Abstract
Associations between birthweight and cardiovascular disease in adult life, are supported by experiments showing that undernutrition in fetal life programmes blood pressure. In rats, the feeding of a maternal low protein (MLP) diet during gestation programmes hypertension. This study aimed to assess the potential for a nutritional insult to impact across several generations. Pregnant female Wistar (F0) rats were fed a control (n=10) or MLP diet (n=10) throughout gestation. At delivery all animals were fed standard laboratory chow diet. At 10 weeks of age, F1 generation offspring were mated to produce a second generation (F2) without any further dietary change. The same procedure produced an F3 generation. Blood pressure in all generations was determined at 4, 6 and 8 weeks of age and nephron number was determined at 10 weeks of age. F1 generation MLP exposed offspring exhibited raised (P<0.001) systolic blood pressure (male 143±4, female 141±4 mmHg) compared with controls (male 132±3, female 134±4 mmHg). Raised blood pressure and reduced nephron number was also noted in the F2 generation (P<0.001) and this intergenerational transmission occurred via both the maternal and paternal lines, as all three possible offspring crosses (MLP × CON, CON × MLP and MLP × MLP) were hypertensive (132±3 mm Hg) compared with controls (CON × CON), (123±2 mmHg). No effect was noted in the F3 generation. It is concluded that fetal protein restriction may play a critical role in determining blood pressure and overall disease risk in a subsequent generation.
Keywords: Intergenerational programming, blood pressure, epigenetic, kidney, pregnancy, rat
Introduction
It is well established that diseases in adult life such as hypertension and cardiovascular disease, and the subsequent development of metabolic syndrome emerge as a consequence of interplay between genetic and environmental factors. Recent research has concentrated on undernutrition in pregnancy and the role that it may play in the onset of disease [1-3]. It has been postulated that undernutrition programmes long-term changes in gene expression, which alter metabolism in the developing fetus and result in cardiovascular abnormalities in later life [4]. Whilst the origins of the metabolic syndrome are clearly multifactorial, nutrition in early life may have a profound influence upon risk and upon the responses of the individual to environmental and lifestyle-related risk factors in adulthood. The expression of genes that either predispose or protect against these conditions will be further modified by interactions between the genotype, early life nutrition and the postnatal environment [5].
Reports of epidemiological associations between birthweight and cardiovascular disease [4, 6, 7] are supported by animal experiments showing that both undernutrition and over-nutrition in fetal life can programme adult blood pressure [8-11]. We have demonstrated that, in rats, the feeding of a maternal low protein diet (MLP) during gestation programmes a lifelong elevation of blood pressure [7, 11-14]. Evidence from both human and animal studies indicates that the kidney, and specifically nephron number, may play an important role in the programming of hypertension [15]. In the MLP rat model of nutritional programming, fetal exposure to undernutrition reduces nephron number by as much as 30% [16].
A number of studies have demonstrated that prenatal undernutrition has the capacity to modulate the epigenetic regulation of gene expression[17-19]. This raises the prospect that periods of undernutrition can establish heritable changes to the epigenome and, as such, the disease programming effects of undernutrition in the fetal period may not be limited to the first generation. Indeed there is emerging evidence from studies of humans and animals to suggest that transgenerational effects may occur, whereby the consequences of deficits in maternal nutrition are subsequently passed onto the grandchildren [20-22]. The aim of this study was to assess the potential for both a prenatal insult of maternal protein restriction and a postnatal high fat challenge to impact across several generations.
Materials and methods
Animal protocols
The experiments described in this report were performed under license from the Home Office in accordance with the 1986 Animals (Scientific Procedures) Act. The study used rats of the Wistar strain, and all animals were housed in plastic cages and subjected to a 12 hour light/dark cycle at a temperature of 20-22°C. The animals had ad libitum access to food and water at all times.
Maternal procedure
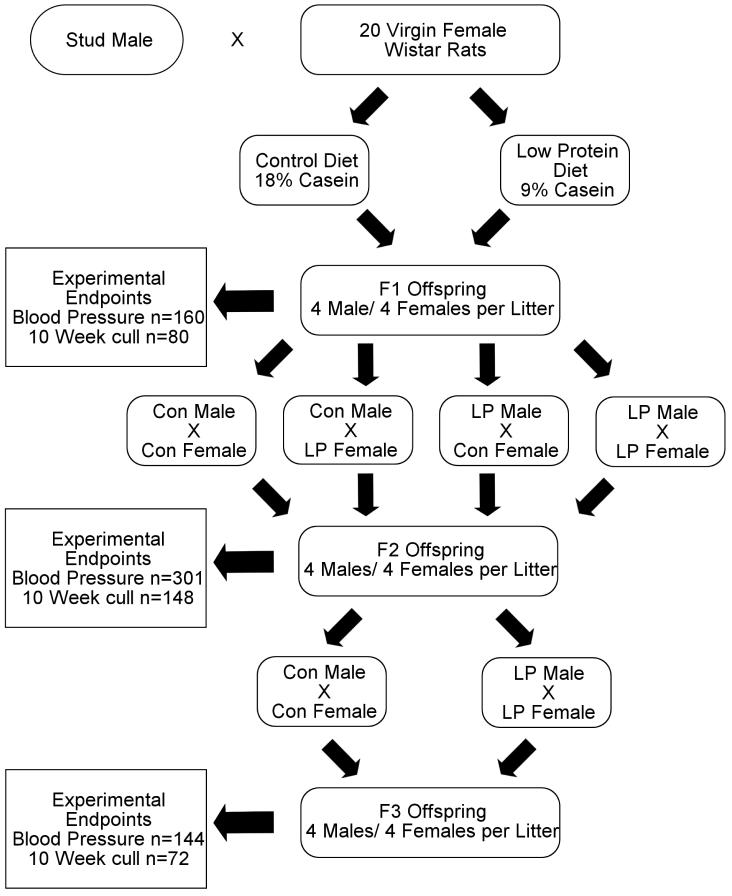
Figure 1 summarises the overall design of this experiment. 20 virgin female Wistar rats (Charles River) were mated with a single stud male at between 180-220g. Upon confirmation of mating by the presence of a semen plug on the cage floor, the rats were allocated to be fed either a 18% (w/w) casein (control) or a 9% (w/w) casein (LP) diet throughout gestation, as described previously [14]. The diets were isoenergetic, the difference in protein-derived energy between the two diets being made up with the addition of carbohydrate (starch-sucrose 2:1 w/w). During pregnancy animals were weighed and food intake was recorded daily. At the time of birth (day 22) animals were transferred to a standard laboratory chow diet (B&K Universal Ltd).
Figure 1. Schematic showing study design.
LP- low protein. Con- Control. Experimental Endpoints- Determination of body composition.
F1 offspring Procedure
At birth, litters were sexed and weighed. All litters were culled to 8 pups (4 male and 4 female) to ensure a standard plane of nutrition. At approximately 3 weeks of age offspring were micro-chipped using the Avid micro-chipping system and allocated into two sub-groups, breeders (B) and nutritional challenge (NC) (2 males and 2 females per group). Breeders and 1 male and 1 female from the NC group were allocated to a standard chow diet (B&K Universal Ltd). Remaining NC animals were allocated to be fed a high fat diet. All animals were housed in single sex groups and had ad libitum access to food and water at all times.
At approximately 10 weeks of age, 2 males and 2 females (B sub-group) from each F1 litter were utilised in a breeding programme for production of the F2 generation. The four animals from each litter allocated for breeding, were crossed with animals from another litter in order to produce the four possible crosses from the F1 generation, (Control male × Control female, LP male × LP female, Control male × LP female and LP male × Control female). Upon confirmation of mating by the presence of a semen plug on the cage floor the female rats were singly housed and fed standard chow diet until delivery of the litter.
F2 offspring Procedure
At birth, litters were sexed and weighed. All litters were culled to 8 pups (4 male and 4 female) to ensure a standard plane of nutrition. At approximately 3 weeks of age offspring were micro-chipped and allocated into B and NC sub-groups as described above. Breeders and 1 male and 1 female from the NC group were allocated to a standard chow diet (B&K Universal Ltd). Remaining NC animals were allocated to be fed a high fat diet. The animals were housed in single sex groups and had ad libitum access to food and water at all times.
At approximately 10 weeks of age, 1 male and 1 female (B sub-group) from each litter were crossed with animals from another litter in order to produce the following crosses based upon the dietary exposures of their parents in the F1 generation: Control male × Control female and LP male × LP female. Upon confirmation of mating by the presence of a semen plug on the cage floor the female rats were singly housed and fed standard chow until delivery of the litter. Male rats were placed back into single sexed housing with their littermates, until studied later in life.
F3 offspring Procedure
At birth, litters were sexed and weighed. All litters were culled to 8 pups (4 male and 4 female) to ensure a standard plane of nutrition. At approximately 3 weeks of age offspring were micro-chipped and allocated to a standard chow diet (B&K Universal Ltd). One male and one female animal were allocated to be fed a high fat diet. The animals were housed in single sex groups and had ad libitum access to food and water at all times.
High Fat Feeding Procedure
Rats (where possible 1 male and 1 female offspring from each NC sub-group per litter in each generation) were allocated to a high fat feeding protocol to assess the impact of this nutritional challenge upon subsequent weight gain and body composition, from approximately 4 weeks of age. The high fat diet was identical to that used in our previous work [23] and contained 29.5% fat (w/w) in the form of lard, and comprised 20% (w/w) protein (casein). The gross energy content of the diet was 25.12 MJ/Kg. A further group (consisting of 1 male and 1 female offspring from each NC group per litter in each generation) were fed a standard laboratory chow diet as a control. This diet contained 19% protein, 4.7% fat and had a gross energy content of 16.39 MJ/Kg. All animals from this group were housed in pairs and provided with ad libitum access to high fat diet or standard laboratory chow. At 5, 7 and 9 weeks of age animals were singly housed and food intake and body weight was monitored for a period of 3 days. Food intake data is shown corrected for body weight as in our previous studies of appetite in this model [24]. This corrects for any influence of body weight upon food intake, and allows comparison between sexes.
Determination of Blood Pressure
Blood pressure was determined in all animals using an indirect tail cuff method, as described previously [25]. Measurements were made using the IITC Life Sciences Model 229 Blood Pressure amplifier/ pump (IITC Inc, Woodlands CA, USA). This instrument allows measurements at a temperature of 27°C, thereby avoiding heat-stress of the animals. All rats were housed at this temperature, in the room where the measurements were made for at least 2 hours prior to testing. Measurements were made at the same time of day following standard procedures to minimise variation due to diurnal changes in blood pressure. Blood pressure was determined at 4, 6 and 8 weeks of age. Measurements were taken in triplicate and an average value was derived for each animal.
Culling of animals
At approximately 10 weeks of age the NC sub-group animals were killed to coincide with matings for the next generation. [using a rising concentration of CO2 and cervical dislocation]. Liver, heart, kidneys, lungs, spleen, thymus, hippocampus, hypothalamus, gonadal fat and perirenal fat were removed, accurately weighed and then snap-frozen in liquid nitrogen and stored at −80°C until used for further analysis. The left kidney was fixed in formalin for later determination of nephron number.
Nephron Number Determination
Nephron number was determined using an adaptation of the acid maceration method of Welham et al., [26]. Although determination of nephron complement via stereology [27] is the gold standard method of analysis, we used maceration in the current study due to the high throughput required. Our results and overall effect match those obtained via stereology in studies of the LP diet in the F1 generation [28]. Formalin fixed kidneys were weighed, cut in half and one portion was then incubated in 1 mol/L hydrochloric acid at 37°C for 30 minutes. Acid was removed and replaced with 5 ml of 50mM phosphate buffered saline (PBS; pH 7.4). The tissue was then homogenised using a bench top homogeniser (Polytron) and a further 5ml of PBS was added to give a final volume of 10ml. The sample was mixed thoroughly by inversion and a 20μl sample was taken and placed on a microscope slide and overlain with a cover slip. A ×10 objective lens was used to count all glomeruli in the sample. This was carried out in triplicate for each tissue sample. The values obtained were averaged and used to calculate the total number of glomeruli per kidney using the following equation.
Determination of circulating metabolites
Cholesterol and Triglyceride Assays
Total plasma cholesterol and plasma triglyceride concentrations were determined using commercially available kits, following the manufacturers' instructions (Thermo).
Glucose Assay
Plasma glucose concentrations were determined in samples from non-fasted animals, using an adaptation of the glucose oxidase method of Trinder [29]. A standard curve was produced by making serial dilutions of the glucose standard (0-2μg glucose). Samples were diluted 1:5 with phosphate buffer and loaded with standards in duplicate onto a microtitre plate in 10μl quantities, 200μl of glucose reagent was then added to each well. The plate was then incubated at 37°C for 15 minutes and then read at an absorbance of 620nm using Magellan, Version 4.0 software and plate reader (Tecan, Sunrise). The inter-assay coefficient of variation was 2.99%.
Statistical Analysis
All data was analysed using the Statistical Package for Social Sciences (SPSS, Inc, Chicago, IL, Version 14.0). Differences between groups were assessed using a mixed model ANOVA (fixed factors, maternal diet, sex and age), unless otherwise indicated in the text. Values are expressed as mean ± S.E.M. P<0.05 was considered as significant. As multiple pups from the same dam were used throughout this study, litter of origin was included as a fixed nested factor in all analyses [30]. Analyses were performed within generations, with no consideration of influences between generation, except for a comparison of blood pressure across the three generations. Within each generation the 18% casein control diet (F1), or the Con × Con breeding cross (F2 and F3) was used as the control group.
Results
Birth outcomes
The number of successful pregnancies, litter size, birthweight and the ratio of male and female offspring was unaffected by maternal diet/ cross in all 3 generations when compared to controls (data not shown). Average litter size was 14 ,with a ratio of 7:6 male to female offspring.
Blood Pressure
F1 Generation
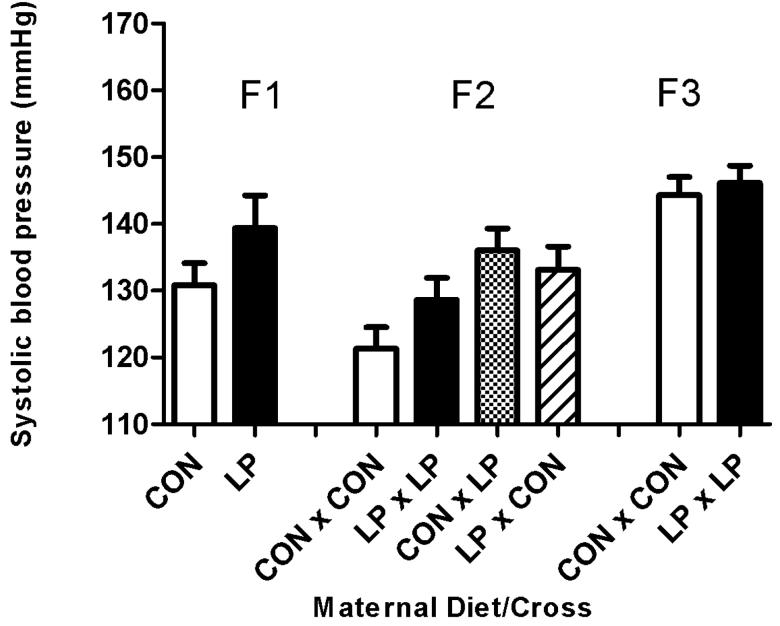
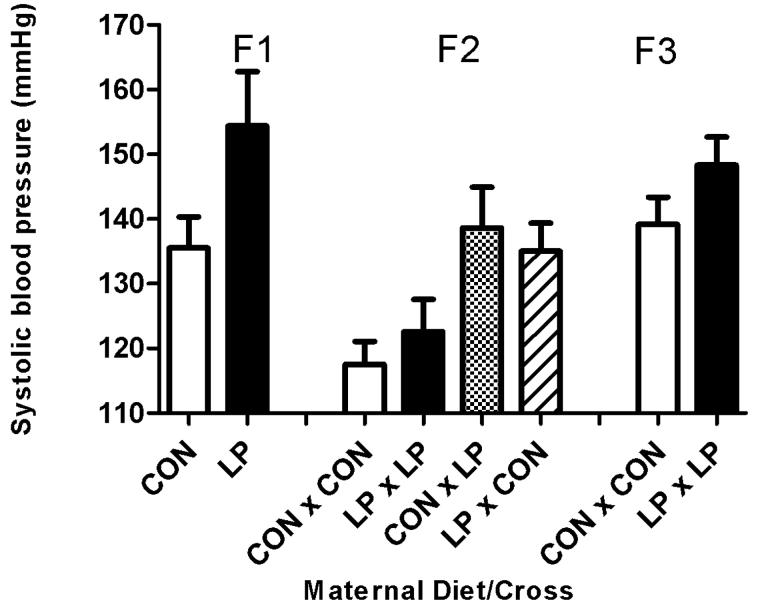
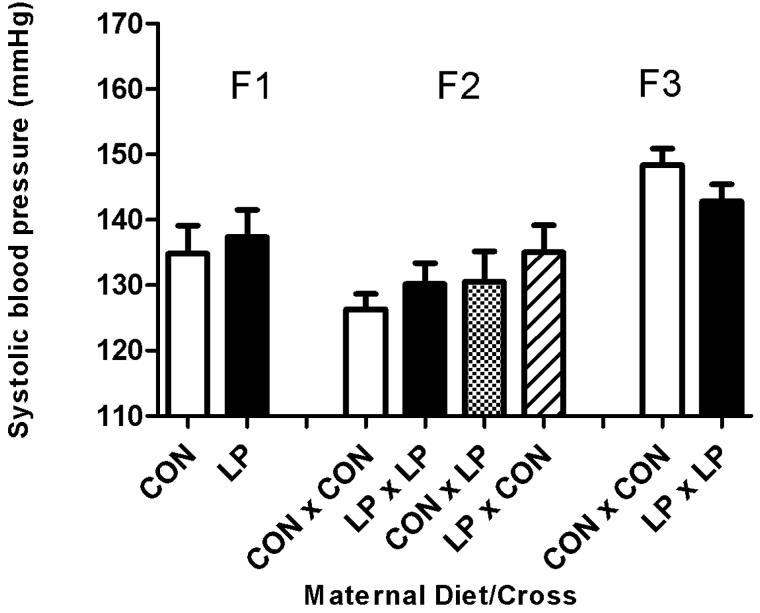
Systolic blood pressure at 8 weeks of age was significantly increased in males fed either a high fat (Figure 2B) diet (19 mmHg higher) or a standard chow (Figure 2A) diet (9 mmHg higher) postnatally, in the offspring (F1) of animals that had been subjected to maternal protein restriction during pregnancy (P<0.001), when compared to control animals of the same age. This effect was also noted in the females (Figure 2C, D) with an increase of 20 mmHg in animals on the high fat diet and an increase of 3 mmHg (P<0.001) in animals maintained on standard chow diet. This trend of increased blood pressure was also apparent at 6 weeks of age (P<0.001), (data not shown). Blood pressure at 4 weeks of age (data not shown) in the F1 generation was significantly lower in animals exposed to maternal protein restriction, relative to controls (P<0.001). This result occurred independent of sex, although a postnatal high fat diet did substantially reduce systolic blood pressure at this age (P<0.002).
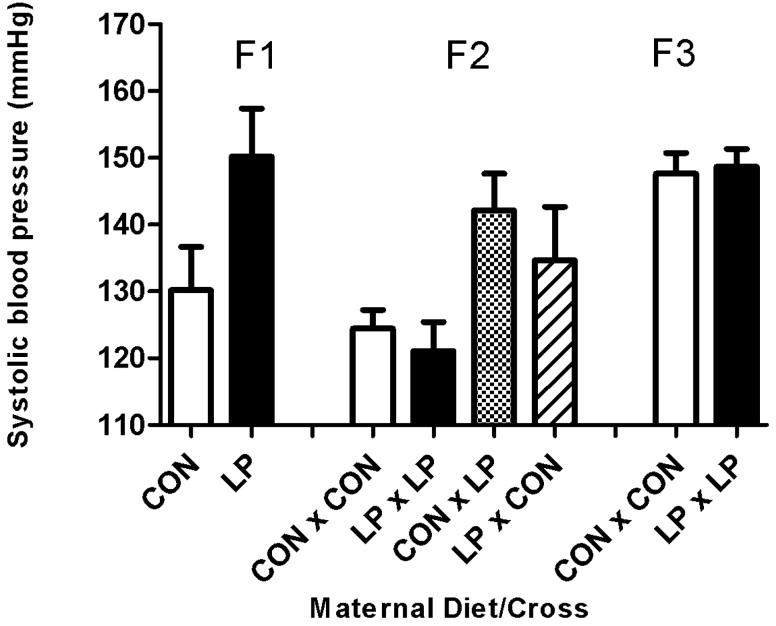
Figure 2. Systolic blood pressure at 8 weeks of age.
Figure 2A: Male standard chow diet. Figure 2B: Male High Fat diet. Figure 2C: Female standard chow diet. Figure 2D: Female High Fat diet. Data are shown as mean ± SEM for n=7 to 35 observations per group. CON; maternal control diet, LP; maternal low protein diet. Analysis of variance indicated significant effects of maternal diet (F1) (P<0.001) and Cross (F2) (P<0.001).
F2 Generation
The systolic blood pressures of the F2 generation were significantly influenced by the original F0 dietary intervention with prenatal LP exposure of either male or female F1 parents increasing blood pressure (Figure 2A-D). Systolic blood pressure at 8 weeks age was significantly increased (P<0.001) in the offspring from all of the breeding crosses where the parents were originally subjected to protein restriction during pregnancy, when compared to controls. The increase in blood pressure was also apparent at 6 weeks of age (P<0.005, data not shown). There was no effect on systolic blood pressure observed at 4 weeks for this generation. There was no influence of sex or any interaction of postnatal diet upon systolic blood pressure at 8 weeks of age.
F3 Generation
The systolic blood pressures of the F3 generation at 8 weeks of age were unaffected by the protein restriction of the original F0 dams. It was noticeable that blood pressures of F3 animals derived from the F0 dams fed the control diet in pregnancy were substantially higher than observed in their F1 equivalent group (Figure 2A-D).
Nephron Number
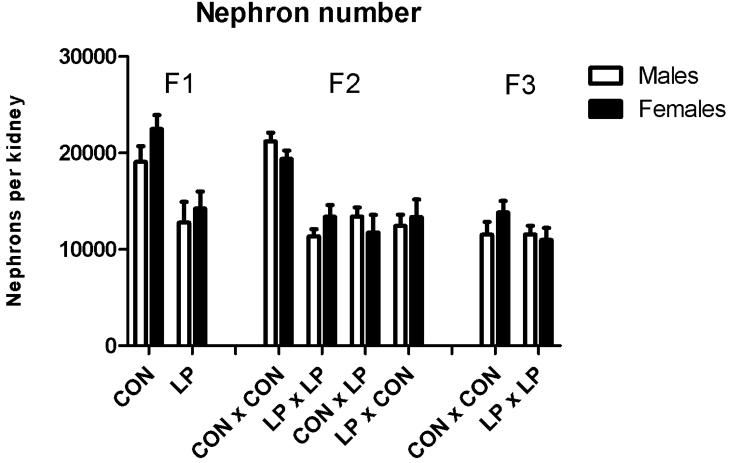
In animals of the F1 generation, total nephron number (Figure 3) was significantly reduced by 33% in males and 35% in females, among the offspring of rats that were subjected to maternal protein restriction during pregnancy (P<0.001). The F2 generation were similarly affected, exhibiting a reduction in nephron number of between 37 and 47% in males and between 31and 39% in females (P<0.05) derived from the breeding crosses where the parents were originally subjected to protein restriction during fetal life. No reduction in nephron number was observed in the F3 generation.
Figure 3. Nephron Number at 10 weeks of age.
Data are shown as mean ± SEM for n=8 to 23 observations per group. CON;maternal control diet, LP; maternal low protein diet. Analysis of variance indicated significant effects of maternal diet (F1) (P<0.001) and Cross (F2) (P<0.05).
Body composition
F1 Generation
Among males of the F1 generation, body weight (Table 1) at cull was similar in control and LP exposed rats that were fed chow diet in both male and female animals. The HF diet did not increase body weight in the control or LP group in females; however, LP exposed male animals exhibited a significantly decreased body weight. All organs (data not shown) and fat pads (Table 1) were of similar size relative to body weight in the chow-fed, male and female animals, irrespective of maternal dietary exposure. HF feeding in the F1 generation, increased the size of thymus in males from both the control and LP groups, and in the LP group produced an enlarged spleen. Heart size relative to body weight was significantly reduced by HF feeding (data not shown). HF exposure postnatally in females, substantially increased liver (P<0.05), lung (P<0.05), spleen (P<0.05), gonadal fat pad (P<0.05) and perirenal fat pad (P<0.05) size relative to body weight in both control and LP groups (data not shown).
Table 1.
Body Fat Deposition.
| Generation | Males |
Females |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal diet/cross |
Postnatal diet |
n | Body wt (g) |
SE | Gonadal fat pad |
SE | Perirenal fat pad |
SE | n | Body wt (g) |
SE | Gonadal fat pad |
SE | Perirenal fat pad |
SE | |
| F1 | Control | Chow | 11 | 383.7 | 19.8 | 0.8 | 0.1 | 0.7 | 0.1 | 9 | 246.9 | 8.4 | 0.8 | 0.1 | 0.6 | 0.1 |
| Control | HF | 10 | 381.7 | 13.6 | 1.0 | 0.1 | 1.0 | 0.1 | 10 | 282.0 | 23.4 | 1.1 | 0.1 | 0.9 | 0.1 | |
| LP | Chow | 8 | 380.8 | 6.9 | 0.7 | 0.0 | 0.7 | 0.1 | 9 | 243.8 | 4.6 | 0.7 | 0.1 | 0.5 | 0.1 | |
| LP | HF | 10 | 356.9 | 15.6 | 0.9 | 0.1 | 0.9 | 0.1 | 11 | 240.0 | 5.8 | 1.2 | 0.1 | 0.9 | 0.1 | |
| F2 | Con × Con | Chow | 11 | 381.3 | 12.9 | 0.7 | 0.0 | 0.7 | 0.1 | 11 | 249.4 | 5.7 | 0.7 | 0.1 | 0.5 | 0.1 |
| Con × Con | HF | 11 | 381.6 | 17.6 | 1.1 | 0.1 | 1.1 | 0.1 | 11 | 244.8 | 7.0 | 1.1 | 0.1 | 0.8 | 0.1 | |
| Con × LP | Chow | 8 | 412.9 | 10.2 | 0.7 | 0.1 | 0.6 | 0.1 | 8 | 239.2 | 8.3 | 0.8 | 0.1 | 0.5 | 0.1 | |
| Con × LP | HF | 8 | 349.3 | 14.1 | 0.9 | 0.1 | 0.8 | 0.1 | 8 | 244.8 | 5.0 | 0.8 | 0.1 | 0.7 | 0.1 | |
| LP × Con | Chow | 6 | 383.3 | 18.7 | 0.7 | 0.1 | 0.7 | 0.1 | 10 | 259.0 | 17.4 | 0.6 | 0.1 | 0.4 | 0.1 | |
| LP × Con | HF | 7 | 350.5 | 10.9 | 0.9 | 0.1 | 0.8 | 0.2 | 8 | 237.0 | 4.3 | 0.9 | 0.1 | 0.6 | 0.1 | |
| LP × LP | Chow | 13 | 391.2 | 14.9 | 0.7 | 0.0 | 0.6 | 0.1 | 7 | 250.6 | 3.9 | 0.6 | 0.1 | 0.5 | 0.1 | |
| LP × LP | HF | 10 | 351.9 | 10.5 | 0.9 | 0.1 | 0.8 | 0.1 | 11 | 228.3 | 8.9 | 0.9 | 0.1 | 0.7 | 0.1 | |
| F3 | Con × Con | Chow | 11 | 401.7 | 8.6 | 0.8 | 0.0 | 0.7 | 0.1 | 9 | 255.9 | 9.7 | 0.7 | 0.1 | 0.5 | 0.1 |
| Con × Con | HF | 9 | 376.1 | 14.8 | 1.0 | 0.1 | 1.0 | 0.1 | 9 | 236.1 | 7.5 | 1.0 | 0.1 | 0.7 | 0.1 | |
| LP × LP | Chow | 6 | 411.2 | 14.2 | 0.7 | 0.1 | 0.6 | 0.1 | 8 | 266.8 | 18.0 | 0.7 | 0.1 | 0.6 | 0.1 | |
| LP × LP | HF | 8 | 377.8 | 10.0 | 0.9 | 0.1 | 0.8 | 0.1 | 8 | 238.7 | 3.6 | 0.9 | 0.1 | 0.7 | 0.1 | |
| P for effect of maternal diet (F1) | <0.05 | - | NS | - | NS | - | - | <0.05 | - | NS | - | NS | - | |||
| P for effect of postnatal diet (F1) | NS | - | <0.05 | - | <0.05 | - | - | NS | - | <0.05 | - | <0.05 | - | |||
|
P for interaction of maternal × postnatal diets (F1) |
<0.05 | - | NS | - | NS | - | - | <0.05 | - | NS | - | NS | - | |||
| P for effect of F2 or F3 cross | F3: <0.05 |
- | F2: <0.05 | - | F2: <0.05 | - | - | F3: <0.05 |
- | F2: <0.05 | - | F2: <0.05 | - | |||
|
P for interaction of F2 or F3 cross × postnatal diet |
F3: <0.05 |
- | F2: <0.05 |
- | NS | - | - | F3: <0.05 |
- | F2: <0.05 | - | NS | - | |||
Data shows mean for n observations per group. For F2 and F3 crosses the dietary origin of the male parent is shown before the female parent, e.g. Con × LP indicates a cross between a male exposed to control diet and a female exposed to LP diet in utero.
F2 Generation
F2 generation body composition did not differ greatly from the F1 generation. Bodyweight at cull was similar among all groups of male animals on standard chow and similar to the F1 generation. HF feeding did not increase body weight in the control group, however there was a trend towards a decreased body weight in the LP exposed animals although this was not statistically significant. F2 female body weight did not differ significantly with regards to parental cross in animals exposed to a postnatal chow diet, however HF feeding postnatally generally decreased body weight (Table 1) in all crosses with exception to the Control × LP cross. All organs (data not shown) and fat pads were of similar size relative to body weight in the chow-fed, male animals. However HF feeding (Table 1) in both the males and females significantly decreased perirenal and gonadal fat deposit size relative to body weight in the animals of all cross groups involving a LP exposed parent (P<0.05).
F3 Generation
In the F3 generation, all organs (data not shown) and fat pads were of similar size relative to body weight in the chow-fed, male and female animals. HF feeding decreased bodyweight, and increased left kidney and heart size relative to body weight relative to their control counterparts.
Circulating Metabolites
Plasma glucose (Table 2) concentrations were unaffected by the original manipulation of the maternal diet, by sex or by high fat feeding in any of the three generations. Plasma cholesterol concentrations were also unaffected by maternal diet/cross in all 3 generations however HF feeding substantially increased plasma cholesterol concentrations in both the F1 and F3 control and LP male groups (P<0.05). Plasma triglyceride concentrations in the F1 generation were increased with HF feeding, in the LP exposed male group 0.93 mmol/L compared with controls 0.57 mmol/L (P<0.05). Plasma triglyceride concentrations were higher in F2 groups, derived from LP fed F0 dams when they were maintained on a standard chow diet, compared with the Con × Con group (P<0.05). Triglyceride concentrations were similar in the F3 generation irrespective of cross.
Table 2.
Circulating Metabolites
| Generation | Males |
Female |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal diet/cross |
Postnatal diet |
n | Glucose mmol/L |
SE | Triglyceride mmol/L |
SE | Cholesterol mmol/L |
SE | n | Glucose mmol/L |
SE | Triglyceride mmol/L |
SE | Cholesterol mmol/L |
SE | |
| F1 | Control | Chow | 11 | 9.2 | 1.7 | 0.7 | 0.2 | 1.7 | 0.1 | 9 | 8.9 | 1.5 | 0.5 | 0.1 | 1.6 | 0.1 |
| Control | HF | 9 | 12.3 | 1.6 | 0.6 | 0.1 | 2.4 | 0.2 | 10 | 9.6 | 1.2 | 0.1 | 0.1 | 1.5 | 0.1 | |
| LP | Chow | 8 | 10.3 | 1.6 | 0.5 | 0.1 | 1.6 | 0.2 | 9 | 9.9 | 1.7 | 0.3 | 0.1 | 1.6 | 0.2 | |
| LP | HF | 9 | 8.9 | 0.8 | 0.9 | 0.2 | 2.6 | 0.2 | 11 | 9.7 | 0.9 | 0.3 | 0.1 | 1.7 | 0.1 | |
| F2 | Con × Con | Chow | 11 | 11.2 | 1.7 | 0.9 | 0.1 | 1.3 | 0.2 | 11 | 12.0 | 1.0 | 0.4 | 0.1 | 1.6 | 0.2 |
| Con × Con | HF | 11 | 12.9 | 1.7 | 0.7 | 0.1 | 2.1 | 0.2 | 11 | 11.2 | 1.1 | 0.2 | 0.1 | 1.8 | 0.1 | |
| Con × LP | Chow | 8 | 10.5 | 1.8 | 1.2 | 0.2 | 1.5 | 0.1 | 8 | 12.4 | 2.0 | 0.3 | 0.1 | 1.6 | 0.2 | |
| Con × LP | HF | 8 | 8.2 | 1.8 | 0.7 | 0.2 | 2.0 | 0.2 | 8 | 12.2 | 0.8 | 0.3 | 0.1 | 1.5 | 0.1 | |
| LP × Con | Chow | 6 | 13.2 | 2.0 | 1.3 | 0.3 | 1.4 | 0.2 | 10 | 10.5 | 2.3 | 0.6 | 0.1 | 1.6 | 0.2 | |
| LP × Con | HF | 7 | 9.3 | 2.4 | 0.5 | 0.1 | 2.1 | 0.1 | 8 | 10.8 | 2.6 | 0.3 | 0.1 | 1.7 | 0.2 | |
| LP × LP | Chow | 13 | 11.5 | 1.4 | 1.0 | 0.2 | 2.1 | 0.4 | 7 | 13.7 | 2.3 | 0.4 | 0.2 | 1.9 | 0.1 | |
| LP × LP | HF | 10 | 10.1 | 1.3 | 0.6 | 0.5 | 1.5 | 0.2 | 11 | 13.0 | 1.4 | 0.4 | 0.2 | 1.3 | 0.4 | |
| F3 | Con × Con | Chow | 11 | 11.8 | 1.0 | 0.6 | 0.1 | 1.5 | 0.2 | 9 | 10.5 | 1.0 | 0.3 | 0.1 | 1.6 | 0.2 |
| Con × Con | HF | 9 | 8.8 | 1.6 | 0.9 | 0.1 | 2.2 | 0.2 | 9 | 10.0 | 1.2 | 0.1 | 0.0 | 1.6 | 0.2 | |
| LP × LP | Chow | 6 | 9.0 | 1.2 | 0.5 | 0.1 | 1.7 | 0.2 | 8 | 11.5 | 4.0 | 0.5 | 0.1 | 1.4 | 0.1 | |
| LP × LP | HF | 8 | 7.8 | 1.1 | 0.7 | 0.1 | 2.0 | 0.2 | 8 | 9.5 | 1.2 | 0.2 | 0.1 | 1.5 | 0.1 | |
| P for effect of maternal diet (F1) | NS | - | NS | - | NS | - | - | NS | - | NS | - | NS | - | |||
| P for effect of postnatal diet (F1) | NS | - | NS | - | P<0.05 | - | - | NS | - | NS | - | P<0.05 | - | |||
|
P for interaction of maternal × postnatal diets (F1) |
NS | - | NS | - | NS | - | - | NS | - | NS | - | NS | - | |||
| P for effect of F2 or F3 cross | NS | - | F2:P<0.05 | - | NS | - | - | NS | - | F2:P<0.05 | - | NS | - | |||
|
P for interaction of F2 or F3 cross × postnatal diet |
NS | - | NS | - | NS | - | - | NS | - | NS | - | NS | - | |||
Data shows mean for n observations per group. For F2 and F3 crosses the dietary origin of the male parent is shown before the female parent, e.g. Con × LP indicates a cross between a male exposed to control diet and a female exposed to LP diet in utero.
Food Intake
Food intake (Table 3) at 5 and 7 weeks of age (data not shown) was unaffected by maternal diet/cross in the F1 and F3 generations although there was an effect of postnatal diet (P<0.001) in the F1 generation with rats consuming roughly double the weight of food per day per kilogram body weight when maintained on a standard chow diet. In the F2 generation (P<0.001) it was apparent that offspring of the LP × Con cross had an increased food intake when on a high fat diet (P<0.001), relative to all other groups at 5 weeks of age. By 9 weeks of age there was an effect of postnatal diet (P<0.002) in all 3 generations whereby animals on standard chow consumed on average more grams per day per kilogram bodyweight than those fed the HF diet.
Table 3.
Food Intake
| Generation | Males |
Females |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal diet/cross |
Postnatal diet |
n | Week 9 | SE | Energy MJ/Kg |
SE | n | Week 9 | SE | Energy MJ/Kg |
SE | |
| F1 | Control | Chow | 11 | 107.6 | 11.7 | 1.8 | 0.2 | 9 | 118.0 | 8.8 | 1.9 | 0.1 |
| Control | HF | 10 | 64.3 | 3.0 | 1.6 | 0.1 | 10 | 83.0 | 5.5 | 2.1 | 0.1 | |
| LP | Chow | 8 | 98.4 | 2.9 | 1.6 | 0.0 | 9 | 118.7 | 5.0 | 1.9 | 0.1 | |
| LP | HF | 10 | 81.5 | 12.2 | 2.0 | 0.1 | 11 | 76.9 | 6.2 | 1.9 | 0.2 | |
| F2 | Con × Con | Chow | 33 | 81.0 | 8.3 | 1.3 | 0.1 | 33 | 89.5 | 10.9 | 1.5 | 0.2 |
| Con × Con | HF | 11 | 74.5 | 15.1 | 1.9 | 0.4 | 11 | 63.5 | 2.1 | 1.6 | 0.1 | |
| Con × LP | Chow | 24 | 99.0 | 3.0 | 1.6 | 0.0 | 24 | 118.8 | 4.6 | 1.9 | 0.1 | |
| Con × LP | HF | 8 | 68.7 | 14.2 | 1.7 | 0.4 | 8 | 67.6 | 4.0 | 1.7 | 0.1 | |
| LP × Con | Chow | 22 | 97.4 | 6.3 | 1.6 | 0.1 | 26 | 112.5 | 6.8 | 1.8 | 0.1 | |
| LP × Con | HF | 7 | 55.6 | 3.2 | 1.4 | 0.1 | 8 | 59.7 | 3.1 | 1.5 | 0.1 | |
| LP × LP | Chow | 35 | 89.6 | 1.8 | 1.5 | 0.0 | 29 | 100.4 | 4.8 | 1.6 | 0.1 | |
| LP × LP | HF | 10 | 59.8 | 2.5 | 1.5 | 0.1 | 11 | 70.2 | 5.6 | 1.8 | 0.1 | |
| F3 | Con × Con | Chow | 11 | 109.0 | 5.8 | 1.8 | 0.1 | 9 | 121.2 | 5.9 | 2.0 | 0.1 |
| Con × Con | HF | 9 | 60.6 | 3.5 | 1.5 | 0.1 | 9 | 63.7 | 2.0 | 1.6 | 0.1 | |
| LP × LP | Chow | 6 | 103.7 | 3.4 | 1.7 | 0.1 | 8 | 122.8 | 4.4 | 2.0 | 0.1 | |
| LP × LP | HF | 8 | 60.4 | 4.6 | 1.5 | 0.1 | 8 | 66.3 | 4.0 | 1.7 | 0.1 | |
| P for effect of maternal diet (F1) | NS | - | NS | - | - | NS | - | NS | - | |||
| P for effect of postnatal diet (F1) | P<0.001 | - | NS | - | - | P<0.001 | - | NS | - | |||
|
P for interaction of maternal × postnatal diets (F1) |
NS | - | NS | - | - | NS | - | NS | - | |||
| P for effect of F2 or F3 cross | NS | - | NS | - | - | NS | - | NS | - | |||
|
P for interaction of F2 or F3 cross × postnatal diet |
NS | - | NS | - | - | NS | - | NS | - | |||
Data shows mean for n observations per group. Data represented as grams per day per kilogram body weight. For F2 and F3 crosses the dietary origin of the male parent is shown before the female parent, e.g. Con × LP indicates a cross between a male exposed to control diet and a female exposed to LP diet in utero.
Discussion
In the present paper we have considered the potential for intrauterine protein restriction to impact upon blood pressure, renal development and body composition across several generations. We have previously characterised the programming effects of feeding of a low protein diet in rat pregnancy upon blood pressure [11, 12, 14], renal function and development [16, 31], body composition [32], feeding behaviour [24], lipid metabolism [23, 33] and body fatness [32] in the first generation (F1). In the present study we have for the first time shown that the effects of a low protein diet during rat gestation may not be limited to the first generation. This unique study has combined measurements of blood pressure with nephron number determination to show that a relatively mild protein restriction has far reaching consequences for further generations.
Much of the work previously undertaken on transgenerational programming has focused purely on a mechanistic viewpoint, without extensive effort to demonstrate that there is intergenerational transmission of any phenotype [34]. The present paper is the first to examine the interplay between blood pressure and renal structure across several generations, and has demonstrated that the effects of a mild-moderate protein restriction and its resultant phenotype is transgenerationally passed from the F1 generation to the F2 generations via both the maternal and paternal lines. It is clear from our findings on blood pressure and nephron number, that the F2 offspring of either male or female rats exposed to low protein diets in utero, develop broadly the same traits as their parents. A major strength of the research design for this study was the use of males from the F1 and F2 generations in the mating crosses to produce the successive generations. This allows us to assess the potential contribution of paternal as well as maternal factors in transgenerational programming. Other studies [35, 36] that have found evidence of transgenerational programming have considered only maternal transmission of phenotypes, as they have utilised stud males unexposed to dietary or hormonal challenges in utero to breed from programmed females. One of the main problems with such studies is that they cannot conclusively demonstrate that maternal traits are transmitted to the next generation via programmed influences on the ovum as changes to the maternal environment and the composition of proteins in the developing embryo may play a key role. Metabolic traits such as glucose intolerance or cardiovascular traits such as raised blood pressure could be programmed as a response to the prevailing maternal environment during an F1 pregnancy. For example, it has been previously shown that LP diets in rat pregnancy and lactation lead to glucose intolerance. F2 offspring derived from glucose intolerant females also show this trait, acquired through glucose spill-over across the placenta [37]. In the current study we can at least conclude that male animals must transmit a programming signal to their offspring via alterations to the sperm genome.
The feeding of a low protein diet during rat gestation has been consistently shown to produce a systolic blood pressure rise of between 7 and 30 mmHg [12] by 4 weeks of age. This increase in blood pressure appears permanent, remaining elevated well into adult life [38]. This phenotype was exhibited by the F1 generation within this study regardless, of their postnatal diet, and was subsequently passed to a second generation (F2) via both the maternal and paternal lines (as all F2 crosses involving low protein result in high blood pressure). The decreased blood pressure observed in the F1 LP exposed animals at 4 weeks of age was atypical of previous research using the maternal low protein diet. However similar outcomes have been observed following maternal restriction of iron [9, 10], where high blood pressure follows a period of lower blood pressure around the time of weaning.
Current thinking is that the transgenerational passage of phenotypic information is likely to involve epigenetic gene regulation. Although the exact mechanism which underpins this phenomenon is poorly understood, DNA methylation [19, 36, 39, 40] and histone modification [17, 18] remain key potential candidates. Currently there is a large and varied volume of work within the programming field that is focussed on these epigenetic mechanisms [41]. Lillycrop and colleagues [42] have, for example, shown that the LP diet leads to hypomethylation of specific gene loci and hence leads to their overexpression. There is evidence that these methylation changes also appear in F2 offspring [41]. Although there is a great deal of controversy surrounding epigenetics it is important to emphasise that the results within this study show that it is the most likely mechanism through which programming has occurred in the male line. Epidemiological evidence from Swedish cohort studies support this hypothesis [43]. Arguments suggesting that maternal blood pressure tracking (familial aggregation) may produce these effects, fall short of the mark as high blood pressure was passed down both the maternal and paternal lines. This argument also cannot explain the decrease in nephron number found in both the F1 and F2 populations. It has been hypothesised that there may be the potential for some form of selection, whereby only fetuses with elevated blood pressures survive the pregnancy insult. This would potentially select for a hypertensive genotype. This “survivor” effect is not plausible within this study as pregnancy outcomes were similar in rats fed to control and LP diets.
The kidney plays a major role in homeostatic regulation of blood pressure and, in rats, the low protein diet has been shown to reduce nephron complement by up to 30% [16, 26]. The present study shows that this reduction in nephron number is passed to a second generation via both parental lines. It is hypothesised that to compensate for nephron deficits at birth and to maintain renal haemodynamic functions, blood pressure within the nephrons is increased to maintain glomerular perfusion. Thus a cycle of progressive nephron loss begins whereby increasing blood pressures result in the loss of more nephrons eventually resulting in hypertension [15]. There is, however, emerging evidence that nephron number and the nutritional programming of blood pressure are independent processes acting in a sex specific manner [44]. Although there is some debate over the relationship between nephron number and subsequent blood pressure, it is clear that the restriction of protein during gestation has the potential to generate a phenotype that features both reduced nephron complement and hypertension and that this phenotype is subsequently passed on to a second generation via both parental lines. Torrens and colleagues [45] have also recently shown, with a design powered only to assess maternal transmission, that high blood pressure can be transmitted to the F2 generation following maternal protein restriction. Within this study there was evidence of an impaired vasodilatory response to acetylcholine, suggesting that in addition to renal programming, endothelial dysfunction may contribute to transgenerational phenomena. Both mechanisms are likely to operate in parallel. Further studies are required to identify the primary mechanisms that drive these physiological processes.
An interesting and unexpected result to arise from this investigation was that the blood pressures of control animals in F3 were elevated, and nephron counts decreased, compared to the F1 equivalents. The reason for this is unknown. We would, however, suggest that these intergenerational differences may effectively be due to in-breeding of a small colony in the F2 and F3 generations. Furthermore, the fact that our study shows that the experience of the mother and grandmother of each animal determines blood pressure, and nephron complement may be of relevance. The grandmaternal influence on the F1 generation will have been different to the grandmaternal influence on the F3 generation, and it is this difference which could explain the changes.
The feeding of a HF diet within this study was utilised to assess the impact of a “western diet” against a background of prenatal undernutrition. Typically a “western diet” has a high saturated fat content and is linked with obesity and the later development of adult diseases such as cardiovascular disease[46, 47]. The HF diet mirrored this composition as it had an overall fat content of 29.5%. interestingly rather than becoming obese the LP animals within this study exhibited a lean phenotype consisting of slightly reduced bodyweight and fat mass compared to chow fed animals at 10 weeks of age. This result was not entirely unexpected as our previous work [33] has demonstrated that up to 9 months of age LP exposed animals are resistant to obesity and exhibit similar plasma triglyceride, cholesterol, glucose, and insulin concentrations to those of controls. By 18 months of age there is an abrupt change in metabolic profile with LP-programmed hypertriglyceridemia and insulin resistance. This indicates that prenatal protein restriction programmes the development of a metabolic syndrome-like phenotype resistant to obesity that develops with senescence [33]. The present study, due to its complexity was unable to examine the development of this aging – related phenotype.
The work within this study demonstrates that the feeding of a maternal low protein diet throughout gestation results in a phenotype that is transgenerationally passed to a second generation via both the maternal and paternal lines. The impact of the initial period of maternal undernutrition was not observed in the F3 generation. Further work will take a mechanistic focus and attempt to identify possible gene targets involved in endothelial cell biology. Possible epigenetic modes of inheritance will be analysed in order to understand the molecular machinery behind the phenotype.
Acknowledgements
This study was supported by the British Heart Foundation (studentship for MH). MH was responsible for acquisition of data, statistical analysis, interpretation of data and is author of the communication. SLE was responsible for study design, statistical analysis, discussion/ interpretation of data and an author of the communication.
The authors gratefully acknowledge the expert technical support of Mr R Plant, Mrs C Armett and Ms S Kirkland.
Footnotes
There are no conflicts of interest to declare.
References
- 1.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Size at birth,childhood growth and obesity in adult life. International Journal of Obesity. 2001;25:735–740. doi: 10.1038/sj.ijo.0801602. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 5.Langley-Evans SC. Developmental programming of health and disease. Proc Nutr Soc. 2006;65:97–105. doi: 10.1079/pns2005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–9. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 7.Langley-Evans SC, Gardner DS, Jackson AA. Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. J Reprod Fertil. 1996;106:307–12. doi: 10.1530/jrf.0.1060307. [DOI] [PubMed] [Google Scholar]
- 8.Bergel E, Belizan JM. A deficient maternal calcium intake during pregnancy increases blood pressure of the offspring in adult rats. Bjog. 2002;109:540–5. [PubMed] [Google Scholar]
- 9.Crowe C, Dandekar P, Fox M, Dhingra K, Bennet L, Hanson MA. The effects of anaemia on heart, placenta and body weight, and blood pressure in fetal and neonatal rats. J Physiol. 1995;488:515–9. doi: 10.1113/jphysiol.1995.sp020986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gambling L, Dunford S, Wallace DI, Zurr G, Solanky N, Srai SK, McArdle H. Iron deficiency during pregnancy affects postnatal blood pressure in the rat. Journal of Physiology. 2003;552:603–610. doi: 10.1113/jphysiol.2003.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–22. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- 12.Langley-Evans SC, Phillips GJ, Jackson AA. In utero exposure to maternal low protein diets induces hypertension in weanling rats, independently of maternal blood pressure changes. Clin Nutr. 1994;13:319–24. doi: 10.1016/0261-5614(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 13.Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CR, Jackson AA, Seckl JR. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–72. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 14.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996;91:607–15. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie HS, Brenner BM. Fewer nephrons at birth: a missing link in the etiology of essential hypertension? American Journal of Kidney Disease. 1995;26:91–98. doi: 10.1016/0272-6386(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 16.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 64:965–74. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 17.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 18.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 23:5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdge GC, Hanson MA, Slater-Jefferies JL, Lillycrop KA. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr. 2007;97:435–9. doi: 10.1017/S0007114507352392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beach RS, Gershwin ME, Hurley LS. Gestational zinc deprivation in mice: persistence of immunodeficiency for three generations. Science. 1982;218:469–471. doi: 10.1126/science.7123244. [DOI] [PubMed] [Google Scholar]
- 21.James WPT. Will feeding mothers prevent the Asian metabolic syndrome epidemic? Journal of Clinical Nutrition. 2002;11:S516–S523. doi: 10.1046/j.1440-6047.11.supp3.12.x. [DOI] [PubMed] [Google Scholar]
- 22.Pembrey M. Imprinting and Transgenerational modulation of gene expression: human growth as a model. Acta Geneticae Medicae et Genellogiae. 1996;45:111–125. doi: 10.1017/s0001566000001197. [DOI] [PubMed] [Google Scholar]
- 23.Erhuma A, Bellinger L, Langley-Evans SC, Bennett AJ. Prenatal exposure to undernutrition and programming of responses to high-fat feeding in the rat. Br J Nutr. 2007;98:517–24. doi: 10.1017/S0007114507721505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellinger L, Lilley C, Langley-Evans SC. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr. 2004;92:513–20. doi: 10.1079/bjn20041224. [DOI] [PubMed] [Google Scholar]
- 25.Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 1998;94:373–81. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- 26.Welham SJ, Wade A, Woolf AS. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int. 2002;61:1231–42. doi: 10.1046/j.1523-1755.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- 27.Bertram JF. Counting in the kidney. Kidney Int. 2001;59(2):792–6. doi: 10.1046/j.1523-1755.2001.059002792.x. [DOI] [PubMed] [Google Scholar]
- 28.Zimanyi MA, Bertram JF, Black JM. Nephron number in the offspring of rats fed a low protein diet during pregnancy. Image Anal Stereol. 2000;19:219–222. [Google Scholar]
- 29.Trinder P. Determination of blood glucose using a oxidase-peroxidase system with a non-carcinogenic chromogen. Journal of Clinical Pathology. 1969;22:161–258. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Festing MF. Design and statistical methods in studies using animal models of development. Ilar J. 2006;47:5–14. doi: 10.1093/ilar.47.1.5. [DOI] [PubMed] [Google Scholar]
- 31.Nwagwu MO, Cook A, Langley-Evans SC. Evidence of progressive deterioration of renal function in rats exposed to a maternal low-protein diet in utero. Br J Nutr. 2000;83:79–85. [PubMed] [Google Scholar]
- 32.Bellinger L, Sculley DV, Langley-Evans SC. Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int J Obes (Lond) 2006;30:729–38. doi: 10.1038/sj.ijo.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ. Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am J Physiol Endocrinol Metab. 2007;292:E1702–14. doi: 10.1152/ajpendo.00605.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anway MD, Rekow SS, Skinner MK. Transgenerational epigenetic programming of the embryonic testis transcriptome. Genomics. 2008;91:30–40. doi: 10.1016/j.ygeno.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–36. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waterland RA, Travisano M, Tahiliani KG. Diet-induced hypermethylation at agouti viable yellow is not inherited transgenerationally through the female. Faseb J. 2007;21:3380–5. doi: 10.1096/fj.07-8229com. [DOI] [PubMed] [Google Scholar]
- 37.Reusens B, Remacle C. Intergenerational effect of an adverse intrauterine environment on perturbation of glucose metabolism. Twin Res. 2001;4:406–11. doi: 10.1375/1369052012597. [DOI] [PubMed] [Google Scholar]
- 38.Langley-Evans SC, Jackson AA. Captopril Normalises systolic boold pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comparative Biochemistry and Physiology. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- 39.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;1:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 40.Van den Veyver IB. Genetic effects of methylation diets. Annu Rev Nutr. 2002;22:255–82. doi: 10.1146/annurev.nutr.22.010402.102932. [DOI] [PubMed] [Google Scholar]
- 41.Burdge GC, Hanson MA, Slater-jefferies JL, Lillycrop KA. Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr. 2007;97:1036–46. doi: 10.1017/S0007114507682920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–73. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth period. Eur J Hum Genet. 2002;10(11):682–8. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 44.McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R85–90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- 45.Torrens C, Poston L, Hanson MA. Transmission of raised blood pressure and endothelial dysfunction to the F2 generation induced by maternal protein restriction in the F0, in the absence of dietary challenge in the F1 generation. Br J Nutr. 2008 doi: 10.1017/S0007114508921747. In Press. [DOI] [PubMed] [Google Scholar]
- 46.Kris-Etherton P, Eckel RH, Howard BV, Jeor S, Bazzarre TL. AHA Science Advisory: Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease. Circulation. 2001;103:1823–5. doi: 10.1161/01.cir.103.13.1823. [DOI] [PubMed] [Google Scholar]
- 47.Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest Suppl. 1990;201:3–23. [PubMed] [Google Scholar]