Abstract
Although recombinant adeno-associated virus (rAAV) has been widely used in lung gene therapy approaches, it remains unclear to what extent commonly used AAV serotypes transduce adult progenitors in the lung. In this study, we evaluated the life span and proliferative capacity of rAAV1-, 2-, and 5-transduced airway cells in mouse lung, using a LacZ-CRE reporter transgenic model and Cre-expressing rAAV. In this model, the expression of CRE recombinase led to permanent genetic marking of transduced cells and their descendants with LacZ. To investigate whether the rAAV-transduced cells included airway progenitors, we injured the airways of rAAV-infected mice with Naphthalene, while simultaneously labeling with 5-bromodeoxyuridine (BrdU) to identify slow-cycling progenitor/stem cells that entered the cell cycle and retained label. Both rAAV5 and rAAV1 vectors were capable of transducing a subset of long-lived Clara cells and alveolar type II (ATII) cells that retained nucleotide label and proliferated following lung injury. Importantly, rAAV1 and 5 appeared to preferentially transduce conducting airway epithelial progenitors that had the capacity to clonally expand, both in culture and in vivo following lung injury. These studies suggest that rAAV may be a useful vector for gene targeting of airway stem/progenitor cells.
Introduction
Recombinant adeno-associated virus (rAAV) is a nonpathogenic parvovirus that has shown great promise for efficient gene delivery to both dividing and nondividing cells of many tissues. With new rAAV serotypes continuing to be identified, the ability to target specific cell types within a given organ is also expanding. It is generally accepted that rAAV genomes integrate at a low frequency and that the majority of genomes remain episomal, as either linear or circular concatamers. Hence, gene therapies with this vector that rely on persistent expression will likely require repeat administration as episomal viral genomes are diluted following cell division. However, some studies have demonstrated that rAAV vectors are capable of persisting in hematopoietic stem/progenitor cells1,2,3 and germline stem cells,4 because of their integration into the genome. These findings suggest that integration may be higher in certain stem/progenitor cell types. Additionally, rAAV has been shown to transduce a number of stem/progenitor cell types from a broad range of organs and tissues, including several human and mouse cell types: ES cells,5 mesenchymal stem cells,6,7,8 cardiac stem cells,9 and liver progenitor cells.10 Hence, rAAV appears to be an effective vehicle for delivering genes to the stem/progenitor cells of multiple organs. In the current study, we sought to evaluate the ability of several rAAV genotypes to transduce stem/progenitor cells in the lungs of mice.
In addition to their utility for expressing gene products, rAAV vectors have been shown to facilitate gene repair/correction through homologous recombination.11 This feature of rAAV has the potential to facilitate long-term gene therapies by direct correction of genetic defects in stem/progenitor cells. Indeed, proof of principle for gene correction with rAAV has been demonstrated in vivo for mouse liver targeting a mutant reporter gene,12,13 and also ex vivo in for human mesenchymal stem cells targeting mutant COL1A2 alleles from individuals with osteogenesis imperfecta.14 These capabilities of rAAV suggest that this vector may be well suited for in vivo gene repair/correction of stem/progenitor cells in the treatment of inherited lung diseases such as cystic fibrosis.
Resident stem/progenitor cells in the adult mouse lung play crucial roles in maintaining an epithelial barrier in the lung following injury and normal cellular turnover.15 Although the efficiency of rAAV-mediated gene transfer to the mouse lung has been extensively evaluated, very little is known about whether rAAV is capable of transducing resident lung stem/progenitor cells. Furthermore, it is unclear whether rAAV-mediated transduction of airway stem/progenitors alters their potential to differentiate into various cell lineages. In this study, we used a Cre/LoxP transgenic reporter mouse model to evaluate the abilities of rAAV1, rAAV2, and rAAV5 to transduce epithelial stem/progenitor cells of the adult lung. Our results demonstrate that the rAAV1 and rAAV5 vectors are indeed able to transduce epithelial slow-cycling stem/progenitor cells that have the capacity to proliferate in vivo following lung injury, and to expand clonally in vitro. These findings suggest that rAAV vectors may be useful for gene repair/correction of airway stem/progenitor cells in the treatment of lung diseases such as cystic fibrosis.
Results
Low-level Cre expression following rAAV transduction is sufficient for Cre-mediated recombination
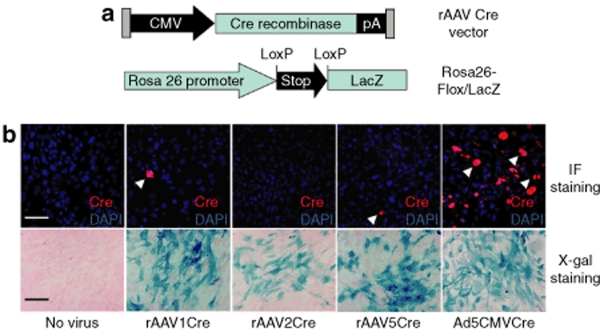
To confirm that the rAAVCre vectors (Figure 1a) are able to mediate functional recombination in a Cre/LoxP system, we infected primary mouse embryonic fibroblasts generated from Rosa26-Flox/LacZ mice with a rAAVCre vector (serotype 1, 2, or 5) or Ad.CMVCre (as a positive-control). Immunofluorescence staining for Cre protein and X-gal staining for β-galactosidase activity were then performed at 96 hours after infection. Although Cre expression in cells transduced with Ad.CMVCre was high, Cre expression in rAAV1Cre, rAAV2Cre, or rAAV5Cre vector– infected fibroblasts was almost undetectable (Figure 1b top panel), consistent with previous findings of inefficient rAAV transduction in fibroblasts.16 Surprisingly, however, X-gal staining of primary mouse embryonic fibroblasts transduced with these viral vectors was generally comparable (Figure 1b bottom panel). Together, these findings indicate that even the low-level Cre expression achieved by these rAAV vectors is sufficient to induce LoxP-mediated recombination.
Figure 1.
rAAVCre mediates efficient recombination in the Cre/LoxP system. (a) Schematic representation of the rAAVCre viral genome and the Rosa26-Flox/LacZ transgene used in the study. (b) rAAV serotype 1, 2, and 5 viruses (rAAV1Cre, rAAV2Cre, or rAAV5Cre) and recombinant adenovirus (Ad5CMVCre)-mediated Cre expression (as detected by immunofluorescence for Cre; upper panels), and Cre-mediated recombination (as detected by X-gal histochemical analysis for LacZ; lower panels), in primary mouse embryonic fibroblasts generated from Rosa26-Flox/LacZ mice. Only low-level Cre expression (arrow heads) was detected at 96 hours after infection in the case of PMEFs infected with rAAVCre viruses (MOI of 10,000 particles/cell), whereas high-level Cre expression was detected at this point in the case of PMEFs infected with the Ad5CMVCre (top panel). Cre-mediated recombination and the recovery of LacZ activity were nevertheless efficient in PMEFs infected with each of the viruses, as determined by X-gal staining at 96 hours after infection (bottom panel). Bar = 100 µm.
rAAV-mediated functional expression of Cre in the adult mouse lung
To investigate the efficiency of rAAVCre transduction to adult mouse lung, we transduced the adult Rosa26-Flox/LacZ mouse lung with 2 × 1010 particles of rAAV1Cre, rAAV2Cre, or rAAV5Cre via intratracheal instillation and analyzed β-galactosidase activity as an index of functional transduction (i.e., expression of the encoded Cre gene). The Rosa26-LacZ transgenic mouse was used as a positive control, and in these animals the entire lung was X-gal positive (Figure 2a,d). As expected, Rosa26-Flox/LacZ lungs instilled with phosphate-buffered saline demonstrated no X-gal staining (Figure 2b,e). Rosa26-Flox/LacZ mouse lungs infected with rAAV5Cre were characterized by strong X-gal staining at one month after infection, in both the conducting airways (bronchi and bronchioles) and alveoli (Figure 2c,f–i). Within the alveoli, transduced cells appeared to predominantly include alveolar type-II (ATII) cells and much less frequently alveolar type-I (ATI) cells (Figure 2g), based on morphology. Within the proximal airways, X-gal positive epithelial cells were also infrequent in the tracheal epithelium (Figure 2h), but more abundant in the primary bronchi (Figure 2i). Transduction with the rAAV1Cre vector resulted in a similar distribution of X-gal staining—with respect to both the alveolar and conducting airways—as that seen in the case of rAAV5Cre, but the levels were significantly lower at the 1-month time point (data not shown). Transduction with the rAAV2Cre vector resulted in significantly less abundant X-gal-positive cells, in both the conducting airways and the alveoli of the lung (data not shown). These findings are consistent with results obtained in previous studies of the adult mouse lung, in which these serotypes of rAAV vectors served as carriers for other reporter genes.17,18,19,20
Figure 2.
rAAVCre mediates functional recombination in the adult mouse lung. Adult Rosa26-Flox/LacZ or Rosa26-LacZ mouse lungs were infected with 2 × 1010 particles of rAAV5Cre or phosphate-buffered saline (PBS) alone, via intratracheal instillation. Whole-mount X-gal staining of the lung was performed at 1 month after this treatment. (a,d) Positive control Rosa26-LacZ mouse lung instilled with PBS; (b,e) negative control Rosa26-Flox/LacZ mouse lung instilled with PBS; (c,f,g–i) Rosa26-Flox/LacZ mouse lung infected with rAAV5Cre virus. (d–i) H & E staining of paraffin sections from X-gal stained lungs. (f) Abundant LacZ-positive cells were found in the distal airway epithelial cells and alveolar cells of Rosa26-Flox/LacZ mouse lungs infected with rAAV5Cre virus and (g) by morphologic criteria, both ATII cells (arrow heads) and ATI cells (arrows) were seen to be positive in alveolar regions of the lung. Also, LacZ-positive epithelial cells were occasionally observed in tracheal epithelia (h), and more frequently in the bronchial epithelia (i). d–f, Bar = 100 µm; g–i, Bar = 50 µm.
rAAV1- and rAAV5-transduced lung epithelial cells survive long-term in vivo
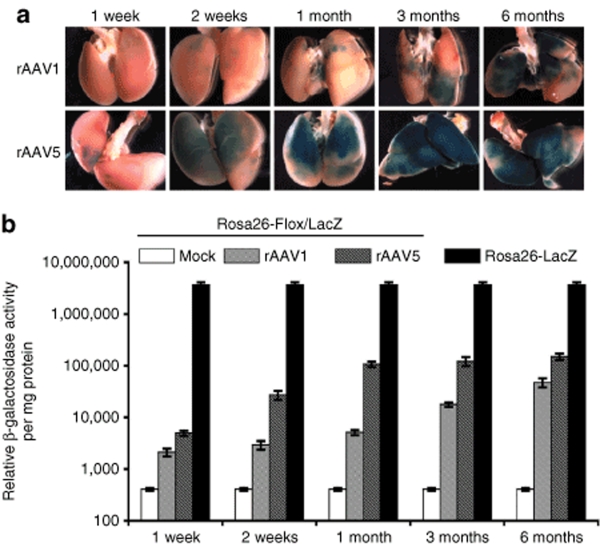
As the expression of Cre recombinase in the Rosa-Flox/LacZ transgenic mouse leads to the genetic marking of transduced cells and their descendants, it provides an opportunity to evaluate the long-term survival of transduced cells in a manner that is not dependent on retention of the viral genome. Given that rAAV genomes remain predominantly episomal, such analysis is not possible without a permanent genetic marker of transduction. To evaluate the longevity and dynamics of β-galactosidase expression in rAAVCre-transduced lungs, we infected 6–10-week-old adult Rosa26-Flox/LacZ mice intratracheally with 2 × 1010 particles of rAAV1Cre or rAAV5Cre. The efficiency of recovery of β-galactosidase activity mediated by these rAAVCre vectors in the lung was examined using X-gal staining, and by measuring relative enzymatic activity at 1 week, 2 weeks, 1 month, 3 months, and 6 months after infection. In the cases of both the rAAV1Cre and rAAV5Cre vectors, X-gal staining (Figure 3a) and β-galactosidase activity (Figure 3b) in lungs steadily increased over a 6-month period following infection, at which point the experiment was terminated. The β-galactosidase activity recovered at 6 months after infection with rAAV1Cre and rAAV5Cre was 3 and 5%, respectively, of that measured in the Rosa26-LacZ reporter mouse lung. Additionally, infection with rAAV5Cre gave rise to more rapid and efficient recovery of β-galactosidase activity than did infection with the rAAV1Cre vector. In contrast, infection with rAAV2Cre resulted in very little β-galactosidase activity above that in the mock-infected control mice (data not shown). In light of the poor transduction with the rAAV2Cre vector, we did not move this vector forward in our analysis of progenitor cell transduction.
Figure 3.
rAAVCre-transduced cells persist long-term in the mouse lung. (a) Whole-mount X-gal staining of Rosa26-Flox/LacZ mouse lungs infected with 2 × 1010 particles of rAAV1Cre or rAAV5Cre virus at 1 week, 2 weeks, 1 month, 3 months, and 6 months after infection. (b) Quantification of β-galactosidase activity from Rosa26-Flox/LacZ mouse lungs infected with rAAVCre viruses. rAAV expression values shown are relative to β-galactosidase activity from Rosa26-LacZ mouse lungs at each time point. Data represent the mean (±SEM) relative β-galactosidase activity per mg/protein from three mice per experimental point (three independent experiments).
rAAV-mediated Cre transduction selectively occurs in SPC-expressing ATII cells and CCSP-expressing Clara cells of the conducting airways
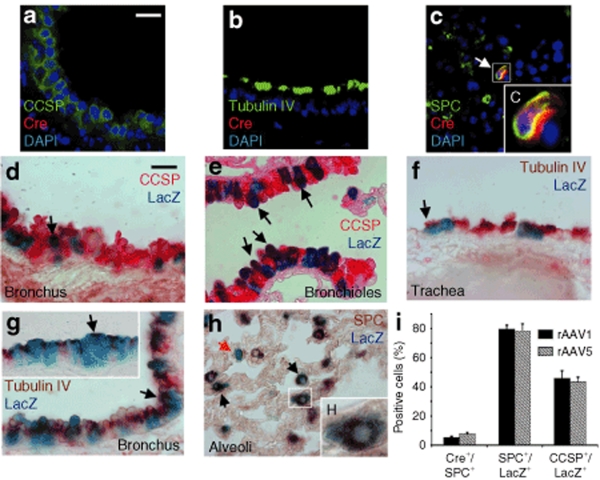
Previous studies on gene transduction mediated by rAAV vectors in animal models have demonstrated a tropism of different rAAV serotypes for distinct airway epithelial cell types.20,21,22,23,24 To investigate rAAV-mediated Cre expression in epithelial cells of the lung, we carried out immunofluorescence colocalization analysis of Cre and epithelial cell-specific markers on the Rosa26-Flox/LacZ mouse lung transduced with 2 × 1010 rAAV1Cre or rAAV5Cre vector particles at 1 month after infection. Although there was no detectable Cre expression in CCSP-positive Clara cells (Figure 4a), Tubulin IV-positive ciliated cells (Figure 4b), or Keratin 14-positive basal cells (data not shown), both the rAAV1Cre- and rAAV5Cre-infected lungs demonstrated 5.3 ± 0.7% and 7.6 ± 1.1% of SPC positive ATII cells expressed detectable CRE, respectively (Figure 4c,i). In contrast, LacZ expression mediated by both rAAV serotypes was commonly seen in CCSP-expressing Clara cells of the bronchi and bronchioles (Figure 4d,e), with the percentage of LacZ expressing cells also positive for CCSP equaling 45.7 ± 5.3% and 43.5 ± 3.1% for rAAV1Cre- and rAAV5Cre-infected lungs, respectively (Figure 4i). In the alveoli, abundant LacZ-expressing cells were SPC positive ATII cells (Figure 4h), with the percentage of LacZ expressing cells also positive for SPC equaling 79.5 ± 2.8% and 78.3 ± 3.1% for rAAV1Cre- and rAAV5Cre-infected lungs, respectively (Figure 4i). Much less frequent LacZ expression was observed in tubulin-IV-expressing ciliated cells in the trachea and bronchi (Figure 4f,g). Additionally, LacZ expression was not observed in basal cells of the tracheal airway at the rAAV vector dose tested here (data not shown), suggesting that this cell type is not efficiently transduced. These data indicated that Clara cells and ATII cells in the adult mouse lung are more susceptible to rAAV1 and rAAV5 transduction than are the other investigated cell types, and that the low-level Cre expression mediated by rAAV vectors is sufficient to stimulate Cre-mediated recombination in these cell types in vivo.
Figure 4.
Alveolar type II and Clara cells are highly susceptible to rAAV transduction in the adult mouse lung. (a–c) Immunofluorescence-based colocalization of Cre and the indicated epithelial cell-specific markers in frozen sections of Rosa26-Flox/LacZ mouse lung transduced with 2 × 1010 particles of rAAV5Cre virus at 1 month after infection. Cre expression was not detected in the (a) CCSP-positive Clara cells or (b) Tubulin IV-positive ciliated cells, but moderate levels of Cre were observed in the (c) SPC-positive ATII cells—boxed region in c is enlarged in the inset C. (d,e) Immunohistochemical staining of the Clara-cell marker CCSP in paraffin sections of X-gal-stained Rosa26-Flox/LacZ lung transduced with rAAV5Cre. LacZ staining was observed in CCSP-positive Clara cells in both the bronchus and bronchioles (arrows). (f,g) Immunohistochemical staining of Tubulin IV-positive ciliated cells in paraffin sections of X-gal-stained Rosa26-Flox/LacZ lungs transduced with rAAV5Cre. LacZ/Tubulin IV double-positive epithelial cells were found infrequently in the (f) tracheal epithelium, and more frequently in the (g) bronchial epithelium (arrows). (h) Immunohistochemical staining of SPC-positive alveolar type II cells in paraffin sections of X-gal-stained Rosa26-Flox/LacZ lungs transduced with rAAV5Cre. The majority of SPC-positive ATII cells were LacZ positive (black arrows) while infrequent SPC−/LacZ+ cells were also observed (red arrow). Boxed region in h is enlarged in the inset. (i) Quantification of cell types expressing either CRE or LacZ. Three types of data are shown in this graph: (i) the percentage of SPC positive cells that also expressed CRE in alveolar regions of the lung (Cre+/SPC+), (ii) the percentage of LacZ positive cells that also expressed SPC in alveolar regions of the lung (SPC+/LacZ+), and (iii) the percentage of LacZ positive cells that also expressed CCSP in bronchioles of the lung (CCSP+/LacZ+). Bars for both fluorescent- and light-level panels = 20 µm. Data in I represent the mean (±SEM) from N = 3 mice per experimental point.
rAAV is capable of transducing epithelial stem/progenitor cells in the adult mouse lung
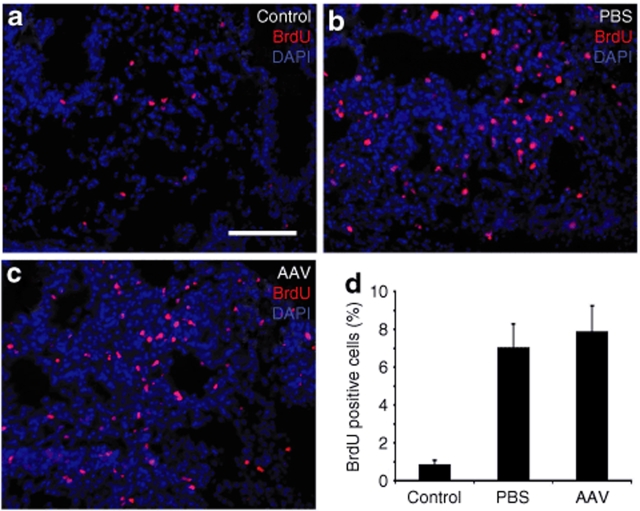
Several lung epithelial cell types have been proposed as potential stem/progenitor cells; these include basal cells, Clara cells, and ATII cells.15,25 The LacZ-positive cells found in the airways of rAAVCre-infected Rosa26-Flox/LacZ lungs are rAAVCre-transduced cells and/or descendants of rAAVCre-transduced epithelial stem/progenitor cells. Thus, the finding that LacZ expression increases over time up to 6 months suggested two possibilities: (i) rAAV-mediated transduction and expression of Cre is a very slow process, and/or (ii) rAAVCre-transduced airway progenitors expand in the lung over time. To determine whether there were rAAV1Cre- or rAAV5Cre-transduced epithelial stem/progenitor cells in the adult Rosa26-Floxed/LacZ mouse lung, we administered 5-bromodeoxyuridine (BrdU) to track slow cycling stem/progenitor in rAAVCre infected animals. The rAAV-transduced LacZ positive stem/progenitor cells were then assessed by detection of a slow cycling stem/progenitor cell phenotype that leads to BrdU label retention. Two procedures for BrdU labeling were used: (1) mice were first infected with rAAVCre and 15 days later the mice were injured with naphthalene and BrdU labeled, or (2) mice were first injured with naphthalene and labeled with BrdU and 60 days later the mice were infected with rAAVCre. Putative stem/progenitor cells that entered the cell cycle and retained the BrdU label and also expressed LacZ were assessed at 60 days after naphthalene injury/BrdU labeling (for Procedure 1), or at 90 days after naphthalene injury/BrdU labeling (for Procedure 2). Because of the slow turnover of epithelial cells and the low frequency of stem cell division in the normal state, injury has previously been required for studies of stem/progenitor cells in lung.15 In the studies presented here, intratracheal instillation of rAAV caused acute injury in the alveolar regions of the lung, as evident from increased BrdU incorporation in alveolar cells shortly following infection (Figure 5). This acute lung injury and enhanced proliferation of alveolar cells was dependent on the intratracheal instillation technique and not the vector. As shown in Figure 5, intratracheal instillation of vehicle (phosphate-buffered saline) led to enhanced BrdU incorporation in alveolar regions of the lung with a labeling index (7.0 ± 1.3%) that was not significantly different from vector administration (7.9 ± 1.4%). Both administration of vehicle or vector led to an approximately tenfold enhancement in the proliferative index over naive control animals (0.86 ± 0.22%). In contrast, the administration of naphthalene was required to induce cellular proliferation and incorporation of BrdU in the conducting airway epithelium (data not shown). These approaches were combined with colocalization studies—of BrdU label retention, epithelial cell-specific markers, and/or β-galactosidase labeling—to characterize the phenotype of potential rAAV-transduced stem/progenitor cells.
Figure 5.
Intratracheal administration of vehicle causes acute alveolar injury. Rosa26-Flox/LacZ were instilled with 20 µl rAAVCre5 vector (2 × 1010 particles) or PBS into the trachea, followed by BrdU labeling (i.p) for three consecutive days beginning on the day of infection. The proliferative index of the lung under each condition was then evaluated by anti-BrdU staining of lung section on the day after the final administration of BrdU. The control mice were administrated only BrdU alone and did not receive intratracheal instillations. (a–c) Alveolar immunofluorescent photomicrographs of BrdU staining (red) and DAPI staining (blue) are shown for each treatment group as labeled. (d) Quantification of proliferative index. Data represent the mean (±SEM) percent BrdU positive nuclei in the alveolar regions of the lung from four mice in each group. Bar in c = 100 µm in panels a–c.
Indeed, both LacZ-positive and -negative label-retaining cells (LRCs) were found in bronchiolar airways (Figure 6a,b,f), at bronchiolar-alveolar duct junctions (Figure 6d,e), and in ATII cells of the alveoli (Figure 6c) in lungs that had been infected with either the rAAV1Cre or rAAV5Cre virus (data only shown for rAAV5). These LacZ+/BrdU+ cells in bronchiolar airways were observed with both protocols of BrdU labeling—with naphthalene injury/labeling before rAAV infection (Figure 6f) or naphthalene injury/labeling after rAAV infection (Figure 6b,d). Immunohistochemical colocalization of LacZ, BrdU and markers specific for various epithelial cell types revealed that a subset of LacZ-positive LRCs was CCSP-positive in bronchioles (Figure 6g) and bronchiolar-alveolar duct junctions (Figure 6h), or SPC-positive in alveoli (Figure 6i). In the bronchioles and bronchiolar-alveolar duct junctions, these infrequently observed LRCs were associated with large transgene-expressing patches of cells, consistent with clonal expansion of rAAV-transduced stem/progenitors in the regenerated epithelia. There was no statistically significant difference in the distribution of LacZ+/BrdU+ LRCs in animals infected with rAAV1Cre or AAV5Cre. In the alveoli, the percentage of LacZ positive cells that retained BrdU label (i.e., were LCRs) following rAAV1 and rAAV5 infection was 0.029 ± 0.012% and 0.031 ± 0.011%, respectively (Figure 6j). In the bronchioles, the percentage of LacZ positive cells classified as LRCs following rAAV1 and rAAV5 infection was 0.042 ± 0.019% and 0.072 ± 0.011%, respectively (Figure 6j). The finding of label retention in both ATII cells and Clara cells that were LacZ-positive is consistent with viral transduction of cells of the mouse lung with known progenitor capacity.15,25
Figure 6.
A subset of rAAV-transduced LacZ-positive ATII cells and Clara cells are BrdU label-retaining cells (LRCs). rAAV5Cre-infected Rosa26-Flox/LacZ mice were pulse labeled with BrdU and/or injured with Naphthalene as described in Materials and Methods. For analysis of the bronchioles, two procedures were followed and included infection with rAAV 15 days before Naphthalene injury/BrdU labeling (Procedure 1) or Naphthalene injury/BrdU labeling 60 days before rAAV infection (Procedure 2). Lungs were then X-gal stained, embedded, and sectioned for immunohistochemical staining of BrdU (LRCs) and/or CCSP in bronchioles at 60 days after injury/BrdU labeling (for Procedure 1), or at 90 days after injury/BrdU labeling (for Procedure 2). For analysis of LRCs in alveolar regions, BrdU labeling was performed at the time of rAAV infection and lungs were harvested 90 days later for colocalization of LacZ, BrdU, and/or SPC. (a–f) Immunohistochemical localization of BrdU in X-gal-stained sections representing the (a, b) bronchiolar epithelium (using Procedure-1 BrdU labeling), (c) alveolar epithelium (BrdU labeling without injury), (d, e) respiratory bronchiolar epithelium at the bronchiolar-alveolar duct junction (BADJ) of the lung (using Procedure-1 BrdU labeling), and (f) bronchiolar epithelium (using Procedure-2 BrdU labeling). a shows an example of a BrdU+/LacZ− cell, while b–f show examples of BrdU+/LacZ+ double-positive cells. b′ and d′ are enlargement of boxed regions in b and d panels, respectively. (g,h) Triple histochemical staining for CCSP, BrdU, and LacZ in g bronchiolar epithelium or h respiratory bronchiolar epithelium at the BADJ. (i) Triple histochemical staining for SPC, BrdU, and LacZ in alveolar epithelium of the lung. Insets depict enlargements of regions as marked by the letters and red arrows in each panel. Inset A depicts a BrdU-positive, LacZ-negative cell; insets B–F depict BrdU/LacZ double-positive cells; insets G–I depict CCSP/BrdU/LacZ and SPC/BrdU/LacZ triple-positive cells. a, b′, d′, e, f, g, h, and i bar = 25 µm; c bar = 50 µm, b and d bar = 100 µm. (j) Quantitative analyses evaluating the percentage of LacZ positive cells also BrdU positive (i.e., LRCs) in alveoli and bronchiolar airways. Data represent the mean (±SEM) from three mice per experimental point.
A subset of rAAV-transduced airway epithelial cells has the potential to expand both in vitro and in vivo
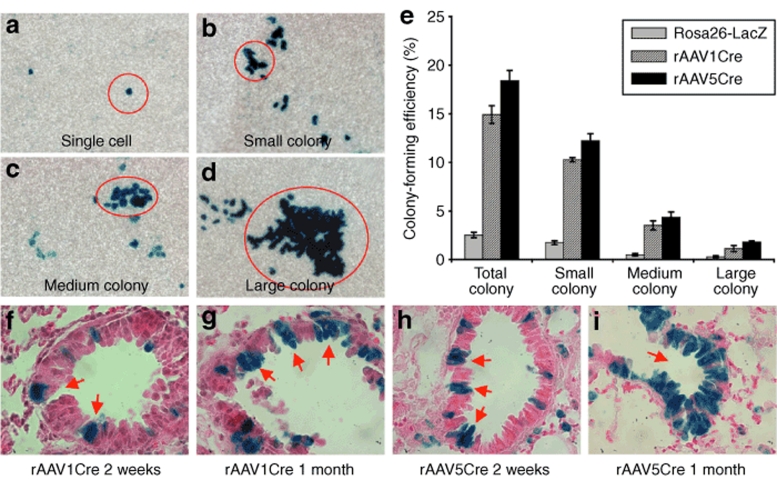
The capacity of airway epithelia cells to expand in vitro has been previously used to assess progenitor capacity, using colony-forming efficiency (CFE) assays that index the number and size of expanded transgene-marked epithelial cell colonies.15,26 This assay is typically performed using proximal tracheobronchial epithelial cells that can be grown at the air–liquid interface (ALI) in mixed cultures, where a subset of cells is marked by a transgene such as LacZ. This method provided the ideal approach to assess and quantify progenitor cells transduced by rAAV in the proximal airway. Although the trachea was only poorly transduced by rAAV in these studies, transduction of extra-lobar bronchi was sufficient to allow for clonal analysis (Supplementary Table S1). In vitro CFE assays using rAAV5Cre- and rAAV1Cre-infected airway epithelial cells from Rosa26-Flox/LacZ mice demonstrated the capacity of a subset of rAAVCre-transduced cells to form LacZ-positive colonies of varying sizes (Figure 7a–d). To quantify the relative abundance of rAAVCre-transduced progenitor cells, controls with mixed cultures containing 1% airway cells from Rosa26-LacZ mice and 99% airway cells from noninfected Rosa26-Flox/LacZ mice were used to determine the baseline distribution of progenitor cell clones of various sizes. In control cultures, 2.5 ± 0.3% of airway epithelial cells from Rosa26-LacZ mice formed colonies, with 0.3 ± 0.1% of clones forming large colonies (Figure 7e). These findings were consistent with the results of previous studies, which had reported a total CFE of 1.7% and a large-colony CFE of 0.1% for Rosa26-LacZ tracheal airway epithelial cells.26 Surprisingly, rAAVCre-transduced airway epithelial cells demonstrated a much higher CFE compared to the cultures generated from Rosa26-LacZ mice (Figure 7e). ALI cultures generated with rAAV1Cre- and rAAV5Cre-transduced airway epithelial cells from Rosa26-Flox/LacZ mice generated colonies at an efficiency of 14.9 ± 0.9% and 18.4 ± 1.1%, respectively, representing a greater than six- to sevenfold enhancement over control Rosa26-LacZ cells. A similar enhancement was seen in the percentage of rAAV-transduced cells that formed large colonies (rAAV1Cre 1.1 ± 0.3% and rAAV5Cre 1.8 ± 0.1%) (Figure 7e). These results suggested that rAAV1 and rAAV5 might selectively transduce airway epithelial cells in vivo with a higher proliferative capacity. Indeed, analysis of the Rosa26-Flox/LacZ mouse lungs transduced with rAAV1Cre or rAAV5Cre suggested that clonal expansion might indeed occur in vivo in the distal airway epithelia (Figure 7f–i). At 2 weeks after infection, small LacZ-positive clusters of epithelial cells were observed (Figure 7f,h), and these expanded into larger LacZ-positive clusters at 1 month after infection (Figure 7g,i). Although the clonal origin of these clusters of transgene-marked cells remains unclear, their appearance is consistent with the expansion of rAAV-transduced progenitors in the airway.
Figure 7.
AAVCre-transduced airway epithelial cells have the capacity to expand in vitro and in vivo. (a–d) Examples of LacZ clone sizes observed in the in vitro colony-forming efficiency (CFE) assay used to index the proliferative capacity of rAAV-transduced airway stem/progenitor cells: (a) single cell, (b) small colony (5–10 cells), (c) medium colony (11–25 cells), and (d) large colony (>25 cells). (e) CFE quantification of rAAVCre-infected tracheobronchial epithelial cells harvested from Rosa26-Flox/LacZ-infected mouse lungs, as compared to the CFE of cultures containing 1% LacZ-positive tracheobronchial epithelial cells from Rosa26-LacZ mice. Data represent the mean (±SEM) %CFE from N = 8 mice in each group, from two independent experiments. (f–i) X-gal histochemical staining of Rosa26-Flox/LacZ mouse lung sections following transduction with rAAV1Cre (f and g) and rAAV5Cre (h and i) in the absence of Naphthalene injury. In both cases, small LacZ-positive clusters of epithelial cells were found at 2 weeks after infection (f and h), and the size of the transgene-positive clusters of cells expanded at 1 month after infection (g and i).
Regeneration of rAAV transduced epithelial cells after airway injury
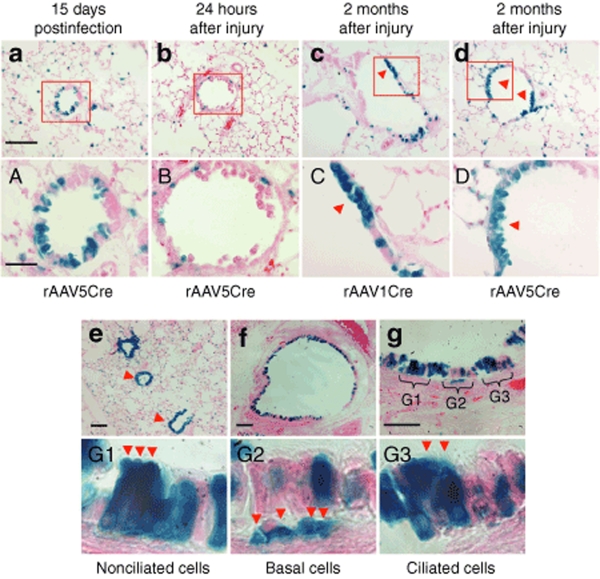
To provide further evidence that rAAV1 and rAAV5 can transduce progenitor cell populations in the mouse airway, we induced airway damage and expansion of epithelial progenitors in rAAVCre-infected Rosa26-Floxed/LacZ mice by administering naphthalene at 15 days after infection. The proliferative capacity of rAAVCre-transduced cells was then evaluated by assessing the formation of large colonies of LacZ-positive cells in the regenerated epithelium at 2 months after injury. Individual LacZ-positive cells and small LacZ-positive colonies were observed in the distal airway of the uninjured lung at 15 days after infection (Figure 8a, A). The administration of naphthalene resulted in the ablation of airway epithelia at 24 hours, but a subset of isolated LacZ-positive cells remained in the distal airway epithelia (Figure 8b, B). Large colonies with LacZ-positive cells in the regenerated epithelia were observed in the naphthalene-injured lungs infected with rAAV1Cre (Figure 8c, C) and rAAV5Cre (Figure 8d, D). Such colonies were also infrequently associated with a single LRC (Figure 6b,d). This result supports the notion that a subset of stem/progenitor cells in the conducting airway epithelia are transduced by rAAV1 and rAAV5, and have the capacity to proliferate following airway injury.
Figure 8.
A subset of rAAV-transduced airway epithelial cells has the capacity to regenerate epithelia after airway injury. (a) Rosa26-Floxed/LacZ mice infected with 2 × 1010 particles of rAAV5Cre virus demonstrated abundant LacZ-positive cells in the distal airways at 15 days after infection. (b) At 24 hours following naphthalene injury, significant portions of the airway epithelium were ablated and few LacZ-positive cells remained in the airway. (c and d) Large LacZ-positive clones (arrowheads) emerged in the regenerated distal airway epithelium in mice infected with either (c) rAAV1Cre or (d) rAAV5Cre at 60 days after naphthalene injury. (A–D) Higher magnification images of the boxed regions in a–d, respectively. a–d bar = 100 µm; A–D bar = 30 µm. (e–g) Transduction of tracheal epithelium with rAAV1 required high doses of virus. X-gal-stained sections from Rosa26-Flox/LacZ mouse lung and trachea infected with 1 × 1012 particles of AAV1Cre at 1 month after infection. (e) Photomicrograph of lung section with bronchioles marked by arrowheads. (f) Photomicrograph of tracheal cross-section. (g) Enlarged photomicrograph showing the tracheal surface of the airway epithelium. (G1–G3) Enlarged photomicrographs of regions marked in g, representing LacZ-positive (G1) nonciliated cells, (G2) basal cells, and (G3) ciliated cells, as indicated by the arrowheads in each panel. e, f, Bar = 100 µm; g, bar = 50 µm.
Efficient transduction of proximal airway epithelial cells requires high rAAV titers
Well-differentiated polarized proximal airway epithelia have proven difficult to transduce from the apical surface using rAAV vectors, both in vitro and in vivo.19,20,27,28 Proximal airway transduction with both rAAV1Cre and rAAV5Cre at doses of 2 × 1010 particles per lung is relatively inefficient, as shown in the above-described studies. To determine whether this efficiency could be increased, we administered rAAV1Cre and rAAV5Cre to Rosa26-Flox/LacZ mice at a dose of 1 × 1012 particles per lung, and revaluated transduction by assessing LacZ-positive epithelial cells in the proximal and distal conducting airway at 1 month after infection. Transduction of tracheal and bronchiolar airway epithelial cells was greatly enhanced by increasing the dose of rAAV1Cre (Figure 8e,f) and rAAV5Cre (data not shown). Histological analysis revealed that several epithelial cell types were LacZ positive (Figure 8g), including nonciliated columnar cells (Figure 8G1), basal cells (Figure 8G2), and ciliated cells (Figure 8G3). Although only the results for rAAV1Cre are shown, similar findings were seen for rAAV5Cre (data not shown). This suggests that efficient rAAV1 and rAAV5 transduction to the proximal (large) airway is highly dependent on vector dosage, consistent with previous findings in rabbit.22
Discussion
Gene manipulation in mice using the Cre-loxP system is a widely used approach for studying gene function in development and disease. Additionally, this system has been effectively used to define lineage relationships among stem/progenitor cells, using cell-specific promoters to drive Cre expression. Alternative approaches to fate mapping in vivo have involved the use of recombinant retroviruses/lentiviruses that lead to the integration of an expressed transgene marker. In contrast to retroviruses/lentiviruses, rAAV mediates transgene expression largely via episomal viral genomes, and hence vector-mediated transgene expression alone cannot be used to track cell fate of transduced progenitors. In this study, we sought to combine viral vector and transgenic Cre-LoxP reporter approaches to study the progenitor cell types that are transduced by several rAAV serotypes. Because gene expression by rAAV vectors is typically fairly low—which makes it difficult to follow transgene expression at the cellular level in tissue sections—this CRE-mediated approach has also provided a clearer understanding of the cell types transduced by rAAV in the mouse airway, and of the capacity of these cell types to remain in and/or expand within the lung over long periods of time. In this regard, previous studies in mouse and rat lungs have demonstrated that rAAV1- and rAAV5-mediated expression of secreted proteins declines by 40 days after infection.29,30 Our study demonstrates that despite a decline in transgene expression over time, lung progenitor cells transduced by rAAV1 or rAAV5 remain resident in the lung and expand over time.
The transduction of various stem/progenitor cell populations by rAAV vectors is affected by specific tropisms of the vector used. For example, rAAV2 has been shown to transduce undifferentiated human and mouse ES cells, whereas the rAAV4 and rAAV5 vectors can transduce only differentiated ES cells.5 rAAV1, on the other hand, seems to be the most efficient at transducing mouse hematopoietic stem cells.3,31 In mesenchymal stromal cells of the baboon, the order of efficiency of rAAV transduction is AAV serotype 2>3>5>1, 4, 6, 8 (ref. 32). Moreover, rAAV2 vectors have been shown to efficiently transduce stem/progenitor cells in other mouse organs,9,33 and also in ATII cells of the neonatal rabbit lung.22 Here we demonstrate that rAAV5 and rAAV1 can efficiently transduce airway epithelial stem/progenitor cells in the conducting airway of the mouse lung. In this context, rAAV5 gave more rapid and higher levels of transduction in the conducting airways than did rAAV1, but both transduced a similar distribution of cell types. Both rAAV1 and rAAV5 serotypes also transduced long-lived ATII progenitors of the alveolar regions of the lung. In contrast, rAAV2 demonstrated much less efficient transduction in adult mouse lung in all regions.
Our studies comparing the colony-forming efficiencies of the proximal airway epithelia of ROSA26-LacZ and ROSA26-LoxP/LacZ mice following transduction with rAAV1 and rAAV5 demonstrated that these rAAV serotypes transduce proximal airway progenitor cells six- to sevenfold more effectively than differentiated cell types that lack the ability to proliferate in vitro. Such findings are consistent with histologic evidence for greater Clara cells transduction, as compared to terminally differentiated ciliated cells, throughout all levels of the conducting airways. In the mouse, Clara cells are considered to be progenitors in the airway, with a subset of variant Clara cells likely representing the stem cell pool in the distal airways.34
The ability of rAAV1 and rAAV5 to transduce lung stem/progenitors suggests that these serotypes can be used to develop gene correction strategies with rAAV in the mouse. rAAV vectors are particularly useful in mediating gene repair via homologous recombination,11,13 and have been successfully used to functionally correct mutated genes in both human and mouse somatic cells, in vitro and also in vivo.12,14,35 Although the efficiency of in vivo gene correction using rAAV remains low in the liver14 and has yet to be studied in the lung, improvements in this technique may indeed make it possible to correct airway stem cells in diseases such as cystic fibrosis. Such an approach could allow for more persistent correction of genetic defects without the need for repeat administration of vector.
While rAAV1 and rAAV5 appear to more efficiently transduce lung progenitors in mice, it should be cautioned that these same serotypes might not perform similarly in the human lung. Significant species-specific differences exist with respect to the efficiency of various rAAV serotypes in transducing human and mouse airways.20 Furthermore, differences in the stem/progenitor cell niches within the proximal airways also appear to exist between mice and humans.15 Hence, during the development of rAAV-mediated therapies to airway stem/progenitors, it will be important to evaluate such methods in several species of animals that may more accurately model both the human tropisms for various rAAV serotypes and human airway stem/progenitor cell biology. Indeed, the proximal airways of both the pig and ferret have been shown to have similar serotype preferences for rAAV,24 and the fact that cystic fibrosis models in these species have also been developed36,37 may position these species uniquely for use in the development of rAAV-mediated stem/progenitor cell therapies to the lung in the treatment of cystic fibrosis.
Materials and Methods
Animals and tissue culture. All animal studies were approved by the University of Iowa Animal Care Committee and were carried out in accordance with the principles and procedures outlined in the NIH guidelines for the care and use of experimental animals. B6.129S4-Gt(ROSA)26Sor/J (Stock number 002073) LacZ reporter mice (called Rosa26-LacZ in this report) and B6.129S4-Gt(ROSA)26Sortm1Sor/J (Stock number 003474) floxed-LacZ reporter mice (called Rosa26-Flox/LacZ in this report) were obtained from Jackson Laboratories (Bar Harbor, ME). Both male and female mice, 6–10 weeks of age, were used in these studies. The 293 cell line was purchased from ATCC and cultured in DMEM medium supplemented with 10%FBS and 1% pen/strep, in a 5% CO2 atmosphere at 37 °C. Rosa26-Flox/LacZ primary mouse embryonic fibroblasts were generated from 15-day embryos as previously described, and were cultured under the same conditions as the 293 cells.35
Generation of rAAVCre recombinase vectors and rAAV infection. rAAV1Cre, rAAV2Cre, and rAAV5Cre viruses were generated by inserting a CMV promoter-directed complete reading frame of the Cre recombinase gene between AAV2 ITRs, and then packaging the proviral pAAV2CMVCre vector into serotype 1 (rAAV1Cre), 2 (rAAV2Cre), or 5 (rAAV5Cre) AAV capsids using a triple plasmid-transfection method in 293 cells as previously described.38 Physical titers of rAAV were determined by slot blot hybridization using a α32P-dCTP labeled Cre cDNA probe. Control recombinant adenoviral Cre virus (Ad.CMVCre) was provided by the Vector Core of the Gene Therapy Center at The University of Iowa. Viral infection of the cells was performed in the absence of serum for 2 hours before returning cells to normal medium. In vivo infection of mouse lung with recombinant viral vectors was performed by intratracheal instillation. Following the administration of a single low dose of ketamine (50 mg/kg) and xylazine (2.5 mg/kg), each mouse was intratracheally instilled with 2 × 1010 (or 1 × 1012 for high-dosage experiment) particles of rAAVCre vector in a volume of 20 µl phosphate-buffered saline. Control mice were intratracheally instilled with phosphate-buffered saline.15
X-gal staining and measurement of β-galactosidase activity. The β-galactosidase activity in the lungs and/or tracheas was evaluated by either X-gal staining or by measurement of relative luminescent units using the Galacto-Light Plus System (Applied Biosystems, Bedford, MA). Designated time points for analysis ranged from 1 week to 6 months after infection. Briefly, the lung and trachea were inflation-fixed for X-gal staining before harvesting at designated time points of 1 week, 2 weeks, 1 month, 3 months, and 6 months following infection. Lung whole-mounts that were X-gal stained were processed for histological analysis in paraffin sections as previously described.39 For the measurement of β-galactosidase activity, lung tissue was homogenized in assay buffer and relative luminescent units were measured according to the manufacturer's protocol.
In vitro analysis of the proliferative capacity of rAAV-transduced proximal airway epithelial cells using a CFE assay. Rosa26-Flox/LacZ mice were infected with rAAV1Cre or rAAV5Cre virus by intratracheal instillation. Tracheobronchial epithelial cells were harvested at 15 days following infection and used to regenerate polarized airway epithelia in ALI cultures as previously described.20 The proliferative capacity of rAAV-transduced airway epithelial cells was measured using an in vitro CFE assay as previously described.15,40 In this assay, the number and size of LacZ-positive colonies after 2 weeks of ALI culture are scored to index the proliferative capacity of LacZ-marked stem/progenitor cells. Controls for baseline % CFE of non-AAV-infected mice used mixed cultures containing 1% Roas26-LacZ and 99% airway epithelial cells from noninfected Rosa26-Flox/LacZ mice. En face X-gal staining of airway epithelia on the filters was performed at 2 weeks after the establishment of the ALI, and the number and sizes of the colonies were assessed and quantified using a dissecting microscope. Colony size classifications were 5–10 cells, 11–25 cells, and >25 cells. The number of colonies in each size group divided by the number of LacZ-positive cells seeded into each culture was used to calculate the % CFE.40
Evaluation of the regenerative capacity of rAAV-transduced airway epithelial cells following lung injury. To investigate whether airway stem/progenitor cells were transduced with rAAV, we labeled slow-cycling nucleotide LRCs using pulse labeling with BrdU as previously described.15 Long-term nucleotide label retention is one index of slow cycling stem/progenitor cells in the airway.34,41 To access LRCs in the alveolar regions of the lung, we labeled rAAV-infected Rosa26-Flox/LacZ mice with BrdU (100 mg/kg body weight, i.p.) for three consecutive days at the time of rAAV1Cre or rAAV5Cre infection. The lungs were harvested for X-gal staining and paraffin embedding at 90 days after infection, and BrdU was colocalized with X-gal staining by immunohistochemical staining with anti-BrdU antibody (Roche, Indianapolis, IN).15 For the assessment of LRCs in the conducting airways, it was necessary to drive expansion of stem/progenitor cells in this region by injuring the airways with naphthalene. Two procedures were used: (i) infection of airways with rAAV before naphthalene injury and BrdU labeling, or (ii) injury of airways with naphthalene and labeling with BrdU before infection of airways with rAAV. Protocols for each procedure were as follows. Procedure 1: At 15 days after infection of Rosa26-Flox/LacZ mice with rAAV1Cre or rAAV5Cre virus (by intratracheal instillation), mice were injured with Naphthalene and BrdU labeled. Lungs were then harvested for analysis at 60 days after Naphthalene injury/BrdU labeling (75 days after infection). Procedure 2: Rosa26-Flox/LacZ mice were first injured with Naphthalene and BrdU labeled. At 60 days after Naphthalene injury/BrdU labeling, the mice were infected with rAAV1Cre or rAAV5Cre virus (by intratracheal instillation). Lungs were then harvested for analysis at 30 days after infection (90 days after Naphthalene injury/BrdU labeling). Naphthalene-mediated lung injury was induced15 by i.p. injection of freshly made naphthalene (Fisher Scientific, dissolved in sterile Mazola corn oil at a concentration of 27.5 mg/ml) at 275 mg/kg body weight. Naphthalene injured mice were injected (i.p.) with BrdU (100 mg/kg body weight) for three consecutive days after naphthalene injury. Lungs were harvested for X-gal staining and then embedded in paraffin. LRCs in paraffin sections were then detected by immunohistochemistry with anti-BrdU antibody.
Immunofluorescence and immunohistochemical staining. To localize Cre recombinase and X-gal-positive cell types, we performed immunofluorescence and/or immunohistochemical staining on frozen and paraffin sections using: mouse anti-Cre antibody (Covance, Berkeley, CA), goat or rabbit anti-CCSP antibody (Upstate, Lake Placid, NY), rabbit anti-SPC antibody (Upstate, Lake Placid, NY), mouse anti-BrdU antibody (Roche, Indianapolis, IN), mouse anti-β-Tubulin IV antibody (BioGenex, San Ramon, CA), and rabbit anti-keratin 14 antibody (NeoMarkers, Fermont, CA). Frozen sections were prepared by embedding mouse lungs and/or tracheas in the Tissue-Tek OCT compound (Sakura, Torrance, CA). Paraffin sections (6 µm) were prepared from X-gal-stained lung and/or tracheal tissues following postfixation in 10% neutral-buffered formalin (Richard-Allan scientific, Kalamazoo, MI) at 4 °C overnight. Immunofluorescence staining was conducted on 6 µm frozen sections, using the appropriate primary and fluorescent-labeled secondary antibodies. Stained sections were mounted in Vectashield Mounting Medium, which contained DAPI to demarcate the nucleus (H-1200, Vector Laboratories, Burlingame, CA). Immunohistochemical staining was performed on either frozen sections or paraffin sections, using the relevant primary antibodies, alkaline phosphatase- or peroxidase-labeled secondary antibodies, and either the Vectastain ABC kit or the Elite ABC kit (Vector Laboratories, Burlingame, CA). Sections were mounted in permount, and staining was visualized using the alkaline phosphatase substrate kit or the DAB kit (Vector Laboratories, Burlingame, CA), as appropriate.
Supplementary MaterialTable S1. Colony forming efficiency data.
Supplementary Material
Colony forming efficiency data.
Acknowledgments
This work was supported by NIH HL58340 and DK047967, the University of Iowa Center for Gene Therapy (DK54759), and a grant from Targeted Genetics Corporation. We do not hold financial interests in Targeted Genetics Corporation and do not receive any form of personal financial income from this company. We also gratefully acknowledge the editorial assistance of Dr Blaumueller.
REFERENCES
- Goodman S, Xiao X, Donahue RE, Moulton A, Miller J, Walsh C, et al. Recombinant adeno-associated virus-mediated gene transfer into hematopoietic progenitor cells. Blood. 1994;84:1492–1500. [PubMed] [Google Scholar]
- Han Z, Zhong L, Maina N, Hu Z, Li X, Chouthai NS, et al. Stable integration of recombinant adeno-associated virus vector genomes after transduction of murine hematopoietic stem cells. Hum Gene Ther. 2008;19:267–278. doi: 10.1089/hum.2007.161. [DOI] [PubMed] [Google Scholar]
- Zhong L, Li W, Li Y, Zhao W, Wu J, Li B, et al. Evaluation of primitive murine hematopoietic stem and progenitor cell transduction in vitro and in vivo by recombinant adeno-associated virus vector serotypes 1 through 5. Hum Gene Ther. 2006;17:321–333. doi: 10.1089/hum.2006.17.321. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Megee S, Zeng W, Destrempes MM, Overton SA, Luo J, et al. Adeno-associated virus (AAV)-mediated transduction of male germ line stem cells results in transgene transmission after germ cell transplantation. Faseb J. 2008;22:374–382. doi: 10.1096/fj.07-8935com. [DOI] [PubMed] [Google Scholar]
- Smith-Arica JR, Thomson AJ, Ansell R, Chiorini J, Davidson B., and , McWhir J. Infection efficiency of human and mouse embryonic stem cells using adenoviral and adeno-associated viral vectors. Cloning Stem Cells. 2003;5:51–62. doi: 10.1089/153623003321512166. [DOI] [PubMed] [Google Scholar]
- Kang Y, Liao WM, Yuan ZH, Sheng PY, Zhang LJ, Yuan XW, et al. In vitro and in vivo induction of bone formation based on adeno-associated virus-mediated BMP-7 gene therapy using human adipose-derived mesenchymal stem cells. Acta Pharmacol Sin. 2007;28:839–849. doi: 10.1111/j.1745-7254.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- Stender S, Murphy M, O'Brien T, Stengaard C, Ulrich-Vinther M, Soballe K, et al. Adeno-associated viral vector transduction of human mesenchymal stem cells Eur Cell Mater 20071393–99.discussion 99 [DOI] [PubMed] [Google Scholar]
- Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X., and , Chu CR. Adeno-associated viral gene transfer of transforming growth factor-β1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14:804–813. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- Barth AS, Kizana E, Smith RR, Terrovitis J, Dong P, Leppo MK, et al. Lentiviral vectors bearing the cardiac promoter of the Na(+)-Ca(2+) exchanger report cardiogenic differentiation in stem cells. Mol Ther. 2008;16:957–964. doi: 10.1038/mt.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Witek RP, Lu Y, Choi YK, Zheng D, Jorgensen M, et al. Ex vivo transduced liver progenitor cells as a platform for gene therapy in mice. Hepatology. 2004;40:918–924. doi: 10.1002/hep.20404. [DOI] [PubMed] [Google Scholar]
- Hendrie PC., and , Russell DW. Gene targeting with viral vectors. Mol Ther. 2005;12:9–17. doi: 10.1016/j.ymthe.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Miller DG, Wang PR, Petek LM, Hirata RK, Sands MS., and , Russell DW. Gene targeting in vivo by adeno-associated virus vectors. Nat Biotechnol. 2006;24:1022–1026. doi: 10.1038/nbt1231. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF. AAV hits the genomic bull's-eye. Nat Biotechnol. 2006;24:949–950. doi: 10.1038/nbt0806-949. [DOI] [PubMed] [Google Scholar]
- Chamberlain JR, Deyle DR, Schwarze U, Wang P, Hirata RK, Li Y, et al. Gene targeting of mutant COL1A2 alleles in mesenchymal stem cells from individuals with osteogenesis imperfecta. Mol Ther. 2008;16:187–193. doi: 10.1038/sj.mt.6300339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Driskell RR., and , Engelhardt JF. Stem cells in the lung. Methods Enzymol. 2006;419:285–321. doi: 10.1016/S0076-6879(06)19012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Qing K, Kwon HJ, Mah C., and , Srivastava A. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J Virol. 2000;74:992–996. doi: 10.1128/jvi.74.2.992-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Ritchie TC, Duan D., and , Engelhardt JF. Recombinant AAV-mediated gene delivery using dual vector heterodimerization. Methods Enzymol. 2002;346:334–357. doi: 10.1016/s0076-6879(02)46065-x. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U., and , Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zak R, Zhang Y, Ding W, Godwin S, Munson K, et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol. 2004;78:2863–2874. doi: 10.1128/JVI.78.6.2863-2874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yan Z, Luo M., and , Engelhardt JF. Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction. Am J Respir Cell Mol Biol. 2006;34:56–64. doi: 10.1165/rcmb.2005-0189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TR, Afione SA, Conrad C, McGrath SA, Solow R, Oka H, et al. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin PL, Chu S, Conrad C, McVeigh U, Ferguson K, Flotte TR, et al. Alveolar stem cell transduction by an adeno-associated viral vector. Gene Ther. 1995;2:623–631. [PubMed] [Google Scholar]
- Fleurence E, Riviere C, Lacaze-Masmonteil T, Franco-Motoya ML, Waszak P, Bourbon J, et al. Comparative efficacy of intratracheal adeno-associated virus administration to newborn rats. Hum Gene Ther. 2005;16:1298–1306. doi: 10.1089/hum.2005.16.1298. [DOI] [PubMed] [Google Scholar]
- Liu X, Luo M, Guo C, Yan Z, Wang Y., and , Engelhardt JF. Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther. 2007;14:1543–1548. doi: 10.1038/sj.gt.3303014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Driskell RR., and , Engelhardt JF. Airway glandular development and stem cells. Curr Top Dev Biol. 2004;64:33–56. doi: 10.1016/S0070-2153(04)64003-8. [DOI] [PubMed] [Google Scholar]
- Schoch KG, Lori A, Burns KA, Eldred T, Olsen JC., and , Randell SH. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol. 2004;286:L631–L642. doi: 10.1152/ajplung.00112.2003. [DOI] [PubMed] [Google Scholar]
- Duan D, Yue Y, Yan Z, McCray PB., Jr, and , Engelhardt JF. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther. 1998;9:2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, Muramatsu S, et al. Specific and efficient transduction of Cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther. 2005;12:725–733. doi: 10.1016/j.ymthe.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Auricchio A, O'Connor E, Weiner D, Gao GP, Hildinger M, Wang L, et al. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J Clin Invest. 2002;110:499–504. doi: 10.1172/JCI15780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalon Z, Bruckheimer EM, Lustig KH, Rogers LC, Peluso RW., and , Burstein H. Secretion of a TNFR:Fc fusion protein following pulmonary administration of pseudotyped adeno-associated virus vectors. J Virol. 2004;78:12355–12365. doi: 10.1128/JVI.78.22.12355-12365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Zhao W, Wu J, Maina N, Han Z., and , Srivastava A. Adeno-associated virus-mediated gene transfer in hematopoietic stem/ progenitor cells as a therapeutic tool. Curr Gene Ther. 2006;6:683–698. doi: 10.2174/156652306779010660. [DOI] [PubMed] [Google Scholar]
- Chng K, Larsen SR, Zhou S, Wright JF, Martiniello-Wilks R., and , Rasko JE. Specific adeno-associated virus serotypes facilitate efficient gene transfer into human and non-human primate mesenchymal stromal cells. J Gene Med. 2007;9:22–32. doi: 10.1002/jgm.990. [DOI] [PubMed] [Google Scholar]
- Surace EM, Auricchio A, Reich SJ, Rex T, Glover E, Pineles S, et al. Delivery of adeno-associated virus vectors to the fetal retina: impact of viral capsid proteins on retinal neuronal progenitor transduction. J Virol. 2003;77:7957–7963. doi: 10.1128/JVI.77.14.7957-7963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM., and , Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Liu X, Yan Z, Luo M, Zak R, Li Z, Driskell RR, et al. Targeted correction of single-base-pair mutations with adeno-associated virus vectors under nonselective conditions. J Virol. 2004;78:4165–4175. doi: 10.1128/JVI.78.8.4165-4175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118:1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Yan Z, Yi Y, Li Z, Lei D, Rogers CS, et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest. 2008;118:1578–1583. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Lei-Butters DC, Liu X, Zhang Y, Zhang L, Luo M, et al. Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia. J Biol Chem. 2006;281:29684–29692. doi: 10.1074/jbc.M604099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Driskell RR, Luo M, Abbott D, Filali M, Cheng N, et al. Characterization of Lef-1 promoter segments that facilitate inductive developmental expression in skin. J Invest Dermatol. 2004;123:264–274. doi: 10.1111/j.0022-202X.2004.23201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zheng D, Liu Y, Sham MH, Tam P, Farzaneh F, et al. Overexpression of soluble TRAIL induces apoptosis in human lung adenocarcinoma and inhibits growth of tumor xenografts in nude mice. Cancer Res. 2005;65:1687–1692. doi: 10.1158/0008-5472.CAN-04-2749. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR., and , Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Colony forming efficiency data.