Abstract
Breast, prostate, pancreatic, colorectal, lung, and head and neck cancers exploit deregulated signaling by ErbB family receptors and their ligands, EOF family peptide growth factors. EGF family members that stimulate the same receptor are able to stimulate divergent biological responses both in cell culture and in vivo. This is analogous to the functional selectivity exhibited by ligands for G-protein coupled receptors. Here we review this literature and propose that this functional selectivity of EGF family members is due to distinctions in the conformation of the liganded receptor and subsequent differences in the sites of receptor tyrosine phosphorylation and receptor coupling to signaling effectors. We also discuss the roles of divergent ligand activity in establishing and maintaining malignant phenotypes. Finally, we discuss the potential of mutant EGF family ligands as cancer chemotherapeutics targeted to ErbB receptors.
Keywords: Epidermal Growth Factor Receptor, EGF, ErbB, receptors, ErbB4, signal transduction, Neuregulins, Transforming Growth Factor alpha, Amphiregulin
1. Introduction
Ligand functional selectivity is defined as divergent ligand activation of signaling pathways through a single receptor. Functionally selective ligands have been identified for the serotonin, opioid, dopamine, vasopressin, and adrenergic receptors (Urban et al., 2007). Ligand binding to these G-protein coupled receptors (GPCRs) results in receptor association with heterotrimeric G-proteins. Ligand functional selectivity appears to be mediated by distinctions in the conformation of liganded receptors and subsequent differential association of the liganded receptors with heterotrimeric G proteins (Urban et al., 2007). The plethora of functionally selective ligands for GPCRs has facilitated the elucidation of the mechanisms by which GPCRs couple to signaling effectors and biological responses. Moreover, functionally selective ligands portend the discovery of GPCR ligands that have therapeutic signaling properties but lack adverse signaling properties (Mailman, 2007).
Like many other receptor tyrosine kinases, ErbB family receptors have numerous peptide ligands encoded by several distinct genes and by alternatively-spliced transcripts. These peptide ligands exhibit differences in receptor affinity and display exquisite receptor binding specificity. Other factors contribute to ligand specificity, including distinctions in the timing of ligand expression, tissue-specific patterns of ligand expression and differences in post-translational cleavage and processing. Accessory molecules and co-receptors such as heparan sulfate proteoglycans may contribute to ligand specificity by sequestering local high concentrations of these growth factors or by controlling their bioavailability, thereby selectively modulating the duration and/or strength of signaling stimulation by those EGF family members that bind these molecules (Britsch, 2007; Citri & Yarden, 2006; Edwin et al., 2006; Higashiyama et al., 2008; Iwamoto & Mekada, 2006; Jones et al., 2003; Mahtouk et al., 2006; Nanba & Higashiyama, 2004; Nishi & Klagsbrun, 2004; Normanno et al., 2001; Ohtsu et al., 2006; Sanderson et al., 2006; Shilo, 2005; Singh & Harris, 2005).
In addition, peptide ligands that display binding for the same ErbB family receptor have recently been shown to exhibit differences in function. This review will discuss the functional selectivity of ErbB receptor ligands, particularly in the context of the roles that functionally-selective ErbB receptor ligands may play in human malignancies and in the context of the roles that functionally-selective ErbB receptor ligands may play in cancer drug discovery.
The ErbB family of receptor tyrosine kinases includes the epidermal growth factor (EOF) receptor (EGFR/ErbBl/HERl), ErbB2/HER2/Neu, ErbB3/HER3, and ErbB4/HER4. Signaling by ErbB receptors is of principal importance in the control of cell fate, influencing proliferation, survival, or differentiation; hence, deregulated signaling by these receptors plays important roles in human malignancies. Currently, both EGFR and ErbB2 are validated targets for cancer chemotherapeutics (Normanno & Gullick, 2006; Plosker & Keam, 2006) that are being used to treat breast, lung, colorectal, and head and neck cancers. However, the development of resistance to chemotherapeutic agents that target ErbB receptors (Blackball et al., 2006; Nahta et al., 2006) has spurred continuing investigation into the mechanisms by which ErbB family receptor signaling is regulated and may be deregulated. Combining multiple therapeutics that target ErbB receptors via independent mechanism of action can result in enhanced responses. Moreover, ErbB inhibitors with different mechanisms of action elicit therapeutic responses in tumors that are refractory to treatment with other therapeutics that target ErbB receptors (Cameron et al., in press; Geyer et al., 2006; Storniolo et al., 2005; Storniolo et al., 2008). Thus, identifying additional agents that can target ErbB family receptors through novel means is an important goal in the treatment of cancer.
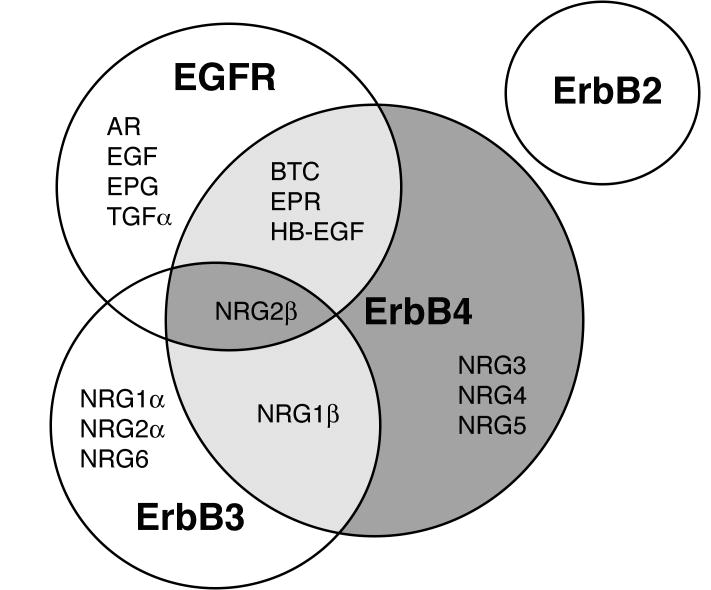
Members of the EGF family of peptide growth factors serve as agonists for ErbB family receptors. They include EGF, transforming growth factor-alpha (TGFα), amphiregulin (AR), betacellulin (BTC), heparin-binding EGF-like growth factor (HB-EGF), epiregulin (EPR), epigen (EPG), and the neuregulins (NRGs) (Figure 1) (Kinugasa et al., 2004; Kochupurakkal et al., 2005; Normanno et al., 2005). Collectively, these agonists regulate the activity of the four ErbB family receptors, each of which appears to make a unique set of contributions to a complicated signaling network (Citri & Yarden, 2006). Tumor cell expression of some EGF family members, most notably TGFα, AR, and HB-EGF, is associated with poorer patient prognosis or resistance to chemotherapeutics (Celikel et al., 2007; Eckstein et al., 2008; Gee et al., 2005; Ishikawa et al., 2005; Ritter et al., 2007; Wang et al., 2007).
Figure 1. Amino acid sequence of the EGF homology domain of selected EGF family peptide growth factors.
(A) Underlined are the six conserved cysteine residues that form the three intramolecular disulfide bridges present in the mature ligands. Selected EGF family peptide growth factors include EGF (NCBI Protein database # NP_001954), TGFα (#AAA61159), AR (#AAA51781), HB-EGF (AAA35956), BTC (#AAB25452), EPR (#BAA22146), EPG (#Q6UW88), NRG1α (#NP_039258), NRG1β (#ABR13844), NRG2α (#NP_004874), NRG2β (#NP_053584), NRG3 (#NP_001010848), NRG4 (#AAH17568), NRG5 (#BAA90820), and NRG6 (#AAC69612). NRG5 is also known as tomoregulin and NRG6 is also known as neuroglycan-C. (B) Carboxyl-terminal NRG2 residues that regulate differences in ligand potency, receptor affinity (residue 43), and intrinsic activity (residue 45) are noted.
Ligand binding causes the homo- and/or heterodimerization of ErbB receptors, leading to the activation of their intracellular tyrosine kinase domains. As a result, ligand binding causes ErbB receptor phosphorylation on cytoplasmic tyrosine residues. This provides a mechanism for coupling to downstream signaling proteins via Src homology-2 (SH2) and phosphotyrosine binding (PTB) domains, which both recognize specific sets of tyrosine phosphorylation sites (Citri & Yarden, 2006; Riese & Stern, 1998; Warren & Landgraf, 2006). The EGF family ligands exhibit a complex pattern of interactions with the four ErbB family receptors; for example, EGFR can bind eight different EGF family members (Figure 2) and Neuregulin 2beta (NRG2β) binds EGFR, ErbB3, and ErbB4 (Figure 2). Given that ErbB2 lacks a EGF family ligand, ErbB3 lacks kinase activity, and the four ErbB receptor display distinct patterns of coupling to signaling effectors (Citri & Yarden, 2006), differences in the affinity of a given EGF family member for each of the four ErbB receptors is a key determinant of ligand signaling specificity (Jones et al., 1999). Here we will review evidence from the literature indicating that specific ErbB ligands that stimulate a given ErbB receptor can direct different biological responses both in cell culture and in vivo. Next, we will propose a novel mechanism that may account for the divergent biological effects exhibited by EGFR and ErbB4 ligands. Finally, we will discuss evidence for this mechanism and discuss how distinctions in ligand activity might be exploited to develop a new class of cancer chemotherapeutics targeted to ErbB receptors.
Figure 2. EGF family ligands bind and activate multiple ErbB receptors.
A Venn diagram illustrates the interactions of the four ErbB family receptors with EGF family members. This figure summarizes published data (Hobbs et al., 2002; Kinugasa et al., 2004; Kochupurakkal et al., 2005; Normanno et al., 2005).
2. EGF Family Ligands Stimulate Different Biological Outcomes From The Same Receptor
In a variety of cultured cell model systems, different EGF family ligands that bind the same receptor can promote divergent biological outcomes. Emerging data indicate that this is true even when the ligands are present at saturating concentrations. Thus, these distinctions in signaling are independent of ligand affinity or potency and appear to reflect differences in ligand intrinsic activity or efficacy. The EGFR ligands TGFα and AR stimulate equivalent levels of DNA synthesis in MDCK cells. AR also stimulates a morphologic change and redistribution of E-cadherin in these cells, but TGFα does not (Chung et al., 2005). In MCF10A human mammary epithelial cells, AR stimulates greater motility and invasiveness than does EGF (Willmarth & Ethier, 2006). Ectopic expression of EGFR in the 32D mouse myeloid cell line permits a saturating concentration of EGF to stimulate EGFR coupling to survival. In contrast, a saturating concentration of Neuregulin 2beta (NRG2β) stimulates EGFR coupling to proliferation in these cells (Gilmore et al., 2006). Finally, EGF, HB-EGF, and TGFα can suppress alcohol-induced apoptosis in human placental cytotrophoblast cells, whereas AR cannot (Wolff et al., 2007).
Different EGF family members can stimulate divergent biological outcomes from the same receptor in animal model systems. Transgenic mice in which AR is expressed in the epidermis from the K14 promoter lack hair follicles and exhibit epidermal hyperplasia, aberrant differentiation, resistance to apoptosis, and increased inflammation characterized by skin plaques (Cook et al., 2004; Cook et al., 1997). In contrast, transgenic mice in which TGFα is expressed from the K14 promoter exhibit only a thicker epidermis and stunted hair growth (Dominey et al., 1993; Vassar & Fuchs, 1991). Transgenic mice that lack AR exhibit more severe stunting of mammary gland outgrowth than do transgenic mice that lack EGF or TGFα. Indeed, AR appears to be the primary EGFR ligand involved in pubertal mammary ductal morphogenesis, whereas EGF and TGFα seem to play more pronounced roles in mammary gland morphogenesis during pregnancy and lactation (Booth & Smith, 2007; McBryan et al., 2008).
The in vitro and in vivo results discussed above are buttressed by emerging data indicating that the expression of specific EGFR ligands in certain tumors is differentially associated with prognosis. EGF expression in breast tumor samples is associated with a more favorable prognosis, whereas TGFα expression is associated with more aggressive tumors (Revillion et al., 2008). Likewise, microarray analyses reveal that early hyperplastic precursors of breast cancer display increased AR transcription and decreased EGF transcription relative to normal breast tissue (Lee et al., 2007). In non-small-cell lung carcinoma (NSCLC) patients, TGFα and AR serum concentrations correlate with tumor aggressiveness, but the serum concentration of EGF does not. In fact, the serum concentration of EGF is significantly higher in healthy individuals than in NSCLC patients (Lemos-Gonzalez et al., 2007). Moreover, NSCLC tumors that are refractory to the EGFR tyrosine kinase inhibitor gefitinib display increased TGFα and AR transcription than do tumors that are sensitive to gefitinib (Kakiuchi et al., 2004). Taken together, these data argue that TGFα and AR stimulate EGFR coupling to tumor cell aggressiveness and chemoresistance, while EGF fails to do so - and may in fact antagonize stimulation of pathogenic signaling by TGFα and AR.
Similarly, individual ErbB4 ligands appear to stimulate ErbB4 coupling to divergent biological responses. Ectopic expression of ErbB4 in the CEM human lymphoid cell line permits the ErbB4 ligands BTC, Neuregulin 1beta (NRG1β), Neuregulin 2beta (NRG2β), and Neuregulin 3 (NRG3) to stimulate similar levels of ErbB4 phosphorylation. However, in these CEM/ErbB4 cells BTC and NRG1 β stimulate greater viability and proliferation than do NRG2β and NRG3 (Sweeney et al., 2000). Ectopic expression of EGFR and ErbB4 in the BaF3 mouse lymphoid cell line permits the ErbB4 ligands NRG1β and NRG2β to stimulate proliferation. However, in these BaF3/EGFR+ErbB4 cells the ErbB4 ligand NRG3 stimulates survival, whereas the ErbB4 ligands Neuregulin 2alpha (NRG2α) and Neuregulin 4 (NRG4) fail to stimulate either survival or proliferation (Hobbs et al., 2002). Likewise, at the neuromuscular junction NRG2β appears to be more effective than NRG2α at stimulating ErbB4 coupling to increased transcription of the acetylcholine receptor (Ponomareva et al., 2005). These functional differences between NRG2α and NRG2β are particularly noteworthy given that these ligands are transcriptional splicing isoforms that differ in the carboxyl-terminus of the canonical EGF homology domain (Normanno et al., 2001; Normanno et al., 2005).
3. Mechanistic Model for Functionally Selective EGF Family Ligands
3.1. EGF family members differentially stimulate receptor coupling to signaling effectors by stimulating receptor phosphorylation on distinct sets of tyrosine residues
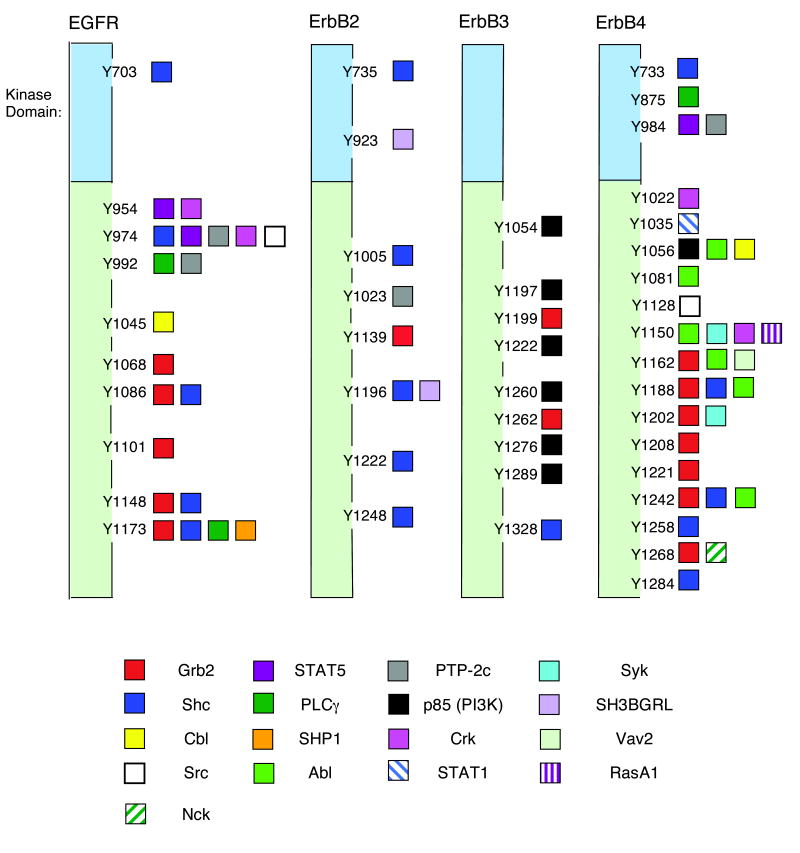
ErbB family receptors couple to a large variety of signaling effectors (Figure 3). We postulate that the complement of signaling effectors recruited to and activated by ligand-stimulated ErbB receptors is specified by the receptor and ligand, thereby accounting for differences in ligand intrinsic activity. For example, AR stimulates NF-κB signaling and Interleukin-1 (IL-1) secretion in MCF10A immortalized breast cells, but EGF does not. This appears to account for the divergent stimulation of motility and invasiveness by AR and EGF (Streicher et al., 2007). TGFα also appears to be functionally distinct from EGF. Both ligands stimulate migration and proliferation in fibroblasts; however, they appear to do so through different EGFR effectors. EGF stimulation of migration and proliferation requires p70s6k and CD44. In contrast, TGFα requires phospholipase Cγ (PLCγ) and integrin αvβ3 (Ellis et al., 2007).
Figure 3. Ligand stimulation of ErbB receptor tyrosine phosphorylation creates docking sites for numerous signaling effectors.
Putative sites of EGFR, ErbB2, ErbB3, and ErbB4 tyrosine phosphorylation are denoted, as well as signaling effectors predicted or shown to bind to these sites of phosphorylation (Cohen et al., 1996; Hellyer et al., 2001; Kaushansky et al., 2008; Keilhack et al., 1998; Rotin et al., 1992; Schulze et al., 2005; Sorkin et al., 1996; Zrihan-Licht et al., 1998). The ErbB receptors are not drawn to scale.
Differences in the sites of ligand-induced EGFR tyrosine phosphorylation may underlie divergent ligand-induced EGFR coupling to signaling effectors and biological responses. For example, EGF stimulates abundant EGFR phosphorylation at Tyr1045 whereas AR does not (Gilmore et al., 2008). Phosphorylation of Tyr1045 creates a canonical binding site for the E3 ubiquitin ligase c-cbl, leading to EGFR ubiquitination and degradation by the 26S proteasome (Levkowitz et al., 1999; Levkowitz et al., 1998). Thus, unlike AR, EGF stimulates c-cbl binding to EGFR, EGFR ubiquitination, and EGFR degradation (Stern et al., 2008). We hypothesize that differential ligand-induced EGFR phosphorylation at Tyr1045 and differential EGFR coupling to cbl-dependent ubiquitination and turnover leads to distinctions in the duration of ligand-induced EGFR signaling, thereby accounting for the inability of EGF to stimulate motility and invasiveness in cells that do respond to AR.
Differences in the sites of ligand-induced receptor phosphorylation may also account for divergence in the intrinsic activity of ErbB4 ligands. The ErbB4 ligands BTC, NRG1β, NRG2β, and NRG3 differentially stimulate ErbB4 coupling to survival and proliferation in CEM/ErbB4 cells and differentially stimulate ErbB4 coupling to Shc, p85, Akt, and Erk1/2. This is accompanied by distinctions in the sites of ligand-induced ErbB4 tyrosine phosphorylation (Sweeney et al., 2000). NRG1β stimulates ErbB4 phosphorylation at nineteen tyrosine residues (Kaushansky et al., 2008); these residues are candidates for sites of ligand-specific tyrosine phosphorylation. However, ligand-specific sites of ErbB4 tyrosine phosphorylation have yet to be identified and the biological relevance of differences in these sites of ErbB4 phosphorylation has yet to be established.
3.2. Differences in the conformation of the liganded receptor extracellular domain may account for distinct patterns of ErbB receptor tyrosine phosphorylation and downstream signaling
The binding of an EGF family member to its cognate ErbB receptor stabilizes the receptor extracellular domains in an extended conformation that exposes a dimerization arm in subdomain II, thereby facilitating dimerization of the extracellular region (Burgess et al., 2003). This is thought to lead to asymmetric dimerization of the receptor intracellular domains, activation of kinase activity, and trans-phosphorylation of cytoplasmic tyrosine residues on one receptor monomer by the kinase domain of the other receptor monomer (Zhang et al., 2006).
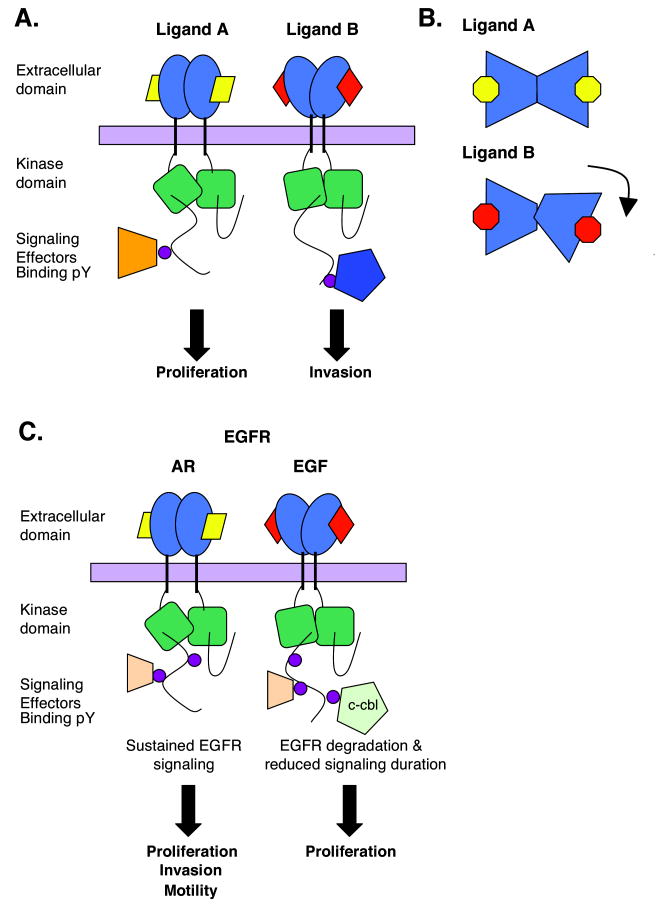
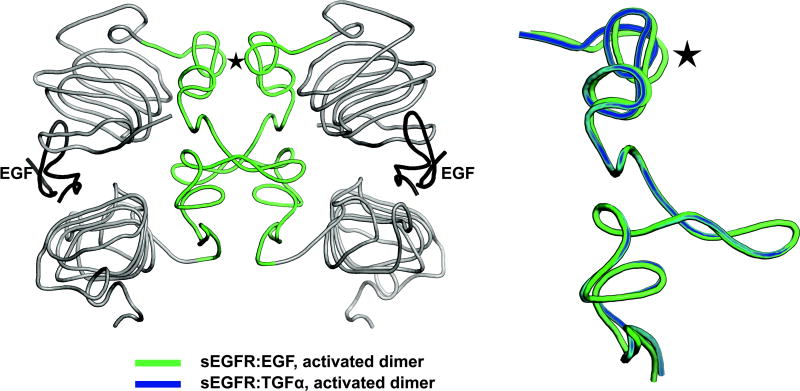
We hypothesize that different ErbB ligands could stabilize the extracellular regions of a given ErbB receptor in subtly distinct conformations. This could alter the details of the interaction between the two intracellular domains in the observed asymmetric dimer, in turn influencing which cytoplasmic tyrosine residues of each receptor monomer are most efficiently phosphorylated by their dimerization partner. Conformationally-distinct dimers might possess different complements of phosphorylated tyrosine residues, leading to activation of unique sets of signaling effectors and distinct biological outcomes (Figure 4). Studies using constitutively active ErbB2 and ErbB4 mutants reveal that artificially manipulating the structural relationship between two receptor monomers within a dimer can result in divergent receptor signaling and coupling to downstream events (Burke & Stern, 1998; Pitfield et al., 2006; Williams et al., 2003). In addition, evidence for ligand-specific receptor conformations can be seen in a comparison of the EGFR extracellular region bound to EGF or TGFα. The conformation of the EGFR extracellular subdomain II is subtly different in the EGFR-EGF and EGFR-TGFα complexes (Figure 5) (Garrett et al., 2002; Harte & Gentry, 1995; Ogiso et al., 2002). Since the dimerization interface extends along this entire domain (Dawson et al., 2005), it appears reasonable to suggest that EGF and TGFα promote different spatial relationships between the EGFR monomers within a receptor dimer. It has been postulated that ligand-specific alterations in the conformation of subdomain II may reflect ligand-specific differences in the buttressing of subdomain II by the EGFR extracellular subdomain III (Dawson et al., 2005). The plausibility of this hypothesis is supported by the fact that the EGFR extracellular subdomain III contains one of the two sites for binding EGF family ligands and is therefore a reasonable potential site for ligand-specific conformational changes. Further efforts are required to fully understand the proximal (the structure of receptors bound to functionally selective ligands), intermediate (sites of differential receptor phosphorylation caused by functionally selective ligands), and distal mechanisms (distinctions in signaling pathway activation and gene expression) that underlie the functional selectivity of EGF family ligands.
Figure 4. Model for differential ligand-induced ErbB receptor coupling to biological responses.
(A) Hypothetical depiction of differences in the conformation of receptor extracellular and intracellular domains and distinctions in the sites of receptor tyrosine phosphorylation following ligand binding. (B) Hypothetical depiction of the difference in the juxtapositioning of receptor extracellular domain monomers within a ligand-induced dimer. (C) Depiction of the distinctions in EGF and AR stimulation of EGFR phosphorylation at Tyr1045, resulting in differences in the binding of c-cbl, alterations in signaling duration, and changes in the biological consequences of EGFR signaling (Gilmore et al., 2008; Stern et al., 2008; Willmarth & Ethier, 2006).
Figure 5. Differences in the conformation of the EGFR extracellular domain following EGF or TGFα binding.
(A) A worm representation of an EGFR extracellular domain dimer, with subdomain II colored green at the dimer interface. (B) A worm representation of EGFR extracellular subdomain II conformation following binding of EGF (green) or TGFα (blue). Subtle differences, particularly at the upper site of dimerization, are marked with a star.
4. Implications of Functionally Selective EGF Family Ligands
4.1. Analyses of the roles that EGF family ligands and ErbB receptors play in tumorigenesis and tumor progression must account for differences in the intrinsic activity of EGF family members
Many studies of ligand-induced ErbB signaling in tumor cell model systems have employed EGF or NRG1 β as representative ligands for EGFR and ErbB4 signaling, respectively. However, since most human cancers express EGFR ligands other than EGF, differences in intrinsic ligand activities have not been captured by these studies and future work designed to elucidate the roles played by specific ErbB ligands in tumorigenesis and tumor progression should be expanded across the ligand family. Moreover, future studies should account for the possibility that ErbB receptor coupling to biological responses in a given tissue or cell may reflect competition between multiple EGF family ligands with different intrinsic activities. For example, increased EGFR coupling to tumor cell motility and invasiveness may be due to an increase in AR concentration. However, because EGF fails to stimulate EGFR coupling to tumor cell motility and invasiveness in some model systems (Willmarth & Ethier, 2006), a decrease in EGF concentration (and consequently, reduced competitive inhibition of AR-induced EGFR signaling) may result in increased EGFR coupling to tumor cell motility and invasiveness. Likewise, in some model systems it appears that NRG2β stimulates ErbB4 coupling to cell proliferation, whereas NRG2α fails to. Consequently, the latter ligand could function as a competitive antagonist of mitogenesis triggered by NRG2β (Wilson et al., 2007). Thus, increased ErbB4 coupling to cell proliferation may be due to an increase in NRG2β concentration or a decrease in NRG2α concentration.
4.2. EGF family ligand point mutants that display reduced intrinsic activity suggest a new paradigm for therapeutic ErbB receptor antagonists
The high degree of amino acid sequence identity between NRG2α and NRG2β suggests that a relatively small number of amino acid residues are responsible for the differences in their intrinsic activities. This hypothesis has been tested by exchanging individual amino acid residues between NRG2α and NRG2β. The NRG2β Q43L mutant, like wild-type NRG2β, potently stimulates ErbB4 tyrosine phosphorylation and appears to retain high affinity binding to ErbB4. However, unlike wild-type NRG2β, NRG2β/Q43L fails to stimulate ErbB4 coupling to cell proliferation (Wilson et al., 2007). Similarly, a variant of NRG2α with the converse L43Q mutation does not exhibit greater affinity for ErbB4, but stimulates ErbB4 coupling to cell proliferation (Wilson et al., 2007). Thus, the intrinsic activity of these EGF family members can be regulated independently of their affinity for their cognate receptor(s) and amino acid residue 43 of NRG2α and NRG2β appears to be a crucial determinant of differences in their intrinsic activities.
The observation that NRG2β/Q43L potently stimulates ErbB4 phosphorylation (Hobbs et al., 2004; Wilson et al., 2007) yet fails to stimulate ErbB4 coupling to cell proliferation (Wilson et al., 2007) makes it plausible to hypothesize that NRG2β/Q43L will competitively antagonize ligand-induced ErbB4 coupling to cell proliferation. If so, a new paradigm for receptor tyrosine kinase antagonists is suggested in which variants of other receptor tyrosine kinases peptide agonists with reduced intrinsic activity but no change in receptor binding affinity might be developed. These would function as therapeutic competitive antagonists of malignant phenotypes induced by endogenous growth factors. Therapeutic effect could be optimized with novel, functionally selective ligands such that only subsets of effectors from a single receptor are activated or inhibited, thereby increasing the therapeutic effect and minimizing adverse effects.
Moreover, our hypothesis that NRG2β/Q43L antagonizes ErbB4 signaling by stabilizing a receptor conformation that cannot couple to certain effectors suggests that it might be possible to develop small molecules that allosterically antagonize ErbB receptor signaling by stabilizing the receptor in an analogous, inactive conformation. We predict that such molecules would be resistant to the effects of ErbB receptor kinase domain mutations that disrupt the activity of small molecule ATP-competitive kinase inhibitors. Accordingly, such molecules would represent a class of receptor antagonists that are uniquely effective against certain tumors that are resistant to ErbB receptor kinase inhibitors. Clearly, further detailed mechanistic understanding of ErbB receptor activation - with a focus on understanding the effects of different ligands - will be required to take full advantage of this possibility, and could lead to a new generation of ErbB-targeted therapeutics.
Acknowledgments
The authors gratefully acknowledge the support of the National Institutes of Health (AR045585, DK067875, CA080770 and CA114209), the United States Army Medical Research and Materiel Command Breast Cancer Research Program (D AMD-17-00-1-0416), and Susan G. Komen for the Cure.
Abbreviations
- AR
Amphiregulin
- BTC
Betacellulin
- EGF
Epidermal Growth Factor
- EGFR
Epidermal Growth Factor Receptor
- EPG
Epigen
- EPR
Epiregulin
- GPCR
G-protein-coupled receptor
- HB-EGF
Heparin-binding EGF-like growth factor
- MDCK
Madin-Darby canine kidney
- NRG
Neuregulin
- NSCLC
Non-small-cell lung carcinoma
- PLCγ
Phospholipase C gamma
- PTB
Phosphotyrosine-binding
- SH2
Src-homology domain type 2
- TGFα
Transforming Growth Factor Alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blackball F, Ranson M, Thatcher N. Where next for gefitinib in patients with lung cancer? Lancet Oncol. 2006;7:499–507. doi: 10.1016/S1470-2045(06)70725-2. [DOI] [PubMed] [Google Scholar]
- Booth BW, Smith GH. Roles of transforming growth factor-alpha in mammary development and disease. Growth Factors. 2007;25:227–235. doi: 10.1080/08977190701750698. [DOI] [PubMed] [Google Scholar]
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- Burke CL, Stern DF. Activation of Neu (ErbB-2) mediated by disulfide bond-induced dimerization reveals a receptor tyrosine kinase dimer interface. Mol Cell Biol. 1998;18:5371–5379. doi: 10.1128/mcb.18.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. doi: 10.1007/s10549-007-9885-0. in press. [DOI] [PubMed] [Google Scholar]
- Celikel C, Eren F, Gulluoglu B, Bekiroglu N, Turhal S. Relation of neuroendocrine cells to transforming growth factor-alpha and epidermal growth factor receptor expression in gastric adenocarcinomas: prognostic implications. Pathol Oncol Res. 2007;13:215–226. doi: 10.1007/BF02893502. [DOI] [PubMed] [Google Scholar]
- Chung E, Graves-Deal R, Franklin JL, Coffey RJ. Differential effects of amphiregulin and TGF-alpha on the morphology of MDCK cells. Exp Cell Res. 2005;309:149–160. doi: 10.1016/j.yexcr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Cohen BD, Green JM, Foy L, Fell HP. HER4-mediated biological and biochemical properties in NIH 3T3 cells. Evidence for HER1 -HER4 heterodimers. J Biol Chem. 1996;271:4813–4818. doi: 10.1074/jbc.271.9.4813. [DOI] [PubMed] [Google Scholar]
- Cook PW, Brown JR, Cornell KA, Pittelkow MR. Suprabasal expression of human amphiregulin in the epidermis of transgenic mice induces a severe, early-onset, psoriasis-like skin pathology: expression of amphiregulin in the basal epidermis is also associated with synovitis. Exp Dermatol. 2004;13:347–356. doi: 10.1111/j.0906-6705.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- Cook PW, Piepkorn M, Clegg CH, Plowman GD, DeMay JM, Brown JR, et al. Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J Clin Invest. 1997;100:2286–2294. doi: 10.1172/JCI119766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM. Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Mol Cell Biol. 2005;25:7734–7742. doi: 10.1128/MCB.25.17.7734-7742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominey AM, Wang XJ, King LE, Jr, Nanney LB, Gagne TA, Sellheyer K, et al. Targeted overexpression of transforming growth factor alpha in the epidermis of transgenic mice elicits hyperplasia, hyperkeratosis, and spontaneous, squamous papillomas. Cell Growth Differ. 1993;4:1071–1082. [PubMed] [Google Scholar]
- Eckstein N, Servan K, Girard L, Cai D, von Jonquieres G, Jaehde U, et al. Epidermal growth factor receptor pathway analysis identifies amphiregulin as a key factor for cisplatin resistance of human breast cancer cells. J Biol Chem. 2008;283:739–750. doi: 10.1074/jbc.M706287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin F, Wiepz GJ, Singh R, Peet CR, Chaturvedi D, Bertics PJ, et al. A historical perspective of the EGF receptor and related systems. Methods Mol Biol. 2006;327:1–24. doi: 10.1385/1-59745-012-x:1. [DOI] [PubMed] [Google Scholar]
- Ellis IR, Schor AM, Schor SL. EGF and TGF-alpha motogenic activities are mediated by the EGF receptor via distinct matrix-dependent mechanisms. Exp Cell Res. 2007;313:732–741. doi: 10.1016/j.yexcr.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110:763–773. doi: 10.1016/s0092-8674(02)00940-6. [DOI] [PubMed] [Google Scholar]
- Gee JM, Robertson JF, Gutteridge E, Ellis IO, Finder SE, Rubini M, et al. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- Gilmore JL, Gallo RM, Riese DJ. The epidermal growth factor receptor (EGFR)-S442F mutant displays increased affinity for neuregulin-2beta and agonist-independent coupling with downstream signalling events. Biochem J. 2006;396:79–88. doi: 10.1042/BJ20051687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JL, Scott JA, Bouizar Z, Robling A, Pitfield SE, Riese DJ, 2nd, et al. Amphiregulin-EGFR signaling regulates PTHrP gene expression in breast cancer cells. Breast Cancer Res Treat. 2008;110:493–505. doi: 10.1007/s10549-007-9748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MT, Gentry LE. Mutations within subdomain II of the extracellular region of epidermal growth factor receptor selectively alter TGF alpha binding. Arch Biochem Biophys. 1995;322:378–389. doi: 10.1006/abbi.1995.1478. [DOI] [PubMed] [Google Scholar]
- Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–42161. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. Membrane-anchored growth factors, the epidermal growth factor family: beyond receptor ligands. Cancer Sci. 2008;99:214–220. doi: 10.1111/j.1349-7006.2007.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs SS, Cameron EM, Hammer RP, Le AT, Gallo RM, Blommel EN, et al. Five carboxyl-terminal residues of neuregulin2 are critical for stimulation of signaling by the ErbB4 receptor tyrosine kinase. Oncogene. 2004;23:883–893. doi: 10.1038/sj.onc.1207250. [DOI] [PubMed] [Google Scholar]
- Hobbs SS, Coffing SL, Le AT, Cameron EM, Williams EE, Andrew M, et al. Neuregulin isoforms exhibit distinct patterns of ErbB family receptor activation. Oncogene. 2002;21:8442–8452. doi: 10.1038/sj.onc.1205960. [DOI] [PubMed] [Google Scholar]
- Ishikawa N, Daigo Y, Takano A, Taniwaki M, Kato T, Hayama S, et al. Increases of amphiregulin and transforming growth factor-alpha in serum as predictors of poor response to gefitinib among patients with advanced non-small cell lung cancers. Cancer Res. 2005;65:9176–9184. doi: 10.1158/0008-5472.CAN-05-1556. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Mekada E. ErbB and HB-EGF signaling in heart development and function. Cell Struct Funct. 2006;31:1–14. doi: 10.1247/csf.31.1. [DOI] [PubMed] [Google Scholar]
- Jones FE, Golding JP, Gassmann M. ErbB4 signaling during breast and neural development: novel genetic models reveal unique ErbB4 activities. Cell Cycle. 2003;2:555–559. [PubMed] [Google Scholar]
- Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEES Lett. 1999;447:227–231. doi: 10.1016/s0014-5793(99)00283-5. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S, Daigo Y, Ishikawa N, Furukawa C, Tsunoda T, Yano S, et al. Prediction of sensitivity of advanced non-small cell lung cancers to gefitinib (Iressa, ZD1839) Hum Mol Genet. 2004;13:3029–3043. doi: 10.1093/hmg/ddh331. [DOI] [PubMed] [Google Scholar]
- Kaushansky A, Gordus A, Budnik BA, Lane WS, Rush J, MacBeath G. System-wide investigation of ErbB4 reveals 19 sites of Tyr phosphorylation that are unusually selective in their recruitment properties. Chem Biol. 2008;15:808–817. doi: 10.1016/j.chembiol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhack H, Tenev T, Nyakatura E, Godovac-Zimmermann J, Nielsen L, Seedorf K, et al. Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SFIP-1 to the epidermal growth factor receptor and attenuation of receptor signaling. J Biol Chem. 1998;273:24839–24846. doi: 10.1074/jbc.273.38.24839. [DOI] [PubMed] [Google Scholar]
- Kinugasa Y, Ishiguro H, Tokita Y, Oohira A, Ohmoto H, Higashiyama S. Neuroglycan C, a novel member of the neuregulin family. Biochem Biophys Res Commun. 2004;321:1045–1049. doi: 10.1016/j.bbrc.2004.07.066. [DOI] [PubMed] [Google Scholar]
- Kochupurakkal BS, Harari D, Di-Segni A, Maik-Rachline G, Lyass L, Gur G, et al. Epigen, the last ligand of ErbB receptors, reveals intricate relationships between affinity and mitogenicity. J Biol Chem. 2005;280:8503–8512. doi: 10.1074/jbc.M413919200. [DOI] [PubMed] [Google Scholar]
- Lee S, Medina D, Tsimelzon A, Mohsin SK, Mao S, Wu Y, et al. Alterations of gene expression in the development of early hyperplastic precursors of breast cancer. Am J Pathol. 2007;171:252–262. doi: 10.2353/ajpath.2007.061010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos-Gonzalez Y, Rodriguez-Berrocal FJ, Cordero OJ, Gomez C, Paez de la Cadena M. Alteration of the serum levels of the epidermal growth factor receptor and its ligands in patients with non-small cell lung cancer and head and neck carcinoma. Br J Cancer. 2007;96:1569–1578. doi: 10.1038/sj.bjc.6603770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, et al. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtouk K, Cremer FW, Reme T, Jourdan M, Baudard M, Moreaux J, et al. Heparan sulphate proteoglycans are essential for the myeloma cell growth activity of EGF-family ligands in multiple myeloma. Oncogene. 2006;25:7180–7191. doi: 10.1038/sj.onc.1209699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryan J, Howlin J, Napoletano S, Martin F. Amphiregulin: role in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:159–169. doi: 10.1007/s10911-008-9075-7. [DOI] [PubMed] [Google Scholar]
- Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- Nanba D, Higashiyama S. Dual intracellular signaling by proteolytic cleavage of membrane-anchored heparin-binding EGF-like growth factor. Cytokine Growth Factor Rev. 2004;15:13–19. doi: 10.1016/j.cytogfr.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Nishi E, Klagsbrun M. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a mediator of multiple physiological and pathological pathways. Growth Factors. 2004;22:253–260. doi: 10.1080/08977190400008448. [DOI] [PubMed] [Google Scholar]
- Normanno N, Bianco C, De Luca A, Salomon DS. The role of EGF-related peptides in tumor growth. Front Biosci. 2001;6:685–707. doi: 10.2741/normano. [DOI] [PubMed] [Google Scholar]
- Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, et al. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets. 2005;6:243–257. doi: 10.2174/1389450053765879. [DOI] [PubMed] [Google Scholar]
- Normanno N, Gullick WJ. Epidermal growth factor receptor tyrosine kinase inhibitors and bone metastases: different mechanisms of action for a novel therapeutic application? Endocr Relat Cancer. 2006;13:3–6. doi: 10.1677/erc.1.01185. [DOI] [PubMed] [Google Scholar]
- Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- Pitfield SE, Bryant I, Penington DJ, Park G, Riese DJ. Phosphorylation of ErbB4 on tyrosine 1056 is critical for ErbB4 coupling to inhibition of colony formation by human mammary cell lines. Oncol Res. 2006;16:179–193. doi: 10.3727/000000006783981134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plosker GL, Keam SJ. Trastuzumab: a review of its use in the management of HER2-positive metastatic and early-stage breast cancer. Drugs. 2006;66:449–475. doi: 10.2165/00003495-200666040-00005. [DOI] [PubMed] [Google Scholar]
- Ponomareva ON, Ma H, Dakour R, Raabe TD, Lai C, Rimer M. Stimulation of acetylcholine receptor transcription by neuregulin-2 requires an N-box response element and is regulated by alternative splicing. Neuroscience. 2005;134:495–503. doi: 10.1016/j.neuroscience.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Revillion F, Lhotellier V, Hornez L, Bonneterre J, Peyrat JP. ErbB/HER ligands in human breast cancer, and relationships with their receptors, the bio-pathological features and prognosis. Ann Oncol. 2008;19:73–80. doi: 10.1093/annonc/mdm431. [DOI] [PubMed] [Google Scholar]
- Riese DJ, 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- Rotin D, Margolis B, Mohammadi M, Daly RJ, Daum G, Li N, et al. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. Embo J. 1992;11:559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson MP, Dempsey PJ, Dunbar AJ. Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors. 2006;24:121–136. doi: 10.1080/08977190600634373. [DOI] [PubMed] [Google Scholar]
- Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:2005.0008. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Mazzotti M, Sorkina T, Scotto L, Beguinot L. Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J Biol Chem. 1996;271:13377–13384. doi: 10.1074/jbc.271.23.13377. [DOI] [PubMed] [Google Scholar]
- Stern KA, Place TL, Lill NL. EGF and amphiregulin differentially regulate Cbl recruitment to endosomes and EGF receptor fate. Biochem J. 2008;410:585–594. doi: 10.1042/BJ20071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storniolo AM, Burris H, Pegram M, Overmoyer B, Miller K, Jones S, et al. A phase I, open-label study of lapatinib ( GW572016) plus trastuzumab; a clinicaly active regimen. J Clin Onc. 2005;23:559. [Google Scholar]
- Storniolo AM, Pegram MD, Overmoyer B, Silverman P, Peacock NW, Jones SF, et al. Phase I dose escalation and pharmacokinetic study of lapatinib in combination with trastuzumab in patients with advanced ErbB2-positive breast cancer. J Clin Onc. 2008;26:3317–3323. doi: 10.1200/JCO.2007.13.5202. [DOI] [PubMed] [Google Scholar]
- Streicher KL, Willmarth NE, Garcia J, Boerner JL, Dewey TG, Ethier SP. Activation of a nuclear factor kappaB/interleukin-1 positive feedback loop by amphiregulin in human breast cancer cells. Mol Cancer Res. 2007;5:847–861. doi: 10.1158/1541-7786.MCR-06-0427. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Lai C, Riese DJ, 2nd, Diamonti AJ, Cantley LC, Carraway KL., 3rd Ligand discrimination in signaling through an ErbB4 receptor homodimer. J Biol Chem. 2000;275:19803–19807. doi: 10.1074/jbc.C901015199. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Vassar R, Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Wang F, Liu R, Lee SW, Sloss CM, Couget J, Cusack JC. Heparin-binding EGF-like growth factor is an early response gene to chemotherapy and contributes to chemotherapy resistance. Oncogene. 2007;26:2006–2016. doi: 10.1038/sj.onc.1209999. [DOI] [PubMed] [Google Scholar]
- Warren CM, Landgraf R. Signaling through ERBB receptors: multiple layers of diversity and control. Cell Signal. 2006;18:923–933. doi: 10.1016/j.cellsig.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Williams EE, Trout LJ, Gallo RM, Pitfield SE, Bryant I, Penington DJ, et al. A constitutively active ErbB4 mutant inhibits drug-resistant colony formation by the DU-145 and PC-3 human prostate tumor cell lines. Cancer Lett. 2003;192:67–74. doi: 10.1016/s0304-3835(02)00690-0. [DOI] [PubMed] [Google Scholar]
- Willmarth NE, Ethier SP. Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J Biol Chem. 2006;281:37728–37737. doi: 10.1074/jbc.M606532200. [DOI] [PubMed] [Google Scholar]
- Wilson KJ, Mill CP, Cameron EM, Hobbs SS, Hammer RP, Riese DJ., 2nd Inter-conversion of neuregulin2 full and partial agonists for ErbB4. Biochem Biophys Res Commun. 2007;364:351–357. doi: 10.1016/j.bbrc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff GS, Chiang PJ, Smith SM, Romero R, Armant DR. Epidermal growth factor-like growth factors prevent apoptosis of alcohol-exposed human placental cytotrophoblast cells. Biol Reprod. 2007;77:53–60. doi: 10.1095/biolreprod.106.057984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Zrihan-Licht S, Deng B, Yarden Y, McShan G, Keydar I, Avraham H. Csk homologous kinase, a novel signaling molecule, directly associates with the activated ErbB-2 receptor in breast cancer cells and inhibits their proliferation. J Biol Chem. 1998;273:4065–4072. doi: 10.1074/jbc.273.7.4065. [DOI] [PubMed] [Google Scholar]