Abstract
Oxysterol-induced macrophage apoptosis may have a role in atherosclerosis. Macrophages lacking the type 2 cannabinoid receptor (CB2) are partially resistant to apoptosis induced by 7-ketocholesterol (7KC). AM-251 and SR144528 are selective antagonists of CB1 and CB2 receptors, respectively. We observed that both compounds reduce 7KC-induced apoptosis in Raw 264.7 macrophages. As oxysterol-induced macrophage apoptosis requires acyl-coenzymeA:cholesterol acyltransferase (ACAT) activity, we tested their affects on ACAT activity. AM-251 and SR144528 both reduced cholesteryl ester synthesis in unstimulated and acetylated LDL-stimulated Raw 264.7 macrophages, CB2 +/+ and CB2−/− peritoneal macrophages, as well as in vitro, in mouse liver microsomes. Consistent with inhibition of ACAT, the development of foam cell characteristics in macrophages by treatment with acetylated LDL was reduced by both compounds. This work is the first evidence that AM-251 and SR144528 are inhibitors of ACAT and as a result, might have anti-atherosclerotic activities independent of their affect on cannabinoid signaling.
Keywords: 7-ketocholesterol, ACAT, apoptosis
During atherogenesis, macrophages in the vascular intima take up modified low density lipoproteins (LDL), such as oxidized LDL (OxLDL), and store much of the LDL-derived cholesterol as cholesteryl esters in cytosolic lipid droplets [1]. Accumulation of cytosolic lipid droplets within macrophages transforms them into the foam cells which define early atherosclerotic lesions known as fatty streaks [2]. Intracellular cholesteryl ester synthesis is carried out by acyl-coenzyme A:cholesterol acyltransferase (ACAT) using cholesterol and fatty-acyl CoA as substrates [3]. Inhibition of ACAT prevents foam cell formation and, in some animal studies, slows the progression of atherosclerosis[4].
Lesional macrophage apoptosis plays several roles during the pathophysiology of atherosclerosis. During early lesion formation, macrophage apoptosis is anti-atherosclerotic [5, 6] while at later stages it likely contributes to plaque destabilization [7]. Lesional macrophages are subject to several apoptotic inducers, including oxLDL, which potently induces apoptosis in cultured macrophages largely as a result of its cytotoxic oxysterol constituents like 7-ketocholesterol (7KC) [8, 9]. Oxysterols are also substrates for ACAT and reduction of ACAT activity in macrophages produces resistance to 7KC-induced apoptosis, indicating that ACAT is a required step in the signaling pathway of oxysterol-induced macrophage apoptosis [10].
Cannabinoids and endocannabinoids function via two G-protein coupled receptors known as CB1 and CB2 [11]. CB1 is mainly expressed in the central nervous system where it mediates the psychoactive effects of cannabinoids [12]. CB2 is expressed by immune cells, including macrophages, and mediates the anti-inflammatory and immunosuppressive effects of cannabinoids [13]. CB2 is expressed by lesional macrophages and Δ9-tetrahydrocannabiol induces lesion regression in mice by a mechanism sensitive to CB2 antagonism [14]. Recently, we observed that CB2 null macrophages are partially resistant to oxLDL/oxysterol-induced apoptosis [15]. During the course of this study, we noted that AM-251, a CB1-selective antagonist [16], in addition to SR144528, a CB2-selective antagonist [17], also increased the survival of macrophages exposed to 7KC. In the present study, we explored the hypothesis that AM-251 and SR144528 reduce 7KC-induced apoptosis by interfering with the ACAT-mediated step of oxysterol-induced apoptosis signaling. Our results demonstrate that both compounds inhibit ACAT activity in vivo and in vitro.
Materials and methods
Cell lines and reagents
Raw 264.7 macrophages were cultured in RPMI-1640 supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2 and 95% air. SR144528 was supplied by Dr. Francis Barth (Sanofi-Aventis). AM-251 and 7-ketocholesterol were obtained from BioMol and Steraloids, respectively. [9, 10-3H]Oleic acid and [Oleoyl-1-14C]-CoA were from American Radiolabeled Chemicals. Mouse liver microsomes and acetylated LDL were purchased from CellzDirect and Biomedical Technologies, respectively. All other reagents and supplies were from Fisher Scientific.
Mice
Resident peritoneal macrophages were isolated by lavage from wild type and CB2 null mice as previously described [15]. All animal procedures were conducted in accordance with the guidelines administered by the Animal Research Facility and the Institutional Animal Care and Usage Committee at East Tennessee State University.
Caspase-3 Assay
Macrophages were seeded (2 × 106/well) in 12-well culture plates. AM-251 or SR144528 were added from 4 mM stock solutions prepared in DMSO, 1h prior to the addition of 7KC from a 2 mg/ml ethanol stock solution. Controls were adjusted to receive equivalent volumes of DMSO and ethanol. After 16 h, caspase-3 activity was determined as previously described [10]. All treatments were done in triplicate and the data presented as the mean RFLU/mg protein ± SD.
APOPercentage Assay
Macrophages, seeded (50,000/well) in 96-well plates and supplemented with AM-251, SR144528 or vehicle alone 1h prior to the addition of 7KC as described above for caspase-3 assays. After 16 h, apoptosis was quantified using the APOPercentage (Biocolor) colorimetric protocol according to the manufacturer’s directions.
Viability Assays
Macrophages were seeded (10,000/well) in 96-well plates and supplemented with AM-251, SR144528 or vehicle only as described above. After 24 h, the viability was determined using a CellTiter 96 Kit (Promega) according to the manufacturer’s instructions.
Neutral Lipid Synthesis
Macrophages (2 × 106) were seeded in 35 mm culture dishes. After 4 h, the cells were rinsed twice with PBS, refed RPMI-1640+0.5% FBS containing varying amounts of AM-251 or SR144528. Controls received an equivalent volume of vehicle. After 1 h, [3H]-oleate (1.0 μCi/ml) or, for determination of acLDL-stimulated ACAT activity, [3H]-oleate (1.0 μCi/ml) + 100 μg/ml acLDL, was added. After 6 h, the cells were rinsed three times with ice-cold PBS+0.2% BSA. Neutral lipids were extracted in hexane:2-propanol (3:2 v/v), subjected to thin layer chromatography (TLC) on silica gel 60 plates and the amount of radiolabel incorporated into cholesterol esters, triacylglycerides, and diacylglycerides was quantified by liquid scintillation counting (LSC) as we previously described [10]. All treatments were done in triplicate and the data presented as the mean dpm/mg protein ± SD.
In Vitro ACAT Assay
A 200 μl assay mixture containing 0.1 M potassium phosphate buffer (pH 7.4), 2.5 mg/ml BSA and 60 μg of microsomal proteins was preincubated with and without inhibitors (added as a 10 μl methanol solution to make final concentrations of 0–20 μM) for 30 min at 37°C. [14C]oleoyl-CoA (0.5 μCi) was then added and the incubation continued for 5 min. The reactions were terminated by adding 1.2 ml of chloroform/methanol (2:1 v/v) and the cholesteryl-[14C]oleate extracted by the method of Bligh and Dyer[18]. The samples were dried under nitrogen, resuspended in hexane, subjected to TLC and the radioactivity of the cholesteryl-[14C]oleate measured by LSC.
Foam cell formation
Raw 264.7 macrophages were seeded at 2 × 105 per well in a 4-chamber slide (LabTek). After attaching, the cells were rinsed twice with PBS and refed RPMI-1640+2% FBS supplemented with and without 8 μM AM-251 or SR144528. All wells were adjusted to receive the same concentrations of vehicle. After 1 h, 100 μg/ml acLDL was added and the incubation continued for 16 h. The cells were rinsed with PBS, fixed in 4% formaldehyde and the intracellular neutral lipids and nuclei were stained with oil red O and hematoxylin, respectively. Lipid droplet formation was examined using a model BX41 Olympus light microscope equipped with a Micropublisher 5.0 cooled RTV digital camera (Qimaging).
Statistical Analysis
Statistical differences were determined by Student t-test with P<0.05 considered statistically significant.
Results
AM-251 and SR144528 inhibit 7KC-induced macrophage apoptosis
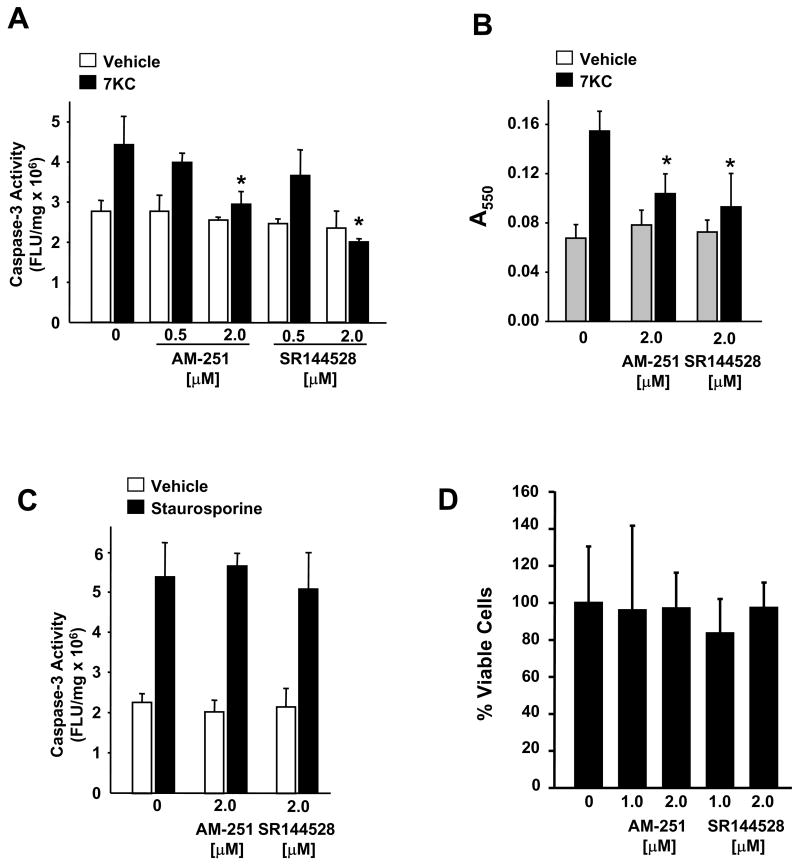
To determine the affect of AM-251 and SR144528 on 7KC-induced apoptosis in Raw 264.7 macrophages we monitored two independent indicators of apoptosis, caspase-3 activity and uptake of APOPercentage dye. In comparison to controls supplemented with vehicle only, Raw 264.7 macrophages supplemented with AM-251 or SR144528 displayed reduced caspase-3 activity (Fig. 1A) and reduced uptake of APOPercetage dye (Fig. 1B) following a 16h treatment with 7KC. In contrast, activation of caspase-3 activity in Raw 264.7 macrophages by staurosporine was not affected by supplementation with either AM-251 or SR144528 (Fig. 1C). At these concentrations the viability of Raw 264.7 macrophages, as determined by a modified MTT assay, was unaffected by either compound (Fig. 1D).
Fig. 1.
AM-251 and SR144528 inhibit 7-ketocholesterol induced apoptosis. Raw 264.7 macrophages were pretreated for 1 h with AM-251 or SR144528, prior to treatment with 7KC (10 μg/ml) as indicated. After 16 h, apoptosis was evaluated by (A) caspase-3 activity assay and (B) APOPercentage assay. (C) Caspase-3 activity induced in Raw 264.7 macrophages after a 6h treatment with staurosporine (1 μM) in the absence and presence of AM-251 or SR144528 as indicated. (D) Cell viability of Raw 264.7 macrophages incubated for 24 h in medium supplemented with AM-251 or SR144528, as indicated, was determined by a modified MTT assay and presented as the percentage ± SD of the mean of the control cells treated with vehicle only. * p<0.05
AM-251 and SR144528 inhibit cholesterol ester synthesis in vivo and in vitro
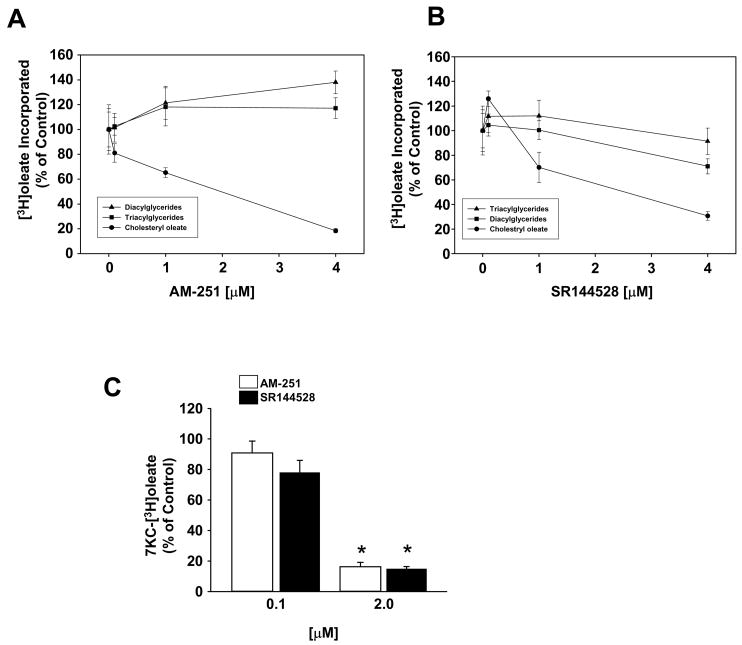
To determined if AM-251 and SR144528 were affecting sterol esterification in macrophages we monitored the ability of SR144528 and AM-251 to inhibit the incorporation of [3H]oleate into neutral lipids; cholesteryl [3H]oleate ([3H]CO), [3H]triacylglycerol ([3H]TG) and [3H]diacylglycerol ([3H]DG). In unstimulated Raw 264.7 macrophages, both SR144528 and AM-251 inhibited [3H]CO synthesis in a dose dependent fashion with IC50 values of ~2.6 μM and ~1.9 μM, respectively (Fig. 2A and 2B). SR144528 showed almost no inhibition of [3H]TG or [3H]DG synthesis even at the highest dose, while AM-251 produced a slight stimulation of [3H]TG and [3H]DG synthesis. Similar to their affect on cholesteryl [3H]oleate formation, both compounds also prevented the incorporation of [3H]oleate into 7-ketocholesteryl-[3H]oleate in macrophages treated with exogenous 7KC (Fig. 2C).
Fig. 2.
AM-251 and SR144528 inhibit sterol esterification in vivo and in vitro. The affect of (A) AM-251 and (B) SR144528 on the incorporation of [3H]oleate into neutral lipids in Raw 264.7 macrophages. Cells were incubated in medium supplemented with increasing concentrations of AM-251 and SR144528 as indicated for 1 h prior to the addition of [3H]oleate (1 μCi/ml). After a 6 h incubation, the total lipids were extracted and the amount of cholesteryl [3H]oleate, [3H]triacylglycerol and [3H]diacylglycerol formed was determined. (C) AM-251 and SR144528 prevent esterification of 7-ketocholesterol. Raw 264.7 macrophages were pretreated for 1 h with the antagonists, as indicated, prior to the addition of [3H]oleate (1 μCi/ml) and 7KC (10 μg/ml). After 6 h, the formation of 7-ketocholesteryl [3H]oleate was determined. Values are the percentage of the mean dpm/mg protein ± SD determined for control cells (vehicle only) for duplicate experiments each performed in triplicate. * p<0.05.
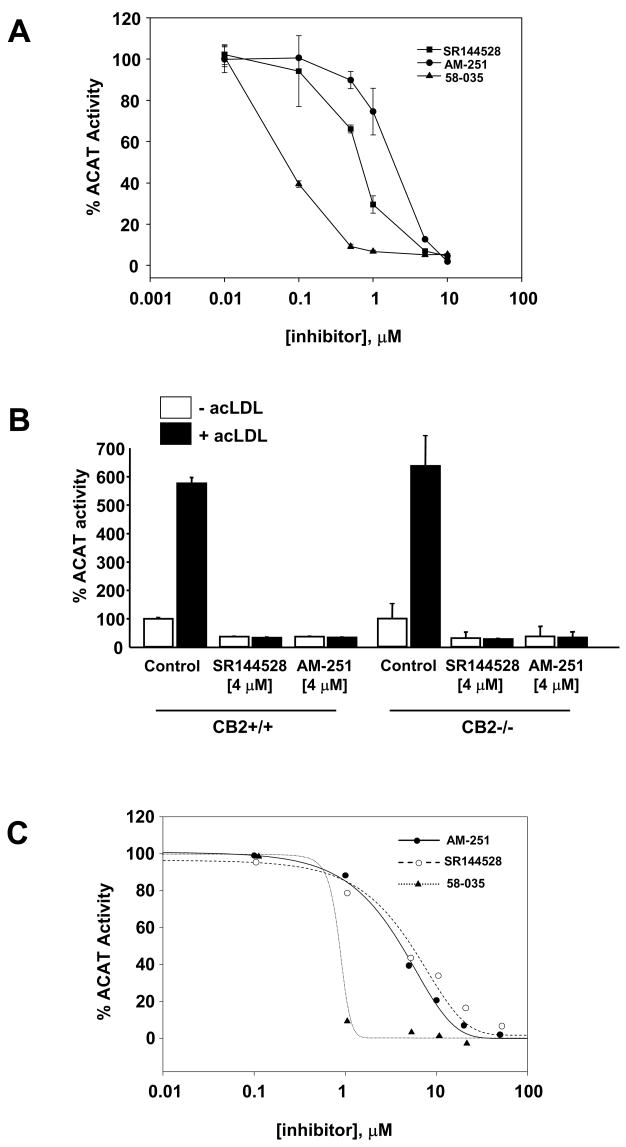
We next examined the ability of AM-251 and SR144528 to inhibit cholesterol ester synthesis in Raw 264.7 macrophages in which ACAT activity was stimulated with acetylated LDL (acLDL) (Fig. 3A). Both compounds displayed dose-dependent inhibition of cholesterol esterification with IC50 values similar to those observed in unstimulated Raw 264.7 cells. In comparison to a prototypical ACAT inhibitor, Sandoz 58–035, both AM-251 and SR144528 were less potent inhibitors of acLDL-stimulated cholesterol esterification in macrophages (Fig. 3A).
Fig. 3.
AM-251 and SR144528 inhibit the stimulation of cholesterol esterification by acLDL independent of CB2 expression. (A) Raw 264.7 macrophages were pretreated with increasing concentrations of AM-251, SR144528 or 58-035 for 1h prior to supplementation with acLDL (100 μg/ml) and [3H]oleate (1 μCi/ml) as indicated. Following a 6 h incubation, the amount of cholesteryl [3H]oleate formed was determined and the ACAT activity expressed as percentage of the mean cholesteryl [3H]oleate dpm/mg realized for controls (vehicle only). Each experiment was performed independently twice, each with triplicate samples. (B) AM-251 and SR144528 inhibit acLDL-stimulated cholesterol esterification in resident peritoneal macrophages from wild type and CB2 null mice. Wild type (CB2+/+) and CB2 null (CB2−/−) resident macrophages were pretreated with AM-251 or SR144528 for 1h prior to the addition of acLDL (100 μg/ml) and [3H]oleate (1 μCi/ml). After 6 h, total lipids were extracted and the amount of cholesteryl [3H]oleate formed was determined. ACAT activity is expressed as the percentage of the mean cholesteryl [3H]oleate dpm/mg realized for controls (vehicle only). Each experiment was performed independently twice, each with triplicate samples. (C) Mouse liver microsomes were preincubated for 30 min with various concentrations (0–20 μM) of the inhibitors as indicated. [14C]Oleoyl-CoA (0.5 μCi) was added and the amount of cholesteryl [14C]oleate formed after a 5 min incubation at 37°C was determined. ACAT activity is expressed as the percentage of cholesteryl [14C]oleate formed in the absence of inhibitors ± SD for triplicate samples. In some cases the error bars are smaller than the symbol and are not visible.
To explore the role of cannabinoid receptor signaling in AM-251 and SR144528 mediated inhibition of sterol esterification, we compared their affects on cholesterol esterification in peritoneal macrophages isolated from CB2+/+ and CB2−/− mice. We observed no affect of CB2 deficiency on the ability of either compound, in the presence or absence of acLDL, to inhibit cellular sterol esterification (Fig. 3B). This result indicates that AM-251 and SR144528 inhibit ACAT activity independent of CB2 expression.
We next evaluated AM-251 and SR144528 for in vitro ACAT inhibitory activity by measuring the formation of cholesteryl [14C]oleate from [14C]oleoyl-CoA in isolated mouse liver microsomes. Preliminary experiments showed the formation of cholesteryl [14C]oleate in mouse liver microsomes was linear up to ~9 minutes, therefore a 5 minute incubation was used for subsequent reactions. AM-251 and SR144528 inhibited microsomal ACAT activity in a concentration-dependent manner with IC50 values of 3.8 ± 1.3 μM and 3.6 ± 1.1 μM, respectively (Fig. 3C). At 10 μM, SR144528 and AM-251 inhibited ACAT activities ~68% and ~77%, respectively. In comparison, 58–035 inhibited ACAT with an IC50 of 0.4 ± 0.2 μM, similar to that reported in the literature [19].
Inhibition of Lipid Droplet Accumulation in Macrophages by AM-251 and SR144528
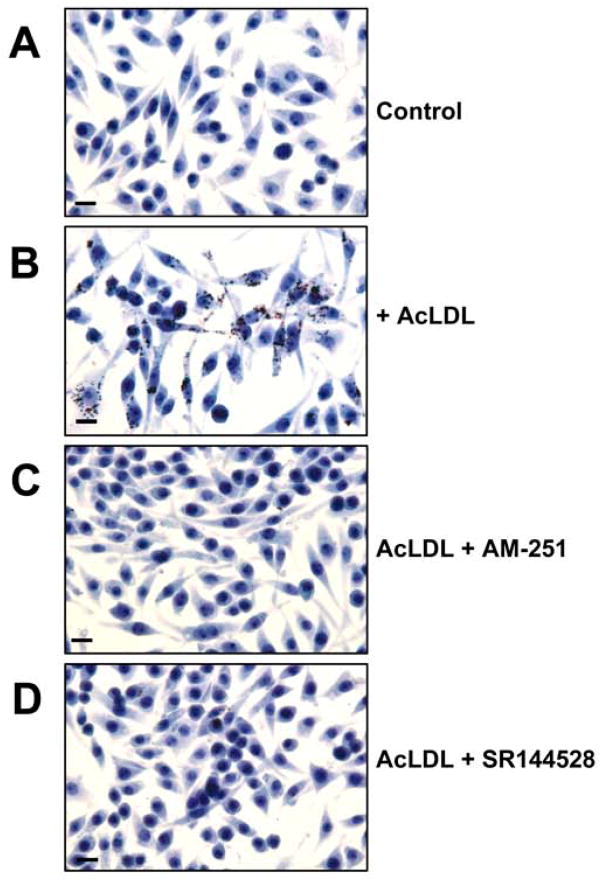
The hallmark of early atherosclerosis is the formation of macrophage-derived foam cells. Cultured macrophages can take on foam cell characteristics when they ingest acLDL via receptor-mediated mechanisms and, in an ACAT-dependent mechanism, store the acLDL-derived cholesterol as cholesteryl esters within lipid droplets in the cytosol. To assess the affect of AM-251 and SR144528 on foam cell formation we stained macrophages with oil red O, a dye selective for intracellular neutral lipids. Lipid droplet formation was undetectable in Raw 264.7 macrophages cultured in the absence of acLDL (Fig. 4A) but readily detectable in those cultured in the presence of acLDL (Fig. 4B). Macrophages cultured in the presence of acLDL and AM-251 (Fig. 4C) or SR144528 (Fig. 4D) displayed substantially reduced accumulation of lipid droplets. Under these conditions we observed no affect on cellular morphology or viability. Similar inhibition of acLDL-stimulated lipid droplet formation by AM-251 and SR144528 was observed with murine peritoneal macrophages (data not shown).
Fig. 4.
Lipid droplet accumulation in Raw 264.7 macrophages is inhibited by AM-251 and SR144528. (A) Cells were cultured for 16 h in medium alone (-acLDL), or medium supplemented with (B) 100 μg/ml acLDL, (C) 100 μg/ml acLDL and 8 μM AM-251 or (D) 100 μg/ml acLDL and 8 μM SR144528. The cells were fixed and the neutral lipids stained with oil red O. Original magnification = 40X. Bar, 10 μm.
Discussion
In this study, we show that AM-251 and SR144528 inhibit 7KC-induced macrophage apoptosis but not staurosporine-induced apoptosis. This suggests that AM-251 and SR144528 selectively inhibit 7KC-induced apoptotic signaling rather than apoptosis in a general. The apoptotic signaling pathway induced by oxLDL/oxysterols in macrophages is dependent upon ACAT-mediated oxysterol esterification[10]. The observation that concentrations of AM-251 and SR144528 necessary to inhibit 7KC-induced apoptosis also blocked sterol esterification in macrophages supports the hypothesis that these compounds prevent 7KC-induced apoptosis, at least partly, as a consequence of their ability to inhibit oxysterol esterification.
CB2 deficiency has been noted to reduce the susceptibility of macrophages to oxysterol-induced apoptosis by a mechanism that is independent, or downstream, of ACAT [15]. Thus, the observation that SR144528 can inhibit ACAT activity in CB2 −/− macrophages suggests that SR144528 may block oxysterol-induced apoptosis by two mechanisms; antagonizing CB2 and inhibiting ACAT. Although ACAT inhibition in CB1 deficient macrophages was not evaluated in this study, it seems unlikely that AM-251 inhibition of 7KC-induced apoptosis is due to affects on CB1 signaling as the concentration of AM-251 required to block apoptosis is nearly two orders of magnitude greater than the reported Ki values for inhibition of CB1 receptor signaling [20].
Although the possibility of additional affects on cholesterol trafficking can not be ruled out by the present study, the ability to inhibit ACAT activity in vitro demonstrates that both compounds are direct inhibitors of ACAT, independent of their ability to antagonize cannabinoid receptors. As inhibition of ACAT slows macrophage foam cell formation [21], it seems reasonable to conclude that the prevention of foam cell formation by AM-251 and SR144528 is a consequence of their inhibition of ACAT.
This is the first report of either AM-251 or SR144528 affecting an enzyme linked to cardiovascular disease or lipoprotein metabolism. However, AM-251 is structurally and pharmacologically similar to another CB1 specific antagonist, SR141716A (Rimonabant, Acomplia™) [22]. The only structural difference is the replacement of a p-iodo group attached to the phenyl substituent at C-5 of the pyrazole ring of AM-251 with a p-chloro group in SR141716A. SR141716A alters some cardiovascular risk factors in both mice [23] and humans [24–28] suggesting a direct pharmacological affect of SR141716A on lipoprotein and lipid metabolism [25, 28]. Anti-atherosclerotic affects of SR141716A are currently under evaluation in human trials [29] and recently, SR141716A was shown to reduce development of atherosclerosis in mice [30]. Interestingly, AM-251, SR144528 and SR141716A all share some structural similarities with a recently identified pharmacophore for ACAT inhibition, the diphenylethane backbone of Sandoz 58-035 [31]. Although the affect of SR141716A on ACAT activity is not yet known, the high structural homology between SR141716A and AM-251 implies that some of the cardiovascular beneficial effects of SR141716A might be due to inhibition of ACAT. Thus, administration of AM251, SR144528 and potentially SR141716A, in addition to having direct affects on cannabinoid signaling, might also influence the progression of atherosclerosis by decreasing serum cholesterol levels through inhibition of ACAT in the liver and suppressing foam cell formation by inhibiting ACAT in macrophages. In addition, affects on plaque stability due to modulation of macrophage apoptosis pathways could also result. The result of which could be either beneficial or harmful depending upon the stage of the lesion. Clearly, additional studies will be needed to fully elucidate the precise mechanisms by which these compounds affect atherosclerosis.
Acknowledgments
Supported by NIH National Heart Lung and Blood Institute grant HL085137 (DPT). The authors wish to thank Dr. Michael Sinensky for his assistance in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quehenberger O. Thematic review series: the immune system and atherogenesis. Molecular mechanisms regulating monocyte recruitment in atherosclerosis. J Lipid Res. 2005;46:1582–1590. doi: 10.1194/jlr.R500008-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1994;14:840–856. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- 3.Chang TY, Chang CC, Cheng D. Acyl-coenzyme A:cholesterol acyltransferase. Annu Rev Biochem. 1997;66:613–638. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki A, Sakai M, Sakamoto Y, Horiuchi S. Acyl-coenzyme A:cholesterol acyltransferase inhibitors for controlling hypercholesterolemia and atherosclerosis. Curr Opin Investig Drugs. 2003;4:1095–1099. [PubMed] [Google Scholar]
- 5.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I. Apoptosis and plaque destabilization in atherosclerosis: the role of macrophage apoptosis induced by cholesterol. Cell Death Differ. 2004;11(Suppl 1):S12–16. doi: 10.1038/sj.cdd.4401444. [DOI] [PubMed] [Google Scholar]
- 8.Chisolm GM, Ma G, Irwin KC, Martin LL, Gunderson KG, Linberg LF, Morel DW, DiCorleto PE. 7 beta-hydroperoxycholest-5-en-3 beta-ol, a component of human atherosclerotic lesions, is the primary cytotoxin of oxidized human low density lipoprotein. Proc Natl Acad Sci U S A. 1994;91:11452–11456. doi: 10.1073/pnas.91.24.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 10.Freeman NE, Rusinol AE, Linton M, Hachey DL, Fazio S, Sinensky MS, Thewke D. Acyl-coenzyme A:cholesterol acyltransferase promotes oxidized LDL/oxysterol-induced apoptosis in macrophages. J Lipid Res. 2005;46:1933–1943. doi: 10.1194/jlr.M500101-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 12.Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- 13.Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- 14.Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- 15.Freeman-Anderson NE, Pickle TG, Netherland CD, Bales A, Buckley NE, Thewke DP. Cannabinoid (CB2). receptor deficiency reduces the susceptibility of macrophages to oxidized LDL/oxysterol-induced apoptosis. J Lipid Res. 2008 doi: 10.1194/jlr.M800105-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee MH, Kim SK. SR144528 as inverse agonist of CB2 cannabinoid receptor. J Vet Sci. 2002;3:179–184. [PubMed] [Google Scholar]
- 17.Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC, Le Fur GL. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- 18.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Clader JW, Berger JG, Burrier RE, Davis HR, Domalski M, Dugar S, Kogan TP, Salisbury B, Vaccaro W. Substituted (1,2-diarylethyl)amide acyl-CoA:cholesterol acyltransferase inhibitors: effect of polar groups on in vitro and in vivo activity. J Med Chem. 1995;38:1600–1607. doi: 10.1021/jm00010a004. [DOI] [PubMed] [Google Scholar]
- 20.Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- 21.Namatame I, Tomoda H, Ishibashi S, Omura S. Antiatherogenic activity of fungal beauveriolides, inhibitors of lipid droplet accumulation in macrophages. Proc Natl Acad Sci U S A. 2004;101:737–742. doi: 10.1073/pnas.0307757100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 23.Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O’Connor SE, Janiak P, Herbert JM. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7:65–72. doi: 10.1111/j.1463-1326.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 24.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 25.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Jama. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 26.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 27.Scheen AJ, Van Gaal LG, Despres JP, Pi-Sunyer X, Golay A, Hanotin C. [Rimonabant improves cardiometabolic risk profile in obese or overweight subjects: overview of RIO studies] Rev Med Suisse. 2006;2:1916–1923. [PubMed] [Google Scholar]
- 28.Hollander P. Endocannabinoid blockade for improving glycemic control and lipids in patients with type 2 diabetes mellitus. Am J Med. 2007;120:S18–28. doi: 10.1016/j.amjmed.2006.11.014. discussion S29–32. [DOI] [PubMed] [Google Scholar]
- 29.Nissen SE, Nicholls SJ, Wolski K, Rodes-Cabau J, Cannon CP, Deanfield JE, Despres JP, Kastelein JJ, Steinhubl SR, Kapadia S, Yasin M, Ruzyllo W, Gaudin C, Job B, Hu B, Bhatt DL, Lincoff AM, Tuzcu EM. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. Jama. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- 30.Dol-Gleizes F, Paumelle R, Visentin V, Mares AM, Desitter P, Hennuyer N, Gilde A, Staels B, Schaeffer P, Bono F. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:12–18. doi: 10.1161/ATVBAHA.108.168757. [DOI] [PubMed] [Google Scholar]
- 31.de Medina P, Payre BL, Bernad J, Bosser I, Pipy B, Silvente-Poirot S, Favre G, Faye JC, Poirot M. Tamoxifen is a potent inhibitor of cholesterol esterification and prevents the formation of foam cells. J Pharmacol Exp Ther. 2004;308:1165–1173. doi: 10.1124/jpet.103.060426. [DOI] [PubMed] [Google Scholar]