Abstract
Stroke has a greater effect on women than men because women have more events and are less likely to recover. Age-specific stroke rates are higher in men, but, because of their longer life expectancy and much higher incidence at older ages, women have more stroke events than men. With the exception of subarachnoid haemorrhage, there is little evidence of sex differences in stroke subtype or severity. Although several reports found that women are less likely to receive some in-hospital interventions, most differences disappear after age and comorbidities are accounted for. However, sex disparities persist in the use of thrombolytic treatment (with alteplase) and lipid testing. Functional outcomes and quality of life after stroke are consistently poorer in women, despite adjustment for baseline differences in age, prestroke function, and comorbidities. Here, we comprehensively review the epidemiology, clinical presentation, medical care, and outcomes of stroke in women.
Introduction
There is growing recognition of the clinical and public health importance of stroke in women.1 Although age-specific stroke incidence and mortality rates are higher in men than in women, stroke affects a greater number of women because of their increased longevity and the fact that stroke event rates increase substantially in the oldest age groups. Moreover, stroke-related outcomes, including disability and quality of life (QOL), are consistently poorer in women than in men, yet the reasons for this are not well understood. The societal impact of poor stroke outcomes in women is compounded by the fact that elderly women are much more likely to live alone and to be socially isolated.
The importance of the differential effect of stroke on women will continue to grow over subsequent decades as an increasingly older population results in an ever greater number of stroke events in women. Because of this oncoming epidemic, the stroke community needs to address the underlying biological, epidemiological, and clinical causes and manifestations of stroke in women. Our aim is to provide a comprehensive review of the published literature on sex differences in stroke, with specific emphasis on the epidemiology, clinical presentation, medical care, and outcomes. In turn, we hope to raise awareness of the important sex differences in stroke, and to identify priority areas for further research.
Descriptive epidemiology
Mortality
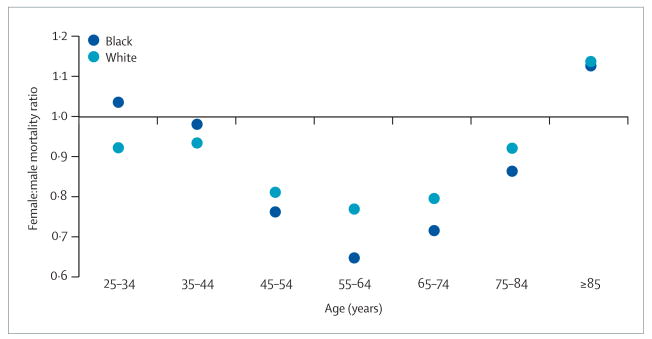
Between 1999 and 2004, the US age-adjusted (year 2000 standard) stroke mortality rate was about 3% lower in white women aged over 25 years (82.4 per 100 000) than in white men (84.8 per 100 000), and 13% lower in black women (111.1 per 100 000) than in black men (128.3 per 100 000; data from the Centers for Disease Control and Prevention WONDER database). However, these sex differences are strongly modified by age (figure 1). Below the age of 45 years, stroke mortality for women and men is similar, but women aged 45–74 years have a substantially lower risk of stroke mortality than do men (about 25–35% lower for black women and 20% lower for white women). This benefit for women declines in older age groups, such that black and white women aged 85 years and older have 12% and 14% higher mortality than do men, respectively (figure 1). Age-adjusted data therefore obscure the complex relation of sex differences at specific ages, and mask the higher stroke mortality for elderly women.
Figure 1. Female:male mortality ratios for stroke by age (USA, 1999–2003).
Data from the US Centers for Disease Control and Prevention WONDER database.
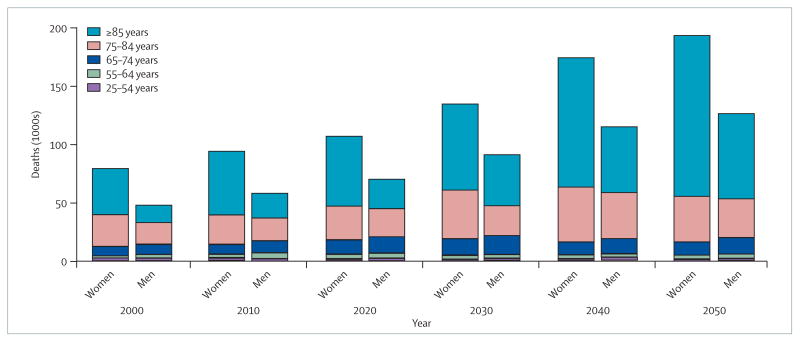
The focus on age-adjusted and age-specific stroke mortality rates also conceals the greater total number of stroke deaths in women. The excess number of deaths in women results from the higher mortality in older women and their disproportionate representation in the population. At the age of 50 years, the female:male population ratio is 1.01, but this increases to 1.19 at 70 years, 1.56 at 80 years, and 2.70 at 90 years.2 Figure 2 illustrates the joint effect of the higher stroke mortality and preponderance of women at older ages for whites (patterns are similar for other racial groups). In 2000, in the USA there were marginally fewer stroke deaths among white women than men below the age of 65 years (13 132 vs 14 629). However, there were approximately 7500 more stroke deaths in women aged 75–84 years, and nearly 26 000 more stroke deaths in women aged over 85 years. The net result of this interplay between the age-specific rates and population size is nearly 80 000 stroke deaths in white women and 48 000 in white men, an excess of 67% in women.
Figure 2. Projected number of deaths from stroke among whites (USA, 2000–2050).
Age-specific and sex-specific mortality estimates were obtained from the US Centers for Disease Control and Prevention WONDER database, and age-specific and sex-specific population projections from the US Census Bureau middle series.2 Projected deaths were calculated by applying age-specific and sex-specific mortality data to the population projections.
The greater burden of stroke deaths in women is predicted to be even higher in the future. Figure 2 applies the current US stroke mortality data to the US Census Bureau population projections,2 and projects sex-specific stroke deaths among whites through to 2050 (again, other race groups have similar patterns). The 32 000 excess stroke deaths in women in 2000 steadily increases to nearly 68 000 by 2050.
Incidence
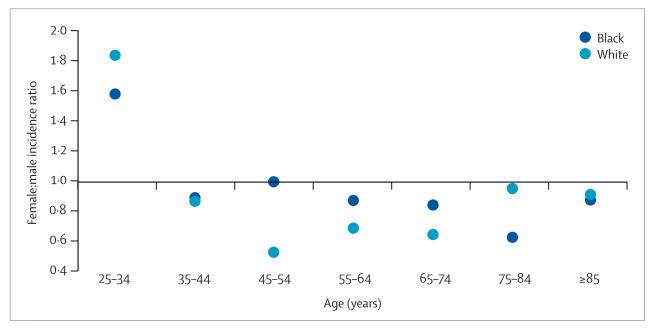
Similar to age-adjusted mortality, women have an overall lower age-adjusted stroke incidence than men.3 Unfortunately, few data are available that describe sex differences in age-specific stroke incidence. Figure 3 shows age-specific female:male incidence ratios in whites and blacks from the Greater Cincinnati–Northern Kentucky Stroke Study (GCNKSS).4 Apart from a higher incidence in women aged 34 years and younger, incidence ratios follow a similar pattern to the mortality ratios (ie, protection for women aged 45–74 years, but not for those aged above 75 years). With fewer blacks in the GCNKSS, the pattern is less consistent. Similarly, a population-based study in Sweden found stroke incidence to be 60% lower for women than men at ages 55–64 years, but by the age of 75 years women had a 50% higher incidence than men.5 The Oxford Vascular Study also showed lower ischaemic stroke incidence for women than men aged 55–74 years, but higher incidence for women aged 85 years and older.6
Figure 3. Female:male incidence ratios for stroke by age.
Data from the Greater Cincinnati–Northern Kentucky Stroke Study.4
The projections describing sex differences in the number of incident strokes are similar to the mortality data. Applying the current GCNKSS stroke incidence to the 2000 US population estimates gives an estimated 82 000 incident stroke events in white women and 49 000 events in white men, and in 2050, an estimated 198 000 events in white women compared with 129 000 events in white men.
Case fatality
Reports assessing sex differences in stroke case fatality are surprisingly variable, with many providing little evidence of a substantial difference,6–10 some showing higher case fatality,11,12 and some reporting lower case fatality in women.13,14 The WHO MONICA Project15 (18 European and Asian populations) showed that 28-day stroke case fatality was mostly either equivalent or higher in women than in men. Higher case fatality in women was shown in the International Stroke Trial,12 which randomly assigned 8003 women and 9367 men to aspirin or heparin, or both; 14-day case fatality in women was 11.0% compared with 8.7% in men, and 6-month case fatality was 24.5% in women and 19.3% in men. However, adjustment for baseline differences in age, stroke severity, atrial fibrillation, and blood pressure resulted in significantly lower 6-month mortality in women (odds ratio [OR] 0.90, 95% CI 0.83–0.98). A similar effect was seen in a national study from Denmark, which showed higher crude stroke case fatality in women, but a 17% lower rate (hazard ratio 0.83, 0.78–0.98) after adjustment for baseline differences in age, stroke severity, subtype, and risk factors.16 These studies suggest that baseline differences in age, stroke characteristics, and cardiovascular risk factors account for much, if not all, of the observed sex differences in case fatality.
Prevalence
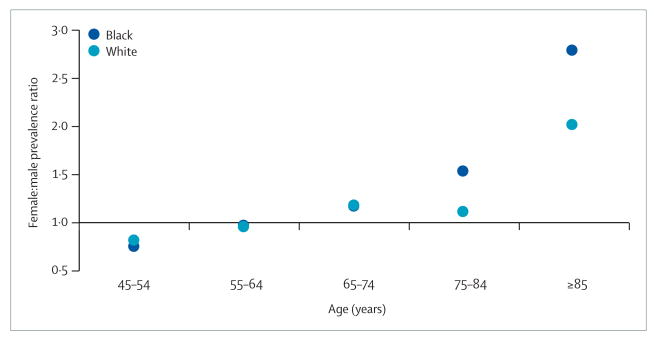
Little information exists on sex differences in stroke prevalence. A 2005 study based on the Behavioral Risk Factor Surveillance System, a nationwide telephone survey of over 352 000 US residents, found a similar stroke prevalence in women (2.5%) and men (2.7%). However, because of the preponderance of women at older ages, the estimated total number of stroke survivors was higher for women (3.1 million) than for men (2.7 million).17 Again, the possibility of age-specific sex differences might give misleading summary measures. Figure 4 provides the female:male ratio of prevalent strokes from previously unreported data from the REGARDS (REasons for Geographic And Racial Differences in Stroke) study.18 For blacks and whites, fewer women than men have prevalent stroke at young ages (45–54 years); however, with increasing age this difference is eliminated, such that among those aged 85 years and older there are almost three times more black women than men with stroke, and double in whites.
Figure 4. Female:male prevalence ratios for stroke by age.
Unpublished data from the REGARDS study18 (personal communication by GH).
Biological origins for sex differences in stroke
The most common biological explanation for sex differences in stroke is related to sex steroid hormones, particularly oestrogen. This hypothesis is supported by robust sex differences in animal models of ischaemic stroke. For example, after middle cerebral artery occlusion in rodents, females have smaller stroke volumes than have males. However, ovariectomised females have similar stroke volumes to males, whereas volumes in ovariectomised females given oestrogen replacement are similar to intact females.19,20 Oestradiol has very potent effects on endothelia that promote dilation and blood flow, whereas testosterone has the opposite effects.21 Similarly, cerebrovascular reactivity is the most robust in premenopausal women, but postmenopausal women have poorer responses than age-matched men. Post-menopausal women receiving oestrogen replacement therapy have reactivity responses similar to pre-menopausal women.22 In addition to vascular effects, oestrogen has anti-inflammatory effects that might be modulated by antioxidant and antiapoptosis effects.23 All of these findings suggest that women are protected by endogenous oestrogens. As a putative neuroprotective agent, oestradiol might be the most widely studied molecule, and yet it has never been tested in patients with acute stroke.1
Human studies of hormone therapy have focused on disease prevention rather than acute treatment. Two randomised trials in women with established cardiovascular disease found no benefit of hormone therapy. The Heart and Estrogen-progestin Replacement Study found that, in postmenopausal women with coronary heart disease, exogenous oestrogen and progesterone did not reduce the risk of coronary events.24 In the Women Estrogen Stroke Trial, exogenous oestrogen did not reduce the risk of stroke or mortality among postmenopausal women with a history of stroke or transient ischaemic attack.25 Among healthy post-menopausal women in the Women's Health Initiative (WHI) study, exogenous oestrogen increased the risk of stroke.26,27 The negative findings of these clinical trials are still poorly understood, but are in sharp contrast to the protective effects of oestradiol seen in animal studies, and the direct protective vascular effects in human studies. These results also conflict with previous epidemiological studies that have shown consistent protective effects against risk for cardiovascular disease,28 although most of these studies have not shown protective effects against stroke. One explanation for the negative results of these trials is that most of the participants were well past menopause at the time of enrolment (eg, mean age of WHI participants was 64 years). A follow-up secondary analysis of the WHI trial showed that, within 5 years of menopause, oestrogen protected against heart disease but not stroke.29 Clearly, many important questions remain about the relation between stroke, menopause, and the use and timing of hormone therapy.
Sex differences in the response to injury and cell death have been shown without the direct influence of sex steroid hormones. For instance, marked sex differences have been observed in cultures of XX (ie, female) and XY (ie, male) cells tested in steroid-free media in vitro. XY cultures seem to be more susceptible to excitotoxic cell death, whereas XX cells are more sensitive to pro-apoptotic (programmed) cell death.1 These findings suggest that the development of new neuroprotective drugs should account for the possibility of sex differences in the mechanism and response to stroke injury. Future research should consider the importance of the neurovascular unit and the signalling processes that occur among the glia, neurons, and endothelium components.30 There might be sex differences in the neurovascular unit that determine the response to therapies such as alteplase. Previous studies have shown greater effectiveness of intravenous therapy in women,31,32 although interestingly, similar differences have not been found for intra-arterial alteplase.33,34
Clinical characteristics at stroke onset
At least two studies have shown that women have worse prestroke disability than men.11,35 Women are also more likely to be living alone or to be in an assisted living arrangement or a nursing home before their stroke event.7,11,35 Men and women with stroke differ with respect to the prevalence of stroke risk factors. Women with stroke are older at onset (by an average of about 4 years), and are more likely to have atrial fibrillation and hypertension, whereas men with stroke are more likely to have a history of heart disease, myocardial infarction, peripheral arterial disease, diabetes, and alcohol and tobacco use.8–12
Although both diabetes and metabolic syndrome are recognised to increase the risk of ischaemic stroke in men and women,36 studies point to both risk factors having a greater effect in women.37,38 For example, a population-based study in Denmark found that type 2 diabetes doubled the risk of stroke in men across all age groups, whereas in women the effect of diabetes on stroke risk was significantly higher (risk ratio [RR] 2.5–6.5).39 Although the prevalence of metabolic syndrome (the clustering of obesity, abdominal obesity, dyslipidaemia, hypertension, and high plasma glucose) is similar in men and women,40 its effect on stroke in women is greater. A recent study found that metabolic syndrome doubled the risk of ischaemic stroke in women but had no effect in men.41 Women with metabolic syndrome are also more likely to develop subclinical atherosclerosis earlier than are men with the syndrome.42 The contribution of specific disorders to metabolic syndrome is different in men and women,43 and this difference seems to modify the risk of vascular disease.44
Migraine is an independent risk factor for stroke. In a recent meta-analysis of 14 observational studies, stroke risk in people with migraine was more than doubled (RR 2.16, 95% CI 1.89–2.48).45 The risk of stroke associated with migraine was even higher in women under the age of 45 years (RR 2.76, 2.17–3.52), and in women who used oral contraceptives (RR 8.72, 5.05–15.05).
Some stroke risk factors are specific to women of reproductive age. A recent meta-analysis concluded that oral contraceptive use increases ischaemic stroke risk by almost three times (RR 2.75, 95% CI 2.24–3.38), although the absolute risk is still small (one stroke per 24 000 women per year).46 Pregnancy results in haemostatic changes, including increases in clotting factors and decreases in anticoagulants and fibrinolytic activity, which increase the risk of thrombosis.47 Overall, the incidence of pregnancy-related stroke is low; a recent estimate derived from the US Nationwide Inpatient Sample was 34.2 per 100 000 deliveries compared with 11 per 100 000 non-pregnant women of childbearing age.48 The risk for all stroke subtypes increases with pregnancy, but the relative risk for intracerebral haemorrhage is higher than that for cerebral infarction.49,50 A Swedish population-based study found that the highest stroke risk was in the peripartum period (ie, 2 days before to 1 day after delivery; RR for cerebral infarction 33.8, 10.5–84.0; RR for intracerebral haemorrhage 95.0, 42.1–194.8), although excess risk persisted into the puerperium period (ie, 2 days to 6 weeks postpartum).49 Certain pregnancy complications further increase stroke risk, including pre-eclampsia, eclampsia, postpartum obstetric haemorrhage, and postpartum infection.48
Ischaemic stroke tends to aggregate in families, with a positive family history conferring a relative risk of stroke of roughly 1.3–1.8.51 More recently, data have been published to suggest that the heritability of stroke might differ for women and men.52,53 Results from a meta-analysis on family history of stroke showed that female probands were more likely to have a positive family history of stroke in any parent than were male probands (pooled OR 1.15, 95% CI 1.03–1.28).53 Female probands were also more likely than male probands to have a history of stroke in their mothers than in their fathers (pooled OR 1.25, 1.15–1.37).53 This finding was in agreement with earlier work from the Oxford Vascular Study.52 Other explanations for these observations include shared environments among family members or differential recall of family history between male and female stroke patients. More research is needed to understand whether sex-specific genetic mechanisms exist for ischaemic stroke.
Overall, women do not seem to have more severe strokes than men, especially after taking into account stroke subtype and age, although the evidence is somewhat contradictory. In two studies that measured stroke severity with the Canadian neurological scale, one found that women had greater severity on presentation,9 whereas the other found no sex difference.10 Two studies that measured severity by use of the National Institutes of Health stroke scale (NIHSS) both found little or no sex difference: in a study from Kansas City, the mean NIHSS score in men and women was 5 and 6, respectively,35 whereas in a study of first-ever ischaemic stroke, the mean NIHSS score was 3.8 and 4.3 in men and women, respectively.54 A large study in Denmark measured severity with the Scandinavian stroke scale and found that women had more severe strokes.16 Other studies have reported small increases in the frequency of symptoms such as coma in women, suggesting a difference in underlying stroke severity.11,12
In terms of stroke type, several studies have shown an increase in the risk of subarachnoid haemorrhage in women.10,14,55 Some studies of ischaemic stroke have shown minor differences in subtype mechanisms. For example, by use of the Oxford Community Stroke Project classification, women were shown to have a higher frequency of total anterior circulation stroke than had men,12 and a lower frequency of posterior circulation strokes.10 However, several other studies have found no evidence of sex differences in these subtypes.11,35,54 In terms of the TOAST (Trial of Org 10172 in Acute Ischemic Stroke56) criteria, at least four studies have found that women have a higher frequency of cardioembolic stroke than men,9,57–59 whereas a study in Texas, USA, found no sex difference.60 The increased risk of cardioembolic stroke is thought to be related to the increased prevalence of atrial fibrillation in older women, and is clinically important because cardioembolic stroke is typically more severe than other ischaemic stroke mechanisms.61
Prehospital and in-hospital delays
Many studies have been undertaken in several countries to identify factors that are associated with the time from stroke onset to arrival in the emergency department (ie, prehospital delay). Nearly all of these studies have found no evidence of clinically important differences in prehospital delay between women and men.10,62–78 Among the few studies that did find evidence of sex differences in prehospital delay, five found that women arrived later than men,79–83 and one found that women arrived sooner.84 One study found that 40% of women arrived within 3 h of onset compared with 47% of men.79 Analysis of data from over 53 000 stroke admissions in a German stroke registry found that the odds of being admitted within 3 h of onset was 10% lower for women than for men.83 Two studies (from Hong Kong80 and Australia81) found that women were less likely to present to the emergency department within 6 h of onset, which is directly opposed to the findings of Lacy and colleagues in New Jersey, USA.84
Two studies have shown that prehospital times are shorter for witnessed stroke events.64,82 A study in North Carolina, USA, found that if the event was witnessed, prehospital delay was substantially shorter than if onset was not witnessed (ie, median delay 2.0 h vs 5.8 h).64 However, this study did not find a longer prehospital delay in women. One can surmise that if a person lives alone, the onset of symptoms is less likely to be witnessed by another person. Women are more likely than men to live alone;10,11 according to the 2003 US census, approximately 8 million women and 2.7 million men live alone in the USA.85 Several studies have found that living alone is associated with increased prehospital delay,62,63,65,66,68 although three studies have found no such relation.72,78,82
Although in-hospital delays have not been studied as extensively as prehospital delays, there is evidence that after arriving in the emergency department, women experience greater delays than do men. Although a few studies have not found significant sex differences in assessment times,63,67,86 four studies found that women have somewhat longer door-to-scan times.87–90 A study from a single hospital in Houston (TX, USA) found that the time from arrival at the emergency department to first contact with a physician (ie, door-to-doctor time) was greater for women than for men.79 Explanations for these delays have not been established, although sex differences in symptoms at presentation have been suggested.91
Sex differences in acute stroke care
In-hospital diagnostic and treatment procedures
Relatively few studies have examined whether sex differences exist in the care of patients with acute stroke. Although some studies have found evidence of differences in the use of specific diagnostic and treatment-related procedures,11,60,92–95 overall the number and magnitude of these differences has been relatively small, indicating that there are not major sex differences in the quality of in-hospital care. However, a European study found that women were less likely to receive brain imaging, carotid ultrasound, and echocardiograms than were men, after adjusting for age.11 A study in Corpus Christi (TX, USA) found that, after adjusting for confounders including age, women with stroke were less likely to receive echocardiography (OR 0.64, 95% CI 0.42–0.98) and carotid imaging (OR 0.57, 0.36–0.91) during their hospital stay.60 Investigators for the Canadian Stroke Registry examined a large number of treatments and procedures and found few differences once age and other baseline differences were accounted for; the only significant finding was that women were less likely to have lipid levels tested while in hospital.10 Unadjusted analysis of data from a hospital-based registry in Michigan, USA, found several significant sex differences in the use of diagnostic procedures, including cardiac monitoring, echocardiography, angiography, and lipid investigation. However, apart from lipid investigation, these differences disappeared after adjustment for age.96
Carotid endarterectomy
Studies in the USA,92–94 Europe,11 and Canada97 have found that women are less likely to undergo carotid endarterectomy than are men. The lower use of carotid endarterectomy in Canadian women was not attributable to differences in age or comorbidity, and therefore seems to represent a true disparity.97 However, another study concluded that the sex disparity in carotid endarter-ectomy was attributable to a higher prevalence of carotid artery disease in men.92 This study, which analysed administrative data from Connecticut, USA, found that although women admitted with stroke had fewer cerebral angiography (7.2% vs 11.8%) and carotid endarterectomy (5.7% vs 10.6%) procedures than did men, these differences disappeared when the data were examined from only those patients with confirmed carotid artery disease. Regardless of any disparities in the use of carotid endarterectomy, women who do receive the surgery seem to gain similar benefits to men.97,98
Stroke-related medications
Some evidence of sex differences exists in the use of stroke-related medications in both inpatient and outpatient settings, although the findings are variable. Use of aspirin and warfarin were lower at admission and at discharge in women in the hospital-based Swedish Riks-Stroke Registry,7 whereas use of antiplatelet medications was lower only among older women (>85 years) in a study of administrative data from Ontario, Canada.8 Data from Scottish primary-care practices showed that the use of antiplatelet drugs, warfarin, and statins were all lower in women stroke survivors,99 whereas data from five Glasgow hospitals (UK) found that women were less likely to be discharged on dual antiplatelet therapy, angiotensin-converting enzyme inhibitors, or statins.100 However, a Michigan registry study found no sex difference in the use of statins at discharge, despite the lower use of lipid testing in women admitted to hospital with acute stroke.96 Finally, a study of Medicare patients in Michigan, USA, found no sex differences in the use of antithrombotic medications among stroke patients at discharge.101
Alteplase treatment
Several studies in the USA, Canada, and Germany have reported on sex differences in intravenous alteplase use.10,83,102–107 Overall, although sex-based comparisons of alteplase treatment are complicated by several factors, including definitions of eligibility for treatment, documentation of contraindications, confounding variables, and the low treatment proportions (typically no higher than 3–4%),108 most studies show that women are less likely to receive alteplase than are men. In studies that provide data on sex-specific treatment, women were between <1% and 4% less likely to receive alteplase than were men.102–104,107 A significant sex difference in the use of alteplase, after adjusting for potentially confounding variables, has been found in five studies.83,102,105–107 In a Michigan registry study, an analysis of all ischaemic stroke patients (n=1584) found that the adjusted OR for intravenous alteplase treatment in women was 0.56 (95% CI 0.4–0.9) compared with men.106 When the analyses were restricted to 323 alteplase-eligible cases (defined as arrival within 3 h of onset with no contraindications) the sex difference remained (adjusted OR 0.4, 0.2–0.8).105 Analysis of data from a large German stroke registry of over 53 000 patients showed that, after adjustment, the OR for intravenous or intra-arterial alteplase in women was 0.87 (0.78–0.96).83 However, when analyses were restricted to the alteplase-eligible subgroup, there was no sex difference in intravenous alteplase use (OR 1.03, 0.89–1.20), although intra-arterial alteplase use remained less common among women (OR 0.59, 0.36–0.95). A recent report from a single academic hospital in Nova Scotia, Canada, examined data from over 2700 stroke patients and found significant sex differences in alteplase use.102 The adjusted OR for alteplase treatment in women was 0.57 (0.35–0.72), and restriction of the analysis to the alteplase-eligible subgroup did not eliminate the sex disparity. Finally, a recent analysis of over 366 000 ischaemic stroke admissions in the US Nationwide Inpatient Sample found that alteplase treatment was given to only 1.4% of men and 0.9% of women.107 After adjustment for age, race, primary payer, Charlson index, and hospital characteristics, alteplase treatment remained significantly lower in women (OR 0.77, 0.72–0.82).
Sex differences in stroke preventive care
There are several sex differences relevant to antiplatelet treatment that are of potential clinical importance. Sex hormones have a differential effect on platelet function, with testosterone promoting platelet activity and oestrogen inhibiting it.109,110 Sex differences in the pharmacology of aspirin, including absorption, bio-availability, and anti-inflammatory and antiplatelet effects,111,112 suggest the possibility of sex-specific effects of aspirin in the prevention of stroke. However, sex-related differences in the efficacy of aspirin have been seen only in primary-prevention stroke trials. A recent meta-analysis of six randomised primary-prevention aspirin trials showed that aspirin in women reduced the risk of ischaemic stroke by 24%, but had no effect in men.113 However, the reverse was seen for myocardial infarction, for which a significant 32% risk reduction was seen in men but no significant effect was observed in women (OR 1.01, 95% CI 0.84–1.21).113 The reasons for these sex-specific effects are unclear. Only two of the six trials included both men and women, and nearly all data specific to women came from the Women's Health Study, which did not include men.112 Differences in trial design and study characteristics, such as the age of participants, aspirin dose, and the length of follow-up, might be explanations, but further work in this area is clearly necessary to resolve these important issues. In contrast to primary prevention, aspirin seems to provide similar benefits in terms of secondary-stroke prevention in both men and women.114
Women with non-valvular atrial fibrillation have nearly double the risk of stroke than men with the same risk factor.115 Use of warfarin anticoagulation for thrombo-embolic prophylaxis in patients with atrial fibrillation has been shown to be less common in women than in men,10,116 even though warfarin substantially lowers the risk of stroke in both men and women (by 60% and 84%, respectively).117 In a recent systematic review of bleeding complications related to anticoagulation therapy for atrial fibrillation, no sex-related differences in the risk of bleeding were found.118 Studies of sex-related differences in the efficacy of specific types or classes of antihypertensive therapies are lacking. The seventh report of the Joint National Committee on high blood pressure found no sex-related differences in the effectiveness of any particular type or class of antihypertensive agent.119
Despite evidence that women admitted to hospital with stroke are less likely to receive lipid testing,10,96 the use of statins at discharge seems to be similar for men and women,120,121 although data from two studies in Scotland indicate lower statin use in women stroke survivors.99,100 Whether statins have similar benefits in terms of stroke protection has been debated;122 however, a recent trial of high-dose statins for secondary stroke prevention found no evidence of any interaction effect by sex.123
Although men and women with more than 75% symptomatic stenosis do benefit from carotid endarterectomy equally,124 women with asymptomatic stenosis do not benefit as much as men do.125 There are sex differences in the characteristics of carotid disease that might explain this discrepancy. One study found that although women had significantly greater carotid stenosis, men had greater plaque area.126 These findings are important because plaque area and not stenosis was found to be a predictor of poor outcome, suggesting that men are at higher risk.126 Histological analysis of carotid endarterectomy samples revealed that, compared with men, women had more stable plaques that are less prone to rupture.127
Functional outcomes and quality of life
Surprisingly few studies have been done with the primary objective of examining sex differences in functional outcomes after stroke. Published studies (table) include those from Europe7,9,11,12,128 and North America.10,35,106,129 A consistent feature of these studies is that women have less favourable outcomes after stroke than do men. Women have more physical impairments and limitations in activities of daily living (ADL), as measured by the Barthel index.10,11,35,106,129 A European study found that women were more likely to be disabled at 3 months (Barthel index <75) after adjusting for age and country.11 In data from the Swedish Riks-Stroke Registry, 54% of women versus 67% of men were independent in primary ADL at 3 months' follow-up.130 In a study of 108 ischaemic stroke survivors from the Framingham Study, 34% of women were disabled at 6 months (Barthel index <60) compared with only 16% of men.129 In the Kansas City Stroke Study, women initially had a 30% lower odds of achieving ADL independence (Barthel index ≥95) by 6 months compared with men, although this difference was greatly diminished after adjusting for age, stroke severity, depression, prestroke physical function, and comorbidites.35 In the Michigan registry study, women had a 63% lower odds of achieving ADL independence (Barthel index ≥95) at 3 months after discharge, and adjustment for potential confounding factors including age and prestroke ambulatory status actually increased this disparity.106
Table. Characteristics and findings of studies examining sex differences in functional outcomes and QOL after acute stroke.
| Time period |
Setting | Study design | n | Outcomes | Follow-up (months) |
Measures of association* (95% CI) |
Adjustment | ||
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | ||||||||
| European studies | |||||||||
| Wyller and co-workers128 | 1992–1993 | Oslo, Norway (single hospital) | Consecutive stroke admissions | 42 | 45 | BI (median scores) | 12 | OR 3.3 (1.2–9.0) | Age |
| Di Carlo and co-workers11 | 1993–1994 | 7 European countries (22 hospitals) | Admissions for first-ever stroke | 2260 | 2239 | Dependent BI (<75), mRS (>1) | 3 | BI: OR 1.4 (1.1–1.8); mRS: OR 1.5 (1.1–1.9) | Age, country |
| Roquer and co-workers9 | 1996–2001 | Barcelona, Spain (single hospital) | Consecutive admissions for first-ever stroke | 772 | 809 | mRS (>2) | 3 | OR 1.9 (1.5–2.4) | None |
| Glader and co-workers7 | 2001 | Riks-Stroke Registry, Sweden (75 hospitals) | National Stroke Quality Registry | 9666 | 9881 | Independent ADL, INH | 3 | ADL: OR 0.7 (0.7–0.8); INH: OR 1.5 (1.3–1.6) | Age |
| Niewada and co-workers12 | 1992–1996 | International Stroke Trial | Clinical trial, ischaemic stroke only | 8003 | 9367 | Death or dependent | 6 | OR 1.4 (1.3–1.5) | Age, consciousness level, stroke subtype, AF, SBP, aspirin use |
| North American studies | |||||||||
| Kelly-Hayes and co-workers129 | 1982–1999 | Framingham Study | Cohort | 63 | 45 | Dependent BI (<60), INH | 6 | BI: OR 2.1 (0.8–6.0) INH: OR 2.7 (1.0–7.9) | Age, stroke subtype |
| Kapral and co-workers10 | 2001–2002 | Canadian Stroke Registry (21 tertiary care hospitals) | Consecutive acute stroke or TIA admissions | 1527 | 1796 | Physical function (SIS-16), HUI | 6 | Lower SIS-16 scores in women (p=0.0001); no difference in HUI | Age, Charlson index, consciousness level, stroke subtype, severity, marital status, living situation |
| Lai and co-workers35 | 1995–1998 | Kansas City Stroke Study (12 hospitals) | Cohort acute stroke admissions | 245 | 214 | Independent BI (≥95), IADL (8 of 9), physical function (SF-36 ≥90) | 6 | BI: HR 0.9 (0.7–1.3); IADL: HR 0.5 (0.3–0.8); SF-36: HR 0.8 (0.4–1.6) | Age, stroke severity, depressive symptoms, pre-stroke physical functioning, comorbidities |
| Gargano and Reeves106 | 2002 | Michigan Stroke Registry (9 hospitals) | Cohort acute stroke admissions | 210 | 163 | Independent BI (≥95), SS-QOL | 3 | BI: OR 0.4 (0.2–0.9); lower QOL in women | Age, race, discharge mRS, ambulatory status, stroke subtype, proxy status, previous stroke |
Women versus men. ADL=activities of daily living. AF=atrial fibrillation. BI=Barthel index. mRS=modified Rankin scale. HR=hazard ratio. HUI=health utilities index. IADL=instrumental activities of daily living. INH=institutionalisation or nursing home. OR=odds ratio. SIS-16=16-item stroke impact scale. SS-QOL=stroke-specific quality-of-life scale. SBP=systolic blood pressure. SF-36=36-item short-form health survey. TIA=transient ischaemic attack.
Only a few reports have looked at sex differences in QOL by use of stroke-specific instruments, such as the stroke impact scale or stroke-specific QOL scale. Almost all the studies show that women have lower overall QOL than do men after stroke. With respect to specific domains, several studies have found lower physical function scores among female stroke survivors. For example, in the Kansas City Stroke Study, women scored significantly lower than men on the 36-item short-form (SF-36) health survey physical functioning scale.35 A study of participants enrolled in an acute ischaemic stroke trial found that women had significantly lower scores on the SF-36 physical functioning and mental health domains 6 months after enrolment, after adjustment for age, baseline stroke severity, and prestroke modified Rankin scale score.58 The Canadian registry reported significantly lower physical function (as measured by the 16-item stroke impact scale) for women than men, although no differences in QOL (measured by the health utilities index) were found 6 months after discharge.10 The stroke-specific QOL scale was used in the Michigan registry study, in which women had significantly lower physical function scores.106 Several studies also showed that women have more depressive symptoms,106,131–133 and are more likely to have clinically diagnosed depression after stroke than men.134 Post-stroke depression is known to hinder functional recovery,35,132 and is associated with increased mortality.131 Depression is also more likely to result in lower scores for other stroke-related QOL domains, such as energy (or fatigue) and social functioning.
The causes of the sex differences in functional outcomes and QOL have yet to be fully elucidated. Differences are most often explained by the fact that, compared with men, women are older, have poorer prestroke function, have more comorbidities such as depression, less social support, and are more likely to be widowed. However, adjustment for these factors does not adequately explain the observed differences in stroke outcomes between men and women.8,11 Stroke severity is often cited as a potential explanation for sex differences in stroke outcomes, although the available data suggest that differences in stroke severity between men and women are small to non-existent. Clearly, more studies that assess stroke survivors in both subjective (eg, health-related QOL) and objective (eg, cognitive functioning, depression) measures are needed to determine the causes of these differences in outcomes.
Rehabilitation and post-stroke recovery
In the USA and elsewhere, women are less likely to be discharged home and are more likely to be discharged to nursing homes and long-term care after a stroke.7,8,10–12,129,135 In Canada, Europe, and the USA, there seem to be no differences in access to physical therapy, speech therapy, or occupational therapy for men and women.8,10,135 Although women have equal access to rehabilitation services, they do not experience the same levels of recovery.8,10,11,135 In the only study that has compared the responsiveness of men and women to rehabilitation care,135 male and female stroke survivors were matched on stroke severity, age, and time since stroke onset. Although both men and women improved after admission into the rehabilitation programme, men were about three times more likely to be independent in stair climbing and ADL (defined as Barthel index ≥95) than were women. These findings were hypothesised to be attributable to differences in muscular strength between men and women.
The findings of sex differences in rehabilitation, post-acute care outcomes, and discharge disposition suggest a complex interplay of demographic factors, psychosocial functioning, pre-existing health state, and disease severity.8,10,11,135 Development of the best rehabilitation recovery programmes will require more specific attention to the unique needs of women. Because of the sex disparities in recovery and outcomes after stroke, women need rehabilitation programmes to focus more on improving their physical functioning and to diagnose and treat depression. Given their greater social isolation, women are also in need of increased social support and counselling.7,136 Providers who deliver rehabilitation programmes for stroke survivors should be aware that strong sex differences in outcomes do exist.
Conclusions and future research
The sex differences in stroke can be summarised as follows: women have more stroke events due to their longer life expectancy and older age at the time of stroke onset. Women with stroke have a higher prevalence of hypertension, atrial fibrillation, and prestroke disability, but have a lower prevalence of heart disease, peripheral vascular disease, and smoking and alcohol use. Women with stroke are less likely to receive intravenous alteplase treatment and lipid testing while in hospital, and after stroke, women have poorer functional outcomes, more depression, and lower QOL than do men.
Search strategy and selection criteria
After searching their own files, each section author did their own MEDLINE search to identify relevant papers with a set of core MeSH terms (“cerebrovascular accident”, “stroke”, “sex”, “sex factors”, “sex ratio”, and “sex distribution”), and with terms relevant to each particular topic. For example, the search string “treatment delay*[tiab] OR triage OR time factors” was used to find time-related or treatment-delay-related papers. The full list of search terms is available from the authors on request. The search period was January, 1980, to April, 2008. The bibliographies of recent articles were also screened to find other previously unidentified articles. Of note, this search strategy is unlikely to have identified all of the published literature on this topic because many publications that include sex-specific data do not include related terms in the title, abstract, or MeSH headings. This Review includes both haemorrhagic and ischaemic stroke, and, where appropriate, findings relevant to specific subtypes (ie, ischaemic stroke, intracerebral haemorrhage, or subarachnoid haemorrhage) are indicated. Only English language articles were included in this Review.
On the basis of this Review, we make the following recommendations regarding future research needs and directions. For basic science research, we suggest that more studies should examine how sex steroids might act as protectants against neurovascular injury. Understanding sex-based differences in the neurovascular unit might lead to a better understanding of the reasons for sex differences in the response to alteplase, for example.31 Clinical research should focus on the study of exogenous oestrogens in perimenopausal women or those with post-surgical menopause. Candidate genetic, inflammatory, or thrombosis markers should be investigated to determine whether sex differences in vascular markers exist and whether they affect outcome.1
In terms of outcomes research, studies are clearly needed to determine the causes of the differences in functional outcomes and health-related QOL after stroke between men and women. Future studies need to assess subjective (ie, QOL) and objective (ie, cognitive functioning, depression, disability) outcome measures in stroke survivors, and more efforts are required to comprehensively document all potentially relevant pre-existing conditions. Finally, more research is needed to assess the responsiveness of women to physical, cognitive, and social interventions during the post-stroke period. These studies might support the development of sex-specific interventions that improve post-stroke recovery for women and reduce their excess burden of disability.
Acknowledgments
We would like to thank John Coffey, librarian at Michigan State University, for his help in developing the MEDLINE search strategy.
Footnotes
For the Centers for Disease Control and Prevention WONDER database see http://wonder.cdc.gov/welcome.html
Contributors: MJR, CDB, and GH developed the overall rationale for the collaboration and developed a detailed outline for the manuscript. MJR was responsible for coordinating the writing group and took primary responsibility for editing and formatting the final version. MJR was also the primary author for the sections on prehospital and in-hospital delays and on future research. CDB was the primary author for the sections on biological origins for sex differences in stroke and on clinical characteristics at stroke onset. GH was the primary author for the section on epidemiology. JWG was the primary author for the section on functional outcomes and quality of life. PWD was the primary author for the section on rehabilitation and post-stroke recovery. GL was the primary author for the section on sex differences in stroke preventive care. AK was responsible for searching for literature, drafting the section on prehospital and in-hospital delays, and manuscript preparation. LL was the primary author for the section on sex differences in acute stroke care. All authors provided editorial comments on draft versions, and approved the final version for submission.
Conflicts of interest: PWD has received consultancy fees from the American Heart Association and GlaxoSmithKline. All other authors have no conflicts of interest.
Contributor Information
Mathew J Reeves, Department of Epidemiology, College of Human Medicine, Michigan State University, East Lansing, MI.
Cheryl D Bushnell, Department of Neurology, Wake Forest University Health Sciences, Winston-Salem, NC.
George Howard, School of Public Health, University of Birmingham, AL.
Julia Warner Gargano, Department of Epidemiology, College of Human Medicine, Michigan State University, East Lansing, MI.
Pamela W Duncan, Department of Community and Family Medicine, Duke University, Durham, NC.
Gwen Lynch, Case-Western Reserve University, Cleveland, OH.
Arya Khatiwoda, Department of Epidemiology, College of Human Medicine, Michigan State University, East Lansing, MI.
Lynda Lisabeth, School of Public Health, University of Michigan, Ann Arbor, MI, USA.
References
- 1.Bushnell CD, Hurn P, Colton C, et al. Advancing the study of stroke in women—summary and recommendations for future research from an NINDS-sponsored multidisciplinary working group. Stroke. 2006;37:2387–99. doi: 10.1161/01.STR.0000236053.37695.15. [DOI] [PubMed] [Google Scholar]
- 2.US Census Bureau. Population projections. US interim projections by age, sex, race, and Hispanic origin: 2000–2050. Summary table 1A. [Sept 10, 2007]; http://www.census.gov/ipc/www/usinterimproj/
- 3.Carandang R, Seshadri S, Beiser A, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–46. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 4.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–31. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 5.Lofmark U, Hammarstrom A. Evidence for age-dependent education-related differences in men and women with first-ever stroke. Neuroepidemiology. 2007;28:135–41. doi: 10.1159/000102141. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–83. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 7.Glader EL, Stegmayr B, Norrving B, et al. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke. 2003;34:1970–75. doi: 10.1161/01.STR.0000083534.81284.C5. [DOI] [PubMed] [Google Scholar]
- 8.Holroyd-Leduc JM, Kapral MK, Austin PC, Tu JV. Sex differences and similarities in the management and outcome of stroke patients. Stroke. 2000;31:1833–37. doi: 10.1161/01.str.31.8.1833. [DOI] [PubMed] [Google Scholar]
- 9.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34:1581–85. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 10.Kapral MK, Fang J, Hill MD, et al. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. 2005;36:809–14. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 11.Di Carlo A, Lamassa M, Baldereschi M, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–19. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 12.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24:123–28. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 13.Benatru I, Rouaud O, Durier J, et al. Stable stroke incidence rates but improved case-fatality 06 in Dijon, France, from 1985 to 2004. Stroke. 2006;37:1674–79. doi: 10.1161/01.STR.0000226979.56456.a8. [DOI] [PubMed] [Google Scholar]
- 14.Sheikh K, Bullock CM. Effect of measurement on sex difference in stroke mortality. Stroke. 2007;38:1085–87. doi: 10.1161/01.STR.0000258103.15708.58. [DOI] [PubMed] [Google Scholar]
- 15.Thorvaldsen P, Asplund K, Kuulasmaa K, Rajakangas AM, Schroll M. Stroke incidence, case-fatality, and mortality in the WHO MONICA Project. Stroke. 1995;26:361–67. doi: 10.1161/01.str.26.3.361. [DOI] [PubMed] [Google Scholar]
- 16.Olsen TS, Dehlendorff C, Andersen KK. Sex-related time-dependent variations in post-stroke survival: evidence of a female stroke survival advantage. Neuroepidemiology. 2007;29:218–25. doi: 10.1159/000112464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neyer JR, Greenlund KJ, Denny CH, et al. Prevalence of stroke—United States, 2005. JAMA. 2007;298:279–81. [Google Scholar]; MMWR Morb Mortal Wkly Rep. 2007;56:469–74. reprinted from. [PubMed] [Google Scholar]
- 18.Howard VJ, Cushman M, Pulley L, et al. The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study: objectives and design. Neuroepidemiology. 2005;25:135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 19.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–65. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 20.McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- 21.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006;101:1252–61. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 22.Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M. Age and sex differences in cerebral hemodynamics: a transcranial Doppler study. Stroke. 1998;29:963–67. doi: 10.1161/01.str.29.5.963. [DOI] [PubMed] [Google Scholar]
- 23.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–35. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 24.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 25.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–49. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 26.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women—The Women's Health Initiative: a randomized trial. JAMA. 2003;289:2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 27.Hendrix SL, Wassertheil-Smoller S, Johnson KC, et al. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–34. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 28.Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–37. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 29.Roussouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]; JAMA. 2008;299:1426. published erratum in. [Google Scholar]
- 30.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–94. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 31.Kent DM, Price LL, Ringleb P, Hill MD, Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke. 2005;36:62–65. doi: 10.1161/01.STR.0000150515.15576.29. [DOI] [PubMed] [Google Scholar]
- 32.Savitz SI, Schlaug G, Caplan L, Selim M. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke. 2005;36:1447–51. doi: 10.1161/01.STR.0000170647.42126.a8. [DOI] [PubMed] [Google Scholar]
- 33.Shah SH, Liebeskind DS, Saver JL, et al. Influence of gender on outcomes after intra-arterial thrombolysis for acute ischemic stroke. Neurology. 2006;66:1745–46. doi: 10.1212/01.wnl.0000218208.31305.84. [DOI] [PubMed] [Google Scholar]
- 34.Arnold M, Kappeler L, Nedeltchev K, et al. Recanalization and outcome after intra-arterial thrombolysis in middle cerebral artery and internal carotid artery occlusion: does sex matter? Stroke. 2007;38:1281–85. doi: 10.1161/01.STR.0000259711.13490.23. [DOI] [PubMed] [Google Scholar]
- 35.Lai SM, Duncan PW, Dew P, Keighley J. Sex differences in stroke recovery. Prev Chronic Dis. 2005;2:A13. [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Circulation. 2006;113:e873–923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 37.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–38. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 38.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–19. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–26. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 40.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–59. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 41.Boden-Albala B, Sacco RL, Lee HS, et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke. 2008;39:30–35. doi: 10.1161/STROKEAHA.107.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iglseder B, Cip P, Malaimare L, Ladurner G, Paulweber B. The metabolic syndrome is a stronger risk factor for early carotid atherosclerosis in women than in men. Stroke. 2005;36:1212–17. doi: 10.1161/01.STR.0000166196.31227.91. [DOI] [PubMed] [Google Scholar]
- 43.Dallongeville J, Cottel D, Arveiler D, et al. The association of metabolic disorders with the metabolic syndrome is different in men and women. Ann Nutr Metab. 2004;48:43–50. doi: 10.1159/000075304. [DOI] [PubMed] [Google Scholar]
- 44.Hanefeld M, Koehler C, Gallo S, Benke I, Ott P. Impact of the individual components of the metabolic syndrome and their different combinations on the prevalence of atherosclerotic vascular disease in type 2 diabetes: the Diabetes in Germany (DIG) study. Cardiovasc Diabetol. 2007;6:13. doi: 10.1186/1475-2840-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330:63. doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta-analysis. JAMA. 2000;284:72–78. doi: 10.1001/jama.284.1.72. [DOI] [PubMed] [Google Scholar]
- 47.Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114:409–14. doi: 10.1016/j.thromres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 48.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–16. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 49.Salonen Ros H, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology. 2001;12:456–60. doi: 10.1097/00001648-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768–74. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke. 2004;35:212–27. doi: 10.1161/01.STR.0000107187.84390.AA. [DOI] [PubMed] [Google Scholar]
- 52.Touze E, Rothwell PM. Heritability of ischaemic stroke in women compared with men: a genetic epidemiological study. Lancet Neurol. 2007;6:125–33. doi: 10.1016/S1474-4422(06)70683-4. [DOI] [PubMed] [Google Scholar]
- 53.Touze E, Rothwell PM. Sex differences in heritability of ischemic stroke: a systematic review and meta-analysis. Stroke. 2008;39:16–23. doi: 10.1161/STROKEAHA.107.484618. [DOI] [PubMed] [Google Scholar]
- 54.Barrett K, Brott T, Brown RJ, et al. Sex differences in stroke severity, symptoms, and deficits after first-ever ischemic stroke. J Stroke Cerebrovasc Dis. 2007;16:34–39. doi: 10.1016/j.jstrokecerebrovasdis.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ayala C, Croft JB, Greenlund KJ, et al. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995–1998. Stroke. 2002;33:1197–201. doi: 10.1161/01.str.0000015028.52771.d1. [DOI] [PubMed] [Google Scholar]
- 56.The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. JAMA. 1998;279:1265–72. [PubMed] [Google Scholar]
- 57.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria—incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–40. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 58.Gray LJ, Sprigg N, Bath PM, et al. Sex differences in quality of life in stroke survivors: data from the tinzaparin in Acute Ischaemic Stroke Trial (TAIST) Stroke. 2007;38:2960–64. doi: 10.1161/STROKEAHA.107.488304. [DOI] [PubMed] [Google Scholar]
- 59.Arboix A, Oliveres M, Garcia-Eroles L, Maragall C, Massons J, Targa C. Acute cerebrovascular disease in women. Eur Neurol. 2001;45:199–205. doi: 10.1159/000052130. [DOI] [PubMed] [Google Scholar]
- 60.Smith M, Lisabeth L, Brown D, Morgenstern L. Gender comparisons of diagnostic evaluation for ischemic stroke patients. Neurology. 2005;65:855–58. doi: 10.1212/01.wnl.0000176054.72325.0f. [DOI] [PubMed] [Google Scholar]
- 61.Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36:1115–19. doi: 10.1161/01.STR.0000166053.83476.4a. [DOI] [PubMed] [Google Scholar]
- 62.Morris DL, Rosamond W, Madden K, Schultz C, Hamilton S. Prehospital and emergency department delays after acute stroke: the Genentech Stroke Presentation Survey. Stroke. 2000;31:2585–90. doi: 10.1161/01.str.31.11.2585. [DOI] [PubMed] [Google Scholar]
- 63.Kothari R, Jauch E, Broderick J, et al. Acute stroke: delays to presentation and emergency department evaluation. Ann Emerg Med. 1999;33:3–8. doi: 10.1016/s0196-0644(99)70431-2. [DOI] [PubMed] [Google Scholar]
- 64.Rosamond WD, Gorton RA, Hinn AR, Hohenhaus SM, Morris DL. Rapid response to stroke symptoms: the Delay in Accessing Stroke Healthcare (DASH) study. Acad Emerg Med. 1998;5:45–51. doi: 10.1111/j.1553-2712.1998.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 65.Casetta I, Granieri E, Gilli G, Lauria G, Tola M, Paolino E. Temporal trend and factors associated with delayed hospital admission of stroke patients. Neuroepidemiology. 1999;18:255–64. doi: 10.1159/000026220. [DOI] [PubMed] [Google Scholar]
- 66.Harper GD, Haigh RA, Potter JF, Castleden CM. Factors delaying hospital admission after stroke in Leicestershire. Stroke. 1992;23:835–38. doi: 10.1161/01.str.23.6.835. [DOI] [PubMed] [Google Scholar]
- 67.Nedeltchev K, Arnold M, Brekenfeld C, et al. Pre- and in-hospital delays from stroke onset to intra-arterial thrombolysis. Stroke. 2003;34:1230–34. doi: 10.1161/01.STR.0000069164.91268.99. [DOI] [PubMed] [Google Scholar]
- 68.Rossnagel K, Jungehulsing GJ, Nolte CH, et al. Out-of-hospital delays in patients with acute stroke. Ann Emerg Med. 2004;44:476–83. doi: 10.1016/j.annemergmed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 69.Azzimondi G, Bassein L, Fiorani L, et al. Variables associated with hospital arrival time after stroke: effect of delay on the clinical efficiency of early treatment. Stroke. 1997;28:537–42. doi: 10.1161/01.str.28.3.537. [DOI] [PubMed] [Google Scholar]; Stroke. 1997;28:1092. published erratum in. [Google Scholar]
- 70.Jorgensen H, Nakayama H, Reith J, Raaschou H, Olsen T. Factors delaying hospital admission in acute stroke: the Copenhagen Stroke Study. Neurology. 1996;47:383–87. doi: 10.1212/wnl.47.2.383. [DOI] [PubMed] [Google Scholar]
- 71.Smith MA, Doliszny K, Shahar E, McGovern P, Arnett D, Luepker R. Delayed hospital arrival for acute stroke: the Minnesota Stroke Survey. Ann Intern Med. 1998;129:190–96. doi: 10.7326/0003-4819-129-3-199808010-00005. [DOI] [PubMed] [Google Scholar]
- 72.Wester P, Radberg J, Lundgren B, Peltonen M. Factors associated with delayed admission to hospital and in-hospital delays in acute stroke and TIA: a prospective, multicenter study. Stroke. 1999;30:40–48. doi: 10.1161/01.str.30.1.40. [DOI] [PubMed] [Google Scholar]
- 73.Zweifler R, Mendizabel J, Cunningham S, Shah A, Rothrock J. Hospital presentation after stroke in a community sample: the Mobile Stroke Project. South Med J. 2002;95:1263–68. [PubMed] [Google Scholar]
- 74.Streifler JY, Davidovitch S, Sendovski U. Factors associated with the time of presentation of acute stroke patients in an Israeli community hospital. Neuroepidemiology. 1998;17:161–66. doi: 10.1159/000026168. [DOI] [PubMed] [Google Scholar]
- 75.Williams LS, Bruno A, Rouch D, Marriott DJ. Stroke patients' knowledge of stroke. Influence on time to presentation. Stroke. 1997;28:912–15. doi: 10.1161/01.str.28.5.912. [DOI] [PubMed] [Google Scholar]
- 76.Goldstein LB, Edwards MG, Wood DP. Delay between stroke onset and emergency department evaluation. Neuroepidemiology. 2001;20:196–200. doi: 10.1159/000054787. [DOI] [PubMed] [Google Scholar]
- 77.Harraf F, Sharma A, Brown MM, Lees KR, Vass RI, Kalra L. A multicentre observational study of presentation and early assessment of acute stroke. BMJ. 2002;325:17. doi: 10.1136/bmj.325.7354.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang K, Tseng M, Tan T. Prehospital delay after acute stroke in Kaohsiung, Taiwan. Stroke. 2004;34:700–04. doi: 10.1161/01.STR.0000117236.90827.17. [DOI] [PubMed] [Google Scholar]
- 79.Menon SC, Pandey DK, Morgenstern LB. Critical factors determining access to acute stroke care. Neurology. 1998;51:427–32. doi: 10.1212/wnl.51.2.427. [DOI] [PubMed] [Google Scholar]
- 80.Cheung RTF. Hong Kong patients' knowledge of stroke does not influence time-to-hospital presentation. J Clin Neurosci. 2001;8:311–14. doi: 10.1054/jocn.2000.0805. [DOI] [PubMed] [Google Scholar]
- 81.Barr J, McKinley S, O'Brien E, Herkes G. Patient recognition of and response to symptoms of TIA or stroke. Neuroepidemiology. 2006;26:168–75. doi: 10.1159/000091659. [DOI] [PubMed] [Google Scholar]
- 82.Mandelzweig L, Goldbourt U, Boyko V, Tanne D. Preceptual, social, and behavioral factors associated with delays in seeking medical care in patients with symptoms of acute care. Stroke. 2006;37:1248–53. doi: 10.1161/01.STR.0000217200.61167.39. [DOI] [PubMed] [Google Scholar]
- 83.Foerch C, Misselwitz B, Humpich M, Steinmetz H, Neumann-Haefelin T, Sitzer M. Sex disparity in the access of elderly patients to acute stroke care. Stroke. 2007;38:2123–26. doi: 10.1161/STROKEAHA.106.478495. [DOI] [PubMed] [Google Scholar]
- 84.Lacy CR, Suh DC, Bueno M, Kostis JB. Delay in presentation and evaluation for acute stroke: Stroke Time Registry for Outcomes Knowledge and Epidemiology (STROKE) Stroke. 2001;32:63–69. doi: 10.1161/01.str.32.1.63. [DOI] [PubMed] [Google Scholar]
- 85.He W, Sengupta M, Velkoff V, DeBarros K. 65+ in the United States. Current population reports; special studies. [June 3, 2008];US Census Bureau. 2005 http://www.census.gov/prod/2006pubs/p23-209.pdf.
- 86.Keskin O, Kalemoglu M, Ulusoy RE. A clinic investigation into prehospital and emergency department delays in acute stroke care. Med Princ Pract. 2005;14:408–12. doi: 10.1159/000088114. [DOI] [PubMed] [Google Scholar]
- 87.Engelstein E, Margulies J, Jeret JS. Lack of t-PA use for acute ischemic stroke in a community hospital: high incidence of exclusion criteria. Am J Emerg Med. 2000;18:257–60. doi: 10.1016/s0735-6757(00)90116-5. [DOI] [PubMed] [Google Scholar]
- 88.Yu RF, San Jose MC, Manzanilla BM, Oris MY, Gan R. Sources and reasons for delays in the care of acute stroke patients. J Neurol Sci. 2002;199:49–54. doi: 10.1016/s0022-510x(02)00103-x. [DOI] [PubMed] [Google Scholar]
- 89.Frankel M, Hinchey J, Schwamm L, et al. Prehospital and hospital delays after stroke onset—United States, 2005–2006. MMWR Morb Mortal Wkly Rep. 2007;56:474–78. [PubMed] [Google Scholar]
- 90.Jungehulsing GJ, Rossnagel K, Nolte CH, et al. Emergency department delays in acute stroke: analysis of time between ED arrival and imaging. Eur J Neurol. 2006;13:225–32. doi: 10.1111/j.1468-1331.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- 91.Labiche LA, Chan W, Saldin KR, Morgenstern LB. Sex and acute stroke presentation. Ann Emerg Med. 2002;40:453–60. doi: 10.1067/mem.2002.128682. [DOI] [PubMed] [Google Scholar]
- 92.Patrick SJ, Concato J, Viscoli C, Chyatte D, Brass LM. Sex differences in the management of patients hospitalized with ischemic cerebrovascular disease. Stroke. 1995;26:577–80. doi: 10.1161/01.str.26.4.577. [DOI] [PubMed] [Google Scholar]
- 93.Ramani S, Byrne-Logan S, Freund K, Ash A, Yu W, Moskowitz M. Gender differences in the treatment of cerebrovascular disease. J Am Geriatr Soc. 2000;48:741–45. doi: 10.1111/j.1532-5415.2000.tb04747.x. [DOI] [PubMed] [Google Scholar]
- 94.Sheikh K, Bullock C. Sex differences in carotid endarterectomy utilization and 30-day postoperative mortality. Neurology. 2003;60:471–76. doi: 10.1212/wnl.60.3.471. [DOI] [PubMed] [Google Scholar]
- 95.Rudd AG, Hoffman A, Down C, Pearson M, Lowe D. Access to stroke care in England, Wales and Northern Ireland: the effect of age, gender and weekend admission. Age Ageing. 2007;36:247–55. doi: 10.1093/ageing/afm007. [DOI] [PubMed] [Google Scholar]
- 96.Gargano J, Wehner S, Reeves MJ. Sex differences in acute stroke care and outcomes in a statewide stroke registry. Stroke. 2008;39:24–29. doi: 10.1161/STROKEAHA.107.493262. [DOI] [PubMed] [Google Scholar]
- 97.Kapral MK, Redelmeier DA. Carotid endarterectomy for women and men. J Womens Health Gend Based Med. 2000;9:987–94. doi: 10.1089/15246090050200015. [DOI] [PubMed] [Google Scholar]
- 98.Kapral MK, Wang H, Austin PC, et al. Sex differences in carotid endarterectomy outcomes: results from the Ontario Carotid Endarterectomy Registry. Stroke. 2003;34:1120–24. doi: 10.1161/01.STR.0000066681.79339.E2. [DOI] [PubMed] [Google Scholar]
- 99.Simpson CR, Wilson C, Hannaford PC, Williams D. Evidence for age and sex differences in the secondary prevention of stroke in Scottish primary care. Stroke. 2005;36:1771–75. doi: 10.1161/01.STR.0000173398.99163.9e. [DOI] [PubMed] [Google Scholar]
- 100.McInnes C, McAlpine C, Walters M. Effect of gender on stroke management in Glasgow. Age Ageing. 2008;37:220–22. doi: 10.1093/ageing/afm153. [DOI] [PubMed] [Google Scholar]
- 101.Lisabeth LD, Roychoudhury C, Brown DL, Levine SR. Do gender and race impact the use of antithrombotic therapy in patients with stroke/TIA? Neurology. 2004;62:2313–15. doi: 10.1212/01.wnl.0000130500.44011.75. [DOI] [PubMed] [Google Scholar]
- 102.Reid JM, Dai D, Gubitz GJ, Kapral MK, Christian C, Phillips SJ. Gender differences in stroke examined in a 10-year cohort of patients admitted to a Canadian teaching hospital. Stroke. 2008;39:1090–95. doi: 10.1161/STROKEAHA.107.495143. [DOI] [PubMed] [Google Scholar]
- 103.Brown DL, Lisabeth LD, Garcia NM, Smith MA, Morgenstern LB. Emergency department evaluation of ischemic stroke and TIA: the BASIC Project. Neurology. 2004;63:2250–54. doi: 10.1212/01.wnl.0000147292.64051.9b. [DOI] [PubMed] [Google Scholar]
- 104.Reed SD, Cramer SC, Blough DK, Meyer K, Jarvik JG. Treatment with tissue plasminogen activator and inpatient mortality rates for patients with ischemic stroke treated in community hospitals. Stroke. 2001;32:1832–39. doi: 10.1161/01.str.32.8.1832. [DOI] [PubMed] [Google Scholar]
- 105.Deng YZ, Reeves MJ, Jacobs BS, et al. IV tissue plasminogen activator use in acute stroke: experience from a statewide registry. Neurology. 2006;66:306–12. doi: 10.1212/01.wnl.0000196478.77152.fc. [DOI] [PubMed] [Google Scholar]
- 106.Gargano JW, Reeves MJ. Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke. 2007;38:2541–48. doi: 10.1161/STROKEAHA.107.485482. [DOI] [PubMed] [Google Scholar]
- 107.Schumacher HC, Bateman BT, Boden-Albala B, et al. Use of thrombolysis in acute ischemic stroke: analysis of the Nationwide Inpatient Sample 1999 to 2004. Ann Emerg Med. 2007;50:99–107. doi: 10.1016/j.annemergmed.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 108.Reeves MJ, Arora S, Broderick JP, et al. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36:1232–40. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- 109.Ajayi AAL, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91:2742–47. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 110.Feuring A, Christ A, Roell A, et al. Alterations in platelet function during the ovarian cycle. Blood Coagul Fibrinolysis. 2002;13:443–47. doi: 10.1097/00001721-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 111.Cavallari LH, Helgason CM, Brace LD, Viana MAG, Nutescu EA. Sex difference in the antiplatelet effect of aspirin in patients with stroke. Ann Pharmacother. 2006;40:812–17. doi: 10.1345/aph.1G569. [DOI] [PubMed] [Google Scholar]
- 112.Chiang N, Hurwitz S, Ridker PM, Serhan CN. Aspirin has a gender-dependent impact on antiinflammatory 15-epi-lipoxin A4 formation: a randomized human trial. Arterioscler Thromb Vasc Biol. 2006;26:E14–17. doi: 10.1161/01.ATV.0000196729.98651.bf. [DOI] [PubMed] [Google Scholar]
- 113.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–13. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 114.Baigent C, Sudlow C, Collins R, Peto R. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–56. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 116.Go AS, Hylek EM, Chang YC, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–92. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 117.Laupacis A, Boysen G, Connolly S, et al. Risk-factors for stroke and efficacy of antithrombotic therapy in atrial-fibrillation: analysis of pooled data from 5 randomized controlled trials. Arch Intern Med. 1994;154:1449–57. [PubMed] [Google Scholar]
- 118.Hughes M, Lip GYH. Risk factors for anticoagulation-related bleeding complications in patients with atrial fibrillation: a systematic review. QJM. 2007;100:599–607. doi: 10.1093/qjmed/hcm076. [DOI] [PubMed] [Google Scholar]
- 119.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]; JAMA. 2003;290:197. published erratum in. [Google Scholar]
- 120.Lalouschek W, Lang W, Greisenegger S, Mullner M. Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack. Stroke. 2003;34:105–10. doi: 10.1161/01.str.0000048865.79221.4d. [DOI] [PubMed] [Google Scholar]
- 121.Mullard AJ, Reeves MJ, Jacobs BS, et al. Lipid testing and lipid-lowering therapy in hospitalized ischemic stroke and transient ischemic attack patients: results from a statewide stroke registry. Stroke. 2006;37:44–49. doi: 10.1161/01.STR.0000195127.12990.43. [DOI] [PubMed] [Google Scholar]
- 122.Dale KM, Coleman CI, Shah SA, Patel AA, Kluger J, White CM. Impact of gender on statin efficacy. Curr Med Res Opin. 2007;23:565–74. doi: 10.1185/030079906X167516. [DOI] [PubMed] [Google Scholar]
- 123.Amarenco P, Bogousslavsky J, Callahan A, III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 124.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 125.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–28. [PubMed] [Google Scholar]
- 126.Iemolo F, Martiniuk A, Steinman DA, Spence JD. Sex differences in carotid plaque and stenosis. Stroke. 2004;35:477–81. doi: 10.1161/01.STR.0000110981.96204.64. [DOI] [PubMed] [Google Scholar]
- 127.Hellings WE, Pasterkamp G, Verhoeven BA, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg. 2007;45:289–97. doi: 10.1016/j.jvs.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 128.Wyller TB, Sodring KM, Sveen U, Ljunggren AE, Bautz-Holter E. Are there gender differences in functional outcome after stroke? Clin Rehabil. 1997;11:171–79. doi: 10.1177/026921559701100211. [DOI] [PubMed] [Google Scholar]
- 129.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–26. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 130.Glader EL, Stegmayr B, Asplund K. Poststroke fatigue: a 2-year follow-up study of stroke patients in Sweden. Stroke. 2002;33:1327–33. doi: 10.1161/01.str.0000014248.28711.d6. [DOI] [PubMed] [Google Scholar]
- 131.Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med. 1998;158:1133–38. doi: 10.1001/archinte.158.10.1133. [DOI] [PubMed] [Google Scholar]
- 132.Herrmann N, Black SE, Lawrence J, Szekely C, Szalai JP. The Sunnybrook Stroke Study: a prospective study of depressive symptoms and functional outcome. Stroke. 1998;29:618–24. doi: 10.1161/01.str.29.3.618. [DOI] [PubMed] [Google Scholar]
- 133.Eriksson M, Asplund K, Glader EL, et al. Self-reported depression and use of antidepressants after stroke: a national survey. Stroke. 2004;35:936–41. doi: 10.1161/01.STR.0000121643.86762.9a. [DOI] [PubMed] [Google Scholar]
- 134.Paradiso S, Robinson RG. Gender differences in poststroke depression. J Neuropsychiatry Clin Neurosci. 1998;10:41–47. doi: 10.1176/jnp.10.1.41. [DOI] [PubMed] [Google Scholar]
- 135.Paolucci S, Bragoni M, Coiro P, et al. Is sex a prognostic factor in stroke rehabilitation? A matched comparison. Stroke. 2006;37:2989–94. doi: 10.1161/01.STR.0000248456.41647.3d. [DOI] [PubMed] [Google Scholar]
- 136.Aberg AC. Gender comparisons of function-related dependence pain and insecurity in geriatric rehabilitation. J Rehabil Med. 2006;38:73–79. [PubMed] [Google Scholar]