probes to vary the avidity effect inherent in the bead based assay format enables rank ordering of clones by affinity in the primary screening step. Further customization of the probes allows screening for reactivity to solubilized integral membrane antigens. The high signal to noise of the assay format is particularly useful for this difficult class of antigens.
Materials and Methods
Immunization Protocols
Anti-peptide libraries were prepared by subcutaneous injection into Balb/c mice of cocktails of 4 or 5 peptides from the extracellular domain of cMet, each conjugated to Keyhole Limpet Hemocyanin (KLH) (Calbiochem). Four mice were used for each peptide pool, with 200 μg of the cocktail injected every other week for 10 weeks. Complete Freund’s adjuvant was used for all injections except the final boost. Spleens were harvested 3 days after the final boost and lymphocytes fused to the myeloma line Ag8.653.P3 by standard methods. For immunizations with recombinant protein (cMet, RON, Protein Kinase C, Nerve Growth Factor), mice were injected in the footpad weekly with 5 μg protein in adjuvant for 5 weeks, with recovery of popliteal lymph nodes 3 days after the final boost. Immunizations with cMet-expressing A549 cells were done with 5 × 106 washed cells that were pelleted, resuspended in a minimal volume, and injected subcutaneously into mice every other week for 10 weeks. All animal work was conducted by Antibody Solutions, Inc (Mountain View, CA), in compliance with animal welfare regulations.
Preparation of Bead Probes
Multihued particles of 6 distinguishable types were used in these experiments. Functionalization was performed by incubating aldehyde derivitized particles with a 3-fold excess of purified protein (determined by total surface area calculation). After overnight incubation, a reductive amination reaction was performed to stabilize the covalent bond between the bead and protein amino groups. Beads were washed to remove unbound protein and then placed into buffered 2% BSA to promote colloidal stability. For peptide-bead probes, the peptides were first conjugated to BSA at a 1:1 mass ratio prior to bead conjugation. Functionalized beads are stored at ~0.1% solids by volume.
CellSpot assay
CellSpot 96 well assay plates were prepared by coating with goat anti-mouse IgG (Jackson ImmunoResearch), 2 μg/ml, and blocking with BSA. Cells were washed and counted prior to being dispensed into the assay plate. For cells that had been growing in bulk culture, any number between 100 and 10,000 cells were placed into each well of a 96-well plate; the optimal number was determined by the frequency of CellSpot-generating cells (i.e. frequency of clones recognized by any of the bead-antigen probes). More than 200 CellSpots per well proved to be difficult to image and analyze due to overlapping footprints. When the frequency of positives was unknown, cells were plated at several dilutions, and the dilution wells with the highest number of good quality CellSpots were used for analysis. Quality in this instance refers to lack of overlap with adjacent cell footprints, and a radially symmetric profile with a characteristic high density of beads at the center declining towards the periphery. After plating, cells were incubated for 2 hours on the assay plate. After incubation, cells were washed out with PBS/0.01% Tween 20, followed by addition of the bead cocktail at a 1:1,000 dilution of the stock suspension. After an overnight incubation at 25°C, beads were gently washed out with PBS/Tween ten times. The plates were fixed with 4% paraformaldehyde, washed with deionized water, washed 3x with methanol, then dried, at which point they became ready for imaging.
Clone Recovery
Once a binned population of cells was identified as containing a clone of interest, the parental population for that bin was expanded and prepared for transfer to a Transwell membrane. Approximately 2,000 cells were resuspended in media and placed into a transwell cup (6-well size, Corning #3450) and centrifuged 5 min at low speed to get all cells directly contacting the membrane. After centrifugation, the remaining media in the cup was drawn off gently and the cells overlayed with 1.2% MeBiol (MeBiol Corp; Tokyo, Japan) in media following the manufacturer’s direction: 1g in 10 mL to create a viscous solution. The cups were placed back into their holders, where fresh media could reach the cells from below the membrane. After an overnight recovery period the cups were placed directly onto a flat polystyrene surface (Nunc OmniTray) that had been coated with capture antibody. Cells were incubated in close proximity to the capture surface for 4–6 hours to allow secreted antibody to diffuse through the membrane and be captured. The cells in their cups were then returned to the incubator, again with media contact from below. The OmniTray was incubated with the bead cocktail and processed for imaging in a manner similar to the standard 96-well assay. The physical (x,y) coordinates of CellSpots of interest were recorded. After returning the transwell cup to the microscope stage, the images of the footprints and the images of the cells were aligned with the assistance of software similar to that used for identifying star constellations in the sky. The colony corresponding to the footprint of interest was recovered using a standard pipettor or micromanipulator. Colonies were usually recovered after 5–7 days of growth in the transwells, at which time they ranged in size from 10–50 cells. Viability after isolation from the semisolid media was good, with over 90% of isolated colonies (10 or more cells) resulting in sustainable cultures.
cMet/RON screen
Immunization strategies included single immunogens or a combination of immunogens. Specifically, custom peptides were prepared (Anaspec; San Jose, CA) for selected regions of cMet IPT or Sema domains, as well as the full extracellular domains (ECD) of cMet or RON expressed and purified as Fc fusion proteins (R&D Systems). cMet/HGF complex was prepared by co-incubation of a 1:1 molar ratio of the two purified proteins (HGF from R&D Systems). Libraries were plated at 20,000 cells/well on 96-well plates, with growth occurring in most wells. Primary screening of these plates by CellSpot identified those producing antibodies of interest. Antibody Solutions, Inc (Mountain View, CA) conducted standard PEG fusion of lymphocytes to form the hybridoma libraries.
Antibody affinity characterization
Hybridomas to Nerve Growth Factor were supplied as coded samples by Antibody Solutions, Inc (Mountain View, CA). Biacore measurements were provided by Biosensor Tools (Salt Lake City, UT). High and low antigen density beads were prepared to modulate avidity. High density: 30% antigen + 70% BSA; low density: 3% antigen + 97% BSA.
Membrane antigen screen
3T12 cells expressing a relatively high level of a confidential membrane antigen (~500,000 copies/cell) were used. The antigen was engineered to have a cytoplasmic HA (hemagglutinin) tag. Both human and murine versions of a related antigen were examined. Cells were extracted in Tris buffered saline containing 4% CHAPS, 10% glycerol, protease inhibitors (Roche mini-complete tablets), and 0.004% DNase (Sigma; St Louis, MO). Anti-HA tag antibody (Abcam, Inc; Cambridge, MA) was conjugated to the appropriate 280 nm combinatorially colored bead, and the beads were incubated with the cellular extract at 4°C for 4 hours. The beads were washed 3 times by centrifugation, and used to probe hybridoma footprints. Libraries were prepared in the same manner as for the cMet/RON screen.
Results
CellSpot™ assay format
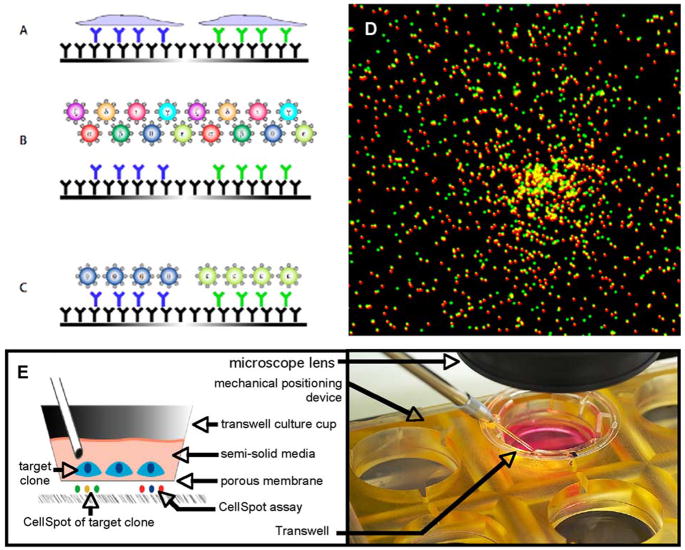
The assay for single cell hybridoma analysis described here has been adapted from our recently described multiplexed cytokine assay (Harriman et al., 2008), an extension of the single parameter T lymphocyte assay called Elispot (Czerkinsky et al., 1988). Hybridoma cells were lightly centrifuged onto a surface coated with an antibody capture reagent (typically an anti-immunoglobulin antibody). Secreted antibodies diffused away from the cell of origin in all directions during the 2 hour cell incubation period and a portion of these were captured in a surrounding footprint of ~200 μm diameter. At the plating densities used, footprints from each cell, termed CellSpots™, remained spatially separate from one another thus forming virtual wells containing purified antibody from the cell of interest. These footprints were probed individually via fluorescence microscopy as if they were physically separate wells. Figure 1(A–C) diagrams the overall assay format.
Figure 1.
(A–C) Diagrammatic summary of single cell antibody footprint assay. (A) Antibody secreted from single cells is captured on an underlying surface coated with anti-Ig capture antibody. (B) Multihue beads are added to the captured footprints, each type carrying a different biological probe. (C) Retained beads at each cell’s footprint define the specificity of that antibody. (D) Example of a CellSpot stained with two bead colors. (E) Membrane handling device used for cell recovery.
To detect antigen bound to the captured antibody footprints, we used 280 nm combinatorially colored latex beads as visualization labels. That is, one bead type had an 8:2 ratio of red to green fluors, while another had a 5:5 ratio and still another a 3:7 ratio, and so forth. As previously described, such beads are well behaved labels, enabling high sensitivity detection of bound analyte attached to the beads. Figure 1(D) shows a typical footprint, visualized with two bead types.
The precursor cytokine assays were performed in a 96-well plate format, and cells were typically discarded afterwards. Performing CellSpot in this modality was also useful for antibody discovery because it allowed us to rapidly survey a number of different libraries generated through alternative immunization protocols and then focus our efforts exclusively on those libraries containing antibodies with desirable binding profiles. Of course, for use as a hybridoma screening tool it was essential to ultimately recover the cells secreting a favorable antibody. For that purpose, we developed a two-step process involving a survey of populations of cells binned in 96 well format, followed by recovery of clones of interest utilizing a specialized CellSpot apparatus.
Library cells were seeded into growth wells, or bins, and grown for 4–5 days, after which time a portion of each bin was transferred to a replica plate. At a typical bin size, an entire hybridoma library was screened, with over-sampling, in under 10 microplates. That is, each original cell had the opportunity to replicate several times. Even slow growing clones were found to generate two or three progeny, providing an internal control on the assay in that each particular phenotype was typically seen at least twice in the same bin. For rare phenotypes, that consistency in pattern was important for distinguishing true positives from random fluctuations. The CellSpot assay was run in this manner and bins containing desired clones were identified, typically in under 1% of the bins. These bins were further expanded for clonal recovery, employing a modified version of the standard CellSpot method. Cells were plated on a membrane placed in close proximity to a capture surface, and allowed to grow into small colonies of 10–50 cells. Antibody diffused through the membrane and was captured on the underlying polystyrene surface. The cells were held in position by gently centrifuging them onto the membrane and then overlaying them with a viscous medium (Mebiol™). Close apposition of the membrane to the underlying plastic capture surface was achieved by the mechanical device shown in Figure 1(E). A commercially available Transwell™ device, normally used to suspend cells above a feeder layer, was used; the device’s bottom surface was a 0.45 μm polyester membrane. For the CellSpot process, the Transwell cup was placed into a customized holder that pressed the membrane down flat against the polystyrene capture surface below.
After the secreted antibody footprint was captured, the membrane with embedded cells was transferred to a cell culture incubator while the footprints on the capture surface were labeled with bead probes and imaged via fluorescence microscopy, a process taking ~24 hours. Computer guided alignment of the secreting cells on the membrane with the digitally imaged footprints enabled recovery of the colonies of interest with high reliability. A standard hand held pipettor with a small diameter plastic tip was used under bright field illumination to harvest the target colonies. The use of MeBiol to hold the cells in place was highly convenient, although it generally resulted in about 10% loss of cell viability. Due to the limited expansion during the binning process, such losses had very little impact on overall recovery of favorable phenotypes as there were generally at least two copies of the cell and one of them was viable.
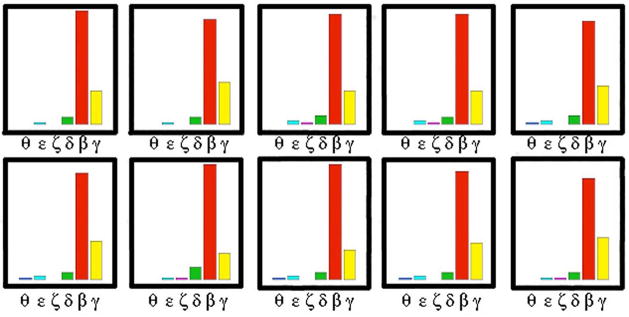
To illustrate the reproducibility of the microscopic assay, CellSpots generated by ten cells of common clonal origin were collected and analyzed. The profiles (bead counts for 6 probes) are shown in Figure 2. The antigen probes in this case were isozymes of Protein Kinase C conjugated to six differently colored beads. Overall reproducibility was high, although variance increased as the total signal diminished. Other assay miniaturization methods, which typically require liquid handling steps, generally become less reproducible as the size scale goes down. The CellSpot assay largely avoided this pitfall by eliminating sub-microliter pipetting steps in both analyte dispensing and detection steps. Antibody was purified in situ using the same kind of affinity capture methods used to purify antibody in bulk. In our examples, a typical hybridoma cell secreted antibody at ~1 pg/cell/day as measured on bulk cultures. We have estimated that half of this secreted antibody was lost by diffusing with a predominant vertical angle. Of the remainder, we estimated that half did not reach the surface within the 200 μm radius of analysis. Of the protein that was within the suitable solid angle for capture, we estimated that half was actually captured. For our standard two hour cell incubation period, then, the amount of antibody captured for subsequent assay was estimated at ~10 fg. This figure correlated well with an independent estimte of sensitivity. Known dilutions of antibody protein were applied to the well for a standard ELISA assay, except using antigen on beads as the detection method. Typically we were able to detect down to 10 pg of antibody in the well, with the lower limit of detection defined as the point that the number of beads captured was three times background level. A 200 μm diameter area (the area of a single cell’s footprint) is ~0.1% of the total area of a single microplate well (inner well diameter at the bottom surface is ~6.35 mm). Accordingly, that level of sensitivity corresponded to ~10 fg of bound antibody. Although neither quantitation method has high precision, the similarity of the result from two independent methods supports the estimate.
Figure 2.
Reproducibility of CellSpot. Histogram of bead counts for six isozymes of Protein Kinase C is shown for ten progeny cells from a single hybridoma. Variance is low except for the isozymes binding very few beads.
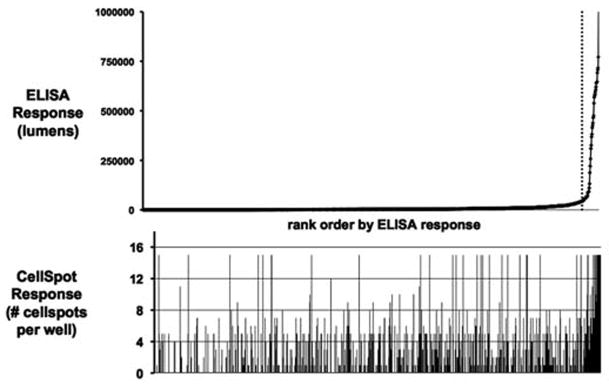
The practical impact of the extremely high sensitivity of the CellSpot assay is illustrated in Figure 3. In this experiment 20 million post-fusion cells were plated for drug selection into 10 plates (20,000 cells/well). Virtually every well had growth after 7 days. A growth titer plate (dilution series) was also set up at the time of initial cell plating, and it showed drug resistant growth down to 2,000 cells/well, indicating that wells plated at 20,000 cells/well should contain 5–10 independent viable clones per well. Therefore, the total library size was estimated to be 5–10,000. The same ~1,000 wells were assayed in parallel by conventional ELISA and by CellSpot. When the wells were rank ordered by ELISA signal, only 5% showed signal above a background threshold. These wells contained the faster growing, higher producing hybridomas, as verified in several examples by correlating footprint size and frequency for progeny of single cells with the absolute cell count and supernatant antibody level determined after bulk culture for several days. The wells which scored positive by ELISA also gave a high score with the CellSpot metric. In the remaining 95% of the wells that scored negative by ELISA, CellSpot detected at least one positive clone in nearly half the wells. The high sensitivity of CellSpot detection did not require high growth or production from the cells. When a desired binding profile was rare, it proved advantageous to look at all antibody candidates, not just those that grew well during drug selection or produce large amounts of antibody.
Figure 3.
High sensitivity of CellSpot. A total of ~1,000 wells, each containing 5–10 independent clones, were screened for binding to a single antigen. Wells were rank ordered by ELISA score on pooled supernatant from all clones in a well (top). Half of the wells that were negative by this metric did in fact contain one or more positive clones as revealed by the CellSpot assay of the same wells (bottom).
In addition to increased sensitivity to a single antigen, CellSpot also provided simultaneous binding data on multiple antigens. In the example in Figure 3, each cell’s secreted antibody was probed with 5 different bead types. To achieve this amount of information using conventional ELISA techniques, a library estimated to contain 10,000 independent clones would need to be plated on 300 plates (to ensure clonality), and 2–3 times that number for over-sampling to be sure of picking up the rare favorable clone. Supernatants from each well would then need to be evaluated on 5 assay plates, resulting in a total of 2–3,000 plates. The CellSpot approach required just 10 assay plates. The huge increase in efficiency is due to multiplexing 5 antigens at a time, along with the ability to obtain clonal binding profiles even though the wells are in fact oligoclonal. That is, each CellSpot was derived from the secreted product of a single cell, and therefore the method inherently generated clonal data. This expansion in the breadth and depth of screening allowed hybridoma libraries to be more completely probed to identify rare clones of interest. In this experiment, 10-fold more clones were found by CellSpot than by ELISA using a single parameter for screening. The additional information content from multiplexing allowed identification of particularly favorable clones from this expanded set of candidates accessible for assay.
Antibody specificity screening
For a discovery program focusing on cancer-associated cell surface tyrosine kinase receptors, we began by testing a variety of immunization strategies on the closely related cMet and RON receptors (Christensen et al., 2005). Peptides (20–25 residues long) were chosen from surface exposed regions of the cMet extracellular domain (ECD), 15 from the SEMA domain and 7 from the IPT domain (Stamos et al., 2004). Mice were immunized with groups of 4 or 5 peptides from nearby sites, or with the entire recombinant ECD. Primary splenocytes or hybridomas from mice from each group were assayed by CellSpot. Multiplexed probes were developed by attaching the entire ECD to one bead and attaching the 22 peptides to differently colored beads using serum albumin as a spacer between bead surface and peptide. For 16 of the 22 peptides, clones were identified that showed high specificity for that peptide relative to others in its immunization set, as well as binding to the full length ECD. Five independent libraries, representing five distinct immunization protocols and an aggregate of 50,000 independent clones, were screened by CellSpot. Since each assay was 6-plex, this was equivalent to 300,000 clonal ELISA assays. The frequency of hits varied widely among the peptides, consistent with the expectation that some portions of the protein are more immunogenic than others; for almost half the peptide epitopes, only a single clone meeting these strict quality criteria was found out of the 50,000 screened. These results are summarized in Figure 4(A). Use of peptides as immunogen is a common practice, as it is generally simpler to prepare adequate amounts for immunization by peptide synthesis than by protein purification. Overall, immunization with the full ECD proved to be a more fruitful approach, however, and the next series of immunizations therefore used the ECD of cMet or RON (fused to immunoglobulin Fc domain), as well as A549 cells expressing cMet.
Figure 4.
(A) Antibody repertoire is summarized for mice immunized with cMet or with peptides (20–25 residues) from cMet conjugated to KLH. Number of clones from different immunization strategies is plotted, where each positive clone shows specificity to a single peptide as well as recognition of the intact protein. Although epitopes differ substantially in immunogenicity, specific recognition of individual peptides was generated for 75% of the probes by one or another strategy. (B) Rare subtype of antibodies against cMet. Hybridomas raised against cMet were evaluated for binding to cMet and to cMet complexed with its natural protein ligand HGF. Many clones (brown points along the 45° line) recognize both cMet alone and the complex with HGF (presumably binding to a site independent of HGF). A second large set of clones (blue points) recognize cMet better than the complex (presumably binding to a site occluded to some degree by presence of HGF). However, a few clones (red points along x-axis, with strongest binders circled) only bind cMet in the presence of HGF (presumably binding to a cryptic epitope that becomes revealed following a conformational change in cMet induced by HGF).
For the second series of immunizations, a total of 6 hybridoma libraries were prepared; 60,000 clones were screened with cMet, RON, and with a complex of cMet and its natural protein ligand HGF. Of these, ~1,000 were positive for at least one of the probes, for a hit rate of ~1.6%. A relatively rare class was clones that only bound cMet in the presence of the ligand HGF, suggesting recognition of a cryptic epitope revealed by a conformational change induced by ligand binding. Only ~5 such clones were observed with high bead counts indicative of tight binding (Figure 4(B)). We have observed analogous preferential binding of an antibody to a ligand-receptor complex for a different receptor tyrosine kinase, and in that instance we verified the antibody’s activity in blocking signaling. Clones binding the cMet-HGF complex were distinguishable from the much larger number of clones that bound cMet equally well in the presence or absence of HGF. Many clones were also observed that bound cMet by itself but failed to bind in the presence of HGF, suggesting that they were binding at the HGF site. These results are consistent with prior antibody discovery efforts against cMet, for which the great majority of antibodies were found to bind to similar epitopes (Jin et al., 2008).
Affinity ranking
Specificity is an important parameter defining antibody quality in many applications, including research, clinical diagnostics and therapeutics. This property is not strictly linked to affinity of the antibody. In typical hybridoma primary screens, the raw ELISA signal reflects an unknown combination of the intrinsic affinity of the antibody and its abundance in the supernatant. A low affinity antibody at high concentration can give the same signal as a high affinity antibody at low abundance. Hybridoma cells grow at different rates and secrete antibody at widely varying levels thus exacerbating the bulk supernatant measurement problem. The CellSpot assay makes measurements on a single cell basis and has proven to be insensitive to these bulk effects.
For the cMet/RON hybridoma screen, we wanted to include an affinity rank ordering criterion along with the specificity criteria. Our first attempt dedicated one bead type for use in quantifying the amount of IgG in the footprint. Normalizing against that amount left the resulting signal proportional to affinity (not shown). Although this method was promising, a better method was developed. The improved method took advantage of the fact that the bead based assay format presents many copies of the antigen, allowing relatively weak affinity antibody producing clones to be detected due to the avidity effect (Causey and Dwyer, 1996) arising from multi-dentate binding between the bead and the immobilized antibody footprint. By diluting the antigen on the bead with BSA, the avidity effect was systematically varied. Low density beads were made at 30% and 3% loading. Using the estimate of ~10 fg IgG in a footprint, the number of interacting antibodies at the lowest antigen loading on the beads was estimated to be a few molecules per bead. As shown in Figure 5(A), two antibodies to the same antigen (Nerve Growth Factor), which differ in affinity by ~10-fold, were probed with the antigen at high density (green beads) and low density (red beads). The weaker affinity antibody bound only the high density beads while the tighter binding antibody bound both bead types.
Figure 5.
(A) Rank ordering of clones can be accomplished by varying the density of antigen on the beads to modulate the avidity. Two clones against Nerve Growth Factor, with known affinity by Biacore, were probed with high and low density antigen beads (green and red respectively). Only the high affinity clone binds both bead types. (B) Rare high affinity clones (red points, circled) identified by the ratio assay method have ~6-fold higher affinity by Biacore (average Kd of 9 nM) than the population average.
By taking the ratio of the bead counts for the differentially loaded beads, a rank ordering by affinity was generated. As an example, Figure 5(B) shows the spectrum of murine hybridoma clone types against RON with regard to binding high and low affinity probes. Many weak binding clones were found, along with a few putative higher affinity clones (circled in the Figure). Antibodies from each group were subjected to Biacore analysis, and the high affinity group was determined to have an average Kd of 9 nM, which was ~6-fold tighter than the predominant group.
Membrane antigen screen
Membrane antigens typically represent difficult antibody targets as compared to soluble antigens. Supplies of antigen for immunization and screening are often limited due to difficulty in recombinantly expressing such antigens. Further, processing of membrane antigens by antigen presenting cells is generally less efficient, often resulting in a very low frequency of positive clones in the immunized repertoire. CellSpot screens large numbers of cells in polyclonal wells while requiring the cells to secrete only femtograms of antibody giving the method a distinct advantage over conventional ELISA in cases when clones are very rare. To test the technology for this class of antigen, hybridomas against a proprietary integral membrane antigen were screened using detergent extracted antigen immobilized on beads. The antigen had been engineered to carry an HA tag on the intracellular domain. Solubilization and extraction via an anti-HA antibody on beads resulted in capture of the antigen in reasonably high yield and purity, as measured by SDS gel electrophoresis and Western blotting (not shown). The small amount of antigen consumed in functionalizing the bead reagents eased the typical antigen supply issue faced by conventional screening.
The noise level was higher for this assay than other CellSpot assays, as the relatively hydrophobic antigen present in solubilized membrane preparations caused increased non-specific binding to plastic assay surfaces. This issue arises in both ELISA and CellSpot assay formats. However, CellSpot’s image based method allows incorporation of noise reduction techniques not available in methods that pool signal from the entire well. As part of the pattern recognition software involved in locating CellSpots for automated assay processing, algorithms were developed that discard CellSpots which do not conform to the shape and radial bead distribution expected from the diffusion process underlying a CellSpot’s formation. Clumps of beads and other assay artifacts did not display the characteristic CellSpot distribution. The elimination of such artifacts greatly enhanced the signal to noise of the assay. In the case of the membrane protein antigen, the specific signal from a small number of positive cells in a well was very small compared to the non-specific background binding to the well itself, a fact that directly impacts measurement by ELISA. Figure 6(A) top panel shows the raw fluorescence signal from a single well of a 96 well plate, with quantification displayed in the middle panel. The boxed areas in the top panel represent the true CellSpots determined by algorithms that identify characteristic shapes such as that shown in the bottom panel. Despite the higher noise level, multiplexing was still feasible for this class of antigens. Figure 6(B) shows staining of two hybridomas, one (left) that binds only the human form of the antigen on green beads while the other (right) binds both human (green beads) and murine (red beads) forms.
Figure 6.
An integral membrane protein antigen was extracted and immobilized on beads, then probed against a hybridoma library. (A) Top panel shows high background of the assay due to non-specific binding of the hydrophobic antigen to the polystyrene well. Middle panel shows quantitation of the signal as height. Bottom panel shows the characteristic profile of a true CellSpot, reflecting radial diffusion of antibody from the secreting cell. (B) CellSpot analysis was performed on hybridomas that bind only the human form of the antigen, or both human and murine forms. Human antigen on green beads stains only the former (left), while the latter binds both human antigen (green beads) and the murine form (red beads), with bead counts for a single cell’s footprint quantified below the images.
Discussion
As antibodies have grown in importance as a therapeutic class, the search methods used to find high quality monoclonal antibodies has become a subject of considerable interest. Recombinant antibodies, and antibody analogs, are relatively easy to generate in large numbers (tens of millions). However, the potential diversity that the intact immune system can generate is immensely greater. In an immunized host, mutation and selection are carried out in a highly parallelized, iterative fashion. The result is a sophisticated sampling of the available diversity, offering the prospect of superior antibodies (Or-Guil et al., 2007). For therapeutic purposes in particular, the selection against self antigens in a mammalian host should result in lower risk of toxic cross-reactivity than for purely in vitro antibody discovery methods using a single target antigen for selection of clones. One approach to screening the naturally immunized repertoire is to convert it into a recombinant form (Wiberg et al., 2006). A more practical and efficient alternative described here is to miniaturize the screening of hybridomas to the point that it becomes feasible to screen large libraries for rare clones with desirable properties.
Biochemical screening of any large library has an intrinsic noise level, a phenomenon encountered in combinatorial chemistry as well as in antibody screening. In general, the assay noise forces a trade off between false positive rate (insufficiently stringent screening) and false negative rate (overly stringent screening). In addition, large libraries typically have a significant frequency of redundant positives. Winnowing out false and redundant positives is conventionally accomplished in secondary assays that are typically more labor intensive than the primary screen. As the library size grows, the secondary screening work increases, putting a premium on selection of higher quality clones in the primary screen. Since specificity is the primary quality criterion for antibodies, conventional single parameter assays run the risk of losing the best overall candidates from consideration.
Flow cytometry is a technique that has been explored for screening antibody producing cells (Carroll and Al-Rubeai, 2005), but this technique is poorly quantitative due to variable immunoglobulin expression on the surface of hybridomas. Also, for clones more rare than ~1/1,000, the utility of flow cytometry declines due to high false positive and false negative rates. Further, although flow cytometry can in principle achieve high multiplexing, in practice, modest multiplexing is more typical due to constraints on signal level (dynamic range) for multiple probes on the confined surface area of a cell. By contrast, CellSpot distributes the antibody from a single cell over an area with a diameter of ~200 μm, providing room for capture of very bright fluorescent beads, with good dynamic range and linearity in dose-response for each probe.
In this regard, the CellSpot approach has an advantage over other single cell analysis and recovery technologies such as the LEAP technology (Cyntellect, Inc; San Diego, CA) or the SLAM technology (ImmGenics, Inc which was acquired by Abgenix, Inc which in turn has been acquired by Amgen, Inc; Thousand Oaks, CA) and the similar technology used in the Clonepix technology (Genetix, Inc; New Milton, Hampshire, United Kingdom). In all of these methods, the antibody visualized is either on the cell surface or present in a narrow halo around the cell, making linear quantitation difficult. The LEAP technology, like flow cytometry, is not as useful for recovery of rare cells, as it is for enriching a broader population, e.g. preserving B cells while killing T cells with focused laser pulses.
Our use of digital microscopy to screen the secreted antibody from single cells created the opportunity for a multiplexed screen with a low false positive rate. This high signal to noise feature was particularly apparent in screening with a solubilized integral membrane protein for which the non-specific binding was too high for effective screening by ELISA. An added benefit of extra data channels from the multiplexing technology is the ability to modulate avidity effects as a way to incorporate affinity ranking into the primary screen. Finally, multiplexing allows parallel screening for related antigens, improving the efficiency of the overall process. In the cMet example, peptides were used as immunogens, following a commonly used strategy to leverage genomic data. That is, peptides are easy to make in sufficient quantity for immunization, whereas protein purification or manufacture is typically more arduous. Although we found better response rates for intact protein immunogens, even the peptide immunizations yielded useful clones at a frequency within reach for CellSpot to find them.
Not surprisingly, the frequency of positive clones declines as additional selection criteria are introduced into the screen. Thus, the high quality threshold set by multiparameter assays defines rare favorable clones, while miniaturization enables adequate throughput to find those rare cells. In the CellSpot method, digitized microscopy is used to achieve both multiplexing and miniaturization. The ability to screen large numbers of individual antibody producing cells against multiple probes concurrently provides several orders of magnitude more data than is practical to collect using conventional hybridoma screening. High quality clones that are too rare to be reliably detected by prior methods thereby become readily identifiable and recoverable.
Acknowledgments
For help on this project, we thank our colleagues Carol Cain, Donna Chen, Neal DeChene, Beverly Freeman, Da Nguyen, John Pease, Jim Quarato, Jason Tseng, Eric Walters, Jiangzhang Zhang.
This work was supported in part by SBIR grant #2R44GM070021-02A1.
Abbreviations
- BSA
bovine serum albumin
- PBS
phosphate buffered saline
- ELISA
enzyme linked immunosorbent assay
- ECD
extracellular domain
- HA
hemagglutinin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babcook JS, Leslie KB, Olsen OA, Salmon RA, Schrader JW. A novel strategy for generating monoclonal antibodies from single, isolated lymphocytes producing antibodies of defined specificities. Proc Natl Acad Sci USA. 1996;93:7843–8. doi: 10.1073/pnas.93.15.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S, Al-Rubeai M. ACSD labelling and magnetic cell separation: a rapid method of separating antibody secreting cells from non-secreting cells. J Immunol Methods. 2005;296:171–8. doi: 10.1016/j.jim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Causey LD, Dwyer DS. Detection of low affinity interactions between peptides and heat shock proteins by chemiluminescence of enhanced avidity reactions (CLEAR) Nat Biotechnol. 1996;14:348–51. doi: 10.1038/nbt0396-348. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C, Andersson G, Ekre HP, Nilsson LA, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- de StGroth SF, Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35:1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- Gross HJ, Verwer B, Houck D, Recktenwald D. Detection of rare cells at a frequency of one per million by flow cytometry. Cytometry. 1993;14:519–26. doi: 10.1002/cyto.990140511. [DOI] [PubMed] [Google Scholar]
- Harriman W, Collarini E, Cromer R, Dutta A, Strandh M, Zhang F, Kauvar L. Multiplexed Elispot Assay. J Immunol. doi: 10.1016/j.jim.2008.11.010. submitted. Methods revision submitted October 14, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–16. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- Jin H, Yang R, Zheng Z, Romero M, Ross J, Bou-Reslan H, Carano RA, Kasman I, Mai E, Young J, Zha J, Zhang Z, Ross S, Schwall R, Colbern G, Merchant M. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res. 2008;68:4360–8. doi: 10.1158/0008-5472.CAN-07-5960. [DOI] [PubMed] [Google Scholar]
- Lerner RA, Kang AS, Bain JD, Burton DR, Barbas CF., 3rd Antibodies without immunization. Science. 1992;258:1313–4. doi: 10.1126/science.1455226. [DOI] [PubMed] [Google Scholar]
- Love JC, Ronan JL, Grotenbreg GM, van der Veen AG, Ploegh HL. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat Biotechnol. 2006;24:703–7. doi: 10.1038/nbt1210. [DOI] [PubMed] [Google Scholar]
- Or-Guil M, Wittenbrink N, Weiser AA, Schuchhardt J. Recirculation of germinal center B cells: a multilevel selection strategy for antibody maturation. Immunol Rev. 2007;216:130–41. doi: 10.1111/j.1600-065X.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6:349–56. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Leinfelder U, Gessner P, Zillikens D, Brocker EB, Zimmermann U. CD19+ B lymphocytes are the major source of human antibody-secreting hybridomas generated by electrofusion. J Immunol Methods. 2001;255:93–102. doi: 10.1016/s0022-1759(01)00431-8. [DOI] [PubMed] [Google Scholar]
- Stamos J, Lazarus RA, Yao X, Kirchhofer D, Wiesmann C. Crystal structure of the HGF beta-chain in complex with the Sema domain of the Met receptor. EMBO J. 2004;23:2325–35. doi: 10.1038/sj.emboj.7600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg FC, Rasmussen SK, Frandsen TP, Rasmussen LK, Tengbjerg K, Coljee VW, Sharon J, Yang CY, Bregenholt S, Nielsen LS, Haurum JS, Tolstrup AB. Production of target-specific recombinant human polyclonal antibodies in mammalian cells. Biotechnol Bioeng. 2006;94:396–405. doi: 10.1002/bit.20865. [DOI] [PubMed] [Google Scholar]