Abstract
Context
Characteristics and factors influencing viral decay under TDF and ADV need to be determined in HIV-HBV co-infected patients.
Patients and methods
This non-randomized, open label study compared the HBV DNA decay in 85 HIV-HBV co-infected patients initiating a combined antiretroviral regimen either including TDF or associated with ADV. The first 6 month-change in viral load was analyzed using mixed-linear models. The adjusted hazards rate ratio, comparing the rates of undetectable HBV-DNA between treatments, was calculated using Cox proportional hazard model.
Results
The HBV-DNA decay, adjusted for baseline HBV viral load, was more pronounced in patients treated with TDF than with ADV (−66% versus −53% at 12 months, p=0.0001). Patients in the TDF group presented a steeper slope of decline, estimated at 1.1 (95% CI: 0.9, 1.3) compared to 0.8 (95% CI: 0.6, 1.0) in the ADV group (p=0.036). The mean time to HBV-DNA undetectability was 19.3 months (95% CI: 16.7 – 22.0) with TDF and 25.9 months (95% CI: 21.1 – 30.7) with ADV. When adjusted for HBe Ag, HBV-DNA and ALT levels at baseline, the influence of treatment on time to HBV-DNA undetectability remained in favor of TDF versus ADV (HR = 2.79; 95 % CI: 1.05–7.40, p=0.039)
Conclusion
The early-phase HBV-DNA decay is more strongly influenced by TDF than by ADV. This is associated with a sustained antiviral activity in the TDF group, in which patients reach the threshold of HBV undetectability at a faster rate and at a larger proportion than those taking ADV.
Keywords: Adenine; analogs & derivatives; pharmacology; therapeutic use; Adult; Antiviral Agents; pharmacology; therapeutic use; DNA, Viral; blood; Drug Therapy, Combination; Female; HIV Infections; complications; drug therapy; virology; Hepatitis B; complications; drug therapy; virology; Hepatitis B virus; drug effects; genetics; Humans; Male; Middle Aged; Phosphonic Acids; pharmacology; therapeutic use; Proportional Hazards Models; Treatment Outcome; Viral Load
Keywords: chronic hepatitis B, HIV infection, modelling, tenofovir, adefovir
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a leading cause of liver disease, affecting almost 400 million people worldwide and accounting for 80% of hepatocellular carcinoma cases [1]. The prevalence of chronic HBV is high among HIV-infected patients (from 5 to 10 % in the Western world to 20 – 30% in Africa and Asia) because both viruses share similar modes of transmission and immunodeficiency has been shown to be a risk-factor for chronic HBV infection [2], [3]. Chronic liver disease significantly contributes to morbidity and mortality among such patients and complicates their therapeutic management. Interferon alpha (IFN) and Pegylated IFN have been efficiently used in HBV-monoinfected patients [4], [5], [6]. However, the use of the former treatment has been questioned by its side effects and a low response-rate among HIV-infected patients, while the latter, although expressing a higher efficacy and tolerance profile, has not yet been evaluated in HIV-HBV co-infected individuals. Significant progress has been recently made by the development of nucleoside or nucleotide analogs of reverse transcriptase that are potent inhibitors of HBV replication. The antiretroviral agent Lamivudine (LAM) was the first nucleoside analog licensed against HBV, but its long-term use has been precluded by the rapid appearance of resistance mutations in the pol gene of HBV, especially in HIV-coinfected patients [7]. Adefovir divipoxil (ADV) is a nucleotide analog more recently licensed for the treatment of HBV, which is active against both wild type and LAM resistant strains of HBV [8], [9]. Tenofovir disoproxil fumarate (TDF), a new nucleotide analogue licensed in 2001 for HIV infection [10], also exhibits an efficacious activity against wild type and LAM-resistant HBV, both in vitro [11] and in vivo [12], [13]. This dual activity against HBV and HIV may provide a more optimal treatment to be included in combined antiretroviral regimens (cART) of HIV-HBV coinfected patients, compared to ADV, which only displays an anti-HBV activity at the dose used to treat chronic HBV infection. Moreover, two recent studies, one non-randomized [14] and one randomized, double-blinded, placebo-controlled trial [15], have suggested that the antiviral activity of TDF may not be inferior to that of ADV. However, the characteristics of HBV dynamics under TDF and ADV have not been established and the factors influencing viral decay need to be determined. In the context of HIV-HBV co-infection, mathematical modeling of dynamics of HBV decay under therapy may bring useful insights on the biology of HBV as well as on the potency of the evaluated therapy and the parameters that might impact its efficacy [13].
We present here a non-randomized, open label study comparing the HBV DNA dynamics in 85 HIV-HBV co-infected patients initiating a combined antiretroviral regimen either including TDF or associated with ADV.
PATIENTS AND METHODS
From May 2002 to May 2003, 308 co-infected patients were enrolled in a French multi-centre prospective cohort to analyze the determinants of liver fibrosis progression during HIV-HBV co-infection [16]. A subset of 85 patients, naïve or pre-treated, who initiated a cART containing tenofovir 300mg daily (n=56), or adefovir 10mg daily (n=29) (both concomitantly with or without lamivudine), was included in the present study. Inclusion criteria were as follows: positive HIV enzyme-linked immunosorbent assay (ELISA) confirmed by a complete Western Blot pattern, HBs antigen seropositivity confirmed by two positive concordant serologies 6 months prior to and at inclusion, available pre-TDF and pre-ADV serum, and a baseline HBV DNA load of at least 1000 copies per mL. All but two patients, treated with ADV as a single drug, started or were already under cART at inclusion. Informed patient consent was obtained from all patients and the study was approved by the Pitié-Salpêtrière hospital ethics committee (Paris, France).
HBV DNA was measured within 10 months prior to inclusion and every 3 months thereafter using a commercial quantitative polymerase chain reaction (PCR) assay (PCR-Amplicor; Roche Diagnostic Systems Meylan, France; detection threshold 200 copies/mL). The DNA chip technology was used to detect HBV DNA polymerase gene mutations and for genotyping [17].
At each HBV DNA assay, transaminases and immunovirological status were determined prospectively: HIV viral load was measured with the branched-DNA technique (b-DNA Quantiplex 3.0 Bayer Diagnostics, Cergy Pontoise, France, detection limit 50 copies/mL), CD4+ and CD8+ cells were counted by flow cytometry, and levels of alanine amino transferase (ALT) and aspartate amino transferase (AST) were measured using standard methods. Markers of hepatitis C virus and hepatitis delta virus were regularly tested during follow up by means of commercial ELISA techniques as recommended by the manufacturers. Demographic characteristics (including evaluation of alcohol consumption) were collected at inclusion, as well as the history of HIV and HBV infections (date of first positive serology, routes of contamination, history of AIDS-defining events). Liver biopsies were performed between 2002 and 2006 according peer-reviewed guidelines determined in 2006 [18]. Liver fibrosis and activity were scored by means of METAVIR (F0 no portal fibrosis, F1 portal fibrosis without septa, F2 portal fibrosis with few septa, F3 portal fibrosis with many septa but no cirrhosis, F4 cirrhosis; A0 no activity, A1 mild activity, A2 moderate activity, A3 severe activity respectively) [19].
Demographic, HIV and HBV infection, and baseline and 12-month biological characteristics were summarized using means (SD) or medians (IQR) for continuous variables and counts (frequencies) for categorical variables. The co-treatment associated with either ADV or TDF was described at inclusion by the following ARV classes: protease inhibitor (PI), nucleoside reverse trancriptase inihibitor (NRTI), nucleoside reverse trancriptase inihibitor (NNRTI). Comparisons were made between treatment groups using Student’s t-test, Fisher’s Exact Test, Pearson Χ2, or Kruskall-Wallis test, as appropriate. Differences between baseline and 12-months were compared within treatment groups using Fisher’s Exact Test, Paired t-test, or Wilcoxon signed-rank test, and between treatment groups using the percent change from baseline for each group and Student’s t-test. Using the time to HBV-DNA undetectability (<200 copies/mL) as the virological endpoint, treatments were compared by Kaplan-Meier curves. The hazards rate ratio, comparing the rates of undetectable HBV-DNA between treatments, was calculated using Cox proportional hazard model, and subsequently adjusted for the following covariates: HBeAg positivity, log HBV DNA and ALT levels at baseline. The proportional hazards-assumption was assessed by testing the interaction term between the time-dependent follow-up and treatment status using a Wald Χ2 Test. Using mixed-linear models, we focused on the first 6 months after treatment initiation in order to analyze the early decline of the biphasic viral decay. We used a simple model implementing a linear treatment-dependent decrease in log10 viral load (VL) throughout time. More specifically, the model for the log viral load in treatment group i, individual j and at time tjk was as follows:
where αi and βi are the treatment-dependent, population-averaged intercept and slope of viral load decrease, aj, bj are random subject-dependent intercept and slope, with joint binormal distribution, and εijk is the error term. In order to examine the influence of baseline HBV-DNA and ALT/AST levels on HBV dynamics, an interaction term including time, slope of viral decline, and respective baseline parameters was added. We also took into account censoring due to viral load below the detection threshold. A global test was performed to determining the difference in intercept and slope due to treatment. Analyses were carried out using the following software: SAS (version 9.1), STATA (version 9.2) and R (version 1.9.1). All statistical significances were determined by a p-value of less than 0.05.
RESULTS
Characteristics of the study population
Data on demographics and virological data are summarized in Table 1. The study population was predominantly male (sex ratio: 27), pretreated (97.7%, median number of molecules = 6, IQR 4), with a mean age of 42.0 years (SD 7.3) and a mean estimated duration of HIV infection of 10.7 years (SD 4.8). Immunodeficiency was mild (mean CD4 T-cell count before ADV or TDF treatment = 465/mm3, SD 265) and HIV replication was controlled (viral load below 50 copies/mL) in 39 (45.9%) patients. Drugs associated with either TDF or ADV at inclusion were as follows: 2 NRTIs, n=6; 1 NRTI + 1 NNRTI, n=9; 1 NRTI + 1 PI, n=31; 1 NRTI + 1 NNRTI + 1 PI, n=37. Two patients with no indication for HIV treatment were treated with ADV as a single drug. The characteristics of HBV infection were similar in both groups, with a mean estimated duration of HBV infection of 7.9 years (SD 4.8) and more than 80% of the patients having a history of treatment with lamivudine (median duration 58 months, range 0 – 116). The mean baseline HBV viral load differed between treatment groups (p = 0.02), and the rates of pre-core and other mutations conferring resistance to Lamivudine were balanced between groups. However, in patients with a histologic assessment of liver fibrosis and necro-inflammatory activity (n=59), advanced fibrosis and cirrhosis were more prevalent in the ADV group (56.5% v. 33.3% in the TDF group, p=0.02), whereas activity was equally distributed between groups (A2–A3 = 47.6% in the ADV group v. 38.9% in the TDF group).
Table 1.
Description of the study population according to treatment group
| Adefovir (n = 29) | Tenofovir (n = 56) | p | |
|---|---|---|---|
| Demographics | |||
| Sex Male/Female (% Males) | 28/1 (96.6) | 54/2 (96.4) | ns |
| Age, years (median, SD) | 41.5 (7.7) | 42.3 (7.2) | ns |
| Country of origin HBV prevalence > 2% (n, %) | 5 (17.2) | 9 (16.1) | ns |
|
Characteristics of HIV infection | |||
| Duration of HIV Infection, years (mean, SD) | 10.7 (5.3) | 10.7 (4.5) | ns |
| History of an AIDS-defining event (n, %) | 5 (17.2) | 9 (16.1) | ns |
| Nb (%) of patients treated with ARV before inclusion patients | 26 (89.7) | 53 (94.6) | ns |
| Other ARV treatments associated at baseline | ns | ||
| 2 NRTI (n, %) | 2 (6.9) | 4 (7.1) | |
| 1 NRTI + 1 NNRTI (n, %) | 3 (10.3) | 6 (10.7) | |
| 1 NRTI + 1 PI (n, %) | 13 (44.8) | 18 (32.1) | |
| 1 NRTI + 1 NNRTI + 1 PI (n, %) | 9 (31.0) | 28 (50.0) | |
| CD4 T cell count, per mm3 (mean, SD) | 482 (298) | 457 (250) | ns |
| Rate of HIV-RNA above detection limit (n, %) | 14 (53.9) | 29 (51.8) | ns |
| HIV-RNA, log10 copies/mL (median, IQR) | 1.88 (1.70–3.81) | 1.84 (1.70–4.14) | ns |
|
Characteristics of HBV infection | |||
| Duration of HBV Infection years (mean, SD) | 8.3 (4.6) | 7.7 (5.0) | ns |
| Presence of serum HBe Ag (n, %) | 25 (86.2) | 50 (89.3) | ns |
| Presence of serum antiHBe Ab (n, %) | 4 (15.4) | 6 (10.9) | ns |
| HBV-DNA, log10 copies/mL (median, IQR) | 8.30 (6.56 – 9.10) | 7.30 (6.00 – 8.30) | ns |
| HCV co-infection (n, %) | 3 (10.3) | 4 (7.1) | ns |
| HDV co-infection (n, %) | 2 (6.9) | 2 (3.6) | ns |
| History of treatment with lamivudine (n, %) | 26 (89.7) | 50 (89.3) | ns |
| Duration of treatment with lamivudine, months(mean, SD) | 69.6 (27.8) | 63.8 (28.1) | ns |
| Co-treatment with lamivudine during study (n, %) | 19 (65.5) | 44 (78.6) | ns |
| History of treatment with Interferon or PegInterferon (n, %) | 5 (17.3) | 4 (7.1) | ns |
| Fibrosis METAVIR score F0–F1 v. F2 v. F3–F4 (n, %) | 2 (8.7) – 8 (34.8) – 13 (56.5) | 14 (38.9) – 10 (27.8) – 12 (33.3) | 0.02 |
| Activity METAVIR score A0–A1 v. A2 v. A3 (n, %) | 22 (52.2) – 9 (39.1) – 2 (8.7) | 22 (61.1) – 11 (30.6) – 3 (8.3) | ns |
| YMDD mutants, n=75 (n, %) | 21 (91.3) | 39 (75.0) | ns |
| Pre Core mutants (W28 mutation), n=65 (n, %) | 3 (30.0) | 9 (16.4) | ns |
| ALT IU/mL (mean, SD) | 134 (150) | 90 (93) | ns |
Continuous variables compared using two-tailed t-test; categorical variables compared using Pearson’s chi-square statistics (with continuity-corrected chi-square statistics where appropriate.
12-month changes in biological parameters
The figure 1 displays the smooth estimates of mean HBV viral load at each time point according to treatment, and table 2 summarizes virological and biochemical changes between baseline and at 12-months. The HBV-DNA decay was more pronounced in patients treated with TDF than with ADV (−66% versus −53% at 12 months, p=0.0001), even after adjustment for baseline HBV viral load (p < 0.001). A higher number of patients had a substantially consistent profile of HBV-DNA levels in the ADV group compared to that in the TDF group: 7 (24.1%) and 4 (7.1%) patients in the respective groups presented a change of less than −2 log10 copies/mL over 12 months (p=0.03). Undetectability (HBV-DNA < 200 copies/mL) was reached by 37/56 (66%) and 8/29 (28%) patients treated with TDF and ADV, respectively (p=0.04). Among patients with detectable HBe antigen at baseline, a similar and significant rate of HBe Ag loss was found (p=0.02) in both groups [4/21 (19%) in ADV and 8/47 (17.0 % in TDF treated patients]. Seroconversion to antiHBe antibodies was noted in 2 (3.9%) and 1 (4.6%) patients treated with TDF or ADV, respectively (p=0.5), whereas no seroconversion for anti-HBs antibodies was observed.
Figure 1. Time course of HBV-DNA during the first 12 months of treatment with TDF (upper line) or ADV (lower line).
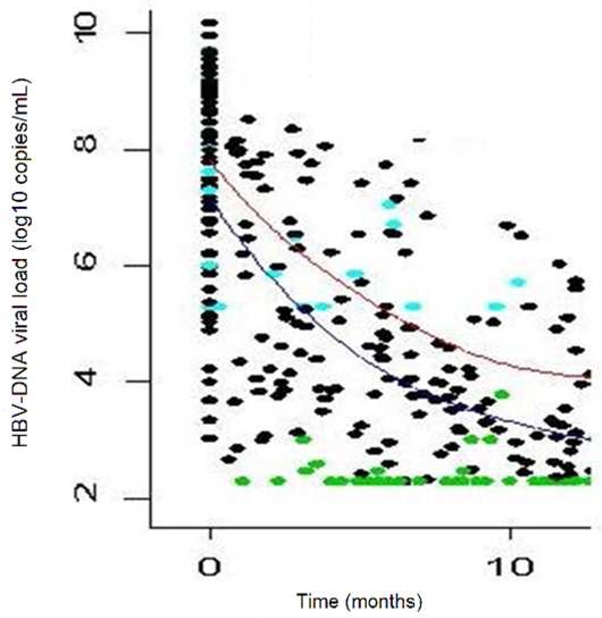
Plain lines are the smooth estimates of the mean HBV load at each time point according to treatment. Undetectable viral loads are shown as green spots and thresholds as blue spots.
Table 2.
12 month evolution of biochemical and virological parameters according to treatment groups
| ADV group (n = 29) | p | TDF group (N = 56) | p | |||
|---|---|---|---|---|---|---|
| baseline | 12 months | baseline | 12 months | |||
| HBV-DNA log10 copies/mL, median (Q1 – Q3)1 | 8.30 (6.56 – 9.10) | 3.87 (2.95 – 5.60) | <0.0001 | 7.30 (6.00 – 8.30) | 2.47 (2.30 – 3.52) | <0.0001 |
| Presence of HBe Antigen (n, %)2 | 21 (87.5) | 17 (70.8) | 0.02 | 47 (88.7) | 40 (75.5) | 0.002 |
| Presence of antiHBe Antibody (n, %)2 | 4 (20.0) | 5 (25.0) | 0.001 | 6 (11.5) | 8 (15.4) | <0.001 |
| ALT IU/L, mean (SD)1 | 134 (150) | 83 (79) | ns | 90 (93) | 46 (29) | 0.001 |
| AST IU/L, mean (SD)1 | 112 (102) | 72 (80) | ns | 66 (74) | 33 (11) | 0.001 |
| g-Glutamyl Transferase IU/L, mean (SD)3 | 136 (223) | 74 (75) | ns | 81 (95) | 60 (67) | ns |
| ALP IU/L, mean (SD)3 | 104 (63) | 101 (57) | ns | 85 (33) | 79 (24) | ns |
| Creatinine Clearance ml/min, mean (SD)3 | 103.8 (17.8) | 94.2 (19.5) | 0.02 | 95.3 (32.5) | 100.0 (23.2) | ns |
| CD4+ cell count/mm3, mean (SD)3 | 482 (298) | 488 (402) | ns | 457 (250) | 501 (238) | 0.01 |
| HIV-RNA log10 copies/mL, median (IQR)1 | 1.88 (1.70–5.20) | 1.70 (1.70–5.67) | ns | 1.85 (1.70–6.19) | 1.70 (1.70–3.84) | <0.0001 |
Wilcoxon signed-rank test;
Fisher’s Exact Test;
Two-tailed paired t-test
ADV-treated patients were more likely to have a baseline ALT/AST level > 100 IU/mL [13 (46%)/7 (25%)] vs. TDF [7 (26%) vs. 5 (19%)] (p=0.03/p=0.05). However, a decrease in liver enzymes from baseline to 12 months was noted in both the ADV (AST: −38%, p=0.1 and ALT: −28%, p=0.05) and TDF group (AST: −49% and ALT: −50%, p<0.0001). Creatinine clearance remained high (> 95 ml/min) and stable throughout treatment in both groups.
The evolution of the immuno-virological status was also different according to treatment groups. There was an increase in CD4 T-cells of 44/mm3 (+8.8%) in patients treated with TDF compared to 6/mm3 (+1.2%) with ADV (p=0.01), where 45 (81.8%) and 16 (55.2%) patients in the respective groups obtained an undetectable HIV viral load (< 50 copies/mL) (p=0.009).
Comparison of HBV DNA kinetics between treatment groups
Mixed linear models were adjusted to the observed data in order to model the HBV-DNA dynamics and analyze the impact of treatment on the early viral decrease (Figure 2). During the first 6 months, there was an overall decrease of HBV load by 1.0 log10 copies/ml (CI 95%: 0.9, 1.2) per month. Individuals in the TDF group presented a significantly more rapid decline, with a slope of decline estimated at 1.1 (95% CI: 0.9, 1.3) compared to 0.8 (95% CI: 0.6, 1.0) in the ADV group (p=0.036). No changes were observed between treatment-dependent slopes when adjusted for baseline HBV-DNA as well as baseline ALT and AST (data not shown).
Figure 2.
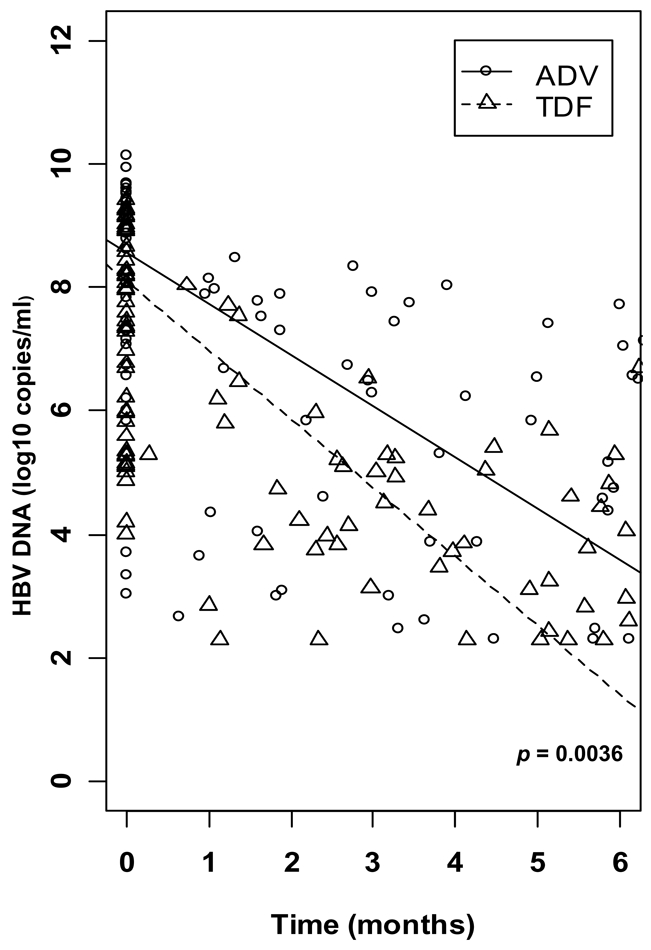
Modelling of HBV-DNA dynamics between inclusion and month 6 according to treatment.
Factors influencing the time to undetectability (HBV-DNA <200 copies/mL)
The median duration of treatment was 775 days (IQR 396 – 1008) for ADV and 1050 days (IQR 797 – 1196) for TDF. In each group, the mean time to HBV-DNA undetectability was 19.3 months (95% CI: 16.7 – 22.0) in presence of TDF and 25.9 months (95% CI: 21.1 – 30.7) in presence of ADV. Success curves representing time-to-undetectability (figure 3) were significantly in favor of TDF v. ADV treatment [log-rank test: p=0.04, HR: 2.22 (95% CI: 0.99 – 4.94)]. In univariate analysis, TDF vs ADV therapy, HBeAg status, HBV-DNA level and ALT at baseline had an impact on HBV time-to-undetectability, whereas no influence was noted for mutations in the YMDD motif, CD4+ cell count, fibrosis score, co-treatment with Lamivudine, and alcohol consumption (Table 3). When adjusted for the presence of HBe Ag, HBV-DNA at baseline and ALT levels, the influence of treatment on time to HBV-DNA undetectability remained in favor of TDF versus ADV (HR = 2.79; 95 % CI: 1.05–7.40, p=0.039) (Table 4).
Figure 3.
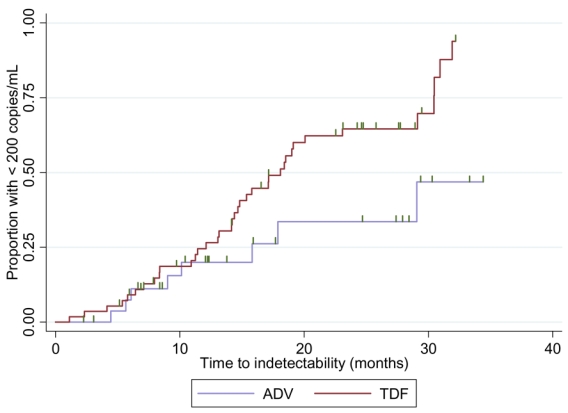
Success curves representing time-to-undetectability (HBV-DNA < 200 copies/mL) according to treatment group.
Table 3.
Factors influencing the time to undectability (HBV-DNA < 200 copies/mL), univariate analysis
| Crude Hazard Ratio | CI 95% | p | |
|---|---|---|---|
| TDF v. ADV | 2.22 | (0.99–4.94) | 0.05 |
| HBV load at baseline | |||
| - per log10 copies/mL HBV-DNA | 0.66 | (0.55–0.80) | <0.001 |
| - < 2000 copies/mL | 1.00 | ** | ** |
| - 2000 – 10,000 copies/mL | 0.12 | (0.03–0.46) | 0.002 |
| - > 10,000 copies/mL | 0.05 | (0.02–0.14) | <0.001 |
| Presence of HBe antigen in serum | 0.13 | (0.04–0.41) | <0.001 |
| Presence of YMDD mutation | 0.77 | (0.29–2.07) | 0.6 |
| Presence of Pre C mutation | 0.60 | (0.28–1.28) | 0.2 |
| HIV load at baseline (per log10 HIV-RNA) | 0.85 | (0.47–1.54) | 0.6 |
| Duration of HIV infection (per year) | 0.95 | (0.88–1.02) | 0.14 |
| CD4+ Cell Count (≥ 402/mm3) | 0.74 | (0.42–1.31) | 0.3 |
| METAVIR Fibrosis F0–F2 vs F3–F4 | 1.72 | (0.78–3.79) | 0.2 |
| ALT level at baseline (≥72 UI/mL = median) | 0.46 | (0.25–0.85) | 0.01 |
| Additional HCV co-infection | 1.42 | (0.44–4.58) | 0.6 |
| Alcohol consumption (cut at the median) | 0.71 | (0.40–1.29) | 0.3 |
Table 4.
Multivariate analysis of factors influencing the time to HBV-DNA undetectability (<200copies/mL)
| Adjusted Hazard Ratio | CI 95% | p | |
|---|---|---|---|
| TDF v. ADV | 2.79 | (1.05–7.40) | 0.039 |
| HBV load at baseline (per log10 copies/mL HBV-DNA) | 0.75 | (0.59–0.94) | 0.01 |
| ALT level at baseline (≥72 UI/mL = median) | 0.58 | (0.32–1.05) | 0.07 |
| Presence of HBe antigen in serum | 0.20 | (0.05–0.79) | 0.02 |
DISCUSSION
The rapid emergence of Lamivudine-resistant HBV strains in HIV-HBV co-infected patients [7], in whom Interferon does not exhibit a sufficient antiviral efficacy [20], has required the development of alternative therapeutic strategies. Two nucleotide reverse transcriptase inhibitors, Adefovir dipivoxil (ADV) and Tenofovir disoproxil fumarate (TDF), exhibit a strong in vivo and in vitro anti-HBV activity on both wild and lamivudine resistant strains [11]. The HBV dynamics study presented herein establishes a greater efficacy of TDF over ADV in terms of early and optimal control of HBV replication, coupled with a greater impact on transaminases levels. Both molecules were well tolerated and no acute renal insufficiency was noted during the clinical follow-up.
The antiviral efficacy of TDF has been recently demonstrated to be non inferior to that of ADV in a randomized controlled trial involving 52 HIV-HBV co-infected patients [15]. Despite this primary endpoint, Peters et al. extended similar findings obtained in the HBV-monoinfected patient population to HIV coinfection, suggesting a stronger anti-HBV activity in TDF-treated patients after an initial period of 48 weeks [14]. We confirm the data presented and demonstrate with mixed linear models that the early-phase HBV-DNA kinetics is more strongly influenced by TDF than by ADV, with a decrease of 1.1 log10 copies/month compared to 0.8 log10 copies/month, respectively. This early difference is associated with a sustained antiviral activity in the TDF group over time, in which patients reach the threshold of HBV undetectability at a faster rate and at a larger proportion than those taking ADV. A recently published case-series of HBV mono-infected patients reported in vivo concordant results on a greater TDF anti-HBV activity [21].
A crucial goal of anti-HBV therapy is to obtain an undetectable viral load at a faster rate in order to limit the emergence of viral resistance and predict an optimal long-term viral efficacy [22]. A sustained antiviral response is also essential to reverse the prognostic indicators of liver disease, such as the progression of liver inflammation and fibrosis [23]. The concomitancy of HIV disease further emphasizes the crucial nature and difficulty in attaining these goals. Indeed, although lamivudine induces an early decline of HBV replication in HBV mono-infected patients [24], the duration of its efficacy is undermined in HIV co-infected patients because of the rapid emergence of resistant HBV strains [7]. During the course of ADV treatment, the viral suppression is progressive, reaching a plateau with few HIV-infected patients presenting an undetectable viral profile [25]. This may help to explain why some case-series studies have reported the occurrence of newly diagnosed HBV strains resistant to ADV in both HBV mono-infected [26] and HIV-HBV co-infected patients [27]. Conversely, HIV-infected patients treated with TDF reached undetectable HBV viral loads after a median of less than one year with a rapid initial phase and a steady second phase of viral decline [13], [28], [29]. Moreover, the description of newly acquired resistance mutations to TDF has been scarce [30] and controversial [31] even though TDF has been used in HIV-HBV co-infected patients since 2003. As a result, recent guidelines recommend TDF containing therapy as the first line ARV regimen for HIV-HBV infected individuals who requires HIV treatment (EACS guidelines 2007). However, ADV remains a therapeutic option for co-infected individuals without need for ARV treatment, since it does not seem to select HIV reverse transcriptase mutations, even at the doses used for its anti-HBV activity [32], [33].
The positive impact of a sustained viral suppression on liver fibrosis is well described in HBV mono-infected patients and is associated with the eventual decrease in the incidence of end stage liver disease [34], [35]. The decrease of the necrotico-inflammatory activity within the liver may subsequently reduce the progression of fibrosis and even induce a reverse-effect on liver fibrosis, which has been reported after treatment with lamivudine and adefovir [24], [8], [9], [36]. Similar data in treated HIV-HBV co-infected patients are currently scarce [37], nevertheless, histologic evaluation of TDF on liver fibrosis will become available through phase II and III trials designed at analysing the efficacy of TDF in HBV mono-infected patients [38], [39]. The suppressive effect of TDF or ADV on HBV replication could in turn enhance the antifibrotic process by affecting known determinants of fibrosis progression, such as hepatic flares and high levels of transaminases [40], [41]. In our study, a higher decrease of transaminase levels in patients treated with TDF was found compared to ADV. In co-infected patients, immunosuppression likely plays an additional role in fibrosis genesis through the persistent expression of pro-inflammatory cytokines, combined with the aberrant activity of stellate liver cells [42]. The restoration of the immune system, as illustrated in our study by the significant increase in CD4 cell count in patients receiving TDF, may help reverse the rate of liver fibrosis due to the anti-HIV-HBV activity of TDF. Furthermore, a superior control of HIV RNA, added to the enhanced CD4 reconstitution, was achieved in patients treated with TDF compared to ADV at doses recommended for HBV infection. However, it is unknown whether the strong, previously-described antiretroviral activity of TDF [10] might in turn act as a mediator in the increase of HBV specific immunity.
In conclusion, the clinical, virological and histological background supporting the use of TDF in HIV-HBV co-infected patients compared to ADV is widening. Recent phase III trials conducted in HBV mono-infected patients showed promising results when using TDF in patients considered difficult to treat (i.e. lamivudine-resistant and precore mutants) [39], [38], whose features are commonly shared with chronic hepatitis B in HIV-infected patients. ADV treatment can remain an indication for HIV-infected patients to whom no antiretroviral therapy is prescribed (absence of AIDS-defining events and CD4 lymphocytes cell count more than 350/mm3), considering that TDF cannot be administered as a single molecule due to its potential to select for HIV resistance mutations.
There is now growing evidence to support an earlier introduction of antiretroviral therapy in HIV-hepatitis co-infected patients. Indeed, recent data suggests the importance of HIV in the fibrogenic process through the binding of HIV-gp120 to CCR5-receptors of hepatic stellate cells, thus triggering an increased expression of collagen and pro-inflammatory chemokines [43]. This would imply a need for an early combined treatment of HIV and hepatitis including TDF, in order to induce a rapid suppression of HBV replication and abate liver disease progression. In any case, the results of recent studies suggest that early viral suppression is a major predictor of treatment success and of prevention of HBV drug resistance [23], [44]. This should be coupled with a close virological monitoring in order to rapidly adapt therapeutic strategies and prevent the emergence of viral resistance.
Acknowledgments
The authors would like to thank the participating centers (Hôpital Saint-Antoine, Hôpital Saint-Louis, hospital Tenon, Hôtel-Dieu Lyon) and their clinicians and researchers who were actively involved in the patients’ recruitment and follow-up, A special thanks to Pascale Tran, Rosa Mouchotte, Manuela Sébire, Nadège Algans for their commitment to the French HIV-HBV cohort.
This study was sponsored by IMEA (Institut de Médecine et d’Epidémiologie Appliquée) and was mostly funded by Sidaction. It also received grants from the ANRS (Agence Nationale de Recherche sur le Sida et les Hépatites), the European Community (HepBvar project contrat QLRT2001-00977), viRgil network (contract LSHM-CT-2004-503359) and Gilead Sciences.
Footnotes
Email contacts: karine.lacombe@sat.aphp.fr, joel.gozlan@sat.aphp.fr, boyd@u707.jussieu.fr, boelle@u707.jussieu.fr, philippe.bonnard@tnn.aphp.fr, jean-michel.molina@sls.aphp.fr, patrick.miailhes@chu-lyon.fr, caroline.lascoux-combe@sls.aphp.fr, lawrence.serfaty@sat.aphp.fr, zoulim@inserm.lyon.fr, pierre-marie.girard@sat.aphp.fr
The results of this paper have been presented at the CROI 2007, Los Angeles, USA.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of Viral Hepatitis. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- 3.Bodsworth N, Cooper D, Donovan B. The influence of human immunodeficiency virus type 1 infection on the development of the hepatitis B virus carrier state. J Infect Dis. 1991;163:1138–1140. doi: 10.1093/infdis/163.5.1138. [DOI] [PubMed] [Google Scholar]
- 4.Krogsgaard K, Bindslev N, Christensen E, et al. The treatment effect of alpha interferon in chronic hepatitis B is independent of pre-treatment variables. Results based on individual patient data from 10 clinical controlled trials. European Concerted Action on Viral Hepatitis (Eurohep) J Hepatol. 1994;21:646–655. doi: 10.1016/s0168-8278(94)80114-2. [DOI] [PubMed] [Google Scholar]
- 5.Marcellin P, Lau GKK, Bonino F, et al. Peginterferon Alfa-2a Alone, Lamivudine Alone, and the Two in Combination in Patients with HBeAg-Negative Chronic Hepatitis B. N Engl J Med. 2004;351:1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 6.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 7.Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30:1302–1306. doi: 10.1002/hep.510300525. [DOI] [PubMed] [Google Scholar]
- 8.Marcellin P, Chang T-T, Lim SG, et al. Adefovir Dipivoxil for the Treatment of Hepatitis B e Antigen-Positive Chronic Hepatitis B. N Engl J Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 9.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir Dipivoxil for the Treatment of Hepatitis B e Antigen-Negative Chronic Hepatitis B. N Engl J Med. 2003;348:800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 10.Schooley RT, Ruane Pa, Myers RAb, et al. Tenofovir DF in antiretroviral-experienced patients: results from a 48-week, randomized, double-blind study. [Article] AIDS. 2002;16:1257–1263. doi: 10.1097/00002030-200206140-00008. [DOI] [PubMed] [Google Scholar]
- 11.Lada O, Benhamou Y, Cahour A, et al. In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir. Antivir Ther. 2004;9:353–363. [PubMed] [Google Scholar]
- 12.Benhamou Y, Tubiana R, Thibault V. Tenofovir Disoproxil Fumarate in Patients with HIV and Lamivudine-Resistant Hepatitis B Virus. N Engl J Med. 2003;348:177–178. doi: 10.1056/NEJM200301093480218. [DOI] [PubMed] [Google Scholar]
- 13.Lacombe K, Gozlan J, Boelle PY, et al. Long-term hepatitis B virus dynamics in HIV-hepatitis B virus-co-infected patients treated with tenofovir disoproxil fumarate. AIDS. 2005;19:907–915. doi: 10.1097/01.aids.0000171404.07995.5c. [DOI] [PubMed] [Google Scholar]
- 14.van Bommel F, Wunsche T, Mauss S, et al. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421–1425. doi: 10.1002/hep.20464. [DOI] [PubMed] [Google Scholar]
- 15.Peters MG, Andersen J, Lynch P, et al. Randomized controlled study of tenofovir and adefovir in chronic hepatitis B virus and HIV infection: ACTG A5127. Hepatology. 2006;44:1110–1116. doi: 10.1002/hep.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacombe K, Massari V, Girard PM, et al. Major role of hepatitis B genotypes in liver fibrosis during coinfection with HIV. AIDS. 2006;20:419–427. doi: 10.1097/01.aids.0000200537.86984.0e. [DOI] [PubMed] [Google Scholar]
- 17.Tran N, Berne R, Chann R, et al. European multicenter evaluation of high-density DNA probe arrays for detection of hepatitis B virus resistance mutations and identification of genotypes. J Clin Microbiol. 2006;44:2792–2800. doi: 10.1128/JCM.00295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benhamou Y. Treatment algorithm for chronic hepatitis B in HIV-infected patients. J Hepatol Proceedings of the 1st European Consensus Conference on the Treatment of Chronic Hepatitis B and C in HIV Co-infected Patients. 2006;44:S90–S94. doi: 10.1016/j.jhep.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 20.Brook M, McDonald J, Karayiannis P, et al. Randomised controlled trial of interferon alfa 2A (rbe) (Roferon-A) for the treatment of chronic hepatitis B virus (HBV) infection: factors that influence response. Gut. 1989;30:1116–1122. doi: 10.1136/gut.30.8.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leemans WF, Janssen HL, Niesters HG, et al. Switching patients with lamivudine resistant chronic hepatitis B virus from tenofovir to adefovir results in less potent HBV-DNA suppression. J Viral Hepat. 2008;15:108–114. doi: 10.1111/j.1365-2893.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 22.Yuen M, Fong D, Wong D, et al. Hepatitis B virus DNA levels at week 4 of lamivudine treatment predict the 5-year ideal response. Hepatology. 2007;46:1695–1703. doi: 10.1002/hep.21939. [DOI] [PubMed] [Google Scholar]
- 23.Lai C, Gane E, Liaw Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 24.Lai C-L, Chien R-N, Leung NWY, et al. A One-Year Trial of Lamivudine for Chronic Hepatitis B. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 25.Benhamou Y, Thibault V, Vig P, et al. Safety and efficacy of adefovir dipivoxil in patients infected with lamivudine-resistant hepatitis B and HIV-1. J Hepatol. 2006;44:62–67. doi: 10.1016/j.jhep.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Chen CH, Wang JH, Lee CM, et al. Virological response and incidence of adefovir resistance in lamivudine-resistant patients treated with adefovir dipivoxil. Antivir Ther. 2006;11:771–778. [PubMed] [Google Scholar]
- 27.Lacombe K, Ollivet A, Gozlan J, et al. A novel hepatitis B virus mutation with resistance to adefovir but not to tenofovir in an HIV-hepatitis B virus-co-infected patient. AIDS. 2006;20:2229–2231. doi: 10.1097/01.aids.0000252061.35422.84. [DOI] [PubMed] [Google Scholar]
- 28.de Vries-Sluijs TE, van der Eijk AA, Hansen BE, et al. Wild type and YMDD variant of hepatitis B virus: no difference in viral kinetics on lamivudine/tenofovir therapy in HIV-HBV co-infected patients. J Clin Virol. 2006;36:60–63. doi: 10.1016/j.jcv.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Benhamou Y, Fleury H, Trimoulet P, et al. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548–555. doi: 10.1002/hep.21055. [DOI] [PubMed] [Google Scholar]
- 30.Sheldon J, Camino N, Rodes B, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther. 2005;10:727–734. [PubMed] [Google Scholar]
- 31.Delaney Wt, Ray A, Yang H, et al. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006;50:2471–2477. doi: 10.1128/AAC.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delaugerre C, Marcelin AG, Thibault V, et al. Human immunodeficiency virus (HIV) Type 1 reverse transcriptase resistance mutations in hepatitis B virus (HBV)-HIV-coinfected patients treated for HBV chronic infection once daily with 10 milligrams of adefovir dipivoxil combined with lamivudine. Antimicrob Agents Chemother. 2002;46:1586–1588. doi: 10.1128/AAC.46.5.1586-1588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheldon JA, Corral A, Rodes B, et al. Risk of selecting K65R in antiretroviral-naive HIV-infected individuals with chronic hepatitis B treated with adefovir. AIDS. 2005;19:2036–2038. doi: 10.1097/01.aids.0000189563.79976.05. [DOI] [PubMed] [Google Scholar]
- 34.Liaw YF. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antivir Ther. 2006;11:669–679. [PubMed] [Google Scholar]
- 35.Villeneuve JP, Condreay LD, Willems B, et al. Lamivudine treatment for de-compensated cirrhosis resulting from chronic hepatitis B. Hepatology. 2000;31:207–210. doi: 10.1002/hep.510310130. [DOI] [PubMed] [Google Scholar]
- 36.Hadziyannis S, Tassopoulos N, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Mallet VO, Dhalluin-Venier V, Verkarre V, et al. Reversibility of cirrhosis in HIV/HBV coinfection. Antivir Ther. 2007;12:279–283. [PubMed] [Google Scholar]
- 38.Marcellin P, Buti M, Krastev Z, et al. A randomized, double-blind, comparison of Tenofovir DF (TDF versus Adefovir Dipivoxil (ADV) for the treatment of HBeAg negative chronic hepatitis B (CHB): Study GS-US-174-0102. Hepatology. 2007;46 abstract LB2. [Google Scholar]
- 39.Heathcote EJ, Gane E, de Man RA, et al. A randomized, double-blind, comparison of Tenofovir DF (TDF versus Adefovir Dipivoxil (ADV) for the treatment of HBeAg positive chronic hepatitis B (CHB): Study GS-US-174-0102. Hepatology. 2007;46 Abstract LB6. [Google Scholar]
- 40.Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009–1022. doi: 10.1053/gast.2001.22461. [DOI] [PubMed] [Google Scholar]
- 41.Pol S, Lebray P, Vallet-Pichard A. HIV infection and hepatic enzyme abnormalities: intricacies of the pathogenic mechanisms. Clin Infect Dis. 2004;38(Suppl 2):S65–72. doi: 10.1086/381499. [DOI] [PubMed] [Google Scholar]
- 42.Rehermann B. Immune responses in hepatitis B virus infection. Semin Liver Dis. 2003;23:21–38. doi: 10.1055/s-2003-37586. [DOI] [PubMed] [Google Scholar]
- 43.Marra F, Bruno R, Galastri S. gp120 induces directional migration of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Hepatology. 2007;46 doi: 10.1136/gut.2008.163287. Abstract A125. [DOI] [PubMed] [Google Scholar]
- 44.Chan HL, Heathcote EJ, Marcellin P, et al. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann Intern Med. 2007;147:745–754. doi: 10.7326/0003-4819-147-11-200712040-00183. [DOI] [PubMed] [Google Scholar]


