Abstract
The modification or degradation of RNAs including miRNAs may play vital roles in regulating RNA functions. The polyadenylation- and exosome-mediated RNA decay is involved in the degradation of plant RNAs including the primary miRNA processing intermediates. However, plant miRNA levels are not affected by exosome depletion. Here, we report the cloning of a large number of 5′ and/or 3′ truncated versions of the known miRNAs from various tissues of Populus trichocarpa (black cottonwood). It suggests that plant miRNAs may be degraded through either 5′ to 3′ or 3′ to 5′ exonucleolytic digestion. We also show that a significant portion of the isolated miRNAs contains, at the 3′-end, one or a few post-transcriptionally added adenylic acid residues, which are distinct in length from the polyadenylate tail added to other plant RNAs for exosome-mediated degradation. Using an in vitro miRNA degradation system, where synthesized miRNA oligos were degraded in extracts of P. trichocarpa cells, we revealed that the adenylated miRNAs were degraded slower than others without adenylation. It indicates that addition of adenylic acid residues on the 3′-end plays a negative role in miRNA degradation. Our results provide new information for understanding the mechanism of miRNA degradation.
INTRODUCTION
RNA degradation is a vital mechanism in the control of RNA quality and quantity in cells. It can be processed from endonucleolytic cleavage as well as 5′ to 3′ or 3′ to 5′ exonucleolytic digestion. The polyadenylation- and exosome-mediated 3′ to 5′ RNA decay, in which RNAs are first polyadenylated by a poly(A) polymerase (PAP) complex and then degraded by an exosome, is extensively involved in the decay of plant RNAs, such as a select subset of mRNAs, tRNAs, snRNAs, snoRNAs, ncRNAs and primary miRNA (pri-miRNA) processing intermediates (1). However, levels of mature miRNAs are not affected by exosome depletion (1). It indicates the existence of a distinct mechanism for miRNA degradation in plants.
miRNAs are generated from pri-miRNAs transcribed from miRNA loci (2). In plants, pri-miRNAs are cleaved by Dicer-like 1 (DCL1) protein in the nucleus to produce miRNA precursors (pre-miRNAs) (3,4). Pre-miRNAs are further processed again by DCL1 into miRNA:miRNA* duplexes, liberating the miRNAs (21 nt). The miRNA is then loaded onto AGO1 in the cytoplasm and guides target RNA silencing through either site-specific cleavage or nondegradative translational repression of the target genes (5–8). In Arabidopsis, the miRNA:miRNA* duplexes are methylated on the 2′OH of their 3′-terminal ribose molecules by a small RNA methyltransferase known as HEN1 (9,10). Silence of the Arabidopsis HEN1 gene led to the addition of one to five uridylic acid residues on the 3′-ends of miRNAs, indicating that the 2′-O-methylation plays a role in retaining the integrity of miRNAs (11).
Here, we report the isolation of a large number of 5′ or 3′ truncated miRNAs from various tissues of Populus trichocarpa (black cottonwood), suggesting the occurrence of exonucleolytic degradation of miRNAs. We also show that a significant portion of the isolated miRNAs contains one or a few post-transcriptionally added adenylic acid residues at the 3′-end. The short adenylate tail of miRNAs is distinct from the longer polyadenylate tail added to other plant RNAs for the exosome-mediated degradation. Results of in vitro miRNA degradation assays revealed that the addition of adenylic acid residues on the 3′-end reduced miRNA degradation rate.
MATERIALS AND METHODS
Cloning and sequencing of small RNAs
Shoot tips (0.2–0.4 cm) (STs), root tips (about 0.3 cm) (RTs) and tissues of the stem cambial zone (CZ) were collected from greenhouse-grown P. trichocarpa (black cottonwood) genotype Nisqually-1 in the fast-growing season. Total RNA was isolated by the CTAB method (12). Small RNA libraries were constructed as previously described (13) with modifications. Three 3′ adaptor oligonucleotides were synthesized. Each of them was used for a tissue-specific library. They were 3′-linker 1 (5′-pCTAGGCACCATGTTCATCACx-3′; p, phosphate; x, 3′-amino-modifier C-7) used for ST small RNA library, 3′-linker 2 (5′-pCATGGCACCATGTTCATCACx-3′) for CZ small RNA library, and 3′-linker 3 (5′-pCTTGGCACCATGTTCATCACx-3′) for RT small RNA library, respectively. A 5′ adaptor oligonucleotide (5′-ATGTCGTGTGaggcaccaaa-3′, RNA/DNA version, lowercase RNA) containing hydroxyl groups at both 5′ and 3′ ends was used in the construction of all three small RNA libraries. RT-primer (5′-GATGAACATGGTGCC-3′) was used for reverse transcription. The 5′ primer used for PCR was PCR-5 (5′-GTCGTGTGAGGCACCAAA-3′). Three 3′ PCR primers were designed and each of them was used for a tissue-specific library. They were PCR-1 (5′-GATGAACATGGTGCCTAG-3′) used for ST small RNA library, PCR-2 (5′-GATGAACATGGTGCCATG-3′) for CZ small RNA library and PCR-3 (5′-GATGAACATGGTGCCAAG-3′) for RT small RNA library, respectively. The BanI digested PCR products were purified by running a polyacrylamide gel and eluted in a buffer containing 0.3 M sodium acetate, pH7.8 and 2 mM Na2EDTA. After precipitated with ethanol, cDNA was dissolved in RNase-free water and then purified using a Centri-spin-20 column (Princeton Separations). The purified cDNA was concatamerized using the T4 DNA ligase. Concatamers with sizes >70 bp were purified using a QIAquick gel extraction kit (QIAGEN). About 2 µg concatamers from each library were mixed, concentrated, and then sent to 454 Life Sciences (http://www.454.com) for sequencing using the GS 20 Sequencing System.
Analysis of miRNA sequences
The resulted sequences information obtained from 454 Life Sciences were parsed and analyzed using PERL scripts to identify the library tags, extract small RNA sequences and calculate the frequency of occurrence of the small RNA in each library. Small RNAs from 7 to 35 nt were search against the P. trichocarpa draft genome assemble v1.0 (http://genome.jgi-psf.org/Poptr1_1/Poptr1_1.home.html) using BLAST (14) to identify poplar genome-matched small RNAs. Sequences in a miRNA family were searched using the Microsoft Office Excel. The adenylated miRNAs were recognized by aligning the genome-unmatched sequences with miRNA precursors (13,15,16). The percentage of adenylated miRNA was calculated by dividing the number of adenylated miRNAs by the total amount of adenylated miRNAs and genome-matched miRNAs of a family.
Preparation of P. trichocarpa extracts
One-gram tissues from the stem CZ of P. trichocarpa were ground to a fine powder in liquid nitrogen. Proteins were extracted with 1.5 ml of 1× degradation buffer [50 mM Tris–HCl, pH 8.0, 10 mM MgCl2, 75 mM KCl, 5 mM DTT, 10 mM ascorbic acid and 30 µl of protease inhibitor cocktail (Sigma)] at 4°C, and then centrifuged at 10 000 g for 20 min at 4°C. Extracts were adjusted to 1.0 µg/µl protein by adding the 1× degradation buffer, and then stored at –80°C in aliquots after freezing in liquid nitrogen.
In vitro miRNA degradation assays
RNA oligos, miR397a-21 (5′-UCAUUGAGUGCAGCGUUGAUG-3′), miR397a-20a (5′-UCAUUGAGUGCAGCGUUGAUA-3′), miR397a-21m (5′-UCAUUGAGUGCAGCGUUGAUmG-3′; mG, 2′-O-methyl G), miR1447-21 (5′-CAGAAUUGCAGUGCCUUGAUU-3′), miR1447-20a (5′-CAGAAUUGCAGUGCCUUGAUA-3′), miR1447-20 (5′-CAGAAUUGCAGUGCCUUGAU-3′) and miR1447-19a (5′-CAGAAUUGCAGUGCCUUGAA-3′), were synthesized and HPLC-purified by IDT, Inc. For in vitro miRNA degradation assays, 5 µl of 1.0 µg/µl miRNA was added to 150 µl extracts and incubated at 25°C. Ten-microliter aliquots were removed after incubating for 0, 10, 20, 40 and 80 min, respectively. Reactions were stopped by immediately adding 10 μl 2× loading dye (8 M urea, 20 mM Tris–HCl, pH 8.0, 1 mM EDTA, pH 8.0, 0.025% xylene cyanol FF and 0.025% bromophenol blue) and denatured at 95°C for 10 min as described (17). RNAs were separated on a 15% denaturing polyacrylamide gel. In order to quantitatively determine the level of nondegraded miRNAs after incubation, the top band from a reaction was cut out. MiRNAs were eluted from gel in 0.3 M NaCl at 4°C for overnight with gentle agitation and precipitated by ethanol in the presence of glycogen. Real-time PCR quantification of miRNAs was performed as previously described (18). DNA oligos, 5′-TCATTGAGTGCAGCGTTGATG-3′, 5′-TCATTGAGTGCAGCGTTGATA-3′, 5′-CAGAATTGCAGTGCCTTGATT-3′, 5′-CAGAATTGCAGTGCCTTGATA-3′, 5′-CAGAATTGCAGTGCCTTGAT-3′ and 5′-CAGAATTGCAGTGCCTTGAA-3′, were used as miRNA specific primers for quantification of miR397a-21, miR397a-20a, miR1447-21, miR1447-20a, miR1447-20 and miR1447-19a, respectively.
RESULTS AND DISCUSSION
Isolation of miRNA sequences and their truncate variants
Small RNAs were isolated from shoot tips (STs), root tips (RTs) and tissues of the stem cambial zone (CZ) of P. trichocarpa (black cottonwood) and sequenced using the high-throughput pyrosequencing developed by 454 Life Sciences (http://www.454.com). A total of 49 993, 36 238 and 39 036 distinct sequences with a size range from 7 to 35 nt were obtained from the ST, CZ and RT small RNA libraries, respectively. Forty-nine percent of total distinct sequences match the genome (16). For the 42 P. trichocarpa miRNA families (miRBase Release 11.0, http://microrna.sanger.ac.uk/sequences/index.shtml), 27 of them had at least 10 counts of genome-matched sequences with sizes of 20–21 nt in our data set (Table 1). Concentrating on these 27 miRNA families, we further identified 5′ and/or 3′ truncate versions of miRNA. Sequences for ptc-MIR397, ptc-MIR1447 and ptc-MIR1450 are shown in Tables 2–4. Although we cannot exclude the possibility that a few truncated miRNAs are the decay product of aberrant pri-miRNA, it is unlikely that the large amount of truncated miRNAs is produced from aberrant pri-miRNA since sequences corresponding to other locations of the pri-miRNA are rare or not found. The truncated miRNAs were also isolated from wild type and hen1 mutant of Arabidopsis and were considered as products of exonuclease activity (11). Thus, these truncate versions are likely the intermediates of miRNA degradation, suggesting that miRNAs may be degraded through either 5′ to 3′ or 3′ to 5′ exonucleolytic digestion.
Table 1.
Counts of the 20–21 nt genome-matched miRNAs of the 27 families isolated from shoot tips (STs), root tips (RTs) and tissues of the stem cambial zone (CZ) of P. trichocarpa
| miRNA family | STs | CZ | RTs | Total |
|---|---|---|---|---|
| ptc-miR408 | 1425 | 1658 | 1888 | 4971 |
| ptc-miR168 | 2182 | 1664 | 1070 | 4916 |
| ptc-miR160 | 2008 | 1164 | 786 | 3958 |
| ptc-miR475 | 707 | 1244 | 320 | 2271 |
| ptc-miR171 | 1746 | 235 | 45 | 2026 |
| ptc-miR398 | 249 | 196 | 1028 | 1473 |
| ptc-miR159 | 733 | 115 | 152 | 1000 |
| ptc-miR1448 | 360 | 61 | 123 | 544 |
| ptc-miR169 | 155 | 110 | 216 | 481 |
| ptc-miR1444 | 192 | 39 | 170 | 401 |
| ptc-miR397 | 40 | 87 | 196 | 323 |
| ptc-miR472 | 85 | 22 | 164 | 271 |
| ptc-miR166 | 81 | 8 | 166 | 255 |
| ptc-miR167 | 75 | 5 | 100 | 180 |
| ptc-miR482 | 69 | 4 | 17 | 90 |
| ptc-miR396 | 0 | 62 | 11 | 73 |
| ptc-miR394 | 31 | 28 | 3 | 62 |
| ptc-miR319 | 32 | 11 | 0 | 43 |
| ptc-miR393 | 6 | 33 | 3 | 42 |
| ptc-miR473 | 2 | 9 | 30 | 41 |
| ptc-miR1447 | 21 | 3 | 14 | 38 |
| ptc-miR1446 | 1 | 9 | 25 | 35 |
| ptc-miR476 | 5 | 10 | 19 | 34 |
| ptc-miR172 | 9 | 6 | 14 | 29 |
| ptc-miR162 | 6 | 1 | 18 | 25 |
| ptc-miR164 | 8 | 16 | 1 | 25 |
| ptc-miR1450 | 4 | 4 | 7 | 15 |
Table 2.
Sequences of the genomic loci of ptc-MIR397 family and the cloned sequences isolated from shoot tips (STs), root tips (RTs) and tissues of the stem cambial zone (CZ) of P. trichocarpa
 |
Adenylic acid residues added on the 3′-ends of miRNAs are shown in bold.
Table 3.
Sequences of the genomic locus of ptc-MIR1447 family and the cloned sequences isolated from shoot tips (STs), root tips (RTs) and tissues of the stem cambial zone (CZ) of P. trichocarpa
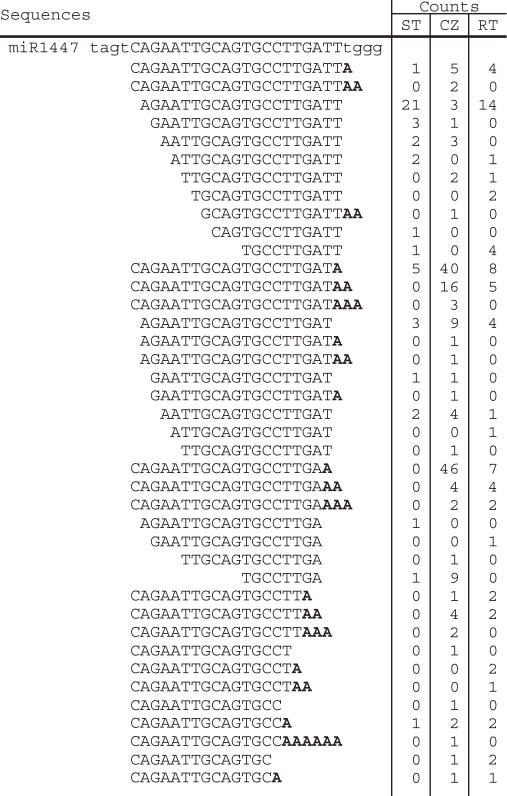 |
Adenylic acid residues added on the 3′-ends of miRNAs are shown in bold.
Table 4.
Sequences of the genomic loci of ptc-MIR1450 family and the cloned sequences isolated from shoot tips (STs), root tips (RTs) and tissues of the stem cambial zone (CZ) of P. trichocarpa
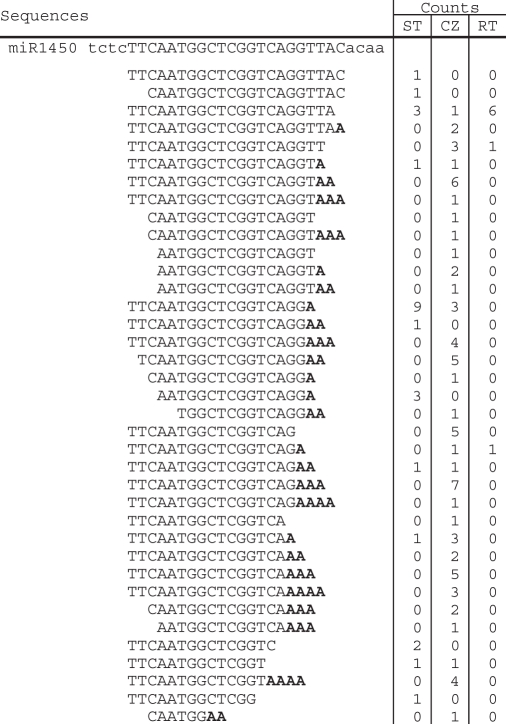 |
Adenylic acid residues added on the 3′-ends of miRNAs are shown in bold.
Identification of adenylated miRNA sequences
Adenylated miRNA species for most of the 27 miRNA families were identified by analyzing the genome-unmatched sequences. Although 15 of the 27 families have low ratios (<5%) of the counts of adenylated miRNAs to the counts of all the miRNAs of a family (hereinafter called the adenylated miRNA percentage), the ratios for the remaining 12 families are greater (from 5 to 72%) (Table 5). It should be emphasized that the adenylic acid residue(s) at the 3′-ends of some genome-matched miRNA sequences could also be post-transcriptionally added, such as 5′-TCATTGAGTGCAGCGTTGA-3′ for miR397a, 5′-AGAATTGCAGTGCCTTGA-3′ for miR1447, and 5′-TTCAATGGCTCGGTCA-3′ for miR1450 (Tables 2–4); however, these miRNAs were not considered as adenylated because it is technically difficult to recognize the source of the adenylic acid residues at their 3′-ends. Thus, the actual counts of adenylated miRNAs might be higher. The high percentage of adenylated miRNAs is unlikely a result of sequencing errors. It is also unlikely that these adenylic acid residues were artificially incorporated during the cloning processes. Adenylated miRNAs have been reported from Arabidopsis (11) and P. trichocarpa (15). Thus, we conclude that adenylation of miRNAs occurred in vivo.
Table 5.
Adenylation of 12 miRNA families isolated from P. trichocarpa
| miRNA family | Genome- matched | Adenylated | Total counts | Adenylation (%) |
|---|---|---|---|---|
| ptc-MIR1450 | 29 | 76 | 105 | 72 |
| ptc-MIR1447 | 106 | 180 | 286 | 63 |
| ptc-MIR397 | 1027 | 546 | 1573 | 35 |
| ptc-MIR1444 | 536 | 122 | 658 | 19 |
| ptc-MIR159 | 3801 | 796 | 4606 | 17 |
| ptc-MIR172 | 72 | 12 | 84 | 14 |
| ptc-MIR396 | 1894 | 282 | 2176 | 13 |
| ptc-MIR166 | 440 | 41 | 481 | 9 |
| ptc-MIR167 | 727 | 45 | 772 | 6 |
| ptc-MIR319 | 1391 | 82 | 1473 | 6 |
| ptc-MIR475 | 3684 | 198 | 3882 | 5 |
| ptc-MIR1448 | 924 | 48 | 972 | 5 |
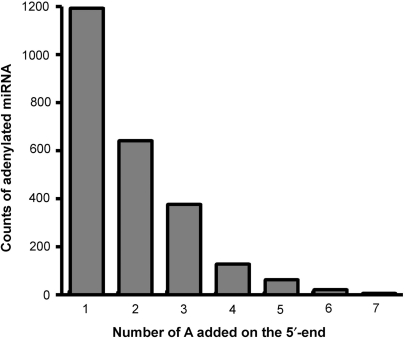
The number of adenylic acid residues added on the 3′-end of a miRNA ranges from one to seven (Figure 1). About half of the adenylated miRNAs have one post-transcriptionally added residue. The percentages for miRNAs with two and three post-transcriptionally added adenylic acid residues are 26% and 15%, respectively. These short tails of adenylic acid residues are obviously distinct in length from the long poly(A) tails added on the 3′-end of other RNAs that are consequently degraded by an exosome (19). Addition of one adenylic acid residue on the 3′-ends was previously observed for other small RNAs, such as signal recognition particle (SRP) RNA and U2 snRNA isolated from yeast, frog and human cells (20,21), suggesting that it occurs among a variety of small RNA species of eukaryotes. However, the function of this adenylation has poorly been characterized.
Figure 1.
MiRNAs with post-transcriptionally added adenylic acid residues. Total counts of adenylated miRNAs for 12 families isolated from P. trichocarpa are shown. The number of adenylic acid residues added on the 3′-end of a miRNA sequence ranges from one to seven, respectively.
Degradation of adenylated miRNAs
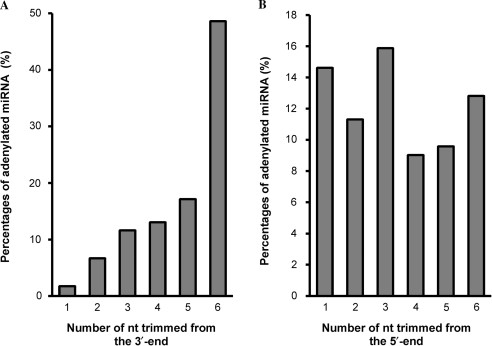
In order to understand the role of miRNA adenylation, we analyzed the 5′ and 3′ truncate ptc-miRNA of the 12 families having the adenylated miRNA percentages >5%. As shown in Figure 2A, <2% of miRNAs are adenylated when 1 nt is trimmed from the 3′-ends; while the adenylated miRNA percentage increases to about 50% when 6 nt are trimmed. Percentages of adenylated miRNAs continuously increase as 1–6 nt are trimmed from the 3′-ends. Such a trend was not observed for sequences with nucleotides trimmed from the 5′-ends (Figure 2B). These results strongly indicate that adenylation is associated with the 3′ to 5′ miRNA degradation. The addition of adenylic acid residues on the 3′-end of a miRNA and the 5′ to 3′exonucleolytic digestion are likely two independent events.
Figure 2.
Association of adenylation with miRNA degradation. The adenylated miRNA percentages are shown for sequences with 1–6 nt trimmed from the 3′- (A) or 5′-ends (B).
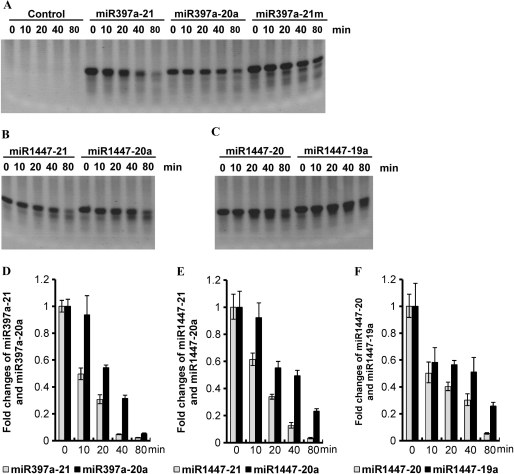
In order to study the role of miRNA adenylation experimentally, we developed an in vitro miRNA degradation system. In this system, RNase-containing extracts were prepared from cells in and around the stem CZ of greenhouse-grown P. trichocarpa. We tested the degradation rates of ptc-miR397a with or without adenylation on the 3′-end. We also studied the degradation of methylated ptc-miR397a, since the 2′-O-methyl group was found at the 3′-end of plant miRNAs produced in vivo and was suggested to play a role in retaining the integrity of miRNAs (11). Our results showed that both adenylation and methylation delayed the degradation of ptc-miR397a degradation (Figure 3A and D). It has been reported that uridylation of plant miRNAs in the absence of methylation could have a protective role against exonucleolytic degradation (17). To compare degradation rates of the adenylated- and the uridylated-miRNAs, we analyzed ptc-miR1447 using our in vitro miRNA degradation system. The 21-nt ptc-miR1447 has two Us at the 3′-end. Replacement of the last U with an A resulted in a delay of degradation (Figure 3B and E). Similar result was also observed for the 20 nt, 3′ truncate version of ptc-miR1447 (Figure 3C and F). Taken together, the adenylated miRNAs are degraded in our in vitro assays slower than others without adenylation. While the in vivo relevance of these observations remains to be formally established, the results suggest that addition of adenylic acid residues on the 3′-end plays a negative role in miRNA degradation.
Figure 3.
In vitro miRNA degradation assays. RNA oligos (1.0 µg per lane) were incubated with RNase-containing extracts (10 µg protein per reaction) prepared from cells in and around the stem cambial zone of greenhouse-grown P. trichocarpa at 25°C for 0, 10, 20, 40 and 80 min, respectively, and then separated on a 15% denaturing polyacrylamide gel (A–C). Reactions without the addition of RNA oligos were used as controls (A). The level of nondegraded miRNAs after incubation was quantitated by the real-time PCR method as previously described (18). Levels of miRNA from reactions incubated for 0 min were arbitrarily set to 1. The data are means of three measurments ± SD (D–F).
Plant tissue-dependence of miRNA adenylation
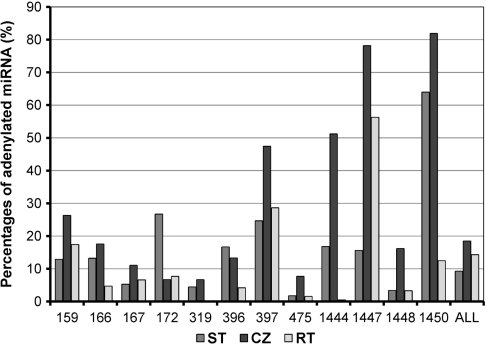
In order to know the tissue-dependency of miRNA adenylation, miRNAs isolated from ST, CZ and RT were analyzed individually. Again, we focused on the 12 miRNA families having percentages of adenylated miRNAs >5%. We found that the adenylated miRNA percentage isolated from CZ (∼19%) is about twice as high as that from ST (∼9%) (Figure 4). Among the 12 miRNA families examined, 10 show the greatest adenylation percentage for miRNAs isolated from CZ of P. trichocarpa (Figure 4). It includes an miRNA (ptc-miR475) expressed highly in CZ and nine having lower expression levels in CZ than in ST and RT (Table 1 and Figure 4), suggesting that the percentage of adenylated miRNA is independent with the expression level of miRNA. Thus, miRNA adenylation seems tissue-dependent. The high percentage of adenylated miRNAs isolated from CZ could be associated with the active metabolism of cells in and around the cambial zone of P. trichocarpa in the fast-growing season, when the tissues were collected. Further study to find the reason causing high percentage of adenylated miRNAs in CZ may help to add new insights into the regulatory mechanism of miRNA degradation.
Figure 4.
Percentages of adenylated miRNAs isolated from shoot tips (ST), root tips (RT) and tissues of the stem cambial zone (CZ). Percentages of adenylated miRNAs from each family and from all of the 12 families are shown. The 12 miRNA families include ptc-MIR159, ptc-MIR166, ptc-MIR167, ptc-MIR172, ptc-MIR319, ptc-MIR396, ptc-MIR397, ptc-MIR475, ptc-MIR1444, ptc-MIR1447, ptc-MIR1448 and ptc-MIR1450.
Ptc-MIR1450, ptc-MIR1447 and ptc-MIR397 have the overall adenylation percentage >35% (Table 5). Ptc-MIR1450 and ptc-MIR1447 are two P. trichocarpa specific miRNA families; while ptc-MIR397 is a conserved miRNA family among various plant species, including Arabidopsis, rice and P. trichocarpa (15,16,22–24). The adenylation percentages for ptc-miR1450 sequences isolated from ST, CZ and RT are 64%, 81.9% and 12.5%, respectively (Figure 4). Similarly, the ptc-miR1447 and ptc-miR397 sequences isolated from CZ have greater adenylation percentage than those isolated from ST and RT (Figure 4). Ptc-miR1450 is predicted to target a leucine-rich repeat transmembrane protein kinase and two function-unknown proteins, and has the highest expression in developing xylem among the detected tissues including leaf and phloem (15). The expression of ptc-miR1450 in xylem is significantly downregulated in plant response to mechanical stress (15). Ptc-miR1447 is also mechanical stress-responsive and target to a leucine-rich transmembrane protein kinase. The other targets of ptc-miR1447 include six ankyrin repeat family proteins, a disease-resistance protein, a translationally controlled tumor protein homolog (TCTP), a β-fructofuranosidase, an oxidoreductase and a function-unknown protein (15). MiR397 has been found to target a large number of laccase genes for cleavage in various plant species, including Arabidopsis and P. trichocarpa (15,25). The plant laccase is the first enzyme shown to be able to polymerize lignin monomers in vitro (26) and has been found to be vital for wood formation (27–31). These results suggest that miR1450 and miR1447 and miR397 play important regulatory roles in xylem development. However, the significance of the high adenylation percentage of these miRNAs is currently unknown.
In summary, although the biogenesis of plant miRNAs has been intensely studied, little is known about the mechanism of miRNA degradation (17). Through the analysis of small RNAs isolated from P. trichocarpa, we found that (i) a large number of 5′ and/or 3′ truncate versions of miRNAs are present; (ii) a significant portion of the truncated miRNAs contains one or a few post-transcriptionally added adenylic acid residues at the 3′-ends; (iii) the percentages of adenylated miRNAs continuously increase as 1–6 nt are trimmed from the 3′-ends; and (iv) adenylation of plant miRNAs could be tissue-dependent. Using an in vitro miRNA degradation system, we showed that adenylation of miRNAs in P. trichocarpa played a protective role as uridylation does in the miRNA methyltrasferase gene-silenced Arabidopsis plants (11,17). Thus, the short adenylate tail adding to a plant miRNA has a distinct function with the poly(A) tail involving in the exosome-mediated RNA decay. PAPs responsible for adding the transient poly(A) tail for RNA degradation have been identified in various organisms (19). They belong to the large noncanonical PAP family and probably have substrate specificity of RNA species (32). However, enzymes associated with miRNA adenylation remain to be elucidated.
FUNDING
The North Carolina State University Forest Biotechnology Industrial Research Consortium. Funding for open access charge: The North Carolina State University Forest Biotechnology Industrial Research Consortium.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We thank J. Jakobek for comments.
REFERENCES
- 1.Chekanova JA, Gregory BD, Reverdatto SV, Chen H, Kumar R, Hooker T, Yazaki J, Li P, Skiba N, Peng Q, et al. Genome-wide high-resolution mapping of exosome substrates reveals hidden features in the Arabidopsis transcriptome. Cell. 2007;131:1340–1353. doi: 10.1016/j.cell.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park W, Li J, Song R, Messing J, Chen X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 2002;12:1484–1495. doi: 10.1016/s0960-9822(02)01017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2:0642–0652. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen X. MicroRNA biogenesis and function in plants. FEBS Lett. 2005;579:5923–5931. doi: 10.1016/j.febslet.2005.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- 8.Mallory AC, Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat. Genet. 2006;38:S31–S36. doi: 10.1038/ng1791. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Ebright YW, Yu B, Chen X. HEN1 recognizes 21-24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide, Nucleic Acids Res. 2006;34:667–675. doi: 10.1093/nar/gkj474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, Steward R, Chen X. Methylation as a crucial step in plant microRNA biogenesis, Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Yang Z, Yu B, Liu J, Chen X. Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr. Biol. 2005;15:1501–1507. doi: 10.1016/j.cub.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993;11:113–116. [Google Scholar]
- 13.Lu S, Sun Y-H, Shi R, Clark C, Li L, Chiang VL. Novel and mechanical stress-responsive microRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell. 2005;17:2186–2203. doi: 10.1105/tpc.105.033456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 15.Lu S, Sun Y-H, Chiang VL. Stress-responsive microRNAs in Populus trichocarpa. Plant J. 2008;55:131–151. doi: 10.1111/j.1365-313X.2008.03497.x. [DOI] [PubMed] [Google Scholar]
- 16.Tuskan GA, DiFazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandran V, Chen X. Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science. 2008;321:1490–1492. doi: 10.1126/science.1163728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques. 2005;39:519–525. doi: 10.2144/000112010. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JT. RNA turnover: unexpected consequences of being tailed. Curr. Biol. 2005;15:R635–R638. doi: 10.1016/j.cub.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Sinha KM, Gu J, Chen Y, Reddy R. Adenylation of small RNAs in human cells. Development of a cell-free system for accurate adenylation on the 3′-end of human signal recognition particle RNA. J. Biol. Chem. 1998;273:6853–6859. doi: 10.1074/jbc.273.12.6853. [DOI] [PubMed] [Google Scholar]
- 21.Perumal K, Gu J, Reddy R. Evolutionary conservation of post-transcriptional 3′ end adenylation of small RNAs: S. cerevisiae signal recognition particle RNA and U2 small nuclear RNA are post-transcriptionally adenylated. Mol. Cell Biochem. 2000;208:99–109. doi: 10.1023/a:1007098122583. [DOI] [PubMed] [Google Scholar]
- 22.Barakat A, Wall KP, Diloretto S, Depamphilis CW, Carlson JE. Conservation and divergence of microRNAs in Populus. BMC Genomics. 2007;8:481. doi: 10.1186/1471-2164-8-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol. Cell. 2004;14:787–799. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Sunkar R, Zhu JK. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell. 2004;16:2001–2019. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel-Ghany SE, Pilon M. J. Biol. Chem. MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. 10.1074/jbc.M801406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freudenberg K. Biosynthesis and constitution of lignin. Nature. 1959;183:1152–1155. doi: 10.1038/1831152a0. [DOI] [PubMed] [Google Scholar]
- 27.Bao W, O’Malley DM, Whetten R, Sederoff RR. A laccase associated with lignification in loblolly pine xylem. Science. 1993;260:672–674. doi: 10.1126/science.260.5108.672. [DOI] [PubMed] [Google Scholar]
- 28.Dean JFD, Eriksson K-EL. Laccase and the deposition of lignin in vascular plants. Holzforschung. 1994;48:21–33. [Google Scholar]
- 29.Driouch A, Laine A-C, Vian B, Faye L. Characterization and localization of laccase forms in stem and cell cultures of sycamore. Plant J. 1992;2:13–24. [Google Scholar]
- 30.Ranocha P, Chabannes M, Chamayou S, Danoun S, Jauneau A, Boudet AM, Goffner D. Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol. 2002;129:145–155. doi: 10.1104/pp.010988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato Y, Wuli B, Sederoff R, Whetten R. Molecular cloning and expression of eight laccase cDNAs in loblolly pine (Pinus taeda) J. Plant Res. 2001;114:147–155. [Google Scholar]
- 32.Zimmer SL, Fei Z, Stern DB. Genome-based analysis of Chlamydomonas reinhardtii exoribonucleases and poly(A) polymerases predicts unexpected organellar and exosomal features. Genetics. 2008;179:125–136. doi: 10.1534/genetics.107.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]