Abstract
The Duffy blood group Ag (dfy) binds selective CXC and CC chemokines at high affinity and is expressed on erythrocytes and endothelial cells. However, it does not transmit a signal via G proteins, as occurs with other seven-transmembrane receptors. We hypothesized that dfy functions as a chemokine reservoir and regulates inflammation by altering soluble chemokine concentrations in the blood and tissue compartments. We determined whether Duffy Ag “loss-of-function” phenotypes (human and murine) are associated with alterations in plasma chemokine concentrations during the innate inflammatory response to LPS. Plasma CXCL8 and CCL2 concentrations from humans homozygous for the GATA-1 box polymorphism, a dfy polymorphism that abrogates erythrocyte chemokine binding, were higher than in heterozygotes following LPS stimulation of their whole blood in vitro. Similarly, dfy−/− mice showed higher plasma MIP-2 concentrations than dfy+/+ mice following LPS stimulation of whole blood in vitro. We then determined the relative contributions of erythrocyte and endothelial Duffy Ag in modifying chemokine concentrations and neutrophil recruitment in the lungs following intratracheal LPS administration in dfy−/− and dfy+/+ mice reconstituted with dfy−/− or dfy+/+ marrow. Mice lacking endothelial dfy expression had higher MIP-2 and keratinocyte chemoattractant concentrations in the airspaces. Mice lacking erythrocyte dfy had higher MIP-2 and keratinocyte chemoattractant concentrations in the lung tissue vascular space, but lower plasma chemokine concentrations associated with attenuated neutrophil recruitment into the airspaces. These data indicate that dfy alters soluble chemokine concentrations in blood and local tissue compartments and enhances systemic bioavailability of chemokines produced during local tissue inflammation.
The observation that RBC bind the majority of CXCL8/IL-8 in whole blood (1) led to the discovery that Duffy Ag, a minor blood group Ag, is a chemokine-binding protein (2). Historically known as the receptor for the malarial parasite Plasmodium vivax (2–4), Duffy Ag demonstrates high-affinity binding to IL-8/CXCL8 with a dissociation constant (Kd) of 5 nM and receptor binding sites estimated at 1000–9000/RBC surface (1, 5). In addition to binding CXCL8/IL-8, Duffy Ag displays high-affinity binding to other CXC chemokines such as CXCL1/growth-related oncogene α and CXCL7/neutrophil-activating peptide 2; Duffy Ag also binds CC chemokines such as CCL2/MCP-1 (CCL2), CCL5/regulated upon activation, normal T expressed and secreted (RANTES) but not CCL3/monocyte inflammatory protein 1α (MIP-1α)3 or CCL4/MIP-1β (6–9).
Unlike all other seven-transmembrane chemokine receptors, Duffy Ag lacks the highly conserved aspartyl-arginyl-tyrosine (DRY-)-G protein-coupling motif located in the second cytoplasmic loop (10, 11), raising the possibility that the Duffy Ag is a negative regulator. In addition, chemokines bound to erythrocyte Duffy Ag are not accessible to neutrophils circulating nearby (1). This established the notion of erythrocyte Duffy Ag as a “sink” for circulating chemokines, binding the majority of intravascular chemokines to prevent excessive activation of neutrophils (1). However, further studies show that the in vivo role of the Duffy Ag is more complicated than the term chemokine sink suggests (12, 13).
Approximately 68% of African Americans and >95% of West Africans lack Duffy Ag on their RBC (10, 14) and, therefore, do not demonstrate chemokine binding on erythrocytes (10, 15). However, Duffy Ag expression has been subsequently identified along splenic sinusoids and postcapillary venules using tissue from individuals lacking erythrocyte Duffy Ag (16). The selective abrogation of erythrocyte Duffy Ag is due to a substitution T-46C in the GATA-1 erythroid-specific promoter region of the Duffy gene (17). Although a promoter polymorphism silences erythrocyte expression of Duffy Ag, organ-specific (i.e., endothelial) Duffy Ag positioned at the site of leukocyte emigration has been conserved in evolution.
Although Duffy Ag binds many inflammatory chemokines with high affinity and up-regulated at tissue sites of inflammation (18–21), its functional role in inflammation is less clear. We hypothesized that Duffy Ag functions as a chemokine reservoir and can regulate inflammatory responses by altering soluble chemokine concentrations in blood and local tissue compartments. To test this hypothesis, we first determined whether Duffy Ag “loss-of-function” phenotypes (human and murine) are associated with alterations in plasma chemokine concentrations following an inflammatory stimulus of whole blood in vitro. We studied the GATA-1 box polymorphism of the human Duffy Ag gene that abrogates erythrocyte Duffy expression (17) and erythrocyte chemokine binding (2, 15). We also studied the FyX mutation arising from the Arg89Cys substitution, which results in significantly less membrane expression of the Duffy Ag, and ~75% less chemokine binding by erythrocytes from homozygous individuals (15). We then determined the in vivo contribution of erythrocyte and endothelial Duffy Ag in modifying neutrophil recruitment and chemokine concentrations in the airspace, lung tissue vascular space, and plasma compartments following an inflammatory stimulus arising from the lungs.
Materials and Methods
Human study subjects
We have previously reported the description of the study subjects (22). Briefly, healthy subjects between 18 and 65 years of age gave written informed consent for the studies and were recruited from the University of Washington community. Exclusion criteria included history of smoking, major chronic illnesses such as coronary artery disease, cancer, chronic obstructive pulmonary disease, and diabetes, active use of anti-inflammatory agents (steroidal or nonsteroidal), or participation in vigorous exercise within the previous 24 h. The human subjects protocol was approved by the University of Washington Institutional Review Committee.
LPS stimulation of human whole blood in vitro
The whole blood assay has been previously described (22). Briefly, whole blood was collected from fasting subjects between 8:00 and 10:00 a.m. Whole blood at a volume of 23.5 ml was anti-coagulated with 2.5 ml of 100 mM citrate (pH 7.2). Escherichia coli 011:B4 LPS at a final concentration of 10 ng/ml was added to anticoagulated whole blood (10 ml) in a 50-ml polypropylene tube, mixed gently, and incubated for 6 h at 37°C. Plasma supernatants were collected following centrifugation at 1000 × g for 10 min and stored at −80°C. Cytokines IL-1β, TNF-α, CXCL8, and CCL2 were measured using a bead-based cytometric immunoassay system (Luminex). Incubation of whole blood with medium alone showed minimal cytokine production (data not shown). We have previously described the temporal stability of the LPS-induced cytokine expression phenotype in whole blood (22).
Animals
The generation of dfy−/− mice has been previously described (16). The dfy−/− mice were backcrossed to the C57BL/6J background. At the N3 generation, a genome scan using 108 markers spanning the 19 autosomes was performed to select male carriers with the targeted mutation (dfy) and highest percentage of inbred recipient background (C57BL/6J) for the next backcross generation. Male carriers were mated with C57BL/6J females at each successive generation until the N6 generation. A homozygous dfy−/− mouse colony was then established, from which the experimental animals were obtained. For the generation of bone marrow-transplanted mice, the B6.SJL-Ptprca/Pep3b/Boy j mouse strain was used as the wild-type (WT) control mice for the experiments, because they are identical to the C57BL/6J mouse at all loci except at the CD45 allele. The B6.SJL-Ptprca/Pep3b/Boy j mouse expresses the CD45.1 allele, whereas the C57BL/6J mouse expresses the CD45.2 allele. The B6.SJL-Ptprca/Pep3b/Boy j mouse will be referred to as WT mice. Age- and sex-matched animals were used in all experiments. Animals were maintained in specific pathogen-free cages. Experiments were conducted in accordance with the Institutional Animal Care and Use Committee at the Veterans Affairs Puget Sound Health Care Systems, the University of Washington, and the University of Pittsburgh.
LPS stimulation of murine whole blood in vitro
Age- and gender-matched dfy−/− and dfy+/+ mice were euthanized by overdose with i.p. pentobarbital followed by immediate removal of whole blood by intracardiac puncture in sterile heparinized 1-ml tuberculin syringes. The white blood cell count and the RBC counts in WT and knockout (KO) mice were similar. Equal volumes of whole blood (400 μl) from individual mice were incubated with LPS E. coli 011:B4 at a final concentration of 10 ng/ml for 6 h at 37°C. Following the 6-h incubation period, plasma supernatants were isolated by centrifugation and stored at −80°C. Cytokines TNF-α, MIP-2, and CCL2 were measured using a bead-based cytometric immunoassay system (Luminex).
Adoptive transfer studies
We have previously reported our method of adoptive transfer (23). Briefly, the dfy−/− (KO) and B6.SJL-Ptprca/Pep3b/Boy j (WT) mice underwent total-body irradiation for a total treatment of 900 cGy. This dose was delivered at a dose rate of 16.8 cGy/min, with half the dose delivered from above and half from below, using a linear accelerator. The mice were returned to their cages and given free access to water and food. TBI recipients received prophylactic Enrofloxacin, 1.8 ml in drinking water from day 1 through day 14. On the day of adoptive transfer (day 0), whole marrow cells were harvested under sterile conditions from nonirradiated mice of each donor strain. Marrow cells were counted, the viability was tested using the trypan blue exclusion method, and prepared at 25 × 106 cells/ml in sterile PBS. Each recipient received 5 × 106 cells in 200 μl by tail vein injection. The following combinations of adoptive transfer were performed: WT→WT, KO→KO, WT→KO, and KO→WT, where donor marrow cells are indicated to the left of the arrow and total body-irradiated recipients are indicated to the right of the arrow.
Intratracheal instillation of LPS
LPS from E. coli 011:B4 (List Biological Laboratories) at 1.5 μg/kg per mouse were instilled intratracheally into mice that were anesthetized using isoflurane. Method of intratracheal instillation has been previously described (13). Mice were euthanized at specified time points of 0, 2, and 4 h by i.p. injection of 120 mg/kg pentobarbital followed by removal of whole blood by intracardiac puncture. Whole blood was collected in sterile heparinized 1-ml tuberculin syringes and placed on ice for immediate processing.
Mouse necropsies and lung tissue processing
Mice were euthanized with 100 mg/kg i.p. pentobarbital followed by intracardiac puncture and removal of whole blood into heparinized 1-ml tuberculin syringes. Our method of mouse necropsy and lung tissue processing has been previously described (24). An aliquot of whole blood was immediately processed for flow cytometry. The rest was centrifuged, plasma isolated, and stored in −80°C. Our method of measuring myeloperoxidase (MPO) activity in lung tissues has been previously described (24). MIP-2 and keratinocyte chemoattractant (KC) concentrations in the bronchoalveolar lavage (BAL) fluid, lung homogenates, and plasma were measured using a bead-based cytometric immunoassay system (Luminex).
Murine Duffy immunohistochemistry
The right lungs were inflated at 15 cm of H2O with 4% paraformaldehyde and paraffin embedded. Lungs were sectioned 6-μm in thickness. A sheep anti-murine Duffy polyclonal Ab was used at 1/50 dilution to immunolocalize Duffy Ag in WT lungs following total body irradiation. The verification of the murine Duffy Ab and method for Duffy immunostaining have been previously described (25). Amino-ethylcarbazole (red chromogen) was used as substrate for peroxidase. Hematoxylin was used as counterstain. We used nonspecific IgG and omission of primary Ab as negative controls.
Quantitative RT-PCR
The left lung and spleen were removed during necropsy, snap frozen immediately in liquid nitrogen, and stored in −80°C until use. Lung and spleen tissue were homogenized and RNA was extracted using the TRIzol method (Invitrogen Life Technologies). The RNA samples were reverse transcribed into cDNA, which served as template for quantitative RT-PCR. Probes and primers for the kc, mip-2, and dfy gene were commercially available, as was the TaqMan Master Mix containing the necessary reagents for gene expression studies (Applied Biosystems). Gene expression was analyzed by the ΔΔ-threshold cycle method, with 18S rRNA as the endogenous control and age-matched unstimulated C57BL/6 mouse lung and spleen serving as the calibrator.
Flow cytometry and determination of chimerism
The B6.SJL-Ptprca/Pep3b/Boy j (WT) mouse expresses the CD45.1 allele, whereas the dfy−/− (KO) mouse on a C57BL/6J background expresses the CD45.2 allele. An aliquot of whole blood at the time of euthanasia was taken from each individual mouse and assessed for the percentage of CD45.1- and CD45.2-expressing cells in peripheral leukocyte populations by flow cytometry. Whole blood from each individual mouse was incubated with PE-anti-monoclonal (m) CD45.1 (BD Pharmingen) and FITC-anti-mCD45.2. Isotype control Abs PE-mouse IgG2a (κ) and FITC-mouse IgG2a were also at a concentration of ~1 μg/106 cells. Following incubation on ice in the dark for at least 30 min, RBC were lysed with Pharm-Lyse according to the manufacturer’s protocol (BD Biosciences), washed with PBS plus 0.2% BSA plus 0.1% sodium azide, and resuspended in this buffer for analysis. Two-color analysis was performed with FITC- and R-PE-conjugated Abs. The percent CD45.1 and CD45.2 was calculated based upon quadrant statistics performed on gated leukocyte populations out of 10,000 total events counted. Data from animals showing adequate reconstitution with donor marrow cells were included in subsequent analyses.
Single nucleotide polymorphism (SNP) genotyping
The single nucleotide polymorphism probes and primers were designed using the Assays by Design software version 2.0 (Applied Biosystems) and the available GenBank sequence AF055992 for the promoter sequence of the Fy gene and the reference sequence NM_002036 for the FyX mutation. The probe for the GATA1 SNP that corresponds with the absence of Duffy Ag expression in erythrocytes was GATA1–1207M2 FAM: CCAAGG TAAGAGCC (reverse) and the GATA1 SNP more prevalent in Caucasians was GATA1–1207V2: VIC CTTCCAAGATAAGAGCC (reverse). The primers are GATA1–1207F: CTGATGGCCCTCATTAGTCCTT and GATA1–1207R: GCTGGGACGGCTGTCA. The probe corresponding to the FyX mutation is FYX-505M2: FAM CTGCCAGCAGAAGA (reverse), and the probe corresponding to WT is FYX-505V2: VIC CTGCCAGCG GAAGA (reverse). The primers are FyX-505F: GCTAGCAGCACTGTC CTCTTC and FyX-505R: GCCAGCCAGGGCAGAG. Long oligonucleotides spanning the polymorphisms were designed and used as positive controls for each assay plate. For quality control, random samples were sequence verified for both polymorphisms.
Results
Influence of the Duffy Ag on chemokine concentrations in the plasma of humans following LPS stimulation of whole blood in vitro
We obtained whole blood from healthy human volunteers. The whole blood samples were incubated with E. coli 011:B4 LPS for 6 h. The plasma supernatants were isolated from the stimulated whole blood samples, and cytokine concentrations were measured. We examined the association among the GATA-1 box and FyX single nucleotide polymorphisms and plasma cytokine concentrations by self-reported race. These populations were in Hardy-Weinburg equilibrium in Caucasian and African American populations (data not shown).
Among 347 Caucasians tested for the GATA-1 box single nucleotide polymorphism, no individuals were homozygous for the polymorphism (GG), 5 individuals were heterozygous (AG), and the other 342 individuals did not have the GATA-1 box polymorphism (AA) (Table I). Because of the codominant expression of the Duffy Ag alleles, individuals heterozygous (AG) for the GATA-1 box polymorphism have previously been shown to have only 50% of the expected amount of erythrocyte chemokine binding, as compared with individuals without the GATA-1 box polymorphism (15). Table I summarizes plasma cytokine concentrations obtained after stimulation of whole blood with LPS in vitro. Among Caucasian individuals, heterozygosity for the GATA-1 box polymorphism (AG) was associated with higher CCL2/MCP-1 concentrations than WT (AA), consistent with reduced chemokine binding on erythrocytes (Table I, p < 0.05). Plasma CXCL8/IL-8 concentrations obtained after stimulation of human whole blood with LPS in vitro were similar in the heterozygous and WT groups. One explanation for the differences in plasma CXCL8 and CCL2 concentrations in the Caucasian GATA-1 box subjects may be that CCL2 is more effective in displacing other ligands from erythrocyte Duffy Ag. This is supported by the fact that others have shown that heterologous displacement of ligand from the Duffy Ag is in some cases more effective than homologous displacement, and we have shown that CCL2 demonstrates lower IC50 than CXCL8 (5, 13).
Table I.
Plasma cytokine concentrations following LPS stimulation of whole blood in vitro according to the GATA-1 box polymorphism in Caucasiansa
| GATA-1 Box Genotype in Caucasians |
||||
|---|---|---|---|---|
| Cytokine | GG (0) | AG (5) | AA (342) | p |
| CXCL8 | NA | 2262 ± 410 | 3115 ± 142 | 0.6795 |
| IL-1β | NA | 10200 ± 3647 | 7474 ± 226 | 0.4949 |
| TNF-α | NA | 5530 ± 1739 | 5168 ± 141 | 0.7945 |
| CCL2 | NA | 2299 ± 261 | 1743 ± 50 | 0.0447 |
CXCL8, IL-1β, TNF-α, and CCL2 concentrations in picograms per monocyte count (×103/μl) and their corresponding SEM are depicted in columns according to genotype. The numbers in parentheses in each genotype column represent the number of individuals tested. NA, Not available. Statistical comparisons made between genotypes for each variable cytokine are depicted in the far right-hand column. Data were analyzed by rank sum Mann-Whitney U test and p ≤ 0.05 defined as significant.
Among self-reported African Americans in our cohort (Table II), the minor allele frequency of the GATA-1 box polymorphism was 0.6, consistent with previous reports (10, 14). All self-identified African Americans in our cohort had at least one allele expressing the GATA-1 box polymorphism (Table II). Among African Americans, homozygosity for the GATA-1 box polymorphism (GG) was associated with ≥2-fold higher plasma concentrations of CCL2/MCP-1 and CXCL8/IL-8 over the heterozygous state (AG) (p = 0.0004 and p = 0.0001, respectively, Table II). In contrast, plasma concentrations of IL-1β and TNF-α obtained after stimulation of human whole blood with LPS in vitro were similar in the homozygous and heterozygous groups.
Table II.
Plasma cytokine concentrations following LPS stimulation of whole blood in vitro according to the GATA-1 box polymorphism in African Americansa
| GATA-1 Box Genotype in African Americans |
||||
|---|---|---|---|---|
| Cytokine | GG (26) | AG (15) | AA (0) | p |
| CXCL8 | 10562 ± 1583 | 2748 ± 763 | NA | 0.0001 |
| IL-1β | 8034 ± 739 | 7705 ± 453 | NA | 0.4168 |
| TNF-α | 5672 ± 341 | 4564 ± 463 | NA | 0.0832 |
| CCL2 | 6551 ± 657 | 2945 ± 505 | NA | 0.0004 |
CXCL8, IL-1β, TNF-α, and CCL2 concentrations in picograms per monocyte count (×103/μl) and their corresponding SEM are depicted in columns according to genotype. The numbers in parentheses in each genotype column represent the number of individuals tested. NA, Not available. Statistical comparisons made between genotypes for each variable cytokine are depicted in the far right-hand column. Data were analyzed by rank sum Mann-Whitney U test and p < 0.05 defined as significant.
Among 295 Caucasians tested for the presence of the FyX single nucleotide polymorphism, no individuals were homozygous for the polymorphism (TT), 6 individuals were heterozygous (CT), and 289 individuals did not have the polymorphism (CC). Plasma CXCL8/IL-8 concentrations were similar in CT and CC groups, although plasma CCL2/MCP-1 concentrations showed a trend toward higher levels in the heterozygous (CT) group (p = 0.07; Table III). Although the GATA-1 box and the FyX single nucleotide polymorphisms were rare in Caucasians within our cohort and the study lacked the required power to detect a difference in CXCL8 concentrations, the GATA-1 box polymorphism was common among African Americans and was associated with ≥2-fold increase in plasma CXCL8/IL-8 as well as CCL2/MCP-1 concentrations when comparing samples from individuals homozygous and heterozygous for the polymorphism. Thus, the findings suggest that erythrocyte Duffy Ag selectively binds and reduces plasma chemokine concentrations in human blood stimulated with LPS in vitro.
Table III.
Plasma cytokine concentrations following LPS stimulation of whole blood in vitro according to the FyX polymorphism in Caucasiansa
| FyX Genotype in Caucasians |
||||
|---|---|---|---|---|
| Cytokine | TT (0) | CT (6) | CC (289) | p |
| CXCL8 | NA | 3770 ± 821 | 3157 ± 186 | 0.2518 |
| IL-1β | NA | 7587 ± 1412 | 7705 ± 453 | 0.8656 |
| TNF-α | NA | 4482 ± 1048 | 5298 ± 312 | 0.2788 |
| CCL2 | NA | 2136 ± 138 | 1796 ± 106 | 0.0676 |
CXCL8, IL-1β, TNF-α, and CCL2 concentrations in picograms per monocyte count (×103/μl) and their corresponding SEM are depicted in columns according to genotype. The numbers in parentheses in each genotype column represent the number of individuals tested. NA, Not available. Statistical comparisons made between genotypes for each variable cytokine are depicted in the far right-hand column. Data were analyzed by rank sum Mann-Whitney U test and p < 0.05 defined as significant.
Plasma chemokine concentrations in dfy−/− and dfy+/+ mice following LPS stimulation of whole blood in vitro
To determine the relationship between plasma chemokine concentrations and Duffy Ag gene mutations in mice, we examined whether dfy−/− (KO) mice demonstrate altered plasma chemokine concentrations as compared with dfy+/+(WT) mice following stimulation of their whole blood with LPS E. coli 011:B4 in vitro for 6 h (Table IV). Although the KO mice lack Duffy Ag protein expression on both erythrocytes and endothelial cells (16), our in vitro whole blood LPS stimulation assay tested the effects of erythrocyte Duffy Ag in isolation of in vivo chemokine clearance mechanisms. Stimulated whole blood from KO mice showed significantly higher plasma MIP-2 concentrations than blood from WT mice (Table IV; p = 0.03). KC concentrations were higher in stimulated blood from KO mice compared with WT mice, but the difference was not statistically significant (p = 0.39). Because plasma chemokine concentrations are dependent on various factors such as the Kd of the chemokines, the competitive interactions of different ligands with the Duffy Ag, the amount of soluble chemokines produced, and the kinetics of chemokine release by inflammatory cells, it is not surprising that the response to LPS seen with MIP-2 and KC levels in the WT and KO are different at the 6-h time point studied. Interestingly, CCL2/MCP-1 plasma concentrations were undetectable in both groups at 6 h poststimulation. The very low levels of soluble CCL2 produced in vitro limit the detection of any differences in LPS responses. Thus, the absence of erythrocyte chemokine-binding function of the Duffy Ag is associated with higher plasma MIP-2 concentrations during the innate inflammatory response to LPS, consistent with the in vitro findings in humans.
Table IV.
Plasma cytokine concentrations in Duffy KO and WT mice following LPS stimulation of whole blood in vitroa
| Duffy Ag Genotype |
|||
|---|---|---|---|
| Cytokine | KO | WT | p |
| MIP-2 | 3728 ± 1605 | 495 ± 270 | 0.03 |
| KC | 2654 ± 578 | 1691 ± 346 | 0.39 |
| TNF-α | 132 ± 37 | 68 ± 42 | 0.12 |
| CCL2 | OOR< | OOR< | NA |
MIP-2, KC, TNF-α, and CCL2 concentrations in picograms per milliliter and their corresponding SEM are depicted in columns according to genotype. OOR<, Out of range and below the sensitivity of the assay. NA, Not available. Statistical comparisons made between genotypes for each variable cytokine are depicted in the far right-hand column. Data were analyzed by rank sum Mann-Whitney U test and p ≤ 0.05 defined as significant. Data reflects n = 4–6 animals per group and are representative of two independent experiments.
Lethally irradiated mice are fully reconstituted with donor marrow cells in the blood compartment at day 60 posttransplantation
Because of the Duffy-dependent alterations in human and mouse plasma chemokine levels during the innate inflammatory response to LPS, we determined the in vivo role of the Duffy Ag in modifying neutrophil recruitment into the airspaces, local tissue, and the systemic inflammatory chemokine response following intratracheal instillation of LPS. We determined the relative contribution of erythrocyte and endothelial (parenchymal) Duffy Ag by transplanting bone marrow cells from either dfy+/+ (WT) and dfy−/− (KO) donor mice into lethally irradiated dfy+/+ (WT) and dfy−/− (KO) recipients by lateral tail vein injection. We generated WTCD45.1→WTCD45.1, KOCD45.2→ KOCD45.2, WTCD45.1→KOCD45.2, and KOCD45.2→WTCD45.1 mice and assessed the allele status of the panleukocyte Ag marker CD45 in whole blood leukocytes as a measure of the degree of reconstitution with donor hemopoietic cells at 60 days posttransplantation. The direction of the arrow reflects the direction of donor marrow cell type transplanted into irradiated host. Thus, the WTCD45.1→WTCD45.1 and KOCD45.2→KOCD45.2 mice reflect the control groups, the WTCD45.1→KOCD45.2 group reflects the absence of endothelial or parenchymal Duffy Ag, and the KOCD45.2→WTCD45.1 group reflects the absence of erythrocyte or hemopoietic Duffy Ag. As shown in Fig. 1, the majority of leukocytes in circulation at day 60 are of donor origin, consistent with our previous results (23).
FIGURE 1.
CD45 allele status of transplanted mice at day 60. Whole blood from transplanted mice was dual labeled with PE-anti-mCD45.1 and FITC-anti-mCD45.2 and analyzed by flow cytometry. The percentages of cells expressing the CD45.1 allele in mice were as follows: WT→WT, 98 ± 0.4%; KO→KO, 0.2 ± 0.1%; WT→KO, 96 ± 0.7%; and KO→WT, 5 ± 0.6%. The percentages of cells expressing the CD45.2 allele in each chimeric mice group were as follows: WT→WT, 0.3 ± 0.1%; KO→KO, 99 ± 0.2%; WT→KO, 2.5 ± 0.3%; and KO→WT, 95 ± 0.6%. There were 10–13 animals analyzed in each group, and this figure shows representative images from each transplanted group. Data from animals showing adequate reconstitution with donor marrow cells were included in subsequent analyses.
We determined Duffy Ag expression in the lungs following total irradiation in WT mice using a murine Duffy-specific Ab (25). Immunoreactivity was present in the lung endothelium (Fig. 2B) using sheep anti-murine Duffy IgG, but absent with nonspecific IgG (Fig. 2A). Duffy Ag immunostaining was also present on erythrocytes but absent on mononuclear cells and polymorphonuclear (PMN) (data not shown). We further characterized the chimeric mice by examining dfy gene expression in their lungs and spleens by real-time PCR (Fig. 2, C and D). Although the WT→WT and KO→WT groups showed similar elevations in dfy gene expression in the lungs when compared with unstimulated C57BL/6 mouse lung and spleen, the KO→KO showed undetectable levels and the WT→KO group showed minimally detectable levels. Dfy gene expression was elevated at the site of LPS stimulation, the lungs (Fig. 2C), but not in the spleen of the four chimeric groups at 2 and 4 h (Fig. 2D). Thus, induction of the dfy gene was largely dependent on the presence of the WT genotype in the recipient mouse (dfy+/+ status of the lung and spleen). The relative dfy gene expression in the lung and spleen of WT→KO mice likely reflect hemopoietic cells derived from the WT donor (Fig. 2, C and D).
FIGURE 2.
Duffy Ag expression in the lungs following total irradiation. A, Negative control immunostaining with nonspecific IgG. B, Duffy immunostaining with sheep anti-murine Duffy Ab. Immunoreactivity is present in endothelium in WT-irradiated mouse lung (indicated by red staining). C, Relative dfy gene expression in the lung following intratracheal LPS instillation. D, Relative dfy gene expression in the spleen following intratracheal LPS instillation. Gene expression was measured using quantitative real-time PCR in lung and spleen homogenates obtained from the four chimeric groups (WT→WT, KO→KO, WT→KO, and KO→KO). Gene expression was quantified using the ΔΔ CT method with 18S rRNA as the endogenous control. Unstimulated age-matched C57BL/6 mouse lung or spleen was used as the calibrator. Data represent three mice at each time for the four groups.
Duffy Ag modifies neutrophil recruitment in the lungs following LPS stimulation
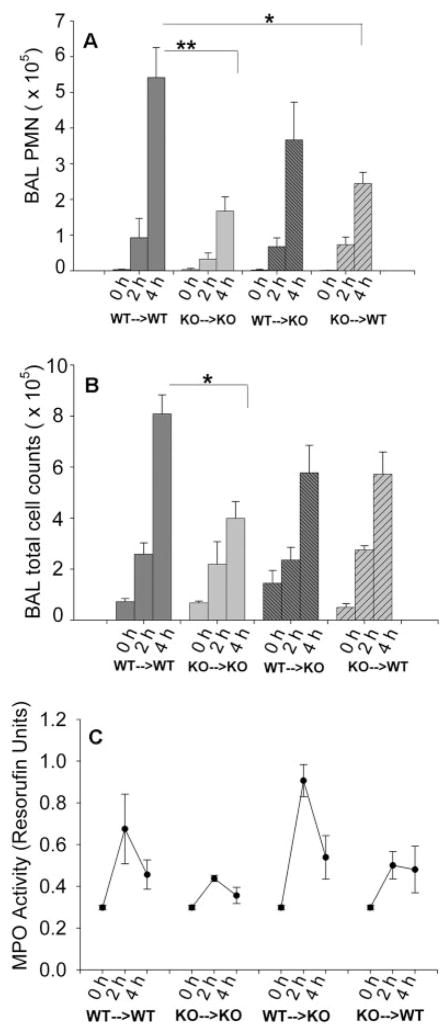
We examined the total and neutrophil cell counts in the BAL and MPO activity in the lung tissue homogenates at 0, 2, and 4 h to determine whether Duffy Ag can modify neutrophil recruitment into the lungs during the innate inflammatory response to LPS, and the relative contribution of erythrocyte and endothelial Duffy Ag. We instilled the mice with low-dose LPS intratracheally (1.5 μg/kg) to initiate inflammatory cell recruitment and endogenous chemokine release in the lungs but not overwhelm systemic inflammatory responses. As expected, there were no significant differences in BAL total protein among the four groups of mice at the time points of 2 and 4 h studied (data not shown), indicating that lung permeability was not differentially altered among the four groups with this dose of LPS. The absence of erythrocyte Duffy Ag was associated with attenuated neutrophil migration into the airspaces, since the KO→KO and KO→WT mice had fewer neutrophils in the airspaces as compared with the WT→WT mice following intratracheal LPS instillation (Fig. 3A; p = 0.014 and p = 0.002, respectively). The WT→KO mice also had lower neutrophils recruitment than the WT→WT mice, but this was not statistically significant (p = 0.08). Thus, mice lacking erythrocyte Duffy Ag showed greater impairment in airspace neutrophil recruitment than mice lacking endothelial Duffy Ag.
FIGURE 3.
BAL PMN, BAL total cell counts, and lung MPO following intratracheal LPS instillation at day 60 posttransplant. Total PMN and total cell counts were measured in the BAL fluid obtained from chimeric mice of various combinations at 0, 2, and 4 h following LPS instillation. A, The KO→KO and KO→WT mice showed significant attenuation of neutrophil recruitment into the airspaces, when compared with the WT→WT group (p = 0.014 and p = 0.002, respectively). B, The KO→KO mice showed significant attenuation of total cell counts in the airspaces, when compared with the WT→WT mice (B, p = 0.02). C, MPO activity of the lungs following intratracheal LPS instillation at day 60 posttransplant. Resorufin activity, an indicator of total peroxidase activity, was measured from left lung homogenates obtained from chimeric mice. Peak MPO activity was noted at 2 h following LPS instillation for each group. The KO→KO and KO→WT groups showed lower MPO activity than the WT→WT and WT→KO groups, although the differences were not statistically significant (p = 0.08, rank sum Mann-Whitney U test). Data represent three to six animals in each time point for each chimeric group, and is representative of two independent experiments.
MPO activity in the lung homogenates was also measured as a marker of total neutrophil content in the lungs (Fig. 3C). In each group, lung MPO activity peaked at 2 h, reflecting neutrophil entrapment in the local tissue vascular space of the lung, an event that occurs earlier than migration into the airspaces. Mice lacking erythrocyte Duffy Ag were also associated with reduced MPO activity in the lungs, although this was not statistically significant (p = 0.08). Thus, the pattern of MPO activity in the lungs was similar to that seen in the BAL cell counts, with lower neutrophil responses seen primarily in the mice lacking erythrocyte Duffy Ag.
MIP-2 and KC concentrations in the airspaces following intratracheal LPS instillation
To gain further insight into the lung neutrophil response in the chimeric mice, we measured chemokines MIP-2 and KC concentrations in the BAL fluid, lung tissue homogenates, and plasma following intratracheal LPS instillation. The MIP-2 concentrations in the BAL fluid were not statistically different among the various groups at 2 h. However, relative to the WT→WT mice (101 ± 16 pg/ml) at the 4-h time point, the MIP-2 concentrations were higher in the BAL of KO→KO (178 ± 23 pg/ml) and WT→KO mice (172 ± 30 pg/ml) (Fig. 4A), suggesting that the lack of endothelial or parenchymal Duffy Ag impairs chemokine removal from the airspaces. The MIP-2 concentration in the BAL fluid of the KO→WT animals at 4 h was 157 ± 18 pg/ml, a concentration that was intermediate between the concentrations of WT→WT and KO→KO animals.
FIGURE 4.
MIP-2 and KC concentrations in the airspaces, lung tissue vascular compartment, and plasma of the chimeric mice groups following intratracheal LPS instillation. A, MIP-2 concentrations in the BAL fluid. B, KC concentrations in the BAL fluid. C, MIP-2 concentrations in the lung tissue vascular compartment. D, KC concentrations in the lung tissue vascular compartment. E, MIP-2 concentrations in the plasma compartment. F, KC concentrations in the plasma compartment. Data represent three to six animals per group and are the combined results of two independent experiments. Data were analyzed by multiple comparisons of the four groups by ANOVA, with p ≤ 0.05 defined as significant. Note the differences in the y-axis for A–F.
Soluble KC concentrations in the BAL fluid were different among the chimeric groups at the 2- and 4-h time points, although only the 2-h time differences were statistically significant. Relative to the KC concentrations in the WT→WT group (356 ± 72 pg/ml at 2 h, 126 ± 40 pg/ml at 4 h), the KC concentrations were higher in the WT→KO group (887 ± 86 pg/ml at 2 h, 244 ± 77 pg/ml at 4 h) (p = 0.02; Fig. 4B). The KO→KO group (711 ± 50 pg/ml at 2 h, 204 ± 26 pg/ml at 4 h) had higher BAL KC concentrations at 2 h as compared with the WT→WT mice, but this was not statistically significant (p = 0.07). Collectively, the absence of Duffy Ag was associated with higher soluble concentrations of MIP-2 and KC in the airspaces at various time points, suggesting that Duffy Ag normally promotes the removal of soluble chemokines within the airspaces of the lungs following an inflammatory stimulus. The lack of endothelial or parenchymal Duffy Ag was associated with higher chemokine concentrations in the airspaces than the lack of erythrocyte Duffy Ag, as the WT→KO mice had higher MIP-2 and KC concentrations in the BAL fluid than KO→WT mice.
MIP-2 and KC concentrations in lung tissue homogenates following intratracheal LPS instillation
MIP-2 and KC concentrations were also measured from the lung tissue homogenates of the chimeric mice. The lung tissue homogenates contain both soluble and tissue-bound chemokines and is a reflection of the local tissue vascular space. Relative to the WT→WT mice (3756 ± 538 pg/ml), the MIP-2 concentrations in the KO→KO group (5681 ± 613 pg/ml) and the KO→WT group (5418 ± 640 pg/ml) were higher at the 2-h time point (p = 0.03 and p = 0.05, respectively; Fig. 4C). Relative to the WT→WT mice (1363 ± 241 at 2 h, 853 ± 231 pg/ml at 4 h), the KC concentrations in the KO→KO group (2549 ± 216 pg/ml at 2 h, 1278 ± 199 pg/ml at 4 h) (p = 0.09 for 2 and 4 h), the KO→WT group (2499 ± 814 pg/ml at 2 h, 1078 ± 237 pg/ml at 4 h) (p = 0.10 for 2 h, p = 0.36 for 4 h), and the WT→KO group (2386 ± 454 pg/ml at 2 h, 982 ± 281 pg/ml at 4 h) (p = 0.17 for 2 h, p = 0.71 for 4 h) were higher but the differences were not statistically significant (Fig. 4D). We did not find significant variations in the relative gene expression of mip-2 and kc in the lung homogenates by real-time PCR to account for the differences in protein concentrations within the chimeric groups (data not shown). Our findings show that the lack of erythrocyte Duffy appeared to be more strongly associated with higher chemokine concentrations in the lung homogenates than the lack of endothelial Duffy Ag, and reflect the prominent role of erythrocyte Duffy Ag in reducing chemokine concentrations from the local tissue vascular space.
MIP-2 and KC concentrations in plasma following intratracheal instillation of LPS
The absence of the Duffy Ag was associated with significantly lower MIP-2 and KC concentrations in the plasma compartment (Fig. 4, E and F). Relative to the MIP-2 concentrations of the WT→WT mice (142 ± 35 at 2 h, 35 ± 3.2 pg/ml at 4 h), the KO→KO (33 ± 3 at 2 h, 22 ± 0.2 pg/ml at 4 h) and KO→WT (50 ± 14 at 2 h, 22 ± 0.8 pg/ml at 4 h) mice showed lower MIP-2 concentrations at 2 and 4 h (p = 0.02 for both 2-h comparisons and p < 0.01 for both 4-h comparisons) (Fig. 4E). The WT→KO group also had lower MIP-2 concentrations (95 ± 23 pg/ml at 2 h, 21 ± 0 pg/ml at 4 h) as compared with WT→WT mice, although only the 4-h comparison was statistically significant (p < 0.01).
The most notable finding in the WT→WT chimeric group was that the plasma KC concentrations were 25-fold higher than plasma MIP-2 concentrations at 2 and 4 h (Fig. 4, E and F). Relative to the KC concentrations in the WT→WT group (3714 ± 413 pg/ml at 2 h, 1510 ± 371 pg/ml at 4 h), the KC concentrations in the KO→KO mice (627 ± 276 pg/ml at 2 h, 268 ± 65 pg/ml at 4 h) and the KO→WT mice (717 ± 166 pg/ml at 2 h, 375 ± 27 pg/ml at 4 h) were significantly lower (p ≤ 0.04 for all comparisons described). Thus, in the absence of erythrocyte Duffy Ag, the plasma MIP-2 and KC concentrations were reduced to a much greater extent than when Duffy was absent from parenchymal cells, because the KO→WT mice had lower MIP-2 and KC concentrations than WT→KO mice. Collectively, the data in the three compartments (airspace, lung tissue vascular space, plasma) show that the net effect of Duffy Ag is to reduce chemokine concentrations in the airspaces and lung tissue vascular space and to increase plasma chemokine concentrations. Our data also show that the Duffy Ag regulates systemic KC concentrations to a greater degree than MIP-2 concentrations, as suggested by the >25-fold higher plasma KC levels in the WT→WT mice. Thus, both erythrocyte and endothelial Duffy Ag participate in reducing local tissue chemokine concentrations following intratracheal LPS administration but erythrocyte Duffy Ag sustains chemokine concentrations in the systemic circulation to enhance neutrophil recruitment into the lungs.
Discussion
We hypothesized that the Duffy Ag functions as a chemokine reservoir and regulates inflammation by altering soluble chemokine concentrations in local tissue and the systemic circulation. We first examined the role of erythrocyte Duffy Ag in altering plasma chemokine concentrations in the blood compartment following LPS stimulation in vitro. This provided a model to examine the effects of the Duffy Ag on soluble chemokine concentrations in one compartment isolated from other compartments of the body during the innate immune response to LPS. In this model, we show that homozygosity for the GATA-1 box polymorphism, a polymorphism that confers the absence of the Duffy Ag on erythrocytes, is associated with significantly higher plasma CXCL8/IL-8 and CCL2/MCP-1 concentrations than heterozygosity for the GATA-1 box polymorphism. These findings are reproduced in mice, in which plasma concentrations of the CXC chemokine MIP-2 are higher in KO mice as compared with WT mice following in vitro stimulation of whole blood with LPS. Thus, the findings indicate that erythrocyte Duffy Ag reduces soluble chemokine concentrations in the blood compartment during an inflammatory state in vitro.
We then examined the in vivo contribution of endothelial and erythrocyte Duffy Ag in modifying local and systemic chemokine concentrations in the setting of an inflammatory stimulus in the lungs. Following intratracheal instillation of LPS, endothelial Duffy Ag reduces soluble chemokine concentrations in the airspaces. Erythrocyte Duffy Ag reduces chemokine concentrations in the local tissue vascular space and enhances systemic chemokine concentrations in plasma. The lack of erythrocyte Duffy Ag, as represented by the KO→WT and the KO→KO mice, is associated with impaired neutrophil migration into the airspaces and lower MPO activity in the lung tissue vascular space. Thus, in the in vivo setting where erythrocytes constantly transit through local tissue vascular beds, the data suggest that the Duffy Ag functions as a chemokine reservoir, binding chemokines locally where concentrations are high during tissue inflammation and releasing chemokines systemically where the concentrations reach levels below the Kd value of chemokines for the Duffy Ag. This, in effect, would result in enhancement of chemokine bioavailability in the systemic circulation produced during local tissue inflammation.
One explanation for why Duffy Ag reduces plasma chemokine concentrations in vitro but enhances plasma chemokine concentrations in vivo is the absence of clearance mechanisms for soluble chemokines in vitro that are normally present in vivo (12, 26). Indeed, Fukuma et al. (12) previously demonstrated that Duffy Ag KO mice showed more rapid clearance of chemokines injected i.v. than their WT counterparts (12). When radiolabeled chemokine was introduced systemically, the KO mice showed rapid accumulation in the liver and kidney, suggesting that these organs may be responsible for clearance of chemokines in circulation. As the authors hypothesized, when the plasma chemokine concentrations decrease below the value, chemokines bound to erythrocyte Kd Duffy Ag may be released (12). This, in turn, would have the effect of maintaining chemokine concentrations in the blood compartment. This is consistent with our in vivo findings. In the absence of chemokine clearance mechanisms such as in vitro, functional KO erythrocytes do not have the capacity to bind chemokines and reduce the soluble chemokine concentrations. Therefore, their plasma chemokine levels are higher.
Our data also show that plasma KC concentrations are >25-fold higher than the MIP-2 concentrations at 2 and 4 h following LPS stimulation in the WT→WT mice (Fig. 4, E and F). We speculate that high systemic concentrations of KC may provide the initial signal for neutrophils, as others have shown the role of KC in the mobilization of bone marrow neutrophils and its importance in neutrophil recruitment into the lungs (27–29). Quinton et al. (30) recently demonstrated that CINC, the rat homolog of KC, is selectively transported from the lungs to the vascular compartment. The identity of this selective chemokine transporter is not known, although Luan et al. (25) have previously noted a strong correlation between the expression of KC and the Duffy Ag in the developing mouse. Our data show that the Duffy Ag is involved in the preferential compartmentalization of KC in the plasma. Our findings are also consistent with the hypothesis set forth by Rot and colleagues (31–33) that endothelial Duffy Ag facilitates chemokine mobilization from the abluminal to luminal direction, as the WT→KO mice show higher BAL concentrations of MIP-2 at 4 h and KC at 2 h (Fig. 4, A and B). However, the effect of either endothelial and/or erythrocyte Duffy Ag on chemokine concentrations in the airspaces and lung tissue vascular compartment is relatively modest, when compared with the effect of erythrocyte Duffy Ag in regulating systemic chemokine concentrations following local tissue inflammation. In an intratracheal model of chemokine instillation, we have previously shown that the net effect of Duffy Ag is to facilitate chemokine-mediated neutrophil recruitment into the lungs (13). Based on the findings in this study, we suggest that enhancement of systemic chemokine bioavailability by the Duffy Ag following intratracheal LPS stimulation facilitates early neutrophil recruitment into the lungs.
Previous studies highlight a complex interaction between the effects of erythrocyte and tissue-specific (endothelial) Duffy Ag on chemokine availability in the circulation and extravascular sites, and suggest that the seemingly contradictory findings in different model systems are not mutually exclusive (12, 32). In contrast to our in vivo findings with low-dose intratracheal LPS, Dawson et al. (34) have shown that high-dose systemic LPS results in more severe lung inflammation in KO mice. One explanation for their finding is that systemic LPS can activate local tissue, particularly endothelium, and greater retention of chemokines produced locally in the lungs of KO mice may provide a greater stimulus for activated neutrophils entrapped in the lung vasculature to transmigrate across the airspaces. Alternatively, overwhelming systemic inflammatory response may significantly impair organs normally responsible for chemokine clearance, rendering KO mice more susceptible to chemokine-mediated organ inflammation. This would, in effect, be compatible with our findings in vitro, where the absence of chemokine clearance mechanisms renders higher chemokine concentrations in the functional KO.
A unifying hypothesis would be that both erythrocyte and endothelial Duffy Ag regulate the kinetics of chemokine bioavailability during inflammation. In organ sites such as the lungs, endothelial Duffy Ag may bind, internalize, and reduce local chemokine concentrations to promote selective chemokine mobilization from the abluminal to luminal surface of the endothelium. As hypothesized by others (31, 33, 35), chemokines are then immobilized by heparin sulfate proteoglycans on the luminal surface of the endothelium and presented to circulating cells expressing cognate chemokine receptors. Erythrocyte Duffy Ag can function as a chemokine reservoir, dampening chemokine effects in the local circulation, and sustaining its concentrations in the systemic compartment.
In summary, our in vitro data show that, under static conditions, erythrocyte Duffy Ag significantly reduces soluble chemokine concentrations produced in blood following an inflammatory stimulus. Our in vivo data show that endothelial Duffy Ag reduces soluble chemokine concentrations from the airspaces following an inflammatory challenge in the lungs. In this model, erythrocyte Duffy Ag reduces chemokine concentrations from local tissue vascular beds and increases their concentrations in plasma to promote airspace neutrophil recruitment during tissue inflammation. We conclude that the Duffy Ag enhances systemic bioavailability of chemokines produced during local tissue inflammation, and functional polymorphisms of the human Duffy Ag may contribute to the interindividual variability in systemic and tissue chemokine responses during inflammatory states.
Acknowledgments
We are indebted to Amy Koski for her assistance with the in vivo LPS stimulation studies. The technical expertise of John Ruzinski and Steve Mongovin is gratefully acknowledged.
Footnotes
This work was supported by National Institutes of Health Grants HL70178 (to J.S.L.), American Heart Associate Pacific Mountain Affiliate Beginning Grant-in-Aid (to J.S.L.), Grant HL72923 (to M.W.W.), Grant HL70840 (to G.M.-B.), and Grant P50 HL73996 (to T.R.M.).
Abbreviations used in this paper: MIP-1, monocyte inflammatory protein 1; BAL, bronchoalveolar lavage; dfy, Duffy blood group Ag; KC, keratinocyte chemoattractant; MPO, myeloperoxidase; WT, wild type; KO, knockout; m, monoclonal; SNP, single nucleotide polymorphism; PMN, polymorphonuclear.
Disclosures The authors have no financial conflict of interest.
References
- 1.Darbonne WC, Rice GC, Mohler MA, Apple T, Hebert CA, Valente AJ, Baker JB. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest. 1991;88:1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, Miller LH. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- 3.Miller LH, Mason SJ, Dvorak JA, McGinniss MH, Rothman IK. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975;189:561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- 4.Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks: the Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 5.Neote K, Darbonne W, Ogez J, Horuk R, Schall TJ. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J Biol Chem. 1993;268:12247–12249. [PubMed] [Google Scholar]
- 6.Horuk R, Colby TJ, Darbonne WC, Schall TJ, Neote K. The human erythrocyte inflammatory peptide (chemokine) receptor: biochemical characterization, solubilization, and development of a binding assay for the soluble receptor. Biochemistry. 1993;32:5733–5738. doi: 10.1021/bi00073a002. [DOI] [PubMed] [Google Scholar]
- 7.Horuk R, Wang ZX, Peiper SC, Hesselgesser J. Identification and characterization of a promiscuous chemokine-binding protein in a human erythroleukemic cell line. J Biol Chem. 1994;269:17730–17733. [PubMed] [Google Scholar]
- 8.Neote K, Mak JY, Kolakowski LF, Schall TJ. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood. 1994;84:44–52. [PubMed] [Google Scholar]
- 9.Szabo MC, Soo KS, Zlotnik A, Schall TJ. Chemokine class differences in binding to the Duffy antigen-erythrocyte chemokine receptor. J Biol Chem. 1995;270:25348–25351. doi: 10.1074/jbc.270.43.25348. [DOI] [PubMed] [Google Scholar]
- 10.Hadley TJ, Peiper SC. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood. 1997;89:3077–3091. [PubMed] [Google Scholar]
- 11.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology: XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 12.Fukuma N, Akimitsu N, Hamamoto H, Kusuhara H, Sugiyama Y, Sekimizu K. A role of the Duffy antigen for the maintenance of plasma chemokine concentrations. Biochem Biophys Res Commun. 2003;303:137–139. doi: 10.1016/s0006-291x(03)00293-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Frevert CW, Wurfel MM, Peiper SC, Wong VA, Ballman KK, Ruzinski JT, Rhim JS, Martin TR, Goodman RB. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J Immunol. 2003;170:5244–5251. doi: 10.4049/jimmunol.170.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanger R, Race RR, Jack J. The Duffy blood groups of New York negroes: the phenotype Fy (a-b-) Br J Haematol. 1955;1:370–374. doi: 10.1111/j.1365-2141.1955.tb05523.x. [DOI] [PubMed] [Google Scholar]
- 15.Yazdanbakhsh K, Rios M, Storry JR, Kosower N, Parasol N, Chaudhuri A, Reid ME. Molecular mechanisms that lead to reduced expression of Duffy antigens. Transfusion. 2000;40:310–320. doi: 10.1046/j.1537-2995.2000.40030310.x. [DOI] [PubMed] [Google Scholar]
- 16.Peiper SC, Wang ZX, Neote K, Martin AW, Showell HJ, Conklyn MJ, Ogborne K, Hadley TJ, Lu ZH, Hesselgesser J, et al. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J Exp Med. 1995;181:1311–1317. doi: 10.1084/jem.181.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tournamille C, Colin Y, Cartron JP, Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet. 1995;10:224–228. doi: 10.1038/ng0695-224. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhuri A, Rodriguez M, Zbrzezna V, Luo H, Pogo AO, Banerjee D. Induction of Duffy gene (FY) in human endothelial cells and in mouse. Cytokine. 2003;21:137–148. doi: 10.1016/s1043-4666(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Frevert CW, Thorning DR, Segerer S, Alpers CE, Cartron JP, Colin Y, Wong VA, Martin TR, Goodman RB. Enhanced expression of Duffy antigen in the lungs during suppurative pneumonia. J Histochem Cytochem. 2003;51:159–166. doi: 10.1177/002215540305100204. [DOI] [PubMed] [Google Scholar]
- 20.Liu XH, Hadley TJ, Xu L, Peiper SC, Ray PE. Up-regulation of Duffy antigen receptor expression in children with renal disease. Kidney Int. 1999;55:1491–1500. doi: 10.1046/j.1523-1755.1999.00385.x. [DOI] [PubMed] [Google Scholar]
- 21.Segerer S, Regele H, Mac KM, Kain R, Cartron JP, Colin Y, Kerjaschki D, Schlondorff D. The Duffy antigen receptor for chemokines is up-regulated during acute renal transplant rejection and crescentic glomerulonephritis. Kidney Int. 2000;58:1546–1556. doi: 10.1046/j.1523-1755.2000.00316.x. [DOI] [PubMed] [Google Scholar]
- 22.Wurfel MM, Park WY, Radella F, Ruzinski J, Sandstrom A, Strout J, Bumgarner RE, Martin TR. Identification of high and low responders to lipopolysaccharide in normal subjects: an unbiased approach to identify modulators of innate immunity. J Immunol. 2005;175:2570–2578. doi: 10.4049/jimmunol.175.4.2570. [DOI] [PubMed] [Google Scholar]
- 23.Matute-Bello G, Lee JS, Frevert CW, Liles WC, Sutlief S, Ballman K, Wong V, Selk A, Martin TR. Optimal timing to repopulation of resident alveolar macrophages with donor cells following total body irradiation and bone marrow transplantation in mice. J Immunol Methods. 2004;292:25–34. doi: 10.1016/j.jim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Frevert CW, Matute-Bello G, Wurfel MM, Wong VA, Lin SM, Ruzinski J, Mongovin S, Goodman RB, Martin TR. TLR-4 pathway mediates the inflammatory response but not bacterial elimination in E. coli pneumonia. Am J Physiol. 2005;289:L731–L738. doi: 10.1152/ajplung.00196.2005. [DOI] [PubMed] [Google Scholar]
- 25.Luan J, Furuta Y, Du J, Richmond A. Developmental expression of two CXC chemokines, MIP-2 and KC, and their receptors. Cytokine. 2001;14:253–263. doi: 10.1006/cyto.2001.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olszyna DP, Prins JM, Dekkers PE, De Jonge E, Speelman P, Van Deventer SJ, Van Der Poll T. Sequential measurements of chemokines in urosepsis and experimental endotoxemia. J Clin Immunol. 1999;19:399–405. doi: 10.1023/a:1020554817047. [DOI] [PubMed] [Google Scholar]
- 27.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154:335–344. [PubMed] [Google Scholar]
- 28.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 29.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 30.Quinton LJ, Nelson S, Zhang P, Boe DM, Happel KI, Pan W, Bagby GJ. Selective transport of cytokine-induced neutrophil chemoattractant from the lung to the blood facilitates pulmonary neutrophil recruitment. Am J Physiol. 2004;286:L465–L472. doi: 10.1152/ajplung.00153.2003. [DOI] [PubMed] [Google Scholar]
- 31.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 32.Rot A. Contribution of Duffy antigen to chemokine function. Cytokine Growth Factor Rev. 2005;16:687–694. doi: 10.1016/j.cytogfr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Rot A, Hub E, Middleton J, Pons F, Rabeck C, Thierer K, Wintle J, Wolff B, Zsak M, Dukor P. Some aspects of IL-8 pathophysiology: III. Chemokine interaction with endothelial cells. J Leukocyte Biol. 1996;59:39–44. doi: 10.1002/jlb.59.1.39. [DOI] [PubMed] [Google Scholar]
- 34.Dawson TC, Lentsch AB, Wang Z, Cowhig JE, Rot A, Maeda N, Peiper SC. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC) Blood. 2000;96:1681–1684. [PubMed] [Google Scholar]
- 35.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]