Abstract
Regulatory T (Treg) cells are essential for maintaining peripheral tolerance, preventing autoimmune diseases and limiting chronic inflammatory diseases. However, they also limit beneficial responses by suppressing sterilizing immunity and limiting anti-tumour immunity. Given that Treg cells can have both beneficial and deleterious effects, there is considerable interest in determining their mechanisms of action. In this Review, we discuss the basic mechanisms used by Treg cells to mediate suppression, and discuss whether one or many of these mechanisms are likely to be crucial for Treg-cell function. In addition, we present the hypothesis that effector T cells may not be ‘innocent’ parties in this suppressive process and might in fact potentiate Treg-cell function.
Several sophisticated regulatory mechanisms are used to maintain immune homeostasis, prevent autoimmunity and moderate inflammation induced by pathogens and environmental insults. Chief amongst these are regulatory T (Treg) cells that are now widely regarded as the primary mediators of peripheral tolerance. Although Treg cells play a pivotal role in preventing autoimmune diseases, such as type 1 diabetes1,2, and limiting chronic inflammatory diseases, such as asthma and inflammatory bowel disease (IBD)3,4, they also block beneficial responses by preventing sterilizing immunity to certain pathogens5,6 and limiting anti-tumour immunity7. A seminal advance in the analysis of Treg cells came with the identification of a key transcription factor, forkhead box P3 (FOXP3), that is required for their development, maintenance and function8,9. Mice and patients that lack FOXP3 develop a profound autoimmune-like lymphoproliferative disease that graphically emphasizes the importance of Treg cells in maintaining peripheral tolerance10-12 (BOX 1). Although FOXP3 has been proposed as the master regulator of Treg cells that controls the expression of multiple genes that mediate their regulatory activity13,14, this has been recently challenged raising the possibility that other transcriptional events may operate upstream of and/or concurrently with FOXP3 to mediate Treg-cell development15.
Box 1. Scurfy mice: misplaced mechanistic expectations?
Mice that carry a spontaneous loss-of-function mutation (known as Scurfy mice) or a deletion of Foxp3 develop a fatal autoimmune-like disease with hyperresponsive CD4+ T cells9,12. More recently Foxp3:diptheria toxin receptor (DTR) knockin mice have allowed for the selective depletion of Treg cells following DT treatment105. These mice have been invaluable for dissecting the role of Foxp3 in Treg-cell function. Given the profound phenotype in these mice, there is a general expectation that genetic disruption of any key Treg-cell inhibitory molecule or mechanism would probably result in a Scurfy-like phenotype. Of course, it is also possible that deletion of a key Treg-cell gene may be more synonymous with DT-mediated Treg-cell depletion where Foxp3 may still serve to prevent expression of proinflammatory cytokines105. Nonetheless, this has lead to the notion that if mutant mice don't have a Scurfy-like or a Treg-cell-depleted phenotype, then the disrupted gene probably isn't important for Treg-cell function. This may not necessarily be correct. Indeed, it is possible that no mouse lacking a Treg-cell inhibitory effector molecule will ever be generated that develops a profound, spontaneous autoimmune disease21. It should be noted that mutant mice that are Helicobacter spp. and/or Citrobacter rodentium positive may have an exacerbated phenotype, as several studies have shown that opportunistic enteric bacteria can significant exacerbate gut pathology4. Ultimately, the occurrence of disease in knockout mice will depend on whether Treg cells rely on a single or multiple suppressive mechanisms. Given the number of genes induced or modulated by FOXP3, it is probable that a programme of intrinsic and extrinsic regulation is induced that involves multiple proteins9,13. Therefore, it would not be surprising if deletion of a single molecule does not provoke the profound Scurfy-like phenotype seen in mice that lack Foxp3.
While Foxp3 has proven to be an invaluable marker for murine Treg cells, its role in human Treg cells is less straightforward (see BOX 2 for a discussion of Treg-cell markers). Humans that lack FOXP3 develop immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX), a severe autoimmune disease that presents early in infancy. Although FOXP3 appears to be required for human Treg-cell development and function, expression of FOXP3 alone is clearly not sufficient as a significant percentage of human activated T cells express FOXP3 and yet do not possess regulatory activity16-20. Furthermore, induction of FOXP3 in human T cells by transforming growth factor-β (TGFβ) does not confer a regulatory phenotype, in contrast to their murine counterparts20. Consequently, FOXP3 is not a good marker for human Treg cells (BOX 2). Whether this distinction is due to intrinsic differences between mouse and human FOXP3 and/or a requirement for an additional cofactor/transcription factor is an important question that needs to be resolved.
Box 2. Treg-cell markers.
Identifying discriminatory cell surface markers for the characterization and isolation of Treg cells has always been a critical goal. Although excellent markers exist for murine Treg cells, this goal has remained elusive for human Treg cells. Traditionally, murine and human Treg cells have been characterized as CD4+CD25+ (also known as interleukin-2 receptor α (IL-2Rα)). Indeed, murine Treg cells can be effectively isolated based on staining for CD4+CD25+CD45RBlow expression. However, the purity of isolated human Treg cells has always been an issue because T cells up-regulate CD25 upon activation106. Indeed, during the influenza or allergy season a substantial proportion of human CD4+ T cells can express CD25. Although the identification of forkhead box P3 (Foxp3) as a key regulator of Treg-cell development and function has facilitated their identification in the mouse8, many activated (non-regulatory) human T cells express FOXP3, precluding it as a useful marker for human Treg cells16-20. Consequently, the search for Treg-cell-specific cell-surface markers, particularly in humans, has continued in earnest with a growing number of candidates proposed (reviewed by Zhao and colleagues107). For instance, it was shown that the expression of CD127 (also known as IL-7R) is down-regulated on Treg cells and that this could be used to increase the purity of human Treg-cell isolation. Indeed, there is a 90% correlation between CD4+CD25+CD127low T cells and FOXP3 expression108,109. In addition, it was recently found that Treg cells expressed a higher level of folate receptor 4 (FR4) compared with activated effector T cells110. It is also important to recognize that Treg cells, like their T helper cell counterparts, may be heterogeneous and thus a collection of cell surface markers could facilitate their isolation and functional characterization. Indeed, such heterogeneity has recently been described based on differential expression of HLA-DR or CCR6102,103. However, the general use of both markers remains to be fully established so it is quite probable that the search for better Treg-cell markers will continue for some time.
Significant progress has been made over the last few years in delineating the molecules and mechanisms that Treg cells use to mediate suppression21,22. In this Review, we outline our current understanding of the mechanisms used by Treg cells to mediate suppression, and the challenges that lie ahead in defining their mode of action. We also discuss whether Treg cells are likely to depend on one, a few or many of these mechanisms. In addition, we propose that effector T cells may have a significant role in boosting and/or modulating Treg-cell function. Unless stated, we focus here primarily on the mechanisms that are used by thymus-derived natural CD4+CD25+ FOXP3+ Treg cells.
Basic mechanisms of Treg-cell function
Defining the mechanisms of Treg-cell function is clearly of crucial importance. Not only would this provide insight into the control processes of peripheral tolerance but it would probably provide a number of potentially important therapeutic targets. Although this quest has been ongoing since interest in Treg cells was reignited in 199523, there has been significant progress in the last few years. From a functional perspective, the various potential suppression mechanisms of Treg cells can be grouped into four basic ‘modes of action’: suppression by inhibitory cytokines, suppression by cytolysis, suppression by metabolic disruption, and suppression by modulation of dendritic-cell (DC) maturation or function (FIG. 1).
Figure 1. Basic mechanisms used by Treg cells.
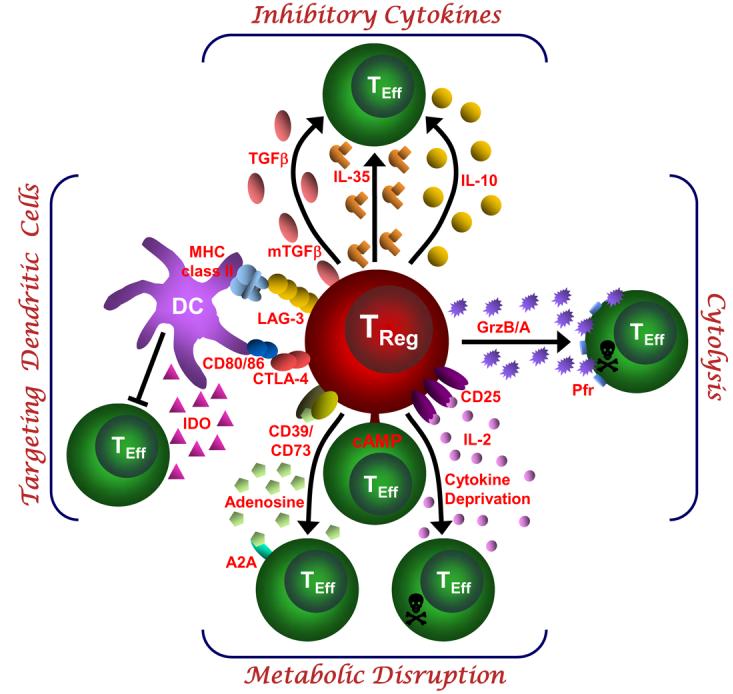
This schematic depicts the various regulatory T (Treg)-cell mechanisms arranged into four groups centred around four basic modes of action. ‘Inhibitory cytokines’ include interleukin-10 (IL-10), interleukin-35 (IL-35) and transforming growth factor-β (TGF-β). ‘Cytolysis’ includes granzyme-A- and granzyme-B-dependent and perforin-dependent killing mechanisms. ‘Metabolic disruption’ includes high affinity IL-2 receptor α (CD25)-dependent cytokine-deprivation-mediated apoptosis, cyclic AMP (cAMP)-mediated inhibition, and CD39- and/or CD73-generated, adenosine–purinergic adenosine receptor (A2A)-mediated immunosuppression. ‘Targeting dendritic cells’ includes mechanisms that modulate DC maturation and/or function such as lymphocyte activation gene-3 (LAG3; also known as CD223)–MHC-class-II-mediated suppression of DC maturation, and cytotoxic T lymphocyte antigen-4 (CTLA4)–CD80/CD86-mediated induction of indoleamine 2,3-dioxygenase (IDO), which is an immunosuppressive molecule, by DCs.
Suppression by inhibitory cytokines
Inhibitory cytokines, such as interleukin-10 (IL-10) and TGFβ, have been the focus of considerable attention as a mechanism of Treg-cell-mediated suppression. There has also been significant interest in their ability to generate induced (also known as adaptive) Treg-cell populations, either naturally in vivo or experimentally as a potential therapeutic modality (BOX 3). Although the general importance of IL-10 and TGFβ as suppressive mediators is undisputed, their contribution to the function of thymus-derived, natural Treg cells is still a matter of debate24. This is partly due to the general perception that Treg cells function in a contact-dependent manner25,26. Indeed, in vitro studies using neutralizing antibodies or T cells that are unable to produce or respond to IL-10 and TGFβ suggested that these cytokines may not be essential for Treg-cell function25-28. However, this contrasts with data from in vivo studies29,30.
Box 3. Induced or adaptive Treg cells: development and mode of action.
Naturally occurring FOXP3+CD4+CD25+ Treg cells develop in the thymus and display a diverse T-cell receptor (TCR) repertoire that is specific for self-antigens111,112. However, Treg cells can also be ‘induced’, ‘adapted’ or ‘converted’ from effector T cells during inflammatory processes in peripheral tissues, or experimentally generated as a possible therapeutic29,113,114. For instance, T regulatory 1 cells (Tr1) and T helper 3 cells (Th3) can be generated experimentally by, and mediate their suppressive activity through interleukin-10 (IL-10) and transforming growth factor-β (TGFβ), respectively114,115. Typically, these regulatory populations do not express FOXP3. In vivo, it has recently been suggested that stimulation of mouse effector T cells by CD103+ dendritic cells (DCs) in the presence of TGFβ and retinoic acid induces the generation of Foxp3+ T cells in the gut-associated lymphoid tissue (GALT)116-121. Furthermore, Treg cells can be preferentially induced in the periphery by exposure to αVβ8-integrin-expressing DCs122 or suppressor of cytokine signalling 3 (Socs3)−/− DCs123. Interestingly, independent of its role in generating induced Treg cells, TGFβ may also have an important role in helping to maintain Foxp3 expression in natural Treg cells124, a process that can be blocked by IL-4 or interferon-γ (IFNγ)125. In contrast to mouse T cells, FOXP3 induction by TCR stimulation in the presence of TGFβ in human T cells does not confer a regulatory phenotype20. The mechanism of action of adaptive Treg cells may not necessarily be restricted to suppressive cytokines. Indeed, human adaptive Treg cells (CD4+CD45RA+ T cells stimulated with CD3- and CD46-specific antibodies) have also been shown to express granzyme B and killing target cells in a perforin-dependent manner126. In contrast to natural Treg cells, induced Treg cells often have a restricted specificity for particular cell types, tumours or foreign antigens127. Therefore, induced Treg cells may be ideally suited to respond to infectious agents. This may also be of particular importance in the GALT and in the tumour microenvironment where TGFβ drives the conversion of induced Treg cells118,128. A significant challenge in deciphering data from in vivo experiments is to assess the contribution of natural Treg cells versus induced Treg cells, and to determine whether inhibitory molecules, such as IL-10 or TGFβ, were derived from the former or the latter (or elsewhere).
In allergy and asthma models, evidence suggests that both natural and antigen-specific Treg cells control disease in a manner that is, in part, dependent on IL-1029 and in some reports dependent on both IL-10 and TGFβ31. Adoptive transfer of allergen-specific Treg cells induced significant IL-10 production by CD4+ effector T cells in the lung following allergen challenge and this Treg-cell-mediated control of disease was reversed by treatment with an IL-10-receptor-specific antibody32. However, suppression of allergic inflammation and airway hyper-reactivity, and increased production of IL-10 still occurred following transfer of IL-10-deficient Treg cells, suggesting that Treg cells can suppress the Th2-driven response to allergens in vivo through an IL-10-dependent mechanism, but that the production of IL-10 by Treg cells themselves is not required for the suppression observed. This contrasts with a recent study suggesting that the Treg-cell-specific ablation of IL-10 expression resulted in increased lung allergic inflammation and hyperreactivity33.
This scenario might occur in other disease models. For instance, the effects of IL-10 can only be partially attributed to Treg-cell-derived IL-10 in the immune response to hepatitis B virus34 and in the allograft tolerance response elicited by splenocytes exposed to non-inherited maternal antigens35. Recently, it was also shown that IL-10 is crucial for the control of various infections in which Treg cells have been reported to be involved including Mycobacterium tuberculosis36, Toxoplasma gondii37, Leishmania major38, and Trichinella spiralis39. However, Treg cells were not the source of IL-10 in all of these infection models.
By contrast, several studies have shown that IL-10 production by Treg cells is essential for the prevention of colitis in mouse models of IBD40. Moreover, it appears that the tumour microenvironment promotes the generation of FOXP3+ Treg cells that mediate IL-10-dependent, cell-contact independent, suppression41. Similarly, in UV-radiation-induced carcinogenesis, IL-10 production by Treg cells appears to be important for blocking anti-tumour immunity42. IL-10 produced by Treg cells also appears to be crucial for IL-10-mediated tolerance in a model of hepatitis induced by concanavalin A43 and tolerance to bacterial and viral superantigens44. In addition, recent papers suggest new roles for Treg-cell-derived IL-10 in the induction of feto-maternal tolerance45 and B-cell-enhanced recovery from experimental autoimmune encephalomyelitis46. Collectively, the picture that appears to be emerging is that the relative importance of Treg-cell-derived IL-10 is very dependent on the target organism or disease and on the experimental system. Furthermore, the Treg-cell-specific deletion of IL-10 did not result in the development of spontaneous systemic autoimmunity, but did result in enhanced pathology in the colon of older mice and in the lungs of mice with induced airway hypersensitivity, suggesting that the function of Treg-cell-derived IL-10 may be restricted to controlling inflammatory responses induced by pathogens or environmental insults33.
While some early in vitro studies using neutralizing antibodies to TGFβ or Treg cells lacking TGFβ25,47 indicated that TGFβ was not required for natural Treg-cell function, other studies, both in vitro and in vivo suggested a critical role for Treg-cell surface bound TGFβ48,49. Therefore, the importance of TGFβ for natural Treg-cell function has also been a controversial topic. Indeed, there has been considerably more focus recently on the importance of TGFβ in the development of induced Treg cells and perhaps in Treg-cell maintenance in general (BOX 3). However, there are studies that suggest that TGFβ produced by Treg cells may directly participate in effector T-cell suppression. For instance, effector T cells that are resistant to TGFβ-mediated suppression cannot be controlled by Treg cells in an IBD model50. In addition, TGFβ produced by Treg cells has been found to be important in the control of the host immune response to M. tuberculosis36, suppression of allergic responses31 and prevention of colitis in an IBD model51. Interestingly, TGFβ produced by Treg cells has also been implicated in limiting anti-tumour immunity in head and neck squamous-cell carcinoma52 and in follicular lymphoma53 by rendering T cells unresponsive to the tumour. TGFβ also appears to limit the anti-tumour activity of cytokine-induced killer cells54.
Membrane-tethered TGFβ can also mediate suppression by Treg cells in a cell-cell contact-dependent manner48. Treg cells can control islet infiltration of CD8+ T cells and delay the progress of diabetes through membrane-tethered TGFβ49. However, experiments using mice deficient in TGFβ-receptor (TGFβR) signalling in effector T cells or using TGFβ or TGFβR blocking reagents failed to show that membrane-tethered TGFβ is required for natural Treg-cell development or function47. More recently, however, interest in membrane-tethered TGFβ has re-surfaced with the description of a previously unappreciated role for it in the tumour microenvironment. TGFβ associated with tumour exosome membranes appears to enhance the suppressive function of Treg cells and skew T cells away from their effector functions and towards regulatory functions55. Furthermore, ovalbumin-induced airway inflammation can be attenuated by heme oxygenase-1 through membrane-tethered TGFβ and IL-10 secretion by Treg cells56, a process that activates the Notch1–HES1 (hairy and enhancer of split 1) axis in target cells57. Thus, in light of the most current data, it now appears that soluble and/or membrane-tethered TGFβ may have a previously unappreciated role in natural Treg-cell function.
Recently, a new inhibitory cytokine, IL-35, has been described that is preferentially expressed by Treg cells and is required for their maximal suppressive activity58. IL-35 is a novel member of the IL-12 heterodimeric cytokine family and is formed by the pairing of Epstein–Barr virus-induced gene 3 (Ebi3), which normally pairs with p28 to form IL-27, and p35 (also known as Il12a), which normally pairs with p40 to form IL-12. Both Ebi3 and Il12a are preferentially expressed by murine Foxp3+ Treg cells58,59, but not resting or active effector T cells, and are significantly upregulated in actively suppressing Treg cells58. As predicted for a heterodimeric cytokine, both Ebi3−/− and Il12a−/− Treg cells had significantly reduced regulatory activity in vitro and failed to control homeostatic proliferation and cure IBD in vivo. This precise phenocopy suggested that IL-35 is required for the maximal suppressive activity of Treg cells. Importantly IL-35 was not only required but sufficient, as ectopic expression of IL-35 conferred regulatory activity on naive T cells and recombinant IL-35 suppressed T cell proliferation in vitro58. Although IL-35 is an exciting addition to the Treg-cell portfolio, there is clearly much that remains to be defined about this cytokine and its contribution to Treg-cell function. For instance, it remains to be determined if IL-35 suppresses the development and/or function of other cell types such as DCs and macrophages.
It is now clear that three inhibitory cytokines, IL-10, IL-35 and TGFβ, are key mediators of Treg-cell function. Although they are all inhibitory, the extent to which they are utilized in distinct pathogenic/homeostatic settings differs suggesting a non-overlapping function, which needs further refinement.
Suppression by cytolysis
Cytolysis mediated through secretion of granzymes had long been considered the forte of natural killer (NK) cells and cytotoxic CD8+ T lymphocytes (CTLs) (reviewed by Lieberman in REF. 60). However, many human CD4+ T cells exhibit cytotoxic activity. Consistent with this, activated human natural Treg cells have been shown to express granzyme A. Furthermore, target cell killing was mediated by granzyme A and perforin through adhesion of CD1861.
By contrast, murine CD4+ T cells are not cytolytic and therefore it was surprising that early gene expression arrays showed that granzyme B expression was up-regulated in murine Treg cells62,63. Noelle and co-workers were the first to report that granzyme-B-deficient murine Treg cells had reduced suppressive activity in vitro64, but this Treg-cell-induced apoptosis appeared to be perforin-independent. The notion that Treg cells might possess cytolytic activity was supported by studies showing that Treg cells can kill B cells in a granzyme B-dependent and partially perforin-dependent manner that results in the suppression of B-cell function65. More recently, Treg cells were shown to suppress the ability of NK cells and CTLs to clear tumours by killing these cells in a granzyme-B- and perforin-dependent manner66. In addition, Noelle and colleagues have unpublished data showing that effector T cells that overexpress a granzyme-B-specific inhibitor, Spi-6, are resistant to Treg-cell-mediated suppression (Randolph Noelle, personal communication). Using a transplantation model in which Treg-cell-mediated tolerance is induced by CD154–CD40 co-stimulatory blockade in conjunction with donor lymphocyte-specific transfusion, they have also shown that the Treg cells that mediate this tolerance are also dependent on granzyme B for their suppressive activity.
Although the majority of research to date regarding Treg-cell-induced cytolysis has been focused on granzyme-B-mediated mechanisms, a recent study has suggested that activated Treg cells induce apoptosis of effector T cells through a TRAIL–DR5 (tumour-necrosis factor-related apoptosis inducing ligand–death receptor 5) pathway67. Furthermore, it has been suggested that galectins can mediate cytolysis in a granzyme- and perforin-independent manner68. These studies emphasize that more work is required to define the cytolytic mechanisms that Treg cells use to mediate suppression.
Suppression by metabolic disruption
Recently, several intriguing suppressive mechanisms have been described that could collectively be referred to as mechanisms that mediate ‘metabolic disruption’ of the effector T-cell target. A long-standing debate in the Treg-cell field is whether the high expression of CD25 empowers Treg cells to ‘consume’ local IL-2 and therefore starve actively dividing effector T cells by depleting the IL-2 they need to survive26,69. Although previous studies suggested that this was not a bone fide Treg-cell mechanism70,71, a recent study has reignited interest in this question by suggesting that Treg cells induce cytokine (specifically IL-2) deprivation-mediated apoptosis72. However, given that a recent report using human Treg cells suggests that IL-2 depletion alone is not required for Treg cells to suppress effector T cells73, more work is clearly required to resolve this debate.
Two new Treg-cell mechanisms have recently been proposed that induce the intracellular or extracellular release of adenosine nucleosides. Concordant expression of the ectoenzymes CD39 and CD73 was shown to generate pericellular adenosine, which suppressed effector T-cell function through activating the adenosine A2A receptor74-76. Interestingly, binding of adenosine to the A2A receptor appears to not only inhibit effector T-cell functions but also to enhance the generation of adaptive Treg cells by inhibiting IL-6 expression while promoting TGFβ secretion77. In addition, adenosine has also been shown to modulate DC maturation and favour a toleragenic phenotype (Peter Ernst, personal communication). Although TGFβ induces Foxp3 and Treg-cell differentiation, IL-6 inhibits the generation of Treg cells and promotes generation of pro-inflammatory Th17 cell development78. Thus, inhibiting IL-6 has important implications in Treg-cell maintenance. Treg cells were also shown to suppress effector T-cell function directly by transferring the potent inhibitory second messenger cyclic AMP into effector T cells via membrane gap junctions79. Although these mechanisms represent interesting additions to the Treg-cell arsenal, further studies will be required to corroborate these exciting findings and assess their relative use by Treg cells.
Suppression by targeting dendritic cells
In addition to directly affecting effector T-cell function, Treg cells might modulate the maturation and/or function of dendritic cells (DCs) required for effector T-cell activation. This has long been considered an attractive idea but there has been only limited data in support80. However, intravital microscopy has revealed direct interactions between Treg cells and DCs in vivo, which was proposed to attenuate effector T-cell activation by DCs81,82. So in what way might DCs be used as a conduit for Treg-cell-mediated suppression? Some time ago, cytotoxic T-lymphocyte antigen 4 (CTLA4) was shown to be constitutively expressed by Treg cells25,83, and by using either CTLA4-specific blocking antibodies or CTLA4-deficient Treg cells it was shown that in the absence of functional CTLA4, Treg-cell-mediated suppression of effector T cells via DCs was reduced84,85. Importantly, it was also shown that Treg cells could condition DCs, through a mechanism dependent on interactions between CTLA4 and CD80 and/or CD86, to express indoleamine 2,3-dioxygenase (IDO), which is a potent regulatory molecule that induces the catabolism of tryptophan into pro-apoptotic metabolites that results in the suppression of effector T cells86,87.
In addition to inducing DCs to produce immunosuppressive molecules, several studies have suggested that Treg cells may also downmodulate the capacity of DCs to activate effector T cells. Ivars and colleagues first reported that Treg cells could downregulate the expression of the co-stimulatory molecules CD80 and CD86 on DCs in vitro88. Several studies have also reported the immunomodulatory effects of Treg cells on DC maturation and/or function85,89-92. Studies with human Treg cells have also indicated that Treg cells may also modulate the function of monocytes and macrophages93,94. Although the precise mechanism by which this is orchestrated remains elusive, this modulation may be mediated through cell-surface molecules such as CTLA4 and/or cytokines such IL-10 and TGFβ.
Recent studies have also suggested that lymphocyte-activation gene 3 (Lag3; also known as CD223) may block DC maturation. Lag3 is a CD4 homologue that binds MHC class II molecules with very high affinity, has a negative regulatory T cell intrinsic function and is required for maximal Treg-cell suppression95,96. Binding of Lag3 to MHC class II molecules expressed by immature DCs induces an immunoreceptor tyrosine-based activation motif (ITAM)-mediated inhibitory signalling pathway, which involves FcγRγ– and extracellular-signal-regulated kinase (ERK)-mediated recruitment of SH2-domain-containing protein tyrosine phosphatase 1 (SHP1), which suppresses DC maturation and immunostimulatory capacity97. It is noteworthy that human MHC class II+ Treg cells, have been shown to be more suppressive than MHC class II− Treg cells, raising the possibility that these cells suppress by ligating LAG3 on activated effector T cells98. Although more work is required to fully elucidate if, and how, Treg cells might suppress effector T-cell function through DCs, this mode of action is attractive, as it may be a more efficient way of suppressing immune responses in vivo given the ∼1:8 ratio of Treg cells to effector T cells, compared with the ∼1:0.8 Treg cell to DC ratio found in the peripheral lymph nodes (as determined by flow cytometry and cell counting of pooled lymph nodes; CJW and DAAV, unpublished observations). Furthermore, it has recently been shown that neuropilin-1 (Nrp-1) promotes prolonged interactions with Treg cells and immature DCs99. Given that Nrp-1 is differentially expressed on Treg cells, this may give them an advantage over naïve T cells in modulating DC function.
Lastly, Treg cells can also influence immune responses by modulating the recruitment and function of other cell types. For instance, Treg-cell-derived IL-9 has been shown to recruit and activate mast cells which were shown to be essential regulatory intermediaries in the establishment of peripheral allograph tolerance100.
Complicating issues
Current dogma dictates that a hallmark of Treg cells is their dependence on direct contact to mediate their inhibitory activity. This has been upheld by in vitro experiments where Treg cells are unable to suppress effector T cell proliferation when the two populations are separated by a permeable membrane25,26. These data led to the notion that Treg-cell-mediated suppression is contact-dependent. However, there are two important issues one should consider when evaluating the Treg-cell mechanisms outlined above in the context of contact-dependency. First, these assays are really a measure of proximity rather than contact. Indeed, soluble mediators are most effective close to the source of their generation. The close proximity maintains high local cytokine concentrations, which has been shown to be important for the function of interleukin-2 (IL-2)101. Therefore, the dilution effect of diffusion across the well may render a soluble mediator ineffective. One should also consider the importance of proximity for labile mediators that might be very effective when Treg cells are close to their target cells but not when far away. For instance, adenosine has a half-life of less than ten seconds.
Second, it is not yet clear how much of the regulatory potency of Treg cells is directed towards DCs/APCs versus effector T cells. Although several studies have shown that Treg cells can directly suppress effector T cells in vitro in the absence of APCs, there is no direct evidence that contact between Treg cells and effector T cells is required for suppression in vivo. Indeed, intravital microscopy experiments suggest that Treg cells are far more frequently found in contact with DCs81,82. Furthermore, it is still not clear what the primary target is for many of the mechanisms described above. For instance, suppression by cytolysis, adenosine or cAMP could be directed against DCs and/or effector T cells. Inhibitory cytokines could also influence both populations. For example, although IL-35 was shown to directly act on effector T cells, an effect on DCs has not been precluded. The one mechanism that might be considered effector T cell exclusive is IL-2 deprivation-mediated apoptosis. Clearly, more work is needed to determine the primary target of Treg-cell suppression, particularly in vivo.
How many mechanisms do Treg cells need?
Although efforts to define the suppressive mechanisms used by Treg cells continue, an important question looms large. Is it likely that all these molecules and mechanisms will be crucial for Treg-cell function? There are three broad possibilities.
One, a single, overriding suppressive mechanism is required by all Treg cells
Until the entire mechanistic panoply of Treg cells is defined, one cannot completely rule out this possibility. However, this possibility would seem unlikely as none of the molecules and/or mechanisms that have been defined to date, when blocked or deleted, result in the complete absence of regulatory activity — a consequence that one might predict would result in a ‘Scurfy-like’ phenotype (BOX 1). So, although Treg cells that lack a single molecule, for instance IL-10, IL-35 or granzyme B, exhibit significantly reduced suppressor function, a scurfy phenotype does not ensue. Given that none of the current Treg-cell mechanisms can exclusively claim this distinction, it seems unlikely that any ‘unknown’ molecules or mechanisms could do so either.
Two, multiple, non-redundant mechanisms are required for maximal Treg-cell function
In the studies conducted to date, Treg cells that lack various suppressive molecules have been shown to be functionally defective. This favours a scenario where there are multiple mechanisms that can be used by Treg cells but they are non-redundant, with each molecule contributing to the mechanistic whole. At present, this possibility would seem plausible. Indeed, this is supported by the recent analysis of mice possessing a Treg-cell-specific ablation of IL-10 expression, in which enhanced pathology was observed following environmental insult33. One would predict that at some point we should be able to generate knockout mice that lack a particular set of genes which results in a complete loss of Treg-cell activity. For this to be truly non-redundant, this list would probably be restricted and small (2–4 genes).
Three, multiple, redundant mechanisms are required for maximal Treg-cell function
With the plethora of regulatory mechanisms described to date and the possibility of more yet to be identified, it is conceivable that there are multiple mechanisms that function redundantly. Such a redundant system would help to mitigate against effector T-cell escape from regulatory control. Also, given the very small size of the Treg-cell population, a sizable arsenal may be required at the height of an effector T-cell attack. Of course, it is possible that a semi-redundant scenario exists.
These possibilities have been discussed from the perspective of there being a single homogeneous Treg-cell population. However, as for helper T cell subsets it remains possible that a few or even many different Treg-cell subsets exist24. Each of these may rely on one or multiple regulatory mechanisms. Several recent studies have provided support for both phenotypic and functional heterogeneity amongst Treg cells. For instance, it has recently been shown that a small sub-population of Treg cells express the chemokine receptor CCR6, which is associated with T cells possessing an effector-memory phenotype102. CCR6+ Treg cells appeared to accumulate in the central nervous systems of mice with experimental autoimmune encephalomyelitis (EAE) suggesting that they may have a prevalent role in controlling responses in inflamed tissues. Heterogeneous expression of HLA-DR has also been suggested to mark different subpopulations of functionally distinct human Treg cells103. Indeed, HLA-DR positive Treg cells were found to be more suppressive than their DR negative counterparts. One might speculate that their enhanced inhibitory activity is due to DR-mediated ligation of the inhibitory molecule LAG3 expressed by activated effector T cells95,96.
So, if multiple suppressor mechanisms exist, how might these be integrated and used productively by Treg cells in vivo? We would propose the following possible models21. First, a ‘hierarchical’ model in which Treg cells possess many mechanisms that could be used but only one or two that are really crucial and consistently important in a variety of regulatory settings. Second, a ‘contextual’ model where different mechanisms become more or less important depending on the background or context in which the Treg cells reside and the type of target cell that they have to repress. For example, some cell types may be inhibited primarily by cytokines, whereas others are most effectively suppressed through lysis by Treg cells. Alternatively, different mechanisms may be more effective in different tissue compartments or in different disease settings. This notion is supported by the recent analysis of mice in which IL-10 expression was specifically ablated in Treg cells33. Whereas Treg-cell-derived IL-10 was not required for the systemic control of autoimmunity, it did seem to be required from the control of inflammatory events at mucosal interfaces such as the lungs and colon. As a clear picture of the available Treg-cell weaponry emerges, an important challenge will be to determine their relative importance and contribution to Treg-cell function in different disease models.
A hypothesis: effector T cells potentiate Treg-cell function?
Most cellular interactions within the immune system are bidirectional, with molecular signals moving in both directions even though the interaction has broader unidirectional intentions (for example, CD4+ T-cell help). However, to date the general perception is that Treg cells suppress and effector T cells capitulate. We hypothesize that this is in fact an incomplete picture and that effector T cells have a very active role in their own functional demise. Three recent observations support this view. First, we have recently examined the molecular signature of activated Treg cells in the presence and absence of effector T cells and were surprised to find that it was strikingly different, with hundreds of genes differentially modulated as a consequence of the presence of effector T cells (C.J.W. and D.A.A.V., unpublished observations). Second, we have shown that Ebi3 and Il12a mRNA are markedly upregulated in Treg cells that were co-cultured with effector T cells, supporting the idea that effector T cells may provide signals which boost IL-35 production in trans58. Third, we found that Treg cells were able to mediate suppression of effector T cells across a permeable membrane when placed in direct contact with effector T cells in the upper chamber of a Transwell™ plate (L.W.C. and D.A.A.V., unpublished observations). Interestingly, this suppression was IL-35 dependent, as Ebi3−/− Treg cells were unable to mediate this ‘long-distance’ suppression. Collectively, these data suggest that it is the ‘induction’, rather than the ‘function’, of Treg-cell suppression that is contact-dependent and that effector T cells have an active role in potentiating Treg-cell-mediated suppression. Therefore, we hypothesize that receptor–ligand interactions between the co-cultured CD4+ effector T cells and Treg cells initiate a signalling pathway that leads to enhanced IL-35 secretion and regulatory activity (FIG. 2). While the molecule that mediates this enhanced Treg-cell suppression is unknown, it is possible that IL-2 may serve this function104. Given the contrasting genetic profiles of activated Treg cells in the presence and absence of effector T cells, it seems possible that this interaction may boost the expression of other regulatory proteins. It may well be that effector T cells unwittingly perform the ultimate act of altruism.
Figure 2. Model for how effector T cells might boost Treg-cell function.
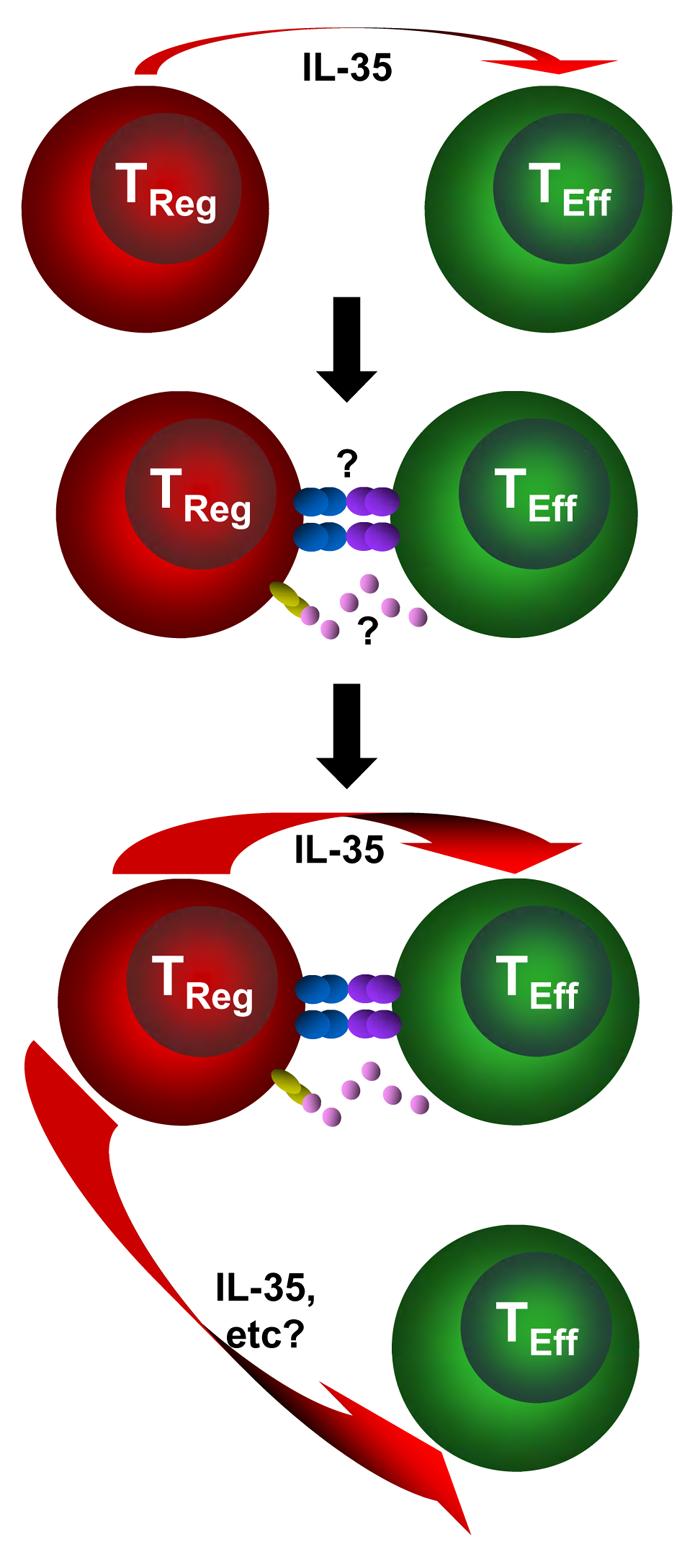
This occurs in three stages. (a) Initial regulatory T (Treg)-cell activation induces production of regulatory factors such as interleukin-35 (IL-35). (b) Treg cells ‘sense’ the presence of recently activated effector T cells through a receptor–ligand interaction (cell surface or soluble). (c) This in turn boosts or potentiates Treg-cell function resulting in the enhanced production of regulatory mediators, such as IL-35, and perhaps the induction of new mediators.
Concluding remarks
Although significant progress has been made over the last few years in defining the mechanisms that Treg cells use to mediate their suppressive function, there is clearly much that remains to be elucidated and many questions persist. First, are there more undiscovered mechanisms and/or molecules that mediate Treg-cell suppression? What is clear is that the transcriptional landscape of Treg cells is very different from naive or activated effector T cells. There are literally thousands of genes that are upregulated (or downregulated) in Treg cells compared with effector T cells. Although it seems unlikely that all or many of these will be crucial for Treg-cell function, it is quite possible that a few undiscovered genes might be important. It should be noted that although we are discussing mechanisms here, it is clear that some of these molecules may perform key Treg-cell functions, such as Treg-cell homing and homeostasis, which are likely to indirectly influence their suppressive capacity in vivo but don't directly contribute to their inhibitory activity. It is also possible that some of these unknown molecules may represent more specific markers for the characterization and isolation of Treg cells, a particularly important issue for the analysis and use of human Treg cells (BOX 2).
Second, which mechanisms are most important? An important but potentially complex challenge will be to determine if a few mechanisms are important in many Treg-cell settings or whether different mechanisms are required in different cellular scenarios. At present it is difficult to assess this objectively as these mechanisms have predominantly been elucidated in different labs using distinct experimental systems and thus none have really been compared in side-by-side experiments. Furthermore, only recently have conditional mutant mice been examined that have a regulatory component specifically deleted in Treg cells33.
It almost goes without saying that although defining the Treg-cell mode of action is of great academic importance, it is also essential in order to develop effective approaches for the clinical manipulation of Treg cells. Given the capacity of Treg cells to control inflammation and autoimmunity, and their implication in blocking effective anti-tumour immunity and preventing sterilizing immunity, it seems probable that a clear understanding of how Treg cells work will present definitive opportunities for therapeutic intervention.
Supplementary Material
Acknowledgments
We wish to thank Randolph Noelle and Peter Ernst for granting permission to cite their unpublished observations. This work is supported by the National Institutes of Health (NIH), the Juvenile Diabetes Research Foundation (JDRF), a Cancer Center Support CORE grant and the American Lebanese Syrian Associated Charities (ALSAC). We appologize to those authors whose work we could not cite due to space limitations.
Glossary
- Peripheral tolerance
The lack of self-responsiveness of mature lymphocytes in the periphery to specific antigens. These mechanisms control potentially self-reactive lymphocytes that have escaped central-tolerance mechanisms. Peripheral tolerance is associated with suppression of production of self-reactive antibodies by B cells and inhibition of self-reactive effector cells, such as cytotoxic T lymphocytes. Regulatory T (Treg) cells constitute one mechanism of peripheral tolerance.
- Type 1 diabetes
A chronic autoimmune disease that is characterized by the T-cell-mediated destruction of β-cells (which secrete insulin) in the pancreas. Individuals with type 1 diabetes develop hyperglycaemia and can develop diabetes-associated complications in multiple organ systems owing to lack of insulin.
- Inflammatory bowel disease (IBD)
A T-cell-mediated inflammatory response that affects the gastrointestinal tract. There are two forms of IBD in humans; Crohn's disease, which can affact any part of the gastrointestinal tract but usually desends from the terminal ileum, and ulcerative colitis (UC), which mainly affects the colon. In the mouse model, most of the inflammation is confined to the large intestines. The target antigen for the pathogenic T cells is unknown.
- Airway hyper-reactivity
Initiated by exposure to a defined stimulus that is usually tolerated by normal individuals and that causes bronchoconstriction and inflammatory-cell infiltration in allergic individuals.
- Experimental autoimmune encephalomyelitis (EAE)
An animal model of the human autoimmune disease multiple sclerosis. EAE is induced in experimental animals by immunization with myelin or peptides derived from myelin. The animals develop a paralytic disease with inflammation and demyelination in the brain and spinal cord.
- Exosomes
Small lipid-bilayer vesicles that are released from activated cells. They comprise either plasma membrane or membrane derived from intracellular vesicles.
- Adenosine nucleosides
Adenosine (C10H13N5O4) is a nucleoside composed of adenine linked to ribose and is a structural component of nucleic acids. It is also the primary molecular component of cAMP (Cyclic adenosine monophosphate – an important intracellular second messenger), AMP, ADP and ATP (a key sourse of chemical energy for many enzymatic reactions).
- Ectoenzymes
An enzyme that is outside the cell memebrane and thus can cleave extracellular substratetes. These are typically teathered to the outside of the cell by a transmembrane domain.
- Intravital microscopy
This is used for examination of biological processes, such as leukocyte–endothelial-cell interactions, in living tissue. In general, translucent tissues are used, such as the mesentery or cremaster muscle, which can be exposed and mounted for microscopic observation.
- Granzymes
A family of serine proteinases that are found primarily in the cytoplasmic granules of cytotoxic T lymphocytes and natural killer cells. They enter target cells through perforin pores, then cleave and activate intracellular caspases and lead to target-cell apoptosis.
- Perforin
A component of cytolytic granules that participates in the permeabilization of plasma membranes, allowing granzymes and other cytotoxic components to enter target cells.
- Sterilizing immunity
An immune response that leads to the compele removal of the pathogen.
Biographies
DARIO VIGNALI
Dario AA Vignali received his Ph.D. from the London School of Hygiene and Tropical Medicine, University of London, England, and his postdoctoral training from the German Cancer Research Centre in Heidelberg, Germany and Harvard University in Cambridge, Massachusetts, USA. He is currently an Associate Member of the Immunology Department at St. Jude Children's Research Hospital in Memphis, Tennessee, USA. His research focuses on various aspects of T cell biology including TCR:CD3 signalling and cell biology, the development and function of regulatory T cells, and type 1 diabetes.
LAUREN COLLISON
Lauren Collison received her Ph.D. from The University of Texas at Austin where her work focused on phospholipid metabolism in aging T cells. She is currently a postdoctoral fellow in the Immunology Department at St. Jude Children's Research Hospital in Memphis, Tennessee, USA. Her research interests are in immunoregulatory mechanisms and she aims to pursue work in the field of translational immunology.
CREG WORKMAN
Creg J Workman received his Ph.D. at the University of Illinois, Champaign, Illinois. He completed his postdoctoral training at St. Jude Children's Research Hospital in Memphis, Tennessee. He is currently a Scientific Manager in Dario Vignali's laboratory where his research interests include mechanisms of regulatory T cell function and type 1 diabetes.
References
- 1.Sakaguchi S, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol. Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 3.Xystrakis E, Boswell SE, Hawrylowicz CM. T regulatory cells and the control of allergic disease. Expert. Opin. Biol. Ther. 2006;6:121–133. doi: 10.1517/14712598.6.2.121. [DOI] [PubMed] [Google Scholar]
- 4.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol. Rev. 2005;204:184–194. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev. Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 6.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol. Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 7.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol. Rev. 2006;212:163–169. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 8.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. References 8 and 9 provided the first direct demonstration that Foxp3 is required for Treg cell development and sufficient to confer regulatory activity on naive T cells. [DOI] [PubMed] [Google Scholar]
- 10.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 11.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 12.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. References 10-12 were the first to identify Foxp3 as the defective gene in IPEX patients and Scurfy mice. [DOI] [PubMed] [Google Scholar]
- 13.Rudensky A. Foxp3 and dominant tolerance. Philos. Trans. R. Soc. Lond B Biol. Sci. 2005;360:1645–1646. doi: 10.1098/rstb.2005.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 15.Hill JA, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Allan SE, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 17.Morgan ME, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum. Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Ioan-Facsinay A, van,d. V, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 19.Gavin MA, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vignali D. How many mechanisms do regulatory T cells need? Eur J Immunol. 2008;38:908–911. doi: 10.1002/eji.200738114. [DOI] [PubMed] [Google Scholar]
- 22.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. This seminal paper reignited interest in ‘suppressor’ cells by demonstrating that a small CD4+CD25+ T cell population had regulatory activity. [PubMed] [Google Scholar]
- 24.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 26.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp. Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonuleit H, et al. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp. Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev. Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 30.Annacker O, Asseman C, Read S,, Powrie F. Interleukin-10 in the regulation of T cell-induced colitis. J. Autoimmun. 2003;20:277–279. doi: 10.1016/s0896-8411(03)00045-3. [DOI] [PubMed] [Google Scholar]
- 31.Joetham A, et al. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 32.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp. Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. This paper revealed the interesting distinction that IL-10 is required for the Treg-cell-mediated control of airway hyperreactivity but is derived from the suppressed effector T cells rather than the Treg cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Stoop JN, et al. Tumor necrosis factor alpha inhibits the suppressive effect of regulatory T cells on the hepatitis B virus-specific immune response. Hepatology. 2007;46:699–705. doi: 10.1002/hep.21761. [DOI] [PubMed] [Google Scholar]
- 35.Molitor-Dart ML, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–6761. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 36.Kursar M, et al. Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol. 2007;178:2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 37.Jankovic D, et al. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp. Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp. Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beiting DP, et al. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- 40.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. This paper demonstrated that Treg cells require IL-10 for their maximal regulatory activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion and characteristics of human T regulatory type 1 cells in co-cultures simulating tumor microenvironment. Cancer Immunol Immunother. 2007;56:1429–1442. doi: 10.1007/s00262-007-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loser K, et al. IL-10 controls ultraviolet-induced carcinogenesis in mice. J Immunol. 2007;179:365–371. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 43.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 44.Ivars F. T cell subset-specific expression of antigen receptor beta chains in alpha chain-transgenic mice. Eur. J. Immunol. 1992;22:635–639. doi: 10.1002/eji.1830220304. [DOI] [PubMed] [Google Scholar]
- 45.Schumacher A, et al. Mechanisms of action of regulatory T cells specific for paternal antigens during pregnancy. Obstet. Gynecol. 2007;110:1137–1145. doi: 10.1097/01.AOG.0000284625.10175.31. [DOI] [PubMed] [Google Scholar]
- 46.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 47.Piccirillo CA, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp. Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. This paper demonstrated that Treg cells require cell surface-bound TGFβ for their maximal regulatory activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fahlen L, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp. Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Strauss L, et al. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin. Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 53.Hilchey SP, De A, Rimsza LM, Bankert RB, Bernstein SH. Follicular lymphoma intratumoral CD4+CD25+GITR+ regulatory T cells potently suppress CD3/CD28-costimulated autologous and allogeneic CD8+ J Immunol. 2007;178:4051–4061. doi: 10.4049/jimmunol.178.7.4051. [DOI] [PubMed] [Google Scholar]
- 54.Li H, et al. CD4 +CD25 + regulatory T cells decreased the antitumor activity of cytokine-induced killer (CIK) cells of lung cancer patients. J Clin. Immunol. 2007;27:317–326. doi: 10.1007/s10875-007-9076-0. [DOI] [PubMed] [Google Scholar]
- 55.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 56.Xia ZW, et al. Heme oxygenase-1 attenuates ovalbumin-induced airway inflammation by up-regulation of foxp3 T-regulatory cells, interleukin-10, and membrane-bound transforming growth factor- 1. Am. J Pathol. 2007;171:1904–1914. doi: 10.2353/ajpath.2007.070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostroukhova M, et al. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin. Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. This paper was the first to describe the inhibitory cytokine IL-35 and its requirement by Treg cells for their maximal regulatory activity. [DOI] [PubMed] [Google Scholar]
- 59.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 60.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev. Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 61.Grossman WJ, et al. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 62.McHugh RS, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 63.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp. Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. This paper was the first to demonstrate that Treg cells have cytolytic capacity and regulate in a granzyme B-dependent manner. Reference 66 subsequently showed that the granzyme-dependent lytic activity of Treg cells was required for their regulatory activity in vivo. [DOI] [PubMed] [Google Scholar]
- 65.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao X, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 67.Ren X, et al. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4(+)CD25(+) regulatory T cells. Cell Death. Differ. 2007;14:2076–2084. doi: 10.1038/sj.cdd.4402220. [DOI] [PubMed] [Google Scholar]
- 68.Toscano MA, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 69.de la RM, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur. J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 70.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 71.Duthoit CT, Mekala DJ, Alli RS, Geiger TL. Uncoupling of IL-2 signaling from cell cycle progression in naive CD4+ T cells by regulatory CD4+CD25+ T lymphocytes. J. Immunol. 2005;174:155–163. doi: 10.4049/jimmunol.174.1.155. [DOI] [PubMed] [Google Scholar]
- 72.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4(+) T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 73.Oberle N, Eberhardt N, Falk CS, Krammer PH, Suri-Payer E. Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J Immunol. 2007;179:3578–3587. doi: 10.4049/jimmunol.179.6.3578. [DOI] [PubMed] [Google Scholar]
- 74.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borsellino G, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 76.Kobie JJ, et al. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. References 74-76 collectively revealed the ability of Treg cells to generate the inhibitory molecule adenosine by selective expression of CD39 and CD73. Reference 79 showed that another inhibitory adenosine nucleosides, cAMP, is directly transferred into effector T cells via gap junctions. [DOI] [PubMed] [Google Scholar]
- 77.Zarek PE, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oukka M. Interplay between pathogenic Th17 and regulatory T cells. Ann. Rheum. Dis. 2007;66(Suppl 3):iii87–iii90. doi: 10.1136/ard.2007.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bopp T, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp. Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr. Opin. Immunol. 2005;17:638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 81.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tadokoro CE, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp. Med. 2006;203:505–511. doi: 10.1084/jem.20050783. References 81 and 82 revealed the importance of Treg cell:DC interactions as a mechanism for blocking effector T cell activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. This paper demonstrated that Treg cells require CTLA4 for their maximal regulatory activity in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Serra P, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 86.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. This paper shows that Treg cells initiate the IDO-mediated catabolism of tryptophan in a CTLA-4-dependent manner. [DOI] [PubMed] [Google Scholar]
- 87.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 88.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 89.Kryczek I, et al. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 90.Lewkowich IP, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp. Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+CD25 high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–5298. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- 92.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 93.Taams LS, et al. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum. Immunol. 2005;66:222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tiemessen MM, et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Workman CJ, Vignali DAA. Negative regulation of T cell homeostasis by LAG-3 (CD223) J. Immunol. 2004;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 96.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 97.Liang B, et al. Regulatory T cells inhibit dendritic cells by LAG-3 engagement of MHC class II. J. Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 98.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 99.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu LF, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 101.Kaplan D. Autocrine secretion and the physiological concentration of cytokines. Immunol. Today. 1996;17:303–304. doi: 10.1016/0167-5699(96)30017-0. [DOI] [PubMed] [Google Scholar]
- 102.Kleinewietfeld M, et al. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 103.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 104.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J. Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 105.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 106.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 107.Yi H, Zhen Y, Jiang L, Zheng J, Zhao Y. The phenotypic characterization of naturally occurring regulatory CD4+CD25+ T cells. Cell Mol. Immunol. 2006;3:189–195. [PubMed] [Google Scholar]
- 108.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp. Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamaguchi T, et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 111.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp. Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsieh CS, et al. Recognition of the Peripheral Self by Naturally Arising CD25+ CD4+ T Cell Receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 113.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 114.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 115.Chen W, et al. Conversion of peripheral CD4+CD25− T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 117.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp. Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur. J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 121.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 122.Travis MA, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsumura Y, et al. Selective expansion of foxp3-positive regulatory T cells and immunosuppression by suppressors of cytokine signaling 3-deficient dendritic cells. J Immunol. 2007;179:2170–2179. doi: 10.4049/jimmunol.179.4.2170. [DOI] [PubMed] [Google Scholar]
- 124.Pyzik M, Piccirillo CA. TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. J Leukoc. Biol. 2007;82:335–346. doi: 10.1189/jlb.1006644. [DOI] [PubMed] [Google Scholar]
- 125.Wei J, et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grossman WJ, et al. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 127.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 128.Liu VC, et al. Tumor evasion of the immune system by converting CD4+ J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


