Abstract
A randomized cross-over trial of HSV-2 suppressive therapy was conducted among 20 HSV-2/HIV-1 co-infected Peruvian women not on antiretroviral therapy. Participants were assigned valacyclovir 500 mg twice daily or placebo for 8 weeks, then after a 2 week washout, the alternative for 8 weeks. Plasma (weekly) and endocervical swabs (thrice weekly) were collected for HIV-1 RNA PCR. HIV-1 plasma levels were significantly lower during the valacyclovir arm compared with the placebo arm (−0.27 log10 copies/ml, p<0.001, a 46% decrease), as were cervical HIV-1 levels (−0.35 log10 copies/swab, p<0.001, a 55% decrease). Suppressive HSV-2 therapy could reduce HIV-1 infectiousness and slow HIV-1 disease progression. (ClinicalTrials.gov number NCT00465205).
Keywords: herpes simplex virus, HIV transmission, women
Introduction
Herpes simplex virus type 2 (HSV-2) is common among persons with HIV-1 (prevalence 50–90%) [1]. HSV-2 reactivation, including asymptomatic shedding without clinically apparent lesions, has been associated with increased concentrations of HIV-1 in plasma and genital secretions [2], suggesting HSV-2 reactivation could heighten HIV-1 infectiousness and accelerate HIV-1 disease progression.
Acyclovir and the related compounds valacyclovir and famciclovir are routinely used as episodic treatment for symptomatic genital ulcer disease due to HSV-2 and as daily suppressive therapy to decrease the frequency of symptomatic HSV-2 reactivation. Among HSV-2/HIV-1 co-infected individuals, HSV suppressive therapy decreases symptomatic HSV-2 reactivation and asymptomatic genital HSV shedding [3, 4]. Moreover, one open-label study found that daily acyclovir was associated with a reduction in plasma HIV-1 levels by ~1/3 log10 [5], and a pooled analysis of 8 studies from the 1990s found that daily high-dose acyclovir, used at that time in combination with mono or dual nucleoside antiretroviral therapy, was associated with a statistically significant survival benefit [6].
Thus, limited data suggest suppressive treatment of HSV-2 could influence HIV-1 replication. We conducted a clinical trial of daily valacyclovir among HSV-2/HIV-1 co-infected Peruvian women not on antiretroviral therapy. The goal of this study was to assess the effect of suppressive therapy for HSV-2 on plasma and genital HIV-1 levels.
Methods
Between March and December 2005, we conducted a randomized, double-blind, placebo-controlled, cross-over trial of HSV-2 suppressive therapy among HSV-2/HIV-1 co-infected women in Lima, Peru. Eligible women were >18 years of age, HIV-1 and HSV-2 seropositive, and had CD4 counts >200 cells/μL (the Peruvian cut-off for antiretroviral therapy at the time of the study). Exclusion criteria included pregnancy, antiretroviral therapy use, anti-HSV medication use, history of seizure or adverse reaction to any anti-HSV medication, creatinine >2.0 mg/dl, or hematocrit <30%.
The institutional review boards of the University of Washington and the Asociación Civil Impacta Salud y Educación approved the protocol. Participants provided written informed consent.
Study drug and randomization
Women were randomly assigned to either valacyclovir 500 mg orally twice daily or matching placebo. Randomization was 1:1, in blocks of 10. After 8 weeks (Weeks 1–8), participants crossed over to the alternative treatment for an additional 8 weeks (Weeks 11–18). Treatment periods were separated by a 2-week washout (Weeks 9–10). Medication was dispensed every two weeks, and adherence was assessed by pill counts. Study drug was supplied by GlaxoSmithKline. Open label valacyclovir 1 g twice daily for three days was dispensed, in addition to study drug, for symptomatic herpes recurrences. Investigators and laboratory technologists were blinded to treatment assignments while data collection and specimen processing were ongoing.
Specimen collection
Each day at home, participants self-collected swabs of the genital and perianal skin into a single cryovial of transport medium, for HSV detection. Participants kept a daily diary of genital symptoms. Three times weekly, women returned to the clinic for speculum pelvic examination collection of a cervical swab specimen for HIV-1; swabs were placed into 500 μL of guanidinium solution (4M guanidinium thiocyanate, 25 mM sodium citrate, 0.5% N-lauroylsarcosine, 0.1 M 2-mercaptoethanol). Once weekly, blood was collected for HIV-1 plasma viral load determination. Samples were frozen at −70°C within 8 hours of collection, with the exception of home-collected swabs for HSV, which were delivered by participants every 2–3 days and then frozen at −70°C.
Laboratory procedures
All study participants were known to be HIV-1 seropositive; HIV-1 serostatus was confirmed by ELISA and Western blot. HSV-2 serostatus was determined by ELISA (HerpeSelect-2, Focus Techologies), later confirmed by Western blot performed at the University of Washington. CD4 counts were determined by standard flow cytometric methods.
Screening for sexually transmitted infections included cervical culture for Neisseria gonorrhoeae on modified Thayer-Martin media. An additional specimen was batch-tested for Neisseria gonorrhoeae and Chlamydia trachomatis (Aptima, Gen-Probe) at the University of Washington at the completion of the study. Light microscopy of a vaginal wet preparation was used to diagnose bacterial vaginosis, Trichomonas vaginalis, and candidiasis. Syphilis was diagnosed by rapid plasma reagin, confirmed by a treponema-specific test (MHA-TP).
Anogenital HSV DNA shedding was detected using previously-described PCR methods [7]. The lower limit of HSV detection was 500 (2.70 log10) copies/swab.
HIV-1 RNA was quantified in plasma and cervical specimens using a TaqMan real-time PCR assay, as previously described [8]. The lower limit of HIV-1 quantification was 120 (2.08 log10) HIV-1 RNA copies/mL for plasma and 188 (2.27 log10) HIV-1 RNA copies/swab for cervical swabs.
Sample size
A sample size of 20 was estimated to provide >80% power to detect a 0.3 log10 copies/swab change in cervical HIV-1, the primary study endpoint, based on variance measures seen in longitudinal studies [9] and allowing for 10% drop-out. The cross-over design was chosen for its efficiency in sample size, as within-person HIV-1 RNA variability is generally less than between-person variability.
Data analysis
Analyses were intent-to-treat and were performed using SPSS 15.0 and S-Plus 7.0. HSV and HIV-1 concentrations were log10 transformed; the midpoint between zero and the lower limit of detection was used for undetectable HIV-1 samples.
Generalized estimating equations with a binomial link and an independent core structure were used to compare the frequency of HSV and HIV-1 detection while on valacyclovir versus placebo. Linear mixed-effects models were used to assess the effect of valacyclovir versus placebo on genital HSV and plasma and genital HIV-1 quantity. To compute the percent change in HIV-1 quantity, linear mixed-effects results were exponentiated and compared to 1.
Results
Twenty HSV-2/HIV-1 co-infected women were enrolled. An additional 19 were screened: ten had CD4 counts <200 cells/μL and nine were HSV-2 seronegative. Median age was 28 years (range 21 to 47) and median CD4 count was 372 cells/μL (range 229 to 850). Nine had previously taken zidovudine monotherapy for prophylaxis against mother-to-child HIV-1 transmission; the most recent use was 84 days before enrollment. One woman had serologic evidence of syphilis; no other genital tract infections were detected. Four used hormonal contraception and one was post-menopausal. Four reported a history of symptomatic herpes, of whom three had used acyclovir for treatment of primary genital herpes. No participant had used acyclovir for treatment or suppression of recurrent genital herpes.
Follow-up
All 20 participants finished the study. Women took a median of 100% (range 99–100%) of dispensed study tablets; this did not differ by arm (p=0.5, McNemar’s test). No serious adverse events were reported. Six women were treated with open-label valacyclovir for herpes recurrences, on a total of 43 study days, including 31 (2.8%) placebo days.
Anogenital HSV shedding
All participants returned 100% of 112 daily self-collected swabs for detection of genital HSV reactivation (2240 swabs total). HSV was detected at least once from all participants; 54 swabs (2.4%) could not be analyzed because of PCR inhibition. HSV was detected significantly less during valacyclovir versus placebo administration (3.7% versus 22.1%, Table 1). By participant, HSV was detected in 0–19.2% and 1.8–69.6% of swabs during valacyclovir and placebo administration, respectively. Among those swabs with detectable HSV, HSV quantity was significantly lower when on valacyclovir compared with placebo (by 0.67 log10 copies/swab). Most HSV shedding was asymptomatic: women reported genital ulcers on 23 (2.1%) valacyclovir days and 49 (4.6%) placebo days.
Table 1.
Rates of detection and quantities of genital HSV and plasma and cervical HIV-1, by study arm.
| n/total (%) or mean ± standard deviation |
||||
|---|---|---|---|---|
| Placebo |
Valacyclovir |
Difference in quantitya (95% confidence interval) |
p-valueb |
|
| Genital HSV detection | 242 / 1094 (22.1%) |
40 / 1092 (3.7%) |
<0.001 | |
| Genital HSV quantity, log10 copies/swabc | 4.80 ± 1.28 | 3.94 ± 1.00 | −0.67 (−1.08, −0.26) |
0.002 |
| Plasma HIV-1 viral load, log10 copies/mL | 4.60 ± 0.76 | 4.34 ± 1.01 | −0.27 (−0.34, −0.20) |
<0.001 |
| Cervical HIV-1 detection | 266 /374 (71.1%) |
182 / 335 (54.3%) |
<0.001 | |
| Cervical HIV-1 viral load, log10 copies/swab | 3.31 ± 1.11 | 2.93 ± 1.06 | −0.35 (−0.46, −0.25) |
<0.001 |
For continuous measures, average differences in quantity between the study arms from linear mixed-effects models.
P-values are from generalized estimating equations models for dichotomous measures and from linear mixed-effects models for continuous measures.
Among those with detectable genital HSV.
Plasma HIV-1 viral load
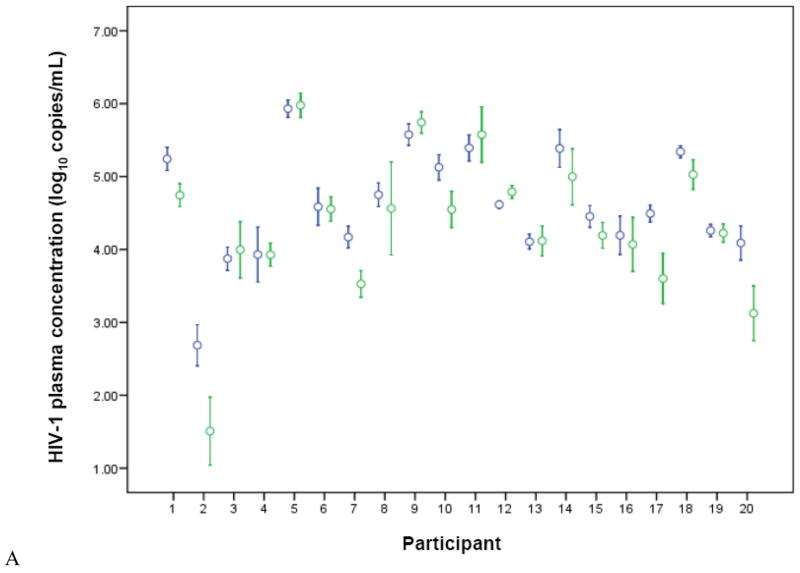
All participants provided once-weekly plasma samples for the entire follow-up period (320 samples total, 2 could not be analyzed). Plasma HIV-1 levels were greater than the limit of detection (60 copies/ml) for 313 of 318 samples (98.4%). Participant-specific plasma HIV-1 levels are shown in Figure 1A. Average HIV-1 plasma viral load was significantly lower, by 0.27 log10 copies/ml, during the valacyclovir arm compared with the placebo arm, a 46% decrease.
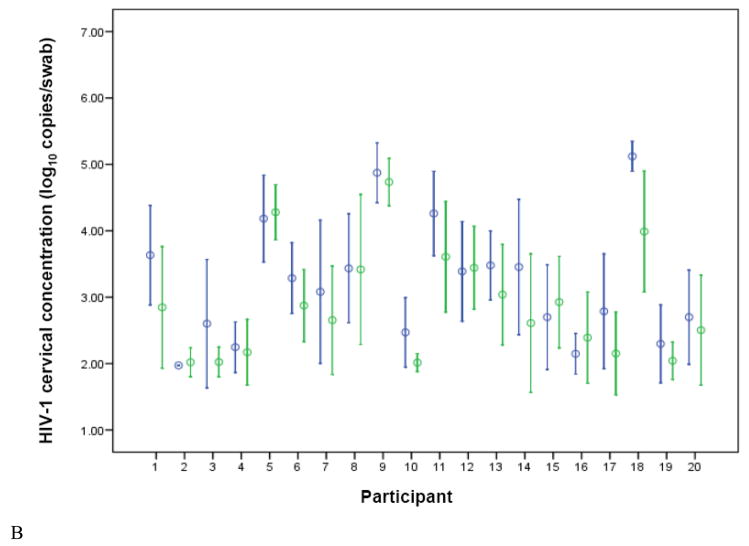
Figure 1.
HIV-1 concentrations in plasma (A) and cervical (B) samples, for each study participant, stratified by treatment arm (blue = placebo, green = valacyclovir). Circles represent the mean and brackets indicate 1 SD. Undetectable levels were imputed at the midpoint between zero and the lower limit of quantification.
Cervical HIV-1 shedding
Participants provided a median of 46 (range 44–48) of 48 possible thrice-weekly endocervical swab samples for HIV-1 quantification. A total of 933 swabs were collected, of which 224 (24.0%) could not be analyzed because of PCR inhibition. Participant-specific cervical HIV-1 levels are shown in Figure 1B. HIV-1 was detected in 54.3% of analyzable swabs during valacyclovir administration versus 71.1% of swabs collected during placebo administration (p<0.001). Average HIV-1 cervical HIV-1 viral load was significantly lower, by 0.35 log10 copies/swab, during the valacyclovir arm compared with the placebo arm, a 55% decrease.
Discussion
In this randomized, cross-over trial, with multiple observations per participant, HSV suppressive therapy reduced plasma and genital HIV-1 levels by ~50% among HSV-2/HIV-1 co-infected women. These findings strongly support the hypothesis that HSV-2 reactivation increases plasma and genital HIV-1 concentrations.
Our results are consistent with those of a recent trial among women from Burkina Faso that found that 12 weeks of HSV suppressive therapy reduced plasma HIV-1 by 0.53 log10 copies/mL and cervicovaginal lavage HIV-1 by 0.29 log10 copies/mL [10]. Among Peruvian men in a cross-over study with an identical design to the present investigation, HSV suppressive therapy reduced plasma and rectal HIV-1 by 0.33 and 0.16 log10 copies/mL, respectively [8]. Together, these studies confirm that short-term HSV suppressive therapy reduces plasma HIV-1 levels by ~0.25–0.5 log10 copies/mL and anogenital HIV-1 by a slightly lesser amount.
The mechanism by which genital HSV reactivation increases systemic and genital HIV-1 levels is not well-understood. In a cross-sectional study among Kenyan women with HIV-1, HSV-2 infection was associated with higher chemokine levels, increased numbers of CCR5+ CD4 cells, and depletion of immature dendritic cells in the cervix, suggesting local immunologic alteration that may increase HIV-1 replication [11]. In vitro studies have found that HSV may directly increase HIV-1 transcription through HSV-encoded proteins that bind to the HIV-LTR [12].
Higher plasma HIV-1 viral load is a strong predictor of faster HIV-1 disease progression and greater infectiousness [13], and genital HIV-1 levels are also likely a marker of infectivity. HIV-1 suppression, through use of combination antiretroviral therapy, leads to higher CD4 counts, lower mortality, and decreased HIV-1 transmission risk [14]. Early studies found zidovudine monotherapy decreased plasma HIV-1 RNA by 0.25–0.5 log10 copies/mL (similar to the effect in the present study) [15], and this modest reduction in HIV-1 levels was accompanied by slower disease progression and decreased mortality [16]. Unlike zidovudine monotherapy, which loses clinical benefits in an individual as zidovudine-resistant HIV-1 variants develop, the benefits of anti-HSV medications on HIV-1 replication may not be attenuated with time, since the effect on HIV-1 is mediated through HSV suppression and acyclovir resistance develops infrequently, even in immunocompromised individuals treated for extended periods [1].
Long-term HSV suppressive therapy is safe among persons with HIV-1 [1]. Studies with comparable doses of acyclovir and valacyclovir (which is rapidly metabolized in vivo to acyclovir) have shown comparable effects on suppressing HSV reactivation [4], although valacyclovir achieves higher peak serum levels than acyclovir. In this study, HSV was detected on 4% of days during the valacyclovir arm, demonstrating that HSV suppression was not complete at a dose of 500 mg twice daily. Moreover, while HSV suppression reduced systemic and genital HIV-1 overall in this study, the magnitude varied across individual participants (Figure 1). Future studies will determine whether higher doses of HSV suppressive therapy might have greater effects on HIV-1 concentrations. Most HSV reactivation in this study was asymptomatic, emphasizing the need for suppressive HSV therapy, rather than episodic treatment in response to clinical HSV disease.
Adherence to the study medication and compliance with sample collection were very high in this study. Other studies have suggested that anti-HSV therapy may have greater effects on HIV-1 levels among those with higher CD4 counts or with longer time on suppressive therapy [10]; however, our small sample size did not permit adequate assessment of these factors. The rate of PCR inhibition in the cervical swab analysis was modest, although this did not prohibit detection of a significant effect of valacyclovir on endocervical HIV-1 levels.
In conclusion, this trial demonstrated that daily valacyclovir for HSV suppression significantly reduced plasma and genital HIV-1 concentrations in HSV-2/HIV-1 co-infected women who did not have severe immunosuppression and were not receiving antiretroviral therapy. Ongoing large clinical trials among HSV-2/HIV-1 co-infected persons will assess whether the short-term effects of HSV suppressive therapy seen in the present study translate into decreased HIV-1 transmission and slower HIV-1 disease progression.
Acknowledgments
We thank the clinical, laboratory, and administrative staff in Lima and Seattle for their valuable contributions to this work – in Lima, Shyla Sánchez for study coordination, Drs. XXX for performing the clinical procedures, and Dr. Esmellin Pérez for pharmacy support, and, in Seattle, Dr. Meredith Potochnic for pharmacy support, Joan Dragavon and the staff of the University of Washington Retrovirus Laboratory for performing HIV-1 PCR testing, Dr. Meei-Li Huang, Stacey Stelke, and the staff of the University of Washington Virology Laboratory for HSV PCR testing, and Dr. Rhoda Morrow and Anne Cent for HSV Western blot testing. We are grateful to the study participants for their time and dedication.
Sponsorship: This study was supported by a research grant from GlaxoSmithKline (research grant R 103) and by the National Institutes of Health (Centers for AIDS Research Clinical Research and Laboratory Core Grants AI-27757 and AI-38858, R37 AI-42528, and HSV Program Project Grant AI-30731).
Footnotes
Potential conflicts of interest: C.C. has received research grant support from the GlaxoSmithKline (GSK) and has served on an advisory board for GSK. J.S. has received grant support from GSK. A.W. has received grant support GSK, Antigenics, 3M, Roche, and Vical; she is a consultant for Novartis, PowderMed, and MediGene and is a speaker for Merck Vaccines. The University of Washington Virology Division Laboratories have received grant funding from GSK and Novartis to perform herpes simplex virus serologic assays and polymerase chain reaction assays for studies funded by these companies. L.C. directs these laboratories. He receives no salary support from these grants.
References
- 1.Strick LB, Wald A, Celum C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin Infect Dis. 2006;43:347–56. doi: 10.1086/505496. [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, McClelland RS, Corey L, et al. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: a randomized clinical trial. J Infect Dis. 2004;189:1466–71. doi: 10.1086/383049. [DOI] [PubMed] [Google Scholar]
- 3.Schacker T, Hu HL, Koelle DM, et al. Famciclovir for the suppression of symptomatic and asymptomatic herpes simplex virus reactivation in HIV-infected persons. A double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:21–8. doi: 10.7326/0003-4819-128-1-199801010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Conant MA, Schacker TW, Murphy RL, Gold J, Crutchfield LT, Crooks RJ. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. Int J STD AIDS. 2002;13:12–21. doi: 10.1258/0956462021924550. [DOI] [PubMed] [Google Scholar]
- 5.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–25. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 6.Ioannidis JP, Collier AC, Cooper DA, et al. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998;178:349–59. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- 7.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–11. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–8. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 9.Coombs RW, Wright DJ, Reichelderfer PS, et al. Variation of human immunodeficiency virus type 1 viral RNA levels in the female genital tract: implications for applying measurements to individual women. Women’s Health Study 001 Team. J Infect Dis. 2001;184:1187–91. doi: 10.1086/323660. [DOI] [PubMed] [Google Scholar]
- 10.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 11.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–98. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 12.Mosca JD, Bednarik DP, Raj NB, et al. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987;325:67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- 13.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 14.Bunnell R, Ekwaru JP, Solberg P, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20:85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- 15.Katzenstein DA, Hammer SM, Hughes MD, et al. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. AIDS Clinical Trials Group Study 175 Virology Study Team. N Engl J Med. 1996;335:1091–8. doi: 10.1056/NEJM199610103351502. [DOI] [PubMed] [Google Scholar]
- 16.Volberding PA, Lagakos SW, Grimes JM, et al. The duration of zidovudine benefit in persons with asymptomatic HIV infection. Prolonged evaluation of protocol 019 of the AIDS Clinical Trials Group. JAMA. 1994;272:437–42. [PubMed] [Google Scholar]