Abstract
AIM: To evaluate the incidence of contrast-induced nephropathy (CIN) in cirrhotic patients and to identify risk factors for the development of CIN.
METHODS: We performed a retrospective review of 216 consecutive patients with cirrhosis who underwent computed tomography (CT) with intravenous contrast at the University of Rochester between the years 2000-2005. We retrospectively examined factors associated with a high risk for CIN, defined as a decrease in creatinine clearance of 25% or greater within one week after receiving contrast.
RESULTS: Twenty-five percent of our patients developed CIN, and 74% of these patients had ascites seen on CT. Of the 75% of patients who did not develop CIN, only 46% had ascites. The presence of ascites was a significant risk factor for the development of CIN (P = 0.0009, OR 3.38, 95% CI 1.55-7.34) in multivariate analysis. Patient age, serum sodium, Model for End-stage Liver Disease score, diuretic use, and the presence of diabetes were not found to be significant risk factors for the development of CIN. Of the patients who developed CIN, 11% developed chronic renal insufficiency, defined as a creatinine clearance less than baseline for 6 wk.
CONCLUSION: Our results suggest that in hospitalized cirrhotic patients, especially those with ascites, the risk of CIN is substantial.
Keywords: Ascites, Cirrhosis, Contrast-induced nephropathy, Renal failure
INTRODUCTION
Renal failure is associated with a high morbidity and mortality in patients with cirrhosis[1–4]. Cirrhotic patients may be particularly predisposed to renal failure because of intravascular volume depletion, hyperaldosteronism, and altered renal hemodynamics[5]. Furthermore, aggressive use of diuretics, repeated large volume paracenteses, and gastrointestinal bleeding often contribute to renal insufficiency in these patients[1,2].
Contrast-induced nephropathy (CIN) is a common cause of renal failure, and is associated with substantial morbidity and mortality[6–9]. Multiple studies in the medical literature have estimated a risk of 2% in low-risk patients, rising to 50% in those with risk factors such as diabetes mellitus (DM), pre-existing renal disease, congestive heart failure, advanced age, anemia, and dehydration[10–15].
Although cirrhosis has been suggested as a risk factor for CIN[13–17], only two studies, to our knowledge, have specifically investigated this issue. A prospective study by Guevara et al[18] did not find an increased susceptibility to CIN in cirrhotic patients. Although this was a well-conducted study, it was conducted under idealized circumstances. Our goal was to conduct a study that may be more representative of the cirrhotic patients we encounter on a frequent basis[18]. The study by Najjar et al[19] was a retrospective study which also did not find an increased prevalence of CIN in cirrhotics. However, the authors did not precisely define CIN, making it difficult to interpret the results of the study[15]. We believe that cirrhosis is a risk factor for the development of CIN, and we aimed to identify risk factors for the development of CIN. We thus performed a retrospective review of cirrhotics who received iodinated contrast during hospitalization for further analysis.
MATERIALS AND METHODS
After approval by the University of Rochester’s Institutional Review Board, we reviewed the charts of 347 patients with cirrhosis who underwent computed tomography (CT) with intravenous contrast during hospitalization at our institution between the years 2000-2005. Inclusion criteria consisted of the presence of cirrhosis, inpatient hospitalization, and the use of iodinated contrast for CT. Patients with pre-existing sepsis, known congestive heart failure, gastrointestinal bleeding, spontaneous bacterial peritonitis (SBP), and chronic kidney disease (CKD), defined as baseline Cr > 18 mg/L, were excluded from our study. Patients who received peri-contrast intravenous sodium chloride, sodium bicarbonate, N-acetylcysteine, as well as those whose diuretic therapy was held during the day of contrast exposure were also excluded from our study.
Clinical data that were reviewed included the Model for End-stage Liver Disease (MELD) score, use of diuretics, serum sodium, a documented diagnosis of diabetes mellitus (DM) in the medical record, and the presence of ascites seen on CT. The use of potentially nephrotoxic medications, such as angiotensin-converting enzyme inhibitors (ACE inhibitors), angiotensin receptor blockers (ARBs), nonsteroidal anti-inflammatory drugs (NSAIDs), and aspirin was documented. The age and race of each patient were also noted. Statistical analysis was performed by faculty of the Department of Biostatistics at the University of Rochester. Multivariate and univariate analyses were performed using logistic regression, and odds ratios were calculated.
All patients received 150 mL of iodinated contrast dye (iohexol, Omnipaque) intravenously per standard radiology protocol. Post-contrast creatinine was then reviewed and compared to baseline values. Serum creatinine on the day of contrast exposure was used to calculate the pre-contrast creatinine clearance (CrCl). Post-contrast CrCl was determined by the highest recorded creatinine value within one week after contrast exposure. The aim of this study was to evaluate the development of CIN, which we defined as a decrease in CrCl of 25% or greater, temporally associated with the use of contrast. CrCl was estimated using the Cockroft-Gault equation. Patients who developed CIN were followed for 6 wk to assess the development of chronic renal insufficiency (CRI), defined as a CrCl less than baseline for 6 wk. The need for dialysis during this time period was also documented.
RESULTS
A total of 216 patients met the inclusion criteria. 34 patients were greater than 65 years of age (16%). The mean age was 53.2 years. 158 patients were Caucasian, 37 were African American, 10 were Hispanic, and 3 were Asian (Table 1). The mean MELD score for all patients was 15.3.
Table 1.
Demographic data of the 216 patients included in the study
| Factor | Patients | Percentage |
| Mean age | 53.2 | N/A |
| > 65 YO | 34 | 16 |
| < 65 YO | 182 | 84 |
| Male | 128 | 59 |
| Female | 88 | 41 |
| Caucasian | 158 | 73 |
| African American | 37 | 17 |
| Hispanic | 10 | 4 |
| Asian | 3 | 1 |
| Unknown | 8 | 5 |
A total of 53 (25%) patients developed CIN. Baseline characteristics of these patients included a mean MELD of 17, mean age of 51.9, and mean serum sodium of 134.7 mmol/L. In 36 of these patients (68%), renal insufficiency persisted for at least one week. A total of 39 (74%) of these patients had ascites (Table 2). Although 6 patients (11%) developed CRI, none of those patients required dialysis during our 6 wk review.
Table 2.
Characteristics of patients who developed CIN (n = 53) vs those who did not develop CIN (n = 163)
| Factor | Percent with CIN | Percent without CIN | P-value |
| Ascites | 74 | 46 | 0.0009 |
| MELD > 15 | 60 | 48 | 0.1774 |
| Age > 65 | 9 | 18 | 0.2171 |
| On diuretics | 66 | 57 | 0.3200 |
| Na ≥ 130 | 91 | 88 | 0.8449 |
A total of 163 patients (75%) did not develop CIN. Their mean MELD score was 14.8, mean age was 53.6, and mean sodium was 135.8 mmol/L. Seventy-five of these patients (46%) had ascites seen on CT (Table 2).
The presence of ascites was a significant risk factor for the development of CIN (P = 0.0009, OR 3.38, 95% CI 1.55-7.34) in multivariate analyses (Table 3, Figure 1). A total of 33 patients were on ACE inhibitors, ARBs, NSAIDs, or aspirin. Ascites remained a significant risk factor when these patients were excluded from this analysis (P = 0.00006, OR 3.98, 95% CI 1.83-8.69). Ascites was also a significant risk factor for the development of CRI, as 5/6 patients (83%) who developed CRI had ascites seen on CT scan. Age, serum sodium, and MELD score were not found to be significantly associated with a higher risk of CIN in multivariate analysis. Our analysis also did not show a significant association between the use of diuretics and an increased risk of CIN in patients with or without ascites (Figure 2).
Table 3.
Relationship of ascites to incidence of CIN
| Factor | Patients | Percentage |
| Ascites | 114 | 53 |
| CIN | 39 | 34 |
| No CIN | 75 | 66 |
| No ascites | 102 | 47 |
| CIN | 14 | 14 |
| No CIN | 88 | 86 |
Ascites was a significant risk factor for the development of CIN (P = 0.0009, OR 3.38, CI 1.55-7.34).
Figure 1.
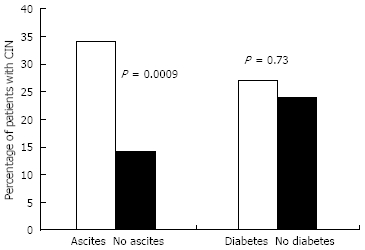
Percentage of patients experiencing CIN in the presence or absence of ascites (P = 0.0009) or diabetes (P = 0.73).
Figure 2.
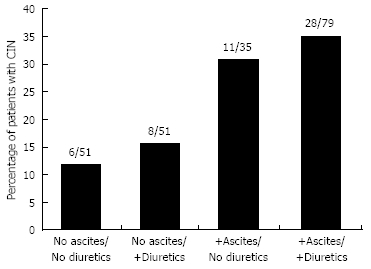
Percentage of patients experiencing CIN in relation to diuretic use and the presence of ascites.
In our study, the presence of DM was not a predisposing factor to CIN (Figure 1). A total of 66 diabetic patients were included in the analysis. The incidence of CIN was 18/66 (27%) in these patients, a nonsignificant difference compared to nondiabetics, where the incidence of CIN was 36/150 (24%). Among the total number of diabetic patients, three had evidence of mild kidney disease prior to the scan, defined as Cr > 15 mg/L. Only one (33%) of these patients developed CIN.
DISCUSSION
Intravenous contrast remains an important cause of acute renal failure in patients who receive CT scans. There is little data on whether the presence of cirrhosis serves as an important risk factor for the development of CIN.
We performed a large retrospective review at our institution of hospitalized cirrhotic patients who received intravenous contrast for CT imaging and found that there was a high rate of CIN. In multivariate analysis, the presence of ascites was a significant risk factor for the development of CIN, conferring over three times the risk compared to the absence of ascites. Factors such as MELD score, serum sodium, diuretic use, the presence of DM, and age failed to show a similar association.
The results of this study are dissimilar to those found in a prospective study by Guevara et al[18], who did not find an increased susceptibility to CIN in cirrhotic patients[18]. However, this study was limited by a relatively small sample size and diuretic therapy was withheld for at least 5 days prior to inclusion in the study. Our study included patients on diuretic therapy, and is therefore reflective of a broad range of cirrhotic patients.
A second retrospective study by Najjar et al[19] comparing 72 cirrhotic patients receiving intravenous contrast with 72 non-cirrhotic controls, revealed the development of CIN in 2 patients with cirrhosis (2.8%) and in 1 patient in the control group (1.4%), a non-significant difference. The authors of this study concluded that cirrhosis may not be a risk factor for CIN. However, the results of this study should be interpreted with caution because the study does not clearly define CIN[19]. Without a precise definition of CIN, it is difficult to interpret the results of this study.
The exact mechanism by which iodinated contrast agents induce renal dysfunction is not entirely understood. The pathophysiology is complex, and a variety of factors act in concert to induce CIN. In vitro and animal studies suggest that damage secondary to iodinated contrast to the kidneys is likely mediated by a combination of toxic or obstructive injury to the renal tubules, ischemic injury by reactive oxygen species, and renal medullary hypoxia. The predominant factor is likely to be renal medullary hypoxia, in which adenosine, calcium, and endothelin bring about intrarenal vasoconstriction after contrast exposure[17,20–25]. Tubular toxicity is also thought to play a role in CIN through both direct nephrotoxicity and tubular obstruction. The generation of reactive oxygen species can cause toxic, ischemic, and immune-mediated direct nephrotoxicity[26–28]. Contrast dye increases urate excretion and leads to the deposition of Tamm-Horsfall proteins within the renal tubules, both of which can cause tubular obstruction[29–31].
Factors such as age, serum sodium, MELD score, and diuretic use were not found to be associated with the development of CIN. It is surprising that there is no statistically significant relationship between diuretic use and CIN in our study. There are a fair number of studies suggesting that peri-contrast hydration can reduce the incidence of CIN[32–36]. Furthermore, prophylactic forced diuresis with furosemide has been shown to augment the risks of CIN[32]. However, there are no data, to our knowledge, on the association between maintenance diuretic therapy and CIN. Although an increased frequency of CIN in the setting of diuretic use is biologically plausible, this relationship should be explored further before any conclusions can be made.
In our analysis, the presence of DM did not confer an increased risk of CIN. Although DM is considered to be a risk factor for CIN, the data on whether this relationship is present independent of underlying renal impairment are conflicting[7,8,37]. Only three patients with DM in our analysis had evidence of mild kidney disease before receiving contrast, and one of these developed CIN. Because our study was retrospective, we were unable to assess our patients for the presence of microalbuminuria or overt proteinuria. Furthermore, our exclusion criteria of heart failure and CKD may have excluded many diabetic patients with significant vascular disease. Our sample may thus have consisted of a higher proportion of patients with uncomplicated diabetes and therefore at a lower risk of CIN.
In those patients that developed CIN, a large proportion (68%) had CIN that persisted for at least one week. 11% of these patients developed CRI as a possible result of contrast exposure. Although none of these patients required dialysis, even transient elevations in creatinine without progression to dialysis have been associated with prolonged hospital stay, adverse cardiac events, and increased mortality[6–9].
There have been multiple studies performed investigating whether certain prophylactic regimens may reduce the risk of CIN. These have included agents such as N-acetylcysteine, diuretics, dopamine, hemofiltration, as well as hydration with sodium chloride or sodium bicarbonate[38]. However, none of these have been performed in cirrhotic patients, and reviews of these trials have given discrepant results. Currently, only the use of low osmolality contrast medium at the lowest dose possible, in conjunction with saline hydration is recommended in a recent review article[17].
The primary limitation of our study is the use of serum creatinine in determining the incidence of CIN. The assessment of renal function is notoriously difficult in patients with cirrhosis, and creatinine is likely a sub-optimal measure of renal function in these patients. Although we assessed CrCl rather than an absolute change in serum creatinine, studies suggest that creatinine-based formulas (e.g. Cockroft-Gault, Modification of Diet in Renal Disease) can only provide a crude approximation of true glomerular filtration rate (GFR) in cirrhotics[38–40]. However, direct measurement of kidney function (e.g. inulin clearance) is impractical in large cohorts, and cystatin C-based equations (e.g. Larsson and Hoek) are also unable to accurately assess GFR in patients with cirrhosis[41]. Furthermore, the prognostic impact of serum creatinine in patients with cirrhosis is well-validated[5,42–45], and its predictive value is reflected by its inclusion in the MELD score. Additionally, numerous studies have used either a 25% rise in serum creatinine or a 25% decrease in estimated GFR to assess the development of CIN[7–9,11,12,34,38]. While not ideal, we feel that using a 25% decrease in CrCl is an adequate means of detecting clinically significant CIN.
Another important limitation of our study is that we defined CIN based solely upon the peak CrCl within one week after receiving contrast. Although patients with heart failure, SBP, sepsis, and CKD were excluded from the study, there are other causes of increases in serum creatinine in cirrhotic patients exposed to intravenous contrast (e.g. hepatorenal syndrome, large volume paracenteses). Fluctuations in serum creatinine are common in the inpatient setting, and often no discernable cause is found for these variations[46]. It is therefore possible that other factors may have contributed to alterations in CrCl in some of our patients. However, in hospitalized patients with no other apparent cause for a decline in renal function temporally associated with the administration of intravenous contrast, it is difficult to exclude CIN as a major contributing factor for this deterioration.
The retrospective nature of our study creates many limitations on our research, most notably the lack of a control group without intravenous contrast administration. The presence of a control group is particularly important when evaluating the incidence of CIN, as fluctuations in serum creatinine can have a multitude of causes. There are only two published studies, to our knowledge, that specifically compare the incidence of post-contrast renal dysfunction with the incidence of renal dysfunction in a control group of patients who did not receive intravenous contrast. Neither of these studies attributed a significant difference in the risk of renal failure to intravenous contrast, suggesting that the risk of CIN may be exaggerated. However, both of these studies were limited by a lack of randomization and a high threshold for the diagnosis of renal dysfunction (defined as a 50% increase in serum creatinine), and it is possible that differences in methodology may account for their findings[47,48]. Nonetheless, future studies assessing the risk of CIN in cirrhotic patients would be strengthened by the inclusion of a parallel control group[49,50].
It is also important to note that although diuretic use did not have an independent association with the development of CIN, there may be an association that our retrospective analysis was not able to elucidate. Patients with ascites are the most likely to receive high doses of diuretic therapy. It is difficult to sub-classify patients on the aggressiveness of their diuretic therapy, and we were thus unable to differentiate between patients on minimal doses of diuretics and those receiving aggressive diuresis. It is possible that high doses of diuretics may be a significant contributing factor to the development of CIN in patients with ascites. In patients with ascites receiving large doses of diuretic therapy, our retrospective study was unable to differentiate whether the development of CIN was from volume depletion from diuretic use or whether ascites was an independent predisposing factor.
In conclusion, our large retrospective study of hospitalized cirrhotic patients revealed a high incidence of CIN, especially in patients with ascites. CIN was associated with a significant percentage of patients progressing to CRI as a likely result of contrast exposure. These results suggest that in hospitalized cirrhotic patients, especially those with ascites, the risk of CIN is substantial. Alternative imaging strategies should be considered, and post-contrast renal function should be meticulously followed. Prospective studies evaluating the risk of contrast-induced nephropathy in cirrhotic patients, with and without the presence of ascites, should be performed for further investigation.
COMMENTS
Background
Contrast-Induced Nephropathy (CIN) is associated with substantial morbidity and mortality, especially in patients with cirrhosis. It is therefore important to determine whether cirrhosis is a risk factor for CIN, as well as to investigate which, if any, cirrhotic patients are particularly prone to CIN.
Research frontiers
The medical implications of CIN are substantial. As a result, there is a significant amount of research taking place on the identification of risk factors for CIN, as well as strategies to prevent its development. However, despite the vast amount of research on CIN, the data on the association between cirrhosis and CIN is scarce.
Applications
The study results suggest that in hospitalized cirrhotic patients, especially those with ascites, the risk of CIN is substantial.
Terminology
CIN: A decrease in CrCl of 25% or greater within one week after contrast exposure; Chronic Renal Insufficiency (CRI): a CrCl less than baseline for 6 wk; Chronic Kidney Disease (CKD): baseline Cr > 18 mg/L; Mild Kidney Disease: baseline Cr > 15 mg/L.
Peer review
Lodhia et al report the findings of a retrospective study that measured the risk of CIN in patients with cirrhosis. They found that the incidence of CIN was increased and that ascites was an independent risk factor for CIN. This is a very interesting and well written study.
Peer reviewer: Mercedes Susan Mandell, MD, PhD, Department of Anesthesiology, University of Colorado Health Sciences Ctr., 12401 E. 17th Ave, B113 Aurora, CO 80045, United States
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
References
- 1.Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 2.Wu CC, Yeung LK, Tsai WS, Tseng CF, Chu P, Huang TY, Lin YF, Lu KC. Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis. Clin Nephrol. 2006;65:28–33. doi: 10.5414/cnp65028. [DOI] [PubMed] [Google Scholar]
- 3.du Cheyron D, Bouchet B, Parienti JJ, Ramakers M, Charbonneau P. The attributable mortality of acute renal failure in critically ill patients with liver cirrhosis. Intensive Care Med. 2005;31:1693–1699. doi: 10.1007/s00134-005-2842-7. [DOI] [PubMed] [Google Scholar]
- 4.Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jiménez W, Arroyo V, Rodés J, Ginès P. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282–1289. doi: 10.1002/hep.20687. [DOI] [PubMed] [Google Scholar]
- 5.Epstien M. Deranged renal function in liver disease. Contrib Nephrol. 1977;7:250–271. [PubMed] [Google Scholar]
- 6.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 7.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 8.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 9.From AM, Bartholmai BJ, Williams AW, Cha SS, McDonald FS. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc. 2008;83:1095–1100. doi: 10.4065/83.10.1095. [DOI] [PubMed] [Google Scholar]
- 10.Mathew R, Haque K, Woothipoom W. Acute renal failure induced by contrast medium: steps towards prevention. BMJ. 2006;333:539–540. doi: 10.1136/bmj.38943.401852.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manske CL, Sprafka JM, Strony JT, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89:615–620. doi: 10.1016/0002-9343(90)90180-l. [DOI] [PubMed] [Google Scholar]
- 12.Nikolsky E, Mehran R, Lasic Z, Mintz GS, Lansky AJ, Na Y, Pocock S, Negoita M, Moussa I, Stone GW, et al. Low hematocrit predicts contrast-induced nephropathy after percutaneous coronary interventions. Kidney Int. 2005;67:706–713. doi: 10.1111/j.1523-1755.2005.67131.x. [DOI] [PubMed] [Google Scholar]
- 13.Abraham PA, Kjellstrand CM. Contrast media nephropathy. In: SG Massry, RJ Glassock., editors. Textbook of nephrology. 2nd ed. Williams & Wilkins: Baltimore; 1989. pp. 851–859. [Google Scholar]
- 14.Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37:233–243. doi: 10.1053/jhep.2003.50084. [DOI] [PubMed] [Google Scholar]
- 15.Barrett BJ, Parfrey PS. Prevention of nephrotoxicity induced by radiocontrast agents. N Engl J Med. 1994;331:1449–1450. doi: 10.1056/NEJM199411243312111. [DOI] [PubMed] [Google Scholar]
- 16.Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol. 1994;5:125–137. doi: 10.1681/ASN.V52125. [DOI] [PubMed] [Google Scholar]
- 17.Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379–386. doi: 10.1056/NEJMcp050801. [DOI] [PubMed] [Google Scholar]
- 18.Guevara M, Fernández-Esparrach G, Alessandria C, Torre A, Terra C, Montañà X, Piera C, Alvarez ML, Jiménez W, Ginès P, et al. Effects of contrast media on renal function in patients with cirrhosis: a prospective study. Hepatology. 2004;40:646–651. doi: 10.1002/hep.20373. [DOI] [PubMed] [Google Scholar]
- 19.Najjar M, Hamad A, Salameh M, Agarwal A, Feinfeld DA. The risk of radiocontrast nephropathy in patients with cirrhosis. Ren Fail. 2002;24:11–18. doi: 10.1081/jdi-120002656. [DOI] [PubMed] [Google Scholar]
- 20.Heyman SN, Reichman J, Brezis M. Pathophysiology of radiocontrast nephropathy: a role for medullary hypoxia. Invest Radiol. 1999;34:685–691. doi: 10.1097/00004424-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Brezis M, Rosen S. Hypoxia of the renal medulla--its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 22.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol. 1994;267:F1059–F1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 23.Humes HD, Hunt DA, White MD. Direct toxic effect of the radiocontrast agent diatrizoate on renal proximal tubule cells. Am J Physiol. 1987;252:F246–F255. doi: 10.1152/ajprenal.1987.252.2.F246. [DOI] [PubMed] [Google Scholar]
- 24.Deray G, Martinez F, Cacoub P, Baumelou B, Baumelou A, Jacobs C. A role for adenosine calcium and ischemia in radiocontrast-induced intrarenal vasoconstriction. Am J Nephrol. 1990;10:316–322. doi: 10.1159/000168126. [DOI] [PubMed] [Google Scholar]
- 25.Heyman SN, Clark BA, Kaiser N, Spokes K, Rosen S, Brezis M, Epstein FH. Radiocontrast agents induce endothelin release in vivo and in vitro. J Am Soc Nephrol. 1992;3:58–65. doi: 10.1681/ASN.V3158. [DOI] [PubMed] [Google Scholar]
- 26.Baud L, Ardaillou R. Reactive oxygen species: production and role in the kidney. Am J Physiol. 1986;251:F765–F776. doi: 10.1152/ajprenal.1986.251.5.F765. [DOI] [PubMed] [Google Scholar]
- 27.Bakris GL, Lass N, Gaber AO, Jones JD, Burnett JC Jr. Radiocontrast medium-induced declines in renal function: a role for oxygen free radicals. Am J Physiol. 1990;258:F115–F120. doi: 10.1152/ajprenal.1990.258.1.F115. [DOI] [PubMed] [Google Scholar]
- 28.Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis. 1997;29:465–477. doi: 10.1016/s0272-6386(97)90212-2. [DOI] [PubMed] [Google Scholar]
- 29.Mudge GH. Uricosuric action of cholecystographic agents. A possible factor in nephrotoxicity. N Engl J Med. 1971;284:929–933. doi: 10.1056/NEJM197104292841701. [DOI] [PubMed] [Google Scholar]
- 30.Postlethwaite AE, Kelley WN. Uricosuric effect of radiocontrast agents. A study in man of four commonly used preparations. Ann Intern Med. 1971;74:845–852. doi: 10.7326/0003-4819-74-6-845. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz RH, Berdon WE, Wagner J, Becker J, Baker DH. Tamm-Horsfall urinary mucoprotein precipitation by urographic contrast agents: in vitro studies. Am J Roentgenol Radium Ther Nucl Med. 1970;108:698–701. doi: 10.2214/ajr.108.4.698. [DOI] [PubMed] [Google Scholar]
- 32.Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 33.Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329–336. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- 34.Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van Moore A, Simonton CA 3rd, Rittase RA, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328–2334. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 35.Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, Goel P, Hewett J. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93:C29–C34. doi: 10.1159/000066641. [DOI] [PubMed] [Google Scholar]
- 36.Mueller-Lenke N, Buerkle G, Klima T, Breidthardt T, Buettner HJ, Mueller C. Incidence of contrast-induced nephropathy with volume supplementation--insights from a large cohort. Med Princ Pract. 2008;17:409–414. doi: 10.1159/000141507. [DOI] [PubMed] [Google Scholar]
- 37.Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, Farid N, McManamon PJ. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–149. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- 38.Brar SS, Shen AY, Jorgensen MB, Kotlewski A, Aharonian VJ, Desai N, Ree M, Shah AI, Burchette RJ. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300:1038–1046. doi: 10.1001/jama.300.9.1038. [DOI] [PubMed] [Google Scholar]
- 39.Ustundag Y, Samsar U, Acikgoz S, Cabuk M, Kiran S, Kulah E, Aydemir S. Analysis of glomerular filtration rate, serum cystatin C levels, and renal resistive index values in cirrhosis patients. Clin Chem Lab Med. 2007;45:890–894. doi: 10.1515/CCLM.2007.130. [DOI] [PubMed] [Google Scholar]
- 40.Cholongitas E, Shusang V, Marelli L, Nair D, Thomas M, Patch D, Burns A, Sweny P, Burroughs AK. Review article: renal function assessment in cirrhosis - difficulties and alternative measurements. Aliment Pharmacol Ther. 2007;26:969–978. doi: 10.1111/j.1365-2036.2007.03443.x. [DOI] [PubMed] [Google Scholar]
- 41.Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21:660–664. doi: 10.1093/ndt/gfi305. [DOI] [PubMed] [Google Scholar]
- 42.Cárdenas A, Ginès P, Uriz J, Bessa X, Salmerón JM, Mas A, Ortega R, Calahorra B, De Las Heras D, Bosch J, et al. Renal failure after upper gastrointestinal bleeding in cirrhosis: incidence, clinical course, predictive factors, and short-term prognosis. Hepatology. 2001;34:671–676. doi: 10.1053/jhep.2001.27830. [DOI] [PubMed] [Google Scholar]
- 43.Terra C, Guevara M, Torre A, Gilabert R, Fernández J, Martín-Llahí M, Baccaro ME, Navasa M, Bru C, Arroyo V, et al. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005;129:1944–1953. doi: 10.1053/j.gastro.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 44.Fraley DS, Burr R, Bernardini J, Angus D, Kramer DJ, Johnson JP. Impact of acute renal failure on mortality in end-stage liver disease with or without transplantation. Kidney Int. 1998;54:518–524. doi: 10.1046/j.1523-1755.1998.00004.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen YC, Tsai MH, Hsu CW, Ho YP, Lien JM, Chang MY, Fang JT, Huang CC, Chen PC. Role of serum creatinine and prognostic scoring systems in assessing hospital mortality in critically ill cirrhotic patients with upper gastrointestinal bleeding. J Nephrol. 2003;16:558–565. [PubMed] [Google Scholar]
- 46.Bagshaw SM, Gibney RT. Conventional markers of kidney function. Crit Care Med. 2008;36:S152–S158. doi: 10.1097/CCM.0b013e318168c613. [DOI] [PubMed] [Google Scholar]
- 47.Cramer BC, Parfrey PS, Hutchinson TA, Baran D, Melanson DM, Ethier RE, Seely JF. Renal function following infusion of radiologic contrast material. A prospective controlled study. Arch Intern Med. 1985;145:87–89. [PubMed] [Google Scholar]
- 48.Heller CA, Knapp J, Halliday J, O'Connell D, Heller RF. Failure to demonstrate contrast nephrotoxicity. Med J Aust. 1991;155:329–332. doi: 10.5694/j.1326-5377.1991.tb142293.x. [DOI] [PubMed] [Google Scholar]
- 49.Rao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology. 2006;239:392–397. doi: 10.1148/radiol.2392050413. [DOI] [PubMed] [Google Scholar]
- 50.Newhouse JH, Kho D, Rao QA, Starren J. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191:376–382. doi: 10.2214/AJR.07.3280. [DOI] [PubMed] [Google Scholar]


