Abstract
Current theories are divided as to whether prospective memory (PM) involves primarily sustained processes such as strategic monitoring, or transient processes such as the retrieval of intentions from memory when a relevant cue is encountered. The current study examined the neural correlates of PM using a functional magnetic resonance imaging design that allows for the decomposition of brain activity into sustained and transient components. Performance of the PM task was primarily associated with sustained responses in a network including anterior prefrontal cortex (lateral Brodmann area 10), and these responses were dissociable from sustained responses associated with active maintenance in working memory. Additionally, the sustained responses in anterior prefrontal cortex correlated with faster response times for prospective responses. Prospective cues also elicited selective transient activity in a region of interest along the right middle temporal gyrus. The results support the conclusion that both sustained and transient processes contribute to efficient PM and provide novel constraints on the functional role of anterior PFC in higher-order cognition.
Keywords: anterior prefrontal cortex, cognitive control, prospective memory, working memory
Introduction
Prospective memory (PM) involves the execution of an intended action at an appropriate time in the future. PM is critical to our day-to-day functioning, as it underlies a variety of real-world tasks such as remembering to pick up groceries on the way home from work, remembering to take medication prior to bed, or remembering to attach a document to an email before clicking the send button. Although the experimental study of PM is now a burgeoning field (McDaniel and Einstein 2007), a number of issues regarding the nature of the processes supporting PM remain to be answered. For example, some investigators have argued that PM is related to working memory (WM) and sustained attention in that it may require both the active maintenance of goal-related information and strategic monitoring of the environment for prospective cues (Burgess et al. 2001; Smith 2003). In contrast, other investigators have demonstrated that prospective remembering can be relatively spontaneous or automatic (McDaniel and Einstein 2000; Einstein et al. 2005). The present study utilized functional magnetic resonance imaging (fMRI) to examine the neural correlates of processes associated with PM. We focused on three questions motivated by previous research: 1) Is PM associated with sustained neural activity associated with strategic monitoring or transient neural activity associated with intention retrieval? 2) Do common or distinct neural networks support sustained processing in PM and WM? and 3) Do common or distinct neural networks support target detection in PM tasks and sustained attention tasks (e.g., oddball tasks)?
A number of investigators have suggested that PM depends on the engagement of strategic monitoring processes that facilitate the detection of prospective cues (i.e., stimuli that prompt the execution of an intended action; Burgess et al. 2001; Guynn 2003; Smith 2003). A behavioral signature of strategic monitoring is the slowing of response time (RT) to an ongoing task when a PM component is added to the task (termed the prospective interference effect: Marsh et al. 2003; Smith 2003). Existing evidence indicates that the prospective interference effect is observed with a variety of ongoing activities and prospective cues (Marsh et al. 2003; Smith 2003; West et al. 2005) and that the presence or magnitude of the effect varies with the characteristics of the prospective component of the task (Marsh et al. 2003; Einstein et al. 2005; Cohen et al. 2008). An open question is whether the prospective interference effect arises from the recruitment of cognitive processes that are tonically activated over the course of task performance (West et al. 2005) or instead from the recruitment of cognitive processes that are engaged transiently during stimulus evaluation (Marsh, Hicks, et al. 2002).
Results from studies using brain-imaging methods can reveal tonic activity associated with strategic monitoring. Studies using positron emission tomography (PET) or blocked fMRI have identified a network of regions associated with PM that includes lateral anterior prefrontal cortex (i.e., Brodmann area [BA] 10 or aPFC; Burgess et al. 2001, 2003, 2008; Simons et al. 2006). In one study, anterior prefrontal cortex (aPFC) activity was observed during a PM task when no PM cues were presented, suggesting that PM engages processes dissociable from those associated with target detection and memory retrieval. However, due to the nature of blocked fMRI and PET designs, it has not been possible to determine whether the PM response reflects truly sustained processes, or instead a transient response that occurs on most trials. The current study used a mixed blocked/event-related fMRI design (Visscher et al. 2003) that permitted the isolation of brain regions that were responsive in either a transient or sustained fashion. This approach enables one to determine whether the addition of a PM component increases 1) sustained responses that span multiple trials (potentially reflecting strategic monitoring; West et al. 2005), 2) transient responses associated with processes that occur within a trial (termed transient, ongoing) that may reflect item checking (Guynn 2003), or both. Current conceptualizations of the processes underlying PM would suggest that both sustained and transient processes are associated with successful PM (Guynn 2003; Smith and Bayen 2005).
The second question addressed in the current study concerns the specificity of neural responses related to PM: does a common neural network support the active maintenance of goal relevant information in WM and PM? Although current theories suggest that both sustained and transient processes support PM, they lead to somewhat different predictions related to the commonality of PM and WM. For example, Smith and Bayen (2005) suggest that strategic monitoring is positively correlated with WM capacity, leading to the suggestion that similar sustained processes may support PM and WM. In contrast, Guynn (2003) suggested that sustained processing in PM reflects a retrieval mode similar to that supporting controlled retrieval in episodic memory (Duzel et al. 1999; Lepage et al. 2000). This latter account leads to the hypothesis that distinct processes may underlie sustained responses associated with PM and WM.
Existing behavioral findings are also somewhat mixed with regard to the similarity of PM and WM. Marsh and Hicks (1998) reported that increasing the rate at which participants needed to generate random numbers (presumably taxing control processes associated with WM) led to a reduction in PM. Other data indicate that PM can be selectively disrupted when PM cues are embedded in task-switching paradigms that require continual updating of task context over time (Marsh, Hancock, et al. 2002; McNerney and West, forthcoming). This finding suggests that the active maintenance or updating of task goals during ongoing task performance may compete for the same processes that support PM. Although these data suggest that control processes may be common across the two domains, decreased PM as a function of an increased demand on attention or WM capacity does not necessarily indicate that active maintenance in WM is the mediating factor. For example, it appears as though performance on one task frequently used to assess active maintenance (the N-Back task) is only weakly correlated with WM span measures thought to index controlled attention (Kane and Conway 2007), suggesting that additional processes may be underlying performance in such complex WM span tasks. Further, a recent case study revealed impaired strategic monitoring across a number of PM tasks in an individual who demonstrated exemplary performance on a variety of WM tasks, suggesting a possible dissociation between the processes supporting PM and WM (West et al. 2007). A significant limitation of existing studies is that behavioral data provide only indirect evidence regarding whether the disruptive effects of increasing WM load during PM are due to competition between common processes. Neuroimaging data can provide direct evidence for the activation of common or distinct neural circuitry supporting PM and WM.
The final question addressed in the current study concerns the nature of processes underlying the detection of PM cues. Conceptual frameworks of PM have borrowed heavily from the recognition memory literature in describing the processes underlying the recognition of PM cues (Einstein and McDaniel 1996; Smith and Bayen 2005). As an example, Einstein and McDaniel (1996; McDaniel and Einstein 2000) suggested that a process of “discrepancy attribution” supports the detection of a PM cue that should trigger the execution of a delayed intention. Specifically, the presentation of a PM cue elicits a discrepancy signal (i.e., that the cue is more or less familiar than expected) that prompts a search of long-term memory. This search of memory results in the intention being retrieved and realized. Data supporting these models lead to the suggestion that similar processes may contribute to target detection in PM and in other tasks. However, evidence from studies utilizing event-related brain potential (ERP) methods indicates that distinct processes may underlie the detection of PM cues and target stimuli. For instance, West and Wymbs (2004) found that the N2pc (a neural correlate of target selection) was elicited by target stimuli for the ongoing task and PM cues, whereas the N300 was elicited by PM cues but not by target stimuli. A similar pattern of data has been observed in a study where PM cues were embedded in a WM task. In that study, the execution of a delayed intention in response to a PM cue was again associated with the N300, whereas target recognition was associated with an increase in the amplitude of the N2 component (West et al. 2006). Taken together, the results of studies utilizing ERPs lead to the suggestion that somewhat distinct processes may contribute to the detection of PM cues and target stimuli. In the current study, we sought to replicate and extend these results by capitalizing on the enhanced spatial resolution and localization potential of fMRI. Specifically, we examined the pattern of neural activity when a given stimulus served as either a PM cue or as a target stimulus in an oddball task. An oddball task was chosen as the appropriate control condition as it allowed us to match the frequency of the PM cues and the oddball targets. Thus, any variation in the neural response to PM cues and oddball targets cannot be attributed to differences in stimulus frequency or novelty.
The current study exploited the power of a hybrid blocked/event-related fMRI design to provide a unique window into the neurocognitive processes underlying PM. We utilized a paradigm that operationalized event-based PM in a manner similar to most other experimental studies of PM wherein participants were required to monitor a stream of events for a low-frequency PM cue that occurred within the context of a demanding ongoing task, but was irrelevant to performance of the ongoing task (Brandimonte et al. 2001; West et al. 2006; McDaniel and Einstein 2007). Specifically, the PM cues were embedded in a N-back WM task, which allowed for the independent manipulation of WM load and the presence or absence of a delayed intention. The PM cues required a response that was distinct from ongoing task responses, and therefore could be behaviorally assessed for accuracy.
We were specifically interested in how the addition of a PM component influenced brain activity during performance of the N-back task. We contrasted the PM task condition with task conditions involving the manipulation of WM load or the detection of an infrequent target stimulus. To identify brain regions associated with WM, we compared high WM load conditions (NB-3Back) to low WM load conditions (NB-1Back) in the N-back task. Such load manipulations are known to produce reliable effects in a standard WM neural circuit (Braver et al. 1997; Cohen et al. 1997; Owen et al. 2005). To examine target detection processes, we used an oddball task that has been used previously to investigate the role of sustained attentional processes on target identification, in conjunction with ERP (Sutton et al. 1965; Duncan-Johnson and Donchin 1977), and fMRI (McCarthy et al. 1997; Linden et al. 1999; Braver et al. 2001) methods. Brain regions associated with PM were identified by asking participants to detect infrequent PM cues while performing an ongoing N-back task (when performed simultaneously, the task will be referred to as the PM-1Back), and comparing the results to the performance of the ongoing task (NB-1Back) or the target detection task (oddball) in isolation. By manipulating WM load independently of PM demands, we were able to directly contrast responses in the neural circuits associated with WM and PM. By including the target detection task, we were able to directly contrast patterns of neural activation associated with infrequent target detection in a more general context with those that are associated with the presentation of a PM cue.
Methods
Participants
Eighteen right-handed participants with no evidence of neurological compromise participated in the study. Participants were 7 males and 11 females with a mean age of 21.8 years (range: 19–29 years). Participants gave informed consent as per guidelines set by the Washington University Medical Center Human Studies Committee and were paid $25 for each hour of participation.
Behavioral Tasks
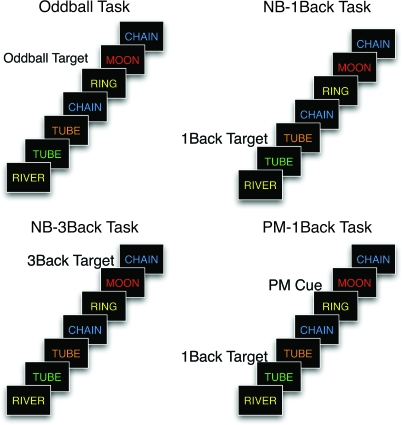
Participants observed stimuli on a display and responded using a hand-held response box. Three different tasks were performed, each involving sequences of visually presented colored words: an oddball detection task, a WM task, and a PM task (see Fig. 1).
Figure 1.
Task design. In the oddball task, an oddball target is defined as a word occurring in a color specified at the beginning of the current task block (in this case, any word appearing in red). Participants respond with their left index finger to these color cues, and with their right index and middle fingers to all other stimuli In the NB-1Back task, a 1-Back target is defined as a word stimulus that has repeated from the previous trial. Participants respond with their right index finger to these stimuli, and with their right middle finger to all other stimuli. In the NB-3Back, a 3-Back target is defined as a word stimulus that is a repeat of the word three trials previously. Participants respond with their right index finger to these stimuli, and with their right middle finger to all other stimuli. In the PM-1Back task, participants respond with their left index finger to color cues, and they perform the NB-1Back task if the current stimulus is not a color cue.
In the oddball task, a target color was specified at the beginning of each block of trials, and participants were asked to respond with their left index finger when a word stimulus occurred in the target color, and with both the index and middle fingers on their right hand when a word stimulus occurred in any other color. A key feature of the oddball task was that the oddball targets occurred with low frequency (∼10%). The use of low-frequency targets in a control task provided an important contrast to the low-frequency PM cues used in the PM task. It was important to include such a control, because target frequency plays a role in determining the cognitive processes engaged during task performance, and further, aPFC is sensitive to this variable (Rugg et al. 1996, 1999).
In the WM task, participants performed a version of an N-back task (Braver et al. 1997; Cohen et al. 1997) in which they determined whether the current stimulus matched the stimulus that occurred N trials previously. In order to manipulate WM load, N was manipulated across blocks (NB-1Back vs. NB-3Back). This manipulation of WM load influences both the importance of active maintenance processes as well as other processes such as updating, response selection, and interference resolution. A critical aspect of the design is that these processes have different temporal dynamics, and as such, differences in their time course can be informative as to their neural substrates (Cohen et al. 1997). For example, the task requires that information be maintained across trials, and therefore, active maintenance should be reflected in sustained responses. However, interference resolution processes are inherently stimulus/response driven, and therefore transient. In the NB-1Back condition, participants responded with their right index finger (target response) if the current word was identical to the one immediately preceding it (i.e., one trial back), and responded with their right middle finger (nontarget response) if the current word was not identical. In the NB-3Back condition, the target was a word that was identical to the one presented three trials back. In the N-back conditions, target trials occurred on approximately 30% of trials to maintain consistency with prior N-back studies (Braver et al. 1997; Cohen et al. 1997).
In the PM task (subsequently referred to as a PM N-Back), participants had to perform the oddball detection and WM tasks simultaneously. Participants were instructed to perform the N-back task (either 1- or 3-back; referred to as the ongoing task), unless a target color appeared, in which case they were to make a response with their left index finger. The requirement to be sensitive to the infrequent target colors while simultaneously performing an ongoing task produces a PM condition that is not present in either control condition; in either task in isolation, participants can simply focus on a given task without needing to be sensitive to additional, frequently irrelevant information (i.e., stimulus color in the N-Back or word identity in the oddball). Participant performance was poor in the PM-3Back condition, suggesting that this condition altered the strategies participants selected. Therefore, only data from the PM-1Back condition is reported here.
Tasks were performed in blocks of 36 trials. Stimuli were pseudorandom sequences of concrete one- or two-syllable words, presented centrally. The stimuli could appear in one of five colors (blue, green, magenta, red, or yellow). The word list assigned to each task condition (oddball, N-back, PM-N back) was counterbalanced across participants. Each block consisted of approximately 10 N-back targets and 4 PM cues or oddball targets. At the beginning of each block of trials, participants were presented with instructions that indicated which level of WM load (IGNORE for oddball, 1-BACK, or 3-BACK), and which color was relevant (IGNORE for N-back, BLUE, GREEN, RED, or YELLOW). Following this instructional cue (5000-ms duration), the task began. Each word was on the screen for 2000 ms and was followed by a variable intertrial interval (ITI) (to enable event-related response estimation), jittered in steps of 2.5 s (minimum ITI 500 ms, with the number of jitters sampled from a geometric distribution with P = 0.6). Participants performed four repetitions of each of the 5 types of blocks.
Prior to the scanning session, participants were given instructions and practice for all tasks to be performed. During practice trials, the experimenter answered any further questions, validated that the instructions were understood, and ensured that the tasks were performed appropriately.
Functional Imaging
Images were acquired on a Siemens 1.5 Tesla Vision System (Erlangen, Germany) with a standard circularly polarized head coil. A pillow and tape were used to minimize head movement. Headphones dampened scanner noise and enabled communication with participants. Both structural and functional images were acquired at each scan. High-resolution (1.25 × 1 × 1) structural images were acquired using a sagittal MP RAGE 3D T1-weighted sequence (time repetition [TR] = 9.7 mm, time echo [TE] = 4, flip = 12°, inversion time [TI]= 300 ms) (Mugler and Brookeman 1990). Functional images were acquired using an asymmetric spin-echo echo-planar sequence (TR = 2500, TE = 50 ms, flip = 90°). Each image consisted of 16 contiguous, 8-mm thick axial slices acquired parallel to the anterior–posterior commissure plane (3.75 × 3.75 mm in-plane), allowing for complete brain coverage. Each run consisted of alternating cycles of fixation (A) and task (B) blocks in an ABABA design. The inclusion of fixation blocks was a feature of the scanning design that enabled on the decomposition of sustained and transient effects (Visscher et al. 2003). Task blocks were 165 s long, whereas Fixation blocks (denoted by a centrally presented crosshair) were 37.5 s in duration. The first four images in each scanning run were discarded used to allow the scanner to stabilize. Each run lasted approximately 7.5 min, and a 2-min delay occurred between runs, during which time participants rested.
Visual stimuli were presented using PsyScope software (Cohen et al. 1993) running on an Apple PowerMac G4. Stimuli were projected onto a screen positioned at the head end of the bore with an AmPro LCD projector (model 150). Participants viewed the screen through a mirror attached to the head coil. A fiber-optic, light-sensitive key press interfaced with the PsyScope Button Box was used to record participants' behavioral performance.
Data Analysis
Behavior
Behavioral data were analyzed to identify specific effects that could be attributed to increases in WM load or PM task demands. Therefore, target and nontarget trials from the NB-1Back condition were used as a baseline. We investigated WM load by comparing NB-1Back to NB-3Back [2 (WM load) × 2 (trial type) repeated-measures ANOVA]. We investigated PM task demands by comparing NB-1Back to PM-1Back [2 (PM task demand) × 2 (trial type) repeated-measures ANOVA]. Additionally, we performed a direct comparison between the high-load conditions [2 (NB-3Back vs. PM-1Back) × 2 (trial type) repeated-measures ANOVA] in order to examine relative effects of task difficulty. The effects of PM task demands on target detection were investigated by comparing performance on PM cue trials during the PM-1Back to performance on oddball-targets during the oddball task (using a two-sample paired t-test). Because these two trial types were directly matched in stimulus parameters and task demands, any differential behavioral effects could be attributed to the addition of the ongoing task. Where appropriate, standard error (SE) and mean-squared error (MSE) are reported.
Imaging
Functional imaging data were preprocessed prior to statistical analysis according to the following procedures: 1) functional slices were temporally aligned to account for timing differences during acquisition; 2) corrected for movement using a rigid-body rotation and translation (Friston et al. 1996; Snyder 1996); 3) scaled to achieve a whole-brain mode value of 1000 for each scanning run; 4) registered to the participants' structural image following transformation of the structural image into the 711-2Y standardized atlas space (Talairach and Tournoux 1988; Ojemann et al. 1997; Buckner et al. 2004), using a 12-dimensional affine transformation (Woods et al. 1992, 1998); 5) spatially interpolated to create 3-mm isotropic voxels; and 6) spatially smoothed with a 9-mm FWHM Gaussian kernel.
A general linear model (GLM) approach (Friston, et al. 1995) was used to estimate parameter values for both sustained and event-related responses. Event-related effects were analyzed by estimating values for the various time points within the hemodynamic response epoch. The duration of this epoch was taken to be 20 s (8 scanning frames). Each trial type (targets, nontargets, and color targets) within each task was modeled with a separate set of 8 regressors. After estimating each time course of activity, the magnitude of each response was computed by cross-correlating each of the time courses with a contrast derived by convolving a boxcar function lasting 2.5 s with a standard hemodynamic response function. Sustained effects were estimated by including regressors modeling the difference between blocks of task and blocks of fixation (convolved with a standard Hemodynamic Response Function). Regressors of no interest to the current questions were included at the beginning and ending of each task block. These regressors were included to account for transition effects that are frequently seen within blocked fMRI experiments, as we did not want them to contribute to the detection of sustained responses (Konishi et al. 2001; Fox et al. 2005; Dosenbach et al. 2006). Additionally, intercept and linear trend covariates were included for each blood oxygenation level–dependent (BOLD) run in order to eliminate mean differences and drift effects within each run. No additional high-pass filtering was performed, as it was likely that high-pass filtering would remove signal associated with the relatively low-frequency sustained effects, and based on simulation, noise with a 1/f distribution is unlikely to covary with sustained regressors (Visscher et al. 2003). Errors were modeled as separate sets of regressors so transient responses reflected only responses to correct trials.
After computing these GLMs for each individual, random-effects group level analyses were performed on the parameter estimates. The group level statistical analysis involved the simultaneous application of multiple contrasts, where each thresholded contrast constitutes a mask, and voxels are identified via the intersection of all masks (Friston et al. 2005; Price and Friston 1997). The inclusion of multiple contrasts (with each set at a relatively liberal threshold) helps to balance the trade-off between power and false-positive protection (i.e., Type II vs. Type I error). The idealized overall α rate for a set of conjunctions was kept constant across conjunctions at P < 0.001 (However, this is likely an underestimate of the actual α level, as each of the masks are not independent). In order for a region of interest (ROI) to be accepted as sensitive to the effect of interest, all voxels within the region were required to meet criterion in all tests (described below). Moreover, an ROI was considered significant only if it contained a cluster of 8 or more contiguous voxels, to increase false-positive protection (Forman et al. 1995; McAvoy et al. 2001).
The set of contrasts used to identify processes involved in PM were employed to insure that there was positive activity during PM-1Back that was distinct from both the demands of the ongoing task (N-back) and the demands of color target detection (oddball). Conceptually, there may be at least two ways in which PM task demands may influence processing: 1) engaging sustained processes that are present across trials; or 2) engaging transient, ongoing processes that are stimulus locked. In order to identify these two potential neural correlates of PM performance, we performed two parallel analyses that identified either sustained or transient, ongoing changes in activation associated with PM. To identify sustained PM activation, each voxel had to meet the following criteria:
Significant sustained response in the PM-1Back relative to fixation
Significantly increased sustained response in the PM-1Back relative to NB-1Back
Significantly increased sustained response in the PM-1Back relative to the oddball task
To identify transient, ongoing PM activation, each voxel had to meet the following criteria:
Significant transient response to the target and nontarget trials in the PM-1Back relative to fixation
Significantly increased transient response to the target and nontarget trials in the PM-1Back relative to target and nontarget trials within the NB-1Back
Significantly increased transient response to nontarget trials in the PM-1Back relative to the nontarget trials within the oddball detection task
We also investigated the processes elicited by the presence of a PM cue in the PM task. The set of contrasts employed for this analysis insured that there was positive activity to PM cues in the PM-1back task, and that this activation was distinct from the both the activation present to ongoing (N-back target and nontarget) trials, and from equivalent low-frequency color target trials that occur within the context of the oddball task. To identify selective PM cue-related activation, each voxel had to meet the following criteria:
Significant transient response to PM cues in the PM task relative to fixation
Significantly increased transient response to PM cues relative to both nontarget and target trials within the PM-1Back task
Significantly increased transient response to PM cues in the PM task relative to low-frequency color targets within the oddball task
Significantly increased difference between the difference between PM cue and NB-nontargets within the PM-1Back task and the difference between oddball targets and nontargets within the oddball task (i.e., condition × trial-type interaction; ensures that the PM cue response is selective to the PM-1Back).
In addition to identifying ROIs associated with PM task demands, a parallel set of analyses was used to identify sustained or transient, ongoing ROIs associated with WM load. In that set of analyses, the NB-3Back was the experimental condition parallel to the PM-1Back condition in the above contrasts. We conducted an overlap analysis to identify those voxels demonstrating WM load effects in the PM analyses, and vice versa. Thus, we attempted to identify 6 networks: 2 networks demonstrating either sustained or transient, ongoing responses to PM task demands but not WM load, 2 networks demonstrating either sustained or transient, ongoing responses to WM load but not PM, and 2 networks demonstrating either sustained or transient, ongoing responses to both PM and WM load.
ROIs identified through these procedures were then subjected to one further constraint; this constraint validated that all effects tested in the voxel-wise conjunction analysis were statistically significant (P < 0.05) at the ROI level. All reported results correspond to the effects identified at the level of the ROI, and all regions described below passed this test. For ROI analyses (and Figs 4– 6), data are expressed in terms of mean percent signal change relative to the fixation trials within a task block.
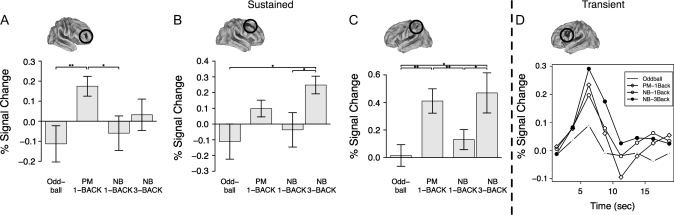
Figure 4.
(A–C) The mean sustained BOLD response for ROIs (A), (B), and (C), respectively. (D) The mean transient, ongoing BOLD responses for ROI D. The y-axis reflects % signal change relative to resting block fixation trials. Within (A–C), moving from left to right, each bar represents the response to the oddball, PM-1Back, NB-1Back, and NB-3Back conditions, respectively. Within (D), each line represents the estimated time course for the average trials within each condition, with symbol reflecting each task (no symbol: oddball, diamonds: PM-1Back, open circles: NB-1Back, filled circles: PM-1Back). For the oddball task, the time course reflects nontarget trials, whereas the time course for all other conditions reflects the average of nontarget and (NB) target trials. Inset surfaces illustrate the ROI represented by the mean responses (ROIs appearing in black and circled). Lines and stars at the top of each bar graph reflect significant contrasts at the level of the ROI: *P < 0.05, **P < 0.01.
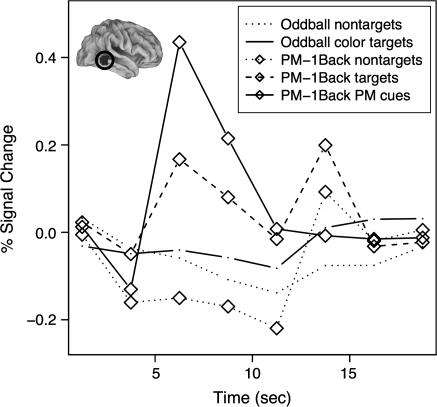
Figure 5.
Pattern of response in a region of right middle temporal cortex. Each line represents the estimated time course within each condition, with symbols representing task (no symbol: oddball task, diamond: PM-1Back) and line style representing trial type (dotted: nontargets, dashed: NB targets, solid: color targets/PM cues). This region shows a selectively increased response to PM cues in the context of a PM task (see Table 3).
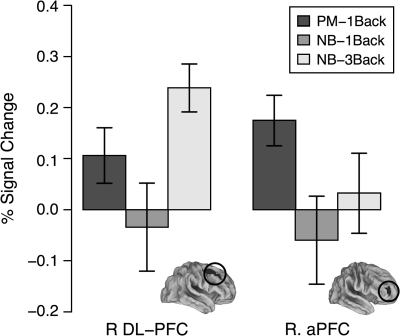
Figure 6.
Pattern of responses to PM and WM load within R. aPFC and R. DL-PFC. These areas were statistically dissociable F2,34 = 6.08, P = 0.006, indicating that the two different regions were associated with different aspects of each task. Focused contrasts revealed that this effect was due to differential responses to NB-3Back and PM-1Back blocks across regions (F1,17 = 7.9, P = 0.012). Specifically, R DL-PFC demonstrated an increased response in the NB-3Back condition relative to the PM-1Back condition (t(17) = 2.08, P = 0.026, one-tailed), whereas R aPFC demonstrated an increased response in the PM-1Back condition relative to the NB-3Back condition (t(17) = 1.83, P = 0.043, one-tailed).
Interactive Effects within aPFC ROIs Sensitive to PM Task Demands
Ramnani and Owen (2004) suggested that a strong test of the hypothesis that aPFC is involved in managing multiple task goals is to determine whether it actually demonstrates a significant overadditive interaction as a function of conjoining multiple task demands. The current design allows for the investigation of such an interaction. If the two demands are conceptualized as two dimensions, one dimension corresponding to target detection processes required by the oddball task relative to fixation, and the other dimension corresponding to WM processes required by the NB-1Back task relative to fixation, then the interaction of these two processes is expressed as:
| (1) |
Because all hemodynamic responses are estimated relative to fixation, the fixation term becomes 0, and the estimate of the interaction becomes:
| (2) |
Thus, any ROIs demonstrating a pattern in which its response in the PM-1Back condition statistically exceeds the sum of its responses in the NB-1Back and oddball conditions would demonstrate the requisite interaction. This contrast was therefore tested within each of the ROIs identified as being sensitive to PM task demands.
Statistical Dissociation of Regions Sensitive to WM and PM Task Demands
To investigate whether the functional contributions of the ROIs associated with PM were statistically dissociable from the effects of WM load, the pattern of activity in each of the sustained PM ROIs was statistically compared with that seen in a right dorsolateral PFC (DL-PFC) ROI identified as being sensitive to WM load (also in terms of sustained response). The right DL-PFC ROI was selected as representative of the canonical WM load effect, as the responses of this region have been replicated across several previous experiments, and it is often considered to be a core region associated with active maintenance of task-goal information during WM (Braver et al. 1997; Cohen et al. 1997; Owen et al. 2005). The presence of a difference between patterns of activity across ROIs would increase the ability to interpret the role of ROIs associated with WM and PM. In order to perform this analysis, the mean sustained responses for the DL-PFC ROI were compared with the sustained responses in each PM ROI with a repeated-measures ANOVA with condition (NB-1Back, NB-3Back, and PM-1Back) and region (PM ROI, DL-PFC ROI) as factors of interest.
Brain–Behavior Relationships
A final analysis was used to test for a significant association between the activity pattern in a given ROI and between-subject variation in task performance. This analysis was a two-stage process, in which zero-order correlations were first investigated in order to determine whether identified ROIs demonstrated a relationship between their responses and behavior (e.g., ROIs associated with PM task demands were correlated with performance on trials during the PM condition). If a significant correlation was found, a second stage analysis was conducted that tested whether the zero-order correlation was selective for that particular condition. In order to determine whether the effect was selective, the relationship between RT and BOLD response in the PM-1Back condition was compared directly to the relationship between RT and BOLD response in the NB-1Back condition using the Z-Based Pearson-Filon (ZPF) statistic (Raghunathan et al. 1996, threshold: ZPF = 1.65, P < 0.05). A statistically reliable difference between the two correlations indicated selectivity. Similarly, in order to determine whether an effect was selective for PM cues, the relationship between RT and BOLD response in the PM-1Back condition was compared directly to the relationship between RT and BOLD response in the oddball condition using the same ZPF statistic.
An outlier analysis was performed in order to identify those observations whose distance from the closest quartile was more than 3 times the interquartile range (this is a conservative application of box-and-whisker plots; Chambers et al. 1983) or had a relative large amount of influence in determining the relationship between RT and % signal change (scaled leverage values > 1.96). Three participants were identified as contributing observations that were consistently deviant from the rest of the population and were removed from the brain–behavior analyses. Thus, analyses regarding the brain–behavior relationships for the PM-1Back task are reported for the remaining 15 participants. The same analyses were also conducted with regard to brain–behavior effects related to WM load. Due to space constraints, these analyses are reported in Supplementary Materials.
Results
Behavior
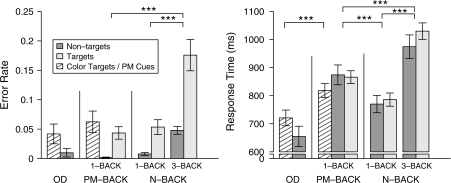
PM task demands had a clear impact on performance, replicating previous findings of a prospective interference effect. The addition of the PM component slowed RT for N-back targets and nontargets by 92 ms on average, F1,17 = 54.6, P < 0.001, MSE = 2790. This slowing did not differ significantly between nontargets and targets (104 ms increase on nontargets, 80 ms increase on targets; 2-way interaction: F1,17 = 1.7, P = 0.2, MSE = 1563). In contrast, error rates on N-back targets and nontargets were not influenced by the addition of the PM component (0.8% decrease across both trial types; nonsignificant main effect and interaction: F1,17 < 1, MSE < 0.001). The effect on RT suggests that the addition of PM task demands led to the recruitment of cognitive operations that influenced the ongoing processing of N-back task stimuli.
Increasing WM load in the N-back task also impacted behavioral performance. Comparing performance in the 3-back and 1-back conditions revealed an increase in error rates for both nontarget and target trials (8.6 % increase across both trial types; F1,17 = 53.3, P < 0.001, MSE = 0.003; see Fig. 2). N-back load interacted with trial type (F1,17 = 10.5, P = 0.005, MSE = 0.002) with the effect of WM load being greater for target trials (12.2%) than for nontarget trials (5.0%). An increase in WM load also slowed RT to both trial types (224 ms increase across both trial types; F1,17 = 96.5, p < 0.001, MSE = 9363), but unlike the error rates, did not differentially affect targets (204 ms increase on nontargets, 243 ms increase on targets; load by trial interaction: F1,17 = 1.0, MSE = 6978). The effect of WM load on both errors and RTs demonstrates that the manipulation was successful: the NB-3Back condition was more demanding than the NB-1Back condition. Furthermore, the increased error rates associated with WM load, but not PM task demands, suggest that different processes may contribute to these two effects.
Figure 2.
Error rate and RT for each condition. Error rates (left panel) increased as a function of WM load, but not PM task demands. RT (right panel) increased as a function of WM load as well as PM task demands. Stars indicate significant effects of PM task demands and WM load (***P < 0.001).
A direct comparison of the two high-load conditions (PM-1Back vs. NB-3Back) revealed that greater error rates and increased RTs were observed in NB-3Back condition (9.4% increase in error rates across trial types; F1,17 = 38.6, P < 0.001, MSE = 0.002; 132 ms average increase in RT across trial types; F1,17 = 23.9, P < 0.001, MSE = 13125). As such, performance was faster and more accurate in the PM-1Back than the NB-3Back, and consequently, difficulty confounds do not pose a problem when interpreting regions where activity is greater for the PM task than the NB task.
Analysis of error rates for PM cues in the PM-1Back relative to targets in the oddball task revealed a nonsignificant difference between the two conditions (2% increase in PM vs. oddball; t(17) = 0.96, P = 0.35, SE = 0.02). In contrast, participants were slower to identify PM cues than oddball targets (98 ms increase; t(17) = 4.4, P < 0.001, SE = 22). This difference in RT indicates that the presence of an additional ongoing task in the PM condition slowed target detection.
Imaging
Sustained Responses to PM Task Demands
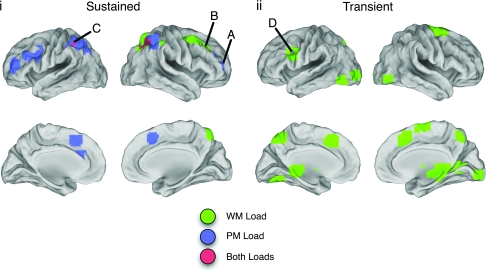
Ten ROIs demonstrated a sustained increase in activity associated with PM task demands that were not activated in relation to WM load. This network included bilateral regions of aPFC (BA 10), more posterior regions of PFC, anterior cingulate cortex, and bilateral parietal cortex (see Figs 3 and 4 and Table 1).
Figure 3.
Panel i illustrates ROIs demonstrating sustained responses to PM load, WM load, or both. Panel ii illustrates ROIs demonstrating transient, ongoing responses to WM load. ROIs in blue demonstrated an increased BOLD response to PM task demands (either sustained or transient ongoing, as indicated by the panel). ROIs in green demonstrated an increased BOLD response to WM load. ROIs in red demonstrated an increased response to PM and WM loads. Labels correspond to ROI labels from Tables 1 and 2.
Table 1.
ROIs displaying sustained responses
| BA | Size (mm3) | Center of mass |
PM-1Back task |
NB-3Back task |
Diss. from | |||||||
| x | y | z | Fixation | Oddball | NB-1Back | Fixation | Oddball | NB-1Back | R DL-PFC | |||
| t(17) | t(17) | t(17) | t(17) | t(17) | t(17) | F(2,34) | ||||||
| ROIs sensitive to PM load in a sustained fashion | ||||||||||||
| L. ant. PFC | 10/46 | 1863 | −29 | 43 | 12 | 5.18*** | 4.01*** | 3.53** | 1.99$ | 2.12* | 1.51 | 2.95$ |
| R. ant. PFC (A) | 10/46 | 675 | 28 | 46 | 9 | 3.53** | 3.02** | 2.8* | 0.41 | 1.2 | 1.11 | 6.08** |
| R. DL-PFC | 46/9 | 216 | 29 | 34 | 22 | 3.37** | 2.8* | 2.42* | 1.78$ | 1.21 | 1.45 | 4.52* |
| L. DL-PFC | 46/9 | 1944 | −29 | 20 | 25 | 4.46*** | 3.13** | 3.46** | 1.63 | 1.19 | 1.43 | 10.88*** |
| L. post. DL-PFC | 6/8 | 324 | −31 | 4 | 39 | 3.51** | 2.35* | 2.39* | 2.46* | 1.81$ | 1.69 | 7.18** |
| ACC | 32/6 | 972 | −3 | 17 | 45 | 4.05*** | 2.66* | 2.5* | 2.75* | 1.99$ | 1.5 | 0.74 |
| L. lat. par. lobe | 7/40 | 6156 | −36 | −53 | 44 | 4.17*** | 3.52** | 3.23** | 2.21* | 1.98$ | 1.77$ | 0.49 |
| R. inf. par. lobe | 7/40 | 1944 | 36 | −54 | 46 | 3.54** | 3.58** | 2.79* | 2.45* | 2.14* | 1.68 | 0.54 |
| L. cerebellum | NA | 243 | −12 | −68 | −31 | 3.03** | 2.85* | 2.95** | 2.22* | 2.22* | 1.68 | 8.58*** |
| R. cerebellum | NA | 243 | 32 | −62 | −30 | 2.89* | 3.63** | 2.58* | 1.18 | 1.67 | 1.58 | 5.93** |
| ROIs sensitive to WM load in a sustained fashion | ||||||||||||
| R. DL-PFC (B) | 9/8 | 243 | 31 | 23 | 38 | 1.87$ | 1.65 | 2.66* | 4.33*** | 2.3* | 2.77* | — |
| R. post. DL-PFC | 6/8 | 729 | 34 | 5 | 52 | 1.86$ | 1.92$ | 1.58 | 4.43*** | 2.78* | 2.61* | — |
| R. sup. par. lobe | 7/19/40 | 3105 | 30 | -65 | 50 | 1.29 | 2.94** | 0.02 | 3.38** | 3.9** | 3.16** | 3.04$ |
| R. sup. par. lobe | 7/40 | 324 | 50 | −45 | 50 | 1.71 | 1.13 | 0.51 | 3.36** | 2.5* | 2.74* | 0.91 |
| L. inf. cerebellum | NA | 270 | −38 | −59 | −43 | −0.23 | 0.68 | 0.05 | 3.39** | 3.55** | 2.71* | 1.13 |
| ROIs sensitive to both PM and WM load in a sustained fashion | ||||||||||||
| L. sup. par. lobe (C) | 7/40 | 432 | −44 | −51 | 51 | 4.64*** | 3.32** | 3.24** | 3.22** | 2.44* | 2.56* | 1.52 |
| R. sup. par. lobe | 7/40 | 351 | 32 | −63 | 43 | 3.75** | 3.06** | 3.34** | 3.07** | 2.77* | 2.43* | 0.51 |
Note: Statistics in columns 7−12 reflect t-statistics for each direct comparison, whereas the last column reflects the F statistic reflecting the ROI × condition interaction. Parentheses next to a region name indicate a label for an illustrated ROI in Figures 3 and 4. Abbreviations: L = left; R = right; Ant = anterior; Inf. = inferior; Lat. = lateral; Post. = posterior; Sup. = superior. ***P < 0.001, **P < 0.01, *P < 0.05, $P < 0.1
Sustained Responses to WM Load
Five ROIs demonstrated an increased sustained response associated with WM load that were not activated in relation to PM task demands. This network included regions within right DL-PFC, the cerebellum, and right parietal cortex (see Figs 3 and 4 and Table 1). Further interrogation of the region in R DL-PFC revealed that it contained two peaks of activation. Separate ROIs around each peak were created to determine whether the two ROIs demonstrated different patterns of responses (see Table 1). The two ROIs exhibited very similar responses, and a direct comparison between the two ROIs revealed that there was neither a main nor an interactive effect of ROI on the BOLD response (both F < 1). Because there were no differences between the smaller ROIs, the larger ROI was retained for further analysis in order to reduce noise in the parameter estimates.
Sustained Effects of WM and PM
Two ROIs located in bilateral posterior parietal cortex demonstrated sustained responses to the increase in WM load and the addition of a PM component (see Figs 3 and 4 and Table 1).
Transient, Ongoing Responses during PM
No ROIs demonstrated a selective transient response to ongoing trials (N-back targets and nontargets) during the PM-1Back. This finding indicates that it is unlikely that individuals were engaged in item checking (Guynn 2003).
Transient Responses to Increased WM Load
Seventeen ROIs demonstrated a selective increased transient, ongoing response for the NB-3Back condition relative to the NB-1Back condition. This widespread and diffuse network included large regions of activation within left posterior PFC, the striatum, the anterior cingulate cortex, and bilateral extrastriate cortex (see Table 2 and Figs 3 and 4).
Table 2.
ROIs displaying transient, ongoing responses
| BA | Size (mm3) | Center of mass |
PM-1Back task |
NB-3Back task |
|||||||
| x | y | z | Fixation |
Oddball |
NB-1Back |
Fixation |
Oddball |
NB-1Back |
|||
| t(17) | t(17) | t(17) | t(17) | t(17) | t(17) | ||||||
| ROIs sensitive to PM load in a transient fashion | |||||||||||
| — | |||||||||||
| ROIs sensitive to WM load in a transient fashion | |||||||||||
| L inf. PFC (D) | 44 | 999 | −47 | 6 | 27 | 3.54** | 2.5* | 0.22 | 4.09*** | 2.73* | 2.81* |
| L. caudate | NA | 621 | −16 | 2 | 5 | 2.97** | 1.21 | −0.11 | 7.12*** | 3.84** | 2.7* |
| R. caudate | NA | 702 | 19 | 5 | 14 | 2.72* | 0.38 | −1.85$ | 5.66*** | 4.05*** | 2.82* |
| ACC | 32/6 | 1404 | 0 | 19 | 44 | 1.9$ | 0.39 | −0.96 | 3.55** | 2.39* | 3.08** |
| R. premotor | 6 | 1134 | 26 | −2 | 63 | 4.31*** | 2.48* | 0.87 | 3.24** | 3.1** | 4.34*** |
| R. premotor | 6 | 540 | 8 | −7 | 63 | 5.21*** | 1.35 | −0.2 | 3.74** | 2.45* | 3.3** |
| Precuneus | 7 | 1512 | −4 | −65 | 50 | 3.11** | 1.44 | 2.21* | 4.38*** | 3.69** | 4.25*** |
| L. extrastriate | 18 | 567 | −30 | −91 | −6 | 7.39*** | −0.82 | −0.07 | 13.22*** | 3.26** | 3.47** |
| L. extrastriate | 18/19 | 621 | −44 | −68 | −19 | 5.69*** | 0.48 | 1.03 | 6.21*** | 2.82* | 3.14** |
| R. estrastriate | 18 | 540 | 20 | −60 | 3 | 1.27 | 0.81 | −0.04 | 4.34*** | 4*** | 2.48* |
| R. extrastriate | 17/18 | 216 | 16 | −88 | −8 | 7.36*** | 0.82 | 0.48 | 8.27*** | 2.86* | 2.7* |
| R. extrastriate | 18/19 | 297 | 48 | −76 | −11 | 3.81** | −0.64 | 0.15 | 9.37*** | 2.88* | 2.89* |
| L. fusiform gyrus | 18/19 | 1296 | −24 | −65 | −21 | 6.03*** | 1.26 | −0.06 | 5.72*** | 3** | 2.97** |
| Reticular form. | NA | 5211 | 2 | −32 | −6 | 4.21*** | −0.1 | −0.85 | 5.01*** | 2.86* | 3.64** |
| L. ant. cerebellum | NA | 432 | −12 | −54 | −47 | 3.89** | 1.47 | 0.77 | 4.05*** | 2.84* | 2.21* |
| L. lat. cerebellum | NA | 270 | −36 | −63 | −45 | 2.33* | 0.34 | 1.75$ | 3.98*** | 2.56* | 2.93** |
| Post. cerebellum | NA | 1026 | −3 | −78 | −41 | 3.34** | 0.09 | 1 | 5.21*** | 3.1** | 3** |
| ROIs sensitive to both PM and WM load in a transient fashion | |||||||||||
| — | |||||||||||
Note: No ROIs were identified as sensitive to PM task demands in a transient, ongoing fashion. Statistics in the last 6 columns reflect t-statistics for each direct comparison. Parentheses next to a region name indicate a label for an illustrated ROI in Figures 3 and 4. Abbreviations: L = left; R = right; Ant = anterior; Inf = Inferior; Lat. = lateral; Post = posterior; ACC = anterior cingulate cortex; PFC = prefrontal cortex. ***P < 0.001, **P < 0.01, *P < 0.05.
Transient, Ongoing Responses to Increases in WM Load and PM Demands
No ROIs demonstrated an increased transient, ongoing response to both WM load and PM task demands.
PM Cue Effects
One ROI in the right middle temporal gyrus demonstrated a selective transient response to PM cues (see Table 3 and Fig. 5). This response was greater on PM cues occurring in the PM-1Back condition than both equivalent target events in the oddball condition and N-back targets in the PM-1Back condition. This suggests that the responses to the PM cues are distinct, and not attributable to low-frequency color stimuli or other target events.
Table 3.
ROI displaying a cue-specific response
| BA | Size (mm3) | Center of mass |
PM cue relative to: |
PM cue vs. PM nontarget relative to: |
||||||
| x | y | z | Fixation | PM-1Back nontarget | PM-1Back target | Oddball PM cues | Oddball PM cue vs. oddball nontarget | |||
| t(17) | t(17) | t(17) | t(17) | t(17) | ||||||
| R. middle temp. gyrus | 21/37 | 459 | 54 | −43 | −5 | 3.38** | 3.76** | 2.57* | 2.95** | 2.91** |
Note: Statistics in the last 5 columns reflect t-statistics for each direct comparison. Abbreviations: R = right; Temp. = temporal. ***P < 0.001, **P < 0.01, *P < 0.05.
Interactive Effects within aPFC ROIs Sensitive to PM Task Demands
Both aPFC ROIs displaying significant sustained responses to PM task demands also demonstrated a greater response when testing for the presence of an overadditive interaction including the WM and oddball conditions (left aPFC: t(17) = 2.7, P = 0.008; Right aPFC: t(17) = 2.35, P = 0.02). Only two of the ROIs that were sensitive to PM task demands in a sustained fashion did not demonstrate a significant interaction contrast, and both approached significance (L pDL-PFC: t(17) = 1.69, P = 0.055; anterior cingulate cortex [ACC]: t(17) = 1.64, P = 0.06, one-tailed).
Dissociable Effects of WM load and PM Task Demands
The patterns of sustained responses within aPFC and DL-PFC were directly compared using an ANOVA with region and condition as factors. The right aPFC ROI was found to be statistically dissociable from right DL-PFC (region × condition interaction; F2,34 = 6.08, P = 0.006), whereas the dissociation within L aPFC approached significance (F2,34 = 2.95, P = 0.066). Focused contrasts revealed that this effect was due to differential responses in the NB-3Back and PM-1Back blocks across regions (F1,17 = 7.9, P = 0.012). Specifically, R DL-PFC demonstrated an increased response in the NB-3Back condition relative to the PM-1Back condition (t(17) = 2.08, P = 0.026, one-tailed), whereas R aPFC demonstrated an increased response in the PM-1Back condition relative to the NB-3Back condition (t(17) = 1.83, P = 0.043, one-tailed). One potential concern is that the NB-3Back condition is more difficult than the PM-1Back condition, and as such, the increased response within R DL-PFC may be attributable to a confound of task difficulty. However, this potential explanation cannot account for the increased response for the PM condition in R aPFC. Given that R DL-PFC shows monotonic effects of WM load in the N-Back task (Braver et al. 1997), it is likely that a similar result would have been obtained even if a more subtle manipulation of WM load had been employed (e.g., using the NB-2Back rather than NB-3Back). This dissociation suggests that the increase in sustained activation during PM conditions cannot be easily interpreted as being due to the same active maintenance processes that occur with increases in WM load.
Five additional PM ROIs were also dissociated from right DL-PFC (see Table 1). Consistent with Cohen et al. 1997, only R DL-PFC was activated in a sustained fashion to WM, whereas distinct bilateral areas of DL-PFC and aPFC responded to PM. Three areas showing sustained PM effects did not dissociate from R DL-PFC (at P < 0.10): ACC and bilateral inferior parietal cortex. Interestingly, these are regions that have typically been found to be sensitive to WM load in prior studies (Cohen et al. 1997; Owen et al. 2005). We provide a possible interpretation of this issue in the Discussion section.
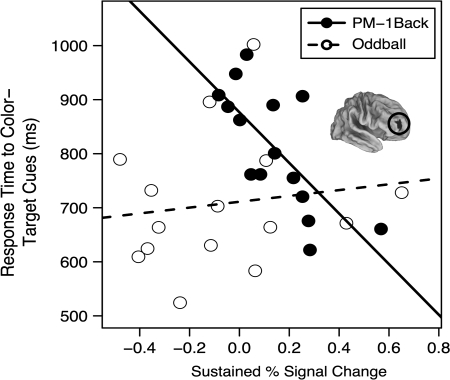
Brain–Behavior Relationships in the PM Task
Three ROIs demonstrating sustained responses to PM task demands also demonstrated significant negative zero-order correlations between the sustained response within the PM-1Back task and RT on trials within that task (no ROIs demonstrated positive correlations with RT). Two ROIs (left lateral parietal cortex and left aPFC) demonstrated a significant negative correlation between the sustained response within the PM-1Back condition and RT on nontarget trials (left parietal: r = −0.50, P = 0.03; left aPFC r = −0.47, P = 0.035, one-tailed). This effect was not selective in the parietal ROI, as a similar relationship (albeit nonsignificant) also existed in the NB-1Back task (non significant ZPF; left parietal: ZPF = 0.55, P = 0.58). However, this effect was selective to the PM condition within left aPFC (ZPF = 2.14, P = 0.015). In R aPFC, a correlation between sustained activity and RT on PM cue trials was observed, such that greater sustained activity was associated with faster responses to PM cues (r = −0.71, P < 0.001; left aPFC also demonstrated a similar relationship: r = −0.50, P = 0.03). In both left and right aPFC, this relationship was selective to the PM task, as increased sustained responses in the oddball task tended to be associated with slowed RT on color target trials (see Fig. 6; ZPF for left aPFC = 1.75, P = 0.04; ZPF for right aPFC = 2.45, P = 0.005). This indicates that for the exact same stimuli and responses (i.e., target button presses made to low-frequency color targets), aPFC activation levels only predicted performance when these stimuli served as PM cues. Two areas were identified as demonstrating significant positive correlations between their sustained activity and error rates on target trials (ACC: r = 0.50, P = 0.05; L pDL-PFC: r = 0.62, P = 0.005). This relationship was consistent across tasks within the ACC (ZPF = 0.2), but was selective for the area of L pDL-PFC (ZPF = 2.07, P = 0.02). Further supporting the dissociation between the WM and PM brain networks, some of the regions selectively sensitive to WM load (e.g., inferior PFC) demonstrated relationships with behavioral performance selectively during the high WM load N-back condition (e.g., NB-3back). These results are presented in detail within the supplementary materials section.
Figure 7.
Correlations between sustained activity and RT to color cues in the PM-1Back and oddball conditions for the ROI in right aPFC (after controlling for outliers). The correlation was significant in the PM-1Back task (r = −0.71, P = 0.003), but not in the oddball task (r = 0.14, P = 0.62). There was a significant difference between the correlations in the two conditions (ZPF = −2.45, P = 0.007). Lines correspond to the least-squares regression line for each condition.
Discussion
The goal of the current study was to investigate three questions regarding the functional neuroanatomy of PM: 1) Is PM supported by processes that are associated with sustained or transient neural recruitment? 2) Do common or distinct processes support sustained processing in PM and WM? and 3) Do common or distinct processes support target detection in PM and sustained attention tasks? The key finding of the study was that PM task demands and WM load were associated with sustained activity in statistically dissociable networks. Specifically, PM was associated with sustained activity in a network that included bilateral aPFC, whereas WM was associated with activation in a network that included DL-PFC. These data extend previous findings that have identified activation within aPFC during a PM task (Burgess et al. 2001) by demonstrating that the recruitment of aPFC was sustained over trials, and further, is distinct from activation related to active maintenance in WM and sustained attention. This set of data addresses the first two questions of interest and provide direct evidence regarding the nature of cognitive processing supporting PM. Specifically, the data indicate that PM elicits sustained neural responses that can be distinguished from sustained responses associated with variation in WM load. Very little existing data speaks to whether PM and WM are dissociable processes (West et al. 2007), and as such, these data are valuable in developing constraints on understanding the control processes underlying PM. Our data suggest that the behavioral costs associated with manipulations such as embedding dual-task or task-switching paradigms within a PM task are not due to simple increases in WM maintenance demands, but may be due to other aspects of the task, such as the increased need to monitor the environment for task-updating signals (Marsh and Hicks 1998; Marsh, Hancock, et al. 2002; McNerney and West, forthcoming). This serves as a key result in the development of our understanding of the mechanisms underlying PM.
The current study also reveals a number of additional novel results. First, the sustained responses observed in left and right aPFC were selectively associated with RT for responses to PM cues within the PM task. Sustained activity within these regions was not correlated with RT for target detection in the oddball task. These data suggest that aPFC supports a sustained process that may serve to maintain an intention in an accessible state, but only under conditions for which the focus of attention is diverted elsewhere (i.e., toward the performance of a separate ongoing task). Under standard conditions in which task-related intentions can be held within the focus of attention, the aPFC might not be playing such a critical role in guiding behavior. Thus, these results are consistent with the idea that the aPFC helps to enable “cognitive branching”—the ability to defer task goals or intentions during a period while a primary task or subgoal is carried out (Koechlin et al. 1999; Braver and Bongiolatti 2002; Koechlin and Hyafil 2007; Koechlin and Summerfield 2007; Reynolds et al. 2006). Second, transient responses in right temporal cortex were selectively associated with the presentation of PM cues. This response to PM cues was selective in that it did not occur to targets within the ongoing task, or when there was no additional ongoing task to be performed (i.e., in the context of the oddball task). As such, this serves to address our third primary question of interest. These data suggest that right temporal cortex ROI was uniquely associated with target detection within the context of PM, and therefore, provide additional support for the hypothesis that unique target detection processes are recruited during the performance of PM tasks (West and Wymbs 2004; West et al. 2006).
The Role of aPFC in PM
Several previous studies have implicated aPFC in the performance of PM tasks (Burgess et al. 2001, 2003; Simons et al. 2006). However, the current study is the first to demonstrate 1) that the responses within aPFC are sustained across multiple trials, and 2) that this sustained response is associated with the efficiency of PM. Further, the current data demonstrate that the response to PM task demands in aPFC reflects an overadditive interaction between the two components that make up the PM task. As such, these data meet the stringent criteria posed by Ramnani and Owen (2004) for the identification and characterization of aPFC responses, and provide additional constraints in the interpretation of the role played by aPFC in higher cognition.
Burgess et al. (2001) suggest that there are at least three possible functions that could account for the recruitment of aPFC under conditions in which a PM cue is expected, regardless of whether a PM cue is encountered. Specifically, they suggest that aPFC activity might reflect a transient, ongoing process that serves to match the current stimulus against a stored internal template of the PM cue, or instead a sustained process that is involved in actively maintaining the PM cue template across trials (so that efficient matching operations can be carried out with each encountered stimulus), or third, that aPFC activity enables a “more abstract cognitive operation such as the constraint of search possibilities…” (Burgess et al. 2001; p. 552). The results of the current study are at odds with the first two explanations, as the aPFC responses in the current study were clearly sustained rather than being locked to each stimulus presentation, and further, these sustained responses were dissociated from sustained responses associated with WM load. As such, this leaves the “more abstract cognitive operation” as a potential function of aPFC. In recent work, Burgess and colleagues have provided a specific suggestion—termed the “gateway hypothesis”—of an abstract cognitive operation that might be performed by aPFC during PM tasks (Burgess et al. 2005). According to this account, medial and lateral aPFC compete to direct attentional resources toward either external stimuli (medial aPFC) or internal representations (i.e., maintained intentions). However it is unclear whether the gateway hypothesis can explain either the dissociation between aPFC and DL-PFC or the task-selective brain–behavior relationships observed in aPFC.
The current data impose two further constraints on theorizing regarding aPFC function. First, the attention directed toward stored intentions appears to be sustained across ITIs and not just instantiated at trial onsets. Second, the activation of aPFC has a direct, beneficial effect on PM. One could imagine that aPFC is not only attending to, but also selecting between multiple internal representations relevant to task performance: one of which is associated with the mappings needed to perform the N-Back task, and one of which is associated with the mappings needed to perform the appropriate response on the infrequent PM cues. To the extent that participants need to manage these multiple internal states, aPFC may be recruited. Likewise, to the extent that aPFC is able to select the relatively infrequently used representation associated with the PM cues (and maintain such a selection), participants should be able to respond more quickly when a PM cue arrives. This ability to select a higher-order, less frequent internal context representation could be conceptualized as a monitoring process that is particularly relevant during multitasking situations in which one must rapidly (although not necessarily frequently) switch the task that is being performed in response to an environmental cue (Pollmann 2001; Braver et al. 2003).
Our characterization of aPFC function suggests that this region will be engaged during a range of multitasking and PM situations. For example, in time-based PM paradigms, the PM response must be executed at a specific time (e.g., every 30 s) rather than in response to specific events. In one recent neuroimaging study of this paradigm, left lateral aPFC was identified (Okuda et al. 2007). Although this study did not decompose sustained and transient processes, it is likely that the aPFC response may reflect sustained processing similar to that identified in the current study. However, it should be noted that aPFC may not be recruited in all PM tasks. Specifically, there are several task dimensions that enable participants to spontaneously retrieve the appropriate intentions when they encounter a PM cue (McDaniel and Einstein 2000; Einstein et al. 2005). Specifically, monitoring costs can be reduced if PM cues are focal in the sense that their processing is encouraged by the ongoing task, if there is a strong association between the PM cue and its associated action, if there is a single PM cue rather than a set of cues, and if the importance of the PM task performance is de-emphasized relative to the importance of performance of the ongoing task. The current study used a set of nonfocal cues (any word appearing in the designated color was a cue, but word color could be ignored in the ongoing task), and therefore, the task had relatively large monitoring demands. As such, the current study's relatively large monitoring demand could account for the ubiquity of sustained effects identified in the current study, and the relative lack of transient, ongoing effects identified in the context of PM task demands. Further study will be needed to determine whether similar responses would be identified as a function of these other variables thought to influence monitoring demands.
Transient Processing of PM Cues
The current study is one of the first studies using fMRI to identify selective areas that are uniquely activated by PM cues solely in the context of a PM task. The ROI identified along the middle temporal gyrus is near areas previously identified as important for the processing of novel stimuli and targets during target detection tasks (Kiehl et al. 2001). Interestingly, this previous task was not a simple oddball task. Rather, within their target-detection task, there are potentially conflicting sources of information in determining the appropriate response following the presentation of an infrequent stimulus (i.e., there are novel nontargets). In this manner, the task used by Kiehl et al more closely parallels the PM condition in which there are multiple contributing factors to the execution of a response other than nontarget (e.g., participants can either produce a WM target response or a PM cue response). Because this ROI demonstrated increased activity to PM cues relative to oddball targets (which have only a single potential response to a novel stimulus), it is likely that this ROI is involved in selecting one of several actions in response to an infrequent stimulus.
WM Load in the Context of the Current Study
The experimental components involving manipulation of WM load provide intrinsically informative and interesting results. The current study investigated which areas demonstrate sustained and transient effects associated with WM load. In a task such as the N-back, active maintenance of goal relevant information occurs between trials, because each new stimulus requires a target decision and requires the updating of WM contents. Thus, similar to the design used by Cohen et al. (1997), brain regions involved in active maintenance processes can be identified via the sustained activation component of the GLM. In contrast, brain regions involved in stimulus-locked processes such as encoding, response selection, and matching can be identified in the transient component of the GLM. As such, the current experiment served to conceptually replicate the effects of WM load in an N-Back task.
This replication was successful; one network including regions of right DL-PFC, bilateral parietal cortex, and the cerebellum was associated with increased sustained responses in the NB-3Back condition relative to the NB-1Back condition. Further, these responses were behaviorally relevant, as participants with increased sustained activity tended to respond more quickly on N-back trials (see Supplementary Materials). As in previous studies (Braver et al. 1997; Cohen et al. 1997), these responses are interpreted as reflecting the active maintenance of information in WM. Likewise, a network consisting primarily of ROIs within sensorimotor brain regions and inferior PFC demonstrated increased transient, ongoing responses to greater WM load (Cohen et al. 1997). Similar to previous studies, these responses are interpreted as reflecting updating and decision processes. These results validate the mixed blocked/event-related design as a means of tapping into task-relevant WM processes, and therefore may be a unique tool to investigate active maintenance processes in the future.
Relationship between WM and PM
Interestingly, there was very little overlap in the patterns of responses identified by the WM and PM manipulations. The lack of overlap appears to be due, at least in part, to the decomposition of sustained and transient responses. In particular, some of the ROIs identified as demonstrating sustained responses in the PM task are typically associated with WM load (e.g., bilateral inferior parietal cortex, left inferior PFC, and ACC: Rypma et al. 1999; Owen et al. 2005; Xu and Chun 2006). In each of these ROIs, task selectivity is due to the fact that these areas demonstrate sustained responses in the PM task, whereas they demonstrate transient responses in the WM task. A blocked design would not be able to distinguish between these different dynamics, and as such, would consider them sensitive to both manipulations. This is one key benefit that the current hybrid blocked/event-related design allows: Although these regions are recruited in both tasks, their temporal dynamics, and thus their functional contribution, appear to be task-dependent and selective. We suggest that, in contrast to aPFC, these regions might be involved in processes that are engaged by both WM and PM demands, but just more strongly (or in a qualitatively different fashion) by PM demands. For example, ACC is commonly associated with transient response conflict (Botvinick et al. 2001), and it was the case that increased WM load did lead to an increase in the transient response of this region (see Table 2, Fig. 3). It may have been the case that the PM-1Back task produced an increase in “task-set” conflict (between the N-back and PM task sets) that may have engaged this region in a more sustained fashion, consistent with prior data from task switching (Braver et al. 2003).
Relationship to Prospective Codes within WM
In previous research, prospective processes have been identified in the context of simple delayed response tasks (Rainer et al. 1999; Curtis et al. 2004; Curtis and D'Esposito 2006). Although the current PM task and previous delayed response tasks share the property that participants may attempt to plan some intended action for the future, they differ in large degree. Namely, in the delayed response tasks used to examine prospective coding in WM, the onset of the target stimuli occurs at a predictable interval after the presentation of a cue, there is no demand to divert attention to other task demands during the delay interval, and participants are always required to execute a response based on information that is being actively maintained in WM. As such, in delayed response paradigms the contents of WM can serve as a top-down bias on prospective action plans. In contrast, in the current study (and PM studies more generally) very different conditions were present during the PM task condition. Participants were unaware, prior to stimulus presentation, whether the upcoming stimulus would be a PM cue or whether it would require an ongoing task (N-back) response. This uncertainty (along with the relative infrequency of PM cues) makes the prospective preparation of a delayed motor plan unlikely. To further mitigate the likelihood of using prospective action plans in the same sense used by previous researchers, the contents and processes associated with the online WM task potentially interfere with those processes required to perform PM (i.e., participants must temporarily stop performing the WM task in order to respond appropriately to presented PM cues). As such, these task differences likely reflect the different neural circuits involved, with more posterior and superior areas of PFC involved in prospective coding in WM as measured by delayed response tasks, and more anterior areas of PFC involved in PM where individuals must maintain access to a task-related intention, but in a form that it does not interfere with performance of the ongoing task.
Conclusions
Despite the ubiquity and importance of PM in daily life, there has been limited investigation of the neural mechanisms that support this form of memory. The current study provides a novel window into the processes involved in successful PM. Specifically, the results demonstrate that, at least in certain task contexts, PM may depend upon sustained activation of aPFC. Further, by replicating previous data regarding the effects of increasing WM load, the current study demonstrates that PM demands are qualitatively distinct from WM demands, and engage a distinct set of brain regions (e.g., aPFC vs. DLPFC). In addition to invoking sustained activation within aPFC and other regions, PM was also associated with a distinct pattern of transient activation within temporal cortex during the presentation of PM cues. This region may be triggered by the detection of salient features of the cue, and may facilitate the reorienting of attentional resources away from the ongoing activity and toward realizing the delayed intention. In sum, within this paradigm successful PM appears to be related to the engagement of primarily sustained, top-down processes supported by aPFC and additional transient, stimulus-driven processes that are recruited when a PM cue is encountered.
Funding
National Institutes of Health grants (P50 MH64445 and RO1 MH66078) to T.S.B.; an National Defense Science and Engineering Graduate Fellowship; and National Institutes of Health Ruth L. Kirschstein National Research Service Award fellowship (F32 MH075300-02) to J.R.R.
Supplementary Material
Acknowledgments
We thank Christine Hoyer for assistance in subject recruitment and testing, and the Cognitive Control and Psychopathology Lab for thoughtful comments and helpful suggestions. Conflict of Interest: None declared.
References
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brandimonte MA, Ferrante D, Feresin C, Delbello R. Dissociating prospective memory from vigilance processes. Psicológica. 2001;22:97–113. [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition, and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of frontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Braver TS. Event-related functional MRI. In: Bandettini P, Moonen C, editors. Functional MRI. Germany: Springer; 1999. pp. 441–452. [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ, Okuda J, Schölvinck ML, Simons JS. On the role of rostral prefrontal cortex (area 10) in prospective memory. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: cognitive, neuroscience, developmental, and applied perspectives. New York: Lawrence Erlbaum Associates; 2008. pp. 235–260. [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41:906–918. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. In: Duncan J, Phillips L, McLeod P, editors. Measuring the mind: speed, control, and age. Oxford: Oxford University Press; 2005. pp. 217–248. [Google Scholar]
- Chambers JM, Cleveland WS, Kleiner B, Tukey PA. Graphical methods for data analysis. Boston: Wadsworth & Brooks; 1983. [Google Scholar]
- Cohen AL, Jaudas A, Gollwitzer PM. Number of cues influences the cost of remembering to remember. Mem Cogn. 2008;36:149–156. doi: 10.3758/mc.36.1.149. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt MR, Provost J. Psyscope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activity during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95:3923–3927. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. On quantifying surprise: the variation of event-related potentials with subject probability. Psychophysiology. 1977;16:53–55. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Duzel E, Cabeza R, Picton TW, Yonelinas AP, Scheich H, Heinze H-J, Tulving E. Task-related and item-related brain processes of memory retrieval. Proc Natl Acad Sci USA. 1999;96:1794–1799. doi: 10.1073/pnas.96.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Prospective memory: remembering a forgotten topic. In: Hermann D, McEvoy C, Hertzog C, Hertel P, Johnson M, editors. Basic and applied memory research: practical applications. Vol. 2. Mahwah (NJ): Erlbaum; 1996. pp. 79–94. [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J. Multiple processes in prospective memory retrieval: factors determining monitoring versus spontaneous retrieval. J Exp Psychol Gen. 2005;134:327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Barch DM, Gusnard DA, Raichle ME. Transient bold responses at block transitions. Neuroimage. 2005;28:956–966. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Guynn MJ. A two-process model of strategic monitoring in event-based prospective memory: activation/retrieval mode and checking. Int J Psychol. 2003;38:245–256. [Google Scholar]
- Kane MJ, Conway ARA, Miura TK, Miura TK, Colflesh GJH. RA, Miura TK, Colflesh GJH. Working memory, attention control, and the N-back task: a question of construct validity. J Exp Psychol Learn Mem Cogn. 2007;33:615–622. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001;38:133–142. [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Konishi S, Donaldson DI, Buckner RL. Transient activation during block transition. Neuroimage. 2001;13:364–374. doi: 10.1006/nimg.2000.0691. [DOI] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci USA. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE, Prvulovic D, Formisano E, Volinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hancock TW, Hicks JL. The demands of an ongoing activity influence the success of event-based prospective memory. Psychon Bull Rev. 2002;9:604–610. doi: 10.3758/bf03196319. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL. Event-based prospective memory and executive control of working memory. J Exp Psychol Learn Mem Cogn. 1998;24:336–349. doi: 10.1037//0278-7393.24.2.336. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Cook GI, Hansen JS, Pallos AL. Interference to ongoing activities covaries with the characteristics of an event-based intention. J Exp Psychol Learn Mem Cogn. 2003;29:861–870. doi: 10.1037/0278-7393.29.5.861. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Watson V. The dynamics of intention retrieval and coordination of action in event-based prospective memory. J Exp Psychol Learn Mem Cogn. 2002;28:652–659. doi: 10.1037//0278-7393.28.4.652. [DOI] [PubMed] [Google Scholar]
- McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment of significant activation in fMRI. Neuroimage. 2001;13:S198. [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: a multiprocess framework. Appl Cogn Psychol. 2000;14:S127–S144. [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory: an overview and synthesis of an emerging field. Thousand Oaks (CA): Sage; 2007. [Google Scholar]
- McNerney MW, West R. An imperfect relationship between prospective memory and the prospective interference effect. Mem Cogn. 2007;35:275–282. doi: 10.3758/bf03193448. [DOI] [PubMed] [Google Scholar]
- Mugler JPI, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP-RAGE) Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- Ojemann J, Akbudak E, Snyder A, McKinstry R, Raichle M, Conturo T. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Yamadori A, Frith CD, Burgess PW. Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time- and event-based prospective memory. Int J Psychophysiol. 2007;64:233–246. doi: 10.1016/j.ijpsycho.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann S. Switching between dimensions, locations, and responses: the role of the left frontopolar cortex. Neuroimage. 2001;14:S118–S124. doi: 10.1006/nimg.2001.0837. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Raghunathan TE, Rosenthal R, Rubin DR. Comparing correlated but nonoverlapping correlations. Psychol Methods. 1996;1:178–183. [Google Scholar]
- Rainer G, Rao SC, Miller EK. Prospective coding for objects in primate prefrontal cortex. J Neurosci. 1999;19:5493–5505. doi: 10.1523/JNEUROSCI.19-13-05493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, McDermott KB, Braver TB. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cereb Cortex. 2006;16:519–528. doi: 10.1093/cercor/bhi131. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM-L, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JDE. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Simons JS, Scholvinck ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44:1388–1397. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: investigating the capacity demands of delayed intention performance. J Exp Psychol Learn Mem Cogn. 2003;29:347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Smith RE, Bayen UJ. The effects of working memory resource availability on prospective memory: a formal modeling approach. Exp Psychol. 2005;52:243–256. doi: 10.1027/1618-3169.52.4.243. [DOI] [PubMed] [Google Scholar]
- Snyder AZ. Difference image versus ratio image error function forms in pet-pet realignment. In: Bailer D, Jones T, editors. Quantification of brain function using pet. San Diego: Academic Press; 1996. [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- West R, Bowry R, Krompinger J. The effects of working memory demands on the neural correlates of prospective memory. Neuropsychologia. 2006;44:197–207. doi: 10.1016/j.neuropsychologia.2005.05.003. [DOI] [PubMed] [Google Scholar]
- West R, Krompinger J, Bowry R. Disruptions of preparatory attention contribute to failures of prospective memory. Psychon Bull Rev. 2005;12:502–507. doi: 10.3758/bf03193795. [DOI] [PubMed] [Google Scholar]
- West R, McNerney MW, Krauss I. Impaired strategic monitoring as the locus of a focal prospective memory deficit. Neurocase. 2007;13:115–126. doi: 10.1080/13554790701399247. [DOI] [PubMed] [Google Scholar]
- West R, Wymbs N. Is detecting prospective cues the same as selecting targets? An ERP study. Cogn Affect Behav Neurosci. 2004;4:354–363. doi: 10.3758/cabn.4.3.354. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing pet images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.