Abstract
CC chemokine ligand 18 (CCL18) was originally discovered as pulmonary and activation-regulated chemokine (PARC), dendritic cell (DC)-chemokine 1 (DC-CK1), alternative macrophage activation-associated CC chemokine-1 (AMAC-1), and macrophage inflammatory protein-4 (MIP-4). CCL18 primarily targets lymphocytes and immature DC, although its agonistic receptor remains unknown so far. CCL18 is mainly expressed by a broad range of monocytes/macrophages and DC. A more profound understanding of the various activation programs and functional phenotypes of these producer cells might give a better insight in the proinflammatory versus anti-inflammatory role of this CC chemokine. It is interesting that CCL18 is constitutively present at high levels in human plasma and likely contributes to the physiological homing of lymphocytes and DC and to the generation of primary immune responses. Furthermore, enhanced CCL18 production has been demonstrated in several diseases, including various malignancies and inflammatory joint, lung, and skin diseases. The lack of a rodent counterpart for human CCL18 sets all hope on primate animal models to further elucidate the importance of CCL18 in vivo. This review will address these different aspects in more detail.
Keywords: dendritic cells, macrophages, chemotaxis, inflammatory, homing
INTRODUCTION
Chemokines constitute a family of chemotactic cytokines that act through seven-transmembrane domain G protein-coupled receptors on their target cells [1–3]. According to the organization of their NH2-terminal Cys residues, chemokines are structurally divided into the CC, CXC, CX3C, and C chemokines. Chemokines are key players in directing the migration and the activation of leukocytes throughout the body, under physiological and immunopathological conditions. Furthermore, they are involved in various other processes, including angiogenesis, hematopoiesis, tumor growth, and metastasis. A first wave of chemokines was mainly identified on the basis of their chemotactic properties [4]. These chemokines are in general highly inducible in multiple cell types and are responsible for the leukocyte infiltration at sites of inflammation. A second generation of chemokines has been discovered since 1996 through bioinformatics. These chemoattractants are mostly implicated in the homeostatic trafficking of lymphocytes and dendritic cells (DC) to those specific tissues where they are constitutively expressed. However, some of these second-generation chemokines, such as the CC chemokine ligand 18 (CCL18), apparently belong to the inflammatory/inducible as well as the constitutive/homeostatic chemokines, depending on the circumstances [4]. This review will focus on the constitutive versus regulated expression pattern of CCL18 and on the biological activities of this CC chemokine under physiological and pathological conditions.
DISCOVERY OF CCL18
By searching the public GenBank expressed sequence tag (EST) database with the cDNA sequence of the human CC chemokine CCL3, Hieshima et al. [5] identified a series of partial cDNA sequences, encoding a polypeptide with significant (64%) sequence identity to CCL3. The full-length cDNA of this new CC chemokine was obtained from human fetal lung. The finding that this chemokine was constitutively expressed at high levels in human lung together with its inducible expression in some human cell lines led to the designation pulmonary and activation-regulated chemokine (PARC). Analogously, Wells and Peitsch [6] discovered, by means of computer-assisted analysis of the EST database, a sequence coding for a new CCL3-like polypeptide, which they named macrophage inflammatory protein-4 (MIP-4). Independently, PARC was also cloned from a cDNA library of monocyte-derived DC and from a cDNA library of macrophages alternatively activated by interleukin (IL)-4 and glucocorticoids (GC) and nominated DC-chemokine 1 (DC-CK1) and alternative macrophage activation-associated CC chemokine-1 (AMAC-1), respectively [7, 8]. Furthermore, Guan et al. [9] isolated, through exon trapping of genomic fragments close to the CCL3 gene, the gene for a novel CCL3-like chemokine, which corresponded to MIP-4. Following the new systematical chemokine nomenclature, PARC/MIP-4/DC-CK1/AMAC-1 has been renamed CCL18 [2].
STRUCTURE OF CCL18 GENE
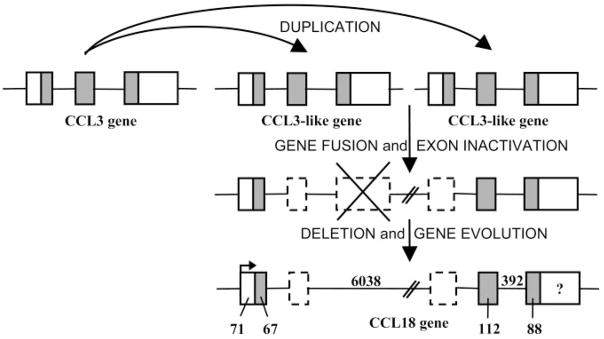
The CCL18 gene was mapped to the major human CC chemokine gene cluster at chromosome 17q11.2, most closely (16 kb) to the CCL3 gene within the subcluster, where other genes of the MIP family of chemokines (e.g., CCL4) are located [5, 9, 10]. The CCL18 gene is featured by the three-exon/two-intron structure conserved among most CC chemokines [9–11]. However, the CCL18 gene is extremely long (7.2 kb vs. general size of 2.0–3.0 kb) as a result of the length of the first intron (6.0 kb), which contains two pseudoexons (Fig. 1). This characteristic, together with the presence of two subsequent regions within the CCL18 gene sharing high-sequence similarity with the CCL3 gene, suggests that the CCL18 gene may have been generated by fusion of two CCL3-like genes with deletion and selective use of some exons (Fig. 1) [10]. Base changes before and after the fusion event might have adapted the gene to a new function.
Fig. 1.
Putative generation mechanism and structure of the human CCL18 gene. It is likely that a twofold duplication of the CCL3 gene led to the generation of two CCL3-like genes. The fusion of the latter in combination with the inactivation of some exons (possibly by base changes around former exon-intron borders) and the deletion of other sequences (marked by a cross) might have created a new transcription unit, i.e., the CCL18 gene [10, 11]. The consensus genomic organization of CCL18 depicted here is composed on the basis of different reports [5, 7–11]. Dotted boxes indicate the pseudoexons, whereas the open and shaded boxes represent the untranslated and translated parts from the actively used exons, respectively. The intron sequences are indicated as horizontal lines between the used exons. Lengths of sequences are in base pairs (bp). The length of the 3′-untranslated region of the CCL18 gene (marked by “?”) varies between 430 bp and 442 bp according to the clones isolated [5, 7–11]. Tasaki et al. [10] demonstrated the transcription-initiation site (arrow) presented here.
As mice appear to contain fewer chemokine genes than humans (e.g., only one murine CCL3 gene in contrast to the existence of several human CCL3-like genes), the gene duplications potentially leading to the CCL18 gene and other CCL3-like genes are likely to have occurred after the diversification of rodents and primates. Indeed, no CCL18 homologue has been found in rodents so far. However, cDNA derived from primate lungs has been shown to hybridize with human CCL18 cDNA present on a microarray, and reverse transcriptase-polymerase chain reaction (RT-PCR)-amplified products were obtained from monkey pulmonary tissue with the use of human CCL18-specific primers [12]. These findings suggested the existence of a primate counterpart for human CCL18. Recently, rhesus macaque CCL18 was cloned and displayed 90% amino acid sequence identity relative to its human homologue [13].
In the promoter region, the sequences 5′ upstream from the TATA box of the CCL3 gene are not well-conserved in the CCL18 gene, which could explain in part a differential expression pattern of the two chemokines [10]. Further analysis revealed the presence of a combined signal transducer and activator of transcription-1 (STAT1)/STAT6-binding element in this part of the CCL18 gene, suggestive for a competitive regulation of CCL18 expression by the interferon-γ (IFN-γ)-activated STAT1 and IL-4-induced STAT6 transcription factors [11]. In addition, the CCL18 promoter contains two putative activator protein-1 and CAAT/enhancer-binding protein (C-EBP) regulatory elements. It has been demonstrated that transcriptional activation of IL-4-dependent genes requires binding of STAT6 and C-EBP to their respective binding elements. The 3′ noncoding region of the CCL18 gene seems to contain nonconsensus polyadenylation signals (TATAAA or AATATA) instead of the canonical AATAAA motif [5, 7]. Moreover, it lacks the AT-rich mRNA destabilization signals.
STRUCTURE OF CCL18 PROTEIN
CCL18 contains an open-reading frame encoding a polypeptide of 89 amino acids. Cleavage of the proposed NH2-terminal hydrophobic signal sequence (20 residues) could give rise to a mature protein of 69 amino acids, lacking a putative N-glycosylation site, with a calculated molecular weight of 7851.2 and an isoelectric point of 9.21 (Table 1) [5, 7–9]. The use of the predicted cleavage site was confirmed by the fact that the sequence of purified recombinant CCL18, produced in insect or COS cells, started with the suggested Ala residue (position 21 of the precursor protein) [5, 7]. Isolation of natural human CCL18 from various sources revealed the occurrence of processed CCL18 isoforms in addition to unglycosylated, intact CCL18(1–69) [14–16]. Indeed, CCL18(1–68), lacking the COOH-terminal Ala, was copurified with CCL18(1–69) from human plasma from healthy donors and from ascitic fluid from ovarian carcinoma patients, whereas the NH2-terminally truncated isoforms CCL18(3–69) and CCL18(4–69) were isolated together with intact CCL18 from the conditioned medium from stimulated peripheral blood mononuclear cells (PBMC) [14–16].
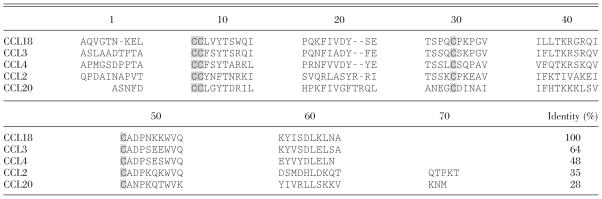
TABLE 1.
Amino Acid Alignment of Mature CCL18 with Other CC Chemokines
|
The amino acid sequence of CCL18 is compared with the primary structure of some other mature human CC chemokines. The four characteristic Cys are marked with gray boxes. Hyphens are inserted to maximize the identity score. The percentage identical amino acids between CCL18 and these other CC chemokines is indicated in the lower right.
The mature CCL18 protein is most closely related to CCL3 (64% identical amino acids) and CCL4 (48%; Table 1). Sequence alignment and computer-assisted, three-dimensional modeling (based on high homology with CCL4) of the CCL18 protein revealed the typical structural characteristics of CC chemokines, including the presence of four positionally conserved Cys residues and a rigid core consisting of a short helical turn followed by a three-stranded, antiparallel β-sheet and a COOH-terminal α-helix. In contrast, the flexible NH2-terminal region preceding the CC motif is considerably different in CCL18 in comparison with other mature CC chemokines [8]. This domain is likely involved in the second phase of the hypothesized “two-step mechanism of chemokine receptor activation”, namely in triggering receptor signaling [17]. However, the region centered around Tyr at position 27 in mature CCL18 is highly conserved among some other CC chemokines (e.g., CCL3, CCL4) and is suggested to play a role in the initial binding of the ligand to its receptor, proposed to be the first step in the activation of chemokine receptors [8, 18].
IN VITRO BIOLOGICAL ACTIVITIES AND RECEPTOR USE OF CCL18
So far, CCL18 is known to trigger a biological response in vitro in T cells, B cells, DC, hematopoietic progenitor cells, fibroblasts, and potentially, in monocytes/macrophages but not in neutrophils (Table 2). The concentrations of CCL18 applied in these reports varied between 0.1 ng/ml and 1000 ng/ml. Helper T cells and cytotoxic T cells showed a similar responsiveness to CCL18, whereas primarily naïve T lymphocytes responded to CCL18 instead of memory T lymphocytes [7, 9]. However, CCL18 also clearly bound 5–10% of skin-homing (CLA+) peripheral blood memory T cells and CLA+ memory T cell lines and directed the migration of the latter in vitro [19]. In addition, Lindhout et al. [20] claimed that CCL18 preferentially attracted naïve or so-called mantle zone B lymphocytes above germinal center B cells. Recently, immature monocyte-derived DC have also been shown to migrate toward CCL18, in contrast to the lack of responsiveness of mature DC [21]. Moreover, CCL18 stimulated collagen production in lung fibroblasts through activation of the extracellular signal-regulated kinase (ERK) pathway [23] and suppressed the proliferation of hematopoietic progenitors [22].
TABLE 2.
In Vitro Biological Activities of CCL18 on Different Human Cell Types
| Cell type | Activity | References |
|---|---|---|
| T lymphocytesa | ||
| T lymphocytes | chemotaxis | [5, 7, 15] |
| calcium | [9] | |
| Helper T lymphocytes (CD4+) | chemotaxis | [7] |
| calcium | [9] | |
| Cytotoxic T lymphocytes (CD8+) | chemotaxis | [7] |
| calcium | [9] | |
| Naïve T lymphocytes (CD45RA+) | chemotaxis | [7] |
| calcium | [9] | |
| Skin-homing memory T lymphocytic cells (CD45RO+, CLA+) | chemotaxis | [19] |
| B lymphocytesa | ||
| B lymphocytes | chemotaxis | [20] |
| Naïve B lymphocytes (CD38−, IgG−) | chemotaxis | [20] |
| Germinal center B lymphocytes (CD39−, IgD−) | chemotaxis | [20] |
| Monocyte-derived immature DCb | chemotaxis | [21] |
| Hematopoietic progenitor cells | inhibition proliferationc | [22] |
| Lung fibroblasts | collagen production | [23] |
T cells and B cells are freshly isolated from peripheral blood or tonsils, respectively, except for skin-homing memory T lymphocytic cells, which are atopic, dermatitis-derived T cell lines.
Immature DC were generated by culturing monocytes for 6 days in the presence of granulocyte macrophage-colony stimulating factor (GM-CSF) and IL-13.
Capacity to inhibit in colony formation assays the proliferation of normal bone marrow-derived hematopoietic progenitor cells {i.e., GM progenitors [colony-forming unit (CFU)-GM], erythroid progenitors [burst-forming unit-erythroid (BFU-E)], and multipotential progenitors [CFU-granulocyte-erythroid-monocyte-megakaryocyte (GEMM)]} in response to multiple growth factors. CLA+, Cutaneous lymphocyte-associated antigen; IgG, immunoglobulin G.
The discrepancy in the NH2-terminal sequence preceding the CC motif between CCL18 and CCL3 could at least partly explain their different spectrum of target cells and receptor selectivity. It is remarkable that no agonistic receptor for CCL18 has been discovered so far. The ability of pertussis toxin to abolish CCL18-responsive T cell and B cell chemotaxis as well as ERK activation in fibroblasts nevertheless points to the involvement of Gαi proteins and G protein-coupled receptors in mediating its biological activity [7, 20, 23]. Hieshima et al. [5] demonstrated the specific binding of CCL18 to freshly isolated T lymphocytes, which was not inhibited in the presence of CCL2, CCL3, CCL4, or CCL5. These data suggested that CCL18 does not share receptors [e.g., CC chemokine receptor 1 (CCR1), CCR2, CCR3, or CCR5] with these chemokines. However, Nibbs et al. [24] showed that CCL18 could significantly inhibit CCR3-mediated eosinophil chemotaxis and calcium mobilization at physiologically relevant concentrations (as low as 100 ng/ml). As CCL18 antagonized the G protein coupling of CCR3 in the presence of CCR3 ligands but only marginally affected the basal G protein coupling in CCR3 transfectants, CCL18 is considered a neutral CCR3 antagonist [25]. Alteration of the extreme NH2-terminal residue of CCL18 from Ala to Met led to a more potent CCR3 antagonistic activity, whereas small changes at the COOH-terminus did not influence this activity [24]. Indeed, the impact of minor modifications of proteins on their biological activity can be drastic, especially for NH2-terminally processed chemokines [26]. In this context, it has to be determined whether intact CCL18(1–69) can also activate monocytes/macrophages, as Schraufstatter et al. [27] observed calcium mobilization, directed migration, and actin polymerization in monocytes/macrophages kept in culture for 3–4 days (but not in freshly isolated monocytes) in response to a recombinant CCL18 form having four extra amino acids at the NH2-terminal part. Moreover, the chromatographical inseparability of the different naturally occurring CCL18 isoforms (see above) impeded the screening for changes in biological activity as a result of the observed NH2- or COOH-terminal cleavage [14–16]. Nevertheless, the natural mixture of CCL18(1–69) and CCL18(1–68), as isolated from ovarian carcinoma ascitic fluid in a 3:1 ratio, significantly attracted freshly isolated T cells and immature DC [15, 21].
Injection of CCL18 into human skin-transplanted severe combined immunodeficiency mice induced the dermal recruitment of human skin-homing memory T cells after their intravenous administration [19]. Furthermore, injection of synthetic CCL18 into the peritoneal cavity of mice resulted in the in vivo accumulation of CD4+ and CD8+ T lymphocytes but not monocytes or granulocytes 24 h later [9]. This chemoattraction in vivo confirmed some of the findings obtained in vitro and indicated that unlike the ligand itself, the human agonistic receptor for CCL18 might have a murine counterpart. This was corroborated by the in vitro migratory capacity of murine B and T lymphocytes, preferentially with a naïve phenotype, toward human CCL18 [28].
CCL18 EXPRESSION
With the use of multitissue Northern blot filters, CCL18 mRNA was initially shown to be constitutively expressed at high levels in lung and at low levels in some lymphoid tissues such as lymph nodes, thymus, and appendix [5]. In situ hybridization demonstrated CCL18 transcription in some alveolar macrophages in the lung and in DC in the T cell areas and germinal centers of lymph nodes and inflamed tonsils [5, 7]. Gene-expression profiling of the tonsillar B cell compartments clarified that CCL18 expression was up-regulated in the germinal centers compared with the mantle zones, where naïve B cells reside [29]. Immunohistochemical stainings revealed the constitutive production of CCL18 protein in some cells in the periarteriolar lymphatic sheets in spleen and in the T cell areas of lymph nodes [20]. More abundant expression of CCL18 protein was detected in the T cell areas and germinal centers of inflamed tonsils [20]. The germinal center-located CCL18-expressing cells did not correspond to tingible body macrophages or follicular DC and most likely represented the so-called “germinal center DC”, to which a function in B cell proliferation, isotype switching, and antibody production has been ascribed [20, 30].
The spectrum of cellular sources for CCL18 was confirmed in vitro to be mainly restricted to leukocytes, in particular, monocytic cells and DC (Table 3). Monocytes/macrophages were shown to constitutively express only low levels of CCL18 [27, 31, 32], but the CCL18 production could be up-regulated in these cells by the classic macrophage activator LPS [5, 27, 31, 33, 34] as well as by other microbial compounds (peptidoglycan and mannan) and by the T cell-derived activation signal CD40-L [34]. Other reports claimed that CCL18 induction required an alternative activation of the monocytes/macrophages by T helper cell type 2 (Th2)-related cytokines, such as IL-4, IL-10, or IL-13, or by GC [7, 8, 32, 35]. Furthermore, this IL-4 induced CCL18 transcription was inhibited in the presence of the Th1-associated cytokine IFN-γ [8]. Alternatively activated macrophages, for which the immunosuppressive alveolar and placental macrophages make good in vivo examples in normal individuals, are generally polarized toward a high capacity for endocytic clearance, a reduced proinflammatory cytokine secretion, and an enhanced release of anti-inflammatory mediators [44–46]. Different environmental stimuli (e.g., IL-4/IL-13 vs. IL-10) are believed to elicit distinct functional phenotypes of alternatively activated macrophages, resulting in their active participation in humoral immunity, anti-inflammatory processes, tolerance induction, and/or tissue repair, partly through the suppression of Th1-mediated immune responses [44–46]. Northern blot analysis of isolated alveolar macrophages from healthy persons, smokers, and asthmatic patients corroborated the aforementioned in vivo expression of CCL18 mRNA in these cells [5, 8]. In addition, blockade of the costimulatory pathway B7/CD28 in mixed lymphocyte reaction cultures led to the generation of IL-10-producing, anergic T cells and of alternatively activated macrophages [47]. The latter expressed CCL18 transcripts and suppressed T cell responses in vitro. These results strengthened the view that alternatively activated macrophages likely contribute to the induction of transplantation tolerance in vivo [47].
TABLE 3.
Different Cellular Sources and Regulators of Human CCL18 Production In Vitro
| Cell type | Expressiona | References | |
|---|---|---|---|
| Normal cells | |||
| Monocytes/macrophages | Constitutiveb,c | [27, 31, 32] | |
| + | LPSc,d; Streptococcus pyogenese; peptidoglycanc,d; mannanc,d; CD40-Lc,d | [5, 27, 31, 33, 34] | |
| +f | IL-4; IL-10; IL-13; GC; IL-4 & GC; IL-4 & TNF-α; IL-4 & GC & TNF-α | [7, 8, 32, 35] | |
| +g,h | IL-4/Tc; SEA/Tc; IL-4/Tc CM | [16] | |
| − | IL-4/IFN-γ; IL-4/GC/IFN-γ | [8] | |
| Alveolar macrophages | Constitutive | [8] | |
| PBMC | Spontaneousc | [14, 34] | |
| + | IL-4g; SEAg; SEBc | [14, 34] | |
| DC (in vitro-derived)i | Constitutive (immature DC)c | [7, 8, 21, 31, 34–38] | |
| +j | Maturation signals (LPSc; TNF-αc; FcγR triggering; FcγR triggering/LPSc; monocyte CM/TNF-α/PGE2) | [31, 37–40] | |
| −j | Maturation signals (LPSc; TNF-αc; CD40-Lc; IFN-γg; SACg; Candida albicansg; influenza virusg; LPS/GCg; LPS/PGE2g LPS/IL-10g; LPS/VitD3g; LPS/IFN-γg; CD40-L/GCg; CD40-L/PGE2g) | [8, 21, 36] | |
| + | IL-4; IL-10c; IL-13; IL-4/GC; VitD3g | [21, 35] | |
| − | GCg; PGE2g | [21] | |
| Langerhans-type DCk | Constitutivec | [34] | |
| + | Peptidoglycanc | [34] | |
| Ex vivo germinal center DC | Constitutivec | [20] | |
| Ex vivo myeloid blood DC | + | Maturation signals (monocyte CM) | [37] |
| + | IL-10g | [21] | |
| Eosinophils | Constitutivec,l | [27] | |
| Chondrocytes | Constitutiveg | [41] | |
| Dermal fibroblasts | + | IFN-γ; IL-4 | [34] |
| Keratinocytes | + | IFN-γ | [34] |
| Tumor cells | |||
| Myeloblastic leukemia cells (KG1) | Constitutive | [42] | |
| + | PMA | [42] | |
| − | PMA/TNF-α | [42] | |
| Myelogenous leukemia cells (K562) | + | PMA | [5] |
| Monocytic leukemia cells (THP-1) | + | PMA/IL-4 | [8] |
| + | Mycobacterium tuberculosis | [43] | |
| Monocytic leukemia cells (U937) | + | PMA | [5] |
LPS, Lipo polysaccharide; CD40-L, CD40 ligand; TNF-α, tumor necrosis factor α; Tc, T lymphocytic cell; SEA or SEB, Staphylococcus aureus enterotoxin A or B; CM, conditioned medium; FcγR, receptor for Fc fragment of IgG; PGE2, prostaglandin E2; SAC, S. aureus Cowan 1; VitD3, vitamin D3; PMA, phorbol 12-myristate 13-acetate.
CCL18 was expressed constitutively or induced (+) upon addition of one stimulator or a combination of substances, separated by “/” in the table. In some cases, the latter led to a synergistic induction, marked by “&” between the two substances. Addition of some regulators reduced CCL18 expression or inhibited the stimulating effect of otherwise inducing agents, in both cases indicated by “−”. Generally, solely CCL18 mRNA expression was investigated, unless indicated differently (seec and g).
Both mRNA and protein expression of CCL18 were demonstrated.
After 6–30 h of incubation of monocytes/macrophages with the inducer.
After 2–24 h S. pyogenes stimulation of monocytes/macrophages, previously cultured for 7 days without stimulators.
Generally after 3–6 days of incubation of monocytes/macrophages with regulators.
Only CCL18 protein release was investigated by enzyme-linked immunosorbent assay (ELISA) or in case of chondrocytes, by antibody microarrays.
Enterotoxin- or IL-4-stimulated adherent monocytes/macrophages were cocultured for 48 h with T lymphocytic cells (lymphocytes or cultured T lymphoblastic cells) or with conditioned medium of cultured T lymphoblastic cells.
Generally, immature DC were derived from monocytes cultured for 6–7 days in the presence of GM-CSF and IL-4, although constitutive CCL18 transcription was already detected after 3 days. Alternatively, other combinations [GM-CSF/IL-13 or fms-like tyrosine kinase 3 (Flt3) ligand/IL-4] have been used [21, 36]. Optionally, the immature DC were incubated for an additional 2–3 days with different regulators.
Contradictory results were obtained.
Langerhans-type DC were generated in vitro by culturing monocytes for 6 days in the presence of GM-CSF, IL-4, and transforming growth factor (TGF)-β, to which TNF-α was added during the last 2 days.
Eosinophils from donors with mild eosinophilia, cultured for 16 h in the presence of IL-5 and GM-CSF to ensure cell survival.
Our ELISA measurements demonstrated that CCL18 protein was spontaneously secreted by PBMC after 48 h and that its release was enhanced selectively by staphylococcal enterotoxins as well as by IL-4 [14]. Pivarcsi et al. [34] recently comfirmed at the transcriptional level the superantigen-regulated CCL18 production by PBMC. Double immunohistochemistry indicated that CD68+ monocytes/macrophages, rather than CD1a+ blood DC, were the major CCL18-producing cells in enterotoxin-induced PBMC [14]. Furthermore, the CCL18 release by enterotoxin- or IL-4-stimulated adherent monocytes/macrophages was enhanced significantly in the presence of lymphocytes, cultured T lymphoblastoid cells, or T cell-conditioned medium [16]. This points clearly to a role for T cell-derived factors in regulating CCL18 production in monocytes/macrophages. Together, these findings also confirm that the expression patterns of CCL18 and the structurally related CCL3 highly diverge, as suggested by their different promoter regions. CCL3 was, for instance, induced in monocytes/macrophages by LPS, but its production was inhibited by IL-4, IL-10, IL-13, and GC [5, 8, 48, 49]. It can be concluded that CCL18 is induced in a broad spectrum of monocyte/macrophage subsets, ranging from classically to alternatively activated macrophages—the latter ones likely assisting in Th2-mediated and/or immunosuppressive responses.
In vitro-generated, immature DC, generally derived from monocytes in the presence of GM-CSF and IL-4, also constitutively expressed CCL18 mRNA and protein (Table 3). Together with CXC chemokine ligand 8 (CXCL8), CCL3, CCL17, CCL19, and CCL22, CCL18 represented, in fact, one of the most abundantly produced chemokines by these cells [21, 34, 37, 40]. However, it is still a matter of debate whether maturation of the immature DC causes up-regulation [31, 37–40] or rather down-modulation [8, 21, 36] of CCL18 expression. It is interesting that immature DC might reach different activation/maturation stages depending on the stimulatory agents within the microenvironment. Signals such as LPS, CD40-L, and FcR triggering are believed to induce full maturation of DC, allowing efficient T cell priming. In contrast, activated DC (e.g., by TNF-α in the absence of pathogens), which are characterized by moderate expression of costimulatory molecules but only low release of proinflammatory cytokines (such as TNF-α, IL-6, and IL-12), have been defined recently as semimature DC, which can likely generate tolerance [50]. Maturation of DC in the presence of the T cell-derived signal CD40-L did not affect or rather strongly down-regulated CCL18 expression [21, 34, 36, 37], whereas enhanced [31, 37, 38, 40] and diminished [8, 21, 36] expression was contradictorily obtained upon TNF-α or LPS treatment of immature DC. As for CD40-L, LPS, and TNF-α, Vulcano et al. [21] also noticed reduced CCL18 release upon culturing of immature DC in the presence of other pathogen-derived, DC-maturing agents such as S. aureus Cowan I, C. albicans, and influenza virus. However, CCL18 production by immature or LPS- or CD40-L-matured DC was not affected by blocking endogenous TNF-α [21]. In contrast, Van Lieshout et al. [51] claimed that inhibition of endogenous TNF-α during LPS-mediated maturation of monocyte-derived DC generated semimature DC, which expressed lower CCL18 mRNA levels compared with cells fully matured with LPS. It is remarkable that alternative activation of immature DC by prolonged culturing in the presence of the Th2 cytokines IL-4, IL-10, or IL-13 resulted in an increase in CCL18 transcription [35], whereas IFN-γ, a Th1-derived costimulator of DC functions, suppressed CCL18 production [21]. In parallel with IL-10, VitD3 also up-regulated CCL18 release, although two other inhibitors of DC differentiation and function, namely the GC dexametasone and PGE2, surprisingly decreased CCL18 secretion [21].
Langerhans-type DC, derived in vitro from monocytes in the presence of GM-CSF and IL-4 as well as TGF-β and TNF-α, also constitutively produced large amounts of CCL18, albeit less than the previously mentioned, immature DC, generated in vitro with GM-CSF and IL-4 only [34]. In contrast, ex vivo-isolated Langerhans cells did not express CCL18, even after in vitro maturation [35, 37]. Although no immunoreactivity could be measured by ELISA, low levels of CCL18 mRNA were detected in myeloid CD11c+ blood DC upon in vitro maturation with monocyte-conditioned medium [37]. Other reports described a lack of CCL18 secretion by myeloid and plasmacytoid blood DC, even after stimulation [52], unless the myeloid blood DC were treated with IL-10 [21]. However, CCL18 mRNA and protein were expressed by freshly isolated germinal center DC [20]. The heterogeneity of DC in terms of origin, morphology, phenotype, activation stage, and function, in vitro and in vivo, suggests that further exploration of the different DC populations might help clarify the expression pattern and role of CCL18 in vivo and vice versa.
Compared with the more abundant CCL18 production by in vitro-generated DC and stimulated monocytes and PBMC, lower CCL18 expression levels were also detected in activated keratinocytes and dermal fibroblasts [34] as well as in eosinophils [27] and normal chondrocytes [41].
POTENTIAL ROLE OF CCL18 IN HOMEOSTASIS AND PATHOLOGY
CCL18 under normal and inflammatory conditions
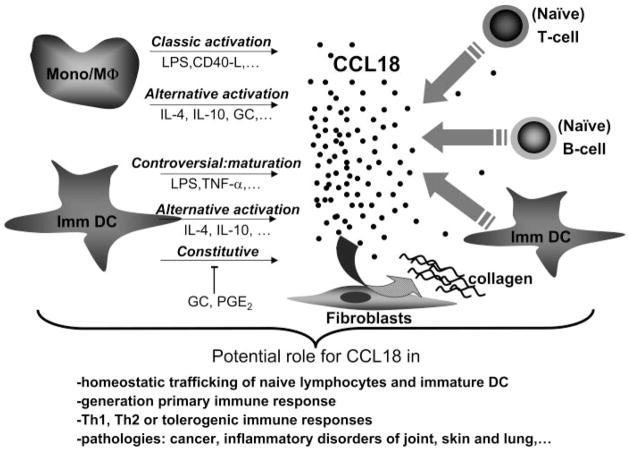
Collectively, the in vivo and in vitro findings concerning the spectrum of target and producer cells of CCL18 (Tables 2 and 3) suggest that this chemokine could participate in the homing of lymphocytes and DC to microanatomical niches of the secondary lymphoid organs. Under steady-state or homeostatic conditions, CCL18-expressing, interdigitating DC in the T cell areas could recruit naïve T cells and perhaps additional immature/semimature DC and hence, induce tolerance, likely in part through the generation of IL-10-producing regulatory T cells [53, 54]. Within a severe inflammatory context, the CCL18-mediated attraction of naïve T cells toward the fully matured, interdigitating DC could assist in mounting a primary immune response [1, 3, 55–57]. These CCL18-producing cells could also attract naïve B cells to the edge of the T cell zones to bring them in closer vicinity of potential antigen and of effector Th2 cells. It is not excluded that the primary activation of the B cells takes place there in a tricellular complex (DC, B cell, and T cell). Furthermore, some of the recently activated B cells might, in response to CCL18, migrate toward the germinal center DC, likely involved in the B cell proliferation and plasma cell differentiation in the germinal centers [1, 3, 55–59].
The data summarized in Tables 2 and 3 also indicate that CCL18 could act in the periphery as a constitutively produced or inducible chemokine to attract lymphocytes, immature DC, or even monocytes toward various sites where (classically or alternatively activated) macrophages and (immature or mature) DC participate in Th1- or Th2-mediated or especially tolerogenic immune responses, e.g., to combat infections, induce wound healing, or counterbalance excess immunological reactions. Moreover, the CCR3 antagonistic activity of CCL18 may limit the recruitment of eosinophils, basophils, and the CCR3-expressing subset of Th2 cells and hence, dampen a local proallergic response [24, 60].
The involvement of CCL18 in the generation of a primary T cell response was recently evidenced in vivo by the revelation of its adjuvant activity during vaccinations requiring strong cell-mediated immunity [28]. The coadministration of CCL18 with malaria vaccines subcutaneously (s.c.) in mice resulted in increased numbers of IFN-γ-secreting, malaria-specific CD8+ T cells and eventually, in enhanced protection against malaria but failed to augment the humoral response. Bruna-Romero et al. [28] argued that CCL18 could exert its adjuvant activity, after its fast draining toward the local lymph nodes, by attracting naïve murine lymphocytes. It is interesting that the enhanced antigen-specific CD8+ T cell responses were not obtained in case the immunogen and CCL18 were injected by a different route or at a different time-point [28]. Therefore, the recently observed chemotactic capacity of CCL18 for immature DC [21] could suggest that the spatial and temporal coadministration of CCL18 and the malaria vaccine might promote the antigen uptake by peripheral immature DC at the site of injection [28]. This could ensure a higher mobilization of antigen-loaded DC toward the draining lymph nodes and might be an additional explanation for the adjuvant activity of CCL18.
It is interesting that rather high basal levels of CCL18 immunoreactivity were consistently detected in normal human plasma (~20 ng/ml) [16]. Therefore, CCL18 seems to join CCL14, CCL16, CXCL4, and CXCL7 on the list of constitutive plasma chemokines [61–64]. Furthermore, the presence of CCL18 has also been demonstrated in several diseases, where the beneficial recruitment of leukocytes often was out of control (Table 4).
TABLE 4.
Involvement of CCL18 in Human Pathology
| Disease | References |
|---|---|
| Acute lymphoblastic leukemia (T-ALL, prepreB-ALL) | [16] |
| Allergic contact hypersensitivity | [65] |
| Atopic dermatitis | [19, 34, 66] |
| Atherosclerosis | [33] |
| Chorioamnionitis (preterm labor) | [67] |
| Chronic hepatitis C infection | [68] |
| Gastric cancer | [69] |
| Gaucher disease | [70–72] |
| Giant cell arteritis | [73] |
| Hypersensitivity pneumonitis | [74] |
| Idiopathic pulmonary fibrosis | [74] |
| Ovarian carcinoma | [15] |
| Rheumatoid arthritis | [14, 40] |
| Scleroderma with lung inflammation | [75] |
| Septic arthritis | [14] |
| Sjogren’s syndrome | [76] |
| Tuberculosis | [77] |
| Vernal keratoconjunctivitis | [78] |
Ovarian cancer and CCL18
Ascitic fluids from ovarian carcinoma patients contained significantly higher CCL18 levels than ascitic fluids originating from diseases other than ovarian carcinoma (120 ng/ml vs. 44 ng/ml) [15]. Furthermore, CCL18 was more abundantly present in ovarian carcinoma ascites than CCL2, CCL3, CCL7, CCL20, and CXCL8 and was proven to be biologically active upon isolation. CCL18 release was not inducible in ovarian and other carcinoma cell lines in vitro. Immunohistochemical staining demonstrated CCL18 expression in tumor-infiltrating cells with monocyte/macrophage morphology but not in the ovarian carcinoma cells [15]. It is interesting that IL-10-producing immunosuppressive macrophages have been detected in ovarian carcinoma tissue and ascitic fluid [79, 80], and these tumor-associated macrophages could correspond to alternatively activated macrophages that may at least partly be responsible for the elevated CCL18 levels. Furthermore, as for many tumor-associated DC, most DC in ascites from patients with peritoneal carcinomatosis were rather immature and inadequate in antigen presentation [81, 82]. Despite the recent report that ovarian tumors are mainly infiltrated by plasmacytoid DC [83] and that this DC subset in the blood from normal subjects lacked CCL18 expression [52], it cannot be excluded that these tumor-associated DC represent an additional source of ascitic CCL18. Hence, CCL18 could be involved in the immunosuppression of a host antitumor response by attracting tumor-infiltrating lymphocytes and additional immature DC toward tolerogenic or suppressive macrophages or DC. In this respect, it has to be awaited whether CCL18 could assist the less abundant ascitic CCL22 and CXCL12 in their tumor-promoting recruitment of regulatory T cells and plasmacytoid DC toward the ovarian carcinoma environment, respectively [83–85].
Gastric cancer and CCL18
Nevertheless, a tumor-suppressing role for CCL18 can certainly not be ruled out. Microarray and quantitative RT-PCR analysis of gastric cancer tissue, for instance, revealed that high CCL18 levels were associated with prolonged survival of gastric cancer patients, independently of tumor stage [69]. Immunohistochemistry and in situ hybridization indicated that CCL18 was expressed by a subpopulation of tumor-associated macrophages that were preferentially located at the tumor invasion front but not by neoplastic or non-neoplastic gastric mucosal cells. Much lower levels of CCL18 expression and CCL18+ macrophages were observed in nontumor mucosa with gastritis. In contrast to the abundance of CCL18+ macrophages, the total number of CD68+ macrophages was not a good prognostic marker for longer survival of the gastric cancer patients [69]. These data suggest a role for CCL18 in the generation of a more efficient anti-tumor response as a result of the attraction and activation of specific immune cells.
Leukemia and CCL18
Children with specific types of acute lymphoblastic leukemia (i.e., T-ALL and prepreB-ALL) were characterized by increased CCL18 serum levels, whereas serum CCL18 levels in acute myeloid leukemia and preB-ALL pediatric patients did not differ significantly from those in control serum samples [16]. In contrast, the serum concentration of another constitutive plasma chemokine, namely CCL14, did not rise above the basal control serum levels in any of these groups of patients. Furthermore, the bone marrow CCL18 levels in leukemic patients were similar to those in serum, and their spinal fluid was devoid of any CCL18. Although lymphocytes or lymphoblastic cells alone could not be triggered to secrete CCL18, coculture of these cells or their conditioned medium with superantigen or IL-4-stimulated monocytes led to significant CCL18 release [16].
Gaucher disease and CCL18
The plasma CCL18 levels were also markedly elevated in patients with Gaucher disease, a lysosomal storage disorder, characterized by the pathologic accumulation of glycolipids in macrophages [71]. It is accompanied by a sustained inflammatory reaction, which could contribute to the observed massive enlargement and/or tissue injury of some organs (e.g., liver and spleen). Subtractive hybridization and Northern blot analysis revealed enhanced CCL18 transcription in Gaucher spleen tissue compared with normal spleen [70]. Immunohistochemistry demonstrated that the glycolipid-loaded macrophages or Gaucher cells were the prominent source of CCL18 in Gaucher spleen tissue and that these cells mostly resemble alternatively activated macrophages, despite their lack of mannose receptor expression [71, 72]. Furthermore, the plasma levels of CCL18 in Gaucher patients proved to be a reliable disease marker to monitor the therapeutic efficacy [71].
Arthritis and CCL18
Synovial fluids represent another type of body fluid in which enhanced CCL18 levels have been discovered under certain pathological conditions. Synovial fluids from septic arthritis and rheumatoid arthritis (RA) patients contained significantly higher CCL18 levels than those from osteoarthritis and crystal-induced arthritis patients (140 ng/ml, 190 ng/ml, 34 ng/ml, and 38 ng/ml, respectively) [14]. In at least 50% of the analyzed septic arthritis patients, the inflammatory joint disease was caused by S. aureus. This pointed clearly to the pathological relevance of the in vitro observation of enterotoxin-mediated CCL18 release by PBMC, especially by CD68+ monocytes/macrophages [14]. Recently, antibody microarrays demonstrated the secretion of CCL18 by normal human chondrocytes [41], although we could not detect any CCL18 protein release by chondrocytes, fibroblasts, or endothelial cells by means of ELISA [14]. Furthermore, immunohistochemistry revealed a lack of CCL18-expressing cells in normal synovial biopsies, whereas many large, irregular, tissue-infiltrating cells stained positive for CCL18 in synovia affected with RA, part of which coexpressed CD68 [14]. Radstake et al. [40] confirmed the elevated CCL18 levels in synovial fluids and tissues from RA patients compared with osteoarthritis patients and healthy subjects. They observed high CCL18 expression in the perivascular regions of RA synovia, partly overlapping with the staining pattern for the mature DC marker DC-lysosome-associated membrane protein. Furthermore, higher CCL18 mRNA and protein amounts were detected in immature and LPS-matured DC upon in vitro generation from monocytes isolated from RA patients instead of from osteoarthritis patients or normal individuals. Whereas FcγR triggering further up-regulated CCL18 expression during LPS-induced maturation of normal monocyte-derived DC, down-regulation of the CCL18 expression was obtained in case the monocytes originally belonged to RA patients [40]. It can at present only be speculated whether CCL18 has pro- or anti-inflammatory properties in septic arthritis and RA. Indeed, CCL18 could contribute to the acute onset of septic arthritis by the chemoattraction of lymphocytes or by exerting other, still unknown activities, such as the stimulation of protease release. In RA, this DC-, B cell-, and T cell-agonistic chemokine could assist in the organization of the characteristic, perivascular lymphocytic aggregates, sometimes containing germinal center-like structures. These aggregates resemble secondary lymphoid follicles and presumably contribute to the pathogenesis of this chronic type of arthritis [86, 87]. However, the presence of CCL18 could as well point to an attempt of the immune system to dampen the synovitis, especially in RA. Indeed, alternatively activated macrophages have been demonstrated in RA synovium [44]. In addition, most of the DC in the inflamed synovial fluid or tissue, except for those present in the perivascular lymphocytic aggregates, are rather immature and express low levels of costimulatory molecules [88–91]. Hence, the possible CCL18 production by alternatively activated macrophages and immature DC and the directed attraction of naïve T cells could help the immune system in its endeavor to suppress Th1-mediated immune responses and to induce tolerance [44, 89]. Nevertheless, the majority of the RA synovial T lymphocytes seems to correspond to effector/memory Th1 cells, which markedly express CXC chemokine receptor 3 and CCR5 [86, 87, 92, 93]. By means of its CCR3 antagonistic activity, CCL18 could also inhibit the recruitment of CCR3-presenting cells [24].
Sjögren’s syndrome and CCL18
It is interesting that CCL18 expression has also been associated with other pathologies marked by the presence of lymphocytic structures, such as Sjögren’s syndrome, chronic hepatitis C infection, and giant cell arteritis. Sjögren’s syndrome is a chronic, autoimmune disease characterized by the progressive destruction and diminished secretory capacity of the salivary and lachrymal glands and by a massive infiltration of mononuclear cells in the gland lesions. RT-PCR analysis confined CCL18 expression to salivary glands of Sjögren’s syndrome patients but not to those of control patients or patients with other autoimmune disorders [76]. In situ hybridization demonstrated that the cells responsible for the CCL18 transcription in Sjögren’s syndrome glands belonged to the infiltrating mononuclear cells and that they correspond to cells resembling DC or surprisingly, B lymphocytes [76].
Hepatitis C and CCL18
Chronic hepatitis C infection is accompanied by inflammation of the portal and periportal areas of the liver. Hepatic CCL18 mRNA levels from patients with chronic hepatitis C infection, measured by quantitative RT-PCR analysis, correlated significantly with the serum alanine aminotransferase levels, which reflect hepatocyte death [68]. In addition, in situ hybridization revealed CCL18 expression by mononuclear cells in the portal area of livers chronically infected with hepatitis C but not in normal livers. It is interesting that this is the region of the liver where naïve T cells were predominantly located and where lymphocytic aggregates similar to primary lymphoid follicles in lymph nodes are characteristically present in case of hepatitis C infection. In contrast, effector/memory T cells were mainly found in the periportal areas at sites of necrosis [68].
Vasculitis and CCL18
Giant cell arteritis is a systemic inflammatory vasculitis of unknown etiology, marked by the formation of granulomatous lymphoid microstructures within the affected arteries. Krupa et al. [73] found that these vascular lesions were enriched with activated, mature DC and that they contained more CCL18 transcripts compared with normal arteries. The accumulation and activation of monocytes/macrophages and T lymphocytes in the arterial tunica intima are likely to play an important role in the development of atherosclerosis. RT-PCR analysis and in situ hybridization demonstrated that CCL18 was expressed in human atherosclerotic plaques but not in normal arteries and that the transcription was restricted to macrophage-rich areas of the lesions [33].
Pulmonary disorders and CCL18
Enhanced CCL18 expression has also been related to various pulmonary conditions. Of 16 chemokines investigated by quantitative RT-PCR (e.g., CCL3, CCL20), CCL18 was the most remarkably and consistently increased chemokine in lungs affected by hypersensitivity pneumonitis or idiopathic pulmonary fibrosis in comparison with control lung tissue [74]. Hypersensitivity pneumonitis is a diffuse inflammatory lung disorder provoked by the inhalation of and sensitization to a variety of organic particles and marked by a strong accumulation of T lymphocytes within the bronchoalveolar structures. Idiopathic pulmonary fibrosis is a chronic interstitial lung disease of unknown etiology, characterized by fibrosis and a usually mild degree of inflammation, which mainly involves lymphocytes. CCL18 transcription was significantly higher in the lungs of hypersensitivity pneumonitis patients than in those of idiopathic pulmonary fibrosis patients. In situ hybridization and immunohistochemistry revealed that the main cellular sources of CCL18 in both disorders corresponded to the interstitial inflammatory cells close to clusters of lymphocytes, i.e., mainly macrophages and occasionally DC, in addition to some alveolar epithelial cells. In contrast, no CCL18 transcripts or protein could be demonstrated in control lung sections. Furthermore, there was a significant correlation between the CCL18 mRNA levels in the affected lung tissues and the percentage of lymphocytes in the bronchoalveolar lavage fluids. Finally, high CCL18 expression was detected in the subacute phase rather than in the chronic phase of hypersensitivity pneumonitis, the former characterized by more severe inflammation and less fibrotic lesions [74]. The finding that CCL18 stimulated collagen production in lung fibroblasts could nevertheless point to a role for CCL18 in lung fibrosis [23]. Although in scleroderma patients with lung inflammation, CCL18 mRNA and protein levels were slightly increased in, respectively, the bronchoalveolar lavage cells and fluids [75]; CCL18 transcription was comparable in bronchoalveolar lavage cells from healthy subjects and patients with pulmonary sarcoidosis [94]. Furthermore, in the latter disease, characterized by a lymphocytic infiltrate, no association could be observed between the CCL18 mRNA expression and the number of lymphocytes in the broncheoalveolar space [94]. The microarray profile and quantitative RT-PCR analysis of differentially expressed genes in a monkey model of allergic asthma suggest that CCL18 might be up-regulated slightly during allergen-induced pulmonary inflammation in monkey lungs [12].
Dermatitis and CCL18
CCL18 is likely to be involved in inflammatory reactions of the skin as well. In situ hybridization demonstrated dermal CCL18 expression during the elicitation phase of allergic contact hypersensitivity, more precisely in perivascular clusters at sites of leukocyte accumulation in the upper dermis [65]. Maximal transcription of CCL18 and some other T cell-attracting chemokines was reached 48–72 h after elicitation of this T lymphocyte-mediated hypersensitivity reaction and coincided with a strong infiltration of T cells. Nomura et al. [66] obtained consistent microarray and real-time RT-PCR data, indicating significant increases of CCL18 levels in atopic dermatitis as compared with skin lesions from Th1-mediated psoriasis and to normal skin. Atopic dermatitis is a biphasic inflammatory skin disease characterized by an initial phase predominated by Th2 cytokines, which switches into a second, Th1-dominated chronic phase. Pivarcsi et al. [34] extended the previous quantitative RT-PCR findings by reporting that CCL18 was the most abundant chemokine among all known chemokines in atopic dermatitis and that CCL18 was specifically and significantly up-regulated in lesional atopic skin when compared with normal skin or lesional skin from other chronic inflammatory skin diseases. Immunohistochemistry confirmed the lack of CCL18 expression in normal skin, psoriatic skin, and nonlesional atopic skin and pointed toward the cells with DC morphology, dispersed within the (epi)dermis or clustered at sites of perivascular infiltrates, and some epidermal keratinocytes and Langerhans cells as the CCL18 source in atopic dermatitis lesions [19, 34]. Serum CCL18 levels as well as the percentages of CCL18-producing monocytes/macrophages and DC in IL-4 stimulated PBMC were also increased in atopic dermatitis patients compared with healthy subjects [19]. In vivo exposure of nonlesional atopic skin to relevant allergen or to staphylococcal enterotoxin B significantly elicited CCL18 expression [34]. It is interesting that many atopic dermatitis patients seem to display S. aureus colonization in their lesions. Analogous to septic arthritis, atopic dermatitis might represent another S. aureus-associated disease in which CCL18 might contribute to the pathogen-triggered initiation and amplification of the inflammatory condition [14, 34].
Other disorders associated with CCL18 expression
In the conjunctiva of patients with vernal keratoconjunctivitis, another Th2-orientated inflammatory process, the number of CCL18+ cells was higher than the numbers of inflammatory cells expressing the Th2 attractants CCL1 or CCL22 and showed the strongest correlation with the numbers of infiltrating CD3+ T cells. Double immunohistochemistry indicated that the CCL18+ cells corresponded to CD68+ monocytes/macrophages [78].
In the case of preterm labor, cDNA array analysis showed that CCL18 is one of the chemokines of which the expression was significantly elevated in the outer gestational membranes affected with chorioamnionitis in comparison with noninflamed choriodecidua [67].
Finally, the human CCL18 gene belongs to a cluster of tuberculosis-susceptibility genes on chromosome 17q11-q21 [77].
CONCLUDING REMARKS
From the moment of its discovery, CCL18 has been postulated to be both a constitutive/homeostatic and an inducible/inflammatory chemokine. Indeed, the spontaneous CCL18 expression by lymphoid tissues and immature DC argues for a potential role of CCL18 in the homing of naïve lymphocytes and immature DC and in the organization of lymphoid structures under physiological conditions (Fig. 2). The considerable CCL18 levels in normal human plasma represent additional evidence for the high constitutive CCL18 expression throughout the body and may reflect a high CCL18 mRNA stability and a low clearance rate of circulating CCL18 protein. However, many in vitro studies have confirmed that the CCL18 expression by its two main producer cell types, monocytes/macrophages and DC, is also highly regulated by diverging stimuli, including pathogen- and T cell-derived signals and immunosuppressive agents (Fig. 2). Furthermore, CCL18 levels were selectively enhanced in different pathological body fluids and in various disease states, such as different malignancies and inflammatory disorders of the skin, lung, and joints. All these findings support the hypothesis that CCL18 expression by mononuclear cells, both in lymphoid and peripheral tissues, might contribute to the active recruitment of lymphocytes and immature DC under inflammatory and pathological conditions. Whether CCL18 could assist in a (Th1- or Th2-mediated) proinflammatory or rather anti-inflammatory/tolerogenic way to limit or promote the inflammation/disease will likely be situation-dependent and definitely will require further investigation. More profound knowledge on the various activation/maturation programs in monocytes/macrophages and DC might help clarifying the expression pattern and the role of CCL18. In particular, more information is needed about the in vivo relevance of the different monocytic and DC subtypes, especially of the alternatively activated macrophages and the semimature DC. Despite the fact that a CCL18 homologue could only be found in primates, pointing to the usefulness of monkey-animal models, the administration of human CCL18 in the context of rodent models might still help to elucidate the in vivo role of human CCL18. In this respect, the involvement of CCL18 in the generation of a primary T cell response was recently demonstrated by the s.c. coinjection of mice with CCL18 and malaria vaccines. The adjuvant activity of CCL18 may result from its peripheral attraction of murine immature DC toward the site of vaccine injection as well as from its recruitment of murine naïve T cells within the draining lymph nodes. It is intriguing that despite its antagonistic activity for CCR3, the true agonistic CCL18 receptor has not been revealed so far. However, the CCL18-stimulated collagen production by lung fibroblasts might represent an additional lead in the search for the CCL18 receptor.
Fig. 2.
Expression and role of CCL18 under physiological and immunopathological conditions. CCL18 is mainly expressed by monocytes/macrophages (Mono/Mϕ) and immature DC (Imm DC), whether or not after additional activation/maturation (marked by thin, straight arrows). The released CCL18 protein can attract lymphocytes and immature DC (depicted by thick, straight arrows) and induce collagen deposition by fibroblasts (curved arrow). In this way, CCL18 can contribute to various normal and immunopathological processes.
Currently, there is still an urgent need for good and selective biomarkers for the early detection, diagnosis, and therapeutic follow-up of many diseases. The enhanced CCL18 levels in selective pathological body fluids, the good prognostic value of high CCL18 expression and CCL18+ macrophage numbers in gastric cancer patients, and the reliability of the plasma CCL18 levels to monitor the therapeutic efficiency in Gaucher patients deserve further validation with the hope that CCL18 might represent a good biomarker, on its own or within a set of biomarkers, for these or other specific pathological conditions.
Acknowledgments
This work was supported by the Fund for Scientific Research of Flanders (FWO-Vlaanderen), the Concerted Research Actions of the Regional Government of Flanders, and the InterUniversity Attraction Pole Initiative of the Belgian Federal Government. We thank Professor G. Laureys (University Gent, Belgium) and Dr. S. Struyf (K.U. Leuven, Belgium) for their critical reading of the manuscript.
References
- 1.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 3.Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol. 2001;78:57–110. doi: 10.1016/s0065-2776(01)78002-9. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- 5.Hieshima K, Imai T, Baba M, Shoudai K, Ishizuka K, Nakagawa T, Tsuruta J, Takeya M, Sakaki Y, Takatsuki K, Miura R, Opdenakker G, Van Damme J, Yoshie O, Nomiyama H. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1α/LD78α and chemotactic for T lymphocytes, but not for monocytes. J Immunol. 1997;159:1140–1149. [PubMed] [Google Scholar]
- 6.Wells TN, Peitsch MC. The chemokine information source: identification and characterization of novel chemokines using the World-WideWeb and expressed sequence tag databases. J Leukoc Biol. 1997;61:545–550. doi: 10.1002/jlb.61.5.545. [DOI] [PubMed] [Google Scholar]
- 7.Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T, Bacon KB, Figdor CG. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713–717. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- 8.Kodelja V, Müller C, Politz O, Hakij N, Orfanos CE, Goerdt S. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1α with a Th2-associated expression pattern. J Immunol. 1998;160:1411–1418. [PubMed] [Google Scholar]
- 9.Guan P, Burghes AHM, Cunningham A, Lira P, Brissette WH, Neote K, McColl SR. Genomic organization and biological characterization of the novel human CC chemokine DC-CK-1/PARC/MIP-4/SCYA18. Genomics. 1999;56:296–302. doi: 10.1006/geno.1998.5635. [DOI] [PubMed] [Google Scholar]
- 10.Tasaki Y, Fukuda S, Iio M, Miura R, Imai T, Sugano S, Yoshie O, Hughes AL, Nomiyama H. Chemokine PARC gene (SCYA18) generated by fusion of two MIP-1α/LD78α-like genes. Genomics. 1999;55:353–357. doi: 10.1006/geno.1998.5670. [DOI] [PubMed] [Google Scholar]
- 11.Politz O, Kodelja V, Guillot P, Orfanos CE, Goerdt S. Pseudoexons and regulatory elements in the genomic sequence of the β-chemokine, alternative macrophage activation-associated CC-chemokine (AMAC)-1. Cytokine. 2000;12:120–126. doi: 10.1006/cyto.1999.0538. [DOI] [PubMed] [Google Scholar]
- 12.Zou J, Young S, Zhu F, Gheyas F, Skeans S, Wan Y, Wang L, Ding W, Billah M, McClanahan T, Coffman RL, Egan R, Umland S. Microarray profile of differentially expressed genes in a monkey model of allergic asthma. Genome Biol. 2002;3:research0020.1. doi: 10.1186/gb-2002-3-5-research0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu S, Schaefer TM, Ghosh M, Fuller CL, Reinhart TA. Molecular cloning and sequencing of 25 different rhesus macaque chemokine cDNAs reveals evolutionary conservation among C, CC, CXC, and CX3C families of chemokines. Cytokine. 2002;18:140–148. doi: 10.1006/cyto.2002.0875. [DOI] [PubMed] [Google Scholar]
- 14.Schutyser E, Struyf S, Wuyts A, Put W, Geboes K, Grillet B, Opdenakker G, Van Damme J. Selective induction of CCL18/PARC by staphylococcal enterotoxins in mononuclear cells and enhanced levels in septic and rheumatoid arthritis. Eur J Immunol. 2001;31:3755–3762. doi: 10.1002/1521-4141(200112)31:12<3755::aid-immu3755>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Schutyser E, Struyf S, Proost P, Opdenakker G, Laureys G, Verhasselt B, Peperstraete L, Van de Putte I, Saccani A, Allavena P, Mantovani A, Van Damme J. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J Biol Chem. 2002;277:24584–24593. doi: 10.1074/jbc.M112275200. [DOI] [PubMed] [Google Scholar]
- 16.Struyf S, Schutyser E, Gouwy M, Gijsbers K, Proost P, Benoit Y, Opdenakker G, Van Damme J, Laureys G. PARC/CCL18 is a plasma CC chemokine with increased levels in childhood acute lymphoblastic leukemia. Am J Pathol. 2003;163:2065–2075. doi: 10.1016/S0002-9440(10)63564-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monteclaro FS, Charo IF. The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1α receptor, confers chemokine selectivity. Evidence for a two-step mechanism for MCP-1 receptor activation. J Biol Chem. 1996;271:19084–19092. doi: 10.1074/jbc.271.32.19084. [DOI] [PubMed] [Google Scholar]
- 18.Clore GM, Gronenborn AM. Three-dimensional structures of α and β chemokines. FASEB J. 1995;9:57–62. doi: 10.1096/fasebj.9.1.7821760. [DOI] [PubMed] [Google Scholar]
- 19.Günther C, Bello-Fernandez C, Kopp T, Kund J, Carballido-Perrig N, Hinteregger S, Fassl S, Schwärzler C, Lametschwandtner G, Stingl G, Biedermann T, Carballido JM. CCL18 is expressed in atopic dermatitis and mediates skin homing of human memory T cells. J Immunol. 2005;174:1723–1728. doi: 10.4049/jimmunol.174.3.1723. [DOI] [PubMed] [Google Scholar]
- 20.Lindhout E, Vissers JLM, Hartgers FC, Huijbens RJF, Scharenborg NM, Figdor CG, Adema GJ. The dendritic cell-specific CC-chemokine DC-CK1 is expressed by germinal center dendritic cells and attracts CD38-negative mantle zone B lymphocytes. J Immunol. 2001;166:3284–3289. doi: 10.4049/jimmunol.166.5.3284. [DOI] [PubMed] [Google Scholar]
- 21.Vulcano M, Struyf S, Scapini P, Cassatella M, Bernasconi S, Bonecchi R, Calleri A, Penna G, Adorini L, Luini W, Mantovani A, Van Damme J, Sozzani S. Unique regulation of CCL18 production by maturing dendritic cells. J Immunol. 2003;170:3843–3849. doi: 10.4049/jimmunol.170.7.3843. [DOI] [PubMed] [Google Scholar]
- 22.Broxmeyer HE, Kim CH, Cooper SH, Hangoc G, Hromas R, Pelus LM. Effects of CC, CXC, C, and CX3C chemokines on proliferation of myeloid progenitor cells, and insights into SDF-1-induced chemotaxis of progenitors. Ann N Y Acad Sci. 1999;872:142–162. doi: 10.1111/j.1749-6632.1999.tb08460.x. [DOI] [PubMed] [Google Scholar]
- 23.Atamas SP, Luzina IG, Choi J, Tsymbalyuk N, Carbonetti NH, Singh IS, Trojanowska M, Jimenez SA, White B. Pulmonary and activation-regulated chemokine stimulates collagen production in lung fibroblasts. Am J Respir Cell Mol Biol. 2003;29:743–749. doi: 10.1165/rcmb.2003-0078OC. [DOI] [PubMed] [Google Scholar]
- 24.Nibbs RJB, Salcedo TW, Campbell JDM, Yao XT, Li Y, Nardelli B, Olsen HS, Morris TS, Proudfoot AEI, Patel VP, Graham GJ. C-C chemokine receptor 3 antagonism by the β-chemokine macrophage inflammatory protein 4, a property strongly enhanced by an amino-terminal alanine-methionine swap. J Immunol. 2000;164:1488–1497. doi: 10.4049/jimmunol.164.3.1488. [DOI] [PubMed] [Google Scholar]
- 25.Wan Y, Jakway JP, Qiu H, Shah H, Garlisi CG, Tian F, Ting P, Hesk D, Egan RW, Billah MM, Umland SP. Identification of full, partial and inverse CC chemokine receptor 3 agonists using [35S]GTPγS binding. Eur J Pharmacol. 2002;456:1–10. doi: 10.1016/s0014-2999(02)02621-3. [DOI] [PubMed] [Google Scholar]
- 26.Struyf S, Proost P, Van Damme J. Regulation of the immune response by the interaction of chemokines and proteases. Adv Immunol. 2003;81:1–44. doi: 10.1016/s0065-2776(03)81001-5. [DOI] [PubMed] [Google Scholar]
- 27.Schraufstatter I, Takamori H, Sikora L, Sriramarao P, DiScipio RG. Eosinophils and monocytes produce pulmonary and activation-regulated chemokine, which activates cultured monocytes/macrophages. Am J Physiol Lung Cell Mol Physiol. 2004;286:L494–L501. doi: 10.1152/ajplung.00323.2002. [DOI] [PubMed] [Google Scholar]
- 28.Bruna-Romero O, Schmieg J, Del Val M, Buschle M, Tsuji M. The dendritic cell-specific chemokine, dendritic cell-derived CC chemokine 1, enhances protective cell-mediated immunity to murine malaria. J Immunol. 2003;170:3195–3203. doi: 10.4049/jimmunol.170.6.3195. [DOI] [PubMed] [Google Scholar]
- 29.Shen Y, Iqbal J, Xiao L, Lynch RC, Rosenwald A, Staudt LM, Sherman S, Dybkaer K, Zhou G, Eudy JD, Delabie J, McKeithan TW, Chan WC. Distinct gene expression profiles in different B-cell compartments in human peripheral lymphoid organs. BMC Immunol. 2004;5:20. doi: 10.1186/1471-2172-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois B, Barthelemy C, Durand I, Liu YJ, Caux C, Briere F. Toward a role of dendritic cells in the germinal center reaction: triggering of B cell proliferation and isotype switching. J Immunol. 1999;162:3428–3436. [PubMed] [Google Scholar]
- 31.Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Song E, Ouyang N, Hörbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 33.Reape TJ, Rayner K, Manning CD, Gee AN, Barnette MS, Burnand KG, Groot PH. Expression and cellular localization of the CC chemokines PARC and ELC in human atherosclerotic plaques. Am J Pathol. 1999;154:365–374. doi: 10.1016/S0002-9440(10)65283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pivarcsi A, Gombert M, Dieu-Nosjean MC, Lauerma A, Kubitza R, Meller S, Rieker J, Muller A, Da Cunha L, Haahtela A, Sonkoly E, Fridman WH, Alenius H, Kemeny L, Ruzicka T, Zlotnik A, Homey B. CC chemokine ligand 18, an atopic dermatitis-associated and dendritic cell-derived chemokine, is regulated by staphylococcal products and allergen exposure. J Immunol. 2004;173:5810–5817. doi: 10.4049/jimmunol.173.9.5810. [DOI] [PubMed] [Google Scholar]
- 35.Kodelja V, Kraft S, Politz O, Hakij N, Treudler R, Orfanos CE, Bieber T, Goerdt S. Langerhans cells do not express alternative macrophage activation-associated CC chemokine (AMAC)-1. Res Immunol. 1998;149:633–637. doi: 10.1016/s0923-2494(99)80029-7. [DOI] [PubMed] [Google Scholar]
- 36.Brossart P, Grunebach F, Stuhler G, Reichardt VL, Mohle R, Kanz L, Brugger W. Generation of functional human dendritic cells from adherent peripheral blood monocytes by CD40 ligation in the absence of granulocyte-macrophage colony-stimulating factor. Blood. 1998;92:4238–4247. [PubMed] [Google Scholar]
- 37.Vissers JL, Hartgers FC, Lindhout E, Teunissen MB, Figdor CG, Adema GJ. Quantitative analysis of chemokine expression by dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2001;69:785–793. [PubMed] [Google Scholar]
- 38.de Vries IJ, Eggert AA, Scharenborg NM, Vissers JL, Lesterhuis WJ, Boerman OC, Punt CJ, Adema GJ, Figdor CG. Phenotypical and functional characterization of clinical grade dendritic cells. J Immunother. 2002;25:429–438. doi: 10.1097/00002371-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Zeidler R, Reisbach G, Wollenberg B, Lang S, Chaubal S, Schmitt B, Lindhofer H. Simultaneous activation of T cells and accessory cells by a new class of intact bispecific antibody results in efficient tumor cell killing. J Immunol. 1999;163:1246–1252. [PubMed] [Google Scholar]
- 40.Radstake TR, van der Voort R, Ten Brummelhuis M, de Waal Malefijt M, Schreurs W, Looman M, Sloetjes A, Figdor CG, Van Den Berg WB, Barrera P, Adema GJ. Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis (RA) and regulation by Fc γ receptors. Ann Rheum Dis. 2004;64:359–367. doi: 10.1136/ard.2003.017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Ceuninck F, Dassencourt L, Anract P. The inflammatory side of human chondrocytes unveiled by antibody microarrays. Biochem Biophys Res Commun. 2004;323:960–969. doi: 10.1016/j.bbrc.2004.08.184. [DOI] [PubMed] [Google Scholar]
- 42.St Louis DC, Woodcock JB, Fransozo G, Blair PJ, Carlson LM, Murillo M, Wells MR, Williams AJ, Smoot DS, Kaushal S, Grimes JL, Harlan DM, Chute JP, June CH, Siebenlist U, Lee KP. Evidence for distinct intracellular signaling pathways in CD34+ progenitor to dendritic cell differentiation from a human cell line model. J Immunol. 1999;162:3237–3248. [PubMed] [Google Scholar]
- 43.Ragno S, Romano M, Howell S, Pappin DJ, Jenner PJ, Colston MJ. Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: a combined transcriptomic and proteomic approach. Immunology. 2001;104:99–108. doi: 10.1046/j.0019-2805.2001.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 45.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Allavena P, Sica A. Tumor-associated macrophages as a prototypic type II polarized phagocyte population: role in tumor progression. Eur J Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Tzachanis D, Berezovskaya A, Nadler LM, Boussiotis VA. Blockade of B7/CD28 in mixed lymphocyte reaction cultures results in the generation of alternatively activated macrophages, which suppress T-cell responses. Blood. 2002;99:1465–1473. doi: 10.1182/blood.v99.4.1465. [DOI] [PubMed] [Google Scholar]
- 48.Standiford TJ, Kunkel SL, Liebler JM, Burdick MD, Gilbert AR, Strieter RM. Gene expression of macrophage inflammatory protein-1α from human blood monocytes and alveolar macrophages is inhibited by interleukin-4. Am J Respir Cell Mol Biol. 1993;9:192–198. doi: 10.1165/ajrcmb/9.2.192. [DOI] [PubMed] [Google Scholar]
- 49.Berkman N, John M, Roesems G, Jose PJ, Barnes PJ, Chung KF. Inhibition of macrophage inflammatory protein-1α expression by IL-10. Differential sensitivities in human blood monocytes and alveolar macrophages. J Immunol. 1995;155:4412–4418. [PubMed] [Google Scholar]
- 50.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 51.van Lieshout TW, Barrera P, Smeets RL, Pesman GJ, van Riel PL, van den Berg WB, Radstake TR. Inhibition of TNF-α during maturation of dendritic cells results in the development of semi-mature DC: a potential mechanism by which TNF-α blockade exerts its benificial effects in rheumatoid arthritis. Ann Rheum Dis. 2005;64:408–414. doi: 10.1136/ard.2004.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Penna G, Vulcano M, Roncari A, Facchetti F, Sozzani S, Adorini L. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol. 2002;169:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- 53.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–F9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 56.Schaniel C, Rolink AG, Melchers F. Attractions and migrations of lymphoid cells in the organization of humoral immune responses. Adv Immunol. 2001;78:111–168. doi: 10.1016/s0065-2776(01)78003-0. [DOI] [PubMed] [Google Scholar]
- 57.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 58.Dubois B, Barthelemy C, Durand I, Liu YJ, Caux C, Briere F. Toward a role of dendritic cells in the germinal center reaction: triggering of B cell proliferation and isotype switching. J Immunol. 1999;162:3428–3436. [PubMed] [Google Scholar]
- 59.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 60.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 61.Schulz-Knappe P, Magert HJ, Dewald B, Meyer M, Cetin Y, Kubbies M, Tomeczkowski J, Kirchhoff K, Raida M, Adermann K. HCC-1, a novel chemokine from human plasma. J Exp Med. 1996;183:295–299. doi: 10.1084/jem.183.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nomiyama H, Hieshima K, Nakayama T, Sakaguchi T, Fujisawa R, Tanase S, Nishiura H, Matsuno K, Takamori H, Tabira Y, Yamamoto T, Miura R, Yoshie O. Human CC chemokine liver-expressed chemokine/CCL16 is a functional ligand for CCR1, CCR2 and CCR5, and constitutively expressed by hepatocytes. Int Immunol. 2001;13:1021–1029. doi: 10.1093/intimm/13.8.1021. [DOI] [PubMed] [Google Scholar]
- 63.Files JC, Malpass TW, Yee EK, Ritchie JL, Harker LA. Studies of human platelet α-granule release in vivo. Blood. 1981;58:607–618. [PubMed] [Google Scholar]
- 64.Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, Flad HD. The β-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol. 2000;67:471–478. doi: 10.1002/jlb.67.4.471. [DOI] [PubMed] [Google Scholar]
- 65.Goebeler M, Trautmann A, Voss A, Bröcker EB, Toksoy A, Gillitzer R. Differential and sequential expression of multiple chemokines during elicitation of allergic contact hypersensitivity. Am J Pathol. 2001;158:431–440. doi: 10.1016/s0002-9440(10)63986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol. 2003;112:1195–1202. doi: 10.1016/j.jaci.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 67.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod. 2002;8:399–408. doi: 10.1093/molehr/8.4.399. [DOI] [PubMed] [Google Scholar]
- 68.Kusano F, Tanaka Y, Marumo F, Sato C. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest. 2000;80:415–422. doi: 10.1038/labinvest.3780046. [DOI] [PubMed] [Google Scholar]
- 69.Leung SY, Yuen ST, Chu KM, Mathy JA, Li R, Chan AS, Law S, Wong J, Chen X, So S. Expression profiling identifies chemokine (C-C motif) ligand 18 as an independent prognostic indicator in gastric cancer. Gastroenterology. 2004;127:457–469. doi: 10.1053/j.gastro.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 70.Moran MT, Schofield JP, Hayman AR, Shi G-P, Young E, Cox TM. Pathologic gene expression in Gaucher disease: up-regulation of cysteine proteinases including osteoclastic cathepsin K. Blood. 2000;96:1969–1978. [PubMed] [Google Scholar]
- 71.Boot RG, Verhoek M, De Fost M, Hollak CE, Maas M, Bleijlevens B, Van Breemen MJ, Van Meurs M, Boven LA, Laman JD, Moran MT, Cox TM, Aerts JM. Marked elevation of the chemokine CCL18/PARC in Gaucher disease: a novel surrogate marker for assessing therapeutic intervention. Blood. 2004;103:33–39. doi: 10.1182/blood-2003-05-1612. [DOI] [PubMed] [Google Scholar]
- 72.Boven LA, Van Meurs M, Boot RG, Mehta A, Boon L, Aerts JM, Laman JD. Gaucher cells demonstrate a distinct macrophage phenotype and resemble alternatively activated macrophages. Am J Clin Pathol. 2004;122:359–369. doi: 10.1309/BG5V-A8JR-DQH1-M7HN. [DOI] [PubMed] [Google Scholar]
- 73.Krupa WM, Dewan M, Jeon MS, Kurtin PJ, Younge BR, Goronzy JJ, Weyand CM. Trapping of misdirected dendritic cells in the granulomatous lesions of giant cell arteritis. Am J Pathol. 2002;161:1815–1823. doi: 10.1016/S0002-9440(10)64458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pardo A, Smith KM, Abrams J, Coffman R, Bustos M, McClanahan TK, Grein J, Murphy EE, Zlotnik A, Selman M. CCL18/DC-CK-1/PARC up-regulation in hypersensitivity pneumonitis. J Leukoc Biol. 2001;70:610–616. [PubMed] [Google Scholar]
- 75.Luzina IG, Atamas SP, Wise R, Wigley FM, Xiao HQ, White B. Gene expression in bronchoalveolar lavage cells from scleroderma patients. Am J Respir Cell Mol Biol. 2002;26:549–557. doi: 10.1165/ajrcmb.26.5.4683. [DOI] [PubMed] [Google Scholar]
- 76.Xanthou G, Polihronis M, Tzioufas AG, Paikos S, Sideras P, Moutsopoulos HM. Lymphoid chemokine messenger RNA expression by epithelial cells in the chronic inflammatory lesion of the salivary glands of Sjögren’s syndrome patients: possible participation in lymphoid structure formation. Arthritis Rheum. 2001;44:408–418. doi: 10.1002/1529-0131(200102)44:2<408::AID-ANR60>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 77.Jamieson SE, Miller EN, Black GF, Peacock CS, Cordell HJ, Howson JM, Shaw MA, Burgner D, Xu W, Lins-Lainson Z, Shaw JJ, Ramos F, Silveira F, Blackwell JM. Evidence for a cluster of genes on chromosome 17q11–q21 controlling susceptibility to tuberculosis and leprosy in Brazilians. Genes Immun. 2004;5:46–57. doi: 10.1038/sj.gene.6364029. [DOI] [PubMed] [Google Scholar]
- 78.El-Asrar AM, Struyf S, Al-Kharashi SA, Missotten L, Van Damme J, Geboes K. Expression of T lymphocyte chemoattractants and activation markers in vernal keratoconjunctivitis. Br J Ophthalmol. 2002;86:1175–1180. doi: 10.1136/bjo.86.10.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loercher AE, Nash MA, Kavanagh JJ, Platsoucas CD, Freedman RS. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cytokine protein expression and proliferation of autologous T cells. J Immunol. 1999;163:6251–6260. [PubMed] [Google Scholar]
- 80.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, Van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-κB activation in tumor-associated macrophages. J Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 81.Melichar B, Savary C, Kudelka AP, Verschraegen C, Kavanagh JJ, Edwards CL, Platsoucas CD, Freedman RS. Lineage-negative human leukocyte antigen-DR+ cells with the phenotype of undifferentiated dendritic cells in patients with carcinoma of the abdomen and pelvis. Clin Cancer Res. 1998;4:799–809. [PubMed] [Google Scholar]
- 82.Gunzer M, Jänich S, Varga G, Grabbe S. Dendritic cells and tumor immunity. Semin Immunol. 2001;13:291–302. doi: 10.1006/smim.2001.0325. [DOI] [PubMed] [Google Scholar]
- 83.Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, Curiel DT, Galanaud P, Capron F, Emilie D, Curiel TJ. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 84.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001;61:4961–4965. [PubMed] [Google Scholar]
- 85.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 86.Godessart N, Kunkel SL. Chemokines in autoimmune disease. Curr Opin Immunol. 2001;13:670–675. doi: 10.1016/s0952-7915(01)00277-1. [DOI] [PubMed] [Google Scholar]
- 87.Loetscher P, Moser B. Homing chemokines in rheumatoid arthritis. Arthritis Res. 2002;4:233–236. doi: 10.1186/ar412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 89.Pettit AR, Thomas R. Dendritic cells: the driving force behind autoimmunity in rheumatoid arthritis? Immunol Cell Biol. 1999;77:420–427. doi: 10.1046/j.1440-1711.1999.00855.x. [DOI] [PubMed] [Google Scholar]
- 90.Stockwin LH, McGonagle D, Martin IG, Blair GE. Dendritic cells: immunological sentinels with a central role in health and disease. Immunol Cell Biol. 2000;78:91–102. doi: 10.1046/j.1440-1711.2000.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002;168:5333–5341. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- 92.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 93.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 94.Mrazek F, Sekerova V, Drabek J, Kolek V, du Bois RM, Petrek M. Expression of the chemokine PARC mRNA in bronchoalveolar cells of patients with sarcoidosis. Immunol Lett. 2002;84:17–22. doi: 10.1016/s0165-2478(02)00130-x. [DOI] [PubMed] [Google Scholar]