Abstract
Many recent studies indicate that dysregulation of autophagy is a common feature of many neurodegenerative diseases. The HIV-1-associated neurological disorder is an acquired cognitive and motor disease that includes a severe neurodegenerative dementia. We find that the neurodegeneration seen in the brain in HIV-1 infection is associated with an inhibition of neuronal autophagy, leading to neuronal demise. Neurons treated with supernatants from SIV-infected microglia develop a decrease in autophagy-inducing proteins, a decrease in neuronal autophagy vesicles, and an increase in sequestosome-1/p62. Examination of brains from HIV-infected individuals and SIV-infected monkeys reveals signs of autophagy dysregulation, associated, respectively, with dementia and encephalitis. Excitotoxic and inflammatory factors could inhibit neuronal autophagy, and stimulation of autophagy with rapamycin prevents such effects. Here we amplify on these findings, and propose that in the setting of HIV-infection, the decreased neuronal autophagy sensitizes cells to pro-apoptotic and other damaging mechanisms, leading to neuronal dysfunction and death. Hence, new therapeutic approaches aimed at boosting neuronal autophagy are conceivable to treat those suffering from the neurological complications of HIV.
Keywords: autophagy, HIV-1 associated neurocognitive disorder, neuron, neurodegeneration, microglia, dementia, AIDS, SIV, NMDA
Inhibition of neuronal autophagy in HIV/SIV infection of the central nervous system
The mechanism of autophagy has received increased attention in recent years because of its recognition as a vital arbiter of death/survival decisions in cells and as a critical defense mechanism in infection, immune surveillance and degenerative states.1–3 Specific attention is being paid to autophagy in neurodegenerative diseases, where it has been implicated in mechanisms of neurodegeneration and in some cases in earlier stages of disease pathogenesis.4 Many studies have shown that autophagy is a protective mechanism in neurodegenerative disorders, and that its inhibition is associated with neurodegeneration.5
The HIV-1-associated neurocognitive disorder (HAND),6, also known as neuroAIDS, occurs in approximately one-third of HIV-1 infected individuals. Symptoms range from minor difficulties to severe dementia. Although highly active anti-retroviral therapy (HAART) has led to a decreased incidence of HAND in infected people with access to therapy, people now live longer following the diagnosis of HAND,7 and the prevalence of HAND may be increasing.8 The severe form, HIV-associated dementia, is an acquired neurodegenerative syndrome. Neurons are not infected by HIV-1, but there is compelling evidence that neurodegeneration results from the production of molecules from activated and infected macrophages/microglia in the brain,9, 10 that includes a number of inflammatory mediators such as cytokines, neurotoxic substances such as inducers of oxidative stress, and potentially injurious viral proteins.11–13 Such glial sources of neurodegeneration, referred to as “non-cell-autonomous neurodegenerative processes” are becoming increasingly recognized to extend beyond infectious diseases and may promote to the progression of a variety of neurodegenerative disorders.14
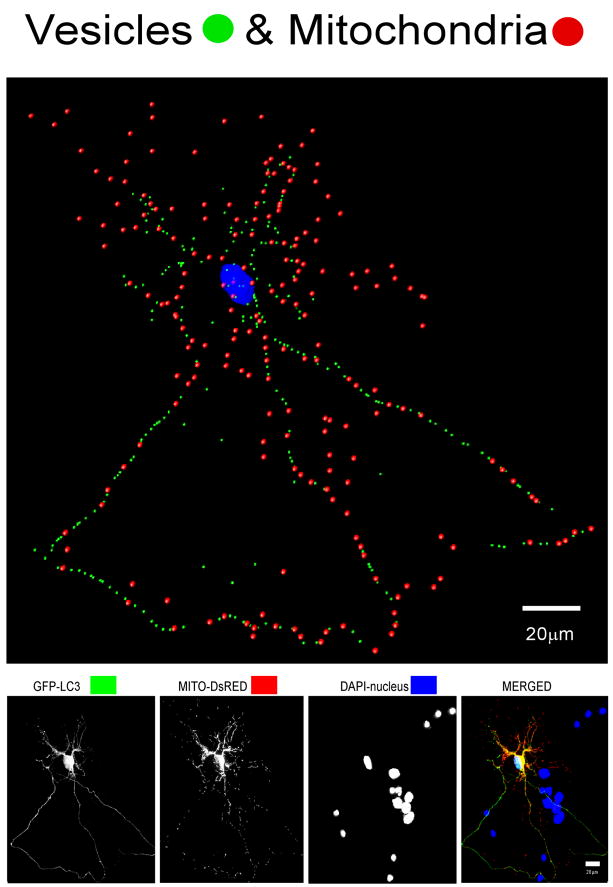
To examine the potential implication of autophagy in this disorder, we used an in vitro model of primary rat neuronal cultures treated with supernatants from microglia derived from simian immunodeficiency virus (SIV)-infected monkeys with encephalitis, an animal model for HIV dementia,15 and compared autophagy parameters with similar cultures treated with supernatants from microglia of uninfected monkeys.16 While many of our measures were well described cellular and biochemical assays, quantifying the autophagic vesicles (AV) in primary cortical neurons, which have extensive neurites extending from the soma in three-dimensions, was difficult. To accomplish this, and distinguish between the processes and the soma of a neuron in the analysis, we utilized laser scanning confocal microscopy and quantitative three-dimensional (3D) analysis. Briefly, once fluorescent optical sections were obtained, they were imported into IMARIS software (BitPlane Inc., MN, USA). The IMARIS macro “filament tracker” was used to automatically outline the GFP-LC3 fluorescence signal in 3D space, define the spatial location of the cell body and all of its neurites, and create a 3D trace of the neuron. A very discriminating signal threshold range is then used to define a real signal above background for precisely outlining and marking positively-labeled vesicles. This range was used throughout the soma and its processes to determine the number and localization of vesicles in neurons under different conditions in order to evaluate their potential changes due to experimental perturbation (Figure 1). While initially the number of AV found, indicative of active autophagy, was surprising, others also recently report evidence of active autophagy in neurons.17
Figure 1.
(Top) IMARIS analysis of confocal images of primary neurons, delineating subcellular structures. Autophagy vesicles (green dots), mitochondria (red dots), and the nucleus (blue) are shown in this neuron. (Bottom) Flattened confocal images of the neuron shown above, which has been co-transfected with GFP-LC3 to label AV and DsRed-Mito to label mitochondria, and reacted with DAPI for nuclear staining.
Our study demonstrates that the total AV (AV in soma and neurites) number is significantly depressed after an exposure to the supernatant from SIV-infected microglia. Combined with additional analyses (decrease in LC3-II and Atg12–Atg5 conjugate levels, increase in sequestosome-1/p62 levels, and reversibility by rapamycin), our experiments reveal an impairment of autophagy in treated neurons.16 The decrease in AV is largely due to loss of AV from the neurites, and is associated with increased neuronal death. We find that such effects can be mimicked by two molecules: N-methyl-D-aspartic acid (NMDA), which mimics the neurotransmitter glutamate at a specific subtype of ionotropic glutamate receptor (NMDA receptor, NMDAR), and tumor necrosis factor alpha (TNF-α); recapitulating the excitotoxic and pro-inflammatory conditions, respectfully, present in the brain during HIV encephalitis. Furthermore, examination of brains from HIV-infected individuals with dementia reveals molecular and histopathological signs of decreased autophagy. In addition to HIV dementia, we also observe similar signs of anomalous autophagy in brains with SIV encephalitis, which are absent from nonencephalitic SIV-infected, or uninfected specimens.
Aggregates of mutant/misfolded proteins are a common finding in many neurodegenerative diseases. Insight from genetics has revealed that in some cases specific gene mutations result in direct alteration of the protein itself, whereas in others the mutations result in altered processing of the protein, both of which can lead to intracellular or extracellular deposition of the resulting abnormal proteins.18 Along with the ubiquitin-proteasome system, autophagy is key in removing such mutant molecules, and defects in these systems may thus contribute to the non-genetic, sporadic versions of neurodegenerative diseases. Whereas deficits in neuronal autophagy have been postulated to develop in vivo, for example in aging,3 here we find that products of microglia inhibit autophagy, and are linked to neuronal death.16 In addition to neuroAIDS, microglia are likely key players in a number of neurodegenerative conditions,19, 20 and we hypothesize that microglia-induced autophagy perturbations may be a more general mechanism contributing to neurodegeneration.
HIV/SIV effects on neuroblastoma cell lines
Autophagy is altered in a number of virally infected cells, including HIV-1, where HIV-1 infection of CD4+ T-lymphocytes inhibits autophagy.21 Yet the relevance to the current findings is unclear, since in this study the cells were infected by HIV-1, whereas neurons are not infectible. However these authors are now assessing the effects of HIV-1 gp120-derived peptides on the neuroblastoma cell line SK-N-SH, and have hypothesized that there is enhanced autophagy in HIV dementia.22 We have also utilized a neuroblastoma cell line in our studies (line SH-SY5Y, a subclone of SK-N-SH). As part of these experiments we examined the effect of exposure of these cells to supernatant from SIV-infected microglia on the number of AV. The cells were examined both in their undifferentiated state, as well as following differentiation with retinoic acid (RA) followed by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA), which induces dopaminergic neuronal differentiation.23 In both cases, exposure to the SIV-infected microglia supernatant significantly enhanced the number of AV per cell (Figure 2).
Figure 2.
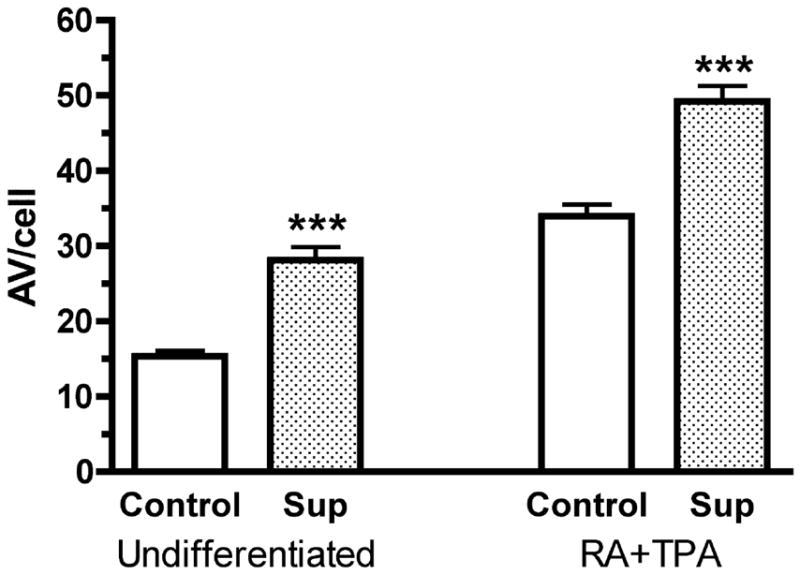
AV counts in undifferentiated (left) and dopaminergic differentiated (RA+TPA) SH-SY5Y cells (right), treated (Sup), or not (control), with supernatant from SIV-infected microglia. In both cases, the number of AV is significantly increased (p<0.001, t-test).
Although the reason for the difference between the effects on primary neurons and the cell line is not known, we believe that there are two likely explanations. First, there are, of course, a large number of differences between neuroblastoma cell lines and primary neurons, and due to the variable degree to which cell lines replicate the properties of specialized cell types such as post-mitotic neurons, caution is needed in applying results from such model systems to the in vivo situation. Although we performed differentiation of the cell line with chemical agents, there are other means to try to make such cells mimic neurons,24 and an ideal cell line system to model neurons is still a significant goal in neuroscience research. Second, the primary neurons we used were cortical glutamatergic neurons. Not only are there a large number of different neuronal subtypes within the cortex, throughout the different brain regions there are a large variety of neurons, all of which have their own specialized functions and likely different responses to damaging agents. Therefore, although we find reduced autophagy in our cortical neuronal cultures, we cannot rule out distinct responses in other types of neurons such as dopaminergic primary neurons.
Excitotoxicity, calpain, and the interplay between autophagy and apoptosis
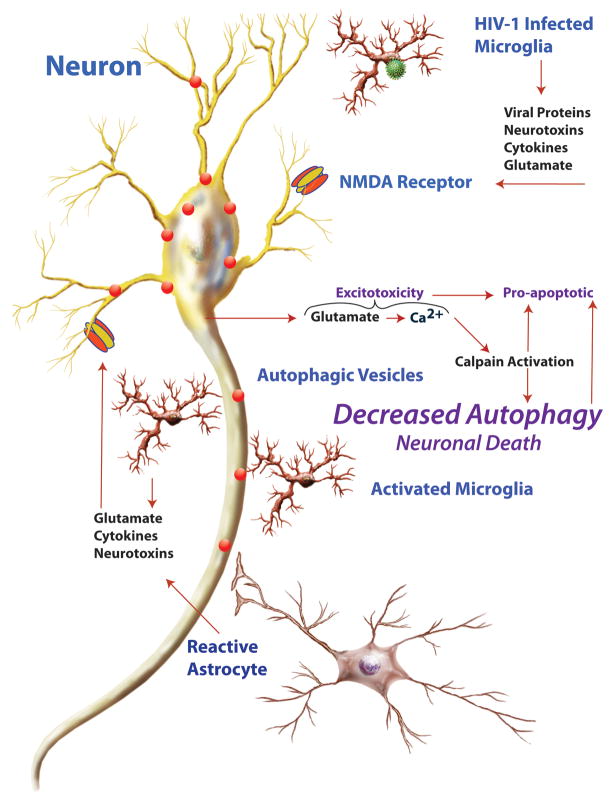
Although our experiments reveal inhibition of autophagy by the SIV-infected microglia supernatant, the mechanism for this inhibition is still unknown. Nevertheless, it is known that neuronal excitotoxicity is a major mechanism in HIV dementia, leading to apoptosis.25 We have reported that the non-competetive NMDA receptor antagonist MK-801 prevents the nuerotoxicity induced by the SIV-infected microglia supernatants.16 Furthermore, we have found that a pharmacological inhibitor of calpain had similar neuroprotective effects (Alirezaei M and Fox HS, unpublished data). Interestingly, calpain is activated and plays a pro-death role in HIV-induced neurodegeneration.26, 27 Excitotoxic agents (mimicked in our study by NMDA treatment) induce Ca2+ influx through glutamate receptors. Ca2+ influx induces calpain activation, which in turn inhibits autophagy.28 Calpain also plays an important role in interactions between autophagy and apoptosis.29, 30 Therefore we hypothesize that neuronal dysfunction and death in HIV infection results from a combination of decreased autophagic and increased apoptotic and other neuronal damaging mechanisms (Figure 3), emanating from the glial cells. As illustrated, in addition to the infected microglia, uninfected but stimulated microglia and astrocytes can also contribute to neuronal injury. Furthermore, such glial factors can act in the absence of infection, and we suggest that such indirect mechanisms may be active in other neurodegenerative disorders. We propose that therapies to stimulate autophagy in neurons can be neuroprotective in neuroAIDS, protecting against pro-apoptotic and other neurotoxic stimuli.
Figure 3.
Schematic illustration of interaction between HIV-infected microglia, other glia, and neurons in neuroAIDS. HIV-infected microglia, as well as activated/reactive microglia and astrocytes, produce molecules that lead to a pro-inflammatory and excitotoxic environment, resulting in an NMDAR-dependent mechanism leading to neuronal injury and death. Glutamate activation of the NMDAR leads to calcium influx into the neuron, calpain activation, suppression of autophagy, and enhancement of apoptotic pathways, leading to neuronal demise.
Acknowledgments
This work was supported by NIH grants DA024467, MH073490 and MH062261.
Footnotes
Addendum to: Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS ONE 2008; 3:e2906
References
- 1.Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev of Pathol. 2008;3:427–55. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 2.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Vicente M, Cuervo AM. Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol. 2007;6:352–61. doi: 10.1016/S1474-4422(07)70076-5. [DOI] [PubMed] [Google Scholar]
- 6.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 2003;17:1539–45. doi: 10.1097/00002030-200307040-00015. [DOI] [PubMed] [Google Scholar]
- 8.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18 (Suppl 1):S75–8. [PubMed] [Google Scholar]
- 9.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12 (Suppl 1):878–92. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 11.Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–22. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, Dickie P, Jhamandas J, Power C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–11. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alirezaei M, Watry DD, Flynn CF, Kiosses WB, Masliah E, Williams BR, Kaul M, Lipton SA, Fox HS. Human immunodeficiency virus-1/surface glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J Neurosci. 2007;27:11047–55. doi: 10.1523/JNEUROSCI.2733-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–60. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burudi EME, Fox HS. Simian Immunodeficiency Virus Model of HIV-Induced Central Nervous System Dysfuntion. In: Buchmeier MJ, Campbell I, editors. Advances in Virus Research. Academic Press; 2001. pp. 431–64. [DOI] [PubMed] [Google Scholar]
- 16.Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS ONE. 2008;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28:6926–37. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–63. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharm Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 20.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS. 2008;22:695–9. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector SA, Zhou D. Autophagy: an overlooked mechanism of HIV-1 pathogenesis and neuroAIDS? Autophagy. 2008;4:704–6. doi: 10.4161/auto.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Presgraves SP, Ahmed T, Borwege S, Joyce JN. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox Res. 2004;5:579–98. doi: 10.1007/BF03033178. [DOI] [PubMed] [Google Scholar]
- 24.Myers TA, Nickerson CA, Kaushal D, Ott CM, Honer Zu Bentrup K, Ramamurthy R, Nelman-Gonzalez M, Pierson DL, Philipp MT. Closing the phenotypic gap between transformed neuronal cell lines in culture and untransformed neurons. J Neurosci Methods. 2008 doi: 10.1016/j.jneumeth.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci. 2006;26:981–90. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, White MG, Akay C, Chodroff RA, Robinson J, Lindl KA, Dichter MA, Qian Y, Mao Z, Kolson DL, Jordan-Sciutto KL. Activation of cyclin-dependent kinase 5 by calpains contributes to human immunodeficiency virus-induced neurotoxicity. J Neurochem. 2007;103:439–55. doi: 10.1111/j.1471-4159.2007.04746.x. [DOI] [PubMed] [Google Scholar]
- 28.Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O’Kane CJ, Floto RA, Rubinsztein DC. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demarchi F, Bertoli C, Copetti T, Tanida I, Brancolini C, Eskelinen EL, Schneider C. Calpain is required for macroautophagy in mammalian cells. J Cell Biol. 2006;175:595–605. doi: 10.1083/jcb.200601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–32. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]