Summary
Broca’s area, a cerebral cortical area located in the inferior frontal gyrus (IFG) of the human brain, has been identified as one of several critical regions associated with the motor planning and execution of language. Anatomically, Broca’s area is most often larger in the left hemisphere, and functional imaging studies in humans indicate significant left-lateralized patterns of activation during language-related tasks [1–3]. If, and to what extent, nonhuman primates, particularly chimpanzees, possess a homologous region that is involved in the production of their own communicative signals remains unknown. Here, we show that portions of the IFG as well as other cortical and subcortical regions in chimpanzees are active during the production of communicative signals. These findings are the first to provide direct evidence of the neuroanatomical structures associated with the production of communicative behaviors in chimpanzees. Significant activation in the left IFG in conjunction with other cortical and subcortical brain areas during the production of communicative signals in chimpanzees suggests that the neurological substrates underlying language production in the human brain may have been present in the common ancestor of humans and chimpanzees.
Results and Discussion
Two cerebral cortical areas in the left hemisphere are most commonly associated with language functions. The first, commonly referred to as Wernicke’s area, is a receptive region for the processing and integration of auditory sensory information [4], and the second, known as Broca’s area, is a productive region concerned with the encoding of vocal signals into meaningful words and sentences [5]. In other words, Broca’s area functions primarily in the planning and execution of speech, whereas Wernicke’s area functions to make sense of the speech that a listener perceives. However, this classic modular view of linguistic processing is now considered somewhat dated [6], and it is evident that both cortical as well as subcortical structures and circuits play essential roles in speech production and perception [7, 8]. Notwithstanding, recent data confirm that left lateralization for linguistic processing is functionally significant [9], modality independent [10–12], and is associated not merely with the perception or production of utterances but with their meaning [13]. It has been suggested that this hemispheric specialization for language evolved from a lateralized manual communication system that arose in a common human and chimpanzee ancestor [14]. Consistent with this theory are data that indicate that chimpanzees intentionally and referentially communicate via manual gestures [15, 16] and, like humans, preferentially use their right hand for communicative gestures [17]. However, more recent data indicate that chimpanzees also use novel sounds to capture the attention of a human and alter the production of their vocal signals as a function of the communicative demands of a situation [18, 19]. These results suggest that chimpanzees intentionally produce manual gestures as well as vocal signals to communicate with humans, and although these signals are manifest in different modalities, their communicative function is the same.
Human-like left hemisphere neuroanatomical asymmetries also have been identified in both the IFG and the posterior temporal lobe of the chimpanzee brain [20, 21], regions considered homologous to Broca’s and Wernicke’s areas, respectively. For the IFG, leftward asymmetry is more pronounced in individuals that preferentially produce manual gestures with their right hand compared to those apes that do not show consistent hand use [22]. Despite this association between hand use for gestures and morphological asymmetries in the chimpanzee IFG, the functional role, if any, of the IFG in chimpanzee communication remains unknown. Indeed, data on the neural systems involved in gestural or vocal production in great apes, most notably chimpanzees, are virtually absent in the scientific literature yet are critical for understanding the evolution of language.
Here, we used positron emission tomography (PET) to examine the neural correlates of the production of communicative gestures and vocal signals in chimpanzees. We chose to focus on communicative signals directed toward humans given that previous research has demonstrated that chimpanzees and other great apes reliably produce manual communicative gestures but only when a human is present and visually oriented toward the subjects [16, 23–28]. In addition, chimpanzees (and other great apes) consistently produce vocalizations and other nonvocal acoustic signals, such as hand clapping, as a means of capturing the attention of an otherwise inattentive social agent [16, 24, 27]. In other words, the production of these communicative signals is initiated by the apes, self-paced, and not bound to a particular context or emotional state.
For this study, three subjects participated in two 40 min behavioral tasks (see Experimental Procedures). Each task began with the subject consuming a radioactive ligand, 18F-fluoro-deoxyglucose (18F-FDG), that had been diluted in a small amount of a sugar-free flavored drink mixture. Subsequently, for the experimental condition (GV), a cache of food was placed outside the subject’s home enclosure with the intermittent presence of a human in order to elicit the production of communicative gestures and vocal signals during the 18F-FDG uptake period. In order to remove the potential influence of general motor actions on PET uptake, the subjects also participated in a second baseline reach and grasp task (BL) in which both the human experimenter and the food were present, but the task did not elicit any communicative behaviors in the apes. In order to isolate the neural correlates of the communicative behaviors, PET data from the BL task were subtracted from the GV task, resulting in a comparison activation (GV > BL). Significant areas of activation (GV > BL) were identified by using paired sample t tests (t ≥ 4.31, p < 0.025; one-tailed test).
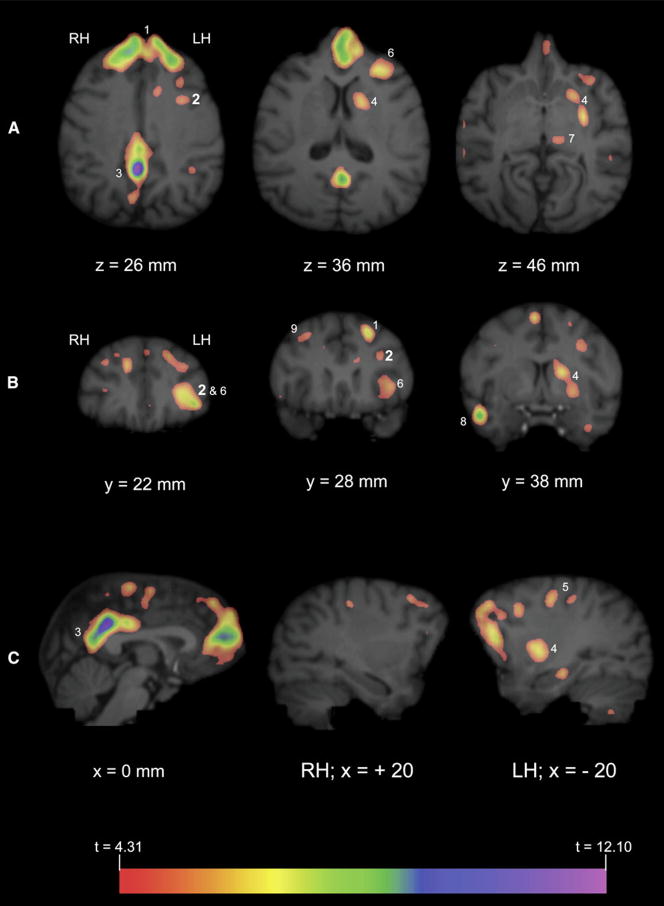
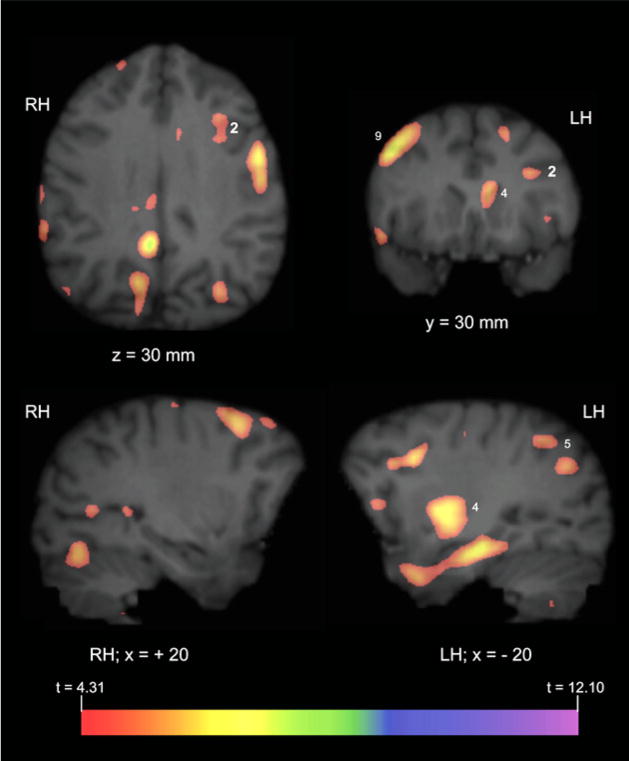
All three subjects produced both gestures and vocalizations in the GV task during the 40 min uptake period (see Table S1 available online), although the frequency of vocal production far exceeded that of gestures. Whole-brain analyses revealed significantly greater activation in the GV condition compared with the BL in a number of brain regions (see Table 1 and Figures 1 and 2), including the left IFG and caudate/putamen, bilateral posterior cingulate gyrus and prefrontal cortex, and right lateral cerebellum (not shown in Figure 2). To determine if the observed activation in the left IFG and other regions were significantly lateralized, the average comparison volume (GV > BL) for all three subjects was flipped on the left-right axis and subtracted from the correctly oriented volume. Significant lateralized areas of activation (t ≥ 4.31) are depicted in Figure 3 overlaid on the MR image of a representative chimpanzee brain. A comparison of these images with the activations depicted in Figure 2 indicate that the results of the lateralization analysis are largely consistent with the whole-brain data. Specifically, significant lateralized activity was observed in the left IFG, left caudate/putamen, and the right middle frontal gyrus.
Table 1.
Significant Areas of Activation, t ≥ 4.31, for GV > BL
| Region | t Statistic | x (mm) | y (mm) | z (mm) |
|---|---|---|---|---|
| R lateral cerebellum | 5.00 | 28 | 76 | 68 |
| R inferior/middle temporal gyrus | 5.52 | 34 | 41 | 59 |
| R superior parietal gyrus | 5.81 | 5 | 75 | 22 |
| R superior frontal gyrus | 5.36 | 5 | 53 | 15 |
| R middle frontal gyrus | 5.19 | 20 | 33 | 18 |
| B superior frontal gyrus | 5.96 | −1 | 15 | 42 |
| 6.16 | −1 | 18 | 27 | |
| B posterior cingulate gyrus | 6.29 | 2 | 76 | 35 |
| L inferior temporal gyrus | 4.68 | −22 | 38 | 67 |
| 4.92 | −38 | 58 | 58 | |
| L parahippocampal gyrus | 5.20 | −18 | 62 | 59 |
| L lateral orbital gyrus | 5.47 | −20 | 27 | 43 |
| L caudate/putamen | 5.17 | −13 | 44 | 44 |
| L inferior frontal gyrus | 4.97 | −18 | 37 | 27 |
| L precentral gyrus | 5.37 | −14 | 56 | 16 |
| L postcentral gyrus | 4.69 | −19 | 66 | 21 |
Figure 1. Three-Dimensional Reconstructions of Magnetic Resonance Images of a Representative Chimpanzee Brain.
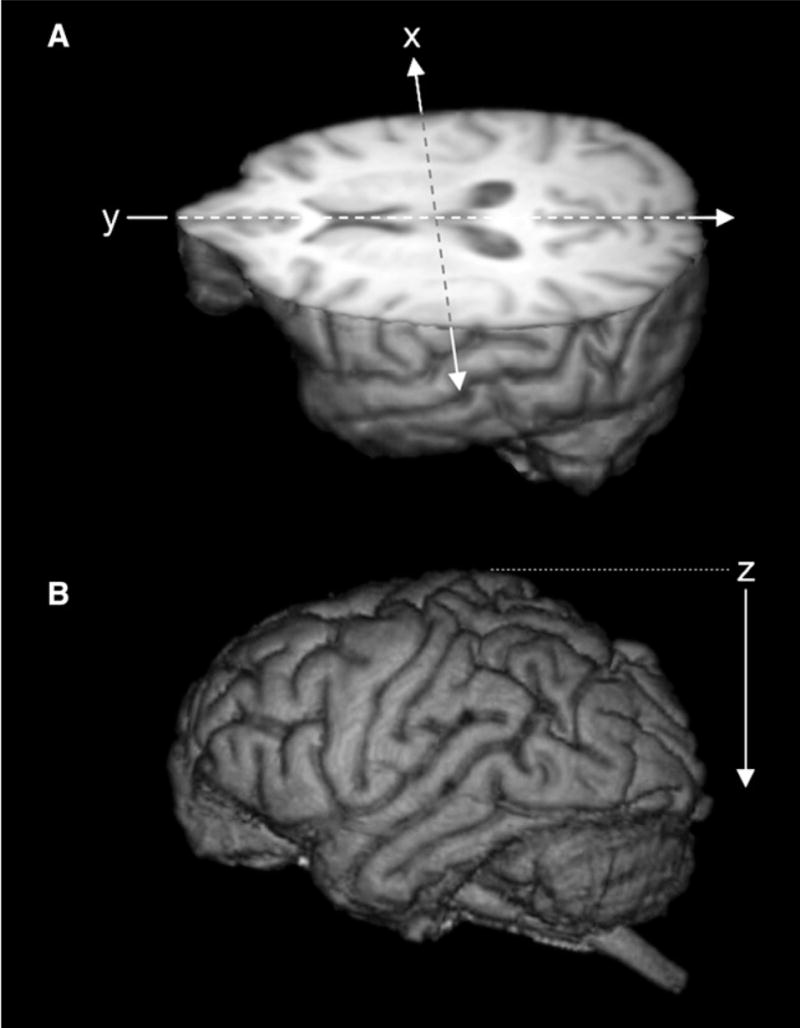
(A) Illustrated is a three-dimensional-rendered MR image of chimpanzee brain cut in to reveal the axial view. “x” and “y” indicate the orthogonal planes (sagittal and coronal, respectively) referred to in Figure 2. Arrow directions refer to ascending slices displayed in Figure 2.
(B) The z axis indicates the axial plane referred to in Figure 2.
Figure 2. Significant Areas of Activation for Communicative Production.
PET activation (GV > BL) was overlaid on MR images of representative chimpanzee brain. “x,” “y,” and “z” refer to the planes described in Figure 1. Measurements refer to the depth from the dorsal tip of the brain (z, dorsal to ventral), distance from frontal pole (y, anterior to posterior), or distance from midsagittal (x, ascending positive values correspond to the right hemisphere, medial to lateral; ascending negative values correspond to the left hemisphere, medial to lateral). Panels display axial (A), coronal (B), and sagittal (C) views of MR images with significant GV > BL activation. Numbers correspond to the following anatomical locations: 1, bilateral superior frontal gyrus; 2, left inferior frontal gyrus (depicted in large bold type); 3, bilateral posterior cingulate gyrus; 4, left caudate/putamen; 5, left medial pre- and postcentral gyrus; 6, left frontal orbital gyrus; 7, left thalamus; 8, right middle temporal gyrus; 9, right middle frontal gyrus. Note that not all areas of activation are labeled in all planes. See Table 1 for a complete list of regions with significant GV > BL activation.
Figure 3. Significant Lateralized Activation.
The average comparison volume (GV > BL) from all three subjects was flipped on the x axis (i.e., right-left) and then subtracted from the correctly oriented volume. A t map volume was then calculated from this subtracted volume (see the Experimental Procedures) and significant areas of activation identified, t ≥ 4.31. The figure depicts PET activation overlaid on MR images of representative chimpanzee brain. “x,” “y,” and “z” and corresponding values refer to the planes and the anatomical locations described in Figures 1 and 2. Values indicate significantly lateralized activity in that hemisphere (i.e., cluster in RH of brain indicates right hemisphere activation is greater than activation in corresponding area in the left hemisphere).
Given the procedural challenges involved with conducting PET with chimpanzees, three subjects were included in our study. This, to some extent, limits the statistical power that would be possible for an individual analysis. Therefore, whole-brain analyses were conducted on the three subjects collectively. The fact that significant areas of activation were identified suggests consistency in activation among the subjects. However, for illustrative purposes, standardized whole-brain PET activations from each individual subject (GV > BL) overlaid on an MR image of a representative chimpanzee brain are available online as supplemental movies.
These results suggest that subcortical and neocortical areas are active concurrently during the production of communicative manual gestures and vocal signals in chimpanzees—a finding that bears some similarities to the neural mechanisms involved in the production of language [7]. Recent studies in macaque monkeys have reported asymmetric activity of cortical regions after passive listening to conspecific vocalizations including the proposed Broca’s area homolog ([29, 30], but see [31] for a critique). However, these studies did not examine the production of communicative signals by the monkeys. Previous research that has focused on vocal production in monkeys found that cortical lesions to the neuroanatomical homolog of Broca’s area had no effect on vocal behavior [32, 33], although stimulation of this area does result in orofacial movements in macaque monkeys [34]. The fact that chimpanzee communicative signaling activates both subcortical and cortical structures, in conjunction with data that indicate these signals are referential and produced intentionally, suggests that the precursors to human language are present at both the behavioral and neuroanatomical levels.
It is important to note that during the uptake period, the chimpanzee subjects produced manual gestures as well as a variety of vocal signals. Therefore, the independent effects of these communicative signals on neural metabolic activity cannot be isolated. Pragmatically, this is challenging because the co-occurrence of gestures and vocalizations is quite common in our subjects [35], and we have not made attempts to specifically train the chimpanzees to produce only one of these behaviors within a given uptake period. Indeed it would be possible to train the chimpanzees to produce one, and only one (e.g., either a gesture or a vocalization, but not both), of these signals or even to produce only a single vocalization type. However, it is possible that this training might compromise the functional communicative relevance of these signals. Therefore, our aim was to capture the neurological correlates of these communicative behaviors in the chimpanzees and not necessarily the signal’s modality of production.
The total number of actions produced in the GV and BL tasks did differ among the subjects and between the tasks (see Table S1). As described above (and in the Experimental Procedures), the chimpanzees in the GV and BL tasks were, more or less, self-paced. Therefore, the number and type of signals produced as well as their modality in the GV and BL tasks and the number of grasping responses in the BL task were not directly under the control of the experimenter during the uptake period. Our rationale for this procedure was to ensure that the subject’s motivation and arousal state did not differ significantly between the GV and BL tasks, enabling us to isolate neuronal metabolic activity related to the production of communicative signals. The number of communicative signals produced by all of the subjects in the GV task far exceeded those produced in the BL task (see Table S1). Moreover, within the BL task, the number of reach and grasp responses produced by each subject far exceeded the number of communicative signals produced. In addition, the chimpanzee subjects did, in fact, produce various types of vocal signals in the GV condition, although the occurrence of attention-getting sounds in this condition far exceeded that of other vocalizations (Table S1). Therefore, although it is not possible to distinguish the relative influence of the communicative modality (vocal or manual gesture) or the call type produced (attention-getting or other types of vocalizations) on the observed neuronal metabolic activity, consideration of the GV > BL tasks succeeded in isolating the communicative behaviors of the subjects relative to their manual motor actions.
Although these data indicate that the left IFG is involved in the production of communicative signals in chimpanzees, cytoarchitectonically, it is not clear what cell types fully comprise this region [36]. Therefore, it is not possible to determine whether or not the neuronal metabolic activity reported in this study corresponds to an area within the chimpanzee IFG that contains Brodmann’s area 44/45 cells—those cells that comprise Broca’s area in humans. In fact, additional areas of significant activation are observed in the frontal orbital gyrus and the frontal pole (Figure 2). Additional work is needed to explore the significance of these areas of activation. Notwithstanding, these data clearly implicate the left IFG and surrounding tissue within the prefrontal cortex and represent the first findings of the neural correlates associated with the production of communicative signals in chimpanzees.
Recently, it has been reported that the left IFG in humans is involved in both speech and American Sign Language production, suggesting that the functional role of this region is modality independent [12]. The fact that during the production of their communicative signals, chimpanzees show significant activation in the left IFG in conjunction with other subcortical regions known to have strong connections to the prefrontal cortex [7, 37, 38] suggests that the neurological substrates underlying language production in the human brain may have been present in the common ancestor of humans and chimpanzees.
Experimental Procedures
All aspects of this study were conducted in accordance with ethical guidelines associated with the care and use of nonhuman primates and with the approval of the Emory University Institutional Animal Care and Use Committee.
Subjects
Subjects were three captive-born chimpanzees including one male and two females between the ages of 14 and 18 years.
Behavioral Tasks
For the communication production task (GV), each subject was separated from his or her social group but remained in his or her home enclosure and consumed the 18F-FDG. A human experimenter would then approach the subject and place a cache of food (1 quart plastic container containing 20–30 small frozen cubes of approximately 2 fl. oz. of sugar-free flavored drink mixture) just outside the subject’s home enclosure at a distance of less than 1 m but beyond the subject’s reach. Previous research in our laboratory has demonstrated that the chimpanzees are likely to produce both manual gestures and vocalizations in these contexts [18, 24, 27]. The human experimenter would remain seated in front of the subject’s enclosure for 2 min. The experimenter would verbally acknowledge the subject’s communicative signals but would not give any of the frozen drink cubes to the subject. At the end of the 2 min block, the experimenter would respond to the subject’s next communicative signal by offering a small frozen-drink cube to the subject. The human experimenter would then leave the area, taking the container of frozen-drink cubes. After a 2 min interval, the experimenter would return with the container of frozen drink cubes, once again placing them in front of the subject’s enclosure. This procedure was repeated for the duration of the uptake period (40 min).
For the unilateral grasping task (BL), subjects were required to unilaterally grasp small stones placed in their cage. As in the GV task, each subject was separated from his or her social group but remained in his or her home enclosure and consumed the ligand. The human experimenter then approached the subject’s enclosure, sat down in front of the subject, and placed 20 small stones inside the enclosure. The subjects had been trained previously to grasp each stone, one by one, and hand them back to the experimenter. After all 20 stones had been returned to the experimenter, the experimenter verbally praised the subject and offered him or her a small frozen-drink cube. A 1 min interval of inactivity was then observed during which the subjects remained seated quietly in their home cage. After the 1 min interval, the human again placed 20 stones in the subject’s enclosure and the procedure was repeated. The total number of grasping responses varied across subjects, but all subjects performed a significant number of grasps during the uptake period (Table S1).
For both the GV and BL conditions, subjects were housed in their home enclosures for the duration of the uptake period. Although physically separated from their social group, the subjects were able to hear conspecifics, and as mentioned above, the human experimenter did provide a limited amount of verbal praise to the subjects during the uptake period. This was done to minimize any stress that would have been associated with placing the chimpanzees in an unfamiliar environment while simultaneously attempting to preserve the authenticity of the communicative interaction. With the exception of the limited speech produced by the experimenter, background noises (e.g., building mechanical equipment), and the rare occurrence of a conspecific vocalization, subjects in both conditions had very limited auditory input. Moreover, the limited sounds that were heard by the subjects did not differ systematically between conditions. Prior to scanning, chimpanzee subjects had been trained with positive-reinforcement techniques to present for an injection. After the behavioral tasks, subjects voluntarily presented for an intramuscular injection of an anesthetic agent and were transported to the PET imaging facility.
PET Procedures
Subjects were administered 18F-fluorodeoxyglucose (18F-FDG) at a dose of 20 mCi. FDG was selected as the ligand because of its relatively long uptake period (~80 min) and long half-life (approximately 110 min). Thus, just as other investigators have done previously, we capitalized on these features of 18F-FDG because they allowed for prolonged behavioral testing during the uptake period and a relatively long time frame to capture neural activity trapped in the cells between the termination of uptake and the interval of time needed to transport and scan the chimpanzees. Previous studies have used nearly identical procedures to scan other nonhuman primate species and have revealed significant and consistent patterns of PET activation [39–41].
Chimpanzees consumed 0.24 ml of 18F-FDG that was diluted in approximately 100 ml of a sugar-free flavored drink mixture. The subjects then participated in the behavioral task for 40 min. After the 40 min uptake period, chimpanzees were asked to voluntarily present for an intramuscular injection of Telazol (4 mg/kg). Once anesthetized, chimpanzees were transported to the PET imaging facility. For the duration of the PET scan, chimpanzees remained anesthetized with Propofol administered intravenously and diluted in lactated ringers at a dose of ~10 mg/kg/hr. After completing PET procedures, the subjects were returned to the Yerkes National Primate Research Center (YNPRC) and temporarily housed in a single cage for approximately 18 hr to allow the effects of the anesthesia to wear off and radioactivity to decay. Subjects were then returned to their home cages with their social group.
The PET images were acquired on a High Resolution Research Tomograph (CPS HRRT; CTI/Siemens, Inc.) approximately 1 hr and 35 min after ingestion of the 18F-FDG. Recall that 40 min of this time period constituted the uptake period; thus, the remaining 55 min constituted the time between the injection of anesthesia, transport to and from the PET imaging, and the PET scan duration (approximately 30 min). Scan procedures were identical for all subjects. Chimpanzees fasted for approximately 5 hr prior to 18F-FDG administration and were rewarded with only minimal amounts of frozen sugar-free flavored-drink cubes during the uptake period. Subjects were placed in the supine position inside the scanner. Six minute transmission scans were followed by 20 min emission scans. Scan parameters were identical for all subjects: axial FOV = 24 cm, transverse FOV = 31.2 cm, and slice thickness = 1.21875 mm. Transaxial spatial resolution FWHM is 2.4 mm at the center and 2.8 mm 10 cm from the center. After scanning, a post reconstruction 2 mm smooth was applied to the images.
MRI Procedures
Magnetic resonance images (MRI) were collected from each subject with a 3.0 Tesla scanner (Siemens Trio) at the YNPRC. T1-weighted images were collected with a 3D gradient echo sequence (pulse repetition = 2300 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320). The archived MRI data were transferred to a PC running Analyze 7.0 (Mayo Clinic) software for postimage processing. MRI scans were then aligned in the axial plane and cut into 1 mm slices with Analyze 7.0.
Image Processing
The individual PET images were spatially aligned to their respective MR images by using 3D voxel registration with a linear transformation (Analyze 7.0, Mayo Clinic). Once aligned, each subject’s MRI was used to outline the brain on the PET image in each and every slice in the axial plane. An average PET activation was then calculated based on the registered activity within these slices. Once the mean activation for the whole brain had been computed, each voxel within that entire volume was divided by the mean activation in order to obtain a normalized PET image. Next, images were smoothed with a low-pass filter with an isotropic 6 mm kernal. Difference volumes were then calculated by subtracting each subject’s normalized BL task from their normalized GV task. The three difference volumes were then spatially registered to one another and a single average PET volume calculated. Whole-brain analysis was conducted for the three subjects collectively by using Analyze 7.0 (Mayo Clinic). Significant areas of activation were identified by calculating a single t-map volume and using a threshold value of t = 4.31. Significant clusters were identified as three or more contiguous voxels on three or more consecutive 2 mm slices in the axial plane with intensity values ≥ 4.31 (i.e., 9 voxels total; 72 mm3).
Supplementary Material
Acknowledgments
The authors would like to thank E. Strobert, J. Orkin, F. Stroud, K. Paul, D. Bonenberger, S. Weissman, and the veterinary and animal care staff of the YNPRC for their support; and M. Jones, D. Votaw, and J. Votaw for their technical assistance with PET imaging. This work was supported in part by National Institutes of Health grants F32DC007823 to J.P.T., NS-36605 and NS-42867 to W.D.H., and RR-00165 to the YNPRC.
Footnotes
Supplemental Data One table and nine movies are available at http://www.current-biology.com/cgi/content/full/18/5/343/DC1/.
References
- 1.Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchanan TW, Lutz K, Mirzazade S, Specht K, Shah NJ, Zilles K, Jancke L. Recognition of emotional prosody and verbal components of spoken language: An fMRI study. Brain Res Cogn Brain Res. 2000;9:227–238. doi: 10.1016/s0926-6410(99)00060-9. [DOI] [PubMed] [Google Scholar]
- 3.Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI Brain. 1999;122:199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- 4.Wernicke C. Der aphasische Symptomenkomplex. Breslau, Germany: Cohn and Weigert; 1874. [Google Scholar]
- 5.Broca P. Du siege de la faculte du langage articule. Bulletin de la Societe d′anthropologie. 1865;6:337–393. [Google Scholar]
- 6.Poeppel D, Hickock G. Towards a new functional anatomy of language. Cognition. 2004;92:1–12. doi: 10.1016/j.cognition.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman P. The evolution of human speech: Its anatomical and neural bases. Curr Anthropol. 2007;48:39–66. [Google Scholar]
- 8.Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR. Functional neuroanatomy of human vocalization: An H215O PET study. Cereb Cortex. 2005;15:1835–1847. doi: 10.1093/cercor/bhi061. [DOI] [PubMed] [Google Scholar]
- 9.Knecht S, Floel A, Drager B, Breitenstein C, Sommer J, Henningsen H, Ringelstein EB, Pascual-Leone A. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat Neurosci. 2002;5:695–699. doi: 10.1038/nn868. [DOI] [PubMed] [Google Scholar]
- 10.Hickok G, Bellugi U, Klima ES. The neural organization of language: Evidence from sign language aphasia. Trends Cogn Sci. 1998;2:129–136. doi: 10.1016/s1364-6613(98)01154-1. [DOI] [PubMed] [Google Scholar]
- 11.Grossi G, Semenza C, Corazza S, Volterra V. Hemispheric specialization for sign language. Neuropsychologia. 1996;34:737–740. doi: 10.1016/0028-3932(96)00008-5. [DOI] [PubMed] [Google Scholar]
- 12.Emmorey K, Mehta S, Grabowski TJ. The neural correlates of sign versus word produciton. Neuroimage. 2007;36:202–208. doi: 10.1016/j.neuroimage.2007.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahn R, Huber W, Drews E, Erberich S, Krings T, Willmes K, Schwarz M. Hemispheric lateralization at different levels of human auditory word processing: A functional magnetic resonance imaging study. Neurosci Lett. 2000;287:195–198. doi: 10.1016/s0304-3940(00)01160-5. [DOI] [PubMed] [Google Scholar]
- 14.Corballis MC. From Hand to Mouth: The Origins of Language. Princeton, NJ: Princeton University Press; 2002. [Google Scholar]
- 15.Leavens DA, Hopkins WD. Intentional communication by chimpanzee (Pan troglodytes): A cross-sectional study of the use of referential gestures. Dev Psychol. 1998;34:813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomasello M, Call J, Nagell K, Olguin R, Carpenter M. The learning and use of gestural signals by young chimpanzees: A trans-generational study. Primates. 1994;35:137–154. [Google Scholar]
- 17.Hopkins WD, Russell J, Freeman H, Buehler N, Reynolds E, Schapiro S. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes) Psychol Sci. 2005;16:487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins WD, Taglialatela JP, Leavens DA. Chimpanzees differentially produce novel vocalizations to capture the attention of a human. Anim Behav. 2007;73:281–286. doi: 10.1016/j.anbehav.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taglialatela JP, Savage-Rumbaugh ES, Baker LA. Vocal production by a language-competent Pan paniscus. Int J Primatol. 2003;24:1–17. [Google Scholar]
- 20.Cantalupo C, Hopkins WD. Asymmetric Broca’s area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee Planum Temporale: Humanlike pattern of Wernicke’s language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- 22.Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. Neuroreport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Call J, Tomasello M. Production and comprehension of referential pointing by orangutans (Pongo pygmaeus) J Comp Psychol. 1994;108:307–317. doi: 10.1037/0735-7036.108.4.307. [DOI] [PubMed] [Google Scholar]
- 24.Hostetter AB, Cantero M, Hopkins WD. Differential use of vocal and gestural communication by chimpanzees (Pan troglodytes) in response to the attentional status of a human (Homo sapiens) J Comp Psychol. 2001;115:337–343. doi: 10.1037//0735-7036.115.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause MA, Fouts RS. Chimpanzee (Pan troglodytes) pointing: Hand shapes, accuracy, and the role of eye gaze. J Comp Psychol. 1997;111:330–336. doi: 10.1037/0735-7036.111.4.330. [DOI] [PubMed] [Google Scholar]
- 26.Leavens DA, Hopkins WD, Bard KA. Indexical and referential pointing in chimpanzees (Pan troglodytes) J Comp Psychol. 1996;110:346–353. doi: 10.1037/0735-7036.110.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leavens DA, Hostetter AB, Wesley MJ, Hopkins WD. Tactical use of unimodal and bimodal communication by chimpanzees, Pan troglodytes. Anim Behav. 2004;67:467–476. [Google Scholar]
- 28.Poss SR, Kuhar C, Stoinski TS, Hopkins WD. Differential use of attentional and visual communicative signaling by orangutans (Pongo pygmaeus) and gorillas (Gorilla gorilla) in response to the attentional status of a human. Am J Primatol. 2006;68:978–992. doi: 10.1002/ajp.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poremba A, Malloy M, Saunders RC, Carson RE, Herscovitch P, Mishkin M. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Nature. 2004;427:448–451. doi: 10.1038/nature02268. [DOI] [PubMed] [Google Scholar]
- 30.Gil-da-Costa R, Martin A, Lopes MA, Munoz M, Fritz J, Braun AR. Species-specific calls activate homologs of Broca’s and Wernicke’s areas in the macaque. Nat Neurosci. 2006;9:1064–1070. doi: 10.1038/nn1741. [DOI] [PubMed] [Google Scholar]
- 31.Ghazanfar AA, Miller CT. Language evolution: Loquacious monkey brains? Curr Biol. 2006;16:879–881. doi: 10.1016/j.cub.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Aitken PG. Cortical control of conditioned and spontaneous vocal behaviour in Rhesus monkeys. Brain Lang. 1981;13:171–184. doi: 10.1016/0093-934x(81)90137-1. [DOI] [PubMed] [Google Scholar]
- 33.Kirzinger A, Jurgens U. Cortical lesion effects and vocalization in the squirrel monkey. Brain Res. 1982;233:299–315. doi: 10.1016/0006-8993(82)91204-5. [DOI] [PubMed] [Google Scholar]
- 34.Petrides M, Cadoret G, Mackey S. Orofacial somatomotor responses in the macaque monkey homologue of Broca’s area. Nature. 2005;435:1235–1238. doi: 10.1038/nature03628. [DOI] [PubMed] [Google Scholar]
- 35.Hopkins WD, Cantero M. From hand to mouth in the evolution of language: The influence of vocal behavior on lateralized hand use in manual gestures by chimpanzees (Pan troglodytes) Dev Sci. 2003;6:55–61. [Google Scholar]
- 36.Sherwood CS, Broadfield DC, Holloway RL, Gannon PJ, Hof PR. Variability of Broca’s area homologue in African great apes: Implication for language evolution. Anat Rec. 2003;217A:276–285. doi: 10.1002/ar.a.10046. [DOI] [PubMed] [Google Scholar]
- 37.Ramnani N. The primate cortico-cerebellar system: structure and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 38.Ramnani N, Behrens TEJ, Johansen-Berg H, Richter MC, Pinsk MA, Andersson JLR, Rudebeck P, Ciccarelli O, Richter W, Thompson AJ, et al. The evolution of prefrontal inputs to the cortico-pontine system: Diffusion imaging evidence from macaque monkeys and humans. Cereb Cortex. 2006;16:811–818. doi: 10.1093/cercor/bhj024. [DOI] [PubMed] [Google Scholar]
- 39.Rilling JK, Barks SK, Parr LA, Preuss TM, Faber TL, Pagnoni G, Bremmer JD, Votaw JR. A comparison of resting-state brain activity in humans and chimpanzees. Proc Natl Acad Sci USA. 2007;104:17146–17151. doi: 10.1073/pnas.0705132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez ZA, Colgan M, Baxter LR, Quintana J, Siegal S, Chatziioannou A, Cherry SR, Mazziotta JC, Phelps ME. Oral 18F-Fluoro-2-Deoxyglucose for primate PET studies without behavioural restraint: Demonstration on principle. Am J Primatol. 1997;42:215–224. doi: 10.1002/(SICI)1098-2345(1997)42:3<215::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman JA, Phillips-Conroy JE, Black KJ, Perlmutter JS. Asymmetric regional cerebral blood flow in sedated baboons measured by positron emission tomography (PET) Am J Phys Anthropol. 2003;121:369–377. doi: 10.1002/ajpa.10181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.