Abstract
Glutamatergic modulation of inhibitory interneurons plays a crucial role in shaping the flow of information in the cerebral cortex. In a cohort of postmortem human brains from schizophrenia (n=20), bipolar disorder (n=20) and normal control (n=20) subjects, we colocalized the mRNA for the N-methyl-D-aspartate (NMDA) receptor NR2A subunit, labeled with [35S], and the mRNA for the γ-aminobutyric acid (GABA) synthesizing enzyme glutamic acid decarboxylase (GAD)67, labeled with digoxigenin. We found that the density of GAD67+ neurons in layers 2–5 of the prefrontal cortex was decreased by 27–36 % in both schizophrenia and bipolar disorder. In addition, the density of the GAD67+/NR2A+ neurons was decreased by 57 % and 49 % in layers 3 and 4, respectively, in schizophrenia, but it was unchanged in bipolar disorder. These findings raise the possibility that glutamatergic innervation of inhibitory interneurons via the NMDA receptor in the prefrontal cortex may be selectively altered in schizophrenia.
1. INTRODUCTION
Normal neurocognitive functioning is contingent upon the integrity of information processing in the cerebral cortex, with specific cortical areas contributing differentially to various aspects of cognition. For instance, the prefrontal cortex (PFC) plays an important role in the temporal organization of behavior, or executive functioning, via working memory [20,22]. Working memory is the ability to temporarily maintain “on-line” internal representations of information in the perceptual, cognitive, and emotive domains that are no longer immediately present, for a brief period of up to tens of seconds, in order to guide future behavior [5]. In other words, the integrity of working memory is critical for the sequential execution of motor or cognitive acts in a goal-directed manner; this capacity forms the basis of a variety of normal daily human activities, such as planning, reasoning, thinking and language. Patients with schizophrenia exhibit impairment in the performance of many of these activities [8]. In fact, working memory and executive functioning deficits are thought to represent core pathophysiologic or perhaps endophenotypic features of schizophrenia [22,41,49]. Although disturbances of the prefrontal functional architecture have also been implicated in bipolar disorder [53], working memory, as measured by the delayed response paradigm, seems to be relatively intact in this illness [40]. Thus, the pathophysiologic nature of neural circuitry disturbances within the PFC may be dissimilar in the two conditions.
Inhibitory interneurons that utilize GABA (γ-aminobutyric acid) as a neurotransmitter play a crucial role in the maintenance of sustained neuronal activation during working memory by dynamically adjusting the conductances of the pyramidal neuronal network. Interestingly, the number of N-methyl-D-aspartate (NMDA) glutamate receptors on GABA neurons appears to be an important determinant of the stability of the network in sustaining working memory [34,54]. GABA neurons receive feedback excitatory modulation via local recurrent excitatory projections from the pyramidal neurons they innervate and, at the same time, they are also targets of feedforward excitatory modulation from axonal projections furnished by other pyramidal neurons, located both within the PFC and in other cortical areas [6,36–38]. Furthermore, projections from the thalamus provide additional excitatory drive to GABA cells [63]. The integrity of working memory and associated functions, such as executive control, depends on the complex interplay of feedback and feedforward mechanisms of modulation of cortical inhibitory activities via activation of glutamate receptors on GABA neurons [11,12,46], which helps to regulate the temporal flow of information between spatially distributed populations of pyramidal neurons in a contextually meaningful manner.
Converging lines of evidence strongly suggest that, in the PFC, functional disturbances of GABA neurons represent a prominent pathophysiologic feature of schizophrenia [1,2,7,13,33,47] and bipolar disorder [24,56,57]. In addition, alterations of glutamatergic modulation of GABA cells could further compromise PFC functions. In fact, in a recent study, we found that in the anterior cingulate cortex the density of GABA cells that expressed the NMDA NR2A subunit was decreased schizophrenia and bipolar disorder [65]. It is currently unknown whether similar changes also occur in other brain regions, such as the PFC. In this study, we used double in situ hybridization to co-localize the mRNA for the NMDA NR2A subunit and that for the GABA synthesizing enzyme glutamic acid decarboxylase (GAD)67, in the PFC in a cohort of 60 human subjects (Figure 1). We found that the pattern of changes in the density of GABA neurons was remarkably similar between schizophrenia and bipolar disorder, but the density of those GABA cells that expressed NR2A was altered in a diagnosis- and laminar-specific manner.
Figure 1.
Photomicrograph of a double-labeled neuron with silver grains superimposed onto the digoxigenin reaction product. Scale bar=5 µm.
2. RESULTS
2.1. GAD67 mRNA-Expressing Neurons
Overall, our analysis revealed a significant diagnosis effect (F2, 57=11.71, p<0.0001; Figure 2). Post-hoc analyses further revealed that, in the schizophrenia subjects, the density of GAD67 mRNA-expressing neurons in layers 2 (t=3.37, p=0.002), 3 (t=3.22, p=0.003), 4 (t=2.66, p=0.01) and 5 (t=2.91, p=0.006) was significantly decreased by 30.2%, 33.2%, 32.2% and 35.9% respectively, compared to the normal control subjects (Figure 2).
Figure 2.
(A) Mean density of all neurons that express GAD67 mRNA. (B) Mean density of GAD67 mRNA-expressing neurons that also express NR2A mRNA. Asterisks indicate statistically significant difference at p=0.01. Error bars represent SEM.
In the subjects with bipolar disorder, the pattern and magnitude of changes in cell density was virtually identical to what was observed in the schizophrenia subjects (Figure 2). Thus, the density of GAD67 mRNA-expressing neurons was significantly decreased by 33.6% and 33.2% in layers 3 (t=2.99, p=0.005) and 4 (t=2.63, p=0.01), respectively. The density of these neurons also appears to be decreased by 26.7% and 32.0% in layers 2 (t=2.13, p=0.04) and 5 (t=1.94, p=0.05), respectively, but these differences did not achieve statistical significance after correction for repeated measures. Because a small number (N=4) of the subjects with bipolar disorder had no history of psychosis, we also analyzed our data with the exclusion of these cases but this did not alter our results.
2.2. NR2A mRNA-Expressing GABA Neurons
A significant effect of diagnosis was detected by our ANOVA model (F2, 57=4.38, p=0.017; Figure 2). During layer-by-layer post hoc analyses, we found that the density of the double-labeled neurons was decreased by 56.8% and 49.4% in layers 3 (t=2.36, p=0.023) and 4 (t=4.10, p=0.0002), respectively, in the schizophrenia subjects, although the reduction in layer 3 did not reach statistical significance after the stringent Bonferroni correction. Cell density also appeared to be decreased by 27% in layer 2 in the subjects with schizophrenia, but this difference was not statistically significant. In the subjects with bipolar disorder, we did not observe any statistically significant differences in neuronal density in any of the cortical layers. Exclusion of the 4 subjects with no history of psychosis also did not affect our results.
2.3. Cortical Thickness Measurements
The average cortical thickness (±S.D.) did not differ (F2, 57=0.46, p=0.63) between the schizophrenia (1,755.0±386.5 mm), bipolar (1,863.6±395.1 mm) and normal control (1,766.8±395.5 mm) groups, suggesting that our observations of reduction in the density of both the single- and double-labeled neurons cannot be explained by differential tissue shrinkage in the 3 subject groups.
2.4. Expression Level of NR2A mRNA in GAD67 mRNA-Expressing Neurons
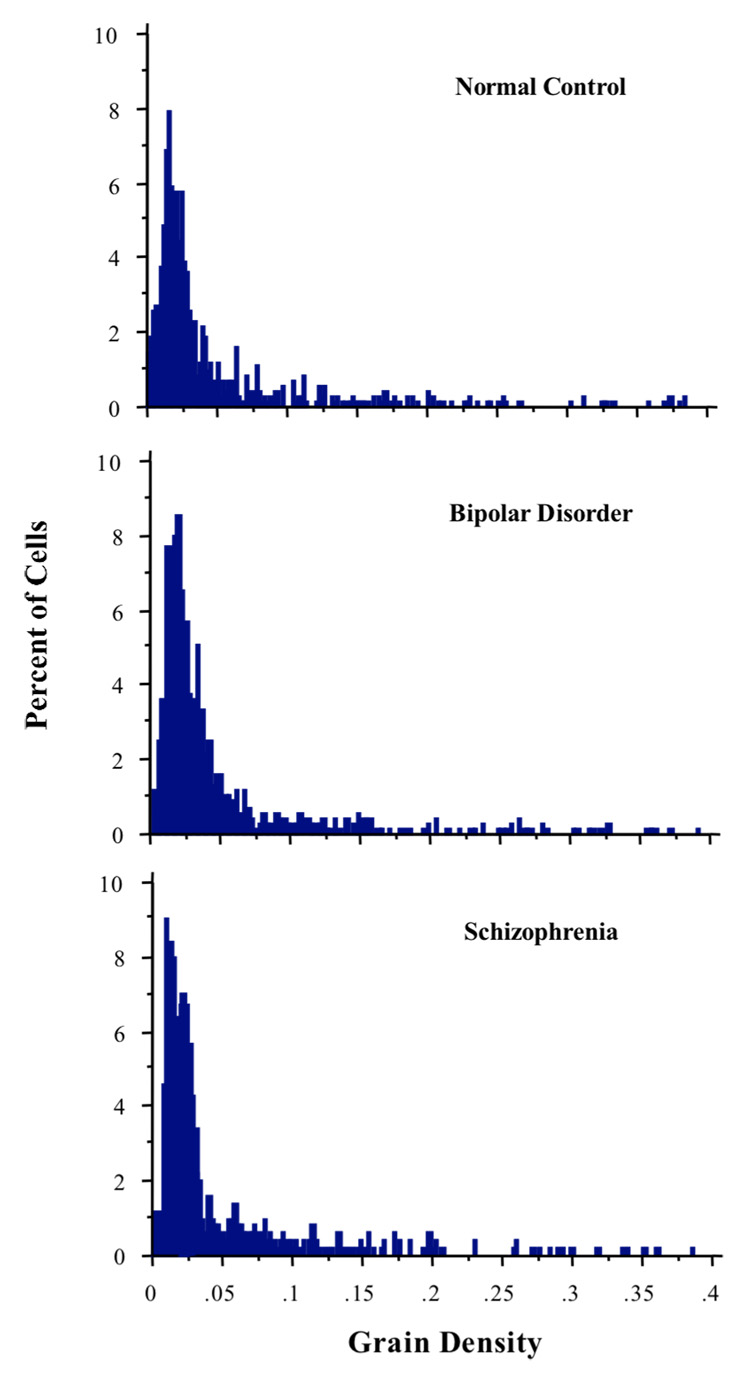
The distributions of the frequency histograms of NR2A mRNA expression level per GAD67+ cell are shown in Figure 3. The Kruskal-Wallis test revealed that the effect of diagnosis on grain density was not statistically significant (H=5.05, p=0.08). Layer-by-layer analyses also failed to reveal any diagnosis effect (data not shown). Finally, the average size of the silver grain clusters also did not differ between the 3 groups.
Figure 3.
Grain density distribution is not significantly different between the 3 diagnosis groups, suggesting that the amount of NR2A mRNA being expressed by GABA interneurons with experimentally detectable transcript level is not altered in schizophrenia or bipolar disorder.
2.5. Potential Confounding Variables
Within individual subject groups and when subject groups were combined, we found no evidence for any statistically significant correlation between any of the continuous variables and neuronal density measures or NR2A mRNA expression level. A small number of subjects with schizophrenia (n=4) or bipolar disorder (n=5) were not on any antipsychotic medications for a period of between 2 weeks and up to years before the time of death. In these subjects, their neuronal densities did not appear to be appreciably higher than neuronal densities in the subjects who were on these medications. Therefore, the observed changes in densities do not appear to be the result of antipsychotic treatment. Similarly, we found no evidence for any correlation between the dosage of divalproex and any of the density measures or NR2A mRNA expression level, suggesting that treatment with this mood stabilizer did not confound our findings. Due to sample size limitations, potential effects of lithium or other mood stabilizers could not be meaningfully evaluated and for the very same reason these medications would not have confounded our results. Finally, no statistically significant differences in neuronal densities or NR2A mRNA expression level were observed between the two sexes or the two hemispheres, either when individual subject groups were analyzed separately or when cases from the control group were combined with those from the schizophrenia or bipolar group.
3. DISCUSSION
Several conclusions can be drawn from the present study. First, the density of GAD67 mRNA-containing neurons appears to be decreased in layers 2–5 of the PFC in schizophrenia. This observation replicates previous findings from several other laboratories [2,24,58] and adds to the consensus that disturbances of GABA neurotransmission represent a key pathophysiologic feature of schizophrenia [1,7,13,33]. Second, the density of GAD67 mRNA-containing neurons in the PFC also appears to be decreased in bipolar disorder. Furthermore, the laminar pattern of reduction is remarkably similar to that in schizophrenia. Third, the density of GABA neurons that express the NMDA NR2A subunit appears to be decreased in the middle cortical layers in schizophrenia, whereas the density of these neurons in bipolar disorder seems to be unaltered. Thus, in the PFC, glutamatergic innervation of GABA interneurons via NMDA channels may be deficient in schizophrenia, but not in bipolar disorder.
3.1. Methodological Considerations
Consistent with previous studies [2,24,58], we found that in 27–36% of the GABA cells, the expression of the mRNA for GAD67 appears to be reduced to a level that is experimentally undetectable in subjects with schizophrenia. Furthermore, NR2A mRNA expression in the GABA cells with unaltered GAD67 expression is significantly reduced. A very important question that cannot be addressed in the present study, however, is whether NR2A expression is also reduced in the GABA cells with undetectable GAD67. Because the expression of the 65 kD-isoform of GAD (GAD65) is not decreased in schizophrenia [24], this question can be indirectly addressed in future studies by comparing the expression of NR2A in GAD67- versus GAD65-expressing neurons.
In the quantification of GAD67+/NR2A+ cells, we included only those with the silver grain density that was 2X above background. In fact, the exclusive majority (>93%) of the double-labeled cells that were counted met this criterion. However, it is possible that the 7% of the cells that were excluded may have contained a small population of GABA cells with very low NR2A expression level. Nevertheless, the possible exclusion of this small contingent of cells should not have affected our conclusions, given the magnitude of change in the density of the double-labeled cells was observed to be in the 50–60% range. Conversely, we cannot rule out the possibility that some of the silver grain clusters included in our analysis may represent non-specific labeling. In this case, we would have underestimated the magnitude of reduction in cell density, but our conclusions should not have been affected.
In any postmortem human brain studies, medications pose a serious potential confound. For instance, in this study, the exclusive majority of the subjects were on psychiatric medications at the time of death, which include antipsychotic drugs in both the schizophrenia and bipolar subjects and mood stabilizers in the bipolar subjects. As described above, our statistical analyses do not support the notion that the observed reduction in the densities of both the GAD67+ and GAD67+/NR2A+ cells is the result of treatment with antipsychotics or mood stabilizers. Furthermore, it has been found that, in monkeys, long-term treatment with haloperidol does not appear to have any effects on the level of GAD67 mRNA expression [58]. Also, the fact that the reduction in the density of GAD67+/NR2A+ cells occurred only in the schizophrenia group even though the majority (N=13) of the subjects with bipolar disorder were also receiving antipsychotic medications seems to argue against the idea that this observation represents the result of antipsychotic treatment. Lastly, many (N=11) of the subjects with bipolar disorder were on a combination of antipsychotics and mood stabilizers. When comparing the density of both the single- and double-labeled cells between these subjects and the rest of the subjects, the two groups did not appear to show any differences, suggesting that the combined use of antipsychotics and mood stabilizers does not appear to have affected our results.
Finally, cigarette smoking is exceedingly prevalent in patients with schizophrenia [9,15]. Unfortunately, the demographic information that is available did not allow us to systematically address this important potential confound. Nevertheless, previous studies in animals suggest that nicotine may actually increase the expression of NR2A in different brain regions, including the cerebral cortex [17,28,59]. If these findings apply to the human brain, we might have actually underestimated the magnitude of NR2A expression reduction in subjects with schizophrenia.
3.2. Disturbances of Inhibitory Interneurons in Schizophrenia and Bipolar Disorder
Our observations are in line with previous studies showing that the density of GAD67 mRNA-expressing neurons appears to be decreased in the PFC [2,24,58] and in the anterior cingulate cortex [65] in schizophrenia. Together, these findings provide clear support for the growing understanding that GABAergic modulation of pyramidal cell activities in the cerebral cortex seems to be significantly altered in this disorder [7,13,33]. There has also been increasing evidence implicating GABA neurotransmission disturbances in bipolar disorder. For instance, using quantitative real-time reverse transcriptase polymerase chain reaction, it was found that the mRNA for GAD67 in homogenized PFC tissues was significantly decreased by up to 50% in the PFC in subjects with bipolar disorder [24]. In the present study, we found that the density of GAD67 mRNA-expressing neurons was decreased in layers 2–5; this pattern was virtually identical to what was observed in schizophrenia and is also consistent with the observations of 2 previous studies [24,57]. Therefore, it appears that disturbances of GABA interneurons in the PFC may represent a common pathophysiologic element in both schizophrenia and bipolar disorder, although the neurobiologic mechanisms that underlie these disturbances remain poorly understood. Because GABA cells are morphologically, neurochemically and functionally heterogeneous, with subpopulations of these neurons involved in different aspects of information processing in the cerebral cortex [16,25,29,35,61], a critical question that will need to be addressed in future studies is whether and if so how subpopulations of GABA interneurons may be differentially affected in the two conditions.
3.3. Disturbances of Glutamatergic Neurotransmission on Inhibitory Interneurons in Schizophrenia
Our observation suggests that glutamatergic innervation of GABA interneurons via NMDA receptors may be decreased in layers 3–4 of the PFC. This observation may at first glance appear to be at odds with previous findings [3] suggesting that NR2A expression in the PFC seems to be unaltered in schizophrenia. However, because GABA cells comprise only about 20 % of all neurons in the PFC, our observation of an approximately 50% decrease in NR2A transcript expressed by GABA cells suggests that NR2A transcript signal is decreased by only about 10% among all of the neurons. If we also take into consideration the amount of neuropil in the PFC, this signal-to-noise ratio will be further diluted and is unlikely to be detectable by film autoradiography [3].
The observed decrease in the density of NR2A+/GAD67+ cells in layers 3 and 4 of the PFC without an apparent decrease in the level of NR2A mRNA expression per GAD67-expressing cells suggests that NR2A mRNA expression in a subpopulation of GABA cells is reduced to an undetectable level. The origin of the glutamatergic terminals that innervate the GABA cells that exhibit decreased NR2A mRNA expression is at present unknown. However, it is known that deep layer 3 and layer 4 together represent the thalamocortical recipient zone in the PFC. In fact, there has been evidence suggesting that thalamocortical inputs to the PFC may be deficient in schizophrenia [4,21,32,39,42,43]. Furthermore, GABA cells in these layers, especially those that contain the calcium binding protein parvalbumin (PV), are known to be targeted by thalamocortical inputs [48,63]. Taken together, our findings are consistent with the idea that thalamocortical innervation of GABA neurons in the PFC may be aberrant in schizophrenia.
Pyramidal neurons in layer 3 of the PFC furnish the intrinsic excitatory circuit [44,64] and approximately 50% of the axon terminals arising from this circuit target GABA neurons [36]. Thus, these terminals may also be a source of the aberrant glutamatergic innervation of the GABA cells that exhibit reduced NR2A expression. It is postulated that this circuit may mediate sustained neuronal activation during working memory through reentrant oscillation of spatially-distributed functionally-segregated pyramidal cell ensembles [23,60]. Because GABA interneurons play a crucial role in regulating the flow of information within and between these pyramidal cell ensembles, altered glutamatergic regulation of GABA neurons may compromise the precise spatiotemporal orchestration that is necessary for reentrant oscillatory dynamics [27,31], resulting in working memory and associated deficits in schizophrenia. In addition, thalamocortical projections may further contribute to reentrant oscillations of the intrinsic excitatory circuit [52]. As such, deficient thalamocortical modulation of GABA neurotransmission could further compromise the functional integrity of this circuit. Interestingly, the generation of neuronal oscillations, especially those in the gamma frequency band, has recently been shown to be disturbed in schizophrenia [10,50,51,55]. Furthermore, it is known that the fast-spiking GABA cells that contain PV are particularly important in mediating gamma band oscillations [14,19,62]. In addition, compared to pyramidal cells and other GABA cells, PV-containing GABA cells contain up to 5-fold the amount of NR2A at both the protein and transcript levels [30]. Taking together, these observations raise an interesting possibility that the PV-containing GABA cells may be among those GABA cells that exhibit drastically reduced NR2A mRNA expression.
3.4. Regional Similarities and Differences in Inhibitory Neural Circuitry Disturbances in Schizophrenia and Bipolar Disorder
The findings that the density of GAD67 mRNA-expressing neurons is reduced in schizophrenia and bipolar disorder in both the PFC [2,24,58] and the anterior cingulate cortex [65] suggest that, in both of these conditions, GABA neurotransmission is compromised across cortical regions. However, while glutamatergic drive to GABA neurons via the NMDA receptor appears to be altered in both schizophrenia and bipolar disorder in the anterior cingulate cortex [65], in the PFC, this alteration may be specific to schizophrenia. This finding is consistent with the idea that NMDA receptors on GABA cells play a key role in regulating sustained neuronal activation [34,54], which forms the physiological substrate of working memory, and that working memory may be preferentially impaired in schizophrenia [40]. In future studies, it will be essential to more precisely delineate the similarities and differences in neural circuitry disturbances in these two cortical regions between schizophrenia and bipolar disorder in terms of cellular and molecular impairments within microcircuits.
4. EXPERIMENTAL PROCEDURE
4.1. Subjects
Sixty postmortem human brains from 20 subjects with schizophrenia, 20 subjects with bipolar disorder and 20 normal control subjects were obtained from the Harvard Brain Tissue Resource Center at McLean Hospital. As shown in Table 1, the 3 subject groups were closely matched for age and postmortem interval (PMI). In addition, the mean freezer storage time (days±S.D.) of brains was not significantly different (F2, 57=1.32, p=0.28) between the normal control (1,822±997), schizophrenia (1,755±878) and bipolar (1,376±931) groups. The mean tissue pH (±S.D.) also did not differ (F2, 57=0.77, p=0.47) between the three groups of subjects (normal control: 6.58±0.25; schizophrenia: 6.53±0.28; bipolar: 6.47±0.22).
Table 1.
Cases Used in the Present Study
| Bipolar disorder | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Age | PMI | Sex | Hemisphere | Race | CE | pH | Psychotropics received at time of death | Cause of death |
| 1 | 83 | 17.5 | M | R | W | 0 | 6.60 | Divalproex | Cardiopulmonary failure |
| 2 | 74 | 22.9 | F | R | W | 100 | U | Divalproex, lorazepam, olanzapine | Cardiopulmonary failure |
| 3 | 26 | 22.8 | F | L | W | 0 | 6.35 | Lithium, lorazepam | Cardiopulmonary failure |
| 4 | 52 | 17.2 | F | R | W | 200 | U | Olanzapine | Hepatic failure |
| 5 | 62 | 13.4 | F | R | W | 0 | U | Divalproex, buproprion, sertraline | Breast cancer |
| 6 | 74 | 7.2 | M | L | W | 100 | 6.70 | Gabapentin, olanzapine | Pneumonia |
| 7 | 85 | 27.5 | F | L | W | 534 | 6.28 | Olanzapine, divalproex | Cardiopulmonary failure |
| 8 | 51 | 31 | M | L | W | 0 | 7.02 | Clonazepam, gbapentin | Suicide by overdose |
| 9 | 69 | 29.5 | M | L | W | 0 | U | Lithium | Pneumonia |
| 10 | 64 | 11 | F | R | W | 800 | 6.26 | Trifluoperazine | Respiratory failure |
| 11 | 42 | 15.8 | F | L | W | 960 | 6.60 | Lithium, divalproex, perphenazine | Medication overdose, rule out suicide |
| 12 | 29 | 10.7 | F | L | W | 200 | 6.70 | Divalproex, lithium, clonazepam, olanzapine | Cardiac arrest |
| 13 | 36 | 9 | M | L | W | U | U | Unknown | Suicide |
| 14 | 73 | 20.8 | F | R | W | 50 | 6.32 | Carbamazepine, risperidone | Sepsis |
| 15 | 74 | 14.3 | M | R | W | 400 | 6.27 | Divalproex, lithium, olanzapine, hydroxyzine prn, lorazepam prn, zolpidem prn | Pneumonia |
| 16 | 62 | 18.7 | F | R | W | 100 | 6.40 | Divalproex, sertraline, risperidone, benztropine, donepezil | Renal failure |
| 17 | 82 | 5.0 | M | L | W | U | 6.37 | Unknown | Cardiopulmonary failure |
| 18 | 40 | 30.8 | M | R | W | 200 | 6.60 | Gabapentin, ziprasidone, citalopram, risperidone, topiramate | Cardiac arrest |
| 19 | 38 | 22 | M | R | W | 200 | 6.24 | Divalproex, paroxetine, olanzapine | Carbon monoxide poisoning |
| 20 | 47 | 16.3 | F | R | W | 50 | U | Divalproex, topiramate, tiagabine, perphenazine, clonazepam, | Cardiopulmonary arrest |
| Mean | 58.1±18.7 | 18.2±7.7 | M:F=10:10 | R:L=11:9 | 212.3±282.8 | 6.48±0.23 | |||
| Schizophrenia | |||||||||
| 21 | 85 | 15.7 | F | R | W | 150 | U | Risperidone, lorazepam | Sepsis |
| 22 | 48 | 33.8 | F | L | W | 450 | 6.63 | Risperidone, divalproex | Cardiac failure |
| 23 | 44 | 19 | M | L | W | 266 | 6.20 | Clozapine | Pneumonia |
| 24 | 89 | 13.5 | F | L | W | 20 | U | Trifluoperazine | Pneumonia |
| 25 | 78 | 13.4 | F | L | W | 750 | 6.81 | Haloperidol, lithium, cogentin | Sinus node disease |
| 26 | 61 | 19.9 | M | R | W | 300 | 6.68 | Clozapine | Sepsis |
| 27 | 61 | 11 | F | R | W | 150 | U | Paroxetine, clonazepam, clozapine | Myocardiac infarction |
| 28 | 84 | 25.8 | F | R | W | 0 | 6.14 | None | Cardiac arrest |
| 29 | 26 | 16 | M | R | W | 357 | 6.75 | Fluphenazine decanoate | Suicide by hanging |
| 30* | 55 | 18 | F | R | W | 0 | 6.52 | None | Oral cancer |
| 31* | 47 | 19.2 | M | R | W | 0 | 6.57 | Clonazepam, hydroxyzine prn | Lung cancer |
| 32 | 73 | 24 | F | R | W | 600 | 6.08 | Risperidone, fluoxetine, chlorazepate, midazolam prn | Lung cancer |
| 33 | 49 | 19 | M | L | W | 500 | 6.60 | Haloperidol decanoate, lorazepam | Suicide by hanging |
| 34 | 63 | 22.3 | M | R | W | 500 | 6.55 | Clozapine, haloperidol, lorazepam, trazadone | Cardiac arrest |
| 45 | 72 | 21.7 | F | R | W | 400 | 6.65 | Risperidone, paroxetine | Ovarian cancer |
| 36 | 66 | 22.1 | M | R | W | 1000 | 6.43 | Haloperidol | Emphysema |
| 37 | 83 | 23.2 | F | R | W | 2000 | 6.91 | Haloperidol decanoate, fluphenazine decanoate | Gastrointestinal bleed |
| 38 | 46 | 18.5 | F | L | W | 200 | 6.31 | Olanzapine, divalproex | Sepsis |
| 39 | 42 | 27.1 | M | R | W | 0 | 6.64 | None | Leukemia |
| 40 | 31 | 14 | M | R | W | 600 | 6.46 | Risperidone, olanzapine, buproprion | Unknown (found dead in home) |
| Mean | 60.2±18.5 | 19.8±5.4 | M:F=10:10 | R:L=14:6 | 412.2±465.7 | 6.53±0.23 | |||
| Normal control | |||||||||
| 41 | 49 | 24.6 | M | L | W | 6.76 | None | Myocardiac infarction | |
| 42 | 37 | 18.8 | M | R | W | 6.68 | None | Electrocution | |
| 43 | 54 | 24.2 | M | L | W | 6.53 | None | Cardiopulmonary arrest | |
| 44 | 78 | 14.1 | F | R | W | 6.22 | None | Myocardial infarction | |
| 45 | 53 | 20.2 | M | R | W | U | None | Cardiopulmonary arrest | |
| 46 | 65 | 24.3 | F | R | W | 6.40 | None | Lung cancer | |
| 47 | 89 | 7.42 | M | R | W | 6.39 | None | Cancer | |
| 48 | 69 | 15.3 | M | R | W | 6.88 | None | Respiratory failure | |
| 49 | 74 | 12.5 | F | L | W | 6.33 | None | Cardiopulmonary arrest | |
| 50 | 66 | 7.4 | F | R | W | 6.03 | None | Cancer | |
| 51 | 42 | 18.3 | M | L | W | 6.78 | None | Myocardial infarction | |
| 52 | 78 | 23.9 | F | R | W | 6.67 | None | Breast cancer | |
| 53 | 40 | 16.6 | M | L | W | 6.24 | None | Myocardiac infarction | |
| 54 | 67 | 22.3 | M | L | W | 6.42 | None | Cardiopulmonary arrest | |
| 55 | 70 | 22.5 | F | L | W | 6.26 | None | Liver cancer | |
| 56 | 66 | 18.7 | M | R | W | 6.76 | None | Myocardial infarction | |
| 57 | 79 | 20.9 | M | L | W | 6.74 | None | Cancer | |
| 58 | 38 | 28.8 | M | L | W | 6.53 | None | Myocardiac infarction | |
| 59 | 30 | 14.8 | M | R | W | U | None | Blunt force trauma | |
| 60 | 70 | 15 | F | R | W | 6.59 | None | Cardiac arrest | |
| Mean | 60.7±16.7 | 18.5±5.7 | M:F=13:7 | R:L=11:9 | 6.51±0.24 | ||||
CE: Chlorpromazine equivalent (in mg); PMI: Postmortem interval; U: Unavailable
Psychiatric diagnoses were established using a retrospective review of medical records and an extensive family questionnaire that included the medical, psychiatric, and social history of the subjects. For the diagnosis of schizophrenia, the criteria of Feighner et al [18] were used and the diagnoses of schizoaffective and bipolar disorder were made according to DSM-III-R criteria. The majority (N=16) of the subjects with bipolar disorder had a history of psychosis, even though they may not have been on antipsychotic medications at the time of death. The medication status of all of the cases can be found in Table 1. None of the subjects were suffering from any substance abuse/dependence disorder at the time of death, as confirmed by review of toxicology reports, medical records and family questionnaires.
4.2. Tissue Preparation
Human brains were blocked and blocks containing Brodmann’s area 9 of the dorsolateral PFC were frozen over liquid nitrogen vapor before being stored at −70 °C. In preparation for in situ hybridization, tissue blocks were sectioned at a thickness of 10 µm on a cryostat. Two sections per subject and therefore six sections per matched triplet were used for the experiment. The six sections from each triplet were mounted on three slides as follows: 1) normal control+schizophrenia, 2) bipolar+normal control, and 3) schizophrenia+bipolar. This method of mounting sections controls for potential variability of hybridization signals due to, among other factors, variability in the thicknesses of emulsion between slides and possibly also on the same slides. All slides were processed at the same time in one single experiment.
4.3. Dual in situ Hybridization
4.3.1. Riboprobe Preparation
4.3.1.1. Radiolabeled cRNA Probe for NR2A mRNA
The cRNA probes for the NR2A subunit (kindly provided by Dr. Christine Konradi, Vanderbilt University) were transcribed in vitro from linearized cDNA subclones encoding the rat NMDA NR2A subunit, as previously described [65]. The specificity of the probe had been verified by northern blot analysis. The probe was derived from a cDNA spanning nucleotides 1185–2154 (Genbank Accession #M91561) within the coding region of the subunit. A corresponding sense probe was used as a control. Radiolabeled cRNA probe was prepared by first drying down [35S]UTP (500 µCi/ml of probe, New England Nuclear, Boston, MA) in a DNA Speed-Vac (Savant, Farmingdale, NY). 100 ng/µl of the cDNA template, 0.1 M dithiothreitol (DTT), 3 U/µl RNasin, 5mM NTPs, 0.8 U/µl of T3 or T7 RNA polymerases (for antisense and sense probe, respectively), and 5X transcription buffer were then added. The transcription mixture was subsequently incubated at 37 °C for 1 hour. The cDNA template was digested by incubating the mixture with R1Q DNase at 37 °C for 15 minutes. Unincorporated NTPs were removed by running the mixture through a Stratagene Nuc-Trap (La Jolla, CA) push column. The eluate was collected and probe concentration was determined by scintillation counting. The probe was then stored at −20 °C until use.
4.3.1.2. DIG-Labeled GAD67 mRNA Probe
DIG-UTP-labeled cRNA probes were transcribed using 100 ng of full-length, linearized human cDNA clones inserted in a bluescript vector (kindly provided by Drs. Allan Tobin and Niranjula Tillakarantne, UCLA) in the presence of 0.1 M DTT, 3 U/µl Rnasin, 0.8 U/µl of T3 and T7 RNA polymerases, 10 mM of ATP, CTP, and GTP, 6.5 mM of UTP, and 3.5 mM of DIG-labeled UTP (Boehringer Mannheim, Indianapolis, IN). The mixture was incubated at 37 °C for 1 hour. cDNA template was digested with RQ1 DNase. This same cRNA probe has been used in 2 previous postmortem studies from our laboratory [26,65].
4.3.2. Hybridization
To ensure adequate tissue penetration, the GAD67 probe was hydrolyzed to 0.8 kb with an equal volume of sodium bicarbonate/carbonate buffer (pH 10.2; 40 mM NaHCO3, 60 mM Na2CO3) at 60 °C for 6–10 minutes. Probes were then reconstituted in a hybridization buffer consisting of 50% formamide, 0.1% yeast tRNA, 10% dextran sulfate, 1X Dehardt’s solution, 0.5 M EDTA, 0.02% SDS, 4X SSC, 10 mM DTT, and 0.1% ssDNA, at a final concentration of 0.4 ng probe/µl hybridization buffer. Before hybridization, mounted tissue sections were air dried and warmed to room temperature. They were then post-fixed in 4% paraformaldehyde for 10 minutes and incubated in 0.1 M TEA for 5 minutes at room temperature before being dehydrated in a graded series of ethanol. Probes were then added to slides for hybridization in a prewarmed, humidified dish. Sections were covered with coverslips and incubated at 55 °C for 12 hours. At the end of hybridization, coverslips were soaked off in 4X SSC in the presence of 100 µl of 2-mercaptolethanol. Tissue was then incubated in 0.5 M NaCl/0.05 M PB for 10 minutes, 0.5 M NaCl with 0.025 mg/ml RNaseA at 37 °C for 30 minutes, followed by a high stringency wash with a solution containing 50% formamide, 0.5 M NaCl/0.05 M PB, and 100 µl 2-mercaptolethanol at 63 °C for 30 minutes. Sections were finally washed overnight in 0.5X SSC with 20 mM 2-mercaptolethanol at room temperature.
4.3.3. Visualization of DIG Labeling
After incubation in blocking buffer (100 mM Tris-HCL, 150 mM NaCl pH 7.5, 3% normal goat serum (NGS), 0.3% Triton X100), sections were incubated overnight at 4 °C in buffer containing 1:200 dilution of alkaline phosphatase-conjugated sheep anti-DIG antibody (Roche Diagnostics, Indianapolis, IN). Sections were then incubated in Vector Red™, which was prepared using the Vector Red™ alkaline phosphatase substrate kit (Vector Laboratories, Burlingame, CA), at room temperature for 40 minutes in complete dark.
4.3.4. Emulsion Autoradiography
It was determined that sufficient autoradiographic signal had developed after the slides were apposed to X-ray film (Kodak Biomax MS) for 10 days. The slides were then dipped in emulsion (Kodak NTB-2), air dried, and stored at 4 °C in darkness for 5 weeks. After development in the dark with Kodak D-19 developer, slides were counterstained with cresyl violet and coverslipped.
4.4. Quantification of GAD67 and NR2A mRNA Expression
All microscopic analyses were conducted by one investigator (EV) under strictly blind condition. [35S]-labeling of NR2A mRNA appeared as clusters of silver grains after processing for emulsion autoradiography. DIG labeling was visualized as a red-brown reaction product under a brightfield microscope equipped with polarizing filters to enhance the optical density of the reaction product (Figure 1). Neurons that were single-labeled with DIG and those that were double-labeled with DIG and [35S] were identified using a Leica Laborlux microscope equipped with a solid state CCD video camera and Bioquant Nova Image Analysis System (R&M Biometrics, Memphis, TN). Using an 100X oil immersion objective lens at a final magnification of 1,000X, the distribution of both single- and double-labeled neurons within a 250 µm-wide column extending from the pial surface to the border between layer 6 and the subcortical white matter were obtained for each section. A total of 120 sections (i.e. 2 sections per subject) were quantified. For the DIG + [35S] double-labeled cells, only those that had a grain density greater than 2x of background grain density (see below) were included. Neighboring sections were stained with cresyl violet for accurate determination of laminar boundaries. All sampling columns were placed within Brodmann’s area 9, which was identified based on known cytoarchitectual criteria [45]. Densities of single- and double-labeled neurons for each cortical layer were then obtained by dividing cell counts by laminar areas. For each subject, density measures from the 2 sections were pooled to give rise to an average measure. Intra-rater reliability, as assessed by counting and recounting profiles within the same column, was established to be consistently between 93–95 % before the actual data collection process was begun and throughout the course of data collection. Quantification was performed on a regular and continuous basis in order to minimize any deviation in the data collection routines. The entire data quantification process was completed in four months.
To quantify the expression level of mRNA for the NR2A subunit in individual GABA cells, the area occupied by each silver grain cluster was outlined using a cursor displayed on the computer monitor. The cluster area was measured by highlighting the grains with a thresholding subroutine. The light intensity was then adjusted to ensure that the size of the grains was neither under- nor over-represented and both the threshold and the light intensity were held constant throughout the entire data collection process. The area covered by autoradiographic grains within the cluster area was automatically computed based on the threshold value and was represented as a pixel count for NR2A transcript expression level. The pixel count was expressed as a function of cluster area. By subtracting the background grain density (i.e. pixel count of the area covered by autoradiographic grains per unit area in µm2 in the white matter), the corrected NR2A expression level was obtained. The average NR2A expression level in GABA interneurons (i.e. cells positive for GAD67 mRNA) for each cortical layer for each case was then computed. For each diagnosis group, a histogram of the distribution of grain density was plotted.
4.5. Statistical Analyses
The densities of GAD67 mRNA+ and GAD67 mRNA+/NR2A mRNA+ neurons were compared between the 3 subject groups across layers 2 through 6, using a repeated-measures analysis of variance (ANOVA), with diagnosis as the between-groups factor, layer as the within-group factor, and repeated measures on layer. For post-hoc testing of differences in group means, two-tailed unpaired t tests were used. Layer 1 was excluded in our analyses because of the very small number of GAD67 mRNA-containing neurons that co-expressed NR2A mRNA in this lamina. The Bonferroni procedure was employed to correct for type 1 error as a result of multiple comparisons (i.e. layers 2, 3, 4, 5, and 6). Therefore, the alpha level for significance for all t tests was p=0.01 (i.e. 0.05/5). In addition, in order to evaluate the effects of potential confounding factors, for continuous variables such as age, PMI, pH, freezer storage time, and exposure to antipsychotic medications or mood stabilizers, simple Pearson correlations were obtained for the individual groups and when cases from the control group were combined with those from the schizophrenia and the bipolar group, respectively. In addition, we used analysis of covariance (ANCOVA) to understand how these confounding variables might have affected our results. Because none of the conclusions derived from our findings were affected by the ANCOVAs, only results from repeated-measures ANOVAs are reported. Potential effects of nominal variables such as hemispheric laterality and sex on our findings were evaluated by using two-tailed unpaired t-tests to compare the density and expression level measures from the two hemispheres and those from the two sexes, both within individual groups and when cases from the control group were combined with those from the schizophrenia and bipolar group, respectively. To detect any differences in the expression of the mRNA for NR2A in GAD67 mRNA-containing neurons, the non-parametric Kruskal-Wallis test was used.
ACKNOWLEDGEMENT
This study was supported by the National Institute of Mental Health (MH 068541).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Brain Res Rev. 2006 doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of General Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 3.Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE, Jr, Jones EG. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. Journal of Neuroscience. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreasen NC, Arndt S, Swayze V, 2nd, Cizadlo T, Flaum M, O'Leary D, Ehrhardt JC, Yuh WT. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 5.Baddeley AD. Working memory. Oxford: Oxford University Press; 1988. [Google Scholar]
- 6.Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neuroscience & Biobehavioral Reviews. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 7.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 8.Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr Clin North Am. 2005;28:613–633. 626. doi: 10.1016/j.psc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29:697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- 10.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical {gamma} synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constantinidis C, Wang XJ. A neural circuit basis for spatial working memory. Neuroscientist. 2004;10:553–565. doi: 10.1177/1073858404268742. [DOI] [PubMed] [Google Scholar]
- 12.Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nature Neuroscience. 2002;5:175–180. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- 13.Costa E, Davis JM, Dong E, Grayson DR, Guidotti A, Tremolizzo L, Veldic M. A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol. 2004;16:1–23. doi: 10.1615/critrevneurobiol.v16.i12.10. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, Maycox PR, Whittington MA, Racca C. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalack GW, Meador-Woodruff JH. Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophr Res. 1996;22:133–141. doi: 10.1016/s0920-9964(96)80441-5. [DOI] [PubMed] [Google Scholar]
- 16.DeFelipe J. Neocortical neuronal diversity: chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cerebral Cortex. 1993;3:273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- 17.Delibas N, Doguc DK, Sutcu R, Eroglu E, Gokalp O. EFFECT of nicotine on hippocampal nicotinic acetylcholine alpha7 receptor and NMDA receptor subunits 2A and 2B expression in young and old rats. Int J Neurosci. 2005;115:1151–1163. doi: 10.1080/00207450590914437. [DOI] [PubMed] [Google Scholar]
- 18.Feighner JP, Robins E, Guze SB, Woodruff RA, Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- 19.Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 20.Fuster JM. The prefrontal cortex. New York: Raven press; 1989. [Google Scholar]
- 21.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 22.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biological Psychiatry. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cerebral Cortex. 2000;10:82–92. doi: 10.1093/cercor/10.1.82. [DOI] [PubMed] [Google Scholar]
- 24.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E, DiGiorgi Gerevini V. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of General Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 26.Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002;59:521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- 27.Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh CY, Leslie FM, Metherate R. Nicotine exposure during a postnatal critical period alters NR2A and NR2B mRNA expression in rat auditory forebrain. Brain Res Dev Brain Res. 2002;133:19–25. doi: 10.1016/s0165-3806(01)00314-5. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 30.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state-and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 32.Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biological Psychiatry. 2001;49:28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- 33.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 34.Lisman JE, Fellous JM, Wang XJ. A role for NMDA-receptor channels in working memory. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- 35.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 36.Melchitzky DS, Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Synaptic targets of the intrinsic axon collaterals of supragranular pyramidal neurons in monkey prefrontal cortex. Journal of Comparative Neurology. 2001;430:209–221. doi: 10.1002/1096-9861(20010205)430:2<209::aid-cne1026>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Melchitzky DS, Lewis DA. Pyramidal neuron local axon terminals in monkey prefrontal cortex: differential targeting of subclasses of GABA neurons. Cereb Cortex. 2003;13:452–460. doi: 10.1093/cercor/13.5.452. [DOI] [PubMed] [Google Scholar]
- 38.Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. Journal of Comparative Neurology. 1998;390:211–224. doi: 10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Pakkenberg B. The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophrenia Research. 1992;7:95–100. doi: 10.1016/0920-9964(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 40.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 41.Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Archives of General Psychiatry. 1995;52:821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- 42.Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Archives of General Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 43.Popken GJ, Bunney WE, Jr, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pucak ML, Levitt JB, Lund JS, Lewis DA. Patterns of intrinsic and associational circuitry in monkey prefrontal cortex. Journal of Comparative Neurology. 1996;376:614–630. doi: 10.1002/(SICI)1096-9861(19961223)376:4<614::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cerebral Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 46.Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. Journal of Neurophysiology. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds GP, Zhang ZJ, Beasley CL. Neurochemical correlates of cortical GABAergic deficits in schizophrenia: selective losses of calcium binding protein immunoreactivity. Brain Res Bull. 2001;55:579–584. doi: 10.1016/s0361-9230(01)00526-3. [DOI] [PubMed] [Google Scholar]
- 48.Rotaru DC, Barrionuevo G, Sesack SR. Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: synaptic relationships to subclasses of interneurons. J Comp Neurol. 2005;490:220–238. doi: 10.1002/cne.20661. [DOI] [PubMed] [Google Scholar]
- 49.Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- 50.Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex. 1997;7:583–604. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- 53.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 54.Tegner J, Compte A, Wang XJ. The dynamical stability of reverberatory neural circuits. Biol Cybern. 2002;87:471–481. doi: 10.1007/s00422-002-0363-9. [DOI] [PubMed] [Google Scholar]
- 55.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 56.Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Archives of General Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 59.Wang F, Chen H, Steketee JD, Sharp BM. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology. 2007;32:103–109. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends in Neurosciences. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- 61.Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A. 2004;101:1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 63.Williams SM, Goldman-Rakic PS, Leranth C. The synaptology of parvalbumin-immunoreactive neurons in the primate prefrontal cortex. Journal of Comparative Neurology. 1992;320:353–369. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- 64.Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 65.Woo TUW, Walsh JP, Benes FM. Density of Glutamic Acid Decarboxylase 67 Messenger RNA-Containing Neurons That Express the N-Methyl-D-Aspartate Receptor Subunit NR2A in the Anterior Cingulate Cortex in Schizophrenia and Bipolar Disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]