Abstract
LL-37 is a human cathelicidin antimicrobial peptide that is released in the skin after injury and acts to defend against infection and modulate the local cellular immune response. We observed in human dermal keloids that fibrosis was inversely related to the expression of cathelicidin and sought to determine how LL-37 influenced expression of types I and III collagen genes in dermal fibroblasts. At nano-molar concentrations, LL-37 inhibited baseline and transforming growth factor-β-induced collagen expression. At these concentrations, LL-37 also induced phosphorylation of extracellular signal-regulated kinase (ERK) within 30 minutes. Activation of ERK, and the activation of a G-protein-dependent pathway, was essential for inhibition of collagen expression as pertussis toxin or an inhibitor of ERK blocked the inhibitory effects of LL-37. c-Jun N-terminal kinase and p38 mitogen-activated protein kinase inhibitors did not alter the effects of cathelicidin. Silencing of the Ets-1 reversed inhibitory effects of LL-37. Taken together, these findings show that LL-37 can directly act on dermal fibroblasts and may have antifibrotic action during the wound repair process.
INTRODUCTION
Antimicrobial peptides are small cationic polypeptides originally discovered for their antimicrobial activity, but recently shown to have many additional functions in immune function (Elsbach, 2003). Cathelicidins have an N-terminal signal peptide, a highly conserved cathelin domain, and variable cationic peptide at the C terminus (Zanetti et al., 1995). The human cathelicidin peptide LL-37 has broad antibacterial activity in vitro and possesses a variety of other biological activities including promoting angiogenesis, wound repair, and chemoattraction of neutrophils, monocytes, and T cells (Gallo et al., 1994; Nizet et al., 2001; Koczulla et al., 2003). The synthesis and deposition of cathelicidins during defense against infection or injury occurs due to its combined production by epithelial cells, neutrophils, mast cells, and other specialized elements of specific to the injured tissue (Di Nardo et al., 2003). Once released, cathelicidin is important in both innate immunity and acquired immunity. In the form of LL-37, this peptide can induce inflammation, epithelial proliferation, and migration, and may have been involved in the response to injury (Gallo et al., 1994; Koczulla et al., 2003). Despite these observations of the significance of LL-37 in the wound repair process, little work has been performed to evaluate the potential influence of this molecule on the extracellular matrix (ECM), in particular collagen synthesis, a critical element of the fibrotic response.
Collagen is the most abundant connective tissue in humans, and collagen fibers provide the tensile strength that allows organs such as the skin to form organized structures. The type I collagen is the most widely distributed and also forms the bulk of skin collagen, accounting for over 80% of dermis. Type III collagen is the second most abundant collagen found in the skin. The triple-helical structure gives collagen many of its unique properties and is essential for normal fibrogenesis. The metabolism of collagen and its regulation are significant to many important diseases that are characterized by excessive accumulation or loss of collagen (Meneghin and Hogaboam, 2007; Wynn, 2007). Excessive accumulation of collagen is known in disorders such as scleroderma, keloids, hypertrophic scars, and also in tumor growth. Outside the skin, pulmonary fibrosis, systemic sclerosis, liver cirrhosis, cardiovascular diseases, and progressive kidney disease are leading causes of morbidity and mortality.
In this study we investigated the expression of cathelicidin in a skin disease associated with fibrosis, keloids, and studied the effect of LL-37 on collagen expression in human dermal fibroblasts (HDF). Our results show that cathelicidin produced during the response to injury may influence the dermal fibrotic response essential for repair and tissue remodeling.
RESULTS
Cathelicidin expression is decreased in keloids
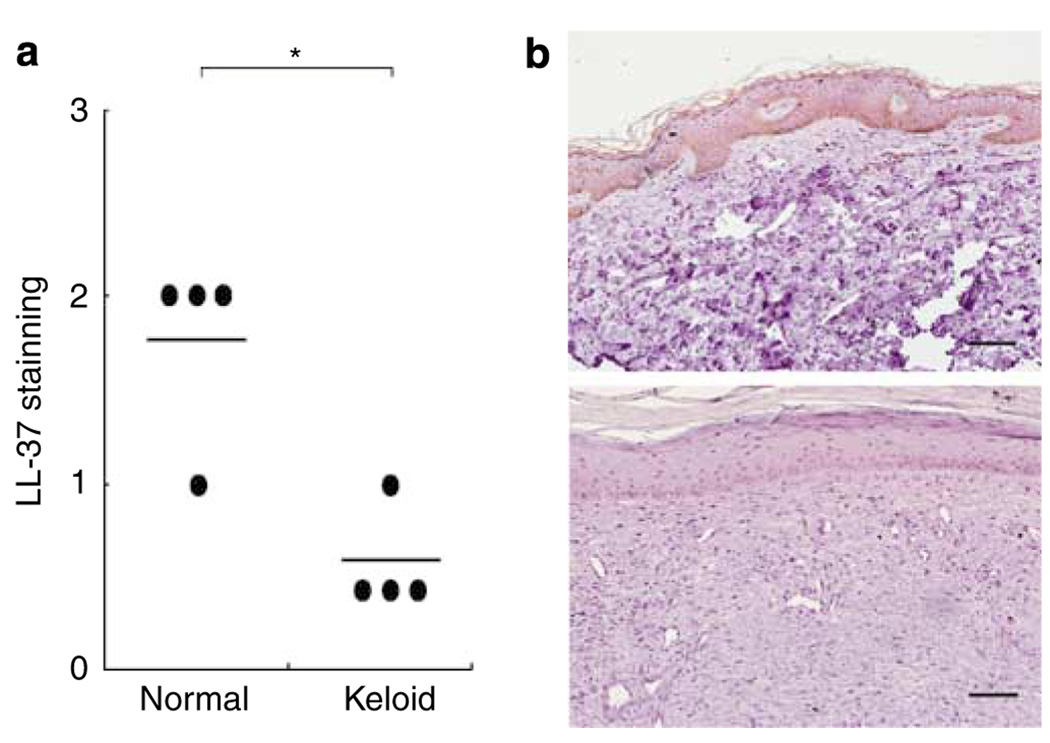
Keloid is a disorder characterized by uncontrolled proliferation of fibrous tissue following injury, and collagen synthesis is markedly increased in keloid lesions. We investigated cathelicidin expression in keloid tissue compared with normal skin by immunostaining with a specific antibody to LL-37 and found that cathelicidin protein expression was lower in the overlying epidermis in keloid patients’ skin than in normal controls (Figure 1). This result suggested that cathelicidin expression was downregulated in keloid patients’ epidermis and that the surrounding cells in this tissue were exposed to a lower level of cathelicidin. The significance of this observation on the production of collagen by the underlying dermal fibroblasts was therefore of interest.
Figure 1. Cathelicidin expression is decreased in the skin of keloid patients.
(a) The intensity of immunostaining with an antibody specific to LL-37 was visually scored on a scale from 0 to 3, with 0 indicating no staining and 3 the most intense staining. *P<0.05 versus normal. (b) Immunostaining for cathelicidin in paraffin-embedded skin sections from normal subjects (upper panel) and patients with keloid. Scale bar: 100 µm.
Effect of LL-37 on collagen production in human dermal fibroblasts
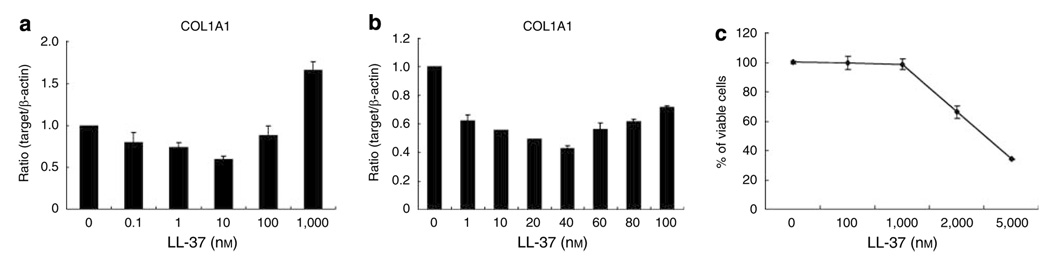
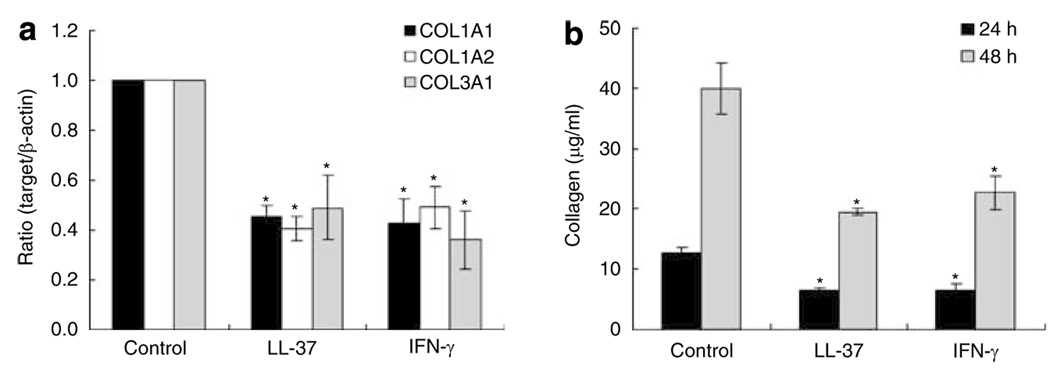
To measure the effect of LL-37 on collagen expression in HDF, cells were treated with various doses of LL-37 for 24 hours and COL1A1 mRNA expression and cell viability were measured. A trend for decreased expression of COL1A1 was seen at the low dose range and increased expression at higher doses that approached the threshold for cell toxicity (Figure 2). Next, experiments were performed with a fixed concentration of LL-37 (40 nm) and the expression of COL1A1, COL1A2, and COL3A1 mRNA were each measured as was collagen protein production as measured by the Sircol collagen assay. COL1A1, COL1A2, and COL3A1 mRNA expression was significantly decreased by LL-37 (Figure 3a). Total soluble collagen was also decreased (Figure 3b) and the degree of inhibition was similar to that seen following exposure to IFN-γ, previously established as an inhibitor of fibroblast collagen synthesis.
Figure 2. Effects of LL-37 on the expression of COL1A1 mRNA in human dermal fibroblasts.
(a, b) HDF cells were treated with indicated concentration of LL-37. After 24 hours, total RNA was extracted, and real-time RT-PCR was performed. Bars indicate mean±SD of three independent experiments performed, each with triplicate samples. (c) Cells were treated with indicated concentration of LL-37 for 24 hours, after which cell viability was measured using an Alama-Blue assay.
Figure 3. Effects of LL-37 on the mRNA expression and production of COL1A1, COL1A2, and COL3A1 in human dermal fibroblasts.
(a) HDF cells were harvested after LL-37 (40 nm) and IFN-γ (1,000 U ml−1) treatment for 24 h. Total RNA was extracted, and cDNA was synthesized for real-time RT-PCR. Bars indicate mean±SD of three independent experiments performed, each with triplicate samples. (b) Cells were cultured in serum-free medium. Protein was determined in supernatants after 48 hours of culture. Total collagen was detected by sircol assay, each performed in triplicate. Data are mean±SD. *P<0.05 versus control.
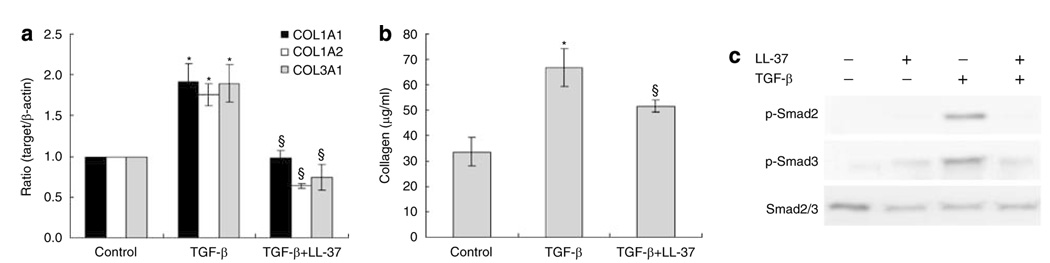
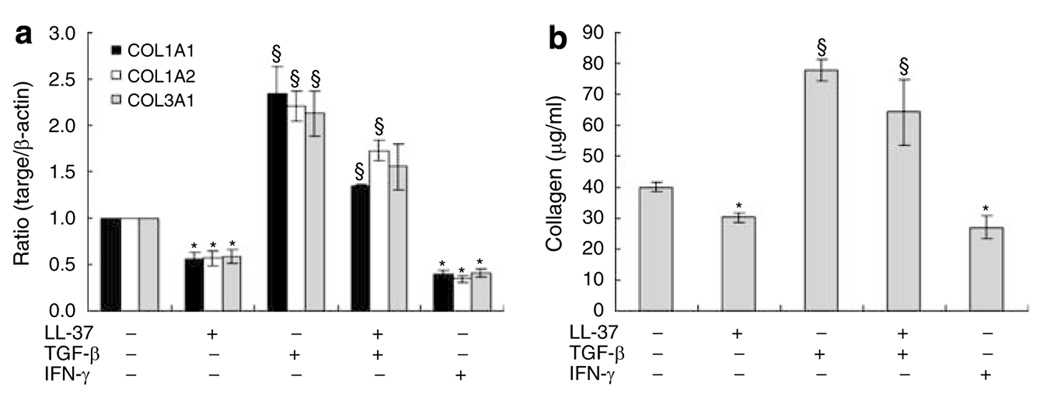
To further evaluate the effects of LL-37 on collagen synthesis, its action on cells stimulated with a pro-fibrotic response was evaluated. Transforming growth factor (TGF)-β is an important participant in the process of fibrosis and pathogenesis of fibrotic disorders by stimulating collagen synthesis in fibroblasts. As expected, treatment of HDF with TGF-β induced collagen gene and protein expression. The presence of low concentrations of LL-37 suppressed TGF-β induced COL1A1, COL1A2, and COL3A1 mRNA expressions and soluble total collagen levels (Figure 4a and b). LL-37 also inhibited TGF-β induction of Smad2 and Smad3 phosphorylation (Figure 4c). To examine the effect on collagen gene transcription, transient cell transfections were performed with the collagen promoter/gene reporter constructs −2,019 COL1A1/Luc. LL-37 had slightly inhibitory effects on the constructs containing −2,019 bp of COL1A1 promoter sequences. Also, LL-37 decreased TGF-β-stimulated COL1A1 promoter activity (Figure S1).
Figure 4. LL-37 inhibited collagen gene expression and production induced by TGF-β.
(a) HDF cells were pretreated with 10 ng ml−1 of TGF-β for 2 hours, before addition of 40 nm of LL-37. After 24 hours, total RNA was extracted, and real-time RT-PCR was performed. Bars indicate mean±SD of three independent experiments performed, each with triplicate samples. (b) Protein was determined in supernatants after 48 hours of culture. Total collagen was detected by sircol assay, each performed in triplicate. Data are mean±SD. *P<0.05 versus control,§P<0.05 versus TGF-β-treated group. (c) LL-37 inhibited TGF-β-induced phosphorylation of Smad. HDF cells were pretreated with TGF-β (10 ng ml−1) for 2 hours, and then challenged with 40 nm LL-37. After 24 hours, whole cell lysates (30 µg per lane) were probed with antibodies against Smad2/3, phospho-Smad2, phospho-Smad3 in Western blots.
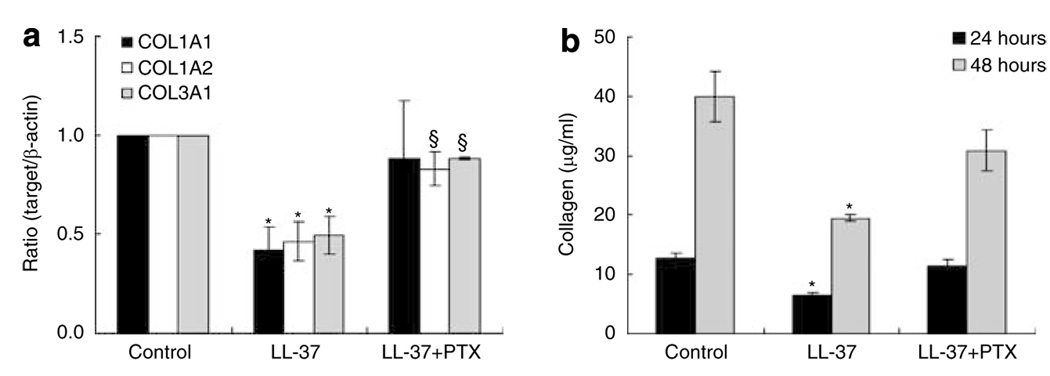
Effect of pertussis toxin on collagen gene expression induced by LL-37
LL-37 is known to act in part through G-protein-coupled receptors (GPCR) to initiate cellular responses including cytokine release and cell migration. To study whether a GPCR is involved in the mediating LL-37 effects on collagen synthesis in HDF, the cells were pretreated with 200 ng ml−1 Pertussis toxin (PTX) before stimulation with 40 nm of LL-37 mRNA levels of COL1A1, COL1A2, and COL3A1 were then analyzed by real-time reverse transcriptase (RT)-PCR. PTX pretreatment reversed inhibitory effects of LL-37 on the production of collagen (Figure 5a and b), showing that GPCR is required for activities of LL-37 on fibroblast.
Figure 5. Effects of pertussis toxin (PTX) on collagen gene expression and production inhibited by LL-37.
HDF cells were pretreated with 200 ng ml−1 of pertussis toxin for 2 hours, before addition of 40 nm LL-37. (a) Total RNA was extracted at 24 hours following LL-37 treatment, and real-time RT-PCR was performed. Bars indicate mean±SD of three independent experiments performed, each with triplicate samples. (b) Protein was determined in supernatants after 48 hours of culture. Total collagen was detected by sircol assay, each performed in triplicate. Data are mean±SD. *P<0.05 versus control, §P<0.05 versus LL-37-treated group.
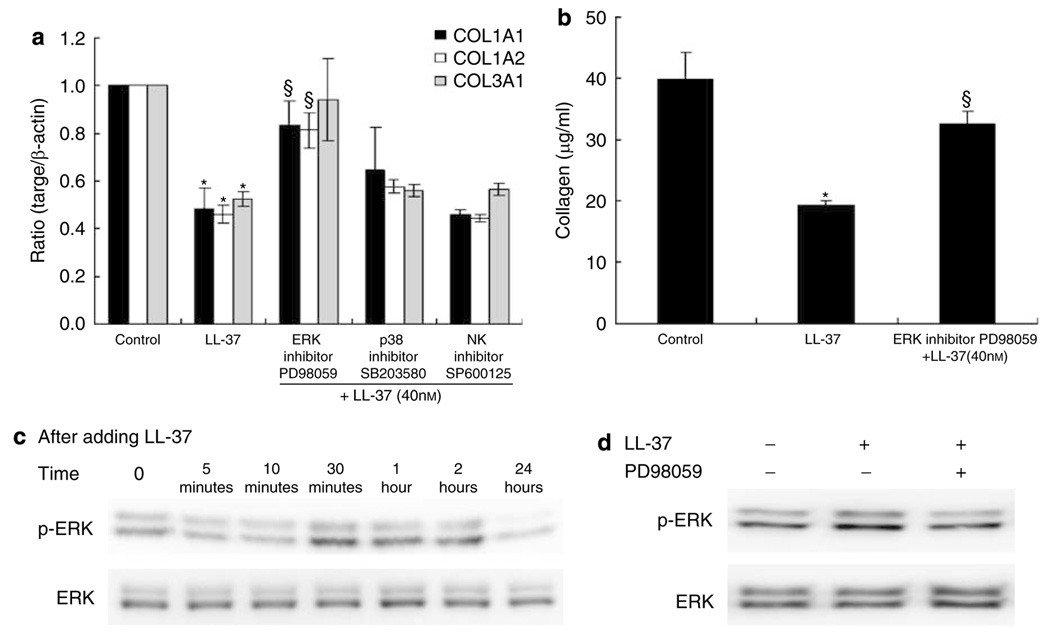
Effect of mitogen-activated protein kinase inhibitor on collagen expression
The mitogen-activated protein kinases (MAPKs) control a wide variety of cellular events including complex cellular programs such as differentiation, proliferation, apoptosis, and processes involved in immune responses. The observation that LL-37 treatment leads to the inhibition of collagen expression prompted us to examine whether MAPK-signaling pathways participate in the regulation of collagen expression. Initial studies were performed to determine the effect of the MAPK inhibitors on mRNA transcription of collagen. HDF were treated with the inhibitors of extracellular signal-regulated kinase (ERK) (PD98059), p38 MAPK (SB203580), and c-Jun N-terminal kinase (JNK) (SP600125) before the addition of 40 nm LL-37. Only PD98059 could reverse the inhibition of LL-37-induced collagen gene and protein production, suggesting that ERK was involved in the signaling pathway in HDF (Figure 6a and b).
Figure 6. LL-37-induced phosphorylation of ERK.
(a) HDF cells were pretreated with 10 µm of ERK inhibitor PD98059, 10 µm of p38 inhibitor SB203580, or 20 µm of JNK inhibitor SP600125 for 1 hours, and then challenged with 40 nm LL-37. After incubations, collagen gene expression was detected by real-time RT-PCR. Bars indicate mean±SD of three independent experiments, each with duplicate samples. (b) Serum-starved cells were treated with LL-37 (40 nm, 48 h) in presence of PD98059 (10 µm). Total collagen was detected by sircol assay, each performed in duplicate. Data are mean±SD. *P<0.05 versus control, §P<0.05 versus LL-37-treated group. (c) Cells were treated with 40 nm of LL-37 for indicated time periods. Phosphorylation of ERK were analyzed by Western blot as described in section “Materials and Methods.” (d) Cells were incubated with PD98059 (10 µm) for 2 hours before addition of LL-37 (40 nm). After 30 minutes cells were harvested, phosphorylation of ERK was analyzed by Western blot.
To directly evaluate the activation of ERK, HDF were treated with LL-37, and the phosphorylation of ERK was analyzed by western blotting using anti-ERK antibodies. LL-37 induced a transient phosphorylation of ERK, and the maximal activation of ERK by LL-37 was observed after 30 minutes (Figure 6c). The ERK inhibitor PD98059 at 10 µm inhibited LL-37-induced phosphorylation of ERK (Figure 6d). Combined, these results indicate that LL-37 downregulates collagen expression by the activation of the ERK pathway, and that this event is dependent on GPCR.
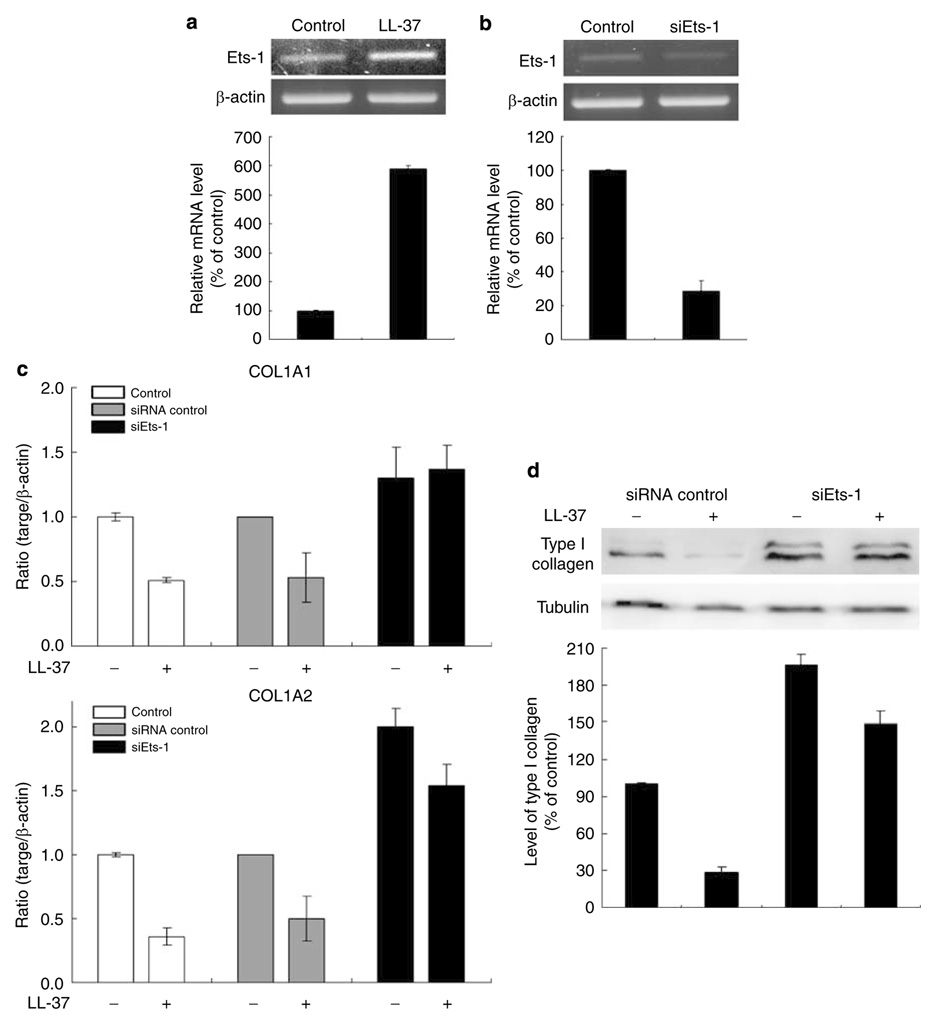
Ets-1 involves in the regulation of collagen expression by LL-37
Recently, the Ets transcription factors have been known to associated with regulation of ECM genes, including type I collagen (Czuwara-Ladykowska et al., 2002; Sherriff-Tadano et al., 2006). We observed Ets-1 involvement in collagen gene expression by LL-37, Ets-1 expression significantly increased in HDF after 24 hours of LL-37 treatment (Figure 7a). Treatment of fibroblasts with Ets-1 small interfering RNA (siRNA) for 24 hours resulted in reduction of Ets-1 mRNA levels by 70% (Figure 7b). In addition, COL1A1 and COL1A2 mRNA levels were examined in HDF treated with Ets-1 siRNA. Silencing of the Ets-1 reversed inhibitory effects of LL-37 on the expression of both COL1A1 and COL1A2 mRNA (Figure 7c). Western blot analysis revealed that type I collagen expression was also reversed by Ets-1 siRNA (Figure 7d). These data suggest that Ets-1 could be involved in negative regulation of type I collagen expression by LL-37.
Figure 7. Effects of Ets-1 on collagen expression inhibited by LL-37.
(a) HDF cells were harvested after LL-37 (40 nm) treatment for 24 h. Total RNA was extracted, and cDNA was synthesized for RT-PCR. (b) Cells were transfected with siEts-1. After 24 hours, total RNA was extracted, and cDNA was synthesized for RT-PCR. The histogram shows Ets-1 mRNA level relative to β-actin by densitometry. Cells were transfected with control siRNA or siEts-1. Cells were harvested after LL-37 treatment for 24 or 48 h. (c) After 24 hours, collagen gene expression was detected by real-time RT-PCR. Bars indicate mean±SD of three independent experiments, each with triplicate samples. (d) After 48 hours cells were harvested, type I collagen production was analyzed by Western blot. The band intensities were quantitated.
Effect of LL-37 on collagen production in human keloid fibroblasts
We also examined whether LL-37 could regulate collagen gene expression in primary human fibroblasts derived from keloids. As shown in Figure 8a, COL1A1, COL1A2, and COL3A1 mRNA expression was decreased by LL-37 in keloid fibroblasts. Total soluble collagen was also decreased (Figure 8b).
Figure 8. LL-37 inhibited collagen gene expression and production induced by TGF-β in primary keloid fibroblasts.
(a) Keloid fibroblast cells were pretreated with or without TGF-β (10 ng ml−1) for 2 hours and incubated in the presence or absence of LL-37 (40 nm) or IFN-γ (1,000 U ml−1) for 24 h. Total RNA was extracted, and cDNA was synthesized for quantitative real-time RT-PCR. Bars indicate mean±SD of three independent experiments performed, each with triplicate samples. (b) Cells were cultured in serum-free medium. Protein was determined in supernatants after 48 hours of culture. Total collagen was detected by sircol assay, each performed in triplicate. Data are mean±SD. *P<0.05 versus control, §P<0.05 versus LL-37-treated group.
DISCUSSION
In the present study, we showed the regulatory effect of LL-37 on production of collagen in HDF. Our results further show that LL-37 inhibits HDF collagen production through GPCR-, ERK-, and Ets-1-dependent pathways. To our knowledge, this is the first report that LL-37 has inhibitory effects on collagen production in fibroblasts. We investigated types I and III collagen because type I collagen accounts for around 80% and type III for most of the other collagens of adult human dermis. LL-37 inhibits COL1A1, COL1A2, and COL3A1 mRNA expression levels similar to that induced by IFN-γ, which is used as antifibrotic treatments in keloid patients. Conversely, TGF-β is crucial in the pathogenesis of fibrosis. In our study, LL-37 also inhibited TGF-β-induced collagen gene expression and soluble collagen production.
TGF-β induces fibrosis through smad or non-smad pathways (Varga and Abraham, 2007). Eight different members of the Smads family have been identified in mammals, and Smad2 and Smad3 are specific mediators in fibrogenesis. LL-37 inhibited TGF-β induction of Smad2 and Smad3 phosphorylation. Non-smad pathways are also important in the regulation of fibrosis. Members of the MAPK family; ERK, p38 MAPK, and JNK, are frequently involved in TGF-β-induced fibrosis. We showed that LL-37 regulates collagen gene expression and soluble collagen production in HDF through ERK pathway.
LL-37 is known to act in part on mammalian cells through binding to formyl peptide receptor-like1, which is one of GPCR. Here we found that the inhibitory effects of LL-37 were reversed after pretreatment of PTX, an inhibitor of GPCR. Therefore, as in other specific cell activation events, the antifibrotic effects of LL-37 on HDF are also regulated through GPCR.
Recent evidence suggests that Ets factor is associated with regulation of ECM genes (Czuwara-Ladykowska et al., 2002; Mizui et al., 2006; Sherriff-Tadano et al., 2006). Mizui et al. demonstrated that overexpression of Ets-1 prevented the TGF-β induction of type I collagen production in cultured mesangial cells. Knockdown of Ets-1 in glomeruli resulted in severe ECM deposition and diseased glomeruli (Mizui et al., 2006). Furthermore, Ets-1 strongly suppressed TGF-β induction of type I collagen and other matrix-related genes (Czuwara-Ladykowska et al., 2002). In the present study, treatment with Ets-1 siRNA blocked the downregulation of type I collagen expression by LL-37. Thus, these results have suggested that Ets-1 mediates the regulation of type I collagen expression inhibited by LL-37.
Böhm et al. showed that one of antimicrobial peptide, α-MSH, has antifibrotic effects in HDF, although α-MSH only suppresses collagen protein levels and not mRNA levels (Böhm and Luger, 2004; Böhm et al., 2004). There are also a few reports about profibrotic effects of α-defensin, which is expressed in human neutrophils, and is also referred to as human neutrophil peptide (Chavakis et al., 2004; Li et al., 2006). Oono et al. showed that synthetic HNP-1 enhanced the expression of pro-a1(I) collagen in cultured HDF (Oono et al., 2002). Yoshioka et al. also demonstrated that α-defensin enhances collagen expression in human lung fibroblasts (Yoshioka et al., 2007), but there has been no report about LL-37.
Fibrotic skin diseases are clinically characterized by thickening of skin due to accumulation of ECM, mainly collagen. Keloid is manifested by localized lesions of considerable cosmetic concern and is associated with significant morbidity in terms of inflammation, infections, pruritus, and pigmentary alterations (Urioste et al., 1999). The primary cause of keloids is currently unknown, but their development is clearly associated with injury to the skin and can be predicted by genetic predisposition of susceptible individuals (Urioste et al., 1999). We observed that LL-37 expression is lower in keloid patients compared with normal control. LL-37 expression is also decreased in skin disease such as atopic dermatitis, which contributes to increased infection in atopic dermatitis patients (Ong et al., 2002). Although LL-37 expression levels have not been known in fibrotic diseases such as keloids, our results could suggest that LL-37 is downregulated in keloid patients, resulting in diminished antifibrotic signaling and excessive collagen production.
In conclusion, we demonstrate that LL-37 inhibits collagen expression in fibroblasts and is associated and dependent on phosphorylation of ERK. These findings may provide insight into immune interactions between LL-37 and the mechanism of fibrosis. Because LL-37 is involved in multiple functions of immune regulation, the ability of LL-37 to inhibit the production of collagen by fibroblasts provides an additional mechanism for the involvement of LL-37 in innate and adaptive immunity and its role in the pathogenesis of skin disorders.
MATERIALS AND METHODS
Cell culture
HDF from neonatal foreskin were obtained from the American Type Culture Collection (CRL-2097, Manassas, VA). The cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco-BRL, Gaithersburg, MD) supplemented with antibiotics (100 U ml−1 penicillin G and 100 µg ml−1 streptomycin) and 10% heat-inactivated fetal bovine serum (Gibco-BRL). This cell line was used for experiments while in their log phase of growth. The cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C and were used at passages 4–11. Keloid fibroblasts were harvested from two volunteer patients. The ethical committee of the Catholic University of Korea approved the study and all patients provided written, informed consent. The study was designed and conducted in accordance with the Declaration of Helsinki. Keloid tissue was washed repeatedly with phosphate-buffered saline (PBS), and minced into 0.5–1 µm pieces. Then the dermal tissue pieces were incubated in a 0.25% collagenase type I solution (Sigma, St Louis, MO) at 37°C for 4 h. Keloid fibroblasts were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 1% antibiotic-antimycotic (100 U ml−1 penicillin G, 100 µg ml−1 streptomycin, and 0.25 µg ml−1 amphotericin B) (Gibco-BRL) at 37°C in a humidified (5% CO2) incubator. Fibroblast cells were subcultured at 80–90% confluence using PBS containing 0.025% trypsin-EDTA. Only cells of passages 3–7 were analyzed in this study.
Cell viability assay
Viability of cells was determined indirectly using Alama-Blue™ (Serotec, UK). Briefly, after the exposure of cells to LL-37 (Phoenix Pharmaceuticals, Belmont, CA), each well (3 × 104 cells per well) was added with 50 µg Alama-Blue solution followed by incubation for 3 hours in the incubator at 37°C. Finally, absorbance was measured at a wavelength of 570 nm, and background absorbance at 600 nm was subtracted.
Cell treatment with inhibitors
Cells were seeded at a density of 5 × 105 cells per 100-mm dish. After 48 hours, the cells were washed with a serum-free medium and then placed in a media without fetal bovine serum for at least 16 hours before experiments. In some experiments, cells were pretreated with 10 µm SB203580 (Sigma), a p38 MAPK inhibitor; 10 µm PD98059 (Sigma), an ERK inhibitor; 20 µm SP600125, a JNK inhibitor; or 200 ng ml−1 PTX (Sigma). We added human LL-37 (40 nm), IFN-γ (1,000 U ml−1), or TGF-β (10 ng ml−1) to the cells for 24 h.
RT-PCR
After treatment, total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA). Two micrograms of total RNA was used for first strand cDNA synthesis using moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI), according to the manufacturer’s instructions. Ets-1 primers were: forward, 5′-ACGATAGTTGTGATCGCCTCA-3′; reverse, 5′-CCATAGCTGGATTGGTCCACT-3′. The PCR amplification consisted of 32 cycles of 94°C for 30 seconds; 58°C for 30 seconds; and 72°C for 1 minute. Human β-actin primers were: forward, 5′-TCACCCACACTGTGCCCATCTACG-3′; reverse, 5′-CAGCGGAACCGCTCATTGCCAATG-3′. The PCR amplification consisted of 30 cycles of 94°C for 30 seconds; 56°C for 30 seconds; and 72°C for 1 minute. 2 × PCR Master mix Solution (i-Taq, iNtRON Biotechnology, Korea) was used to perform RT-PCR reactions. The PCR products subjected to electrophoresis on 1.5% agarose gel were visualized with ethidium bromide.
Quantitative real-time PCR
Total RNA and the cDNAs were generated as described above. Real-time PCR was performed with a Rotor gene 3,000 instrument (Corbett Life Science, Sidney, AU) using SYBR Premix Ex Taq (Takara, Shiga, Japan). Reaction mixtures were incubated for 40 cycles at 95°C for 15 seconds; 58°C for 45 seconds; and 72°C for 20 seconds. The relative amounts of gene expression were calculated by using the expression of β-actin as an internal standard. Primers used for PCR reactions are shown in Table 1.
Table 1.
Primer sequences used in this study
| Name | Forward sequence | Reverse sequence |
|---|---|---|
| COL1A1 | 5′-GGGCAAGACAGTGATTGAATA-3′ | 5′-ACGTCGAAGCCGAATTCCT-3′ |
| COL1A2 | 5′-TCTGGATGGATTGAAGGGACA-3′ | 5′-CCAACACGTCCTCTCTCACC-3′ |
| COL3A1 | 5′-AGGTCCTGCGGGTAACACT-3′ | 5′-ACTTTCACCCTTGACACCCTG-3′ |
| β-actin | 5′-TCACCCACACTGTGCCCATCTACG-3′ | 5′-CAGCGGAACCGCTCATTGCCAATG-3′ |
RNAi and transfection
HDF was transfected with either 40 pmol control siRNA (SantaCruz, CA) or Ets-1 siRNA (SantaCruz) using the Nucleofector apparatus (AMAXA, Köln, Germany), as described by the manufacturer. After 16 hours incubation in serum-free Dulbecco’s modified Eagle’s medium, cells were incubated in the presence or absence of 40 nm LL-37. Twenty-four hours later, cells were harvested to total RNAs to examine the effect on collagen expression. After 48 hours, cell extracts were subjected to western blot analysis with anti-type I collagen (Southern Biotechnology, Birmingham, AL) and γ-tubulin (SantaCruz) antibodies.
Measurement of total collagens
Sircol soluble collagen assay (Biocolor Ltd., Newtownabbey, UK) was used to quantify total soluble collagens. Briefly, collected supernatant was centrifuged at 1,500 r.p.m. for 5 minutes to drop ECM, followed by mixing 100 µl supernatant with 1 ml of Sircol dye for 30 minutes and centrifuging at 10,000 r.p.m. for 10 minutes to drop formed collagen-dye complex. After removing suspension, the pellets were dissolved in 1 ml Sircol alkali reagent and vortexed. Relative absorbance was measured at 540 nm.
Western blot analysis
Whole cell lysates were prepared by extracting proteins using a buffer containing 50 mm Tris-HCl (pH 7.5), 1 mm EDTA, 1 mm EGTA, 150 mm NaCl, 1% Nonidet P-40, 0.1% SDS, 1 mm Na3VO4, 1 µg ml−1 leupeptin, and 1 mm freshly added phenylmethylsulfornyl fluoride. The protein concentration was measured using the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories). Proteins of 30 µg of whole cell lysate were directly separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked for 1 hour in PBS containing 0.1% (v/v) Tween 20 and 5% (w/v) non-fat milk proteins. Blocked membranes were then incubated with primary antibodies (anti-phospho-Smad2, anti-phospho-Smad3, anti-Smad2/3, anti-phospho-ERK, or anti-ERK rabbit polyclonal antibodies at the final concentration of 1:1000, Cell Signaling, Beverly, MA) overnight at 4°C, washed three times (15 minutes each) with PBS containing 0.1% Tween 20, and incubated with a horseradish peroxidase-conjugated anti-goat, or anti-rabbit secondary antibody (1:2,000) for 1 hour at room temperature. The bands were visualized by an enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ). The band intensities were quantified using the Bio-Rad imaging system, and the quantity of the phosphorylated proteins was expressed as ratio of the phosphorylated over total protein in each case.
Immunohistochemistry
Five-micrometer-thick formalin-fixed, paraffin embedded biopsy sections were obtained from five keloid patient samples and from six control specimens. The study was approved by the ethical committee of the Catholic University of Korea and all patients filed an informed consent. We detected human LL-37 expression in tissue sections using anti-cathelicidin LL-37 primary antibody or preimmune serum as described previously (Dorschner et al., 2001). The paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated in a graded series of alcohol. The antibody was diluted in PBS plus 0.1% Triton X-100 at a ratio of 1:300, and was incubated in a wet chamber overnight at 4°C. The antigen was detected using the streptavidin-biotin-peroxidase detection system (Cap-plus Detection Kit, Zymed Laboratories Inc., San Francisco, CA) with 3-amino-9-ethylcarbazole as chromogen and Mayer haematoxylin as the counterstain. Normal rabbit IgG (Zymed Laboratories Inc.) was used for negative control. The degree of expression was graded as follows: 3 for strongly positive, 2 for moderately positive, 1 for weakly positive, and 0 for negative.
Statistical analysis
The statistical significance was estimated using Student’s t-test. Mean differences were considered to be significant when P<0.05. The results are shown as the mean±SD.
Supplementary Material
Figure S1. Effects of LL-37 on the activity of COL1A1 promoter constructs in HDF cells.
ACKNOWLEDGMENTS
This work was supported by the SRC/ERC program of MOST/KOSEF (No. R11-2005-017) and the Korea Research Foundation Grant funded by Korea Government (MOEHRD, Basic Research Promotion Fund, KRF-2007-531-E00045) to H.J.P and a V.A. Merit award and NIH grants AI052453, and AR45676 to R.L.G. We also thank Dr Kyung Moon Kim, Hei Sung Kim, and Seok Bean Song for help in preparation of the final paper.
Abbreviations
- GPCR
G-protein-coupled receptors
- HDFs
human dermal fibroblasts
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Böhm M, Luger TA. Melanocortins in fibroblast biology-current update and future perspective for dermatology. Exp Dermatol. 2004;13 Suppl 4:16–21. doi: 10.1111/j.1600-0625.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Böhm M, Raghunath M, Sunderkotter C, Schiller M, Stander S, Brzoska T, et al. Collagen metabolism is a novel target of the neuro-peptide alpha-melanocyte-stimulating hormone. J Biol Chem. 2004;279:6959–6966. doi: 10.1074/jbc.M312549200. [DOI] [PubMed] [Google Scholar]
- Chavakis T, Cines DB, Rhee JS, Liang OD, Schubert U, Hammes HP, et al. Regulation of neovascularization by human neutrophil peptides (alpha-defensins): a link between inflammation and angiogenesis. FASEB J. 2004;18:1306–1308. doi: 10.1096/fj.03-1009fje. [DOI] [PubMed] [Google Scholar]
- Czuwara-Ladykowska J, Sementchenko VI, Watson DK, Trojanowska M. Ets1 is an effector of the transforming growth factor β (TGF-β) signaling pathway and an antagonist of the profibrotic effects of TGF-β. J Biol Chem. 2002;277:20399–20408. doi: 10.1074/jbc.M200206200. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Vitiello A, Gallo RL. Mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin antimicrobial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- Elsbach P. What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J Clin Invest. 2003;111:1643–1645. doi: 10.1172/JCI18761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, et al. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci USA. 1994;91:11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczulla R, Degenfeld VG, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/Hcap-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Raghunath M, Tan D, Lareu RR, Chen Z, Beuerman RW. Defensins HNP1 and HBD2 stimulation of wound-associated responses in human conjunctival fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:3811–3819. doi: 10.1167/iovs.05-1360. [DOI] [PubMed] [Google Scholar]
- Meneghin A, Hogaboam CM. Infectious disease, the innate immune response, and fibrosis. J Clin Invest. 2007;117:530–538. doi: 10.1172/JCI30595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizui M, Isaka Y, Takabatake Y, Sato Y, Kawachi H, Shimizu F, et al. Transcription factor Ets-1 is essential for mesangial matrix remodeling. Kidney Int. 2006;70:298–305. doi: 10.1038/sj.ki.5001541. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Oono T, Shirafuji Y, Huh WK, Akiyama H, Iwatsuki K. Effects of human neutrophil peptide-1 on the expression of interstitial collagenase and type I collagen in human dermal fibroblasts. Arch Dermatol Res. 2002;294:185–189. doi: 10.1007/s00403-002-0310-6. [DOI] [PubMed] [Google Scholar]
- Sherriff-Tadano R, Ohta A, Morito F, Mitamura M, Haruta Y, Koarada S, et al. Antifibrotic effects of hepatocyte growth factor on scleroderma fibroblasts and analysis of its mechanism. Mod Rheumatol. 2006;16:364–371. doi: 10.1007/s10165-006-0525-z. [DOI] [PubMed] [Google Scholar]
- Urioste SS, Arndt KA, Dover JS. Keloids and hypertrophic scars: review and treatment strategies. Semin Cutan Med Surg. 1999;18:159–171. doi: 10.1016/s1085-5629(99)80040-6. [DOI] [PubMed] [Google Scholar]
- Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka S, Mukae H, Ishii H, Kakugawa T, Ishimoto H, Sakamoto N, et al. Alpha-defensin enhances expression of HSP47 and collagen-1 in human lung fibroblasts. Life Sci. 2007;80:1839–1845. doi: 10.1016/j.lfs.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effects of LL-37 on the activity of COL1A1 promoter constructs in HDF cells.