Abstract
Both emotion and sleep are independently known to modulate declarative memory. Memory can be facilitated by emotion, leading to enhanced consolidation across increasing time delays. Sleep also facilitates offline memory processing, resulting in superior recall the next day. Here we explore whether rapid eye movement (REM) sleep, and aspects of its unique neurophysiology, underlie these convergent influences on memory. Using a nap paradigm, we measured the consolidation of neutral and negative emotional memories, and the association with REM-sleep electrophysiology. Subjects that napped showed a consolidation benefit for emotional but not neutral memories. The No-Nap control group showed no evidence of a consolidation benefit for either memory type. Within the Nap group, the extent of emotional memory facilitation was significantly correlated with the amount of REM sleep and also with right-dominant prefrontal theta power during REM. Together, these data support the role of REM-sleep neurobiology in the consolidation of emotional human memories, findings that have direct translational implications for affective psychiatric and mood disorders.
Keywords: consolidation, emotion, memory, REM, prefrontal, theta
Introduction
Over the last decade, diverse studies spanning descriptive levels have offered converging evidence that sleep plays a critical role in memory processing and brain plasticity (Walker and Stickgold 2006). These findings indicate that sleep, and its varied stages, contribute to latent processes of both declarative and procedural memory consolidation (Walker and Stickgold 2004; Marshall and Born 2007). Aspects of the relationship between declarative memory and sleep have, however, been questioned based on earlier studies that were equivocal—some confirming a role for sleep, others refuting it (Ellenbogen et al. 2006). Thus, the role of sleep in facilitating declarative memory remains an active topic of debate.
Independent of the field of sleep, there is a growing literature demonstrating that memory processing is modulated by the emotional strength of the material being learned (Cahill 2000; McGaugh 2004; Phelps 2004). Under certain conditions, memories including affective content persist more strongly over time than memories lacking emotional tone (Kensinger 2004). Moreover, this behavioral benefit has been related to specific changes in neurochemical and neurophysiological states in certain subcortical and cortical networks (Cahill 2000; McGaugh 2000; Pare et al. 2002; McGaugh 2004; Phelps 2004). Most interestingly, considerable overlap exists between the putative neurobiological mechanisms that orchestrate emotional memory consolidation (Cahill 2000; McGaugh 2004; Phelps 2004) and those that are engaged during rapid eye movement (REM) sleep, including prominent oscillatory activity in the theta frequency band range, raised levels of acetylcholine (ACh), and the re-emergence of coupled hippocampal and amygdala network activity (Hobson and Pace-Schott 2002; Pare et al. 2002; Hu et al. 2006).
Despite these parallel lines of evidence, there has been a paucity of research examining the interaction between sleep and emotional memory consolidation in the human brain. Instead, investigations have been limited largely to animal models, principally focusing on the sleep-dependent sensitivity of contextual fear and shock avoidance learning. For example, daytime training on such tasks commonly triggers alterations in sleep-stage and sleep-architecture characteristics, particularly REM (e.g., Smith et al. 1980; Hennevin and Hars 1987; Ambrosini et al. 1988; Mandai et al. 1989; Ambrosini et al. 1993; Sanford et al. 2001, 2003), considered to reflect a homeostatic demand on REM-dependent consolidation mechanisms. Furthermore, total as well as selective REM-sleep deprivation after learning disrupts consolidation and impairs next day memory retention (e.g., Pearlman 1969; Fishbein et al. 1974; Shiromani et al. 1979; Smith and Lapp 1986; Hennevin and Hars 1987; Oniani et al. 1987; Marti-Nicolovius et al. 1988; Smith and Kelly 1988; Beaulieu and Godbout 2000; Graves et al. 2003). Such findings suggest that, in rodents, consolidation of affective learning displays a sensitivity to, and even dependency on, sleep (and REM in particular).
An alternative explanation for the memory impairments reported in several of these earlier animal studies has been the increased stress of prolong wakefulness, rather than the lack of sleep itself. But more recent studies have demonstrated that selective deprivation of specific sleep stages, and even specific sleep-stage time windows (some located many hours to days after training), still inhibits memory consolidation (Smith and Butler 1982; Smith and Kelly 1988). Furthermore, Smith et al. (1991) have shown that administration of protein synthesis inhibitors (which block cellular consolidation cascades) during specific REM-sleep windows in rats prevents behavioral improvement following sleep, without requiring deprivation. Such findings make arguments of sleep deprivation-induced effects of stress on memory consolidation far less tenable.
To date, several reports have investigated the influence of sleep on emotional memory consolidation in humans, demonstrating a retention advantage across periods containing sleep relative to equivalent time periods awake (Hu et al. 2006; Payne et al. forthcoming), and particularly late-night sleep, rich in REM (Wagner et al. 2001, 2006). However, no study has yet examined the relationship between specific sleep-stages and emotional memory consolidation, or the associated underlying neurophysiological correlates that accompany sleep. Using a nap paradigm, here we explore the consolidation of neutral and negative emotional memories, and test the hypothesis that affective memories are selectively facilitated by REM sleep, and specifically oscillations in the theta frequency range.
Materials and Methods
Participants
Thirty-one subjects between the ages of 18 and 30 were assigned to either a Nap group (n = 15; 7 males, mean age 24.3 [SD ± 2.0]) or No-Nap group (n=16, 8 males, mean age 23.1 [SD ± 1.4]). Subjects had no prior history of drug or alcohol abuse, neurological, psychiatric or sleep disorders. Subjects were not considered as habitual nappers based on a sleep habit questionnaire obtained at initial screen, indicating one or less naps per week on average. Subjects maintained a regular sleep schedule 1 week prior to the study and abstained from caffeine, nonexperimental naps, and alcohol throughout the course of the study. The study was approved by the local human studies committee and conducted according to the principles expressed in the Declaration of Helsinki, with all subjects providing written informed consent.
Experimental Design: Nap and No-Nap Groups
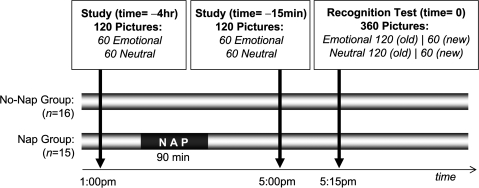
Both groups performed 2 study sessions, in which they learned emotionally negative (unpleasant valence, high arousal) and neutral (neutral valence, low arousal) picture stimuli, selected from a standardized picture set (Lang 1997). The study sessions occurred 4 h prior and 15 min prior to a recognition memory test (Fig. 1). At the recognition test, both sets of previously studied items from the 4- and 15-min study sessions were presented, together with intermixed foils (new emotional and neutral stimuli not previously seen), with subjects indicating whether they believed the stimuli to be old (from both study sessions) or new (not seen before). Offline consolidation was indexed as the difference in recognition memory score for items from the 4-h study session compared with items from the 15-min study session (Fig. 1). Following the first study session, but prior to the second, those in the Nap group obtained a 90-min sleep opportunity (1:15 PM ± 20 min), recorded with polysomnography (PSG), whereas those in the No-Nap group remained awake. Thus, items from the first (4 h) study session transitioned through different brain-states in each group prior to testing, containing sleep in the Nap group and no sleep in the No-Nap group, yet experienced identical brain-state conditions following the second (15 min) study session prior to testing (Fig. 1). During the interval between the “study” and “test” sessions, subjects were allowed to leave the lab and go about their normal daily activities, with the exception of the 90-min sleep opportunity in the Nap group.
Figure 1.
Experimental design. Subjects in each group viewed a series of picture slides (half emotional, half neutral) at 2 study sessions; 4 h prior (1 PM) and 15 min prior (5 PM) to a recognition memory test (5:15 PM). Between study sessions, the Nap group was given a 90 min sleep opportunity, whereas subjects in the No-Nap group remained awake. The nap period was recorded with digitized PSG. Therefore, 2 different “aged” memory sets were examined (4 h old and 15 min old), with stimuli from the first study session (4-h study) transitioning through different brain-states in each group prior to testing—sleep in the Nap group and no sleep in the No-Nap group—yet experienced identical brain-state conditions following the second study session (15-min study), prior to testing.
Experimental Task
The computerized task was composed of pictures selected from the International Affective Picture System (IAPS), a series of stimuli with standardized emotional ratings (Lang 1997). A total of 360 picture stimuli were compiled, matched in terms of visual stimulus characteristics (including faces, human figures and luminance), and which varied in arousal and valence strength on a scale from 1 to 9 (Lang 1997). Half of the stimuli were classified as negatively “emotional” (arousal mean ± SD 5.77 ± 0.59, valence mean ± SD 3.89 ± 1.98), the other half “neutral” (arousal mean ± SD 3.80 ± 0.67, valence mean ± SD 5.61 ± 1.28). The 360 stimuli were split into 3 sets of 120 balanced pictures: 60 emotional and 60 neutral stimuli, submatched for arousal and valence strength according to the above classifications. At each of the 2 study sessions, subjects viewed a set of the 120 stimuli. At the later recognition test, that subjects were aware of, the original 240 stimuli were presented (120 from each of the study sessions), together with the remaining 120 “new” stimuli (foils) intermixed (Fig. 1). Subjects viewed the stimuli on a 17″ CRT monitor at full width and height. The order presentation of the stimuli was pseudorandom, with no more than 3 stimuli of either emotional or neutral categories being presented in succession.
Each of the study session trials began with the presentation of an initial fixation-crosshair (500 ms), followed by the target picture (1000 ms), followed by a blank screen (500 ms), after which a “respond” screen was shown, indicating that subjects had to make a decision as to whether the picture represented an indoor or outdoor scene. The next trial began after the keyboard response.
At the subsequent recognition test, each of the 360 trials (240 original pictures, 120 new foils) began with the fixation-crosshair (500 ms), followed by the picture stimulus presentation (1000 ms), after which a “respond” screen was shown, indicating that subjects had to make their right-handed recognition keyboard choice of old or new. The next trial did not begin until subjects made a recognition judgment. From these choices, 4 response categories were possible: correct old judgments (“hits”), incorrect old judgments (“misses”), correct new judgments (“correct rejections”), and incorrect new judgments (“false alarms”), with recognition accuracy (d′) calculated according to signal detection theory (i.e. the difference between the z-transformed (normalized) probabilities of hit and false alarm rates: d′ = z(hit rate) − z(false alarm rate) where hit rate (HR) and false alarm rate (FAR) are the Hit and False Alarm Rates, respectively (Macmillan and Creelman 1991). The extent of offline consolidation was indexed as the difference in recognition memory score (d′) for items from the 4hr study session compared with items from the 15-min study session (i.e., [4 h score–15 min score]).
PSG Recording and Electrophysiological Analysis
PSG recording was performed in accordance with standardized techniques, using digital electroencephalography (EEG), electromyography, and eletrooculography signals acquired with a Grass Colleague system (sampling rate: 256Hz, high- and low-pass filter 0.3 and 35 Hz, respectively, notch filter 60 Hz). A mastoid referenced PSG electrode montage was utilized, composed of EEG sites F3 and C3 (referenced to A2), and F4 and C4 (referenced to A1). Each sleep epoch was scored blind to subjects behavioral task performance in accordance with standard criteria (Rechtschaffen and Kales 1968), with the exception of epoch length, which was set at 20s to conform with our spectral analysis window length (see below). The PSG recording was scored visually, epoch by epoch, as either NREM stages 1-4, REM sleep, awake or movement time. Slow-wave sleep (SWS) was calculated as NREM stages 3 and 4 combined.
Quantitative EEG analysis was performed by custom Matlab scripts (The MathWorks Inc, Natick, MA), built within the EEGLAB toolbox ([http://www.sccn.ucsd.edu/eeglab/]). Following removal of visually identified epochs containing muscle, cardiac and eye movement artifacts, spectral analysis was applied to each 4-s EEG epoch from stage 1, stage 2, SWS and REM sleep. One participant was excluded from the analysis due to poor quality recording in combination with excessive artifact components. Spectral power density was estimated for each epoch using Welch's averaged modified periodogram (linear detrending, 50% overlap and Hamming windowing, Matlab, MathWorks, Inc., MA). The frequency resolution was set at 0.25 Hz, with a frequency range up to 30 Hz analyzed. Spectral power density of 4 frequency bands was averaged in accordance with International Federation of Clinical Neurophysiology digital standards (Nuwer et al. 1998; Cantero et al. 2003); delta (0.5–3.0 Hz), theta (4.0–7.0 Hz), alpha (9.0–13.0 Hz), and beta (16.0–30.0 Hz).
Results
Memory Recognition Performance (d′)
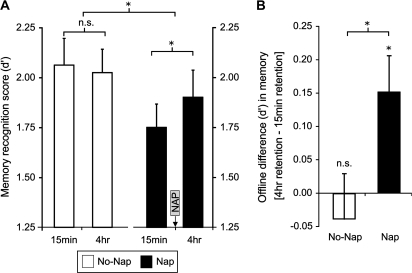
There was a significant overall 3-way ANOVA interaction between Group (Nap, No-Nap) × Memory Type (emotional, neutral) × Memory Age (4 h, 15 min); (ANOVA F3,28 = 4.49, P = 0.006). There was no main effect of Group ([Nap, No-Nap]; ANOVA F1,29 = 1.46, P = 0.24) nor a main effect of Memory Age ([4 h, 15 min]; ANOVA F1,29 = 1.56, P = 0.22). However, and in accordance with our hypothesis, there was a selective offline sleep facilitation of emotional memory (Fig. 2A), as demonstrated by a significant Group [Nap, No-Nap] x Memory Age [4 h, 15 min] interaction ANOVA (F1,29 = 4.33, P = 0.04). Specifically, in the Nap group, there was superior retention of emotional items studied 4 h prior to testing (which passed through the brain-state of sleep) compared with items studied 15 min prior to testing (paired t-test, t(14) = 2.49, P = 0.02). This significant offline benefit was also evident when quantified as the subtracted difference in recognition memory ([4 h memory retention–15 min memory retention]; Fig. 2B). In marked contrast, no such evidence of an offline emotional memory benefit was observed in the No-Nap group, with recognition performance for items from the 4 h and 15 min study sessions being nearly identical (paired t-test, t(15) = 0.57, P = 0.58; Fig. 2A,B). Data values for group HR and Correct Rejection values, forming the basis for the d′ scores, are described in Table 1, with HR similarly showing only a selective retention benefit for emotional items studied at 4 h relative to 15 min in the Nap group (t-test, t(15) = 2.40, P = 0.03), and not in the No-Nap group (t-test, t(15) = 0.25, P = 0.80).
Figure 2.
(A) Recognition memory score (d′) for emotional items studied 4 h or 15 min prior to the test session in the No-Nap and Nap groups. (B) The difference in recognition memory between the 4-h and 15-min study sessions (4 h score–15 min score) for the No-Nap and Nap groups (i.e., the offline consolidation difference for items studied 4 h vs. 15 min prior to the recognition test). *P < 0.05; n.s., nonsignificant. Error bars represent standard error of the mean.
Table 1.
Memory performance in the Nap and No-Nap Group—proportion correct (HR) as a function of study time prior to testing, together with proportion of false alarms (FA rate)
| Emotional | Neutral | |||||
| HR (4 h) | HR (15 min) | FAR | HR (4 h) | HR (15 min) | FAR | |
| Nap group | ||||||
| Mean | 0.93 | 0.88 | 0.30 | 0.87 | 0.88 | 0.21 |
| SEM | 0.02 | 0.02 | 0.05 | 0.03 | 0.03 | 0.04 |
| No-nap group | ||||||
| Mean | 0.91 | 0.92 | 0.28 | 0.83 | 0.85 | 0.20 |
| SEM | 0.02 | 0.02 | 0.03 | 0.03 | 0.04 | 0.03 |
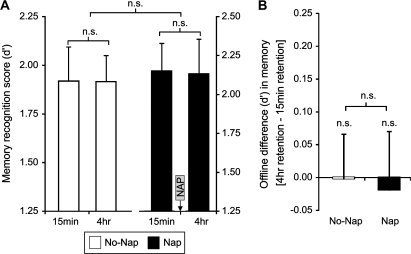
Unlike the differential group profiles of offline memory change for emotional stimuli, there was no significant interaction observed between the 2 groups, and across time, for neutral items (Group [Nap, No-Nap] × Memory Age [4 h, 15 min] interaction ANOVA (F1,29 = 0.02, P = 0.88; Fig. 3A,B). There was also no main effect of either Group or Memory Age (ANOVA, both F1,29 < 0.79, P > 0.38). Within-group comparisons further confirmed this lack of difference between the 4 h and 15 min offline retention periods for neutral memory in the Nap group (paired t-test, t(14) = 0.22, P = 0.83) and No-Nap group (paired t-test, t(15) = 0.03, P = 0.97).
Figure 3.
(A) Recognition memory score (d′) for neutral items studied 4 h or 15 min prior to the test session in the No-Nap and Nap groups. (B) The difference in recognition memory between the 4-h and 15-min study sessions [4 h score–15 min score] for the No-Nap and Nap group (i.e., the offline consolidation difference for items studied 4 h vs. 15 min prior to the recognition test). n.s., nonsignificant. Error bars represent standard error of the mean.
Response times for the 15-min and 4-h study sessions, as well as for the recognition test sessions, are provided in Tables 2 and 3, and demonstrated no significant differences between groups. Therefore, a selective offline consolidation benefit for emotional memory was observed in the Nap group; a difference that was not observed across the simple passage of time, as evidenced by the lack of performance difference for emotional memory in the No-Nap group. Furthermore, this nap-related improvement was only observed for emotional and not neutral memory.
Table 2.
Mean reaction times (ms) across different memory categories in the No-Nap and Nap groups for the 4-h and 15-min study sessions, respectively
| Study | |||||
| Study session (4 h) | Study session (15 min) | ||||
| Emotional | Neutral | Emotional | Neutral | ||
| No-Nap | Mean | 917 | 856 | 936 | 899 |
| SEM | 171 | 117 | 146 | 120 | |
| Nap | Mean | 895 | 773 | 859 | 785 |
| SEM | 119 | 96 | 126 | 110 | |
Note: There were no significant differences in reaction time between the 2 groups for any response type, for either emotional or neutral stimuli (all P > 0.36).
Table 3.
Mean reaction times (ms) across different memory categories and response types in the No-Nap and Nap groups at recognition testing
| Test | |||||||||
| Emotional | Neutral | ||||||||
| Hits | Misses | False alarms | Correct rejections | Hits | Misses | False alarms | Correct rejections | ||
| No-Nap | Mean | 854 | 1065 | 1113 | 883 | 864 | 755 | 1144 | 737 |
| SEM | 113 | 203 | 206 | 127 | 109 | 111 | 179 | 96 | |
| Nap | Mean | 725 | 1148 | 891 | 850 | 854 | 752 | 808 | 631 |
| SEM | 90 | 228 | 129 | 114 | 125 | 119 | 109 | 86 | |
Note: There were no significant differences in reaction times between the 2 groups for any response type, for either emotional or neutral stimuli (all P > 0.11).
It is conceivable that the observed consolidation benefit was dependent on the brain-state of sleep occurring immediately following learning (i.e., the sleep period occurring soon after the first (4 h) study session in the Nap group). To investigate whether this same sleep-dependent emotion memory effect would occur following a more prolonged postlearning delay, a second experiment was performed in an addition group of subjects (n = 17; 8 males, mean age 22.9 [SD ± 1.4]; screened as described above). Subjects performed 4 study sessions using the same task parameters and stimulus set (60 slides at each study session; half emotional half neutral) across 3 days: 1 PM day-1 (48 h prior to testing), 1 PM day-2 (24 h prior to testing), 1 PM day-3 (4 h prior to testing) and 5 PM day-3; 15 min prior to recognition testing at 5:15 PM. Thus, subjects learned the same total number of picture slides to those in the Nap and No-Nap groups, except learning occurred across 4 rather than 2 sessions, with 2 of the learned picture sets passing through an offline consolidation period containing sleep prior to testing (48- and 24-h study sets), whereas 2 of the picture sets did not experience sleep in the offline time before testing (4-h and 15-min study sets). Importantly, the 2 sets of learned information that did pass through sleep did so many hours after learning (1 PM on each day; an average of 10.5-h postlearning, based on sleep logs). These data, provided in Supplemental Figure 1, again demonstrated a specific offline advantage for the retention of emotional memory following periods of sleep (either across one or 2 nights), and that this benefit was evident even when the proximity of learning to the onset of sleep was delayed by many hours. Thus, the facilitation of emotional memory observed in the Nap group appears to be related to the presence of sleep, independent of its proximal relationship to the initial study session.
Sleep-Stage Correlations
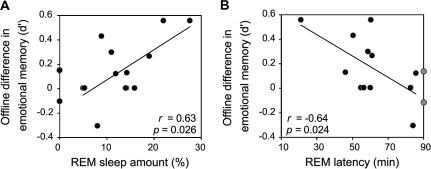
To further examine the relationship between the emotional memory benefit and our experimental REM-sleep hypothesis, sleep-stage values were correlated with the offline difference in emotional recognition memory ([4 h retention–15 min retention]; that is, the values represented in Fig. 2B, filled bar) in the Nap group. Sleep-stage amounts are summarized in Table 4. Of the 15 subjects, 13 achieved REM sleep. As demonstrated in Figure 4, and in accordance with our hypothesis, there was a significant positive relationship between the offline emotional memory benefit and the amount of REM sleep obtained across subjects, both REM percent (R = 0.63, P < 0.03) and REM minutes (R = 0.52, P < 0.05). Furthermore, there was a strong inverse relationship between the offline emotional memory benefit and REM latency (R = –0.64, P < 0.03); indicating that the faster subjects entered REM sleep (shorter latency to REM), the greater the emotional memory advantage. It should be noted, however, that these correlations narrowly missed significance when corrected for multiple comparisons at the most conservative Bonferroni threshold (P = 0.016).
Table 4.
Nap sleep-stage time (min) and percentage in the nap group (mean ± SEM)
| Sleep time (min) | Percentage | |
| Total nap time | 83.27 ± 4.18 | |
| Sleep latency | 10.92 ± 6.07 | |
| REM latency | 59.80 ± 4.76 | |
| Stage 1 | 11.06 ± 3.21 | 12.56 ± 4.69 |
| Stage 2 | 18.18 ± 2.55 | 21.77 ± 3.03 |
| Stage 3 | 14.33 ± 1.92 | 17.42 ± 2.85 |
| Stage 4 | 29.45 ± 3.25 | 36.53 ± 4.41 |
| SWS | 43.78 ± 4.31 | 53.95 ± 6.24 |
| REM | 10.24 ± 1.77 | 11.72 ± 1.99 |
Figure 4.
Correlation between the amount of offline emotional memory benefit in the Nap group (i.e. the d′ difference expressed in Fig. 2B) and (A) REM-sleep amount (%), and (B) Speed of entry into REM sleep (REM latency, in minutes). Filled circles represent all subjects that achieved REM sleep during the nap. Gray circles represent subjects that did not achieve REM during the nap, and were assigned a 90-min REM latency. Person's r-value and significance (P) displayed in figures, with statistical values and regression lines pertaining only to subjects that achieved REM.
This latter REM latency association alone did not, however, differentiate whether it is the time to achieve REM following the onset of sleep, or the time it takes to achieve REM sleep following the completion of memory encoding, which includes time spent awake prior to the onset of sleep. We therefore correlated total time from the end of the 4 h encoding session with the extent of emotional memory improvement (i.e., time awake + REM latency). This analysis revealed a lower and nonsignificant association (R = 0.35, P = 0.20), suggesting that the emotional consolidation “demand” for REM begins upon the initiation of sleep, rather than upon the completion of encoding. Future studies with greater power will be required to fully dissect this observed effect.
We further investigated whether the 2 subjects that did not achieve REM sleep would conform to these correlation distributions by assigning them a zero percent REM amount, and a 90-min REM latency (the duration of the nap opportunity). These REM-absent subjects expressed little offline emotional memory change across the nap, fitting the predictive distributions (Fig. 4A,B). Furthermore, with these subjects added, the strength of the respective correlations only increased (REM amount R = 0.61, P < 0.03; REM latency R = −0.63, P < 0.01).
No relationships were evident between the offline improvement in emotional memory and other sleep stages (stage 2, SWS or total sleep time; all P > 0.23, although insufficient variation in total sleep time may have precluded adequate correlative power for the measure of total sleep time). No significant associations were observed between the offline difference in neutral memory and any sleep parameter (all P > 0.54).
Rather than the nap affording an offline consolidation benefit, an alternative explanation is that sleep confers a detrimental postnap effect on encoding ability at the 15-min study session, artificially inflating the difference between 4-h and 15-min test performance. This would appear unlikely considering that emotional recognition memory performance (d′) at 15 min in the Nap group was not significantly different to that of the No-Nap group (P = 0.12), and was similarly true for the alternative recognition memory measure of HR (P = 0.69; Figs 2A, 3A and Table 1). Nevertheless, to explore this possibility, we correlated absolute emotional recognition memory performance for items studied at the 15-min session (rather than the subtracted difference between 15-min and 4-h performance) with sleep measures; an analysis which, according to the above alternative hypothesis, would predict negative relationships. No evidence for such a relationship between prior sleep and recognition memory performance for items studied at the 15 min session was apparent (stage 2, SWS or REM; all P > 0.18—also note that none of the correlations were in the negative direction). Therefore, a selective offline emotional memory benefit was expressed in the Nap group; the extent of which was strongly correlated with both the amount of REM sleep obtained during the nap, and the speed of entry into REM sleep.
Spectral EEG Analyses
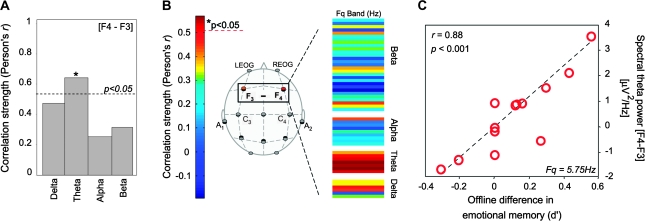
We finally sought to determine whether unique electrophysiological oscillations during REM were as, if not more, accurate in predicting the amount of emotional memory consolidation. We focused a priori on REM-sleep theta-band activity (4.0–7.0 Hz) because of the emerging relationship between affective memory processing and theta oscillations in limbic and prefrontal regions (Pare et al. 2002; Jones and Wilson 2005). Based on the right prefrontal-dominant representation of object versus verbal episodic declarative memory (Tulving et al. 1994; Kelley et al. 1998; Wagner et al. 1998; McDermott et al. 1999), we explored local electrophysiological signatures associated with the relative activity difference between the left versus right-frontal regions (activity at electrode F4 subtracted from that at F3, or [F4 − F3]); representing a specific local measure of sleep oscillatory activity within subjects (Huber et al. 2004; Nishida and Walker 2007).
The extent of right-lateralized prefrontal theta activity ([F4 − F3]) demonstrated a significant and positive correlation with the offline emotional memory benefit (Fig. 5A ; R = 0.61, P < 0.03, but did not reach the conservative Bonferroni corrected threshold for multiple comparisons; P = 0.013). Indeed, this right-sided association between theta power and emotional memory improvement was also evident when examining each electrode independently (see Supplemental Figs 2 and 3). The theta correlation was not observed at central electrode sites ([C4 − C3] or either electrode by themselves; all P > 0.20). No other frequency band at frontal (or central) regions correlated with the extent of offline improvement in emotional memory (all P > 0.15), and no association between REM-sleep theta power was observed with the offline change in neutral memory at frontal or central regions (all P > 0.14).
Figure 5.
(A) Correlation strength (Person's r-value) between offline benefit for emotional memory in the Nap group (i.e., the d′ benefit expressed in Fig. 2B) and the relative right versus left prefrontal spectral-band power ([F4 − F3]), illustrating a significant positive correlation in the theta-band range with the extent of offline emotional memory consolidation (R = 0.61, P = 0.03), (B) a more fine-grained analysis of this same correlation for incremental power spectrum densities within each band, expressed in average 0.5-Hz bins. Correlation strength is represented by the color range, demonstrating significant correlations within the Theta frequency band, and (C) exhibiting a maximum significance at the 5.75-Hz bin, displayed on right figure panel.
To further examine whether these broad EEG bands may have been obscuring more discrete spectral frequency correlations outside of the theta range, we performed a fine-grained separation of the spectrum in 0.5-Hz interval bins. However, as shown in Figure 5B, only frequency bins in the theta spectrum displayed significant correlations with the extent of emotional consolidation, many of which remaining significant following Bonferroni correction (threshold P = 0.001; see Supplemental Fig. 3 for individual electrode data).
Discussion
Using a nap paradigm, here we demonstrate the selective offline benefit of sleep on the consolidation of negative emotional memories. Furthermore, this offline emotional memory advantage correlated with the amount of REM sleep, and specifically the extent of right-dominant prefrontal theta power during REM.
An established literature demonstrates that memory processing can be modulated by the emotional strength of the material being learned (for reviews, see McGaugh 2004). These studies show that memories associated with the evocation of emotion persist more strongly than memories lacking affective tone (LaBar and Phelps 1998; Cahill 2000; Kensinger 2004; McGaugh 2004; Phelps 2004). Most relevant, the effects of emotion on memory retention are known to paradoxically increase as the delay between encoding and retrieval increases (hours/days) (Kleinsmith and Kaplan 1963; Walker and Tarte 1963; Levonian 1972; LaBar and Phelps 1998; Sharot and Phelps 2004), suggesting that emotion influences slow, time-dependent consolidation processes.
To date, a number of studies have investigated the interaction between offline time and sleep on affective memory consolidation. For example, Hu et al. (2006) compared the offline consolidation of emotionally arousing and nonarousing picture-stimuli following a 12 h period across the day or across a night containing sleep. A selective emotional memory benefit was observed only across a night containing sleep and not simply across the simple passage of time, as evidenced by inferior neutral and emotional memory performance across the day. Wagner and colleagues (Wagner et al. 2001) have also shown that sleep selectively favors the retention of previously learned emotional texts relative to neutral texts, and that this affective memory benefit is only present following late-night sleep (a time period rich in stage-2 NREM and REM sleep), an effect that can persist for several years (Wagner et al. 2006). Most recently, it has been shown that sleep preferentially and selectively consolidates emotional objects embedded within a scene, rather than the image as a whole (Payne et al. in press).
Here we similarly describe an offline sleep benefit for emotional memory consolidation but also demonstrate that this effect is evident even following an interval containing a short (90 min) sleep epoch, relative to an equivalent time period awake. Importantly, this effect is revealed when comparing memory performance at identical circadian study-test time points. An alternative explanation for these offline improvements could be attributed to interference from continued waking activities in the No-Nap group, not present during the sleep period in the Nap group, resulting in a passive rather than proactive sleep-state favoring consolidation. However, we find this explanation to be unlikely for several reasons. The lack of interference in the Nap group should result in global consolidation benefits for both emotional and neutral memory categories. Contrary, the sleep benefit was only seen for emotional stimuli. Indeed, one may predict that affective stimuli, being more emotional and potent, should be less susceptible to interference across the day, and result in more similar consolidation benefits to those observed in the No-Nap group. Instead, the opposite was found. Furthermore, the advantage in emotional memory was not proportional to total sleep duration (indexing the total interference-free time in the Nap group), but instead, with a specific type of sleep (REM; incidentally, the stage associated with the greatest amount of potentially interfering mental activity (Hobson and Pace-Schott 2002). Most compelling, however, was that the emotional memory benefit correlated with a specific electrophysiological oscillation, strongly suggesting an active mechanistic role for sleep in consolidation (and not simply a passive state, lacking interference).
The current findings go beyond demonstrating that emotional memory is preferentially modulated across periods of sleep, and to our knowledge, provide the first demonstration that the extent of emotional memory consolidation is associated with REM-sleep characteristics—both amount and speed of entry. Importantly, this REM relationship was specific to emotional memory with no detectable relationship observed for neutral memory. Furthermore, the emotional memory benefit was selective to REM, with no other sleep-stage measure demonstrating an association with offline performance improvement.
Corroborating these correlations, it has previously been hypothesized that REM sleep represents a brain-state particularly amenable to emotional memory consolidation, based on its unique biology (Pare et al. 2002; Hu et al. 2006). Neurochemically, levels of limbic and forebrain ACh are markedly elevated during REM (Vazquez and Baghdoyan 2001), reportedly quadruple those seen during NREM and double those measured in quite waking (Marrosu et al. 1995). Considering the known importance of ACh in the long-term consolidation of emotional learning (McGaugh 2004), this procholinergic REM state may result in a selective memory facilitation of affective memories, similar to that reported using experimental manipulations of ACh (Power 2004). Moreover, by processing such memories in a brain-state that is largely devoid of aminergic tone (Pace-Schott and Hobson 2002), particularly noradrenergic input from the locus coeruleus, the modulation of negative emotional experiences during REM may help depotentiate and ultimately ameliorate the autonomic charge originally acquired at the time of learning, negating a long-term state of chronic anxiety.
Neurophysiologically, these alterations may be reflected in (or caused by) changes of synchronized oscillatory activity between limbic (including amygdala and hippocampal) and neocortical regions during REM sleep (Pare et al. 2002; Jones and Wilson 2005). Cooperation between these structures plays a role in the modulation of affective experiences (Pare et al. 2002), leading to the possibility that synchronous activity within these networks during REM sleep may modulate plastic changes essential to emotional memory consolidation. Complimentary to such a model, it has also been demonstrated that learning and later successful recollection of human emotional episodic memories rely on interactions between the hippocampus and amygdala—the degree to which accurately predicts the extent of latent memory retention (Kilpatrick and Cahill 2003; Dolcos et al. 2004; Dolcos et al. 2005).
Here we demonstrate that the offline facilitation of emotional memory is not simply correlated with the amount and latency of REM sleep, but specifically with an electrophysiological signature of REM sleep—spectral activity in the theta-band range. Furthermore, this relationship was topographically organized, with the biased extent of right-dominant theta power being most predictive of the amount of emotional memory improvement, a relationship consistent with the right-sided anatomical distribution of object (vs. verbal) memory (Kelley et al. 1998; Wagner et al. 1998; McDermott et al. 1999) and also the right-frontal dominant relationship with negative affective processing (Davidson 2002). Although the functional association between emotional memory and REM-sleep electrophysiology remains unclear, coordinated theta oscillations have been proposed to constitute a mechanism allowing disparate brain regions that initially encoded information to selectively interact offline, in a coupled relationship, and by doing so, promote the strengthening of specific memory representations across distributed networks (Buzsaki 2002; Jones and Wilson 2005). It is therefore interesting to speculate whether surface EEG theta correlations observed in the current study, complimentary to those recorded at a cellular level (Jones and Wilson 2005), may represent the large-scale cooperation between connected subcortical limbic structures and prefrontal regions (Sotres-Bayon et al. 2004; Jones and Wilson 2005), the extent of which predicts the amount of offline emotional memory processing and postsleep benefit.
It should be noted, however, that theta activity is not exclusive to REM sleep, and has been observed during periods of sleep–wake transition as well as during quite wakefulness (e.g., Cantero et al. 2003). We did not record EEG activity in the no-nap group, and therefore do not have an index of theta activity during wakefulness in these participants. Although it is likely that an amount of theta activity will have been present, it does not appear to benefit emotional memory consolidation in a similar manner to that observed during REM, because no emotional memory advantage was observed in those that remained awake. Therefore, although these findings in no way dismiss the possibility that theta activity may be present across brain-states, they do suggest that theta activity, in combination with the REM-sleep state, preferentially facilitates emotional memory consolidation.
Although neutral memory was not enhanced following the nap, we are not suggesting that nonemotional declarative memories do not benefit from sleep. There is now substantial evidence indicating that a full night of nocturnal sleep modulates emotion-free declarative memories, and is most commonly associated with NREM SWS characteristics (Marshall and Born 2007). Furthermore, our current study focused principally on a short epoch of sleep (but see data in Supplemental Fig. 1), which, although containing NREM SWS, may not have been sufficient to trigger robust neutral memory consolidation benefits.
In the broader context, this REM-sleep modulation of negative aversive memories may hold implications for the mechanistic understanding and treatment of mood disorders, including major depression. Depression is commonly associated with alterations in REM sleep, including a faster progression into REM (reduced REM latency) and an increase in the amount of REM (Tsuno et al. 2005; Armitage 2007). Considering the REM association with negative emotional memory reported here, such REM abnormalities in depression may represent a maladaptive consolidation process of prior negative affective experiences, which, due to the increased REM amount and faster speed of entry into REM, could selectively and disproportionately reinforce negative memories at night, thereby potentiating the mood disorder. Likewise, post-traumatic stress disorder (PTSD) is also associated with a dysregulation of REM sleep, with reports of increased sympathetic autonomic tone (Harvey et al. 2003; Mellman and Hipolito 2006). There may similarly be an adverse consequence to such trauma-induced REM-sleep changes in PTSD, which if they persist, could counter-productively amplify, rather than ameliorate, the acquired affective experience. Such basic research findings may help the growing translational appreciation of the interaction between affective mood disorders and sleep physiology.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institutes of Health (MH069935); the Howard Hughes Medical Institute; and the American Academy of Sleep Medicine. Funding to pay the Open Access publication charges for this article were provided by The Berkeley Research Impact Initiative.
Supplementary Material
Acknowledgments
We wish to thank Jose L. Cantero and Elizabeth Kensinger for guidance and thoughtful insights. Conflict of Interest: None declared.
References
- Ambrosini MV, Mariucci G, Colarieti L, Bruschelli G, Carobi C, Giuditta A. The structure of sleep is related to the learning ability of rats. Eur J Neurosci. 1993;5:269–275. doi: 10.1111/j.1460-9568.1993.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Ambrosini MV, Sadile AG, Gironi Carnevale UA, Mattiaccio M, Giuditta A. The sequential hypothesis on sleep function. I. Evidence that the structure of sleep depends on the nature of the previous waking experience. Braz J Med Biol Res. 1988;21:141–145. doi: 10.1016/0031-9384(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand. 2007;(Suppl):104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu I, Godbout R. Spatial learning on the Morris Water Maze Test after a short-term paradoxical sleep deprivation in the rat. Brain Cogn. 2000;43:27–31. [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cahill L. Neurobiological mechanisms of emotionally influenced, long-term memory. Prog Brain Res. 2000;126:29–37. doi: 10.1016/S0079-6123(00)26004-4. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J Neurosci. 2003;23:10897–10903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci USA. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Payne JD, Stickgold R. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Curr Opin Neurobiol. 2006;16:716–722. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Fishbein W, Kastaniotis C, Chattman D. Paradoxical sleep: prolonged augmentation following learning. Brain Res. 1974;79:61–75. doi: 10.1016/0006-8993(74)90566-6. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23:377–407. doi: 10.1016/s0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Hars B. Is increase in post-learning paradoxical sleep modified by cueing? Behav Brain Res. 1987;24:243–249. doi: 10.1016/0166-4328(87)90062-3. [DOI] [PubMed] [Google Scholar]
- Hobson J, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Neurosci Rev. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- Hu P, Stylos-Allen M, Walker MP. Sleep facilitates consolidation of emotionally arousing declarative memory. Psychol Sci. 2006;17:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering emotional experiences: the contribution of valence and arousal. Rev Neurosci. 2004;15:241–251. doi: 10.1515/revneuro.2004.15.4.241. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. J Exp Psychol. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: role of the medial temporal lobe in humans. Psychol Sci. 1998;9:490–493. [Google Scholar]
- Lang PJ. International Affective Picture System (IAPS): technical manual and affective ratings. Gainesville (FL): University of Florida; 1997. [Google Scholar]
- Levonian E. Retention over time in relation to arousal during learning: an explanation of discrepant results. Acta Psychol. 1972;36:290–321. doi: 10.1016/0001-6918(72)90013-3. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: a user's guide. New York: Cambridge University Press; 1991. [Google Scholar]
- Mandai O, Guerrien A, Sockeel P, Dujardin K, Leconte P. REM sleep modifications following a Morse code learning session in humans. Physiol Behav. 1989;46:759–762. doi: 10.1016/0031-9384(89)90344-2. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fà M, Giagheddu M, Imperato A, Gessa GL. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Marti-Nicolovius M, Portell-Cortes I, Morgado-Bernal I. Improvement of shuttle-box avoidance following post-training treatment in paradoxical sleep deprivation platforms in rats. Physiol Behav. 1988;43:93–98. doi: 10.1016/0031-9384(88)90103-5. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Buckner RL, Petersen SE, Kelley WM, Sanders AL. Set- and code-specific activation in frontal cortex: an fMRI study of encoding and retrieval of faces and words. J Cogn Neurosci. 1999;11:631–640. doi: 10.1162/089892999563698. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Hipolito MM. Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr. 2006;11:611–615. doi: 10.1017/s1092852900013663. [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PloS One. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwer MR, Comi G, Emerson R, Fuglsang-Frederiksen A, Guérit JM, Hinrichs H, Ikeda A, Luccas FJ, Rappelsburger P. IFCN standards for digital recording of clinical EEG. International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1998;106:259–261. doi: 10.1016/s0013-4694(97)00106-5. [DOI] [PubMed] [Google Scholar]
- Oniani TN, Lortkipanidze ND, Maisuradze LM. Interaction between learning and paradoxical sleep in cats. Neurosci Behav Physiol. 1987;17:304–310. doi: 10.1007/BF01183059. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Pare D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Kensinger EA Forthcoming. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman CA. Effect of rapid eye movement (dreaming) sleep deprivation on retention of avoidance learning in rats. 1969 Rep No 563. Rep US Nav Submar Med Cent: 1–4. [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Power AE. Muscarinic cholinergic contribution to memory consolidation: with attention to involvement of the basolateral amygdala. Curr Med Chem. 2004;11:987–996. doi: 10.2174/0929867043455558. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda (MD): U.S. Department of Health; 1968. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Silvestri AJ, Ross RJ, Morrison AR. Influence of fear conditioning on elicited ponto-geniculo-occipital waves and rapid eye movement sleep. Arch Ital Biol. 2001;139:169–183. [PubMed] [Google Scholar]
- Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003;33:43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- Sharot T, Phelps EA. How arousal modulates memory: disentangling the effects of attention and retention. Cogn Affect Behav Neurosci. 2004;4:294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Shiromani P, Gutwein BM, Fishbein W. Development of learning and memory in mice after brief paradoxical sleep deprivation. Physiol Behav. 1979;22:971–978. doi: 10.1016/0031-9384(79)90343-3. [DOI] [PubMed] [Google Scholar]
- Smith C, Butler S. Paradoxical sleep at selective times following training is necessary for learning. Physiol Behav. 1982;29:469–473. doi: 10.1016/0031-9384(82)90268-2. [DOI] [PubMed] [Google Scholar]
- Smith C, Kelly G. Paradoxical sleep deprivation applied two days after end of training retards learning. Physiol Behav. 1988;43:213–216. doi: 10.1016/0031-9384(88)90240-5. [DOI] [PubMed] [Google Scholar]
- Smith C, Lapp L. Prolonged increases in both PS and number of REMS following a shuttle avoidance task. Physiol Behav. 1986;36:1053–1057. doi: 10.1016/0031-9384(86)90479-8. [DOI] [PubMed] [Google Scholar]
- Smith C, Tenn C, Annett R. Some biochemical and behavioural aspects of the paradoxical sleep window. Can J Psychol. 1991;45:115–124. doi: 10.1037/h0084279. [DOI] [PubMed] [Google Scholar]
- Smith C, Young J, Young W. Prolonged increases in paradoxical sleep during and after avoidance-task acquisition. Sleep. 1980;3:67–81. [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez J, Baghdoyan HA. Basal forebrain acetylcholine release during REM sleep is significantly greater than during waking. Am J Physiol Regul Integr Comp Physiol. 2001;280:R598–R601. doi: 10.1152/ajpregu.2001.280.2.R598. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry. 2006;60:788–790. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Walker EL, Tarte RD. Memory storage as a function of arousal and time with homogeneous and heterogeneous lists. J Verbal Learn Verbal Behav. 1963;2:113–119. [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory and plasticity. Annu Rev Psychol. 2006;10:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.