Abstract
Drosophila vision is mediated by inputs from three types of photoreceptor neurons: R1–R6 mediate achromatic motion detection while R7 and R8 constitute two chromatic channels. Neural circuits for processing chromatic information are not known. Here we identified the first-order interneurons downstream of the chromatic channels. Serial-EM revealed that small-field projection neurons Tm5 and Tm9 receive direct synaptic input from R7 and R8, respectively, and indirect input from R1–R6, qualifying them to function as color-opponent neurons. Wide-field Dm8 amacrine neurons receive input from 13–16 UV-sensing R7s and provide output to projection neurons. Using a combinatorial expression system to manipulate activity in different neuron subtypes, we determined that Dm8 neurons are both necessary and sufficient for phototaxis to ultraviolet in preference to green light. We propose that Dm8 sacrifices spatial resolution for sensitivity by relaying signals from multiple R7s to projection neurons, which then provide output to higher visual centers.
Introduction
Many animals respond differentially to light of different wavelengths: for example, most flying insects exhibit positive phototactic responses but prefer ultraviolet (UV) to visible light, whereas zebra fish are strongly phototactic to ultraviolet/blue and red light but weakly to green (Menzel, 1979; Menzel and Backhaus, 1991; Orger and Baier, 2005). Unlike true color vision, which distinguishes lights of different spectral compositions (hues) independently of their intensities, spectral preferences are strongly intensity-dependent and innate, probably reflecting each species’ ecophysiological needs. Thus, water fleas (Daphnia magna) avoid harmful UV but are attracted to green light, which characterizes abundant food sources (Storz and Paul, 1998). Daylight is rich in UV, so flying insects’ preference for UV over visible light is probably related to the so-called open-space response, the attraction towards open, bright gaps and away from dim, closed sites (Goldsmith, 1961; Hu and Stark, 1977). The receptor mechanisms for spectral preference has been well studied in flying insects, especially in Drosophila (Heisenberg and Buchner, 1977). Two or more photoreceptor types with distinct spectral responses are required to detect different wavelengths of light, and mutant flies lacking UV-sensing photoreceptors exhibit aberrant preference for green light (Hu and Stark, 1977). However, the post-receptoral mechanisms of spectral preference are entirely unknown. Furthermore, it is not clear how spectral preference is related to true color vision. Color-mixing experiments suggest that color vision spectral preference are independent in honeybees (Menzel and Greggers, 1985). In Drosophila, however, spectral preference experiments have revealed that the phototactic response towards UV is significantly enhanced by the presence of visible light, suggesting a “color” contrast effect in spectral preference behavior (Schümperli, 1973; Fischbach 1979). Identifying and characterizing the neural circuits that process chromatic information is the first step to understanding the post-receptoral mechanisms of spectral preference and thus color vision.
With recent advances in genetic techniques that manipulate neuronal function, Drosophila has re-emerged as a model system for studying neural circuits and functions. In particular, the Gal4/UAS expression system combined with the temperature-sensitive allele of shibire makes it possible to examine the behavioral consequences of reversibly inactivating specific subsets of neurons (Kitamoto, 2001). Such interventions allow direct comparisons between the connections of a neuron and its function, thereby establishing causality (reviewed in Luo et al., 2008).
The Drosophila visual system comprises the compound eye and four successive optic neuropils (lamina, medulla, lobula and lobula plate; Figure 1A). The compound eye itself has some 750 ommatidia, populated by two types of photoreceptors. The outer photoreceptors R1–R6, which are in many ways equivalent to vertebrate rod cells, express Rh1 opsin (O’Tousa et al., 1985) and respond to a broad spectrum of light (Hardie, 1979), and are thus presumed to be achromatic. The inner photoreceptor neurons R7 and R8 have complex opsin expression patterns (reviewed in Mikeladze-Dvali et al., 2005): R7s express one of two ultraviolet (UV)-sensitive opsins, Rh3 and Rh4, while beneath R7 the R8s coordinately express blue-sensitive Rh5 or green-sensitive Rh6 opsins (Salcedo et al., 1999). The achromatic R1–R6 channel mediates motion detection (Heisenberg and Buchner, 1977, Yamaguchi et al., 2008). R1–R6 innervate the lamina, where the achromatic channel input diverges to three or more pathways mediated by three types of lamina neurons, L1–L3. Their synaptic connections have been analyzed exhaustively at the electron microscopic (EM) level (Meinertzhagen and O’Neil, 1991; Meinertzhagen and Sorra, 2001). Genetic dissection indicates that these three pathways serve different functions in motion detection and orientation (Rister et al., 2007). Much like vertebrate cones, R7 and R8 photoreceptors are thought to constitute chromatic channels that are functionally required for spectral preference behaviors (Heisenberg and Buchner, 1977). The axons of R7 and R8 penetrate the lamina and directly innervate the distal medulla, where until now their synaptic connections have been completely unknown.
Figure 1. The histamine chloride channel Ort is expressed in subsets of lamina and medulla neurons.
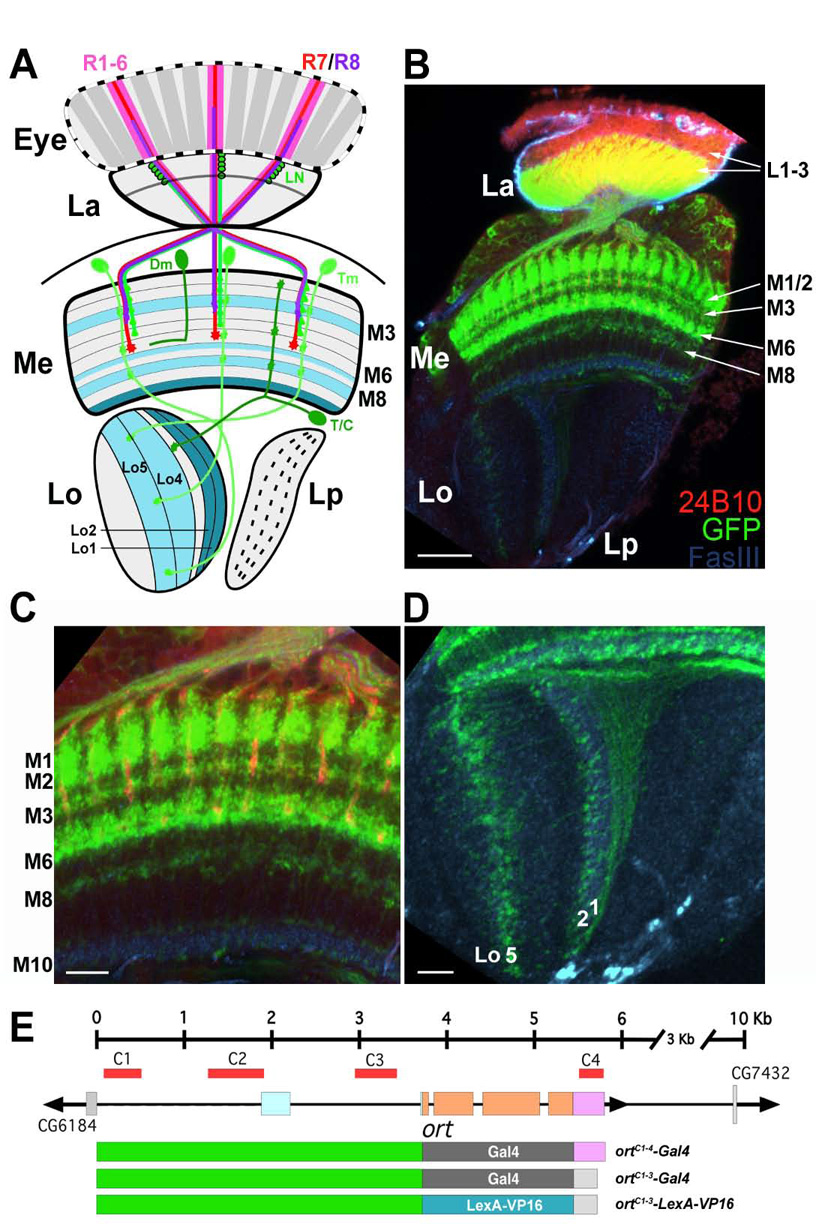
(A) A schematic illustration of the Drosophila visual system, including the eye (Eye) and four optic neuropils: lamina (La), medulla (Me), lobula (Lo), and lobula plate (Lp). The outer photoreceptors, R1–R6 (pink), terminate in the lamina and synapse with lamina neurons (LN: green). The central photoreceptors, R7 (red) and R8 (purple), project axons to the medulla strata M6 and M3, respectively. Three selected types of medulla neurons are shown: transmedulla (Tm) neurons arborize in various medulla strata and project axons to distinct lobula strata; distal medulla (Dm) amacrine neurons extend processes in distal medulla strata; T and C (T/C) neurons extend axons into the medulla and lobula (T2 neurons) or lamina (C2 neurons, not shown). Medulla and lobula strata marked by anti-FasIII antibody are colored cyan.
(B) The ort promoter driver labels subsets of medulla neurons. The ortC1-3-LexA::VP16 driver was used to drive the expression of rCD2::GFP, a membrane-tethered GFP marker (green) in the lamina and medulla neurons that are postsynaptic to photoreceptors. Photoreceptor axons were visualized using MAb24B10 antibody (red). Anti-FasIII antibody (blue), which labels distinct medulla and lobula strata, was used as a stratumspecific landmark.
(C,D) High magnification views of (B) showing the medulla (C) and lobula neuropil (D).
(D) The GFP-labelled transmedulla neurons project axons to strata Lo1, Lo2, and Lo5 of the lobula (Lo1, 2 and 5), forming a topographic map.
(E) Promoter analysis of the ort gene. The ort genomic structure shown as a linear cartoon with boxes representing exons and lines representing introns and intergenic sequences. Comparative genomic analysis identifies four blocks of sequences, C1–C4 (red, shown above the genomic structure), that are highly conserved among twelve species of Drosophila (see Figure S2A). The ort promotor (C1–C3) with the ort or hs70 3’ UTR region (purple and grey, respectively) was fused to either the yeast transcription factor Gal4 (dark grey box) or the chimeric transcription factor LexA::VP16 (blue box) to generate various ort promoter drivers (as indicated). Orange box: coding region; cyan box: 5’-UTR; purple box: 3’-UTR.
Scale bar: 20 µm in (B); 10 µm in (C, D).
The medulla, the largest and most heavily populated optic neuropil, is organized into strata (M1–M10) and columns (Fischbach and Dittrich, 1989; Campos-Ortega and Strausfeld 1972), in a manner reminiscent of the mammalian cortex. All visual information converges upon the distal strata of the medulla: the axons of R7 and R8 directly innervate strata M6 and M3, respectively, while L1–L3 transmit information from the R1–R6 channel to multiple medulla strata (M1/5, M2, and M3, respectively). The R7, R8, and L1–L3, which view a single point in visual space innervate a single medulla column (Meinertzhagen, 1976) and there establish a retinotopic pixel. Previous Golgi studies have revealed about 60 morphologically distinct types of medulla neurons (Fischbach and Dittrich, 1989). Each arborizes in a stereotypic pattern within specific strata of the medulla, and projects an axon to a distinct stratum of the medulla, lobula or lobula plate. The distinct morphological forms of different types of medulla neurons reflect, at least in part, their diverse patterns of gene expression (Morante and Desplan, 2008). Although it is widely presumed that the medulla incorporates key neural substrates for processing color and motion information, little is known about its synaptic circuits and their functions. EM analyses of synaptic circuits have not been possible because of the complexity of this neuropil, while electrophysiological investigations are technically challenging because of the small size of neurons.
In this study, we investigate the chromatic visual circuits in the medulla. Using a combination of transgenic and histological approaches, we identify the first-order interneurons in the medulla that receive direct synaptic inputs from the chromatic channels, R7 and R8. We then subdivide these neurons based on their use of neurotransmitters and gene expression patterns. By systematically inactivating and restoring the activity of specific neuron subtypes, we identify the neurons that are necessary and sufficient to drive a fly’s phototactic preference to UV.
Results
The histamine chloride channel Ort is required for UV/green spectral preference
Previous electrophysiological and histological studies have demonstrated that Drosophila photoreceptor neurons are histaminergic (Hardie, 1987; Sarthy, 1991) and that R7 and R8 photoreceptors provide the predominant histamine-immunoreactive input to the medulla (Pollack and Hofbauer, 1991). Two ionotropic histamine-gated channels, Ort (ora transientless; HisCl2) and HisCl1 have been identified (Gengs et al., 2002; Gisselmann et al., 2002; Zheng et al., 2002; Witte et al., 2002; Pantazis et al., 2008). Mutants for ort exhibit defects in motion detection and their electroretinograms (ERGs), indicating that Ort is required to transmit R1–R6 input to the first-order interneurons (Gengs et al., 2002). To test whether Ort is required for visually guided behavior, we first examined flies’ phototaxis towards either UV or green light in preference to dark (see Experimental Procedures for details). This phototactic response is mediated primarily by the more sensitive, broad-spectrum photoreceptors, R1–R6, although R7 cells also contribute to UV, but not green, phototaxis under the light-adapted condition (Figure S1A, B; Figure 2C, D; Fischbach, 1979). We found that wild-type flies exhibited stronger phototaxis towards UV than towards green light by approximately one order of magnitude, and that light-adaptation, when compared with dark-adaptation, reduced the sensitivity to UV and green light by approximately two orders of magnitude (Figure 2A). In contrast, strong transallelic combination ort1/ortUS2515 mutant flies exhibited much weaker phototaxis towards either UV or green light (by about three and two orders of magnitude, respectively) as compared with wild-type. In negative geotaxis assays, ort mutants exhibited no apparent motor defects (Figure S1C), suggesting that their reduced phototaxis was not a motor system defect but rather a visual deficit. In addition, the ort mutation affected UV phototaxis more severely than green phototaxis. We speculate that Ort plays a role in relaying signals from UV-sensing R7s to their first-order interneurons, and that HisCl1 may participate in phototaxis, especially towards green light (see below).
Figure 2. Ort-expressing neurons mediate phototaxis and a normal preference for UV.
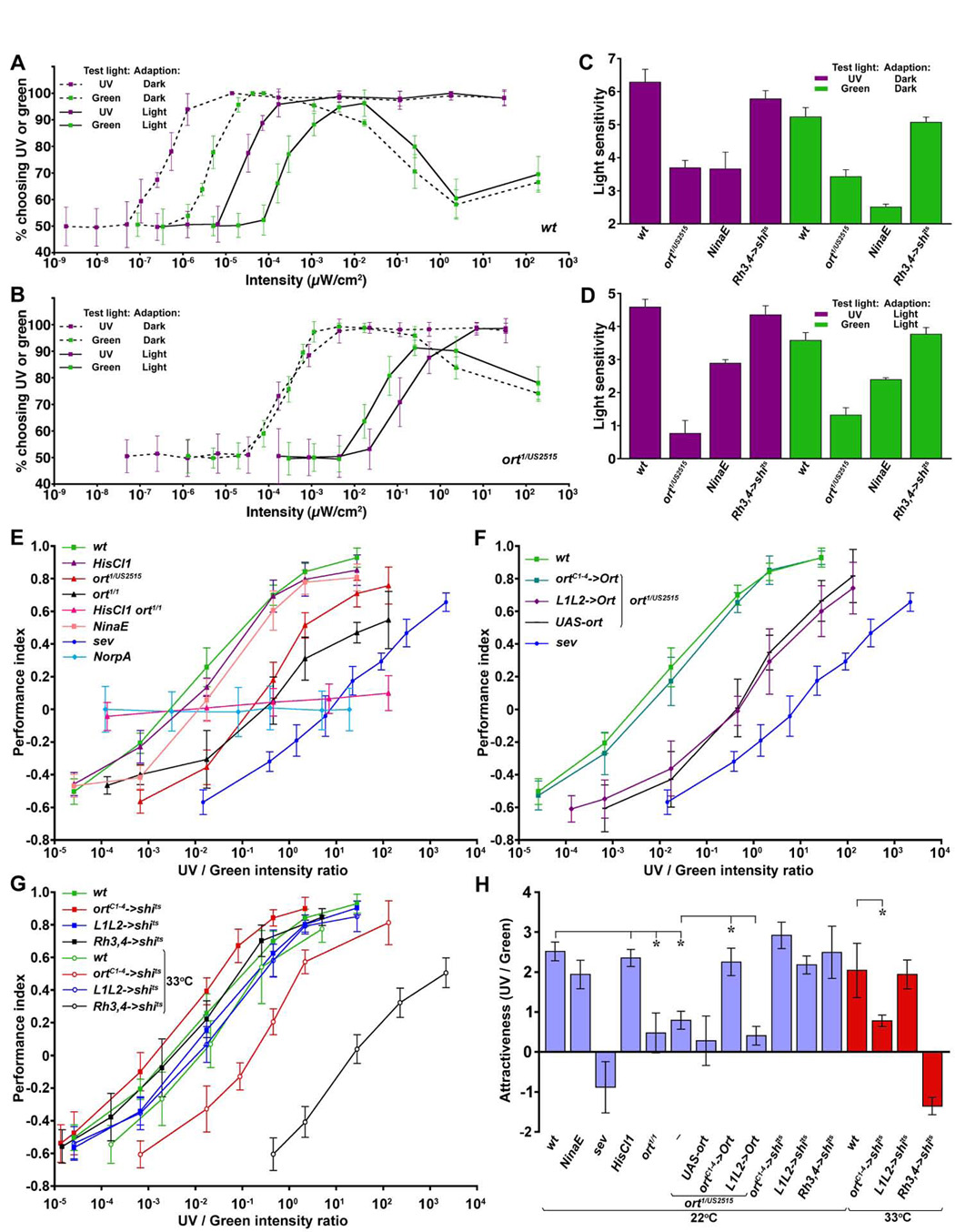
(A–D) Wild-type (A), ort (B) and various mutant flies were tested for fast phototaxis towards UV or green light. The intensity-response curves were measured by recording the percentage of flies choosing UV or green light of various intensities over dark. Light intensity was shown as a logarithmic scale and error bars (standard deviations) represent the variations among trials.
(A) Wild-type flies exhibited phototactic responses to UV in a simple intensity-dependent fashion, resulting in a sigmoidal intensity-response curve. In contrast, phototactic response towards green light was not monotonous because the response was reduced at high intensities of green light. Compared with dark adaptation (dotted lines), light adaptation (solid lines) decreased sensitivity to both UV and green light by approximately two orders of magnitude.
(B) Compared with wild-type, ort mutants exhibited a significantly reduced phototactic response under dark- or light-adapted conditions. In ort mutants, phototaxis towards UV appeared to be affected more severely than that towards green light.
(C–D) Histograms of light sensitivity of wild-type and various mutant flies under dark-(C) or light-adapted (D) conditions. Light sensitivity, defined as the negative logarithm of the minimal light intensity required to attract 75% of the test flies, was calculated from the intensity-response curves. Note that fast phototaxis towards UV or green light was driven primarily by the broad-spectrum R1–R6 photoreceptors since this behavior was significantly affected by the inactivation of R1–R6 (NinaE mutants), but not R7 cells (Rh3,4->shits).
(E–H) Wild-type, ort, HisCl1, and various mutant flies tested for phototactic preference to UV over green light. The intensity-response curves were measured by varying UV intensity while keeping the green light intensity constant (see Experimental Procedures for details). (E–G) The P.I. for each genotype was calculated from the numbers of flies choosing UV (NUV) or green (NG) light by the following formula: P.I. = [NUV − NG] / [NUV + NG]. The UV/green intensity ratio (E–G) is shown as a logarithmic scale. Error bars (standard deviations) represent the variations among trials.
(E) ort mutants had a reduced preference for UV. Wild-type (wt) flies exhibited phototactic preference to UV in an intensity-dependent fashion, resulting in a sigmoidal intensity-response curve. For ort mutants (ort1/1 and ort1/US2515), the intensity-response curve was shifted to the right. Note that normal UV preference requires R7s but not R1–R6 as sevenless (sevE2) mutants, but not NinaE mutants, exhibited low UV preference. HisCl1 ort double-null mutants, like norpA36, a phototransduction mutant, chose UV and green light indiscriminately over a broad range of UV/green intensity ratios.
(F) The expression of Ort driven by ortC1-4-Gal4 restored normal UV preference in ort mutants. In contrast, UAS-ort alone or reinstating Ort function in the achromatic channels L1 and L2 failed to restore UV preference in ort mutants. Positive control (wt) and negative control (sev) were from those described in (E).
(G) Ort-expressing neurons are required for normal UV preference. Shits1 expressed in Ort-expressing neurons or R7s blocks their synaptic transmission. At a restrictive temperature (33°C), ortC1-4->shits1 flies exhibited lower UV preference compared with wild-type controls. Inactivating R7s using Rh3,4->shits1 resulted in an even greater reduction in UV preference. In contrast, inactivating L1 and L2 using L1L2-> shits1 did not affect UV preference. Wild-type control (wt) at 22°C was from that described in (E).
(H) Histogram of the relative attractiveness of UV over green light (AttrUV/G) for each genotype. AttrUV/G was calculated from the UV/green intensity ratio at which flies exhibited phototaxis to UV and green lights with equal frequency (isoluminance point, P.I.=0), based on the following formula: AttrUV/G = −log (UV/green ratio at the isoluminance point). The difference between the AttrUV/G of the wild-type and ort mutants (or ortC1-4->shits1 flies) was statistically significant (*p<0.00001), whereas the difference between the wild-type and rescued ort mutants (or HisCl1 mutants) was not (p>0.1).
To assess whether Ort is required to transmit chromatic input mediated by R7 and R8, we used a quantitative spectral-preference assay. This spectral-preference assay tests the phototaxis towards UV in preference to green (see Experimental Procedures for details) and depends on R7, but not significantly on R1–R6, function (Figure 2E, G; Jacob et. al., 1977, Fischbach, 1979). This behavior depends on the circuit comparing UV and green light and likely reflects salience of UV and green lights rather than a simple linear summation of their phototactic responses. We found that wild-type flies preferred short-wavelength UV to longer-wavelength green light in an intensity-dependent fashion (Figure 2E). In contrast, homozygous null ort1 mutants and strong transallelic combination ort1/ortUS2515 mutants (as well as other allelic combinations, ortP306/ortUS2515 and ort1/ortP306, data not shown) all exhibited reduced UV preference. Over five orders of magnitude in the ratio of UV/green intensities, the proportion of ort mutant flies that chose UV was significantly lower than that for wild-type flies (Figure 2E). To quantify the UV preference, we determined the isoluminance point, the UV/green intensity ratio at which flies found light of either wavelength equally “attractive”, and used the negative logarithm of the intensity ratio as a measure of UV attractiveness (AttrUV/G; Figure 2H). The UV attractiveness for ort mutants (AttrUV/G=0.47±0.50 for ort1 and 0.79±0.22 for ort1/ortUS2515; mean±SD) was significantly lower than that for wild-type flies (AttrUV/G=2.52±0.23) but higher than that for sevenless mutants (AttrUV/G=−0.88±0.64), which lack UV-sensing R7s entirely (Tomlinson and Ready, 1986) (p<0.00001, Student’s t-test; Figure 2E,H).
Given that ort null mutants still exhibited phototaxis, we examined whether the other histamine receptor, HisCl1, might have contributed to UV preference. We found that HisCl1134 null mutants exhibited UV preference indistinguishable from the wild-type (p>0.1). In contrast, strong allelic combination HisCl1134 ort1/HisCl1134 ortP306 double-mutants showed weak phototaxis towards green light (data not shown), while double-null HisCl1134 ort1 mutants, like the phototransduction mutant NorpA, failed to discriminate between wavelengths in the UV and green (Figure 2E). We conclude that Ort is essential for optimal UV preference while HisCl1 plays at most a minor and partially redundant role. We note that double-null HisCl1134 ort1 mutants were not entirely blind and still exhibited very weak fast phototaxis (data not shown), suggesting that there might be residual synaptic transmission between photoreceptors and the first-order interneurons despite of the absence of these two known histamine receptors.
The histamine chloride channel Ort is expressed in subsets of lamina and medulla neurons
We reasoned that the first-order interneurons must express the histamine receptor Ort in order to respond to their inputs from histaminergic R7 and R8 terminals. To identify these first-order interneurons, we determined the ort promoter region using comparative genomic sequence analysis (Odenwald et al., 2005). In the ort locus, we found four blocks of non-coding sequence that are highly conserved among 12 species of Drosophila (Figure S2A). The first three sequence blocks (designated C1–C3) are localized to the intergenic region and the first intron and are therefore likely to contain critical cis-elements (Figure 1E; Figure S2A). We generated ort-promoter constructs driving Gal4 or LexA::VP16, designating these ortC1-3-Gal4 and ortC1-3-LexA::VP16. Both driver systems drove expression patterns in identical subsets of neurons in the lamina, medulla cortices and in the deep C and T neurons of the lobula complex (Figure 1B–D), except that ortC1-3-Gal4 drove somewhat patchy expression with lower intensity (data not shown). The fourth block of conserved sequences, located at 3’UTR, contains putative microRNA binding sites (Figure S2A) and, as examined in ortC1-4-Gal4, did not drive expression in additional cells (data not shown), suggesting that it does not contain critical cis-elements. Overall, the expression patterns of these ort promoter constructs resembled previously published ort expression patterns from in situ hybridization (Witte et al., 2002).
We also performed comparative genomic sequence analysis for the HisCl1 locus and identified two blocks of highly conserved sequence (C1 and C2), located in the first introns of the HisCl1 gene and its neighboring gene (CG17360) (Figure S2B). We generated a HisCl1-Gal4 construct that included these conserved sequences (Figure S3A). We found that HisCl1-Gal4 drove strong expression in the lamina epithelial glia cells (as recently also reported by Pantazis et al., 2008) and medulla cells that are not well characterized (Figure S3B–D). This result is consistent with previous EM data that lamina epithelial glia enwrap each cartridge and are postsynaptic to R1–R6 (Meinertzhagen and O’Neil, 1991). Insofar as both the behavioral requirement and expression pattern indicate that Ort but not HisCl1 plays a critical role in the visual system, we focused on Ort in the following analyses.
Ort-expressing neurons are required for visually driven behaviors
We next examined whether using the ort-promoter Gal4 drivers to express Ort was sufficient to rescue defects in the visual behavior ort mutants. We found that ortC1-4-Gal4-driven Ort expression restored a preference for UV (AttrUV/G=2.25±0.34) in ort mutants to the wild-type level (2.52±0.23; Figure 2F,H). Since Ort, but not HisCl1, is also required in lamina neurons for normal ERG and motion detection responses (Figure S4B, C; Gengs et al., 2002; Rister et al., 2007), we examined the rescued flies for these functions too. We found that expressing Ort in ort mutants using ortC1-3-Gal4 restored, at least qualitatively, the ‘on’- and ‘off’-transients of the ERG, which report transmission in the lamina (Coombe, 1986), as well as the optomotor behavior (Figure S4B–D). These findings are consistent with the observation that ort-Gal4 drove reporter expression in lamina neurons L1–L3 (Figure S4A and below). In contrast, expressing Ort in lamina neurons L1 and L2 using an L1/L2-specific driver (L1L2-A-Gal4) rescued both the ERG, at least qualitatively, and optomotor defects (Figure S4B,D), but not the UV preference (AttrUV/G=0.41±0.23; Figure 2F, H), of ort mutants. Thus, the actions of the ort-Gal4 drivers recapitulated the endogenous Ort expression pattern in the first-order interneurons of R1–R6 and R7.
We next examined whether the Ort-expressing neurons were required for UV reference and motion detection. We found that ortC1-4-Gal4 or ortC1-3-LexA::VP16 driving a temperature-sensitive allele of shibire, shits1, so as to block synaptic transmission in specific neurons (Kitamoto, 2001), significantly reduced the UV attractiveness at non-permissive, but not permissive, temperatures (AttrUV/G=0.78±0.14 at 33°C and 2.92±0.33 at 22°C for ortC1-4: Figure 2G, H; AttrUV/G=0.65±0.20 at 33°C and 2.22±0.40 for ortC1-3: data not shown; p<0.0001). This reduction was smaller than that caused by inactivating the R7s alone (AttrUV/G=−1.36±0.22 at 33°C and 2.49±0.65 at 22°C; Figure 2G, H). These results suggest that Ort-expressing neurons might mediate both UV and green phototaxis, presumably by relaying R7 and R8 channel signals, although we cannot rule out the existence of an ort-independent UV-sensing pathway (see below and Discussion). Similarly, inactivating Ort-expressing neurons abolished the flies’ ability to detect motion (Figure S4D). Thus, we may conclude that Ort-expressing neurons are required for both spectral preference and motion detection.
Ort is expressed in a subset of projection neurons in the medulla
To identify the Ort-expressing neurons that could be synaptic targets of the R7 and R8 channels, we employed a single-cell mosaic technique based on the flip-out genetic method previously described (Wong et al., 2002). In this system, we used the ortC1-3-Gal4 flies that also carried the transgenes UAS>CD2,y+>CD8-GFP and hs-Flp. The flipase activity induced by brief heat-shock at the second- or third-instar larval stages excised the FLP-out cassette in small random populations of cells, thereby allowing Gal4 to drive the expression of the CD8-GFP marker. From over 1000 brain samples, we examined 459 clones of transmedulla neurons, the projection neurons that arborize in the medulla and project axons to the lobula. To identify the exact medulla and lobula strata in which these processes extended, we screened expression patterns of a series of known cell-adhesion molecules and found three useful stratum-specific markers, FasIII, Connectin, and Capricious (Figure S5; Shinza-Kameda et al., 2006). In particular, anti-FasIII immunolabeled medulla and lobula strata of interest and, with MAb24B10 immunolabeling, was used primarily to identify the medulla and lobula strata. Based on the morphologies and stratum-specific locations of the arborization and axon terminals, we could readily assign four types of Ort-expressing projection neurons to types previously described from Golgi impregnation (Fischbach and Dittrich, 1989). These were Tm2, Tm5, Tm9, and Tm20 (Figure 3). In addition, the ort-promoter driver labeled, albeit at a lower frequency, centrifugal neurons C2 and T2, and three types of medulla neurons with processes solely in the medulla, Dm8, other amacrine-like and also glia-like cells (Figure S6 and below). All of these cells were identified multiple times in at least two independent ort-Gal4 lines, but given the sampling nature of the single-cell mosaic technique, we can not exclude the possibility that we might have missed some very rare Ort-expressing neurons. The amacrine-like and glia-like cells had not been previously described from Golgi impregnation (Fischbach and Dittrich, 1989), suggesting that there are even more classes of medulla cell types than those previously reported.
Figure 3. Ort is expressed in subsets of transmedulla neurons.
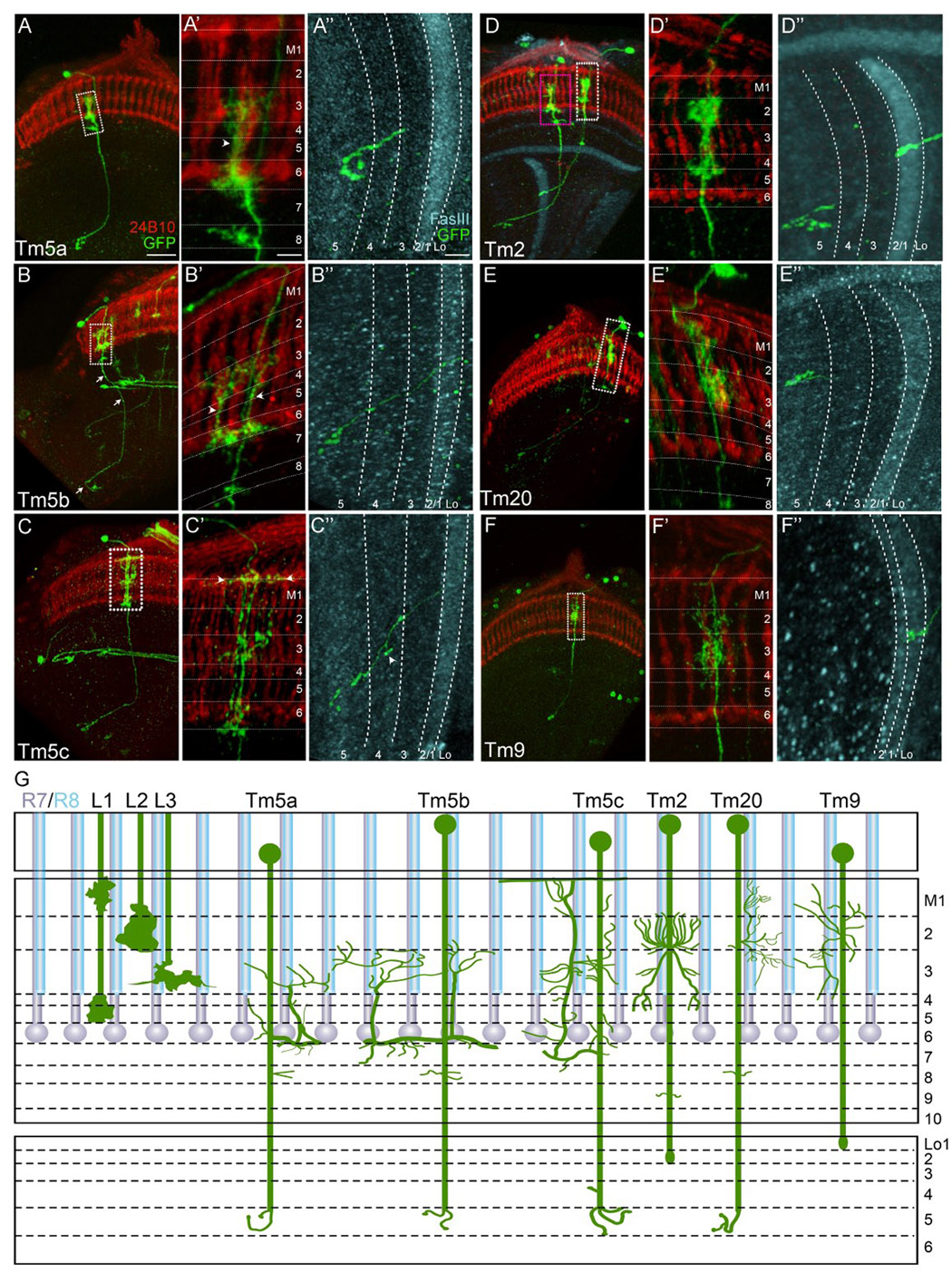
Axonal and dendritic projections (green) of single Ort-expressing transmedulla neurons were examined in flies carrying ort1–3-Gal4, hs-Flp and UAS>CD2,y+>CD8-GFP transgenes. R7 and R8 photoreceptor axons, visualized with MAb24B10 antibody (red), served as landmarks for medulla columns. Medulla and lobula strata were identified using R7 and R8 terminals and anti-FasIII immunolabeling (cyan, see Figure S5). Four Tm types, including Tm5, Tm2, Tm20, Tm9, were identified based on their dendritic morphologies (A’–F’) and stratum-specific axon terminations (A”– F”). Tm5 was further categorized into three subtypes: Tm5a, b, and c (A–C; see text for details).
(A-A’’) The Tm5a neuron extends a single main dendritic branch (arrowhead), which runs along the photoreceptor terminals and extends multiple fine processes in strata M3 and M6 (A’). Its axon terminal in the Lo5 stratum is hook-shaped (A”).
(B–B”) The Tm5b neuron extends 2 or 3 main dendritic branches (arrowheads) with fine processes spanning ~5 columns in strata M3, M6, and also M8, (B’); its axon terminates in stratum Lo5 (B”).
(C–C”) The Tm5c neuron extends a single main dendritic branch with multiple fine processes, which span multiple columns in strata M3 and M6. The most distinguishable features of Tm5c are the dendritic arbors in the superficial part of the M1 stratum (arrowhead, C’) and the presence of axon terminals (arrowhead, C”) in both strata Lo4 and Lo5.
(D–F”) Tm2 (D–D”), Tm20 (E–E”), and Tm9 (F–F”) form type-specific dendritic arbors largely confined to a single medulla column, and project their axons to specific lobula strata.
(A’–F’, A”–F”) High magnification views of (A–F) in the medulla (A’–F’) and lobula (A”–F”), respectively. Scale bars: in (A), 20 µm (for A–F); in (A’), 5 µm (for A’–F’); in (A”), 5 µm (for A”-F”).
(G) Schematic diagram illustrating the dendritic and axonal morphologies of Tm neurons. All are shown in dorso-ventral view (as in A–F”) except Tm2, which is in approximately medio-lateral view.
The Ort-expressing Tm neurons exhibited type-specific patterns of arborization and axon projection. Tm5 neurons extended dendrite-like processes in medulla strata M3 and M6, where R8/L3 and R7 axons terminated, respectively, and they projected axons to terminate in stratum Lo5 in the lobula. This pattern suggests that they relay information from the R7 and R8 or L3 channels to the lobula. The Tm5 neurons could be readily divided into three subtypes, Tm5a, b, and c, based on their unique dendritic patterns (see Figure 3A–C”), the spread of their medulla arborization, and their gene expression patterns. Tm5a (n=125) and Tm5b (n=44) had medulla arborizations of different sizes and shapes (Figure 3A–B”, G); whereas Tm5c (n=20) had dendritic processes in M1, in addition to strata M3 and M5, and the axon projected to both the Lo4 and Lo5 strata. The distinct morphology of Tm5c correlated with its unique expression of the vesicular glutamate transporter (see below). Tm9 (n=43) and Tm20 (n=67) extended type-specific dendrite-like processes in strata M1–M3 and projected axons to distinct lobula strata (Figure 3E–F”, G). Tm20, like Tm5, projected to Lo5 while Tm9 projected to Lo1, suggesting that Tm9 and Tm20 relay information from R8 and (via lamina neurons) R1–R6, to different strata of the lobula (Figure 3E–F”, G). In medulla strata M1–M3, Tm2 (n=160) extended dendrite-like processes which did not appear to make significant contacts with R7 or R8 terminals (Figure 3D–D”, G).
Tm projection neurons relay both chromatic and achromatic channel information to the proximal medulla and the lobula
To determine if the Ort-expressing Tm neurons indeed received synaptic input from photoreceptors, we undertook serial EM reconstructions of Tm9 (two cells), Tm2 (five cells), and parts of a single Tm5 cell that resemble Tm5a, as well as the afferent input terminals that innervate the medulla, including R7, R8 and L1–L5 (Figure 4G; Takemura et al., 2008). The fine dendritic arbor of Tm20 has so far eluded reconstruction. We found that Tm9 received direct synaptic contacts from both R8 and L3 (Figure 4I, J) and the Tm5 received direct synaptic contacts from R7 and L3 (Figure 4H, J). Thus, Tm9 and Tm5 cells were postsynaptic to both the chromatic channels and an achromatic channel. Tm2, by contrast, received synaptic contacts from L2 and L4 but not, despite its Ort expression, R7 or R8 (data not shown). However, we cannot exclude the possibility that Tm2 responds to paracrine release of histamine from the R8 terminal, or an unidentified histamine input in the lobula.
Figure 4. Ort-expressing Tm neurons receive multi-channel inputs in the medulla and are presynaptic at both the medulla and lobula.
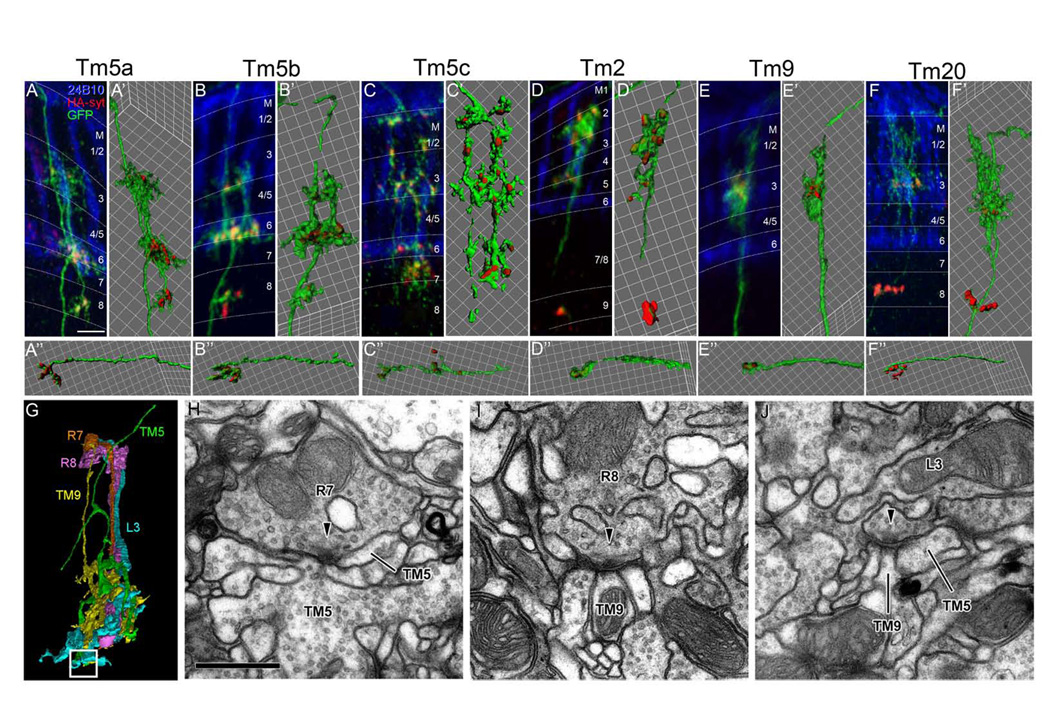
(A–F) The distribution of presynaptic terminals of single Ort-expressing Tm neurons was examined in flies carrying ort1–3-Gal4, hs-Flp, TubP->Gal80>, UAS-mCD8GFP (green) and UAS-synaptotagmin-HA (red). Localization of the presynaptic reporter, Synaptotagmin-HA, was visualized using anti-HA antibody. R7 and R8 photoreceptors were visualized using MAb24B10 antibody (blue). Tm cell types are as indicated. IsoSurface representations of medulla arborization (A’–F’) and lobula terminals (A-F”) were generated using Imaris software. Synaptotagmin-HA was localized to the tips of the axon terminals and dendritic arbors, the latter especially in the proximal medulla strata (M7 for Tm5c; M8 for Tm5a, Tm5b, and Tm20; M9 for Tm2).
(G) Profiles of R7, R8, L3, Tm5 and Tm9 reconstructed in three dimensions from a single medulla column. The white square box indicates the contact site between L3 and both Tm5 and Tm9 shown in (J). Although the partially reconstructed profile resembles Tm5a, the subtype reconstructed is still not certain.
(H–J) Synaptic contacts between R7 and Tm5 (H), R8 and Tm9 (I), and L3 and both Tm9 and Tm5 (J) Arrowheads point to T-bar ribbons in presynaptic elements, in the presumed direction of transmission.
Scale bar: in (A), 5 µm (for A–F); in (H), 500nm (for H–J)
To determine whether Tm neurons could relay information from the medulla to the lobula, we localized a marker for presynaptic sites to the Tm neurons using a flipase-based genetic mosaic method. In this system, heat-shock induced expression of flipase, which in turn removed the Gal80, allowing the ort-Gal4 to drive expression of a presynaptic marker, an HA-tagged synaptotagmin (syt-HA), and the mCD8-GFP marker in a small number of neurons. We found, as expected, that in all Ort-expressing Tm neurons, syt-HA was localized to the axon terminals in the lobula, indicating that these Tm neurons are qualified to be presynaptic in this neuropil (Figure 4A”–F”). Surprisingly, we observed that syt-HA was also localized to the dendrite-like processes in the medulla, especially in strata M7–M10, suggesting that many of these processes contain not only post- but also presynaptic sites (Figure 4A–F’). Especially, the processes of Tm5a, Tm5b, and Tm20 in stratum M8 were heavily decorated with syt-HA, suggesting that this stratum might be a significant output layer for these neurons (Figure 4A’, B’, F’).
Subcategorization of Ort-expressing neurons based on neurotransmitter usage
We reasoned that Ort-expressing neurons might be divided into several groups based on their differential release of other neurotransmitters. To test this possibility, we used a series of promoter-Gal4 and enhancer trap lines driving the CD8 marker to label neurons with glutamatergic, cholinergic, GABAergic, serotonergic and dopaminergic phenotypes in the medulla (see Experimental Procedures for details). To determine whether these neurons also express Ort, and are thus likely to receive histaminergic input, we expressed in the same animals, the rCD2::GFP marker using the ortC1-3-LexA::VP16 driver (Figure S7A–C”’). By overlaying two expression patterns, we found that many Ort-expressing neurons also expressed cholinergic or glutamatergic markers, while few did so for a GABAergic (Figure S7C–C”’) and none appeared to do so for serotonergic or dopaminergic phenotypes (Figure S7D, E). In particular, we found that a group of neurons labeled by both the vesicular glutamate transporter (vGlutOK371) and ort-Gal4 drivers extended processes in the M6 stratum where R7 axons terminate, suggesting that R7’s target neurons might be glutamatergic (Figure S7A–A”’).
To identify candidate R7 target neurons, we employed a combinatorial gene expression system, the Split-Gal4 system (Luan et al., 2006), to restrict Gal4 activity to glutamatergic Ort-expressing neurons. In this system, ort and vGlut promoters drive expression of the Gal4DBD (Gal4 DNA binding domain-leucine zipper) and dVP16AD (a codon-optimized VP16 trans-activation domain-leucine zipper), respectively. Thus, Gal4 activity was reconstituted only in the neurons that expressed both Ort and vGlut. We generated a dVP16AD enhancer trap vector and substituted it for the Gal4 enhancer trap in the vGlut locus (see Experimental Procedures for details). The resulting hemidriver, vGlutOK371-dVP16AD, in combination with a general neuronal hemidriver, elav-Gal4DBD, drove expression in a pattern essentially identical to that driven by vGlutOK371-Gal4, indicating that the vGlutOK371-dVP16AD enhancer trap recapitulated the expression pattern of the vGlutOK371-Gal4 driver (data not shown). The combination of the vGlutOK371-dVP16AD and ortC1-3-Gal4DBD hemidrivers (designated ortC1-3∩vGlut) gave rise to expression in a subset of Ort-expressing neurons in the optic lobe, namely those that express a glutamate phenotype and are thus likely to be glutamatergic (Figure 5A). Single-cell mosaic analysis (using hs-Flp and UAS>CD2>mCD8GFP) revealed that the combinatorial ortC1-3∩vGlut driver was expressed in Dm8, Tm5c, and L1 neurons, as well as in the medulla glia-like cells (data not shown). In contrast, cha∩ortC1-3, the combination of cha-Gal4DBD (choline acetyltransferase-Gal4DBD) and ortC1-3-Gal4AD hemidrivers, drove expression in the Ort-expressing neurons that expressed a cholinergic phenotype (Figure 5B), including L2, Tm2, Tm9, and Tm20 (data not shown). Notable among these findings, L1 and L2, paired lamina neurons that receive closely matched R1–R6 input in the lamina, express different neurotransmitter phenotypes (L1: glutamate; L2: acetylcholine).
Figure 5. Glutamatergic and cholinergic Ort-expressing neurons confer UV and green preference, respectively.
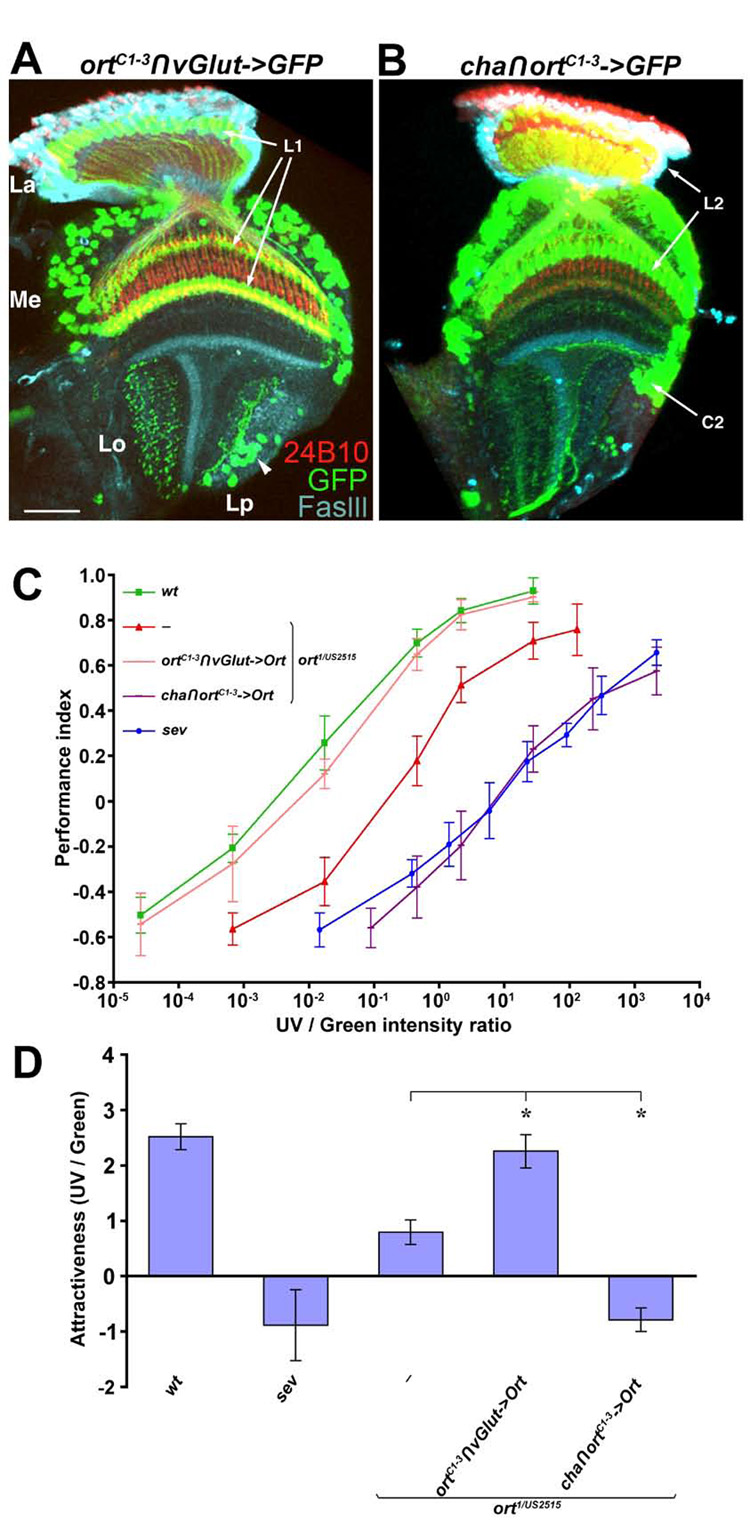
(A,B) The combinatorial drivers, ortC1-3∩vGlut (A) and cha∩ortC1-3 (B) are expressed in distinct neuron subsets in the adult optic lobe. The ortC1-3∩vGlut and cha∩ortC1-3 drivers express the EGFP marker (green) in those Ort-expressing neurons with either a glutamatergic or cholinergic phenotype. The ortC1-3∩vGlut driver labeled L1, Tm5c, and Dm8 neurons while the cha∩ortC1-3 driver was expressed in L2, C2, Tm2, Tm9 and Tm20. Lobula plate neurons (arrowhead, A), which do not normally express Ort, were also labeled by the combinatorial drivers. Photoreceptor axons visualized with MAb24B10 antibody (red); specific neuropil strata marked with FasIII antibody (cyan). Scale bar: 20 µm in (A) for (A–B).
(C–D) Sufficiency of glutamatergic or cholinergic Ort-expressing neurons for UV and green light preference. Restoring Ort function in phenotypically glutamatergic Ort-expressing neurons (ortC1-3∩vGlut->Ort) rescued the UV phototatic defects in ort mutant flies, while restoring phenotypically cholinergic Ort-expressing neurons (cha∩ortC1-3->Ort) rendered a stronger green preference.
(C) Intensity-response curves for UV/green spectral preference were measured as described in Figure 2. ort1/US2515, wild-type and negative control sev are from those described in Figure 2E.
(D) Histogram of the relative attractiveness of UV over green light (AttrUV/G) calculated from (C). The differences between ort mutants and those rescued with ortC1-3∩vGlut->Ort (and cha∩ortC1-3->Ort) are highly significant (*p<0.00001). Error bars indicate standard deviations. ort1/US2515, wild-type and negative control sev data are from those reported in Figure 2E.
The amacrine Dm8 neuron is both necessary and sufficient for optimal UV preference
To determine whether glutamatergic Ort-expressing neurons confer UV preference in flies, we examined whether expressing Ort in these neurons is sufficient to restore normal UV preference in ort mutants. We found that expressing Ort using the combinatorial ortC1-3∩vGlut driver restored normal UV preference in ort mutants (AttrUV/G=2.26±0.30) (Figure 5C,D). In contrast, expressing Ort in cholinergic Ort-expressing neurons using the cha∩ortC1-3 driver further reduced UV preference (AttrUV/G= −0.79±0.21), suggesting that the cholinergic Ort-expressing neurons reduce UV attraction or, more likely, enhance green attraction (Figure 5C,D). Although the cha∩ortC1-3 and ortC1-3∩vGlut drivers were expressed in specific subsets of Ort-expressing neurons in the optic lobe, they showed additional expression outside the visual system, and expressing shits1 with either driver caused non-specific motor defects at the non-permissive temperature (data not shown). Although we could not test whether the glutamatergic Ort-expressing neurons were required for UV preference, our rescue results indicated that the candidate glutamatergic Ort-expressing neurons, which included Dm8 and Tm5c, were involved in UV preference.
To distinguish whether Dm8 or Tm5c is required for UV preference, we dissected the ort promoter and generated three promoter-Gal4 lines, each of which contained one of the three highly conserved regions (C1–C3) of the ort promoter (Figure 6A). We found that the second and the third conserved regions (C2 and C3) gave rise to the expression in two different subsets of Ort-expressing neurons (Figure 6B,C) while C1 alone gave no detectable expression (data not shown). Using single-cell analysis, we found that ortC2-Gal4 drove expression in Dm8 and L1–L3 but not in any Tm neurons, while ortC3-Gal4 was expressed in L2, Tm2, Tm9, C2, and Mi1 neurons (data not shown). All these neurons except Mi1 expressed Ort, suggesting that the C2 and C3 fragments of the ort promoter drove expression in distinct subsets of the Ort-expressing neurons, but that the combination of all conserved regions was required to suppress Ort expression in Mi1.
Figure 6. Wide-field Dm8 amacrine neurons are required for UV preference.
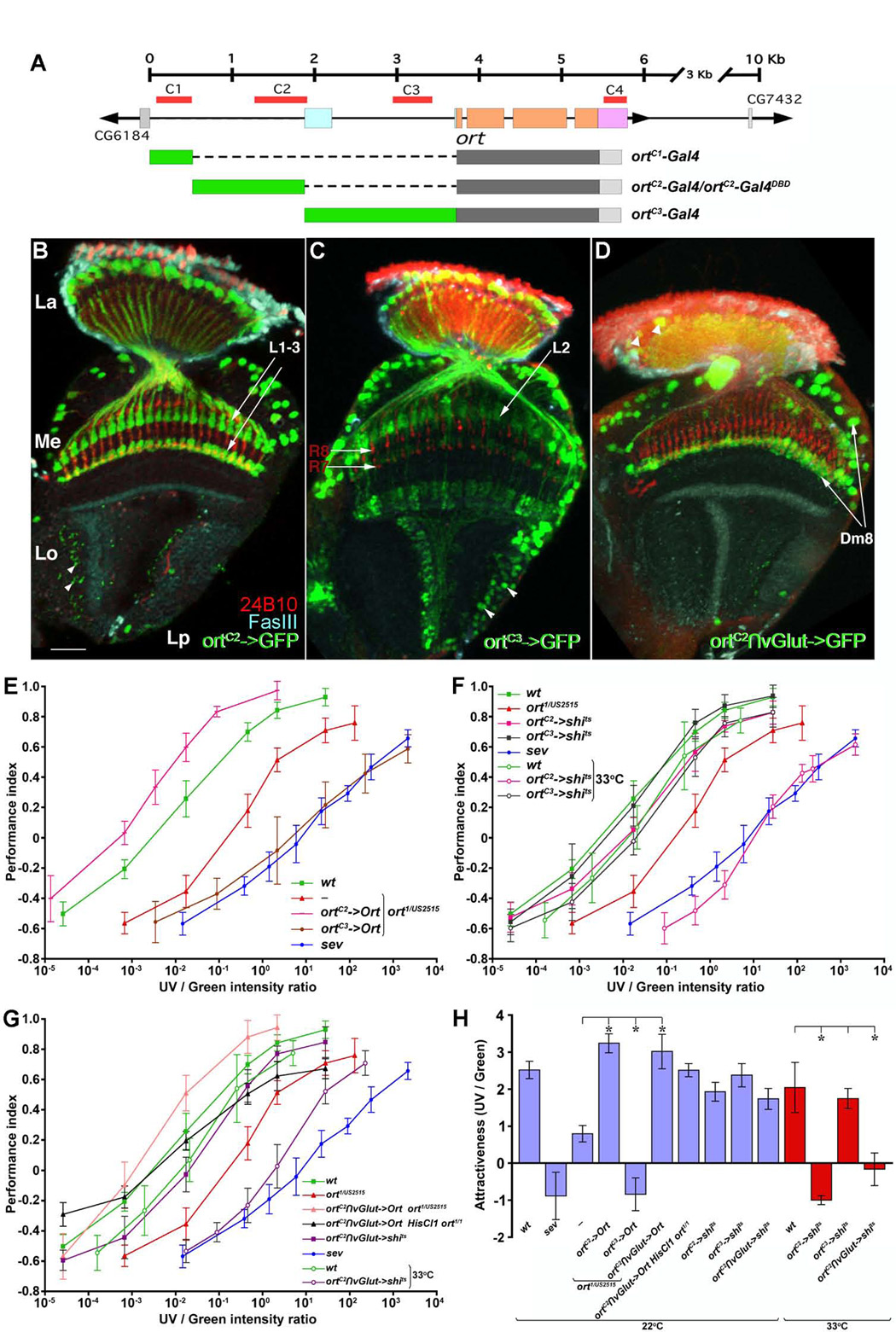
(A) Various ort promoter constructs containing different multispecies-conserved regions (C1–C3). The ort locus structure is as described in Figure 1E.
(B–D) Expression patterns of ortC2-GAL4 (B), ortC3-GAL4 (C) and ortC2∩vGlut (D) drivers in adult optic lobes. These drivers were used to express the mCD8-GFP marker in different subsets of Ort-expressing neurons (see text for details). A few neurons in the lobula and lobula plate (arrowheads), which do not normally express Ort, were labeled by ortC2-GAL4 and ortC3-GAL4, respectively (B, C). (D) The combinatorial driver ortC2∩vGlut labeled Dm8 as well as sparse L1 cells (arrowheads in lamina cortex). Photoreceptor axons visualized with MAb24B10 antibody (red); specific medulla and lobula strata immunolabeled with anti-FasIII (cyan). Scale bar: 20 µm in (B), for (B–D). (E–H) Restoring or blocking different subsets of Ort-expressing neurons affects UV/green preference. Intensity-response curves were measured as described in Figure 2.
(E) Sufficiency of ortC2 and ortC3 neurons for UV/green preference. Restoring the function to the ortC2 neuron subset rescued UV preference in ort mutants while restoring it to the ortC3 subset rendered a stronger green preference. ort1/US2515, wild-type and negative control sev data are from Figure 2E.
(F) Requirement for ortC2 and ortC3 neurons for UV/green preference. Blocking the ortC2, but not ortC3, neuron subset in wild-type background reduced UV preference. ort1/US2515, wild-type and negative control sev data are from Figure 2E.
(G) Requirement for, and sufficiency of, the Dm8 neurons for UV/green preference. Restoring Ort expression in the Dm8 neurons using the ortC2∩vGlut driver rescued UV preference defects in ort or HisCl1 ort double-null mutants. Conversely, inactivating the Dm8 neurons caused a significant reduction in UV preference. ort1/US2515, wild-type and negative control sev data are from Figure 2E.
(H) Histogram of the relative attractiveness of UV over green light (AttrUV/G) calculated from (E–G). The differences between ort mutants and after ort function is rescued in ortC2->Ort (or ortC3->Ort or ortC2∩vGlut->Ort) are statistically significant (*p<0.00001), as are those between the wild-type and ortC2->shits1 (or ortC2∩vGlut->shits1). Error bars indicate standard deviations.
We next examined whether the ortC2 or ortC3 neuron subsets were sufficient and/or required for UV preference. We found that expressing Ort using the ortC2-Gal4 driver in ort mutants was sufficient to restore UV preference at least up to the wild-type level (AttrUV/G=3.23±0.26; Figure 6E, H). Because the lamina neurons L1 and L2 are neither necessary nor sufficient for UV preference (Figure 2F–H), this finding suggested that the Dm8 neurons alone are sufficient to drive a fly’s normal preference for UV. Conversely, we tested whether these neurons were required for UV preference using shits1. We found that flies carrying ortC2->shits1 exhibited strongly attenuated UV preference at the non-permissive, but not permissive, temperatures (AttrUV/G= −1.00±0.12 at 33°C and 1.93±0.25 at 22°C; Figure 6F, H), indicating that the ortC2 subset is required for normal UV preference. In contrast, restoring the ortC3 subset activity further reduced UV preference (AttrUV/G= −0.84±0.44), suggesting that the ortC3 subset inhibits UV sensing, or enhances green-sensing pathways. Moreover, blocking the activity of the ortC3 subset using shits1 did not confer a stronger UV preference (AttrUV/G=1.74±0.27 at 33°C and 2.4±0.31 at 22°C), suggesting that the ortC3 subset is sufficient but likely not required for phototactic preference to green light (Figure 6F, H).
The preceding evidence indicated that the two lines, ortC2 and ort∩vGlut, together identified the Dm8 neurons both functionally and anatomically as a substrate for UV preference. To test this possibility directly, we generated an ortC2-Gal4DBD hemidriver and combined it with the vGlut-dVP16AD hemidriver. We found that the combinatorial driver ortC2∩vGlut was expressed in most Dm8 neurons as well as in a small number of L1 neurons and glia-like cells (Figure 6D). Restoring the expression of Ort in Dm8 in ort or HisCl1 ort double-null mutants completely restored normal UV preference (AttrUV/G=3.02±0.47 and 2.51±0.18, respectively). Conversely, flies carrying ortC2∩vGlut->shits1 exhibited reduced UV preference at the non-permissive, but not permissive, temperature (AttrUV/G= −0.17±0.44 at 33°C and 1.74±0.28 at 22°C; Figure 6G, H). Thus, the Dm8 are necessary and sufficient for a fly’s normal preference for UV.
Amacrine neuron Dm8 receives direct synaptic input from multiple R7s
Finally, using the single-cell mosaic method we examined the morphology of the Dm8 neurons (Figure 7A–A’). We found that in stratum M6 the Dm8 neurons extended web-like processes, which extensively overlapped 13–16 R7 terminals (average 14.6±0.99, n=15, mean ±SD; Figure 7A–A’). To determine whether Dm8 receives direct synaptic input from R7, we expressed an EM marker, HRP-CD2, in the Dm8 neurons using the ortC2–Gal4 driver and examined their synaptic structure at the EM level (Figure 7C). We found that most R7 synapses are triads and that Dm8 contributes to at least one of the three postsynaptic elements in essentially all R7 synapses. Cumulatively, Dm8 contributes to ~38% (18 out of 47 identified) of the elements postsynaptic to R7s, suggesting that Dm8 is a major synaptic target for these photoreceptors. In addition, we reconstructed processes of three Dm8 neurons spanning seven medulla columns. We found that Dm8 processes tiled the M6 stratum with partial overlapping so that each R7 terminal was presynaptic to one or two Dm8 neurons (Figure 7D). Examining the presynaptic structures of the Dm8 neurons at EM and light microscopic levels, revealed that the Dm8 neurons were also presynaptic to small-field medulla neurons in stratum M6, including Tm5 (Figure 7B, E–H) and at a few contacts to a cell that resembles Tm9. In summary, the wide-field Dm8 neuron serves as a major target neuron for R7 input and provides output locally in stratum M6 to small-field projection neurons.
Figure 7. Amacrine Dm8 neurons receive direct synaptic input from multiple R7 neurons.
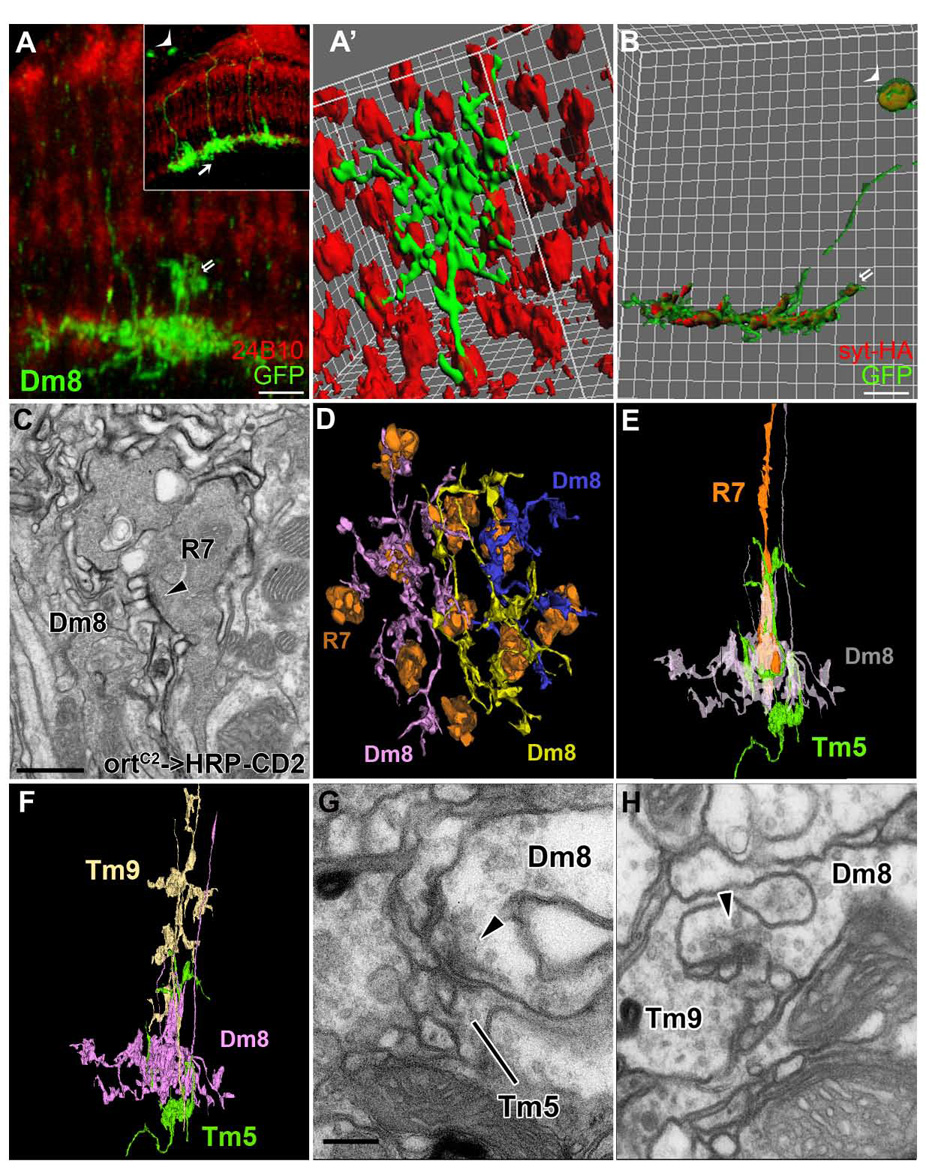
(A–A’) Single Dm8 neuron clones were generated using ortC2-Gal4, hs-Flp, and UAS->CD2>mCD8GFP and visualized with anti-GFP antibody (green). Photoreceptor axons were visualized with MAb24B10 antibody (red). Dm8 neurons extend large processes in medulla stratum M6 (arrow, inset) where they are postsynaptic to 13–16 R7s (A’) and presynaptic to Tm5s. In addition, each Dm8 extends small centrifugal processes to stratum M4 where they are presynaptic to Tm9 (double arrows, A, B). Demonstration of synaptic relations is shown from EM in later panels. (Inset) A low magnification view of (A). The arrowhead and arrow indicate the Dm8 neuron shown in (A). (A’) Isosurface representation of processes of a single Dm8 neuron in a proximo-distal view.
(B) Distribution of presynaptic sites of a single Dm8 neuron. Presynaptic reporter synaptotagmin-HA (red) was localized to the Dm8 processes in strata M6 and M4 (double arrows).
(C) Dm8 is postsynaptic to R7s. A single EM section from stratum M6 shows Dm8 processes marked by an EM marker HRP-CD2 and stained with DAB. R7 terminal was identified based on its vesicle-laden ultrastructure, the presence of capitate projections (not shown) and its location in stratum M6. Presynaptic T-bar ribbon (arrowhead) in R7 profile is juxtaposed to postsynaptic elements of Dm8 with electron-dense membranes.
(D) Serial-EM reconstruction of processes of three Dm8 neurons (pink, yellow and blue) and corresponding R7 terminals (orange). The processes of Dm8 neurons tile stratum M6 with partial overlapping so that each R7 is presynaptic to one or two Dm8 cells.
(E–F) Profiles of R7 (orange), Dm8 (pink), Tm5 (green) and Tm9 (beige) reconstructed from a single medulla column.
(G,H) Single EM sections show that Dm8 is presynaptic to Tm5 (G) and Tm9 (H). Presynaptic T-bar ribbons in Dm8, indicated by arrowheads, point in the presumed direction of transmission.
Scale bar: 5 µm in (A, B); 500 nm in (C); 200 nm in (G) for (G,H)
Discussion
Anatomical and functional mapping of chromatic visual circuits
Previous studies using serial-section EM determined the detailed synaptic connections between R1–R6 photoreceptors and their target neurons in the lamina neuropil (Meinertzhagen and O’Neil, 1991; Meinertzhagen and Sorra, 2001). Based on this circuit information, a recent functional study provided considerable insight into the neural mechanisms of motion detection (Rister et al., 2007). In contrast, little was known about the synaptic target neurons of the R7 and R8 photoreceptors and the chromatic pathways their connection patterns subserve. This deficit reflected our inability until recently to penetrate the medulla‘s complexity (Fischbach and Dittrich, 1989). In this study, we made use of prior knowledge of neurotransmitters and their receptors in the visual system to design corresponding promoter constructs that identify the first-order interneurons. We then labeled these neurons with genetically encoded markers and analyzed their morphology and synaptic connections at the light and electron microscopic levels. Finally, we combined promoter dissection and the Split-Gal4 system with neurotransmitter hemidrivers to target particular neuron subtypes. We envision that the same combinatorial approach can be applied to dissect other complex neural circuits.
Projection neurons integrate chromatic and achromatic channel inputs and relay information to the higher visual centers
In this study, we identified four types of transmedulla neurons, Tm5a/b/c, Tm9, Tm20 and Tm2, that express Ort and are therefore qualified to receive direct input from R7 or R8. These Tm neurons arborize in the medulla and project axons to the lobula, suggesting that they relay spectral information from the medulla to the lobula. Supporting this interpretation, we found that HA-syt, a presynaptic marker, is indeed localized to their terminals in the lobula. These data support previous suggestions that the lobula plays a key role in processing chromatic information for color vision (Bausenwein et al., 1992). Lobula stratum 5 appears most critical for color vision because it receives all three subtypes of Tm5 neurons as well as Tm20. Moreover, we observed that HA-syt also localized to the dendrite-like processes of all Tm neurons in the proximal medulla, suggesting the presence of presynaptic sites at this level, too. Especially, Tm5a, Tm5b, and Tm20 all extend processes with this presynaptic marker in medulla stratum M8, supporting a previous notion that this stratum might receive chromatic information (Bausenwein et al., 1992).
All three subtypes of Tm5 neurons extend processes in medulla strata M6 and M3, suggesting that there they might be postsynaptic to R7 and to R8 or L3. Using serial EM, we partially reconstructed a Tm5 subtype that receives direct synaptic input from both the chromatic UV channel of R7 and the achromatic channel of L3. Serial EM also revealed that Tm9 receives inputs from the chromatic green/blue channel of R8 as well as the achromatic L3 channel. It is tempting to speculate that the Tm9 and Tm5 neurons function as color-opponent neurons by subtracting the L3-mediated luminance signal from the R7/R8 chromatic signal (Figure 8). While the detailed neural mechanism must await electrophysiological studies, these anatomical data provide direct evidence that the achromatic and chromatic channels are not segregated, as previously proposed (Strausfeld and Lee, 1991). Instead they converge on the first/second-order interneurons, early in the visual pathway.
Figure 8. Medulla circuits in chromatic information processing.
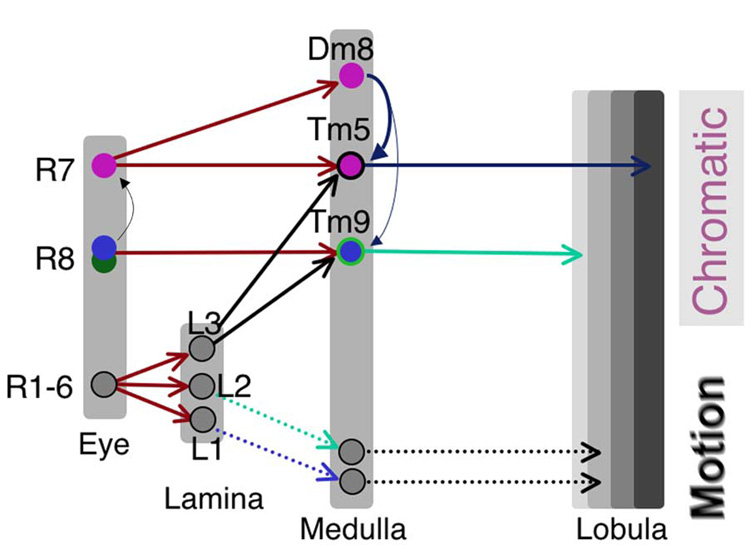
Summary diagram of synaptic connections between photoreceptor neurons and their first-order interneurons. Small-field projection neurons, Tm5 and Tm9, receive inputs from the chromatic channels, R7 and R8, respectively, as well as from the achromatic channel L3. Wide-field amacrine neuron Dm8 receives input from multiple R7s and is presynaptic to Tm5 and Tm9. In addition, R7 receives direct input from R8.
The amacrine neuron Dm8 is required for UV preference
Using a quantitative spectral preference test, we determined that in flies the Dm8 neurons are both necessary and sufficient to confer the animals’ UV preference. Each Dm8 receives direct synaptic input from ~14 UV-sensing R7s. By pooling multiple R7 inputs, the Dm8 neurons may achieve high UV sensitivity at the cost of spatial resolution. Consistent with this notion, Dm8 is a main postsynaptic partner for R7 terminals: essentially all of R7’s presynaptic sites contain at least one Dm8 postsynaptic element. The processes of Dm8 and their synapses with R7s are largely restricted to the medulla stratum M6. The stratum-specific arborization of Dm8 readily explains why R7 photoreceptors that fail to project axons to the M6 stratum are incapable of conferring UV preference (Lee et al., 2001; Clandinin et al., 2001).
Dm8 itself has no direct output to higher visual centers in the lobula; instead it is presynaptic to small-field projection neurons, such as Tm5 and possibly Tm9, in the medulla (Figure 8). Thus, Dm8 provides lateral connections linking projection neurons. The morphologies and connections of Dm8 are thus reminiscent of those made by horizontal and amacrine cells in the vertebrate retina (Dowling, 1987). The vertebrate horizontal cells form reciprocal synapses with multiple cones, and in the case where the cones are of different spectral types, the horizontal cells can establish color opponency, as demonstrated in the goldfish retina (Stell et al., 1975). Dm8 in Drosophila receives inputs from both Rh3- and Rh4-expressing R7s, but does not provide feedback to photoreceptor terminals, suggesting that Dm8 is unlikely to contribute to color opponency, at least not in a way analogous to vertebrate horizontal cells. Vertebrate amacrine cells have diverse subtypes, which carry out very different functions, including correlating firing among ganglion cells, modulating center-surround balance of the ganglion cells and direction selectivity (MacNeil and Masland, 1998; Meister et al., 1995; Yoshida et al., 2001; Nirenberg and Meister, 1997; He and Masland, 1997). The amacrine cells in vertebrate retina receive inputs from bipolar cells and provide the main synaptic input to ganglion cells. It is thus interesting to note that while direct synaptic connections from R7s to Tm5 projection neurons exists, the indirect information flow from R7, to Dm8, and then to Tm5, is both necessary and sufficient to confer UV preference, as suggested by our inactivating and restoring experiments (Figure 8). We hypothesize that the direct and indirect pathways function at different UV intensity levels: Dm8 pools multiple R7 inputs to detect low intensity UV in the presence of high-intensity visible light, while under high intensity UV, Tm5 receives direct input from R7 and mediates true color vision. Further studies using electrophysiology or functional imaging would be required to determine the neural mechanisms of Dm8.
The spectral preference assay used in this study and others measure relative “attractiveness” of UV and green light and therefore depends on the visual subsystems sensing UV and green light as well as the interactions between these subsystems (Schümperli, 1973; Fischbach 1979). While in simple phototaxis assays, the broad-spectrum and most sensitive photoreceptors, R1–R6, dominate simple phototactic response to both UV and green light, they, as well as their first-order interneurons L1 and L2, appear to play an insignificant or redundant role in spectral preference. Thus, R8 alone, or together with R1–R6, provides the sensory input to promote green phototaxis and/or to antagonize UV attraction. The first-order interneurons that relay R8 input in this context have yet to be identified. While our anatomical analysis revealed that Tm9 receives direct synaptic input from R8, the behavioral studies provided only weak and circumstantial evidence for its role in spectral preference. Expressing Ort using the cha∩ortC1-3 or ortC3-Gal4 driver significantly reduced UV preference in ort mutants, and Tm9 is covered by both drivers. Furthermore, inactivating Tm9 using the ortC3 driver and shits1 did not affect UV preference, suggesting that other neurons, such as Tm20, might function redundantly. Verification of these suggestions must await the isolation of Tm9-and Tm20-specific drivers, and the corresponding behavioral studies to assay the effects of perturbing activity in these neurons. It is worth noting that Ort-expressing neurons do not include any Dm8-like wide-field neurons for R8s, and restoring activity in the ortC3 neuron subset is sufficient to confer stronger green preference in ort mutants. It is thus tempting to speculate that Dm8 circuits evolved uniquely to meet the ecological need to detect dim UV against a background of ample visible light.
Experimental Procedures
Comparative genomic analyses
were carried out as previously reported (Odenwald et al., 2005; Yavatkar et al., 2008). See Supplementary Experimental Procedures for details.
Generation of ort and HisCl1 promoter constructs and transgenic flies
Ort and HisCl1 promoter fragments were PCR amplified from genomic DNA of wild-type Oregon-R flies and were used to generate various ort promoter-Gal4, LexA:VP16 and HisCl1-Gal4 constructs (Figure 1, Figure 6 and Figure S3). Cloning procedures are described in Supplementary Experimental Procedures. Transgenic flies were generated using standard injection procedures by Rainbow Transgenic Flies, Inc. (Newbury Park, California).
Fly Stocks
The genotypes of fly lines and rearing conditions are described in Supplementary Experimental Procedures.
Generation of single-neuron clones
To label single neurons, we used the hs-Flp and UAS>CD2,y+>mCD8-GFP transgenes (Wong et al., 2002) in combination with various ort promoter-Gal4 or split-Gal4 drivers. To assess, in single neurons, the distribution of the presynaptic marker, HA-tagged synaptotagmin (a generous gift from Drs. Christopher Potter and Liqun Luo), we used a similar flip-out strategy to that described above but implemented the strategy using Tub>Gal80>. Flies carrying the transgenes hs-Flp, UAS-Syt-HA, UAS-mCD8GFP, Tub>Gal80>, and ortC1-3-Gal4 (or ortC2-Gal4) were used. Larvae of suitable genotypes at the late second- or early third-instar stage were briefly heat-shocked at 38°C for 3 min to remove the flip-out cassette (>CD2, y+> or >Gal80>) in a small number of neurons.
Confocal imaging of whole-mount brains
Confocal imaging was performed as described previously (Ting et al., 2005). See Supplementary Experimental Procedures for details.
Electron microscopy
UAS-HRP-CD2 (a generous gift from Dr. Liquin Luo) was used to visualize Gal4-driven expression in identified neurons that was targeted to the plasmalemma (Larsen et al., 2003). To reveal HRP activity at the EM level, we used the DAB method, and after dissecting brains from the head capsule, exposed them to DAB as previously reported (Clements et al., 2008). Serial-section EM of the medulla was then undertaken, also as previously reported (Takemura et al., 2008). Cells that expressed HRP had an electron-dense reaction product at their membranes. 3D EM reconstructions of Tm5, Tm2, Tm9 and Dm8 profiles were carried out based on an unlabeled series of 672 60-nm sections, which included the outer six strata of the medulla, as described previously (Takemura et al., 2008)
UV/Green Spectral Preference and Phototaxis Assays
The forced two-choice assay for testing a fly’s phototaxis preference to UV or green light has been described previously (Ting et al., 2007). Fast phototaxis assay was performed as for the spectral preference assay except that only one light source was used. Detailed procedures are given in Supplementary Experimental Procedures
Head Yaw Optomotor Response Assay
The head yaw optomotor assay has been described (Rister et al., 2007). The modifications are described in Supplementary Experimental Procedures.
Electrophysiological Recording
Electroretinogram (ERG) recordings were taken using an electrophysiology set up provided by Dr. Howard Nash (NIH) as described previously (Rajaram et al., 2005).
Supplementary Material
Acknowledgments
We thank Drs. William Pak, Liquin Luo, Christopher Potter, Martin Heisenberg, Tzumin Lee, Robert White, Akinao Nose, Howard Nash, Jaeseob Kim, Gero Miesenbock and Alan Wong for reagents. We thank Drs. Claude Desplan and Javier Morante for communicating results prior to publication. We thank Dr. Howard Nash and Robert Scott for helping with ERG recordings and for providing the optomotor equipment. We thank Tom Pohida and Randall Pursley for optimizing the pattern-recognition software, and Jimmie Powell and Howard Metger for instrument fabrication. We thank Mr. Louis Dye and Dr. James Russell at NICHD’s Microscopy and Image Core for assistance with EM image collection. We thank Drs. Alan Hinnebusch, Mark Stopfer, and Henry Levin for helpful discussion and Margaret Dieringer and Laura Lee for editing and manuscript handling. This work was supported by the Intramural Research Program of the NIH, National Institute of Child Health and Human Development (grant HD008748-03 to C.-H.L.), by the German Science Foundation (GRK 1156 and SFB 554 to J.R.) and by a grant from the National Eye Institute of the NIH (EY-03592 to I.A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bausenwein B, Dittrich AP, Fischbach KF. The optic lobe of Drosophila melanogaster. II. Pathways in the medulla. Cell Tissue Res. 1992;267:17–28. doi: 10.1007/BF00318687. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Strausfeld NJ. The columnar organization of the second synaptic region of the visual system of Musca domestica. L. I. Receptor terminals in the medulla. Z Zellforsch Mikrosk Anat. 1972;124:561–585. doi: 10.1007/BF00335258. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Lee C-H, Herman T, Lee RC, Yang AY, Ovasapyan S, Zipursky SL. Drosophila LAR regulates R1–R6 and R7 target specificity in the visual system. Neuron. 2001;32:237–248. doi: 10.1016/s0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Clements J, Lu Z, Gehring WJ, Meinertzhagen IA, Callaerts P. Central projections of photoreceptor axons originating from ectopic eyes in Drosophila. Proc Natl Acad Sci USA. 2008;105:8968–8973. doi: 10.1073/pnas.0803254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe PE, Heisenberg M. The structural brain mutant Vacuolar medulla of Drosophila melanogaster with specific behavioral defects and cell degeneration in the adult. J Neurogenet. 1986;3:135–158. doi: 10.3109/01677068609106845. [DOI] [PubMed] [Google Scholar]
- Dowling JE. The Retina. Cambridge: Harvard University Press; 1987. [Google Scholar]
- Fischbach KF. Simultaneous and successive colour contrast expressed in "slow" phototactic behaviour of walking Drosophila melanogaster. J Comp Physiol. 1979;130:161–171. [Google Scholar]
- Fischbach KF, Dittrich AP. The optic lobe of Drosophila melangaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 1989;258:441–475. [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gengs C, Leung HT, Skingsley DR, Iovchev MI, Yin Z, Semenov EP, Burg MG, Hardie RC, Pak WL. The target of Drosophila photoreceptor synaptic transmission is a histamine-gated chloride channel encoded by ort (hclA) J Biol Chem. 2002;277:42113–42120. doi: 10.1074/jbc.M207133200. [DOI] [PubMed] [Google Scholar]
- Gisselmann G, Pusch H, Hovemann BT, Hatt H. Two cDNAs coding for histamine-gated ion channels in D. melanogaster. Nat Neurosci. 2002;5:11–12. doi: 10.1038/nn787. [DOI] [PubMed] [Google Scholar]
- Goldsmith TH. The color vision of insects. Baltimore: The Johns Hopkins University Press; 1961. [Google Scholar]
- Hardie RC. Electrophysiological analysis of the fly retina. I: Comparative properties of R1-6 and R7 and R8. J Comp Physiol. 1979;129:19–33. [Google Scholar]
- Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? J Comp Physiol. 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- He S, Masland RH. Retinal direction selectivity after targeted laser ablation of starburst amacrine cells. Nature. 1997;389:378–382. doi: 10.1038/38723. [DOI] [PubMed] [Google Scholar]
- Heisenberg M, Buchner E. The role of retinula cell types in the visual behavior of Drosophila melanogaster. J Comp Physiol. 1977;117:127–162. [Google Scholar]
- Hong ST, Bang S, Paik D, Kang J, Hwang S, Jeon K, Chun B, Hyun S, Lee Y, Kim J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci. 2006;26:7245–7256. doi: 10.1523/JNEUROSCI.5426-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu KG, Stark WS. Specific receptor input into spectral preference in Drosophila. J Comp Physiol. 1977;121:241–252. [Google Scholar]
- Jacob KG, Willmund R, Folkers E, Fischbach KF, Spatz HCh. T-maze phototaxis of Drosophila melanogaster and several mutants in the visual systems. J Comp Physiol. 1977;116:209–225. [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Larsen CW, Hirst E, Alexandre C, Vincent JP. Segment boundary formation in Drosophila embryos. Development. 2003;130:5625–5635. doi: 10.1242/dev.00867. [DOI] [PubMed] [Google Scholar]
- Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- Mahr A, Aberle H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr Patterns. 2006;6:299–309. doi: 10.1016/j.modgep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA. The organization of perpendicular fibre pathways in the insect optic lobe. Philos Trans R Soc Lond B Biol Sci. 1976;274:555–594. doi: 10.1098/rstb.1976.0064. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, O'Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Sorra KE. Synaptic organisation in the fly's optic lamina: few cells, many synapses and divergent microcircuits. Progr Brain Res. 2001;131:53–69. doi: 10.1016/s0079-6123(01)31007-5. [DOI] [PubMed] [Google Scholar]
- Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270:1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- Menzel R. Spectral sensitivity and colour vision in invertebrates. In: Autrum H, editor. Handbook of sensory physiology. vol VII/6A. New York: Springer Verlag: Springer, Berlin Heidelberg New York; 1979. pp. 503–580. [Google Scholar]
- Menzel R, Greggers U. Natural phototaxis and its relationship to colour vision in honeybees. J comp Physiol. 1985;157:311–321. [Google Scholar]
- Menzel R, Backhaus W. Colour vision in insects. In: Gouras P, editor. Vision and visual dysfunction. The perception of colour. London: MacMillan; 1991. pp. 262–288. [Google Scholar]
- Mikeladze-Dvali T, Desplan C, Pistillo D. Flipping coins in the fly retina. Curr Top Dev Biol. 2005;69:1–15. doi: 10.1016/S0070-2153(05)69001-1. [DOI] [PubMed] [Google Scholar]
- Morante J, Desplan C. The color-vision circuit in the medulla of Drosophila. Curr Biol. 2008;18:553–565. doi: 10.1016/j.cub.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenbock G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Nirenberg S, Meister M. The light response of retinal ganglion cells is truncated by a displaced amacrine circuit. Neuron. 1997;18:637–650. doi: 10.1016/s0896-6273(00)80304-9. [DOI] [PubMed] [Google Scholar]
- Odenwald WF, Rasband W, Kuzin A, Brody T. EVOPRINTER, a multigenomic comparative tool for rapid identification of functionally important DNA. Proc Natl Acad Sci USA. 2005;102:14700–14705. doi: 10.1073/pnas.0506915102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orger MB, Baier H. Channeling of red and green cone inputs to the zebrafish optomotor response. Vis Neurosci. 2005;22:275–281. doi: 10.1017/S0952523805223039. [DOI] [PubMed] [Google Scholar]
- O'Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Pantazis A, Segaran A, Liu CH, Nikolaev A, Rister J, Thum AS, Roeder T, Semenov E, Juusola M, Hardie RC. Distinct roles for two histamine receptors (hclA and hclB) at the Drosophila photoreceptor synapse. J. Neurosci. 2008;28:7250–7259. doi: 10.1523/JNEUROSCI.1654-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Scott RL, Nash HA. Retrograde signaling from the brain to the retina modulates the termination of the light response in Drosophila. Proc Natl Acad Sci USA. 2005;102:17840–17845. doi: 10.1073/pnas.0508858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister J, Pauls D, Schnell B, Ting CY, Lee CH, Sinakevitch I, Morante J, Strausfeld NJ, Ito K, Heisenberg M. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Huber A, Henrich S, Chadwell LV, Chou WH, Paulsen R, Britt SG. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J Neurosci. 1999;19:10716–10726. doi: 10.1523/JNEUROSCI.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy PV. Histamine: a neurotransmitter candidate for Drosophila photoreceptors. J Neurochem. 1991;57:1757–1768. doi: 10.1111/j.1471-4159.1991.tb06378.x. [DOI] [PubMed] [Google Scholar]
- Schümperli RA. Evidence for color vision in Drosophila melanogaster through spontaneous phototactic choice behaviour. J Comp Physiol. 1973;86:77–94. [Google Scholar]
- Sepp KJ, Auld VJ. Conversion of lacZ enhancer trap lines to GAL4 lines using targeted transposition in Drosophila melanogaster. Genetics. 1999;151:1093–1101. doi: 10.1093/genetics/151.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinza-Kameda M, Takasu E, Sakurai K, Hayashi S, Nose A. Regulation of layer-specific targeting by reciprocal expression of a cell adhesion molecule, capricious. Neuron. 2006;49:205–213. doi: 10.1016/j.neuron.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Stell WK, Lightfood DO, Wheeler TG, Leeper HF. Goldfish retina: functional polarization of cone horizontal cell dendrites and synapses. Science. 1975;190:989–990. doi: 10.1126/science.1188380. [DOI] [PubMed] [Google Scholar]
- Storz UC, Paul RJ. Phototaxis in water fleas (Daphnia magna) is differently influenced by visible and UV light. J Comp Physiol A. 1998;183:709–717. [Google Scholar]
- Strausfeld NJ, Lee JK. Neuronal basis for parallel visual processing in the fly. Vis Neurosci. 1991;7:13–33. doi: 10.1017/s0952523800010919. [DOI] [PubMed] [Google Scholar]
- Takemura S, Lu Z, Meinerzhagen IA. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. J Comp Neurol. 2008;509:493–513. doi: 10.1002/cne.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CY, Herman T, Yonekura S, Gao S, Wang J, Serpe M, O'Connor MB, Zipursky SL, Lee CH. Tiling of R7 axons in the Drosophila visual System is mediated both by transduction of an Activin signal to the nucleus and by mutual repulsion. Neuron. 2007;56:793–806. doi: 10.1016/j.neuron.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CY, Yonekura S, Chung P, Hsu SN, Robertson HM, Chiba A, Lee CH. Drosophila N-cadherin functions in the first stage of the two-stage layer-selection process of R7 photoreceptor afferents. Development. 2005;132:953–963. doi: 10.1242/dev.01661. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Ready DF. Sevenless: A Cell-Specific Homeotic Mutation of the Drosophila eye. Science. 1986;231:400–402. doi: 10.1126/science.231.4736.400. [DOI] [PubMed] [Google Scholar]
- Witte I, Kreienkamp HJ, Gewecke M, Roeder T. Putative histamine-gated chloride channel subunits of the insect visual system and thoracic ganglion. J Neurochem. 2002;83:504–514. doi: 10.1046/j.1471-4159.2002.01076.x. [DOI] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Wolf R, Desplan C, Heisenberg M. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Kitamoto T, Salvaterra PM. Localization of choline acetyltransferase-expressing neurons in the larval visual system of Drosophila melanogaster. Cell Tissue Res. 1995;282:193–202. doi: 10.1007/BF00319111. [DOI] [PubMed] [Google Scholar]
- Yavatkar AS, Lin Y, Ross J, Fann Y, Brody T, Odenwald WF. Rapid detection and curation of conserved DNA via enhanced-BLAT and EvoPrinterHD analysis. BMC Genomics. 2008;9:106–118. doi: 10.1186/1471-2164-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Hirschberg B, Yuan J, Wang AP, Hunt DC, Ludmerer SW, Schmatz DM, Cully DF. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J Biol Chem. 2002;277:2000–2005. doi: 10.1074/jbc.M107635200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


