Abstract
Mean diffusivity (MD), the rotationally invariant magnitude of water diffusion that is greater in CSF and smaller in organized brain tissue, has been suggested to reflect schizophrenia-associated cortical atrophy. Regional changes, associations with CSF, and the effects of genetic predisposition towards schizophrenia, however, remain uncertain. Six-direction DTI and high-resolution structural images were obtained from 26 schizophrenia patients, 36 unaffected first-degree patient relatives, 20 control subjects and 32 control relatives (N = 114). Registration procedures aligned DTI data across imaging modalities. MD was averaged within lobar regions and the cingulate and superior temporal gyri. CSF volume and MD were highly correlated. Significant bilateral temporal, and superior temporal MD increases were observed in schizophrenia compared to unrelated control probands. First-degree relatives of schizophrenia probands showed larger MD measures compared to controls within bilateral superior temporal regions with CSF volume correction. Superior temporal lobe brain tissue deficits and proximal CSF enlargements are widely documented in schizophrenia. Larger MD indices in patients and their relatives may thus reflect similar pathophysiological mechanisms. However, persistence of regional MD effects after controlling for CSF volume, suggests that MD is a sensitive biological marker of disease and genetic liability, characterizing at least partially distinct aspects of brain structural integrity.
Keywords: Diffusion tensor imaging, superior temporal gyrus, brain structure, genetic predisposition
1. Introduction
A substantial amount of imaging evidence supports that schizophrenia affects multiple brain systems. When brain abnormalities similar to those detected in schizophrenia patients are also observed in unaffected biological relatives of patients, differences in structural and/or functional anatomy may indicate markers of schizophrenia-related genetic susceptibility. However, the identification of imaging endophenotypes that show robust disease effects and that indicate a genetic vulnerability towards the disorder has proved challenging. Advanced analysis strategies exploiting different imaging modalities may increase the likelihood of detecting subtle brain changes that serve as biological markers for schizophrenia and schizophrenia-related genetic predisposition, as well as elucidate the pathophysiological mechanisms underlying the disease.
Cerebrospinal fluid (CSF) enlargements are amongst the most replicated structural brain abnormalities observed in chronic schizophrenia (Shenton et al., 2001; Narr et al., 2006). CSF increases are also reported in healthy biological relatives of schizophrenia patients (Seidman et al., 1997; Cannon et al., 1998; Sharma et al., 1998; Staal et al., 2000; Styner et al., 2005), suggesting schizophrenia-related genetic factors contribute to increases in brain CSF compartments, although this hypothesis is not always supported (Cannon et al., 1998; van Haren et al., 2004). During normal brain maturation and aging, brain tissue loss is accompanied by increases in CSF. In schizophrenia, CSF enlargements are similarly associated with brain tissue reductions (Suddath et al., 1989; Gaser et al., 2004). However, although reduced brain tissue volumes, particularly of gray matter, appear characteristic of the disorder, effect sizes are small and regional findings lack consistency (Ward et al., 1996; Shenton et al., 2001). That is, while changes in gray matter properties (volume, density and/or laminar thickness) in medial and superior lateral temporal regions are most replicated, gray matter changes in frontal, parietal and occipital sensory, motor and/or association cortices are in less agreement across studies (Shenton et al., 2001; Kuperberg et al., 2003; Honea et al., 2005; Narr et al., 2005a; Narr et al., 2005b). Variations of CSF, which complement modest global and/or regional changes in gray or white matter, may thus provide a more reliable biomarker for the disease (Narr et al., 2003).
Recently, diffusion tensor imaging (DTI) methods have been employed to isolate schizophrenia-related changes in brain structure not measurable with other imaging modalities. With DTI, the magnetic resonance (MR) signal is made sensitive to the random diffusion of water molecules. When using at least six non-collinear gradient directions, the diffusion tensor in each image voxel can be calculated to estimate the magnitude and the principal directions of diffusion (Basser et al., 1994). Since water diffusion is more likely to be anisotropic in ordered white matter and less anisotropic within gray matter, CSF, or where fibers are disorganized, rotationally invariant scalar measures derived from the diffusion tensor can be used as a metric of white matter integrity. Mean diffusivity (MD), the trace of the diffusion tensor divided by three (or the directionally averaged apparent diffusion coefficient (ADC)), is another measure that may be extracted from DTI data or from any set of diffusion-weighted images with three or more directions to characterize brain structure. Unlike diffusion anisotropy measures, which are higher in coherent white matter, MD is greater in CSF where water diffusion is less restricted by cellular fibers and structure (Basser and Pierpaoli 1996).
To date, most schizophrenia DTI studies have focused on estimating changes in white matter integrity using fractional anisotropy (FA) indices (Kubicki et al., 2007). Although fewer investigations have examined MD as a means to characterize changes in brain morphology, and in spite of small sample sizes, prior evidence suggests MD increases occur within the cerebral tissue of schizophrenia subjects. For example, five recent studies using whole-brain voxel-based methods report MD increases in some overlapping brain regions (Ardekani et al., 2005; DeLisi et al., 2006; Rose et al., 2006; Shin et al., 2006; White et al., 2007). Specifically, Ardekani et al. (2005) reported larger MD in the insular, temporal (lateral and medial) and occipital regions in schizophrenia (n = 15) compared to healthy subjects (n = 15). This result was similar to the findings of Shin et al. (2006), who showed increased MD in temporo-frontal regions in patients (n = 19) compared to controls (n = 21). Similarly, MD increases in temporal (parahippocampal and superior temporal) regions were identified by Rose and colleagues (2006), although clusters of significance distributed in medial frontal, parietal and subcortical regions were further observed in patients (n = 12) with respect to controls (n = 12). The only published study assessing MD in individuals at high-risk for schizophrenia (n = 15), in addition to schizophrenia patients (n = 15) and controls (n = 25), identified clusters of greater MD in left frontal, parahippocampal and occipital regions in both high-risk and schizophrenia subjects compared to controls (DeLisi et al., 2006). Finally, MD increases in posterior hippocampal regions have been implicated in child and adolescent onset schizophrenia (n = 15), when compared to demographically similar healthy subjects (n = 15) (White et al., 2007).
Although prior evidence suggests that schizophrenia-related MD increases occur in frontal and lateral and medial temporal regions most consistently, considerable variations in the spatial location of findings exist at the voxel-level. Inter-subject variation in brain structure, the lower resolution of DTI data and accompanying registration errors may all influence the ability to map the precise location of MD effects at the voxel level. Examining MD changes in larger search regions, however, may circumvent some of these issues and benefit from increases in statistical power. Thus, in this study we applied a regions-of-interest (ROI) approach to investigate local MD changes in schizophrenia. Using spatially aligned high-resolution T1 MR imaging data, MD was measured in lobar regions as well as the cingulate and superior temporal gyrus; cortical regions that are amongst those most implicated in the disorder (Shenton et al., 2001; Narr et al., 2005b). Since CSF enlargements are widely observed in schizophrenia and may reflect the same neuropathological processes assessed by MD, we further aimed to establish the relationships between these measures, and examined MD changes before and after removing the variance associated with CSF volume from the data. Relationships between MD and gray matter and white matter volume were additionally assessed. Finally, since some evidence suggests that CSF enlargements and possibly MD changes indicate a genetic predisposition for the disorder (DeLisi et al., 2006), we newly investigated the presence of disease as well as potential genetic-liability effects by studying schizophrenia patients, unaffected first-degree relatives of patients, healthy control subjects and control first-degree relatives (N = 114).
2. Methods
2.1 Subjects
Subjects included 26 adult-onset schizophrenia patients, 36 unaffected first-degree relatives of patients, 20 healthy control subjects and 32 control first-degree relatives recruited from 59 nuclear families. Table 1 provides the demographic and clinical details of subjects. Exclusion criteria for all subjects included neurological disorders (e.g., temporal lobe epilepsy) and mental retardation and any evidence of drug abuse or alcoholism within at least six months prior to assessment. Patients were additionally excluded if there was any evidence that a past history of substance abuse triggered the psychotic episode, interfered with diagnosis or was a factor in the course of illness. Control subjects were excluded if they had any past history of alcohol abuse. Schizophrenia diagnosis was confirmed by consensus as determined by DSM-IV criteria using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1994) and by informant information. Clinical symptoms were assessed using the expanded 24-item Brief Psychiatric Rating Scale (BPRS; Ventura et al., 2000). Family members of schizophrenia patients were included if they were a first-degree biological relative of a patient with schizophrenia and met the inclusion criteria for all subjects. Community control probands were recruited with demographic profiles similar to those of the schizophrenia probands. Control subjects and first-degree relatives of controls were screened by clinical interview using the SCID-NP to exclude schizophrenia and schizoaffective disorder and potential schizophrenia spectrum disorders (schizotypal, paranoid, avoidant, and schizoid personality disorders).
Table 1.
Demographic Information
| Schizophrenia Family Members | Control Family Members | |||
|---|---|---|---|---|
| Probands (n = 26) |
Relatives (n = 36) |
Probands (n = 20) |
Relatives (n = 32) |
|
| Relationship (parents/siblings) | -- | 25/11 | -- | 20/12 |
| Sex (M/F) | 17/9 | 16/20 | 8/12 | 20/12 |
| Age (mean ± SD) | 32.15 ± 9.17 | 47.14 ± 14.88 | 290.00 ± 8.55 | 46.09 ± 16.54 |
| Socioeconomic Index* (mean ± SD) | 27.27 ± 9.63 | 43.09 ± 22.25 | 40.79 ± 15.76 | 39.84 ± 20.03 |
| Years of Education (mean ± SD) | 13.96 ± 1.86 | 14.46 ± 3.83 | 15.30 ± 1.92 | 14.34 ± 3.39 |
| Age of Onset (mean ± SD) | 22.12 ± 4.17 | -- | -- | -- |
| Duration of Illness (mean ± SD) | 10.04 ± 7.64 | -- | -- | -- |
| BPRS Thought Disturbance (positive symptom factor) (mean ± SD) | 1.72 ± 0.94 | |||
| BPRS Anergia (negative symptom factor) (mean ± SD) | 1.52 ± 0.42 | |||
| Total 24-item BPRS (mean ± SD) | 39.06 ± 7.42 | |||
Socioeconomic status was estimated using the Duncan Total Socioeconomic Index (SEI). These index scores were not applicable for n = 10 schizophrenia probands (9 unemployed, 1 on disability), n = 2 patient relatives (1 unemployed, 1 high school student), n = 3 control probands (3 unemployed), and n = 6 control relatives (1 unemployed, 4 retired, 1 high school student). BRPS factor scores were computed according to Guy et al., 1976.
Patients with schizophrenia were recruited through admissions and referrals from the UCLA Aftercare Research Program and local public and private psychiatric hospitals and clinics in the Los Angeles area. Patients were currently receiving standard antipsychotic medication treatments (risperidone: n = 9, olanzapine: n = 4, ziprasidone: n = 2, aripiprazole: n = 7, haldol: n = 1, clozapine: n = 1, quetiapine: n = 1, fluphenazine: n = 1). Community control subjects were recruited using lists provided by a survey research company and telephone contact. The UCLA Institutional Review Board (IRB) approved all research procedures and informed written consent was obtained from all subjects.
2.2 Image Acquisition and Preprocessing
Whole brain DTI data was obtained on a Siemens 1.5T Sonata system where diffusion was measured in six non-collinear directions including four averages (3×3×3 mm3, b-values 0, 1000; 50 axial brain slices oriented along the AC-PC line). High-resolution T1-weighted structural MR data, collected on the same 1.5T Siemens scanner, included a 3D MP-RAGE sequence also with four averages (NEX) (FOV: 256; 1×1×1 mm3; TR = 1900 ms; TE = 4.38; Flip Angle: 15°).
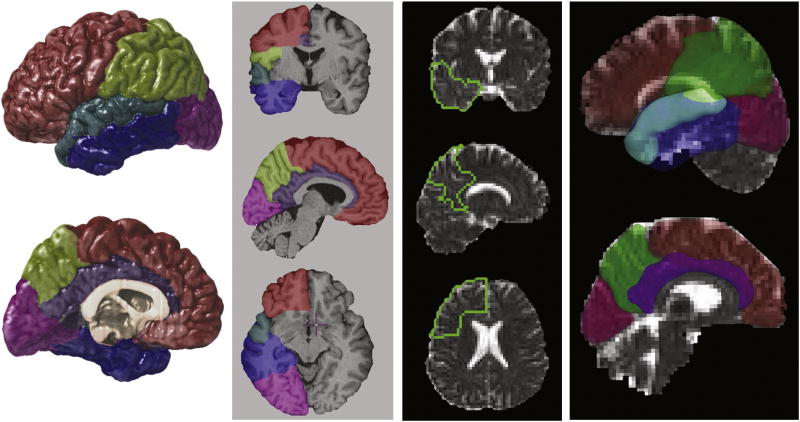
Extra-cortical tissue was removed from the high-resolution structural MR data using BET (Smith 2002) where small errors in automated processing were manually corrected on a slice-by-slice basis. Image volumes were then corrected for RF inhomogeneities (Sled and Pike 1998), and for head tilt and orientation using a three-translation and three-rotation rigid-body transformation (without scaling) (Woods et al., 1998a; Woods et al., 1998b). Frontal, temporal, parietal and occipital as well as superior temporal and cingulate gyrus ROIs were defined using established anatomic protocols (Marquardt et al., 2005; Taylor et al., 2005) and sulcal/gyral landmarks for which intra- and inter-rater reliability has been previously established (Narr et al., 2005a; Narr et al., 2005b) [Figure 1]. Scalp-edited structural data was classified on a voxel-wise basis into the categories of gray matter, white matter and CSF using a partial volume method (Shattuck et al., 2001). Volumes of each tissue compartment (gray matter, white matter and CSF) and CSF volume estimates from each ROI were obtained for all subjects.
Figure 1.
Regions of interest (ROIs), including the frontal, temporal, parietal and occipital lobe and the cingulate and superior temporal gyrus, were defined in T1-weighted structural MR imaging data (left). A twelve-parameter affine registration method aligned the ROIs to corresponding DTI b0 volumes. The ROIs are shown superimposed on MD images from one subject (right).
Siemens hardware coupled with pulse sequence design was used to ensure very low eddy current distortions in the data. Specifically, our diffusion preparation used a dual bipolar diffusion gradient and a double spin echo, thus achieving a high robustness against eddy currents. For DTI data processing, affine six-parameter registrations were used to align DTI images within subjects to reduce any residual eddy current effects, which were not apparent based on visual inspection, and to correct for subject motion across data acquisition. That is, each diffusion-weighted image was registered to the b0 image of each average, and the b0 images from each average were registered to the first b0 image obtained from each subject (Woods et al., 1998a; Woods et al., 1998b). Twelve parameter affine registrations were then used to align the DTI images from each subject to a template b0 image obtained from averaging the b0 images from a single healthy control subject. Software developed locally reconstructed the diffusion tensor at each voxel, providing measures of the three principal diffusion constants (eigenvalues) and diffusion directions (eigenvectors) (Basser et al., 1994). MD was estimated for each voxel according to the formula given in (Basser and Pierpaoli 1996). In order to obtain average measures of MD within each ROI, twelve parameter affine registrations were used to align the high-resolution T1-weighted volumes and structural ROIs to native DTI space (Woods et al., 1998a; Woods et al., 1998b). ROIs derived from the T1 volumes were thus aligned to the diffusion images of each subject. MD measures were then obtained within frontal, temporal, parietal, occipital, superior temporal and cingulate gyrus ROIs for each subject [Figure 1].
2.3 Statistical Analysis
Pearson's correlation analyses were performed to examine the relationships between whole brain MD and whole brain CSF, gray and white matter volumes across all subjects. Relationships between regional CSF volumes, obtained from the tissue segmented structural MR data, and regional MD indices were additionally examined.
To address our hypothesis that regional MD increases occur in schizophrenia, as well as to a lesser extent in biological relatives of patients, with respect to comparison subjects, we employed two analysis strategies. To identify regional schizophrenia effects, we compared schizophrenia probands to unrelated control probands thereby also circumventing the need to model the covariances between related subjects. To establish the presence of schizophrenia-related genetic liability effects, we examined MD changes in family members of schizophrenia patients compared to healthy controls and their relatives, taking into account the relatedness of individuals.
Schizophrenia effects were assessed using disease status (schizophrenia probands versus unrelated control probands) as a fixed factor and MD measures obtained from each ROI, not shown to deviate from normality, as dependent variables. Sex and age were used as covariates, and all statistical tests were performed both with and without controlling for regional CSF volumes. Additional correction for total brain volume was not employed given that MD, representing the overall magnitude of diffusion, is not expected to change as a function of brain size. Since six separate regions were examined, and examination of regional measures in each hemisphere was not considered to constitute an independent hypothesis, a Bonferroni-corrected two-tailed alpha level of P < 0.008 was adopted as the new threshold of significance.
To examine schizophrenia genetic-liability effects, mixed-model ANOVAs, comparing relatives of patients with control probands and relatives, were performed for each ROI including family membership as a random factor. Statistical analyses again controlled for sex and age, and results were examined both with and without covarying for CSF volume. Since first-degree family members of schizophrenia patients share only half their genes on average with patient probands, the magnitude of genetic-liability effects are expected to be of lesser magnitude than those effects observed in schizophrenia. Thus, Bonferroni correction was considered too conservative. Instead, for the examination of genetic liability effects, an uncorrected two-tailed alpha level of P < 0.05 was adopted as the threshold of significance. Since healthy control probands were included in the examinations of schizophrenia and genetic liability effects as a means to maximize statistical power, post-hoc analyses comparing only patient and control relatives were additionally performed to provide complete independence of these two analyses.
Finally, for descriptive purposes, the analyses described above were applied to determine whether regional CSF volume changes with and without controlling for the variance in overall CSF volume occur within the same regions showing schizophrenia and schizophrenia-related genetic liability effects for MD. In addition, for regions showing both significant disease and genetic liability effects of MD, we performed post-hoc analyses of regional gray matter volume changes.
3. Results
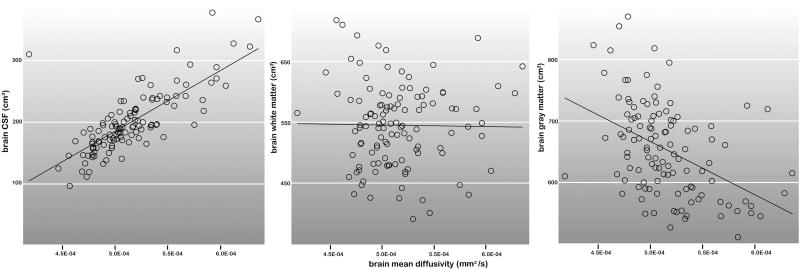
MD and CSF measures obtained from the entire brain (Pearson's r = .80, df = 113, P < 0.0001) and for each region of interest (r: .39-.82, df = 113, P < 0.0001) were highly correlated. Whole brain MD and gray matter volumes also showed significant relationships (r = -.44, df = 113, P < 0.0001), though to a lesser degree. Relationships between MD and white matter volumes were not significant (r = -.01, df = 113, P > 0.10). The relationships of whole brain MD with each brain tissue compartment are plotted in Figure 2.
Figure 2.
The relationship between MD and each tissue compartment (CSF, white matter and gray matter) for the entire brain are shown across all groups (N = 114).
Schizophrenia probands exhibited significantly larger MD indices within the left, F(1, 45) = 8.02, P < 0.007, and right temporal lobe, F(1,45) = 11.73, P < 0.001, and within the left, F(1,45) = 12.31, P < 0.001, and right, F(1,45) = 19.32, P < 0.0001, superior temporal gyrus, compared to unrelated control probands. Effects in the same regions were pronounced after covarying for CSF volume, (F(1,44) = 14. 89; F(1,44) = 22.39; F(1,44) = 25.17; F(1,44) = 28.58, all P < 0.0001, for left and right temporal, and superior temporal gyrus regions respectively).
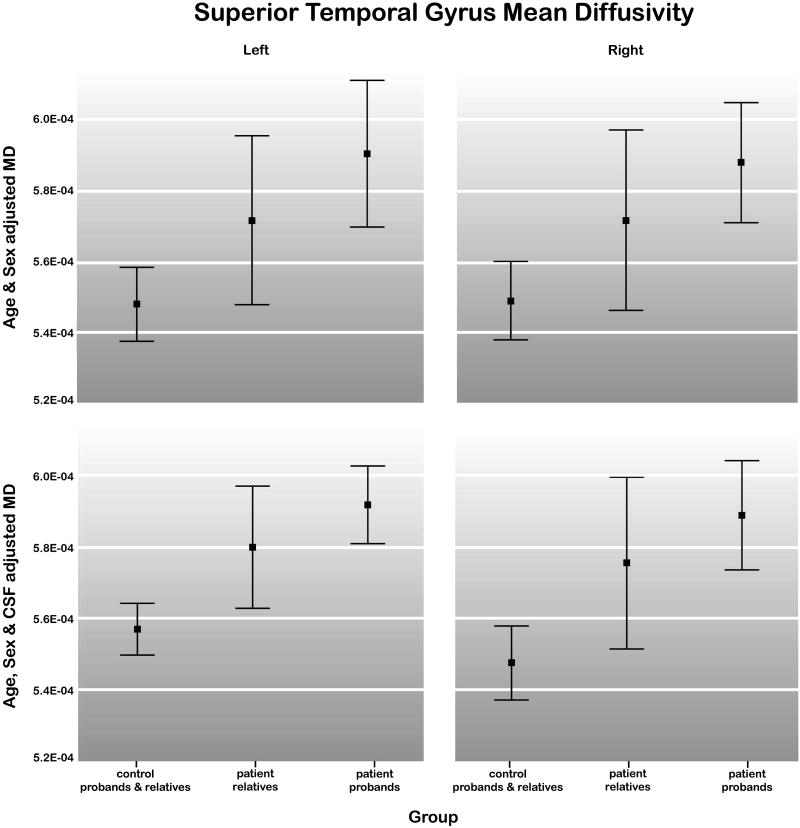
First-degree relatives from families with a schizophrenia proband showed increased MD with respect to control subjects and relatives in the left and right superior temporal gyrus after covarying for CSF volume, F(1,84) = 7.05 and F(1,84) = 5.90 respectively, both P < 0.01. These effects were at trend level significance without covarying for CSF (P < 0.10). Genetic liability findings were similar when control relatives and patient relatives were compared without including control probands in the analysis. That is, patient relatives showed larger MD indices in the left (F(1,64) = 4.14, P < 0.05) and marginally in the right (F(1,64) = 3.86, P < 0.054) superior temporal gyrus with CSF volume correction compared to control relatives. Significant schizophrenia and genetic liability effects for MD were absent for the other ROIs examined. Figure 3 shows the 95% confidence intervals of the means for sex and age-regressed superior temporal gyrus MD within control probands and relatives, relatives of schizophrenia patients and schizophrenia probands with and without CSF volume correction.
Figure 3.
Means and 95% confidence intervals for left and right superior temporal gyrus MD within control probands and relatives (n = 52), patient relatives (n = 36) and schizophrenia probands (n = 26). Results are shown after adjusting the data for sex and age (top) and for sex, age and CSF volume (bottom).
For examination of regional CSF volumes, schizophrenia probands showed greater CSF within superior temporal gyrus regions bilaterally (left: (F(1,44) = 12.59; right: F(1,44) = 4.70, P < 0.03) after correction for overall CSF volume, and within left superior temporal gyrus regions F(1,45) = 5.53, P < 0.02 without correction. Significant schizophrenia liability effects were observed for the left superior temporal gyrus after covarying for global CSF volume, F(1,83) = 6.70, P < 0.01. Means and standard deviations for sex and age-adjusted MD and CSF volume measures are provided in Table 2 within groups defined by disease status and family membership.
Table 2.
Means and Standard Deviations (SD) for sex and age-adjusted regional MD (mm2/s) and CSF volume (cm3) measures.
| Patient Family Members | Control Family Members | |||||||
|---|---|---|---|---|---|---|---|---|
| MD (Mean ± SD) | CSF (Mean ± SD) | MD (Mean ± SD) | CSF (Mean ± SD) | |||||
| ROI | Proband (n = 26) |
Relative (n = 36) |
Proband (n = 26) |
Relative (n = 36) |
Proband (n = 20) |
Relative (n = 32) |
Proband (n = 20) |
Relative (n = 32) |
| All Brain | 5.17E-04 ± 2.81E-05 | 5.15E-04 ± 4.39E-05 | 197.09 ± 37.19 | 197.38 ± 48.71 | 5.10E-04 ± 2.00E-05 | 5.18E-04 ± 3.06E-05 | 194.97 ± 28.22 | 208.32 ± 37.66 |
| Cingulate L | 4.72E-04 ± 2.67E-05 | 4.77E-04 ± 5.06E-05 | 2.98 ± 0.71 | 3.15 ± 1.02 | 4.77E-04 ± 2.18E-05 | 4.88E-04 ± 3.65E-05 | 3.21 ± 0.60 | 3.29 ± 0.98 |
| Cingulate R | 4.95E-04 ± 3.36E-05 | 4.96E-04 ± 5.10E-05 | 2.78 ± 0.72 | 2.90 ± 1.04 | 4.99E-04 ± 2.77E-05 | 5.05E-04 ± 4.45E-05 | 2.86 ± 0.54 | 2.95 ± 0.88 |
| Frontal L | 5.17E-04 ± 4.66E-05 | 5.22E-04 ± 6.38E-05 | 39.59 ± 9.97 | 40.02 ± 12.50 | 5.11E-04 ± 3.12E-05 | 5.17E-04 ± 4.60E-05 | 39.65 ± 6.75 | 41.30 ± 9.54 |
| Frontal R | 5.18E-04 ± 4.89E-05 | 5.21E-04 ± 6.33E-05 | 40.60 ± 9.28 | 41.12 ± 11.83 | 5.11E-04 ± 3.42E-05 | 5.22E-04 ± 4.68E-05 | 41.54 ± 7.95 | 42.98 ± 9.68 |
| Temporal La | 5.46E-04 ± 3.01E-05 | 5.46E-04 ± 2.99E-05 | 16.39 ± 4.06 | 16.16 ± 4.52 | 5.24E-04 ± 1.09E-05 | 5.32E-04 ± 2.09E-05 | 14.92 ± 2.97 | 16.36 ± 3.27 |
| Temporal Ra | 5.24E-04 ± 1.84E-05 | 5.24E-04 ± 1.82E-05 | 16.18 ± 3.38 | 16.80 ± 3.96 | 5.06E-04 ± 1.47E-05 | 5.15E-04 ± 2.25E-05 | 16.23 ± 3.24 | 17.44 ± 3.10 |
| STG La,b,c,d | 5.94E-04 ± 4.34E-05 | 5.91E-04 ± 3.24E-05 | 7.53 ± 1.78 | 7.33 ± 2.17 | 5.53E-04 ± 2.63E-05 | 5.66E-04 ± 3.37E-05 | 6.48 ± 1.30 | 7.11 ± 1.57 |
| STG Ra,b,c | 5.76E-04 ± 3.23E-05 | 5.76E-04 ± 3.24E-05 | 6.26 ± 1.38 | 6.17 ± 1.66 | 5.38E-04 ± 2.63E-05 | 5.52E-04 ± 3.37E-05 | 5.65 ± 1.03 | 6.13 ± 1.33 |
| Parietal L | 5.21E-04 ± 3.02E-05 | 5.21E-04 ± 2.99E-05 | 22.26 ± 4.98 | 22.47 ± 6.40 | 5.16E-04 ± 2.65E-05 | 5.26E-04 ± 3.65E-05 | 21.96 ± 4.94 | 24.09 ± 5.01 |
| Parietal R | 5.04E-04 ± 2.83E-05 | 5.04E-04 ± 2.79E-05 | 20.94 ± 4.11 | 21.58 ± 5.73 | 5.02E-04 ± 2.89E-05 | 5.14E-04 ± 3.66E-05 | 21.91 ± 4.70 | 23.41 ± 4.65 |
| Occip L | 4.84E-04 ± 2.87E-05 | 4.87E-04 ± 3.10E-05 | 5.45 ± 1.69 | 5.03 ± 1.41 | 4.82E-04 ± 2.03E-05 | 4.89E-04 ± 2.90E-05 | 5.24 ± 1.14 | 5.86 ± 1.59 |
| Occip R | 5.00E-04 ± 3.08E-05 | 4.98E-04 ± 2.87E-05 | 6.38 ± 1.81 | 5.93 ± 1.67 | 4.98E-04 ± 1.94E-05 | 5.02E-04 ± 3.04E-05 | 6.17 ± 1.46 | 6.65 ± 1.64 |
Means reflect values that have been residualized for age and sex where the residuals have been added to the overall mean for the entire sample so that relative group differences in MD and CSF are apparent.
Significant schizophrenia and
genetic liability effects for MD.
Significant schizophrenia effects and
genetic liability effects for CSF volume, STG = superior temporal gyrus, Occip = occipital, L = left, R = right.
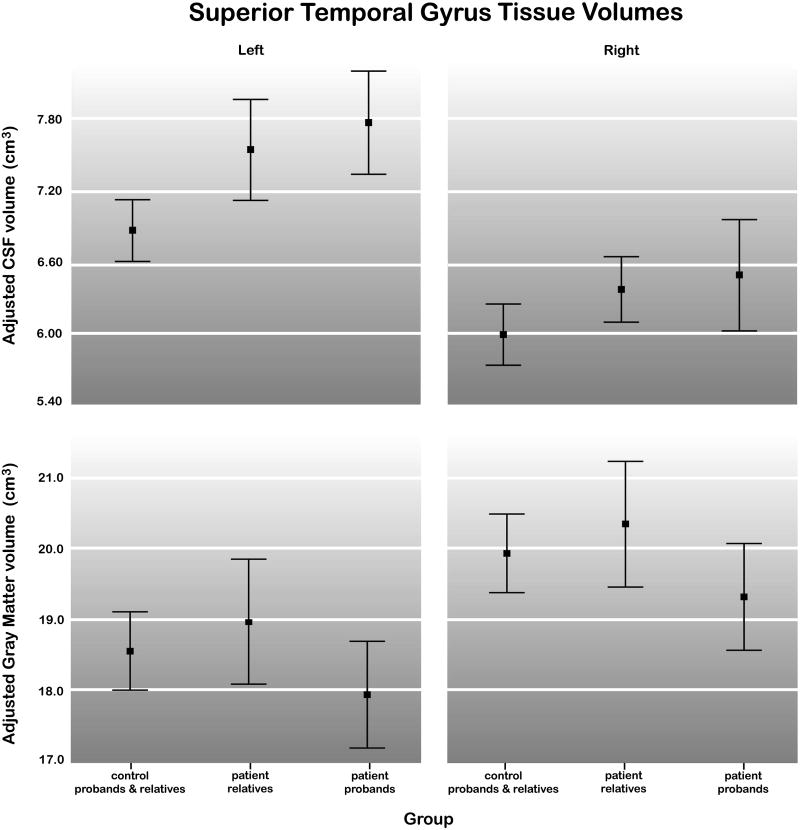
Finally, post-hoc analyses of gray matter volumes within the superior temporal gyrus showed significant schizophrenia effects for the right hemisphere, F(1, 44) = 4.22, P < 0.04 after correction for overall gray matter volume (age and sex-adjusted mean ± SD for schizophrenia probands: 18.44 ± 1.72 cm3 and control probands: 18.76 ± 2.19 cm3), but not in the left hemisphere, P>0.05 (mean ± SD for schizophrenia probands: 19.95 ± 2.10 cm3 and control probands: 19.91 ± 2.30 cm3). Relatives of patients with schizophrenia failed to exhibit significant differences in superior temporal gyrus gray matter volumes compared to healthy subjects, P > 0.10. Figure 4 shows the means and 95% confidence intervals for sex and age-regressed superior temporal gyrus CSF and gray matter volumes within control probands and relatives, relatives of schizophrenia patients and schizophrenia probands.
Figure 4.
Means and 95% confidence intervals for left and right superior temporal gyrus CSF (top) and gray matter (bottom) within control probands and relatives (n = 52), patient relatives (n = 36) and schizophrenia probands (n = 26). Results are shown after adjusting the data for sex, age and whole brain CSF (top) and sex, age and whole brain gray matter (bottom).
4. Discussion
Our data shows that MD increases within superior temporal regions serve as a biological marker for schizophrenia and schizophrenia-related genetic liability. Although it is possible that the superior temporal MD increases observed in schizophrenia patients and first-degree relatives of patients may result from harmful environmental effects shared by schizophrenia probands and their relatives, the inclusion of both parents and siblings for analyses renders this hypothesis less likely. However, the examination of twin cohorts or siblings raised apart, or the assessment of genetic liability as a quantitative measure in multiply affected families (McDonald et al., 2004), may be necessary to empirically confirm the role of genetic versus shared-environmental influences on increased MD. Our findings also demonstrate that MD and CSF, and to a lesser degree MD and gray matter volume, are highly correlated. These relationships suggest that diffusion and brain tissue measures obtained from different imaging modalities may reflect the same underlying neuropathology in schizophrenia. However, disease and schizophrenia liability effects of MD remain after removing the variance associated with changes in CSF volume, where MD effects were present in some regions even in the absence of significant regional CSF changes. Thus, MD appears to characterize some distinct aspects of brain structural integrity in schizophrenia.
Although MD increases within the temporal lobe, particularly the superior temporal gyrus, may serve as a unique biomarker of disease processes in schizophrenia, MD may also act to isolate changes in brain structure that are measurable, but less sensitive when surveyed with other imaging modalities. In support of this hypothesis, changes in MD have been observed in patients with head trauma even in the absence of observable abnormalities in conventional MR data (Rugg-Gunn et al., 2001; Chappell et al., 2006; Salmond et al., 2006). MD thus appears to serve as a marker for disturbances in tissue microstructure that stem from neuronal swelling or shrinkage, changes in the extracellular space, and/or loss of axons and dendritic fibers. Reports of neuronal atrophy, changes in neuronal density and reduced neuropil have been documented in many schizophrenia postmortem studies (Selemon and Goldman-Rakic 1999; Broadbelt et al., 2002; Black et al., 2004; Glantz et al., 2006). Changes in the cellular or interstitial fluid compartments and reductions of neuropil may similarly account for the MD changes, and perhaps to a lesser extent for changes in signal intensities segmenting as CSF or gray matter measured from T1-weighted imaging data, in schizophrenia.
The significant MD increases in schizophrenia may also reflect sulcal and subarachnoid CSF enlargements, separate from or in conjunction with, abnormalities in tissue microstructure. Notably, larger CSF to brain tissue ratios and increased sulcal CSF are widely reported in schizophrenia (Shenton et al., 2001). While fewer studies have investigated the regional specificity of extra-cortical CSF changes, prominent increases in subarachnoid and sulcal CSF surrounding perisylvian cortices, including the superior temporal gyrus bilaterally, have been documented in independent schizophrenia samples when CSF changes were examined at very high spatial resolution (Narr et al., 2003; Narr et al., 2006). These observations are compatible with our findings of larger superior temporal CSF volumes shown by schizophrenia patients and biological relatives of patients. Other ROI studies similarly document CSF increases in temporal regions in schizophrenia patients (Zipursky et al., 1994; Cannon et al., 1998; Sullivan et al., 1998; Hietala et al., 2003), and in healthy siblings of patients (Cannon et al., 1998), although CSF increases other brain regions are also reported (Andreasen et al., 1994; Woods et al., 1996; Cannon et al., 1998).
Gray matter reductions along with proximal increases of CSF manifest during normative brain maturation and aging (Courchesne et al., 2000). Some investigators have suggested that MD, which we have shown is positively associated with CSF and negatively associated with gray matter, may act as a surrogate marker for detecting cortical atrophy in schizophrenia (Ardekani et al., 2003; Ardekani et al., 2005; DeLisi et al., 2006). Although cortical gray matter abnormalities have been reported across several sensory, motor and association regions in schizophrenia, temporal lobe deficits, especially of superior temporal regions, are particularly reproducible in schizophrenia imaging studies (Shenton et al., 2001). In our study, post-hoc analyses showed significant reductions of right superior temporal gyrus gray matter volume in patients compared to controls, although effects were at trend level significance for the left hemisphere and relatives of schizophrenia probands did not show significant evidence of superior temporal gray matter changes in either hemisphere. These findings thus support the conjecture that MD is a more sensitive measure for isolating regional changes in cerebral structure that signify atrophy and/or disturbances in tissue microstructure.
The majority of prior DTI studies in schizophrenia have focused on examining scalar measures of diffusion anisotropy, particularly FA. Although FA changes (mostly reductions) have been reported in distributed brain regions (Kubicki et al., 2007), inconsistencies in results, perhaps attributable to different methodological approaches applied in small cohorts, are frequent. Although future studies are necessary to better understand how MD relates to diffusion anisotropy measures that act as a marker for white matter integrity, our data show that MD and white matter volume measures are poorly correlated. Our findings of increased MD within temporal/superior temporal regions in schizophrenia patients are compatible with findings from prior studies using voxel-wise methods of analysis (Ardekani et al., 2003; DeLisi et al., 2006; Rose et al., 2006; Shin et al., 2006; White et al., 2007). Although MD increases have been documented in several different brain regions with only some overlap across studies, all investigations reported increased MD in temporal (medial and/or lateral) lobe regions in schizophrenia. Only one prior investigation has examined MD in family members at risk for developing schizophrenia (DeLisi et al., 2006). Results showed increased MD in left parahippocampal and lingual gyrus regions (temporo-occipital lobe juncture) and within small clusters in the left frontal lobe in both patients and high-risk subjects, compared to controls. Notably, when ventricular size was measured in the same subjects, only schizophrenia patients were shown to differ from controls. Though subject relatedness was not addressed and voxel-based methods were employed in this prior investigation, these results lend support to the hypothesis that MD may be a more sensitive measure than CSF volume assessments for the early prediction of schizophrenia and/or genetic liability.
There are some limitations to this investigation that may also account for discrepancies observed in regional MD results across studies. Firstly, the relatively low spatial resolution of our DTI data may allow less precise measurement of MD within ROIs. Confounds relating to the use of larger voxel sizes, however, are arguably more severe when comparisons are made at the voxel level. Notwithstanding, very localized changes of MD discernable in voxel-based studies may remain undetected when using larger ROIs. Since our study focused on examining cortical ROIs, we were not able to detect MD changes that may be specific to the thalamus or hippocampus, regions that are widely implicated in structural and functional neuropathology of schizophrenia (Andreasen et al., 1999; Narr et al., 2002; Narr et al., 2004; McDonald et al., 2005; Rose et al., 2006). However, since these smaller subcortical regions are defined primarily by gray matter, diffusion imaging data with greater spatial and angular resolution may be necessary to detect changes within these structures. Partial volume effects due to CSF (Alexander et al., 2001) or other tissue types may also contribute to discrepant regional MD findings in schizophrenia. Although we controlled for CSF volumes, it is possible that CSF changes still act to influence MD measurements. Future studies may address this issue by using FLAIR-DTI sequence to suppress effects of CSF directly. Although our study groups were large relative to prior DTI investigations, subjects were not optimally matched for sex. However, differences in subject demographics were addressed statistically. Furthermore, although we combined control probands and relatives to maximize statistical power when assessing genetic liability effects for MD, post-hoc analyses comparing patient and control relatives exclusively were shown to reveal similar results. Previous research has shown that type and duration of medication treatments influence brain structure in schizophrenia, e.g., (Lieberman et al., 2005). Notably, our observation of regional MD changes in biological relatives of patients similar to those observed in patients suggests that MD effects are not attributable to medication effects or complicating factors relating to the illness specifically.
Our findings, examined within the context of prior schizophrenia studies exploiting different imaging modalities, support that MD reflects both unique aspects of structural brain integrity in schizophrenia, but is also related to structural changes detected in CSF (and to a lesser extent with gray matter) through a common biological mechanism. Changes in MR signal, however, appear to manifest as more robust MD differences. These findings may thus also indicate that MD is a more sensitive marker of brain tissue deficits than signal intensity variations measured in T1-weighted imaging data. Our results support that MD increases within superior temporal cortices, regions that are widely implicated in the disorder, serve as a biomarker for schizophrenia and schizophrenia-related genetic predisposition.
Acknowledgments
This work was generously supported by research grants from the National Center for Research Resources (P41 RR13642), the National Institute of Mental Health (R01 MH49716 and P50 MH066286), the NIH Roadmap for Medical Research (U54 RR021813 entitled Center for Computational Biology (CCB)), and a Career Development Award (K01 MH073990, to KLN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. Analysis of partial volume effects in diffusion-tensor MRI. Magnetic Resonance in Medicine. 2001;45:770–780. doi: 10.1002/mrm.1105. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V, 2nd, O'Leary DS, Ehrhardt JC, Yuh WT. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. Journal of the American Medical Association. 1994;272:1763–1769. [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biological Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Bappal A, D'Angelo D, Ashtari M, Lencz T, Szeszko PR, Butler PD, Javitt DC, Lim KO, Hrabe J, Nierenberg J, Branch CA, Hoptman MJ. Brain morphometry using diffusion-weighted magnetic resonance imaging: Application to schizophrenia. Neuroreport. 2005;16:1455–1459. doi: 10.1097/01.wnr.0000177001.27569.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. American Journal of Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophrenia Research. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TGM, Huttunen M, Loennqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjoeld-Nordenstam CG, Gur RE, Yan M. Regional grey matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Archives of General Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Chappell MH, Ulug AM, Zhang L, Heitger MH, Jordan BD, Zimmerman RD, Watts R. Distribution of microstructural damage in the brains of professional boxers: A diffusion MRI study. Journal of Magnetic Resonance Imaging. 2006;24:537–542. doi: 10.1002/jmri.20656. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Szulc KU, Bertisch H, Majcher M, Brown K, Bappal A, Branch CA, Ardekani BA. Early detection of schizophrenia by diffusion weighted imaging. Psychiatry Research. 2006;148:61–66. doi: 10.1016/j.pscychresns.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV avis I disorders, patient version (SCID-P) (version 2) New York State Psychiatric Institute, Biometrics Research; 1994. [Google Scholar]
- Gaser C, Nenadic I, Buchsbaum BR, Hazlett EA, Buchsbaum MS. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. American Journal of Psychiatry. 2004;161:154–156. doi: 10.1176/appi.ajp.161.1.154. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophrenia Research. 2006;81:47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU assessment manual for psychopharmacology. Department of Health, Education, and Welfare Publication No. ADM 76–338. Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- Hietala J, Cannon TD, van Erp TG, Syvalahti E, Vilkman H, Laakso A, Vahlberg T, Alakare B, Rakkolainen V, Salokangas RK. Regional brain morphology and duration of illness in never-medicated first-episode patients with schizophrenia. Schizophrenia Research. 2003;64:79–81. doi: 10.1016/s0920-9964(03)00065-3. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: A meta-analysis of voxel-based morphometry studies. American Journal of Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. Journal of Psychiatry Research. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Archives of General Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Marquardt RK, Levitt JG, Blanton RE, Caplan R, Asarnow R, Siddarth P, Fadale D, McCracken JT, Toga AW. Abnormal development of the anterior cingulate in childhood-onset schizophrenia: A preliminary quantitative mri study. Psychiatry Research. 2005;138:221–233. doi: 10.1016/j.pscychresns.2005.01.001. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore E, Sham P, Chitnis X, Suckling J, MacCabe J, Walshe M, Murray RM. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: Computational morphometry study. British Journal of Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, Murray RM. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Archives of General Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, DeLuca H, Thompson PM. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cerebral Cortex. 2005a;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Woods RP, Thompson PM, Szeszko P, Robinson D, Ballmaier M, Messenger B, Wang Y, Toga AW. Regional specificity of cerebrospinal fluid abnormalities in first episode schizophrenia. Psychiatry Research. 2006;146:21–33. doi: 10.1016/j.pscychresns.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Narr KL, Sharma T, Woods RP, Thompson PM, Sowell ER, Rex D, Kim S, Asuncion D, Jang S, Mazziotta J, Toga AW. Increases in regional subarachnoid CSF without apparent cortical gray matter deficits in schizophrenia: Modulating effects of sex and age. American Journal of Psychiatry. 2003;160:2169–2180. doi: 10.1176/appi.ajp.160.12.2169. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, Sevy S, Wang Y, Schrock K, Bilder RM. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biological Psychiatry. 2005b;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Narr KL, van Erp TG, Cannon TD, Woods RP, Thompson PM, Jang S, Blanton R, Poutanen VP, Huttunen M, Lonnqvist J, Standerksjold-Nordenstam CG, Kaprio J, Mazziotta JC, Toga AW. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiology of Disease. 2002;11:83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- Rose SE, Chalk JB, Janke AL, Strudwick MW, Windus LC, Hannah DE, McGrath JJ, Pantelis C, Wood SJ, Mowry BJ. Evidence of altered prefrontal-thalamic circuitry in schizophrenia: An optimized diffusion MRI study. Neuroimage. 2006;32:16–22. doi: 10.1016/j.neuroimage.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn FJ, Symms MR, Barker GJ, Greenwood R, Duncan JS. Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. Journal of Neurology Neurosurgery Psychiatry. 2001;70:530–533. doi: 10.1136/jnnp.70.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond CH, Menon DK, Chatfield DA, Williams GB, Pena A, Sahakian BJ, Pickard JD. Diffusion tensor imaging in chronic head injury survivors: Correlations with learning and memory indices. Neuroimage. 2006;29:117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Matsuda G, Hoge EA, Kennedy D, Makris N, Caviness VS, Tsuang MT. Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: A pilot magnetic resonance imaging study. American Journal of Medical Genetics. 1997;74:507–514. doi: 10.1002/(sici)1096-8628(19970919)74:5<507::aid-ajmg11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Sharma T, Lancaster E, Lee D, Lewis S, Sigmundsson T, Takei N, Gurling H, Barta P, Pearlson G, Murray R. Brain changes in schizophrenia. Volumetric mri study of families multiply affected with schizophrenia--the maudsley family study 5. British Journal of Psychiatry. 1998;173:132–138. doi: 10.1192/bjp.173.2.132. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of mri findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YW, Kwon JS, Ha TH, Park HJ, Kim DJ, Hong SB, Moon WJ, Lee JM, Kim IY, Kim SI, Chung EC. Increased water diffusivity in the frontal and temporal cortices of schizophrenic patients. Neuroimage. 2006;30:1285–1291. doi: 10.1016/j.neuroimage.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Sled JG, Pike GB. Standing-wave and RF penetration artifacts caused by elliptic geometry: An electrodynamic analysis of MRI. IEEE Transactions on Medical Imaging. 1998;17:653–662. doi: 10.1109/42.730409. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn ML, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. American Journal of Psychiatry. 2000;157:416–421. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- Styner M, Lieberman JA, McClure RK, Weinberger DR, Jones DW, Gerig G. Morphometric analysis of lateral ventricles in schizophrenia and healthy controls regarding genetic and disease-specific factors. Proceedings of the National Academy of Sciences U S A. 2005;102:4872–4877. doi: 10.1073/pnas.0501117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR, Jr, Weinberger DR. Temporal lobe pathology in schizophrenia: A quantitative magnetic resonance imaging study. American Journal of Psychiatry. 1989;146:464–472. doi: 10.1176/ajp.146.4.464. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Lim KO, Mathalon D, Marsh L, Beal DM, Harris D, Hoff AL, Faustman WO, Pfefferbaum A. A profile of cortical gray matter volume deficits characteristic of schizophrenia. Cerebral Cortex. 1998;8:117–124. doi: 10.1093/cercor/8.2.117. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Blanton RE, Levitt JG, Caplan R, Nobel D, Toga AW. Superior temporal gyrus differences in childhood-onset schizophrenia. Schizophrenia Research. 2005;73:235–241. doi: 10.1016/j.schres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Picchioni MM, McDonald C, Marshall N, Davis N, Ribchester T, Hulshoff Pol HE, Sharma T, Sham P, Kahn RS, Murray R. A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biological Psychiatry. 2004;56:454–461. doi: 10.1016/j.biopsych.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: A principal components analysis of the 24-item brief psychiatric rating scale. Psychiatry Research. 2000;97:129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Ward KE, Friedman L, Wise A, Schulz SC. Meta-analysis of brain and cranial size in schizophrenia. Schizophrenia Research. 1996;22:197–213. doi: 10.1016/s0920-9964(96)00076-x. [DOI] [PubMed] [Google Scholar]
- White T, Kendi AT, Lehericy S, Kendi M, Karatekin C, Guimaraes A, Davenport N, Schulz SC, Lim KO. Disruption of hippocampal connectivity in children and adolescents with schizophrenia--a voxel-based diffusion tensor imaging study. Schizophrenia Research. 2007;90:302–307. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Woods BT, Yurgelun-Todd D, Goldstein JM, Seidman LJ, Tsuang MT. MRI brain abnormalities in chronic schizophrenia: One process or more? Biological Psychiatry. 1996;40:585–596. doi: 10.1016/0006-3223(95)00478-5. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998a;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998b;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, Murphy GM, Csernansky JG, Pfefferbaum A. Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biological Psychiatry. 1994;35:501–516. doi: 10.1016/0006-3223(94)90097-3. [DOI] [PubMed] [Google Scholar]