Abstract
Assigning a gene's function to specific pathways used for classical conditioning, such as conditioned stimulus (CS) and unconditioned stimulus (US) pathway, is important for understanding the fundamental molecular and cellular mechanisms underlying memory formation. Prior studies have shown that the GABA receptor RDL inhibits aversive olfactory learning via its role in the Drosophila mushroom bodies (MBs). Here, we describe the results of further behavioral tests to further define the pathway involvement of RDL. The expression level of Rdl in the MBs influenced both appetitive and aversive olfactory learning, suggesting that it functions by suppressing a common pathway used for both forms of olfactory learning. Rdl knock down failed to enhance learning in animals carrying mutations in genes of the cAMP signaling pathway, such as rutabaga and NF1, suggesting that RDL works up stream of these functions in CS/US integration. Finally, knocking down Rdl or over expressing the dopamine receptor dDA1 in the MBs enhanced olfactory learning, but no significant additional enhancement was detected with both manipulations. The combined data suggest that RDL suppresses olfactory learning via CS pathway involvement.
Keywords: GABAA receptor, olfactory learning, Drosophila, conditioned stimulus, mushroom body, reward
Introduction
Classical conditioning is a type of associative learning that can occur when a conditioned stimulus (CS) is integrated with a positive or negative unconditioned stimulus (US) by pairing the two stimuli to generate a conditioned response (CR) after such pairing. Numerous studies in different systems have identified many genes important for this type of learning (Kandel, 2001; Davis, 2005; Murakami, 2007), but little progress has been made in restricting the function of these genes to the CS pathway, the US pathway, or the integration step that occurs upon CS/US pairing. Placing the function of these genes into the appropriate pathways that support learning is required for assembling a comprehensive framework of learning and to understand the fundamental molecular and cellular mechanisms for learning and memory to occur.
Genes involved in learning and memory have conserved functions between phyla (Davis, 2005). The powerful molecular biology and genetic tools available for Drosophila melanogaster have provided great utility for studying the mechanisms of learning and memory formation with this organism (Davis, 2005; Vosshall, 2007). In flies, like other insects, the mushroom bodies (MBs) are the principle brain structures involved in olfactory associative learning and this provides a cellular focus to restrict the function of genes to particular pathways (Davis, 2005; McGuire et al., 2005).
The gene Rdl encodes a GABAA receptor that is expressed at relatively high levels in the MBs (Harrison et al., 1996; Liu et al., 2007). Recent studies demonstrated that over expression of Rdl in the MBs during adulthood impairs aversive olfactory learning where flies are trained to associate an odor CS with the US of electric shock. Knocking down Rdl in the MBs enhances this type of learning. Functional imaging reveals that over expression of Rdl inhibits the calcium responses in the MBs toward odor stimuli while knock down of Rdl enhances these responses. Interestingly, the level of Rdl expression has no effect on the response observed in MBs when the flies receive electric shock stimuli (Liu et al., 2007). These observations lead to the hypothesis that the GABAA receptor RDL may specifically suppress the CS input into the MBs rather than playing a general inhibitory role by suppressing both the CS and US pathways. To further test this hypothesis, we assayed flies with Rdl over expression or knock down using different olfactory learning paradigms and studied the interaction of Rdl with other known learning and memory genes.
Materials and Methods
Fly culture and maintenance.
The flies were cultured on standard medium at 25°C, 60% relative humidity and a 12 h light/dark cycle. Flies used for behavior tests were out-crossed into the w(CS10) (Canton-S flies carrying the w1118 mutation) background, which was used as a wild-type control in the lab. The UAS-Rdl and UAS-Rdl-RNAi lines were generated as previously described (Liu et al., 2007). Learning mutants: rut1 carries a point mutation in the rut gene (Livingstone et al., 1984), rut2080 is associated with a P element insertion (Levin et al., 1992), and NF1P1 carries a deletion that removes all of the NF1 gene except for the first exon (Guo et al., 2000). UAS-dDA1 is a transgene that carries the cDNA of the dDA1 gene down stream of the UAS sequence.
Immunoblotting.
The rabbit anti-RDL antiserum was generated as previous described (Liu et al., 2007). Fly heads were collected and homogenized over liquid nitrogen and then dissolved in Laemmli sample buffer with 5% β-mercaptoethanol (Bio-Rad Laboratories). The supernatant equivalent to 3 fly heads was loaded into each lane of the precast SDS-PAGE gel (Bio-Rad Laboratories). Two independent repeat experiments were performed with flies from different crosses. After electrophoresis, the protein was transferred onto a PVDF membrane (Bio-Rad Laboratories) and blotted with 1:100 anti-RDL or 1:2000 dilution of mouse anti-Dynamin (BD Biosciences) antibodies and secondary antibodies (Abcam), and the signal was detected using the ECL Plus Western Blotting Reagent Pack (Amersham Biosciences). The average grayscale intensity of the relevant protein band was measured with NIH ImageJ software and a background region of identical size was subtracted. For each line, the RDL/Dynamin ratio was normalized to the wild-type (ELAV/+) sample.
Aversive and appetitive olfactory learning assay.
We performed the aversive olfactory learning assay as previously described (Liu et al., 2007). Briefly, the flies were exposed sequentially to two odors [benzaldehyde (BEN) and 3-octanol (OCT)] for 1 min each. Only the first odor (CS+) was paired with electric shock pulses (US). Immediately after training, the flies were loaded into a T-maze where they faced the choice between two arms, each containing one of the two odors. The flies' avoidance toward the odor previously paired with shock was calculated as the performance index (P.I.), which was the number of flies that responded correctly minus the number of flies that responded incorrectly, divided by total number of the flies. To eliminate naive odor bias, each trial was composed of two simultaneous half trials where we trained one group to associate BEN with shock and the other to associate OCT with shock, and the complete P.I. was the average of these two half P.I.s. We varied the number of shocks used in the training as previously described (Liu et al., 2007) to measure the memory strength as a function of different training intensities. The electric shock pulses were evenly distributed through the 1 min CS+ exposure, with the last shock always at the end of the CS+. For appetitive olfactory learning, flies were collected and kept in food-less vials with a small amount of water in the cotton caps and starved for different periods of time as described in the text. During training, the flies were exposed sequentially to the two training odors for 1.5 min each. The first odor was paired with a dried filter paper (4.5 × 7.8 cm) previously loaded with 400 μl of sucrose solutions of varying concentrations, while the second odor was paired with dried filter paper previously loaded with water only. Testing was identical to aversive learning. All chemicals were purchased from Sigma-Aldrich.
Statistical analysis.
We performed statistical analyses using StatView software (SAS Institute). For comparisons among multiple groups, one-way ANOVA was performed followed by planned pairwise comparisons between relevant groups using Fisher's PLSD test. Unpaired Student's t tests were used for comparisons between two groups. One-sample Wilcoxon signed-rank tests were used to determine whether performance scores were significantly different from zero.
Results
Knocking down Rdl enhances aversive olfactory learning
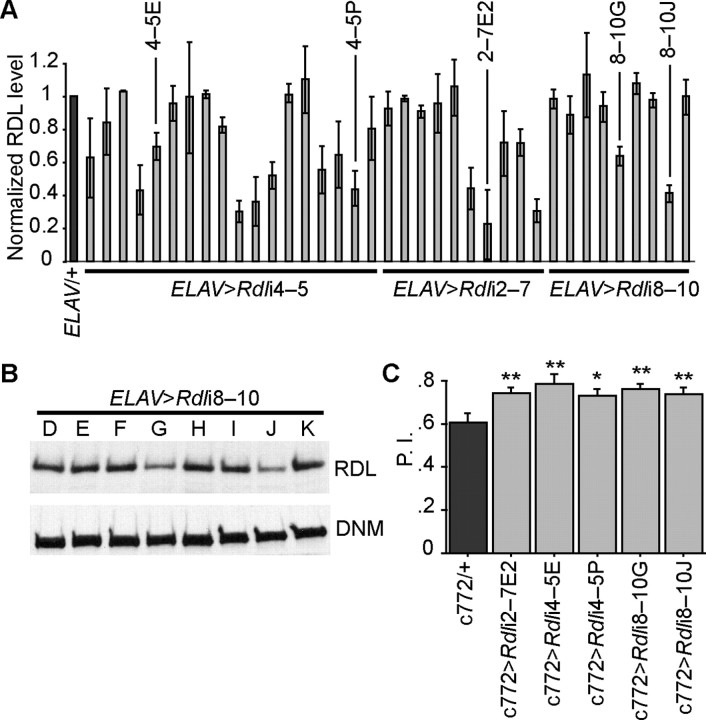
We constructed 3 different UAS-RNAi constructs against different exons of the Rdl gene and generated 37 independent transgenic lines carrying these constructs (Liu et al., 2007). To identify lines with the most pronounced effect, we crossed all 37 lines with a pan-neuronal Gal4 driver, ELAV-Gal4 (Luo et al., 1994), and prepared immunoblots of bi-genic progeny using an anti-RDL antibody (Liu et al., 2007). The lines exhibited significant variation in their ability to knock down the expression of RDL protein, ranging from 20 to 100% of the control (Fig. 1A,B). We selected 5 lines that showed a significant knock down of Rdl and crossed them to a MB Gal4 driver, c772-Gal4. We pursued experiments centered on the use of this driver because: (1) The driver is rather specific to the MBs, but has some expression in the intrinsic neurons of the antennal lobe. (2) Prior experiments have proven its utility in similar studies. Over expression of Rdl by the c772 driver impairs learning and this effect is due to over expression in the MBs rather than antennal lobes (Liu et al., 2007). (3) The c772 MB driver is expressed relatively late during development, thus avoiding the lethality associated with driving transgenes with other MB drivers (Liu et al., 2007). All 5 Rdl RNAi lines driven by the c772 driver displayed a significantly enhanced performance after aversive olfactory conditioning where they were trained to associate odors with electric shock (Fig. 1C). We have used several of these lines in this study to facilitate the genetic construction of several different variants. These results confirm and extend our previous conclusion that RDL inhibits aversive olfactory learning (Liu et al., 2007).
Figure 1.
Knocking down Rdl enhances aversive olfactory learning. A, Summary of the relative Rdl expression levels as determined by Western blotting in 37 independent Rdl RNAi lines driven by the pan-neuronal ELAV-Gal4 driver. Three different Rdl RNAi constructs were prepared that targeted exons 4–5, 2–7, and 8–10 (Rdli4–5, Rdli2–7, and Rdli8–10, respectively). The RDL/Dynamin signal ratio was normalized to the ELAV/+ control. Five lines (labeled) with large effects on RDL expression were identified for subsequent behavioral studies. Means ± SD are shown, n = 2 for each line. B, Representative Western blot images showing several Rdli8–10 lines driven by the ELAV-Gal4 driver. Note the significant reduction in the RDL signals for lines G and J. C, Aversive olfactory learning scores for selected Rdl RNAi lines driven by the MB driver, c772-Gal4. Flies were trained using 3 pulses of electric shock during a CS+ odor presentation of 1 min. All of the Rdl knock down lines showed a significantly enhanced performance index (P.I.) comparing with c772-Gal4 alone control. n = 6 for each group. Means ± SEM; *p < 0.05; **p < 0.01 (Fisher's PLSD).
RDL inhibits appetitive olfactory learning
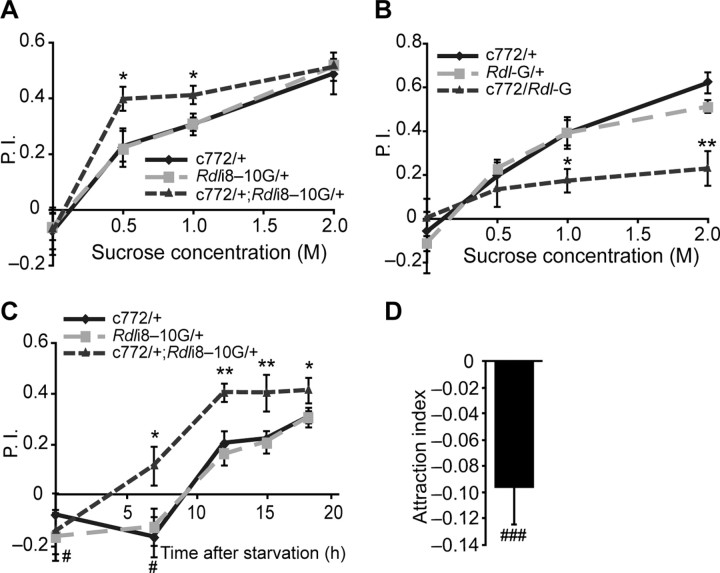
To further understand RDL's role in olfactory learning, we trained and tested the flies using an appetitive olfactory learning paradigm. Instead of using electric shock as the US, we trained starved flies to associate a sucrose reward (US) with odors (CS) (Tempel et al., 1983; Schwaerzel et al., 2003; Kim et al., 2007a). We trained the flies with different concentrations of sucrose to test the learning as a function of US intensity, since recent studies have shown that higher sucrose concentrations produce higher learning performance in both larval and adult Drosophila (Kim et al., 2007b; Schipanski et al., 2008). All of the groups showed increasing performance scores with increasing concentration of sucrose, but the flies with an Rdl knock down in the MBs showed improved performance compared with controls at 0.5 m and 1.0 m sucrose (Fig. 2A). In contrast, flies that over expressed Rdl in the same neurons showed poor performance compared with controls at 1.0 m and 2.0 m sucrose (Fig. 2B). Control experiments demonstrated that these flies exhibited normal responses to odors and sucrose for all the groups tested (Table 1), indicating that the changes in performance reflected changes in learning rather than sensation. Motivational drive is a critical factor for appetitive learning and memory (Krashes and Waddell, 2008). To gain insights into this variable, we starved flies for various periods of time and then tested their learning performance. Generally, flies starved for increasing periods of time exhibited an increasing performance; the Rdl knock down group again showed enhanced performance over controls after 7, 12, 15, and 18 h of starvation (Fig. 2C). These results indicate that RDL inhibits appetitive olfactory learning, like aversive olfactory learning.
Figure 2.
RDL inhibits appetitive olfactory learning. A, Flies were starved for 18 h and trained using different concentrations of sucrose presented with a 1.5 min exposure to the conditioned odor. Flies carrying the c772-Gal4 driver and the Rdli8–10G transgene exhibited an enhanced performance. B, Flies carrying the c772-Gal4 driver and a UAS-Rdl-G transgene exhibited poor performance in the appetitive learning assay described in (A). C, Flies carrying the c772-Gal4 driver and the Rdli8–10G transgene exhibited an enhanced performance after appetitive olfactory conditioning with 1 m sucrose when starved for 7, 12, 15, and 18 h. The performance scores for all three groups with no starvation and for the two control groups after 7 h starvation were significantly lower than zero. D, Wild-type naive flies without starvation showed a significant avoidance of a T-maze arm that contained 1 m sucrose when given a choice of a second arm without sucrose. Means ± SEM are shown for all panels. For panels A–C, n = 6 for each group under each condition; n = 15 for panel D. A–C, *p < 0.05; **p < 0.01 (Fisher's PLSD); C, D, #p < 0.05; ###p < 0.001 (one-sample Wilcoxon signed-rank test, μ0 = 0).
Table 1.
Odor avoidance and sucrose attraction controls for the genotypes assayed for learning
| Genotype | BEN avoidance | OCT avoidance | Sucrose attraction |
|---|---|---|---|
| c772/+ | 0.654 ± 0.041 | 0.619 ± 0.067 | 0.388 ± 0.065 |
| Rdl-G/+ | 0.678 ± 0.052 | 0.611 ± 0.054 | 0.412 ± 0.041 |
| c772/Rdl-G | 0.679 ± 0.057 | 0.578 ± 0.044 | 0.414 ± 0.026 |
| c772/+ | 0.686 ± 0.033 | 0.609 ± 0.043 | 0.332 ± 0.042 |
| Rdli8–10G/+ | 0.643 ± 0.048 | 0.630 ± 0.047 | 0.373 ± 0.055 |
| c772/+;Rdli8–10G/+ | 0.610 ± 0.059 | 0.566 ± 0.046 | 0.345 ± 0.058 |
Flies were exposed to odors and sucrose of the same concentrations as used in the learning assays (0.2% BEN, 0.4% OCT, and 1 m sucrose) during the control experiments. There were no statistically significant differences among any of the groups for each of the individual experiments (separated by table boundaries). n = 8 for each group. Means ± SEM are shown.
Interestingly, we found that for all 3 groups without starvation, and for the 2 control groups with 7 h of starvation, the performance scores were significantly below zero, suggesting that sucrose is an aversive cue without starvation, although the sucrose was presented to the flies in dry form on a filter paper (Fig. 2C). To eliminate the possibility that this aversiveness was due to an aspect of experimental handling such as tapping and transferring the flies during training, we performed the learning assay using well fed, wild-type flies with and without sucrose. The flies trained with sucrose exhibited a performance score that was significantly less than zero (–0.183 ± 0.048, p < 0.001), whereas flies trained and tested under identical conditions but without sucrose exhibited a performance score not significantly different from zero (–0.023 ± 0.049, p > 0.05). These data further suggested that sucrose is aversive to well fed flies. We confirmed this by directly measuring the naive preference of wild-type flies without starvation to a T-maze arm containing sucrose to one without sucrose. Flies exhibited a slight avoidance of the arm containing sucrose (Fig. 2D). This observation is consistent with a recent report that pregnant female flies choose egg-laying sites without sucrose over those that contain sucrose (Yang et al., 2008). We conclude that dry, sucrose-laced filter paper is aversive to well fed flies and can be associated with other sensory stimuli like odors. Starvation overcomes this aversion such that the dry, sucrose-laced filter paper becomes an appetitive cue.
Interaction of Rdl and other learning and memory genes
Since RDL inhibits both aversive and appetitive olfactory learning, it must affect pathways common to both types of learning. The two types of olfactory learning differ in their US pathways, so RDL could either affect the CS pathway that conveys odor information, or potentially a common point downstream of the CS pathway and CS/US integration. To dissect these two possibilities, we performed several experiments to study RDL's role relative to other known gene products involved in olfactory learning.
The cAMP signaling pathway plays an essential role in the MBs to mediate olfactory memory formation in Drosophila (Davis, 2005; McGuire et al., 2005). The rutabaga (rut) gene encodes an adenylyl cyclase thought to be involved in the acquisition rather than consolidation of learning (Dudai et al., 1988). The activity of Rut is dependent on both Ca2+/calmodulin and a G-protein pathway (Levin et al., 1992), which could correspond to the CS and US inputs into the MBs, similar to the model proposed for classical conditioning in Aplysia (Abrams et al., 1991). The simultaneous activation by Ca2+/calmodulin and G-protein stimulation makes Rut a potential coincidence detector for the convergence of the conditioned and unconditioned stimuli in the MBs (Dudai et al., 1988; McGuire et al., 2005). If RDL inhibits olfactory learning by participating in a process upstream from this CS/US integration, then Rdl knock down should have no effect on the performance of rut mutants. If, however, RDL inhibits olfactory learning by participating in a process downstream from this CS/US integration, then Rdl knock down should potentiate the olfactory learning of rut mutants as it does in wild-type flies.
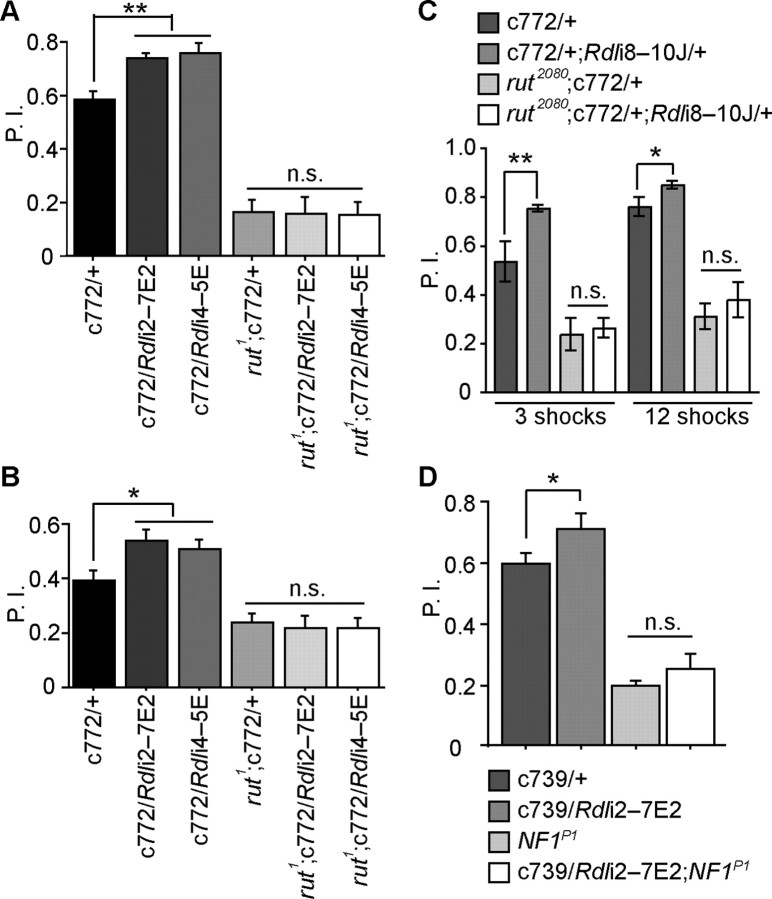
We found that while a knock down of Rdl on wild-type background enhanced both aversive and appetitive olfactory learning, it failed to enhance either form of olfactory learning on the rut1 mutant background (Fig. 3A,B). The rut1 allele is a point mutation that abolishes the enzymatic activity of the protein (Levin et al., 1992). To rule out the possibility that this effect was allele specific, we also tested Rdl knock down on the rut2080 background, which carries a P element insertion that disrupts the transcription of the rut gene. Again, the learning enhancement normally observed with Rdl knock down was abolished by this allele of the rut gene (Fig. 3C). These results suggest that the inhibitory effects of RDL on learning occurs upstream of the actions of the Rut adenylyl cyclase.
Figure 3.
Knocking down Rdl does not enhance learning in learning mutants defective in the cAMP pathway. A, Flies were trained using 3 pulses of electric shock during a CS+ odor presentation of 1 min. Flies carrying either Rdl RNAi2–7E2 or RNAi4–5E driven by c772-Gal4 showed significantly enhanced aversive learning. When combined with rut1, they failed to show any enhancement. B, Flies were starved for 16 h and trained using 1 m sucrose during a CS+ odor presentation of 1.5 min. While flies carrying the two Rdl RNAi lines driven by the c772-Gal4 driver showed significantly enhanced appetitive learning, they failed to show any learning enhancement when combined with rut1. C, Flies were trained using 3 or 12 pulses of electric shock during a CS+ odor presentation of 1 min. While flies carrying the c772-Gal4 driver and the Rdli8–10J transgene showed enhanced aversive learning on the wild-type background, they failed to show any learning enhancement on the rut2080 mutant background. D, Flies were trained using 3 pulses of electric shock during a CS+ odor presentation of 1 min. While flies carrying another MB driver c739-Gal4 and the Rdli2–7E2 transgene showed enhanced aversive learning on the wild-type background, they failed to show any learning enhancement on the NF1P1 mutant background. For all panels, n = 6 for each group under each condition. Means ± SEM; *p < 0.05; **p < 0.01 (Fisher's PLSD); n.s., not significant.
The tumor-suppressor gene Neurofibromatosis 1 (NF1) encodes a protein that has Ras-GAP activity along with stimulatory activity for the rut-encoded adenylyl cyclase (Guo et al., 1997, 2000; Tong et al., 2002). NF1 mutants exhibit impaired performance after aversive olfactory conditioning (The et al., 1997; Guo et al., 2000; Ho et al., 2007). NF1P1 is a mutation lacking most of the NF1 gene due to a P element mediated imprecise excision (Guo et al., 2000). When we tested the effects of Rdl knock down with another MB driver c739-Gal4, which exhibits a high level of expression in the MB α/β neurons and low expression in the antennal lobes (Stocker et al., 1997; Armstrong et al., 1998; Akalal et al., 2006), we again observed an enhancement of performance after aversive learning (Fig. 3D). However, the c739-Gal4/Rdl-RNAi combination failed to enhance the performance of NF1P1 mutants. This suggests that NF1, like the rut-encoded adenylyl cyclase, functions downstream of RDL in learning. The combined results argue that RDL functions independently of the US pathway, and upstream of the Rut/NF1-dependent integration steps. We conclude, therefore, that the effects of RDL are specific to the CS pathway.
In Drosophila, the dDA1 gene encodes a dopamine receptor highly expressed in the MBs (Kim et al., 2003), and dDA1 mutation completely abolishes olfactory learning (Kim et al., 2007a). Previous results also show that both RDL and dDA1 are involved in memory acquisition but not memory stability (Kim et al., 2007a; Liu et al., 2007). Furthermore, it has been suggested that the dopaminergic pathway conveys US information used for aversive olfactory learning (Davis, 1993; Han et al., 1996; Schwaerzel et al., 2003). If this is true, then simultaneously potentiating the CS pathway with knock down of Rdl and the US pathway by over expressing dDA1 might produce even higher levels of learning.
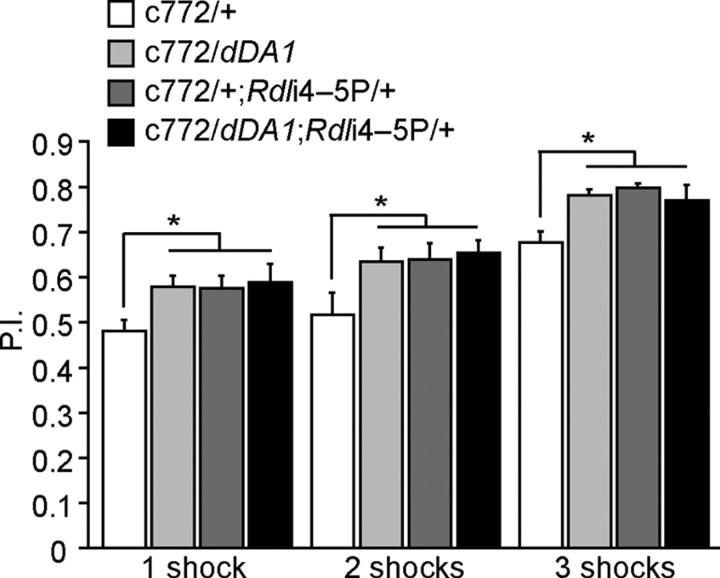
We tested the effects of manipulating the expression level of these receptors in the MBs. Over expression of dDA1 alone enhanced aversive olfactory learning, similar to the effects of Rdl knock down (Fig. 4). This observation indicates that the dDA1 receptor expression level in a wild-type background limits performance. Combining the two transgenes did not produce a further significant enhancement over the effects of either single transgene. These results are consistent with the possibility that the over expression of the dDA1 receptor or knock down of RDL enhances learning through distinct mechanisms, but that either is sufficient to produce ceiling levels of performance. Alternatively, the dDA1 receptor may play some role in the CS pathway, thus overlapping the effects of RDL.
Figure 4.
Knocking down Rdl and over expressing dDA1 enhances learning nonsynergistically. Flies were trained using 1, 2, and 3 pulses of electric shock during a CS+ odor presentation of 1 min. Flies carrying the c772-Gal4 driver and either the Rdli4–5P or the UAS-dDA1 transgene exhibited enhanced learning. Combining both transgenes failed to produce a further enhancement over either single treatment. n = 12 for each group for 1 and 3 shocks; n = 18 for the 2 shock groups. Means ± SEM; *p < 0.05; **p < 0.01 (Fisher's PLSD).
Discussion
The level of Rdl expression in the MBs affects the calcium response observed in these neurons when animals are presented with odor but not shock stimulus (Liu et al., 2007). This provided the basis for hypothesizing that RDL might specifically regulate the CS pathway for olfactory learning. Data presented here showed that the level of Rdl expression the MBs influenced both aversive and appetitive olfactory learning, which share a common CS pathway. Thus, these observations are consistent with the CS pathway-specific hypothesis. Rdl knock down failed to produce enhanced learning when combined with mutations of either the rut or NF1 gene, both of which may be involved in the process of integration of CS and US information. This observation argues against the possibility that RDL acts downstream of CS/US integration, providing further support for RDL's role in the CS pathway.
Prior experiments have shown that blocking neurotransmitter release from dopaminergic neurons impairs aversive olfactory learning but not appetitive olfactory learning, while blocking the synthesis of octopamine impairs appetitive olfactory learning but not aversive olfactory learning (Schwaerzel et al., 2003). This is consistent with the simple model (Davis, 1993; Han et al., 1996, 1998) that the neuromodulators are involved in US pathways for learning, with octopamine delivering only appetitive US (sugar) and dopamine delivering only aversive US (electric shock). This model also suggests that increasing the expression level of dDA1 will increase aversive US input, and thereby enhance aversive learning, as long as other factors such as dopamine release are not limiting. We tested this possibility and provided evidence for increased performance with increased expression of dDA1 in the MBs. Since knocking down Rdl increases the CS signal, it follows that combining over expression of dDA1 with knock down of Rdl might enhance learning synergistically, and produce an even greater enhancement of learning. However, we did not detect any synergism between these two: although dDA1 over expression alone and Rdl knock down alone both enhanced olfactory learning, the combined treatments failed to produce a significantly higher performance score than either treatment alone (Fig. 4). Two possible hypotheses can account for these results. The learning enhancement of either treatment produces performance close to ceiling levels, where no further enhancement can be detected. Alternatively, the dDA1 receptor, and thus the dopamine system, plays some role in the CS pathway that overlaps with RDL, such that the two learning enhancing effects do not sum. We prefer the later possibility for two reasons. First, functional imaging of the dopaminergic neurons projecting to the MBs using calcium reporters has revealed that these neurons respond not only to shock stimuli presented to the fly, but also to odor stimuli (Riemensperger et al., 2005) (M. Mao and R. L. Davis, unpublished observations). This indicates that the response properties of these neurons are not specific to the US pathway, which is predicted by the “US pathway only” hypothesis. Rather, dopaminergic neurons respond to the CS and are therefore intertwined in some way with the CS pathway. Second, flies mutant for the dDA1 gene exhibit impairment in both aversive and appetitive olfactory learning, both of which can be rescued by expressing dDA1 in the MBs (Kim et al., 2007a). This observation suggests that dDA1 may play a role in the CS pathway like RDL. An overriding conclusion is that the model (Davis, 1993; Han et al., 1996, 1998) envisioning aversive and appetitive specific US pathway roles for dopamine and octopamine, respectively, is overly simplistic
Our results suggest that the GABAA receptor RDL regulates the CS pathway in Drosophila olfactory learning. The conclusion that the GABAA receptor modulates the CS pathway for learning is not limited to either insects or learning supported by olfactory cues. During taste aversion learning in mice, pre-exposure to the CS of the tastant alone causes latent inhibition where the mice show reduced learning to the CS after pairing the CS with the US. This phenomenon is distinctly absent in male mice carrying a point mutation in the α5 subunit of the GABAA receptor, which is highly expressed in the hippocampus (Gerdjikov et al., 2008). Since CS information is the only stimulus presented during the pre-exposure period, these results support the role of GABAA receptors in regulating the CS pathway. Extinction is another type of learning where repeated exposure to the CS alone after CS/US conditioning reduces the CR. Systemic administration of a GABAA receptor antagonist blocks the development and expression of extinction in rats during contextual fear learning (Harris and Westbrook, 1998). Since extinction trials are composed of the CS exposure by itself, these results also indicate that GABAA receptors modulate the CS pathway. Moreover, other studies have shown that the surface expression of GABAA receptors increases in the basolateral amygdala after extinction trials following fear conditioning (Chhatwal et al., 2005). These results indicate that CS exposure alone during extinction is sufficient to modulate the cellular trafficking of GABAA receptors, again indicating a role for GABAA receptors in the CS pathway. Our results, together with these previous studies, strongly indicate that GABAA receptors regulate the CS pathway for associative learning.
A role for GABAA receptors in suppressing learning by regulating the CS pathway has at least two broad implications. First, it suggests that the receptors provide a gate to the association center (MBs). Other molecules may also provide similar gates, but learning must overcome this negative influence for memory formation to occur. This gate is probably nonspecific relative to odor type, that is, the GABAA receptor gate suppresses learning to most or all odors. It follows that learning must mobilize cellular mechanisms for overriding the gate. These could be at the level of the presynaptic GABAergic neurons, such that the presynaptic neurons release less neurotransmitter after learning, or they could be at the level of the postsynaptic receptor, with receptor expression, sensitivity, or conductance altered by learning. We have previously provided evidence for a reduced presynaptic release following learning (Liu and Davis, 2009), but postsynaptic mechanisms may occur as well (Chhatwal et al., 2005). Second, events or processes that alter the salience of the CS and its ability to enter into associations might function via altering the presynaptic GABAergic release or the postsynaptic GABAA receptors. For instance, spaced conditioning is generally more effective in producing long-lasting memories compared with massed conditioning (Tully et al., 1994; Beck et al., 2000). It is possible that the rest period between spaced conditioning trials allows for receptor desensitization, producing a more effective subsequent training trial. Memory acquisition becomes more difficult with age. It could be that aging alters the fluidity of the GABAA receptor gate, making acquisition more difficult.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS19904 to R.L.D. and the R. P. Doherty-Welch Chair in Science at the Baylor College of Medicine. M.E.B. is supported by a Children's Tumor Foundation Young Investigator Award and by National Institute of General Medical Sciences Grant T32GM008307. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
References
- Abrams TW, Karl KA, Kandel ER. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J Neurosci. 1991;11:2655–2665. doi: 10.1523/JNEUROSCI.11-09-02655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalal DB, Wilson CF, Zong L, Tanaka NK, Ito K, Davis RL. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem. 2006;13:659–668. doi: 10.1101/lm.221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JD, de Belle JS, Wang Z, Kaiser K. Metamorphosis of the mushroom bodies; large-scale rearrangements of the neural substrates for associative learning and memory in Drosophila. Learn Mem. 1998;5:102–114. [PMC free article] [PubMed] [Google Scholar]
- Beck CD, Schroeder B, Davis RL. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci. 2000;20:2944–2953. doi: 10.1523/JNEUROSCI.20-08-02944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Mushroom bodies and Drosophila learning. Neuron. 1993;11:1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Corfas G, Hazvi S. What is the possible contribution of Ca2+ -stimulated adenylate cyclase to acquisition, consolidation and retention of an associative olfactory memory in Drosophila. J Com Physiol [A] 1988;162:101–109. doi: 10.1007/BF01342707. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Rudolph U, Keist R, Möhler H, Feldon J, Yee BK. Hippocampal alpha 5 subunit-containing GABA A receptors are involved in the development of the latent inhibition effect. Neurobiol Learn Mem. 2008;89:87–94. doi: 10.1016/j.nlm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Guo HF, The I, Hannan F, Bernards A, Zhong Y. Requirement of Drosophila NF1 for activation of adenylyl cyclase by PACAP38-like neuropeptides. Science. 1997;276:795–798. doi: 10.1126/science.276.5313.795. [DOI] [PubMed] [Google Scholar]
- Guo HF, Tong J, Hannan F, Luo L, Zhong Y. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature. 2000;403:895–898. doi: 10.1038/35002593. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Grotewiel MS, Davis RL. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology. 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Harrison JB, Chen HH, Sattelle E, Barker PJ, Huskisson NS, Rauh JJ, Bai D, Sattelle DB. Immunocytochemical mapping of a C-terminus anti-peptide antibody to the GABA receptor subunit, RDL in the nervous system in Drosophila melanogaster. Cell Tissue Res. 1996;284:269–278. doi: 10.1007/s004410050587. [DOI] [PubMed] [Google Scholar]
- Ho IS, Hannan F, Guo HF, Hakker I, Zhong Y. Distinct functional domains of neurofibromatosis type 1 regulate immediate versus long-term memory formation. J Neurosci. 2007;27:6852–6857. doi: 10.1523/JNEUROSCI.0933-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Seong CS, Han KA. Expression of a D1 dopamine receptor dDA1/DmDOP1 in the central nervous system of Drosophila melanogaster. Gene Expr Patterns. 2003;3:237–245. doi: 10.1016/s1567-133x(02)00098-4. [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007a;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA. Classical reward conditioning in Drosophila melanogaster. Genes Brain Behav. 2007b;6:201–207. doi: 10.1111/j.1601-183X.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LR, Han PL, Hwang PM, Feinstein PG, Davis RL, Reed RR. The Drosophila learning and memory gene rutabaga encodes a Ca2+/calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12:53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Krause WC, Davis RL. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron. 2007;56:1090–1102. doi: 10.1016/j.neuron.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;137:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Murakami S. Caenorhabditis elegans as a model system to study aging of learning and memory. Mol Neurobiol. 2007;35:85–94. doi: 10.1007/BF02700625. [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Schipanski A, Yarali A, Niewalda T, Gerber B. Behavioral analyses of sugar processing in choice, feeding, and learning in larval Drosophila. Chem Senses. 2008;33:563–573. doi: 10.1093/chemse/bjn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The I, Hannigan GE, Cowley GS, Reginald S, Zhong Y, Gusella JF, Hariharan IK, Bernards A. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science. 1997;276:791–794. doi: 10.1126/science.276.5313.791. [DOI] [PubMed] [Google Scholar]
- Tong J, Hannan F, Zhu Y, Bernards A, Zhong Y. Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nat Neurosci. 2002;5:95–96. doi: 10.1038/nn792. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Vosshall LB. Into the mind of a fly. Nature. 2007;450:193–197. doi: 10.1038/nature06335. [DOI] [PubMed] [Google Scholar]
- Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319:1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]