Abstract
Blood pressure normally decreases during the night. Absence of this phenomenon (non-dipping) is associated with increased cardiovascular risk. Altered autonomic and endocrine circadian rhythms are suspected to play a role. Patients with peripheral autonomic failure offer a unique opportunity to study this phenomenon because approximately 50% develop supine hypertension despite very low autonomic function. The purpose of this study was to define the prevalence of dipping in these patients, and to determine if dipping is associated with less severe autonomic impairment, or exaggerated nocturnal sodium excretion. We collected blood pressure and urine from 8PM-8AM in 41 peripheral autonomic failure patients with supine hypertension. Dipping (systolic BP fall ≥ 10% during 12AM-6AM from baseline [8PM–10PM]) occurred in 34% of patients, with an average decrease of −44±4mm Hg at 4 AM. Systolic BP, averaged from 12AM-6AM, decreased to normotensive levels in 50% (n=7) of dippers and 15% (n=4) of non-dippers. There were no significant differences in severity of autonomic failure, nocturnal diuresis or natriuresis (0.18±0.01 in dippers vs. 0.18±0.01mEq/mg creatinine in non-dippers; p=0.522) between groups. At 8AM, orthostatic hypotension was similar between groups (−84/−35±9/4 in dippers vs. −93/−39±6/3 in non-dippers, p=0.356 for SBP). In conclusion, dipping was observed in a third of patients with peripheral autonomic failure, so that a significant percentage of patients would not require treatment for supine hypertension. Dipping was not associated with increased nocturnal urinary sodium or volume excretion, or less severe autonomic failure. Thus, mechanisms independent of autonomic pathways contribute to BP dipping in these patients.
Keywords: dipping, supine hypertension, autonomic failure, circadian rhythm, autonomic nervous system, natriuresis
Blood pressure (BP) normally follows a circadian pattern characterized by a decline of ≥ 10% in mean BP levels from day to night (dipping). This phenomenon results from exogenous patterns of activity, stress and posture during the 24h,1,2 as well as endogenous circadian rhythms in autonomic nervous and endocrine systems.3 Alterations in these intrinsic circadian rhythms can result in the absence of the nocturnal BP decline (non-dipping). This altered pattern is commonly seen in patients with essential hypertension, several forms of secondary hypertension and disorders of the autonomic nervous system. The clinical relevance of this phenomenon lies in the fact that non-dipping has been associated with increased frequency of hypertensive target organ damage (brain, heart and kidney), as well as cerebrovascular and cardiovascular events in hypertensive patients 4–7.
The autonomic nervous system is the main suspect in mediating the intrinsic circadian variation in BP. In support of this, non-dipping has been reported in autonomic failure (AF) patients. 8,9 The underlying mechanism, however, is not clear and it is not known if alterations in circadian regulation of diuresis and natriuresis, known to be reversed in these patients,10 could contribute to the abnormal BP profile.
Primary AF can occur with central nervous system manifestations (multiple system atrophy) or with peripheral autonomic impairment (pure autonomic failure, PAF, and Parkinson’s disease, PD+). Both are characterized by severe orthostatic hypotension and, in about half of patients, supine hypertension.11 Patients with peripheral forms of AF are of particular interest because their supine hypertension is not due to residual sympathetic activity ,12 and they have very low levels of plasma norepinephrine and plasma renin activity.11
In the present study, we took advantage of the unique characteristics of these patients to determine the proportion of patients who dip during the night, and to examine whether dipping is associated with less severe autonomic impairment or exaggerated nighttime urinary sodium or water excretion.
METHODS
Subjects
We studied 41 patients with peripheral autonomic failure: thirty-three with PAF (21 men, 72 ± 1 yr, body mass index 24.97 ± 0.58 Kg/m2) and eight with PD+ (4 men, 73 ± 2 yr, body mass index 24.69 ± 1.43 Kg/m2). Patients were diagnosed following the criteria of the American Autonomic Society.13 All patients had supine hypertension defined as supine systolic BP>150 mm Hg at 8 PM. Patients with secondary forms of AF (e.g. diabetes mellitus or amyloidosis) were excluded. All studies were approved by our Institutional Review Board, and written informed consent was obtained from each subject before study entry.
General Protocol
Patients were admitted to the General Clinical Research Center at Vanderbilt University Medical Center. Medications affecting the autonomic nervous system, BP and blood volume were discontinued for ≥ 5 half-lives before admission. Patients were placed on a diet consisting of low monoamine, caffeine-free food containing 150 milliequivalents of sodium and 70 milliequivalents of potassium per day. Studies were conducted ≥ 2.5 hours after a meal. The screening consisted of a medical history, physical examination, 12-lead electrocardiogram, and laboratory assessments. Standardized autonomic function tests were performed to assess the severity of autonomic impairment, as described previously.14 BP and heart rate (HR) were obtained using an automated oscillometric sphygmomanometer (Dinamap, GE Medical Systems Information Technologies, Milwaukee, Wis), finger photoplethysmography (Finapres, Ohmeda) and continuous ECG. Baseline data was digitized for power spectral analysis of heart-rate variability in 22 patients (17 PAF and 5 PD+).
Plasma norepinephrine, plasma renin activity and plasma aldosterone were determined in the morning after the patient remained in the supine position overnight and again after 30 min in the upright position (or as long as tolerated). Blood samples were collected from a heparin lock placed at least 30 min before the first blood draw.
Overnight Blood Pressure Monitoring
Patients were studied for 12 hours starting at 8 PM. Patients were instructed to remain supine throughout the night with the head of the bed elevated 10 degrees. Fluid intake was restricted and BP was measured at 2-hour intervals by an automated sphygmomanometer (Dinamap, GE Medical Systems Information Technologies). At 8 AM, BP and HR were determined after 1 minute in the upright position or as long as tolerated. Urine was collected for 12 hours, from 8 PM to 8 AM, for determination of volume, sodium and creatinine.
Dipping Definition
Overnight BP monitoring was arbitrarily divided into two parts: a baseline period (“daytime”) from 8 PM to 10 PM and a sleeping period (“nighttime”) from midnight to 6 AM.15 Non-dipping was defined as a fall in average sleeping systolic BP < 10% from baseline.
Overnight Medication Trial with Nitroglycerin
To compare the magnitude of blood pressure dipping to that achieved with pharmacological treatment for supine hypertension, a subset of 15 non-dippers was randomized, in a single-blinded, crossover fashion to receive on separate days either transdermal nitroglycerin 0.1 mg/hr (Nitro-Dur patch, Key Pharmaceuticals, Kenilworth, NJ) or placebo patch placed at 8PM and removed at 6AM. Supine BP and HR were measured as described above.
Spectral Analysis
Beat to- beat R–R intervals were digitized and analyzed to determine the power spectra in the low frequency (LF: 0.04 to 0.15 Hz), and high frequency (HF: 0.15 to , 0.40 Hz) ranges, as previously described.16
Laboratory Measurements
Plasma norepinephrine levels were determined by high performance liquid chromatography with electrochemical detection. Plasma renin enzymatic activity was assayed by conversion of angiotensinogen to angiotensin I by radioimmunoassay. Serum aldosterone was measured by radioimmunoassay.
Statistical methods
The main outcome variable was the mean systolic blood pressure during the sleeping period. All values are presented as mean ± SEM. Normal distribution of data was assessed by the Kolmogorov-Smirnov test. If data did not have a normal distribution, non parametric statistical tests (Mann-Whitney U test for independent groups or Wilcoxon signed-rank test for two related groups) were used. Differences in mean BP and mean HR at baseline, sleeping period and at 8AM between dippers and non-dippers were analyzed by unpaired t-tests. Differences in BP and HR within each group were analyzed by paired t-tests.
Comparisons between dippers and non-dippers were analyzed by Student’s t-tests, if they had normal distribution. Otherwise, non parametric tests were used. Pearson’s chi-square test was used for nominal variables.
All tests were two-tailed, and a p-value of <0.05 was considered significant. Analyses were performed with SPSS statistical software (SPSS version 16.0, SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline Characteristics and Blood Pressure Monitoring
Patient characteristics are shown in Table 1. A dipper pattern was observed in 14 patients (34%; 10 PAF, 4 PD+) and a non-dipper pattern in 27 patients (66%; 23PAF, 4 PD+). There were no significant differences in age, gender distribution, duration of disease, history of essential hypertension, body mass index or plasma creatinine between the 2 groups. Of the 41 patients with peripheral autonomic failure and supine hypertension, only 8 (20%) patients had a past medical history of essential hypertension: 3 (21%) dippers and 5 (19%) non-dippers.
Table 1.
Clinical Characteristics of Dippers and Non Dippers.*
| Clinical Characteristics | Dippers 34% (n=14) |
Non-dippers 66% (n=27) |
p value |
|---|---|---|---|
| Age (yr) | 70 ± 2 | 72 ± 2 | 0.526 |
| Gender (male/female) | 7 / 7 | 18 / 9 | 0.300 |
| Duration of disease (yr) | 5.9 ± 0.9 | 7.7 ± 1.1 | 0.536 |
| PMH of HTN (n=8) | 21 % (3) | 19 % (5) | 0.824 |
| BMI (Kg/m2) | 24 ± 1 | 25 ± 1 | 0.350 |
| Plasma Creatinine (mg/dL) | 1.06 ± 0.05 | 1.21 ± 0.08 | 0.454 |
| BP (mm Hg) and HR (bpm) monitoring | |||
| 8PM- 10PM (Baseline) | |||
| SBP | 182 ± 4 | 175 ± 3 | 0.215 |
| DBP | 93 ± 2 | 90 ± 2 | 0.434 |
| HR | 71 ± 2 | 67 ± 1 | 0.106 |
| 12AM-6AM (Sleeping period) | |||
| SBP | 147 ± 5 | 171 ± 4 | 0.001 |
| DBP | 81 ± 3 | 89 ± 2 | 0.039 |
| HR | 68 ± 2 | 65 ± 1 | 0.274 |
| 8AM | |||
| SBP | 163 ± 6 | 175 ± 5 | 0.161 |
| DBP | 88 ± 3 | 92 ± 3 | 0.395 |
| HR | 72 ± 3 | 68 ± 2 | 0.152 |
| Normalization of Nighttime SBP† | 50 % (7) | 15 % (4) | 0.016 |
Values expressed as mean ± SEM. Number of patients is in parentheses.PMH, past medical history in years; HTN, essential hypertension; BMI, body mass index; BP, blood pressure; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Percentage of patients with a SBP, averaged between 12 AM and 6 AM, of <150 mm Hg.
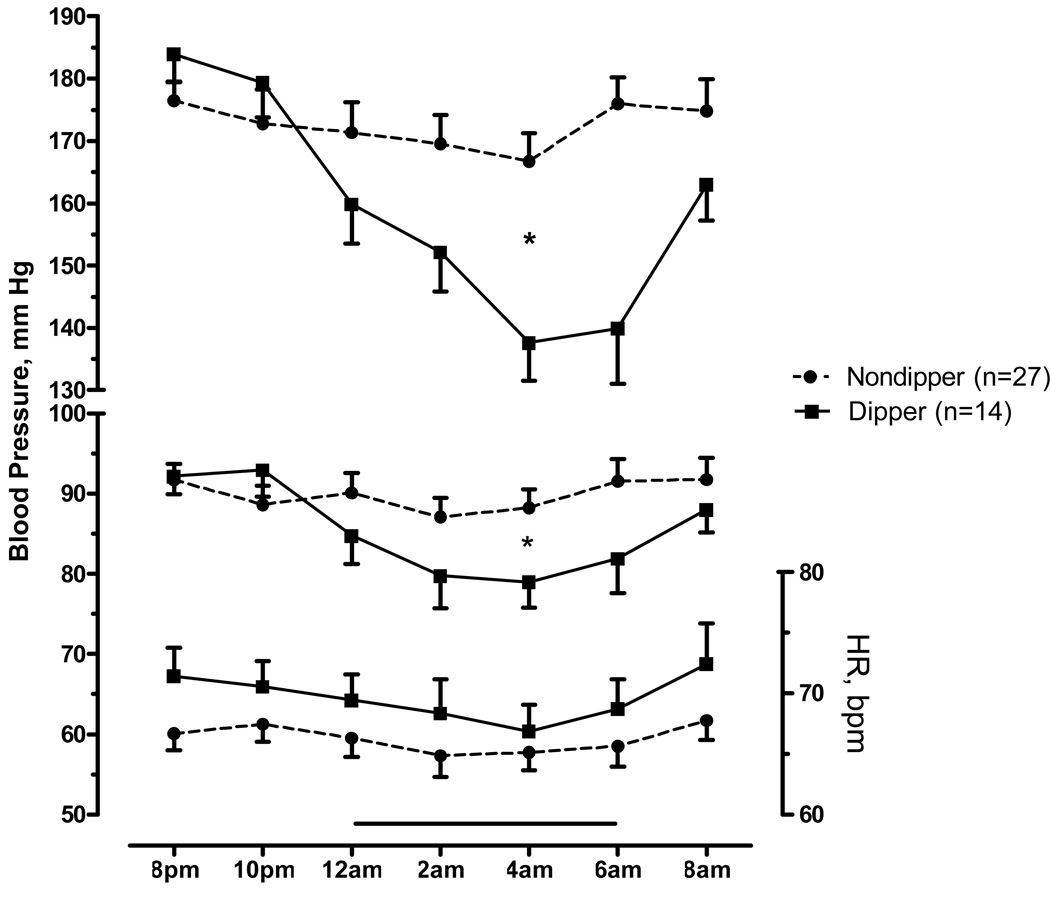
The mean systolic BP at baseline (8PM to 10PM) was non-significantly higher in dippers (Table 1, p=0.215). By definition, mean systolic BP during the sleeping period was lower in dippers (p=0.001). The maximal decrease in systolic BP was −44±4 mm Hg at 4 AM in dippers (from 182±4 mm Hg at baseline to 138±6 mm Hg; figure 1), whereas non-dippers decreased −8±4 mm Hg (from 175±3 mm Hg to 167± 5 mm Hg). Among dippers, 50% (n=7) decreased their mean systolic BP during the sleeping period to normotensive levels (SBP<150 mmHg) compared to 15% (n=4) of non-dippers (p=0.016). At 8 AM, the mean systolic BP was similar between groups (p=0.161), but dippers tended to have lower systolic BP values. The pattern of diastolic BP was similar to the systolic BP, with a significantly lower diastolic BP during the sleeping period in dippers (Figure 1, p=0.039); mean diastolic BP decreased from 93±2 to 81±3 mmHg during the sleep period; p=0.001 by paired t-test). Mean heart rate at baseline, sleeping period, and at 8 AM did not differ significantly between dippers and non-dippers; however, dippers tended to have higher HR in all periods. There was a small but consistent decrease in HR during the sleeping period compared to baseline in dippers (71±2 vs. 68±2; p=0.044 by paired t-test). Non-dippers had a similar pattern but did not reach statistical significance (67±1 vs. 65±1; p=0.066 by paired t-test).
Figure 1.
Nighttime systolic and diastolic blood pressure (left axis), and heart rate (right axis) averaged every two hours from 8PM to 8AM in dippers (solid lines) and non-dippers (discontinued lines). Baseline ("daytime") period was defined from 8PM to 10PM and the sleeping period ("nighttime") from 12AM to 6AM (horizontal bar). Values are expressed as means±SEM.
* p<0.05, for the difference in mean BP in the sleeping period between dippers and non-dippers.
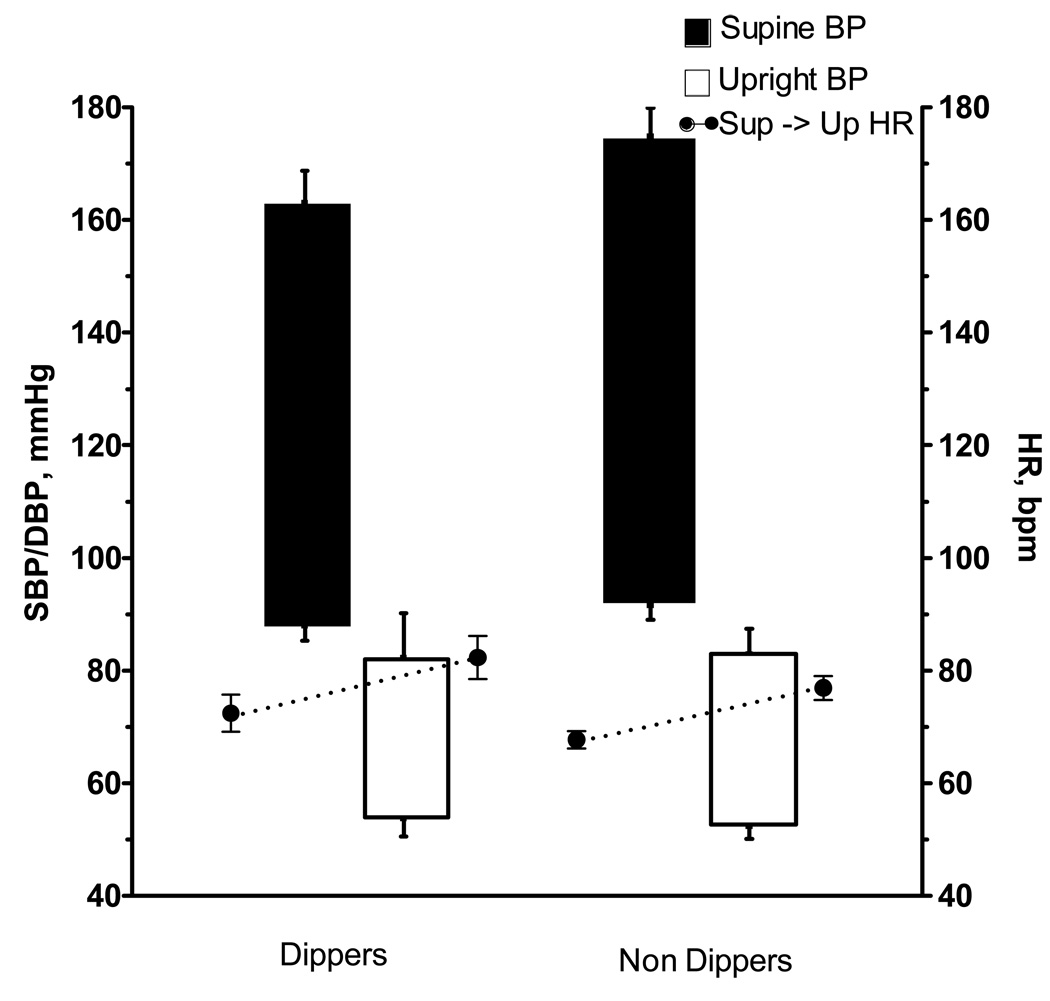
At 8 AM, a similar proportion of dippers and non-dippers were unable to stand up because of profound orthostatic symptoms (7% vs. 11%, respectively). In the remaining patients, both groups had a similar profound decrease in systolic and diastolic BP (−84/−35±9/4 in dippers vs. −93/−39±6/3 mm Hg in non-dippers, p=0.356 for SBP and p=0.494 for DBP) without an adequate increase in HR (10±4 in dippers vs. 10±2 bpm in non-dippers, p=0.892, Figure 2).
Figure 2.
Floating bargraphs denote systolic and diastolic blood pressure in the supine posture (closed bars) and after one minute upright (open bars), taken at 8AM in dippers (left panel) and non-dippers (right panel). Corresponding heart rates are denoted by the closed circles.
Autonomic Testing, Power Spectral Densities and Neurohormonal Determinations
The results of the autonomic testing, power spectral densities and neurohormonal determinations are presented in Table 2. Supine BP was 164±7/88±4 and 170±5/92±3 mm Hg in dippers and non-dippers, respectively (p=0.551 for SBP and p=0.388 for DBP). Supine HR was 74±3 and 68±2 bpm in dippers and non-dippers, respectively (p=0.075). With standing, both groups had a similar profound decrease in BP without an adequate increase in HR. Valsalva maneuver was performed in 39 patients and all had a significant decrease in systolic BP during phase II, which was similar between the 2 groups. Phase IV BP overshoot (increase in systolic BP > 10 mm Hg from Baseline) was absent in all dipper patients, whereas it was present in 2 non-dipper patients (both had PD+). These 2 patients had disabling orthostatic hypotension and had significant abnormalities in the remaining autonomic tests. Both dippers and non-dippers had markedly reduced sinus arrhythmia, Valsalva heart rate ratio, and BP response to pain stimulus (cold pressor test). There were no differences between groups.
Table 2.
Autonomic Testing, Power Spectral Densities and Neurohormonal Determinations in Dipper and Non-dipper Patients*
| Tests | Dippers | n | Non-dippers | n | p value | Controls† |
|---|---|---|---|---|---|---|
| Orthostatic Stress Test | ||||||
| Δ SBP (mmHg) | −75 ± 13 | 14 | −92 ± 5 | 27 | 0.277 | ≤ 20 |
| Δ DBP (mmHg) | −34 ± 6 | 14 | −42 ± 3 | 27 | 0.536 | ≤ 10 |
| Δ HR (bpm) | 13 ± 3 | 14 | 12 ± 2 | 27 | 0.967 | 5–10 |
| SA ratio | 1.06 ± 0.01 | 14 | 1.06 ± 0.01 | 27 | 0.729 | 1.2±0.1 |
| Valsalva Maneuver | ||||||
| Phase II, Δ SBP (mmHg) | −72 ± 8 | 12 | −63 ± 4 | 27 | 0.394 | ≤ 20 |
| Phase IV overshoot (present)‡ | 0 % | 12 | 7 % (2) | 27 | 0.218 | present |
| Valsalva ratio | 1.11 ± 0.03 | 13 | 1.11 ± 0.02 | 26 | 0.561 | 1.5 ± 0.2 |
| Cold pressor (Δ SBP, mmHg) | 8 ± 4 | 14 | 7 ± 2 | 23 | 0.683 | 24 ± 13 |
| LF RRI (ms2) | 30 ± 16 | 10 | 24 ± 6 | 12 | 0.843 | 496 ± 73 |
| HF RRI (ms2) | 11 ± 3 | 10 | 14 ± 3 | 12 | 0.510 | 585 ± 190 |
| Plasma Norepinephrine (pg/mL) | ||||||
| Supine | 107 ± 13 | 12 | 112 ± 15 | 25 | 0.758 | |
| Upright | 179 ± 30 | 12 | 231 ± 40 | 25 | 0.871 | |
| Plasma Renin (ng/mL/hr) | ||||||
| Supine | 0.22 ± 0.07 | 12 | 0.25 ± 0.05 | 24 | 0.617 | 0.3–0.7§ |
| Upright | 0.40 ± 0.10 | 12 | 0.24 ± 0.04 | 23 | 0.098 | 0.7–.3§ |
| Plasma Aldosterone (ng/dL) | ||||||
| Supine | 4.95 ± 1.05 | 12 | 4.23 ± 0.55 | 23 | 0.793 | 1–16§ |
| Upright | 8.88 ± 1.90 | 12 | 8.82 ± 1.20 | 22 | 0.652 | 4–31§ |
Values are expressed as mean ± SEM. Blood pressure and heart rate changes in the orthostatic stress test are given as the changes between supine and standing. ΔSBP, systolic blood pressure change; ΔDBP, diastolic blood pressure change; ΔHR, heart rate change; SA ratio, sinus-arrhythmia ratio. Blood pressure responses during phase II of the Valsalva maneuver are given as the blood pressure change compared with baseline. LF; low frequency; HF high frequency; RRI, R-R interval.
Control values are from the Autonomic Dysfunction Center Database at Vanderbilt University.
Phase IV of the Valsalva maneuver expressed as the percentage of subjects who increased systolic BP >10 mm Hg from Baseline. Number of subjects is in parentheses.
Normal reference values from The Vanderbilt Clinic laboratory.
Spectral analysis of heart-rate variability was available in 22 patients, ten dippers and twelve non-dippers. Both had a markedly blunted HF and LF components compared to normal controls (Table 2), and there were no differences between groups. Normalized units for the HF and LF components were not calculated because total power was at noise levels found in normal subjects in whom autonomic tone was eliminated with ganglionic blockade.17
Supine and upright plasma norepinephrine did not differ significantly between groups (Table 2). Supine and upright plasma renin activity was low and similar between groups (p=0.617 and p=0.098, respectively), but dippers tended to have higher upright levels. There were no significant differences in supine and upright plasma aldosterone between the 2 groups.
Nocturnal urinary volume and sodium excretion
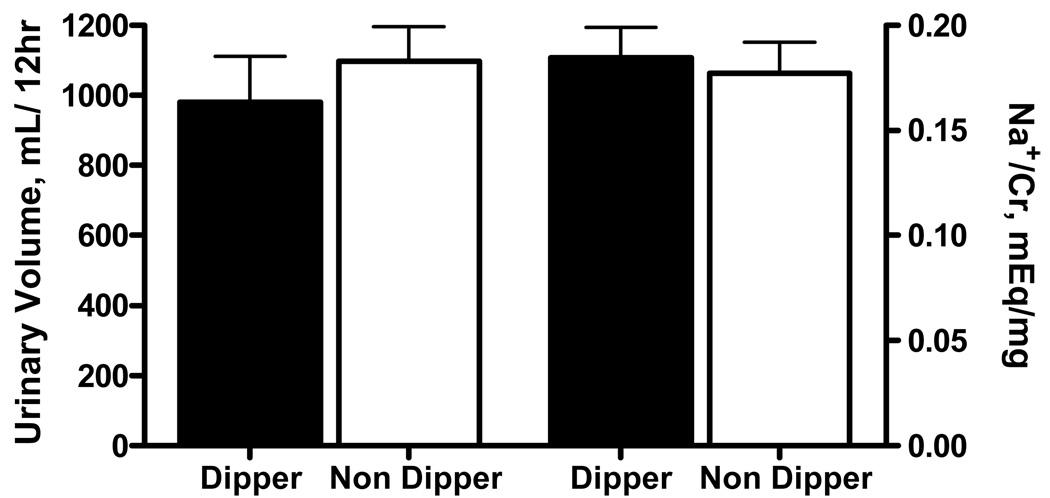
Complete nighttime urine collections were obtained in 35 patients: 11 dippers and 24 non-dippers. Nighttime urinary volume did not differ significantly between dippers and non-dippers (979±133 vs. 1097±99 ml, respectively; p=0.644, Figure 3). Likewise, urinary sodium excretion was similar between the 2 groups (0.184±0.01in dippers vs. 0.177±0.01mEq/mg creatinine in non-dippers; p=0.522).
Figure 3.
Bargraphs denote urinary volume (left) and sodium excretion (right, corrected for creatinine) collected from 8PM to 8 AM in dippers and non-dippers.
Overnight BP Reduction with Dipping and Nitroglycerin
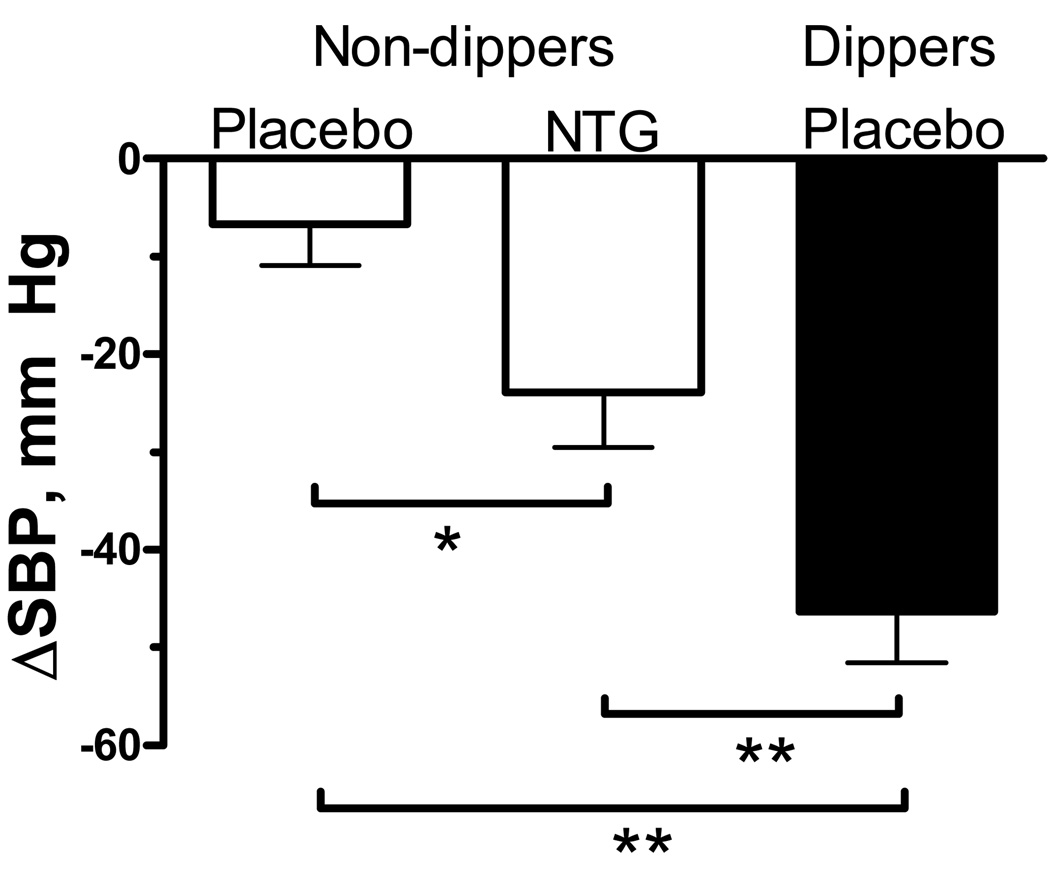
Average supine systolic BP at baseline was similar in dippers and non-dippers receiving placebo or nitroglycerin (182±4, 179±5 and 171±5 mm Hg, respectively). The mean decrease in systolic BP at 4AM (maximal decrease) was significantly greater in dippers compared to non-dippers receiving nitroglycerin or placebo (−46±5 vs. −24±6 and −7±4 mmHg, respectively; p<0.01, figure 4).
Figure 4.
Change in mean systolic BP (SBP) from 8PM to 4AM (maximal decrease) in non-dippers (white bars) treated with placebo or nitroglycerin (NTG), and in dippers (black bar) treated with placebo.
*p=0.048 by Wilcoxon signed-rank test.
**p<0.01 by Man-Whitney U test.
DISCUSSION
Blood pressure fluctuates with a pattern that follows a circadian rhythm, with a peak in the early morning hours, and a trough during sleep. Circadian rhythms typically originate in “master oscillators” located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus. How this hypothalamic rhythm is translated into changes in blood pressure is not entirely known, but the autonomic nervous system is suspected to play a role; sympathetic activity is also modulated by hypothalamic centers, and follows a circadian pattern similar to that of blood pressure.
Absence of this circadian rhythm in blood pressure (“non-dipping”) is more commonly seen in hypertensives, and is a risk factor for the development of end-organ damage and poor cardiovascular outcomes. 4–7 It has been proposed that non-dipping in hypertensive patients is associated with an increase in sympathetic activity 18, or a failure of sympathetic activity to decrease during the night. 19
Here we report that patients with pure autonomic failure have a very high prevalence (66%) of non-dipping, even though they are characterized by very low and fixed sympathetic activity. These results demonstrate that increased sympathetic tone is not essential in the pathogenesis of the non-dipping phenomenon, and suggest that a more important determinant is the failure to modulate sympathetic activity.
Reversal of the normal circadian blood pressure pattern has been previously reported in autonomic failure.8,9,20–22 It is not, therefore, unexpected that we found a high incidence of non-dipping in our patients. The other side of this coin, and perhaps a more interesting finding of this study, is that blood pressure spontaneously decreased during the night (“dipping”) in one third of patients with severe peripheral autonomic failure and supine hypertension; 34% of our patients had a nocturnal fall in blood pressure, with a decrease in systolic blood pressure of −44±4 mm Hg at 4 AM.
It should be noted that the classical definition of dipping, which is based on the average blood pressures during daytime and nighttime hours, cannot be readily applied to our patients. Patients with autonomic failure are very sensitive to stimuli that would normally produce little, if any, effect in blood pressure. For example, upright posture lowered systolic blood pressure by almost 90 mm Hg in our patients. Meals have similar dramatic hypotensive effect, whereas water drinking can produce substantial increases in blood pressure. Daytime blood pressure, therefore, is extremely variable due to seemingly trivial external factors that are difficult to control. Hence, it is difficult to rely on standard 24 hr blood pressure monitoring to determine whether an intrinsic circadian pattern of blood pressure is still present in these patients. Previous studies have highlighted the fact that an abnormal 24 hr blood pressure pattern in autonomic failure could be due simply to the fact that orthostatic hypotension will lower the average daytime blood pressure.22 Even when daytime activities were carefully controlled, postprandial hypotension confounded the blood pressure measured during the scheduled supine periods.8
This constraint required us to define the “daytime” (baseline) period from 8PM to 10PM, and “nighttime” (sleeping) period from 12AM to 6AM. The latter approach has been shown to provide an accurate estimate of blood pressure during sleep, whereas wider intervals may overestimate the true sleeping BP.15,23,24 Future studies would benefit from careful 24 hr monitoring in these patients, to include periods of supine rest at intervals during the day, with strict control of physical activities, meals, water ingestion and other confounding factors. This would test the validity of our ad hoc definition of dipping. Nonetheless, the clear separation in blood pressure between our “dipping” and “non-dipping” groups supports the adequacy of our definition. Furthermore, careful examination of individual data from previous studies shows that in some of those patients blood pressure peaked early in the evening and decreased thereafter. This dipping phenomenon was indeed previously recognized by some investigators,8,9,20 but has not been systematically studied before. Thus, despite differences in the precise definition of dipping, it is clear that this phenomenon is present in a significant minority of AF patients.
The mechanism underlying this blood pressure dipping was not apparent from our studies, but our results showed that it was not due to less severe autonomic impairment, and was not associated with increased urinary sodium or volume excretion during the night. It is unlikely that dipping in our patients was related to residual sympathetic tone because we studied patients with extreme cases of peripheral sympathetic and parasympathetic impairment known to have very low residual sympathetic activity.12 Furthermore, there were no significant differences in the severity of the autonomic impairment between dippers and non-dippers (Table 2). Also, both groups of patients had very low levels of plasma renin activity, consistent with previous findings showing low and unresponsive plasma renin activity in PAF patients.25 In contrast, central regulatory pathways are intact in these patients, as evidenced by their preserved baroreflex-mediated release of vasopressin. 26 It is possible; therefore, that release of vasoactive hormones, following a conserved circadian pattern, could have explained their blood pressure dipping. This hypothesis, however, remains to be tested.
Autonomic failure patients have a reversal of the diurnal variation in urinary output, with urinary volumes being typically twice as large during the night as during the day. 10,27 Supine hypertension is thought to contribute to this phenomenon by inducing pressure natriuresis. Because AF patients are extremely sensitive to changes in plasma volume, 28 we speculated that dippers would have a greater diuresis as the underlying cause for their fall in blood pressure. This hypothesis proved to be incorrect, because we found no significant difference in nocturnal urinary sodium or water excretion between dippers and non-dippers. Furthermore, we would expect a sustained fall in blood pressure throughout the night if volume loss was the determinant of dipping. Instead, we observed a restoration of blood pressure at the end of the nighttime period, following a circadian pattern.
Even though the mechanism of dipping is not apparent from our results, our findings have implications for the treatment of these patients. Patients with PAF have a relatively good survival prognosis, but the presence of supine hypertension has been associated with end-organ damage, such as ventricular hypertrophy. 29 It is justifiable, therefore, to treat hypertension in these patients. Nitroglycerin patch, applied at bedtime and removed in the morning, is an effective agent to control supine hypertension.10,11,30 Unfortunately, nitroglycerin does not decrease nighttime diuresis and, therefore, does not improve orthostatic intolerance in the morning. Furthermore, treatment of supine hypertension is not without risks. In particular, pharmacologic treatment of supine hypertension could potentially aggravate orthostatic hypotension during the night, increasing the risk of falls in patients. Treatment of supine hypertension, therefore, should be only directed to patients who would benefit the most. On the other hand, we found that 27% of all patients spontaneously normalized their BP during the night and the magnitude of this BP reduction was even higher than that achieved with nitroglycerin in non-dippers. This suggests that the decision for treatment of supine hypertension should be based on overnight BP monitoring rather than a single daytime measure. Antihypertensive treatment in this setting could be not only unnecessary but potentially harmful to these patients.
Perspectives
Circadian variation in sympathetic tone is likely to be important in the genesis of normal BP dipping, but our results suggest that it is not essential, because it was observed in patients with the severest form of autonomic failure. Other factors, not yet discovered, are likely to contribute to normal dipping. Patients with AF offer a unique opportunity to explore these potential mechanisms further, given that hemodynamic effects are magnified in these patients because of the extreme sensitivity they have to any pressor or depressor stimuli. Our results also highlight the importance of individualizing treatment in these patients; notably, BP normalized in a significant percentage of our patients with severe supine hypertension, precluding the need to treat this aspect of their disease
Acknowledgements
The authors would like to acknowledge the patients that volunteered for these studies and the Clinical Research Center Nurses who made this study possible.
Sources of Funding
This work was supported by National Institutes of Health grants RO1, NS055670, PO1 HL56693, and UL1 RR024975.
Footnotes
Disclosures
None.
References
- 1.James GD, Pickering TG. The influence of behavioral factors on the daily variation of blood pressure. Am J Hypertens. 1993;6:170S–173S. doi: 10.1093/ajh/6.6.170s. [DOI] [PubMed] [Google Scholar]
- 2.Chau NP, Mallion JM, de GR, Ruche E, Siche JP, Pelen O, Mathern G. Twenty-four-hour ambulatory blood pressure in shift workers. Circulation. 1989;80:341–347. doi: 10.1161/01.cir.80.2.341. [DOI] [PubMed] [Google Scholar]
- 3.Smolensky MH, Haus E. Circadian rhythms and clinical medicine with applications to hypertension. Am J Hypertens. 2001;14:280S–290S. doi: 10.1016/s0895-7061(01)02175-6. [DOI] [PubMed] [Google Scholar]
- 4.Ingelsson E, Bjorklund-Bodegard K, Lind L, Arnlov J, Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 6.Verdecchia P, Schillaci G, Gatteschi C, Zampi I, Battistelli M, Bartoccini C, Porcellati C. Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation. 1993;88:986–992. doi: 10.1161/01.cir.88.3.986. [DOI] [PubMed] [Google Scholar]
- 7.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 8.Omboni S, Smit AA, van Lieshout JJ, Settels JJ, Langewouters GJ, Wieling W. Mechanisms underlying the impairment in orthostatic tolerance after nocturnal recumbency in patients with autonomic failure. Clin Sci (Lond) 2001;101:609–618. [PubMed] [Google Scholar]
- 9.Mann S, Altman DG, Raftery EB, Bannister R. Circadian variation of blood pressure in autonomic failure. Circulation. 1983;68:477–483. doi: 10.1161/01.cir.68.3.477. [DOI] [PubMed] [Google Scholar]
- 10.Jordan J, Shannon JR, Pohar B, Paranjape SY, Robertson D, Robertson RM, Biaggioni I. Contrasting effects of vasodilators on blood pressure and sodium balance in the hypertension of autonomic failure. J Am Soc Nephrol. 1999;10:35–42. doi: 10.1681/ASN.V10135. [DOI] [PubMed] [Google Scholar]
- 11.Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. The Hypertension of Autonomic Failure and Its Treatment. Hypertension. 1997;30:1062–1067. doi: 10.1161/01.hyp.30.5.1062. [DOI] [PubMed] [Google Scholar]
- 12.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. doi: 10.1161/01.cir.101.23.2710. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–126. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 14.Mosqueda-Garcia R. Evaluation of autonomic failure. In: Robertson D, Biaggioni I, editors. Disorders of the Autonomic Nervous System. London: Harwood Academic Press; 1995. pp. 25–59. [Google Scholar]
- 15.Staessen JA, Bieniaszewski L, O'Brien E, Gosse P, Hayashi H, Imai Y, Kawasaki T, Otsuka K, Palatini P, Thijs L, Fagard R The "Ad Hoc' Working Group. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. Hypertension. 1997;29:30–39. doi: 10.1161/01.hyp.29.1.30. [DOI] [PubMed] [Google Scholar]
- 16.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 17.Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, Luft FC, Robertson D, Biaggioni I. The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade. J Hypertens. 2003;21:1677–1686. doi: 10.1097/00004872-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Grassi G, Seravalle G, Quarti-Trevano F, Dell'oro R, Bombelli M, Cuspidi C, Facchetti R, Bolla G, Mancia G. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension. 2008;52:925–931. doi: 10.1161/HYPERTENSIONAHA.108.116368. [DOI] [PubMed] [Google Scholar]
- 19.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens. 2002;15:111–118. doi: 10.1016/s0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- 20.Tulen JH, Man in 't Veld AJ, van Steenis HG, Mechelse K. Sleep patterns and blood pressure variability in patients with pure autonomic failure. Clin Auton Res. 1991;1:309–315. doi: 10.1007/BF01819837. [DOI] [PubMed] [Google Scholar]
- 21.Plaschke M, Trenkwalder P, Dahlheim H, Lechner C, Trenkwalder C. Twenty-four-hour blood pressure profile and blood pressure responses to head-up tilt tests in Parkinson's disease and multiple system atrophy. J Hypertens. 1998;16:1433–1441. doi: 10.1097/00004872-199816100-00006. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho MJ, van den Meiracker AH, Boomsma F, Lima M, Freitas J, Veld AJ, Falcao De FA. Diurnal blood pressure variation in progressive autonomic failure. Hypertension. 2000;35:892–897. doi: 10.1161/01.hyp.35.4.892. [DOI] [PubMed] [Google Scholar]
- 23.Fagard R, Brguljan J, Thijs L, Staessen J. Prediction of the actual awake and asleep blood pressures by various methods of 24 h pressure analysis. J Hypertens. 1996;14:557–563. doi: 10.1097/00004872-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 24.van Ittersum FJ, Ijzerman RG, Stehouwer CD, Donker AJ. Analysis of twenty-four-hour ambulatory blood pressure monitoring: what time period to assess blood pressures during waking and sleeping? J Hypertens. 1995;13:1053–1058. doi: 10.1097/00004872-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Biaggioni I, Garcia F, Inagami T, Haile V. Hyporeninemic normoaldosteronism in severe autonomic failure. J Clin Endocrinol Metab. 1993;76:580–586. doi: 10.1210/jcem.76.3.7680352. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann H, Oribe E, Miller M, Knott P, Wiltshire-Clement M, Yahr MD. Hypotension-induced vasopressin release distinguishes between pure autonomic failure and multiple system atrophy with autonomic failure. Neurology. 1992;42:590–593. doi: 10.1212/wnl.42.3.590. [DOI] [PubMed] [Google Scholar]
- 27.Jordan J, Shannon JR, Biaggioni I, Norman R, Black BK, Robertson D. Contrasting actions of pressor agents in severe autonomic failure. Am J Med. 1998;105:116–124. doi: 10.1016/s0002-9343(98)00193-4. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox CS, Puritz R, Lightman SL, Bannister R, Aminoff MJ. Plasma volume regulation in patients with progressive autonomic failure during changes in salt intake or posture. J Lab Clin Med. 1984;104:331–339. [PubMed] [Google Scholar]
- 29.Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet. 2000;355:725–726. doi: 10.1016/S0140-6736(99)05320-9. [DOI] [PubMed] [Google Scholar]
- 30.Shibao C, Gamboa A, Abraham R, Raj SR, Diedrich A, Black B, Robertson D, Biaggioni I. Clonidine for the treatment of supine hypertension and pressure natriuresis in autonomic failure. Hypertension. 2006;47:522–526. doi: 10.1161/01.HYP.0000199982.71858.11. [DOI] [PubMed] [Google Scholar]