Abstract
Peripheral capsaicin treatment induces molecular changes that sensitize the responses of nociceptive neurons in the spinal dorsal horn. The current studies demonstrate that capsaicin also undermines the adaptive plasticity of the spinal cord, rendering the system incapable of learning a simple instrumental task. In these studies, male rats are transected at the second thoracic vertebra and are tested 24 to 48 hours later. During testing, subjects receive shock to one hindleg when it is extended (controllable stimulation). Rats quickly learn to maintain the leg in a flexed position. Rats that have been injected with capsaicin (1% or 3%) in the hindpaw fail to learn, even when tested on the leg contralateral to the injection. This learning deficit lasts at least 24 hours. Interestingly, training with controllable electrical stimulation prior to capsaicin administration protects the spinal cord against the maladaptive effects. Rats pretrained with controllable stimulation do not display a learning deficit or tactile allodynia. Moreover, controllable stimulation, combined with naltrexone, reverses the capsaicin-induced deficit. These data suggest that peripheral inflammation, accompanying spinal cord injuries, might have an adverse effect on recovery.
Keywords: spinal cord injury, instrumental learning, recovery of function, capsaicin, central sensitization
Recovery of function after a spinal cord injury depends, in part, upon the capacity of the compromised system to establish new functional connections. The modification of central circuits in treadmill training, for example, is used to facilitate the recovery of locomotor function in humans (Wernig, Muller, Nanassy, & Cagol, 1995). Evidence that these changes in locomotor behavior are spinally mediated comes from studies showing that subjects with complete spinal transections can produce weight-supported stepping with training (Bigbee et al., 2007; Edgerton, Roy, De Leon, Tillarkaratne, & Hodgson, 1997; Edgerton, Roy, Hodgson, Gregor, & De Guzman, 1991; Edgerton, Tillakaratne, Bigbee, de Leon, & Roy, 2004; Forssberg & Grillner, 1973; Hodgson, Roy, De Leon, Dobkin, & Edgerton, 1994; Rossignol et al., 1996). In these subjects, locomotor behavior is supported by a central pattern generator (Grillner, 1981) located in the lumbar-sacral segments of the spinal cord (Barthelemy, Leblond, Provencher, & Rossignol, 2006; Langlet, Leblond, & Rossignol, 2005; Lavrov et al., 2006;). Furthermore, the spinal system can adapt reflexive behaviors with experience. Edgerton and colleagues showed that when a spinally transected rat, trained to step on a treadmill, encounters an obstacle (a bar) as it swings a leg forward, the spinal cord learns to produce a greater flexion response that reduces contact with the obstacle (Edgerton, Roy, & de Leon, 2001). The sensory input provided from the paw contacting the obstacle causes enhanced flexion during the swing phase. These data indicate that the spinal cord has the capacity to generate adaptive behavioral changes in response to externally applied stimuli.
After injury, neural plasticity may also result in maladaptive changes at a spinal level. Peripheral inflammation or injury can generate “central sensitization” in the spinal cord. Central sensitization results in the potentiation of responses of nociceptive neurons in the spinal dorsal horn (Simone et al., 1991; Willis, 2001). Behaviorally it is manifest as allodynia (increased reactivity to innocuous sensory stimuli) and hyperalgesia (increased reactivity to noxious sensory stimuli), both of which are characteristic of neuropathic pain. As spinal cord injuries are often accompanied by peripheral tissue damage, and subsequently inflammation, this consequence of plasticity represents a significant concern for the clinical population.
Data from our laboratory suggest that adaptive and maladaptive changes linked to spinal plasticity may interact (Ferguson, Crown, & Grau, 2006). We use a simple instrumental (response-outcome) learning task as an index of spinal plasticity. In previous studies, we have shown that the isolated spinal cord encodes the relationship between an extended leg position and electrical stimulation of the tibialis anterior muscle (Grau, Barstow, & Joynes, 1998). If transected (second thoracic vertebrae) rats are shocked whenever they extend the leg, they rapidly learn to maintain a flexed leg position, thereby minimizing net shock exposure. This change in behavior is not due to shock exposure per se. Using a Master-Yoke paradigm rats are exposed to exactly the same amount of shock; one rat (Master) is given shock only when the leg is extended (controllable shock), while the Yoked partner receives shock whenever his `Master' does. For the Yoked rats, shock and leg position are uncorrelated (uncontrollable shock). Under these conditions, only Master rats exhibit an increase in flexion duration, our measure of learning (Grau et al., 1998). When subjects are subsequently tested under common conditions with responsecontingent shock, Yoked rats fail to learn, and this learning deficit lasts for up to 48 hrs (Crown, Ferguson, Joynes, & Grau, 2002a). Conversely, rats that have previously received training with controllable shock exhibit a savings effect that enables learning when subjects are tested using a more difficult response criterion (Crown, Ferguson, Joynes, & Grau, 2002b). The essential spinal circuits underlying instrumental learning appear to be localized to the L4-S2 region of the spinal cord (Liu et al., 2005)
Recent work suggests that uncontrollable stimulation may disrupt learning because it induces a state akin to central sensitization. Supporting this, Ferguson et al., (2006) showed that exposure to uncontrollable electrical stimulation enhances reactivity to innocuous mechanical stimulation (allodynia), an index of central sensitization (Coderre & Melzack, 1985; Willis, 2001; Woolf, 1983). In addition, a treatment that produces allodynia (intradermal carrageenan) undermined instrumental learning (Ferguson et al., 2006). Peripheral tissue damage early in development, which produces a lasting mechanical hypersensitivity, also produces a long-term disruption in instrumental learning (Young, Baumbauer, Elliot, & Joynes, 2007). In other studies we have shown that, like central sensitization, the learning deficit induced with uncontrollable electrical stimulation depends on an NMDA-mediated process (Ferguson et al., 2006; Joynes, Janjua, & Grau, 2004), intracellular protein kinase C signaling (Bolding, Hook, Ferguson, & Grau, 2003), and protein synthesis (Patton, Hook, Ferguson, Crown, & Grau, 2004).
Interestingly, it appears that training with controllable electrical stimulation can both protect the spinal cord against the effects of uncontrollable electrical stimulation, and reverse the learning deficit produced by uncontrollable training. Crown and Grau (2001) found that rats trained with controllable shock prior to exposure to uncontrollable shock do not develop a learning deficit. Perhaps more clinically relevant was the finding that the learning deficit can be reversed by training with controllable shock combined with naltrexone a pharmacological agent that blocks the expression of the deficit (Crown & Grau, 2001). Given the significant similarities between effects of uncontrollable shock and central sensitization, we hypothesize that instrumental training will also protect against the detrimental effects of capsaicin on spinal plasticity.
The studies reported in this article further explore the interaction between central sensitization and the adaptive plasticity of the isolated spinal cord. In these studies, rather than carrageenan, we use capsaicin to induce central sensitization. Capsaicin induces a robust form of central sensitization that lasts for up to several hours (LaMotte, Shain, Simone, & Tsai, 1991; Simone Baumann, & LaMotte, 1989; Willis, 2001) depending on the dose used. Capsaicin also primarily engages C-fibers (which release glutamate and aspartate into the dorsal horn), and the molecular mechanisms underlying its effects are well understood (Willis, 2001, 2002). The present experiments, therefore, not only provide converging evidence for Ferguson et al. (2006), they also allow us to examine what type of nociceptive fibers (Aδ or C) are necessary for the induction of the learning deficit and may provide further insight into the molecular mechanisms mediating plasticity. In these studies, we hypothesize that capsaicin will undermine the adaptive plasticity of the isolated spinal cord (Experiment 1), producing a learning deficit that outlasts the acute peripheral effects of the manipulation (Experiment 2). Based on previous studies we also predict that controllable instrumental training can be used to protect (Experiments 3 and 4) and reinstate (Experiments 5 and 6) behavioral plasticity after the induction of a learning deficit with capsaicin.
General Method
Subjects
The subjects were male Sprague-Dawley rats obtained from Harlan (Houston, TX). They were approximately 100 to 120 days old and weighed between 360 and 460 g. Subjects were individually housed with food and water available ad libitum. They were maintained on a 12-hour light-dark cycle and were tested during the last 6 hours of the light cycle.
Surgery
Rats were anesthetized with pentobarbital (50 mg/kg i.p.). To stabilize and position the rat's body for surgery, its head was held in a stereotaxic instrument and a small gauze “pillow” was placed under its chest. After the second thoracic vertebra (T2) was localized by palpation, an anterior-posterior incision (approximately 1.5 cm long) was made on the back over T2. Next, the tissue and bone at T2 was cleared away and the exposed spinal cord was transected through cauterization. The void produced was filled with Gelfoam (Harvard Apparatus, Holliston, MA) and the wound was closed with Michel Clips (Fine Science Tools, Foster City, CA).
Subjects in Experiments 5 and 6 had an intrathecal cannula lowered into the lumbar region of the spinal cord following the procedure of Yaksh and Rudy (1976). After the spinal cord was transected, a segment of polyethylene tubing (25 cm; PE-10) fitted with a 0.23 cm (diameter) stainless steel wire (SWGX-090, Small Parts) was inserted 9 cm down the spinal cord. The tubing was inserted into the subarachnoid space, between the dura and the white matter, so as to lie on the dorsal surface of the cord. The exposed end of the tubing was secured to the adjacent tissue using an adhesive (cranoacrylate). The wire was then pulled from the tubing and the wound caudal to the exposed tubing was closed using Michel clips.
Immediately following surgery, the rats were injected with 0.9% saline (2.5 ml i.p.). To prevent muscular damage due to unnatural extension during recovery, rats' legs were gently secured in a natural flexed position with a piece of porous tape (1.3 cm width) wrapped once around their body and legs. Subjects were maintained in a temperature-controlled room (25.5°C) during recovery. Bladders were expressed twice daily, and immediately before any behavioral procedures were performed. For all experiments, the recovery period between surgery and behavioral training or testing was approximately 24 h.
Transections were confirmed by (a) visually inspecting the cord during surgery, (b) observing behavior after recovery to ensure complete paralysis below the forelimbs and no vocalization when exposed to leg shock, and (c) examining the cord post-mortem in a randomly selected subset of subjects.
Capsaicin Injections
Capsaicin (Sigma-Aldrich, St. Louis, MO) was dissolved in 50 μL of vehicle (Tween 80 (7%) and saline (93%)). The drug was injected into the dorsal surface of the foot (50 μL, s.c.) to ensure that the resulting edema did not impact training or testing procedures, which involved taping a contact electrode to the plantar surface of the foot.
Apparatus
The apparatus used in the experiments is described in detail in Grau et al. (1998). Briefly, instrumental training was conducted while the rats were loosely restrained in tubes (23.5 cm long × 8 cm internal diameter). Two slots (5.6 cm long × 1.8 cm wide) were cut 4 cm apart and 1.5 cm from the end of the tube, allowing the rat's hindlegs to hand freely (see Grau et al., 1998). Leg shock was applied by attaching one lead from a BRS/LVE shock generator (Model SG-903, Laurel, MD) to a stainless steel wire inserted through the skin over the tibia, 1.5 cm from the tarsals. The other lead was attached to a stainless steel wire that was inserted 0.4 cm into the tibialis anterior muscle, 1.7 cm above the other electrode.
The position of the hindleg was monitored with a contact electrode constructed from a stainless steel rod. The contact electrode was taped to the plantar surface of the rat's foot directly in front of the plantar protuberance. A fine wire was attached to the end of the rod extending from the rear of the foot and was connected to a digital input monitored by a Macintosh computer. A plastic rectangular dish containing a salt solution was placed approximately 7.5 cm below the restraining tube. A ground wire was connected to a 1 mm stainless steel rod that was placed in the solution. When the contact electrode that was attached to the rat's paw touched the solution it completed an electrical circuit monitored by the computer. The state of this circuit was sampled at a rate of 30 HZ.
The flexion force produced by the tibialis anterior muscle was measured before testing to standardize this variable across subjects. Flexion force was measured using a strain guage that was connected to the rat's foot using a monofilament plastic line. A 300ms shock was applied to the leg, and the intensity was adjusted to a level that produced a 0.4 N flexion response for each subject prior to testing.
Tactile reactivity was tested in the Plexiglas tubes described above. It was assessed using von-Frey stimuli made from nylon monofilaments (Semmes-Weinstein Anesthesiometer; Stoelting Co., Chicago, IL), which were applied to the plantar surface of the hindpaw. Tactile data are reported using the linear monofilament number scale provided by the manufacturer: Intensity = log10 (10,000 × g). We performed analyses on this scale because an analysis of variance (ANOVA) assumes linearity. For the range of monofilaments used, this linear scale is more conservative than the logarithmic changes in grams or mN.
Procedure
Before the rats were placed in the restraining tubes the rear legs were shaved and marked for placement of the shock leads. The stainless steel wire electrode was then inserted over the tibia and the rats were placed in the restraining tubes. The rats were secured in the tubes with the wire `belt' and the leg was taped to minimize lateral leg movements. The contact electrode was taped to the rats paw. Then one lead from the shock generator was attached to the electrode placed over the tibia. The shock generator was set to deliver a 0.1 mA shock and the tibialis anterior muscle (1.7 cm above the first electrode) was probed to find a site that elicited a vigorous flexion response. The second wire electrode was then inserted laterally into the tibialis anterior muscle. The electrode placements were checked by verifying that a single, intense (3 s, 1.6 mA) shock elicited a flexion response of at least 0.8 N. The shock intensity was then adjusted to elicit a 0.4 N flexion force. The line used to connect the rat's leg to the strain gauge was removed. Finally, 3 short (1.5 s) shock pulses were applied, and the level of the salt solution was adjusted so that the tip of the contact electrode was submerged 4 mm beneath the surface.
During instrumental testing, shock was applied to the tibialis anterior muscle whenever the contact electrode attached to the rat's foot touched the underlying salt solution. This response-contingent (controllable) shock was terminated when the contact electrode left the solution. Leg position was monitored by a Macintosh computer at a sampling rate of 30 Hz.
Behavioral Measures
Three behavioral measures were used to assess a subject's capacity to learn the response-outcome relationship, or instrumental response: response number, response duration, and time in solution (see Grau et al., 1998). Performance was measured over time in 30 1-min time bins. The computer monitoring leg position recorded an increase in response number whenever the contact electrode left the salt solution. Response duration was derived from time in solution and response number using the following equation: Response Durationi (60 s - time in solutioni)/(Response Numberi + 1), where i was the current time bin.
Statistics
All results were analyzed using ANOVAs. In all instances, a criterion of p < .05 was used to assess statistical significance. More specific group differences were established by means of post hoc Duncan New Multiple Range tests when appropriate.
Results
Experiment 1: Dose-Response Relationship
Previous work has shown that just 6 minutes of uncontrollable nociceptive electrical stimulation to the tail or leg produces a behavioral deficit in spinal rats (Crown et al., 2002a). If this behavioral deficit is related to the induction of central sensitization, capsaicin should also produce a learning deficit.
Method
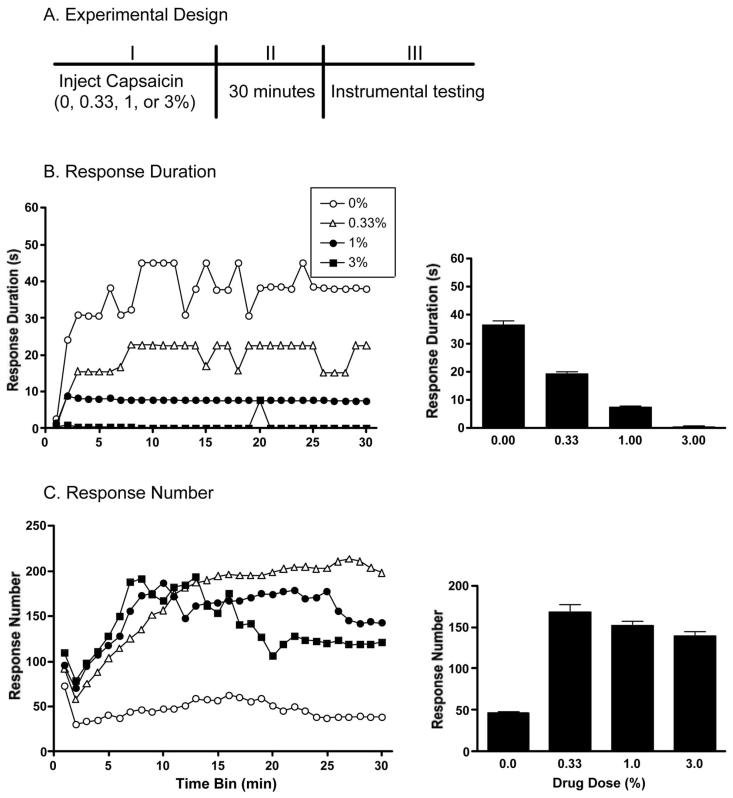
Thirty-two spinalized rats were randomly assigned to one of 4 drug conditions (0, 0.33, 1, or 3% capsaicin). These doses were chosen based on previous studies (e.g., Dougherty, Palecek, Paleckova, Sorkin, & Willis, 1992; Fang, Wu, Lin, & Willis, 2002; Zou, Lin, & Willis, 2002) detailing the behavioral and molecular effects of capsaicin in a model of central sensitization. A subcutaneous injection of the appropriate dose (50 μL volume) was made into the dorsal surface of the left or right foot. Twenty minutes after the injection the rats were placed into the instrumental testing apparatus and prepared as described in the “General Methods” section. Preparing the subjects took approximately 10 minutes, leaving 30 minutes for the capsaicin to take effect. All subjects were then tested with controllable (response-contingent) shock for 30 minutes on the leg contralateral to the injection. An overview of the experimental design is shown in Figure 1A.
Figure 1.
The design of Experiment 1 is outlined in Panel (A). There was a dose-dependent effect of capsaicin on instrumental learning. Subjects treated vehicle or 0.33% capsaicin learned the instrumental relationship between leg position and shock. They displayed an increase in flexion duration across time (B). Both the 1% and 3% doses of capsaicin produced a learning deficit, and did not show a change in response duration throughout the 30-minute testing period. The mean (± SEM) response duration for each group is shown in the right panel of 1B. The learning deficit was not caused by an inability to perform the behavioral response. Subjects treated capsaicin displayed more responses (leg flexions) than those treated with vehicle (C).
Results
To assess whether capsaicin affected baseline behavioral reactivity, we compared the shock intensity needed to produce a 0.4 N flexion force and the duration of the first flexion response across groups. Shock intensity values ranged from 0.44 ± 0.03 to 0.50 ± 0.02 mA. Mean (± SE) initial response durations ranged from 0.12 ± 0.0 to 0.14 ± 0.04 s. The groups did not differ on either measure. Independent ANOVAs confirmed that these differences did not reach significance, both Fs (3, 28) < 1.0, p > .05.
As can be seen in Figure 1, subjects treated with vehicle displayed an increase in response duration, indicative of learning, across trials. There was a dose-dependent decrease in response duration with capsaicin exposure. An ANOVA revealed a main effect of dose on response duration, F(3, 28)= 4.72, p < .01. Duncan New Multiple Range post hoc tests revealed that the response durations displayed by subjects treated with 1% and 3% capsaicin differed significantly (p < .05) from vehicle controls. Subjects treated with 0.33% capsaicin did not differ from the other capsaicin groups or from the vehicle-treated subjects. There was also a significant effect of time on response duration, F(3, 29)= 2.96, p < .001, but the interaction between dose and time was not significant, F(3, 87)= 1.21, p > .05.
As in prior studies (Grau et al., 1998), learning (as indicated by an increase in response duration) was accompanied by a decrease in response number (Figure 1C). Capsaicin treatment disrupted learning, but did not interfere with the capacity to exhibit a shock-induced flexion. Subjects treated with capsaicin displayed more responses than those treated with vehicle. Although there was no main effect of dose on response number, F(3, 29)= 1.97, p > .05, an ANOVA confirmed a significant effect of time, F(3, 29)= 3.58, p < .001, and a significant Dose × Time interaction, F(3, 87)= 1.36, p < .05.
Summary
Capsaicin produces a dose-dependent learning deficit at 30 minutes postinjection. Subjects treated with 1% or 3% capsaicin were unable to learn the instrumental relationship between shock and leg position on the limb contralateral to the capsaicin injection. This suggests that capsaicin has induced a change at a central level that undermines the adaptive capacity of the spinal cord.
Experiment 2: Long-Term Effects of Treatment
Uncontrollable shock induces a learning deficit that lasts for 24 to 48 hours. To test whether capsaicin has long-term effects on plasticity, like uncontrollable shock, Experiment 2 assessed learning 24 hours after capsaicin administration. As in Experiment 1, learning was assessed on the limb contralateral to the capsaicin injection. Here, as in other studies using intermittent shock to induce a deficit, contralateral transfer helps to rule out some peripheral accounts of the effects (Joynes, Ferguson, Crown, Patton, & Grau, 2003). The present experiment will provide further evidence on this issue because the peripheral consequences of capsaicin treatment should wane within a few hours (LaMotte et al., 1991; Simone et al., 1989; Willis, 2001). Effects on learning 24 hours later, therefore, should reflect changes at a central level. To explore the relationship to other behavioral correlates of central sensitization, we also assessed tactile reactivity immediately after the injection of capsaicin and prior to testing 24 hours later.
Method
Twenty-four spinalized rats were randomly assigned to one of four groups (n = 6). Groups received a subcutaneous injection of either 3% capsaicin or vehicle and were tested 30 minutes or 24 hours later. This results in a 2 (capsaicin or vehicle) × 2 (30 minutes or 24 hours) experimental design (Figure 2A). All rats were prepared for instrumental testing as described earlier. They were tested for 30 minutes with response-contingent shock on the leg contralateral to the capsaicin injection. Tactile reactivity was examined immediately prior to testing.
Figure 2.
The learning deficit induced with 3% capsaicin lasted for at least 24 hours. The experimental design is presented in Panel (A). As shown in Panel (B), subjects treated with 3% capsaicin (filled circles and bars) displayed significantly reduced response durations, compared with vehicle controls (open circles and bars), at both 30 minutes and 24 hours after injection. Mean (± SEM) response durations for each group are shown in the far right panel of 2B. As found for Experiment 1, capsaicin treated groups displayed more responses than the vehicle treated subjects (C).
Results
Instrumental learning
As found for Experiment 1, capsaicin and the time of testing did not affect the baseline behavioral reactivity of the subjects. Initial flexion durations ranged from 0.12 ± 0.01 to 0.15 ± 0.01 s. The shock intensity needed to induce a 0.4 N flexion ranged from 0.45 ± 0.02 to 0.5 ± 0.02 mA. Independent ANOVAs confirmed that the groups did not differ on either measure prior to testing (both Fs < 1.0, p < .05).
As shown in Figure 2B, the rats treated with vehicle displayed an increase in response duration, indicative of learning, over the 30 minute testing period. Capsaicin disrupted learning, independent of whether it was given 30 minutes or 24 hours before testing (Figure 2B). There was a main effect of drug on flexion duration, F(1, 20)= 6.62, p < .05, but no effect of time of testing, F(1, 20)= 1.28, p > .05. There was also no interaction between drug and time of testing, F(1, 20) < 1.0, p > .05.
Capsaicin did not undermine the subject's capacity to perform the flexion response (Figure 2C). There was no significant main effect of drug, the time of testing, or any interaction between the two variables, all Fs (1, 20) < 2.43, p > .05. In fact, neither capsaicin nor uncontrollable shock impeded the subject's capacity to exhibit the behavioral response in subsequent experiments. Given this, these data are not reported in Experiments 3 through 6.
Tactile Reactivity
As can be seen in Figure 3, capsaicin treatment enhanced mechanical reactivity on both the injected and noninjected limbs. The mean reactivity thresholds (± SE) for capsaicin treated subjects on the injected limb were 5.90 (± 0.08), compared with 6.24 (± 0.08) for the vehicle controls. On the noninjected limb, reactivity thresholds for capsaicin treated subjects were 6.0 (± 0.05), whereas vehicle controls displayed significantly higher thresholds of 6.29 (± 0.08). There was a main effect of drug on mechanical reactivity thresholds, F(1, 20)= 10.72, p < .01, but no effect of time of testing, F(1, 20) < 1.0, p > .05. There was also no significant difference between the thresholds recorded across the limbs, F(1, 20)= 2.21, p > .05, or any interaction between the effects of drug treatment and the time of testing, F(1, 20) < 1.0, p > .05.
Figure 3.
Three percent capsaicin (filled bars), compared with vehicle controls (open bars), significantly reduced mechanical reactivity thresholds on both the injected (left panel) and noninjected (right panel) legs. Mean (± SEM) reactivity thresholds are depicted at 30 minutes and 24 hours. There was a significant effect of drug at both timepoints.
Summary
These data indicate that capsaicin has a long-term effect on the capacity for instrumental learning, suggesting that changes in plasticity have occurred at spinal loci. Moreover, the changes in plasticity parallel the expression of tactile allodynia, an index of central sensitization.
Experiment 3: Pretraining and 3% Capsaicin
In a previous study, we showed that prior training with controllable shock can block the induction of the learning deficit with uncontrollable shock exposure (Crown & Grau, 2001). Experiment 3 examined whether prior exposure to controllable shock also attenuates the learning deficit induced with capsaicin treatment.
Method
The experiment treatments were conducted in two phases. Initially, 48 spinalized rats were randomly assigned to one of three groups (n = 16). One group was exposed to controllable legshock for 30 minutes. These rats (Master) were given shock to the tibialis anterior muscle whenever their leg was extended and they could terminate the shock by flexing their leg. A second group of rats (Yoked) received legshock at the same time and for the same duration as their Master counterparts, but they had no control over the stimulation (they received shock irrespective of leg position). The remaining group of rats (Unshocked) were restrained for the 30 min period, but were not shocked.
Immediately after the first phase of behavioral training, half of the rats in each training condition (n = 8) were given a subcutaneous injection (in the foot of the trained leg) of capsaicin (3%). The remaining rats were injected with the vehicle. Groups were divided so that the mean amount of shock that the subjects were exposed to during training was approximately equivalent across conditions (all Fs < 1.0, p > .05). This results in a 3 (training condition) × 2 (capsaicin or vehicle) experimental design (Figure 4A). In the second phase of this experiment, 24 hrs after the capsaicin injection, all of the rats were tested with controllable shock on the leg contralateral to the injection.
Figure 4.
Prior training with controllable electrical stimulation reduced the capsaicin-induced learning deficit when subjects were tested 24 hours later (see Experimental design, Panel A). As shown in Panel B, there was a main effect of training on response durations. Vehicle-treated (Left panel) Master subjects displayed significantly longer response durations than Yoked rats and did not differ from the Unshocked controls. Mean response durations (± SEM) are shown in the far right panel.
Tactile reactivity was assessed with von Frey stimuli at four time points: (1) before training (baseline), (2) after training, (3) after the injection of capsaicin or vehicle, and (4) before testing. To ensure that variation in baseline scores did not influence the results, a change from baseline threshold was computed for each subject, and subsequent analyses were performed on this measure.
Results
Instrumental Learning
Phase I: Training
In training, the shock intensity needed to induce a flexion of 0.4 N ranged from 0.47 ± 0.03 to 0.54 ± 0.03 mA. Initial flexion durations ranged from 0.10 ± 0.02 to 0.15 ± 0.05 s. These group differences did not approach statistical significance (both Fs < 1.0, p > .05).
Commensurate with previous studies, Master rats displayed a progressive increase in flexion duration across the 30 min training period, whereas Yoked rats did not (data not shown). There was a main effect of training condition, F(1, 30)= 4.49, p < .05; time, F(1, 29)= 7.14, p < .005; and a significant Training Condition × Time interaction, F(1, 29)= 3.17, p < .005.
Phase II: Testing
Twenty-fours after training, the capacity for instrumental learning was assessed on the contralateral limb. First, we examined whether training affected baseline behavioral reactivity to shock. The shock intensity needed to induce a 0.4 N flexion ranged from 0.38 ± 0.01 to 0.29 ± 0.04 mA. Initial flexion durations ranged from 0.12 ± 0.003 s to 1.38 ± 1.27 s. These group differences were not statistically significant (all Fs ≤ 2.81, p > .05).
As can be seen in Figure 4B, vehicle-treated Yoked rats displayed significantly lower response durations than Master rats during testing. Further, rats treated with capsaicin did not learn as well as the vehicle controls. There were significant main effects of drug treatment, F(1, 42) = 7.58, p < .01, and pretraining condition, F(2, 42) = 6.50, p < .01. Post hoc analyses comparing pretraining conditions only (data collapsed across the capsaicin and vehicle treated groups) showed that Master rats had significantly higher response durations than Yoked rats (p < .05). There was no significant difference between the response durations displayed by the Unshocked controls compared to either Master or Yoked rats. Prior training with controllable stimulation also appeared to attenuate the effects of capsaicin, but there was no significant interaction between drug and training condition for response duration, F(2, 42) = 1.49, p > .05.
Tactile Reactivity
Baseline thresholds ranged from 6.33 (± 0.11) to 6.45 (± 0.00) on the injected leg, and from 6.15 (± 0.07) to 6.43 (± 0.07) on the noninjected leg. There were no significant differences in the thresholds recorded across the groups, F(2, 42) = 1.01, p > .05. There was also no effect of the controllable shock training on reactivity thresholds, F(2, 45) = 2.05, p > .05.
The injection of capsaicin did, however, affect reactivity thresholds. As can be seen in Figure 5A (Post-Injection), all of the capsaicin-treated subjects displayed an allodynic response, or lowered motor reactivity thresholds, on the injected leg. There was a significant main effect of capsaicin on tactile thresholds, F(1, 42)= 19.97, p < .0001, but no effect of training, F(2, 42)= 2.02, p > .15. There was also a significant three-way interaction between leg (injected or not injected), training condition and drug, F(2, 42)= 6.02, p < .005. To further explore this 3-way interaction, we conducted independent ANOVAs on the vehicle and capsaicin treated groups. In the vehicle-treated subjects, there was no effect of training condition on tactile reactivity, F(2, 21) = 1.20, p > .05, or any interaction between training condition and leg, F(2, 21) = 1.56, p > .05. For the capsaicin treated subjects, however, there was an interaction between leg and training condition, F(2, 21) = 5.94, p < .01. As can be seen in Figure 5A, capsaicin-treated rats displayed an allodynic response on the leg injected with capsaicin (right panel). On the noninjected leg (5B, right panel), however, Master rats did not show a change from baseline reactivity while both Yoked and Unshocked rats displayed allodynia. It appears that Master training induced a change in spinal neural circuitry that protects against the development of allodynia on the limb contralateral to the peripheral injury.
Figure 5.
Three percent capsaicin significantly reduced tactile reactivity thresholds on both the injected and noninjected limbs (A and B respectively, right panels). Interestingly, Master training protected the spinal cord against the effects of capsaicin on the noninjected limb when assessed in the Post-Injection period (B, right panel). This significant effect is highlighted with the hatched box. There was no effect of training condition in the vehicle-treated subjects, although there was a tendency for Yoked training to reduce tactile reactivity thresholds on the trained and injected leg (A, left panel).
By the time of testing, the protective effects of Master training on allodynic responses were reduced (Figure 5B, right panel). Immediately prior to testing, capsaicin-treated rats displayed significantly lower motor reactivity thresholds to von Frey stimuli, F(1, 42) = 30.54, p < .0001. There was no effect of training condition, F(2, 42) < 1.0, p > .05, nor a Drug × Training Condition interaction, F(2, 42) = 1.54, p > .05. For the capsaicin treated groups, all subjects displayed a lower reactivity threshold on the injected, compared to the noninjected leg, F(1, 21) = 7.37, p < .01, but there was no interaction between leg and training condition, F(1, 21) < 1.0, p > .05. In the vehicle-treated subjects, there was no effect of training condition, F(1, 21 < 1.0, p > .05, or any Training Condition × Leg interaction, F(1, 21) = 3.19, p > .05.
Summary
We found that a high concentration of capsaicin produced a lasting impairment and it appeared that training with controllable shock lessened this effect. However, individual comparisons with the yoked and unshocked capsaicin treated groups were not significant, F(2, 15) = 1.53, p > .05. A high concentration of capsaicin may produce such a robust deficit that it is relatively insensitive to modulating variables.
Experiment 4: Pretraining and 1% Capsaicin
The present experiment examined whether instrumental control impacts the deficit produced by a lower concentration (1%) of capsaicin. As Yoked training undermined learning in both capsaicin and vehicle treated rats (Experiment 3), Experiment 4 focused on a comparison of Master and Unshocked rats.
Method
As in Experiment 3, this experiment involved two phases. In the first phase 24 spinally transected rats were randomly assigned to 2 groups (n = 12). One group (Master) was exposed to controllable legshock for 30 minutes. The remaining rats (Unshocked) were restrained for the 30-minute period, but were not shocked.
After the first phase of behavioral training, half of the rats in each training condition (n = 6) were given a subcutaneous injection of capsaicin (1%) in the leg ipsilateral to training. The remaining rats were injected with vehicle. This results in a 2 (training condition) × 2 (capsaicin or vehicle) experimental design (Figure 6A). In Phase 2 of the experiment, 24 hrs after the injection, all of the rats were tested with controllable shock for 30 minutes on the contralateral limb.
Figure 6.
Master training protected the spinal cord against the loss of plasticity observed with an injection of 1% capsaicin. For Experiment 4, subjects were trained with controllable shock, or nothing, prior to the 1% capsaicin injection (A). Master and Unshocked rats treated with vehicle learned, displaying an increased response duration across time (B, left panel). Unshocked subjects treated with 1% capsaicin failed to learn, but those pretrained with controllable electrical stimulation learned at a level commensurate with vehicle-treated controls (B, center panel). Mean response durations (± SEM) are shown in the far right panel for each condition.
Tactile reactivity was assessed with von Frey stimuli 1) before training (baseline), 2) after training, 3) after the injection of capsaicin or vehicle, and 4) before testing.
Results
Instrumental Learning
Phase I: Training
The two Master groups, treated with controllable shock, did not differ on measures of the duration of the first flexion response or the shock intensity required to elicit a flexion force of 0.4 N (both Fs < 1.0, p > .05). During training, Master rats exhibited an increase in flexion duration over time, F(29, 290) = 6.00, p < .001, and the two groups exhibited comparable learning, F(1, 10) < 1.0, p > .05.
Phase II: Testing
Prior to testing, the average shock intensity needed to induce a flexion of 0.4 N ranged from 0.34 ± 0.02 to 0.36 ± 0.02 mA. Initial shock durations ranged from 0.14 ± 0.01s to 0.36 ± 0.23 s. Independent ANOVAs revealed no differences between groups (both Fs < 1.0, p > .05).
As shown in Figure 6B, both the Master and Unshocked rats treated with vehicle learned in the testing period. Master rats treated with capsaicin also learned, but the Unshocked rats treated with capsaicin failed. There was a significant main effect of drug on flexion duration, F(1, 20) = 25.59, p < .0001, and a main effect of training condition, F(1, 20) = 11.28, p < .01. There was also a significant Drug × Training Condition interaction, F(1, 20) = 18.62, p < .001. Training with controllable shock appears to have protected the spinal cord against the detrimental effects of a moderate dose of capsaicin.
Tactile Reactivity
Baseline reactivity thresholds ranged from 6.43 (± 0.05) to 6.58 (± 0.03) on the injected leg, and from 6.52 (± 0.05) to 6.58 (± 0.02) on the non-injected leg. The reactivity thresholds did not differ across groups, F(1, 20) < 1.0, p > .05. Master training did not influence tactile reactivity, F(1, 20) < 1.0, p > (Figure 7).
Figure 7.
Master training protected the spinal cord circuitry against the development of long-term allodynia. As found for the 3% dose, 1% capsaicin significantly lowered reactivity thresholds, on the injected leg, 5 min after the injection of capsaicin (A, right panel, Post-injection). Capsaicin-treated rats that trained with controllable shock, however, resembled vehicle treated rats for the pre-testing assessment on both the injected and noninjected legs (right panels, Pre-testing). This effect is highlighted with the hatched boxes. Unshocked rats that were treated with capsaicin were allodynic in the Pre-testing period. Vehicle-treated rats did not display any change in tactile reactivity throughout the assessment periods.
As found in Experiment 3, the injection of capsaicin significantly lowered tactile reactivity thresholds (Figure 7), F(1, 20) = 12.58, p < .01. Moreover, all subjects displayed lowered reactivity thresholds on the injected leg (Figure 7A), compared to the noninjected leg, F(1, 20) = 18.97, p < .001. There was no effect of training condition, F(1, 20)= 3.22, p > .05, or any Drug × Training Condition interaction at the post-injection time point, F(1, 20) < 1.0, p > .05.
Interestingly, by the time of testing master subjects treated with capsaicin resembled vehicle treated rats (Pretesting, Figure 7). Only Unshocked rats treated with capsaicin displayed a significant allodynic response. Master training appears to induce a change in spinal neural circuitry that protects against the development of long-term allodynia with peripheral injury. There was a significant interaction between drug treatment and training condition, F(1, 20) = 19.55, p < .001; Figure 7. There were also significant main effects of drug and training condition on tactile reactivity thresholds at this time, both Fs (1, 20) < 66.59, p < .0001. There was a significant effect of leg, F(1, 20) = 24.24, p < .0001 and a Leg × Drug Treatment interaction, F(1, 20) = 21.44, p < .001 prior to testing; all subjects displayed lower reactivity thresholds on the treated leg.
Summary
The results of Experiment 4 indicate that training with electrical stimulation paradigms can be used to modulate the consequences of a moderate form of peripheral inflammation. Moreover, it suggests that there is a common mechanism mediating the effects of uncontrollable electrical stimulation and the afferent barrage induced with capsaicin.
Experiment 5: Naltrexone Blocks Expression of the Deficit
We have previously shown that naltrexone can be used to block the expression of the learning deficit induced with uncontrollable electrical stimulation (Joynes & Grau, 2004), temporarily restoring plasticity for re-training the spinal cord. The capacity to reinstate plasticity after peripheral inflammation would be clinically important for therapies that aim to reestablish adaptive neural circuits following spinal injury, including treadmill training and functional electrical stimulation paradigms. Experiment 5 tests the hypothesis that naltrexone could be used to block the expression of the learning deficit induced with capsaicin.
Method
Twenty-four spinalized rats were randomly assigned to one of 4 groups (n = 6). Half of the subjects were given a subcutaneous (s.c.) injection of capsaicin (1%) while the remaining subjects received a s.c. injection of the vehicle. Six hours after the injection, subjects were given an intrathecal (i.t.) injection of naltrexone (7 μg in 1 μl) or vehicle and were tested with controllable shock. This results in a 2 (capsaicin or vehicle) × 2 (naltrexone or vehicle) experimental design (Figure 8A).
Figure 8.
The design of Experiment 5 is outlined in Panel (A). As found in prior studies, rats treated with s.c. vehicle displayed an increase in response duration across the testing period, indicative of learning (B, left panel). Rats treated with s.c. 1% capsaicin and i.t. naltrexone (B, center panel), 6 hrs later, also learned at a level commensurate with s.c. vehicle controls (B, left panel). Rats treated with s.c. 1% capsaicin and i.t. saline did not learn; they displayed no change in response duration across the testing period (B, center panel). Naltrexone blocked the expression of the learning deficit induced with 1% capsaicin. Mean response durations (± SEM) are shown in the far right panel for each condition.
Tactile reactivity was assessed with von Frey stimuli (1) before the injection of capsaicin or vehicle (baseline), (2) after the injection of capsaicin or vehicle, and (3) before testing.
Results
Instrumental Learning
The shock intensity needed to induce a flexion of 0.4 N ranged from 0.32 ± 0.02 to 0.36 ± 0.02 mA. Initial flexion durations ranged from 0.21 ± 0.01 to 0.25 ± 0.07 s. There was no effect of capsaicin or naltrexone treatment on either dependent variable (both Fs < 1.37, p > .05).
As shown in Figure 8B, subjects that did not receive capsaicin (s.c. Vehicle) exhibited an increase in flexion duration across the 30 minutes of testing. Capsaicin treatment (s.c. 1% Capsaicin) disrupted learning in the i.t. saline controls. This learning deficit was reversed by i.t. naltrexone. There was a significant main effect of capsaicin treatment on flexion duration, F(1, 20) = 8.21, p < .01, and an interaction between capsaicin and i.t. naltrexone, F(1, 20) = 4.38, p < .05. There was no main effect of naltrexone alone, F(1, 20) = 2.24, p > .05.
Tactile Reactivity
Baseline tactile reactivity ranged from 6.42 (± 0.06) to 6.61 (± 0.02) across groups. This difference, due to chance, was statistically significant (Fs > 5.09, p < .05). To control for these baseline differences, all subsequent analyses were performed on a change from baseline score.
In contrast to the previous experiments, capsaicin per se produced a modest effect that did not reach statistical significance, both Fs (1, 20) < 3.16, p > .05. There was a significant main effect of leg (injected versus not injected) on tactile reactivity prior to testing, F(1,18) = 14.69, p < .001, but no differences emerged across groups. All subjects displayed lower reactivity thresholds on the leg ipsilateral to the injection prior to testing. No other comparisons were significant.
Summary
As found for uncontrollable electrical stimulation, naltrexone blocked the expression of the learning deficit induced with capsaicin, and allowed for adaptive learning in the isolated cord. Perhaps because of the relatively low concentration of capsaicin used in this study, we did not observe a significant effect of capsaicin on tactile reactivity immediately after injection. Naltrexone treatment may have masked the effects of capsaicin prior to testing: both the s.c. capsaicin and vehicle treated groups displayed lower reactivity thresholds prior to testing. Indeed, in Experiment 4, more robust allodynia was observed in unshocked rats (using 1% capsaicin) 24 hrs after the injection.
Experiment 6: Controllable Stimulation as a Therapy
A previous study suggests that naltrexone alone does not have a restorative effect. Using uncontrollable electrical stimulation, Crown and Grau (2001) induced a learning deficit in spinalized rats. They then gave naltrexone alone or combined drug treatment with instrumental training. Twenty-four hours later only the rats given both naltrexone and instrumental training were able to learn. Naltrexone alone did not eliminate the learning deficit. The longterm reinstatement of plasticity appeared to depend on controllable shock training.
Experiment 6 examined whether controllable stimulation was also critical for the restoration of plasticity after the injection of capsaicin. We compared rats trained with naltrexone and controllable shock to those treated with naltrexone alone. The capacity for instrumental learning was assessed 18 hours after naltrexone administration, to ensure that the drug was no longer pharmacologically active.
Method
Thirty-two subjects were randomly assigned to one of 4 groups (n = 8). Initially, half of the subjects were given a s.c. injection of capsaicin (1%) while the remaining subjects received an injection of the vehicle. Six hours after the injection, half of the capsaicin treated groups, and half of the vehicle controls, were given naltrexone and trained with controllable electrical stimulation (Master). The remaining subjects (Unshocked) were given naltrexone alone (Unshocked). Eighteen hours later all subjects were tested with 30 minutes of controllable electrical stimulation. (The Master rats, treated with i.t. naltrexone, in Experiment 5 were used as subjects in this study also. Therefore, training data is not reported in the results of this experiment.) A summary of this experimental design is given in Figure 9A. As a further control, a fifth group of subjects (n = 8) were included in an initial analysis to verify that naltrexone alone did not affect the maintenance of the learning deficit. These subjects were treated with capsaicin, then 6 hours later were given the i.t. vehicle alone (Unshocked). They were tested 18 hours later.
Figure 9.
Experiment 6 examined the effects of controllable stimulation training on the learning deficit induced with 1% capsaicin (A). Controllable stimulation training, combined with naltrexone, reversed the deficit. Master subjects, treated with s.c. capsaicin and i.t. naltrexone, displayed an increase in response duration across the testing period (B, center panel). There were no significant differences in the response durations displayed by these subjects and either group of s.c. vehicle controls (B, left panel). Subjects that received s.c. capsaicin and i.t. naltrexone without controllable stimulation training, displayed significantly lower response durations than their trained counterparts (B, center panel). Mean response durations (± SEM) are shown in the far right panel for each condition.
Baseline tactile reactivity was assessed before the capsaicin injection. Changes in reactivity were assessed (1) 5 min after injection, (2) immediately prior to training, (3) after training, and (4) prior to testing.
Results
Instrumental Learning
The shock intensity needed to induce a flexion force of 0.4 N ranged from 0.53 ± 0.02 to 0.54 ± 0.03 mA. Initial shock durations ranged from 0.13 ± 0.00 to 0.16 ± 0.01 s. Neither drug treatment (capsaicin or vehicle) nor training condition (Master or Unshocked) affected these measures of baseline reactivity (both Fs < 1.0, p > .05).
First, to examine the suffiency of naltrexone alone as a therapy, we compared the responses of the two groups of unshocked capsaicin-treated rats. One of these groups had been treated with i.t. naltrexone while the other had received i.t. vehicle. There was no long-term effect of i.t. naltrexone treatment on the capsaicininduced learning deficit, F(1, 21) < 1.0, p > .05; data not shown.
Having established that naltrexone alone did not affect the maintenance of the learning deficit, we assessed whether combining naltrexone treatment with instrumental training could attenuate the capsaicin-induced deficit. As usual, rats that did not receive capsaicin treatment (vehicle) exhibited a progressive increase in response duration over the 30 minutes of testing. Rats that received capsaicin followed by naltrexone alone exhibited poor learning when tested 24 hours later. Rats that received training with controllable shock after capsaicin treatment did not exhibit a learning deficit. This yielded a significant Drug × Training Condition interaction, F(1, 28) = 4.19, p < .05; Figure 9. The main effects of drug and training condition alone were not significant, both Fs (1, 28) < 1.99, p > .05. As can be seen in Figure 9B, the rats given capsaicin and treated with controllable shock showed even higher levels of learning than the vehicle treated controls.
Tactile Reactivity
Baseline reactivity thresholds did not differ significantly across groups, F(1, 26) < 1.0, p > .05. Moreover, as found for Experiment 5, capsaicin did not alter mechanical reactivity thresholds prior to, or after, instrumental training, all Fs (1, 26) < 2.06, p > .05. Capsaicin treated rats did show slight allodynia in the injected leg prior to testing, F(1, 26) = 5.93, p < .05, but this was not affected by instrumental training, F(1, 26) = 1.06, p > .05, data not shown).
Summary
As found for uncontrollable electrical stimulation, naltrexone alone did not eliminate the learning deficit. Instead, controllable shock training, combined with the opioid antagonist, is necessary for the long-term reinstatement of plasticity.
Discussion
Central Sensitization and Learning
Our previous studies have shown that uncontrollable electrical stimulation undermines the adaptive plasticity of the spinal cord, and renders the system incapable of instrumental conditioning (Grau et al., 1998, 2006; Grau & Hook, 2006). Ferguson et al. (2006) suggested that this learning deficit is due to the development of a state akin to central sensitization. Supporting this, they demonstrated that intradermal formalin treatment undermines learning (Ferguson et al., 2006). The studies reported here support these findings and, by using capsaicin treatment, implicate C-fibers in the induction of the deficit. Specifically, we found that peripheral capsaicin treatment undermines instrumental learning in a dose-dependent manner (Experiment 1), and this loss of adaptive plasticity lasts at least 24 hours (Experiment 2). Pretraining with controllable shock attenuates the deficit, and this effect is most evident when a moderate concentration of capsaicin (1%) is used (Experiment 4). Moreover, as found for uncontrollable electrical stimulation (Joynes & Grau, 2004; Crown & Grau, 2001), naltrexone can reverse the expression of the deficit induced (Experiment 5) and, in combination with behavioral training, decrease the long-term consequences of capsaicin treatment (Experiment 6).
These findings are important, in part, because they demonstrate that our work using uncontrollable electrical stimulation generalizes to more naturalistic physiological states. We have used electrical stimulation previously because it allows for precise control over the activation of nociceptive fibers and the elicitation of behavior. Objective control over the timing of stimulation is not only relevant to instrumental learning, it is also important for functional electrical stimulation (FES) therapies. Also, electrical stimulation allowed us to study the effects of nociceptive input without inducing lasting peripheral tissue damage. A criticism of this approach, however, has been that electrical stimulation is an artificial stimulus and, as such, it was not clear whether the consequences of uncontrollable shock had a natural analog. The present experiment suggests that it does. Peripheral inflammation has similar behavioral consequences to uncontrollable electrical stimulation.
Like the deficit induced with uncontrollable electrical stimulation, the learning deficit observed after inflammation was temporarily reversed by the administration of an opioid antagonist. These data are consistent with other recent studies that implicate the opioid receptor in the expression of the learning deficit (Washburn, Maultsby, & Grau, 2005). Moreover, they concur with studies linking the maintenance of central sensitization to an upregulation of dynorphin within the dorsal spinal cord (Draisci, Kajander, Dubner, Bennett, & Iadarola, 1991; Dubner & Ruda, 1992; Kajander, Sahara, Iadarola, & Bennett, 1990;). Extending these studies, our data also suggest that blocking the opiate system may be clinically useful in a sensitized spinal system. When we blocked the expression of the capsaicin-induced deficit with naltrexone, we were able to retrain the spinal cord and reinstate adaptive plasticity. Controllable electrical stimulation was integral to this therapy (Experiment 6). Controllable electrical stimulation may, therefore, provide a means for modulating the plasticity, as well as guiding the development, of spinal neural circuits (Hook & Grau, 2007). These observations have important clinical implications with regards to the application of functional electrical stimulation. They suggest that response-contingent feedback, in an electrical stimulation paradigm, can influence the induction and the maintenance of central sensitization and thereby influence the development of neuropathic pain.
Impact on Mechanical Allodynia
Tests of mechanical reactivity demonstrated that a high concentration of capsaicin (3%) induced a robust state of allodynia. Interestingly, instrumental control slowed the development of allodynia after the injection of 3% capsaicin, and caused the allodynia observed after a 1% dose to decay more rapidly (Experiment 4). The effects of controllable stimulation seen in Experiments 3 and 4 suggest that the induction of tactile allodynia is modulated by neural circuits involved in instrumental training. However, in contrast to the pre-training data, it is less clear whether therapy using controllable stimulation affects allodynia. As discussed previously, this may be due, in part, to use of a low concentration of capsaicin (1%) that produced a relatively weak allodynia in the untrained subjects (Experiments 5 and 6).
While instrumental training modulated the induction of allodynia, when given before capsaicin, training per se had little effect on tactile reactivity. Controllable stimulation did not appear to reduce tactile reactivity, and uncontrollable stimulation did not produce allodynia. These findings are in contrast to an earlier report by Ferguson et al. (2006) who showed that 6 minutes of intermittent shock enhanced mechanical reactivity. This discrepancy may be partially explained by methodological differences. Specifically, the procedures used across these studies differed in the amount of shock applied and the time between testing and training. Whereas Ferguson et al. (2006) applied a fixed amount of stimulation, the current study involved Master training and the amount of shock applied to the Yoked rat, receiving uncontrollable stimulation, depended on the performance of the Master. Also, in cases where the Master rat rapidly learned, few shocks occurred after the first 10 minutes of training and, as a consequence, approximately 20 minutes would elapse between shock administration and the post-training assessment of tactile reactivity. In the study of Ferguson et al. (2006), tactile reactivity was assessed immediately after training with uncontrollable shock. Another key difference between these studies concerns experimental power; the study of Ferguson et al. (2006) used group sizes that were roughly 2 times larger than those used in the present study. Despite this discrepancy it is clear that a high concentration of capsaicin (3%) produces a more robust allodynia than uncontrollable shock. The effect of shock appears to be more akin to the 1% capsaicin concentration, which also induced an allodynia of a modest magnitude.
Neurochemical/Neurophysiological Mechanisms
There appear to be strong parallels between the effects of uncontrollable stimulation and central sensitization at multiple levels of analysis. At a molecular level, the learning deficit has been linked to a kappa opioid mediated process (Joynes & Grau, 2004). The deficit induced with uncontrollable electrical stimulation also depends on NMDA receptor activation (Ferguson et al., 2006; Joynes et al., 2004), metabotropic group I glutamate receptors (Ferguson et al., 2004), and the neurokinin I (NKI) substance P receptor (Baumbauer, Young, Hoy, & Joynes, 2007). Ferguson, Washburn, Crown, & Grau (2003) also found that administration of the GABA antagonist, bicuculline, attenuated the learning deficit in a dose-dependent fashion. Intracellular processes that are integral to the induction of the deficit involve protein kinase C (PKC) activation (Bolding et al., 2003) and protein synthesis (Patton et al., 2004; Baumbauer, Young, Hoy, France, & Joynes, 2006). Paralleling these findings, the development of central sensitization depends on NMDA (Dougherty et al., 1992; Soliman, Yu, & Coderre, 2005; Zou et al., 2002), metabotropic group I glutamate (Fang, Wu, Zhang, Lin, & Willis, 2003; Neugebauer, Chen, & Willis, 2000; Soliman et al., 2005), and NK1 receptors (Dougherty, Palecek, Paleckova, & Willis, 1994). The maintenance of central sensitization is also correlated with an upregulation of the kappa opioid, dynorphin (Draisci et al., 1991; Dubner & Ruda, 1992; Kajander et al., 1990), and the present studies show that the learning deficit induced with capsaicin can be reversed with an opioid antagonist. Inflammation also increases GABA immunoreactivity within spinal laminae I to III, and the allodynia characteristic of central sensitization is attenuated by the GABA-A antagonist bicuculline (Castro-Lopez, Tavares, Tolle, & Coimbra, 1994; Sluka, Willis, & Westlund, 1993, 1994). Numerous studies have also demonstrated the necessity of PKC activation in the induction of central sensitization (Igwe & Chronwall, 2001; Kawasaki et al., 2004; Lin, Peng, & Willis, 1996; Sluka, Rees, Chen, Tsuruoka, & Willis, 1997; Zhou, Li, & Zhao, 2003; Zou, Lin, & Willis, 2004). It appears that at a cellular level the effects of uncontrollable stimulation and central sensitization depend on remarkably similar molecular processes.
Contrasting with the deficit, our data suggest that adaptive response modifications and inflammation have opposing effects on plasticity and nociceptive processing. Whereas adaptive response modifications appear to enable the learning of selective responses (instrumental learning) and inhibit the generalized increase in mechanical reactivity observed as a consequence of capsaicin exposure, inflammation enhances mechanical reactivity and inhibits instrumental learning. Previous research has established that, at a molecular level, both processes involve a form of NMDARmediated plasticity (Dougherty et al., 1992; Ferguson et al., 2006; Joynes et al., 2004; Soliman et al., 2005; Zou et al., 2002;). We have previously hypothesized that the maladaptive plasticity involved in central sensitization induces a diffuse state of overexcitation in the spinal cord that saturates plasticity and precludes the subsequent learning of selective response-outcome relations (Ferguson et al., 2006). The studies reported here support a complementary prediction: that instrumental learning induces a state that counters the development and/or maintenance of over-excitation. Specifically, our data suggest that instrumental learning protects the cord against the diffuse increase in neural excitability, producing molecular changes that prevent the induction of the deficit and, potentially, reduce the excitotoxic effects resulting from central sensitization at the cellular level.
In our laboratory, we have started to explore the differences between controllable and uncontrollable electrical stimulation at the neurochemical level. In collaboration with Gomez-Pinilla et al. (2007), we found that training with controllable shock increased BDNF mRNA expression, while uncontrollable stimulation downregulated expression. We proposed that this training-induced increase in BDNF expression might enable learning when Master subjects were tested with a more difficult response criterion. Supporting this, we found that a microinjection of BDNF fostered learning and a BDNF inhibitor blocked the enabling effects of prior training with controllable shock (Huie, Gomez-Pinilla, Ying, Edgerton, & Grau, 2005). Intrathecal administration of BDNF also blocked the development of the learning deficit induced with uncontrollable shock (Huie, Hoy, Miranda, & Grau, 2007). These data suggest that BDNF might mediate the beneficial effects of controllable stimulation.
The effects of controllable and uncontrollable electrical stimulation may also depend on different fiber types. The finding that capsaicin led to a learning deficit implicates C-fibers and the release of Substance P in the maladaptive loss of plasticity. Indeed, antagonism of the Substance P (neurokinin 1 [NK1]) receptor blocks the induction of the learning deficit (Baumbauer et al., 2007). Conversely, an intrathecal microinjection of a Substance P analog inhibits learning (Baumbauer et al., 2007). However, while Substance P inhibits learning, administration of an NK1 receptor antagonist does not interfere with learning. This suggests that the acquisition of an instrumental response does not depend on C-fiber activity, and implicates myelinated A-fibers instead. It would appear that if A-fiber input is correlated with a regular signal of limb position it leads to learning and inhibits some of the consequences of C-fiber activity (e.g., the induction of central sensitization and the learning deficit).
This adaptive effect also seems to depend, in part, upon an appropriate level of stimulation. The stimulation must be intense enough to elicit a robust behavioral response, or the subjects habituate to the stimulus (Grau et al., 1998). Our previous studies have shown that intermediate shock intensities (i.e., intensities that elicit a flexion force of 0.4 N) produce optimal learning, as well as a learning deficit in yoked rats (Grau et al., 1998). Assuming that the induction of the deficit requires C-fiber activity, these data suggest that the intensity of stimulation required for learning is suprathreshold for C-fibers. We hypothesize that during adaptive learning A-fiber input must be modulating the effects of C-fiber stimulation.
Our studies have shown that the induction of adaptive learning undermines the development of the learning deficit, and vice versa. Therefore, if learning depends on A-fiber activity, the loss of adaptive plasticity must also involve modification of these circuits. We know that with peripheral inflammation Aβ-fibers can undergo a phenotypic switch and begin to act like C-fibers; they express Substance P and BDNF (Mannion et al., 1999; Neumann, Doubell, Leslie, & Woolf, 1996). Consequently, innocuous mechanical stimuli that activate Aβ-fibers now elicit pain. The longterm effects of the learning deficit induced with capsaicin may depend on such a modification of A-fiber activity.
Implications
Overall, the data reported here have significant clinical implications. Noxious input from a central or peripheral injury may undermine the functional plasticity of the spinal system, and the changes that they produce may affect long-term recovery. To restore function after a spinal injury it is necessary to not only preserve or regenerate spinal neurons, but also to retain the capacity to establish functional neural circuits that can modulate behavioral output. In fact, these data may explain the divergent effects of behavioral locomotor training exercises, with some patients benefiting from the training while others do not. Our data suggest that in the presence of central sensitization, behavioral training exercises will not modify spinal circuits and facilitate recovery. However, our results suggest that behavioral training could, if coupled with naltrexone treatment, engage adaptive plasticity in the spinal cord and counter the adverse effects of central sensitization. Indeed, the reinstatement of adaptive plasticity in the injured spinal system may not only facilitate recovery, it may also be an important step in the treatment of neuropathic pain.
Acknowledgments
This study was supported by NS41548, NS51433, and DA020596. We would like to thank Kyle Baumbauer, Sohum Desai, Kevin Hoy, Denise Puga, and Stephanie Washburn for their comments on an earlier draft of this manuscript. A portion of the data from this study has been previously presented in abstract form.
References
- Barthelemy D, Leblond H, Provencher J, Rossignol S. Nonlocomotor and locomotor hindlimb responses evoked by electrical microstimulation of the lumbar cord in spinalized cats. Journal of Neurophysiology. 2006;96:3273–3292. doi: 10.1152/jn.00203.2006. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Jr., France JL, Joynes RL. Intrathecal infusions of anisomycin impact the learning deficit but not the learning effect observed in spinal rats that have received instrumental training. Behavioural Brain Research. 2006;173:299–309. doi: 10.1016/j.bbr.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Jr., Joynes RL. Intrathecal administration of neurokinin 1 and neurokinin 2 receptor antagonists undermines the savings effect in spinal rats seen in an instrumental learning paradigm. Behavioral Neuroscience. 2007;121:186–199. doi: 10.1037/0735-7044.121.1.186. [DOI] [PubMed] [Google Scholar]
- Bigbee AJ, Crown ED, Ferguson AR, Roy RR, Tillakaratne NJK, Tobin AJ, et al. Two chronic motor training paradigms differentially alter the potential to perform a novel motor learning task in spinally transected rats. Behavioural Brain Research. 2007;180:95–101. doi: 10.1016/j.bbr.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolding KA, Hook MA, Ferguson AR, Grau JW. Abstract Viewer/Itinerary Planner [CD-ROM] Society for Neuroscience; Washington, DC: 2003. The general protein kinase C inhibitor, bisindolylmaleimide-I, dosedependently prevents a shock-induced behavioral deficit in spinalized rats. [Google Scholar]
- Castro-Lopes JM, Tavares I, Tolle TR, Coimbra A. Carrageenan-induced inflammation of the hind foot provokes arise of GABA-immunoreactive cells in the rat spinal cord that is prevented by peripheral neurectomy or neonatal capsaicin treatment. Pain. 1994;56:193–201. doi: 10.1016/0304-3959(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. Increased pain sensitivity following heat injury involves a central mechanism. Behavioural Brain Research. 1985;15:259–262. doi: 10.1016/0166-4328(85)90181-0. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behavioral Neuroscience. 2002a;116:1032–1051. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord. II. Evidence for central mediation. Physiology and Behavior. 2002b;77:259–267. doi: 10.1016/s0031-9384(02)00859-4. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Preserving and restoring behavioral potential within the spinal cord using an instrumental training paradigm. Journal of Neurophysiology. 2001;86:845–855. doi: 10.1152/jn.2001.86.2.845. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Sorkin LS, Willis WD. The role of NMDA and non-NMDA excitatory amino acid receptors in the excitation of primate spinothalamic tract neurons by mechanical, chemical, thermal, and electrical stimuli. Journal of Neuroscience. 1992;12:3025–3041. doi: 10.1523/JNEUROSCI.12-08-03025.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Willis WD. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. Journal of Neurophysiology. 1994;72:1464–1475. doi: 10.1152/jn.1994.72.4.1464. [DOI] [PubMed] [Google Scholar]
- Draisci G, Kajander KC, Dubner R, Bennett GJ, Iadarola MJ. Up-regulation of opioid gene expression in spinal cord evoked by experimental nerve injuries and inflammation. Brain Research. 1991;560:186–192. doi: 10.1016/0006-8993(91)91231-o. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends in Neuroscience. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, de Leon RD. Neural Darwinism in the mammalian spinal cord. In: Patterson MM, Grau JW, editors. Spinal cord plasticity: Alterations in reflex function. Kluwer Academic; Boston: 2001. pp. 185–206. [Google Scholar]
- Edgerton VR, Roy RR, de Leon RD, Tillakaratne N, Hodgson JA. Does motor learning occur in the spinal cord? Neuroscientist. 1997;3:287–294. [Google Scholar]
- Edgerton VR, Roy RR, Hodgson JA, Gregor RJ, De Guzman CP. Restorative neurology, plasticity of motoneuronal connections. Elselvier; New York: 1991. Recovery of full weight-supporting locomotion of the hindlimbs after complete thoracic spinalization of adult and neonatal cats; pp. 405–418. [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annual Review of Neuroscience. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. Journal of Neuroscience. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Wu J, Zhang X, Lin Q, Willis WD. Increased phosphorylation of the GluR1 subunit of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor in rats following intradermal injection of capsaicin. Neuroscience. 2003;122:237–245. doi: 10.1016/s0306-4522(03)00526-8. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Washburn SN, Crown ED, Grau JW. GABAA receptor activation is involved in non-contingent shock inhibition of instrumental conditioning in spinal rat. Behavioral Neuro-science. 2003;117:799–812. doi: 10.1037/0735-7044.117.4.799. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S. The locomotion of the acute spinal cat injected with clonidine i.v. Brain Research. 1973;50:184–186. doi: 10.1016/0006-8993(73)90606-9. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Huie JR, Ying Z, Ferguson A, Crown ED, Baumbauer KM, Edgerton VR, Grau JW. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience. 2007;148:893–906. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behavioral Neuro-science. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental Learning Within the Spinal Cord: Underlying Mechanisms and Implications for Recovery After Injury. Behavioral and Cognitive Neuroscience Reviews. 2006;5:1–48. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grau JW, Hook MA. Spinal neurons exhibit a surprising capacity to learn and a hidden vulnerability when freed from the brain's control. Current Neurology and Neuroscience Reports. 2006;6:177–180. doi: 10.1007/s11910-006-0001-3. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brookhart JM, Mountcastle VB, editors. Handbook of physiology. The nervous system II. American Physiological Society; Bethseda, MD: 1981. pp. 1179–1236. [Google Scholar]
- Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Medicine and Science in Sports and Exercise. 1994;26:1491–1497. [PubMed] [Google Scholar]
- Hook MA, Grau JW. An animal model of functional electrical stimulation: Evidence that the central nervous system modulates the consequences of training. Spinal Cord. 2007;45:702–712. doi: 10.1038/sj.sc.3102096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie JR, Gomez-Pinilla F, Ying Z, Edgerton VR, Grau JW. BDNF enables learning in the rat spinal cord. Program No. 175.16. 2005 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2005. [Google Scholar]
- Huie JR, Hoy KC, Miranda RA, Grau JW. Instrumental learning in the spinalized rat: A therapeutic effect of BDNF. 2007 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2007. [Google Scholar]
- Igwe OJ, Chronwall BM. Hyperalgesia induced by peripheral inflammation is mediated by protein kinase C betaII isozyme in the rat spinal cord. Neuroscience. 2001;104:875–890. doi: 10.1016/s0306-4522(01)00107-5. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Ferguson AR, Crown ED, Patton BC, Grau JW. Instrumental learning within the spinal cord: V. Evidence the behavioral deficit observed after noncontingent nociceptive stimulation reflects an intraspinal modification. Behavioral Brain Research. 2003;141:159–170. doi: 10.1016/s0166-4328(02)00372-8. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Grau JW. Instrumental learning within the spinal cord: III. Prior exposure to noncontingent shock induces a behavioral deficit that is blocked by an opioid antagonist. Neurobiology of Learning and Memory. 2004;82:35–51. doi: 10.1016/j.nlm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Janjua K, Grau JW. Instrumental learning within the spinal cord: VI. The NMDA receptor antagonist, AP5, disrupts the acquisition and maintenance of an acquired flexion response. Behavioral Brain Research. 2004;154:431–438. doi: 10.1016/j.bbr.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ. Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 1990;11:719–728. doi: 10.1016/0196-9781(90)90187-a. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, et al. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphor-ylation in dorsal horn neurons, leading to central sensitization. Journal of Neuroscience. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: Psychophysical studies of underlying mechanisms. Journal of Neurophysiology. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- Langlet C, Leblond H, Rossignol S. Mid-lumbar segments are needed for the expression of locomotion in chronic spinal cats. Journal of Neurophysiology. 2005;93:2474–2488. doi: 10.1152/jn.00909.2004. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, et al. Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability. Journal of Neurophysiology. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- Lin Q, Peng YB, Willis WD. Possible role of protein kinase C in the sensitization of primate spinothalamic tract neurons. Journal of Neuroscience. 1996;16:3026–3034. doi: 10.1523/JNEUROSCI.16-09-03026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GT, Ferguson AR, Crown ED, Bopp AC, Miranda RC, Grau JW. Instrumental learning within the rat spinal cord: Localization of the essential neural circuit. Behavioral Neuroscience. 2005;119:538–547. doi: 10.1037/0735-7044.119.2.538. [DOI] [PubMed] [Google Scholar]
- Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, et al. Neurotrophins: Peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hyper-sensitivity. Proceedings of the National Academy of Sciences USA. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Chen PS, Willis WD. Groups II and III metabotropic glutamate receptors differentially modulate brief and prolonged nociception in primate STT cells. Journal of Neurophysiology. 2000;84:2998–3009. doi: 10.1152/jn.2000.84.6.2998. [DOI] [PubMed] [Google Scholar]
- Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- Patton BC, Hook MA, Ferguson AR, Crown ED, Grau JW. The behavioral deficit observed following noncontingent shock in spinalized rats is prevented by the protein synthesis inhibitor cycloheximide. Behavioral Neuroscience. 2004;118:653–658. doi: 10.1037/0735-7044.118.3.653. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Chau C, Brustein E, Belanger M, Barbeau H, Drew T. Locomotor capacities after complete and partial lesions of the spinal cord. Acta neurobiologiae experimentalis. 1996;56:449–463. doi: 10.55782/ane-1996-1148. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, et al. Neurogenic hyperalgesia: Central neural correlates in responses of spinothalamic tract neurons. Journal of Neurophysiology. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD. Inhibitors of G-proteins and protein kinases reduce the sensitization to mechanical stimulation and the desensitization to heat of spinothalamic tract neurons induced by intradermal injection of capsaicin in the primate. Experimental Brain Research. 1997;115:15–24. doi: 10.1007/pl00005675. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Willis WD, Westlund KN. Joint inflammation and hyperalgesia are reduced by spinal bicuculline. Neuroreport. 1993;5:109–112. doi: 10.1097/00001756-199311180-00003. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Willis WD, Westlund KN. Inflammationinducedrelease of excitatory amino acids is prevented by spinal administration of a GABAA but not by a GABAB receptor antagonist in rats. The Journal of Pharmacology and Experimental Therapeutics. 1994;271:76–82. [PubMed] [Google Scholar]
- Soliman AC, Yu JS, Coderre TJ. mGlu and NMDA receptor contributions to capsaicin-induced thermal and mechanical hypersensitivity. Neuropharmacology. 2005;48:325–332. doi: 10.1016/j.neuropharm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Washburn SN, Maultsby ML, Grau JW. The K2 opioid receptor agonist GR89696 has both adverse and beneficial effects on spinal cord plasticity. Program No. 396.19. 2005 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2005. [Google Scholar]
- Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on `rules of spinal locomotion' is effective in spinal cord injured persons. European Journal of Neuroscience. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Willis WD. Role of neurotransmitters in sensitization of pain responses. Annals of the New York Academy of the Sciences. 2001;933:142–156. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- Willis WD. Long-term potentiation in spinothalamic neurons. Brain Research Reviews. 2002;40:202–214. doi: 10.1016/s0165-0173(02)00202-3. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiology and Behavior. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Young EE, Baumbauer KM, Elliot A, Joynes RL. The impact of neonatal injury on spinally mediated instrumental learning in adult rats. Behavioral Neuroscience. 2007 doi: 10.1037/0735-7044.121.5.1095. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Li GD, Zhao ZQ. State-dependent phosphorylation of epsilon-isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. Journal of Neurochemistry. 2003;85:571–580. doi: 10.1046/j.1471-4159.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience. 2002;115:775–786. doi: 10.1016/s0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Research. 2004;1020:95–105. doi: 10.1016/j.brainres.2004.06.017. [DOI] [PubMed] [Google Scholar]