Abstract
In this report, we have analyzed the human T cell repertoire derived in vivo from a single T cell precursor. A unique case of X-linked severe combined immunodeficiency in which a reverse mutation occurred in an early T cell precursor was analyzed to this end. It was determined that at least 1,000 T cell clones with unique T cell receptor-β sequences were generated from this precursor. This diversity seems to be stable over time and provides protection from infections in vivo. A similar estimation was obtained in an in vitro murine model of T cell generation from a single cell precursor. Overall, our results document the large diversity potential of T cell precursors and provide a rationale for gene therapy of the block of T cell development.
T cell immunity relies on the efficient recognition of a wide set of foreign peptides. Such capacity is provided by the clonal distribution of a large T cell receptor (TCR) diversity on individual CD4 and CD8 T lymphocytes (1). Several mechanisms are involved in the generation of TCR diversity. The earliest step involves stages of IL-7-dependent intense proliferation from hematopoietic stem cells to committed T cell precursors (2). Then, within the thymus, T cell precursors undergo TCR gene rearrangements by the imprecise junctions of V, (D), and J gene segments together with the addition of N nucleotides (3). Finally, self peptides in the thymus and in the periphery end the shaping of T cell diversity (4, 5). Inherited or acquired T cell deficiencies are generally reflected by high susceptibility to opportunistic infections and dramatically reduced life expectancy (6–8). Promising therapies include gene transfer to rescue autologous hematopoietic progenitors such as cycling common lymphoid progenitors (9), endowed with the capacity of generating a new T cell pool. Such approaches will depend strictly on the diversity and the stability of this pool to allow a sufficient and durable protection from infections in vivo. However, the number of T cell progenitors required for generating a functional T cell repertoire is largely unknown.
We have previously described an unusual case of X-linked severe combined immunodeficiency (10). The disease caused by a mutation in the γc cytokine receptor subunit was diagnosed on the basis of family history, clinical symptoms, and evidence of a genetic defect in the γc gene (C115R) in B cell lines. Although such mutation is known to abrogate T cell development (11), T lymphocytes were in close to normal numbers in this patient. In contrast to polymorphonuclear cells, CD19+, and CD14+ cells, the CD3+ population (previously shown to derive from the patient; ref. 10) did not bear the mutation in the γc gene and therefore originates from a reverse mutation. Because such an event is statistically highly unlikely, it is reasonable to assume that it occurred in a single cell. This assumption is reinforced by the absence of reverse mutation in all other patients with X-linked severe combined immunodeficiency studied and in particular in those who bear the same deleterious mutation (12). The absence of natural killer cells and the presence of distinct TCR as determined by TCR-BV (BV) staining strongly suggested that the reverse mutation had occurred after T/natural killer commitment but before TCRβ rearrangement. Thus, in this case, the progeny of the revertant T cell precursor has constituted the T cell pool of this patient. This phenomenon offers the unique opportunity to quantify the TCR diversity generated by a single T cell progenitor in vivo.
Materials and Methods
T Cell Repertoire Analyses.
The patient presented in this report has been described (10). T cell repertoires were analyzed by using immunoscope technology (13, 14). Briefly, RNA was extracted from blood samples containing 5 × 106 T lymphocytes. The cDNA was prepared and amplified with primers specific for one of the TCR-BV subfamilies and for the TCR-BC (BC) segment. All primers used in this study have been described elsewhere (15). PCR products were then subjected to a run-off reaction by using a nested fluorescent primer specific for the BC segment. Analysis of these products on an automated sequencer revealed several fragments spaced by three nucleotides, corresponding to the various sizes of TCRβ rearrangements present in the sample. By using the immunoscope software, the size and the intensity of each fragment were recorded and expressed as a profile corresponding to the complementary determining region 3 (CDR3) size distribution. For several BV segments, BV-BC PCR products were also subjected to run-off reactions by using fluorescent primers specific for one of the TCR-BJ (BJ) segments. TCR sequencing was performed essentially as described (16). Briefly, TCRβ chains bearing a given BV-BJ combination were amplified by PCR and cloned in Escherichia coli by using the Zero Blunt Topo cloning kit (Invitrogen). Sequence reactions were performed by using the M13(-20) primer and the ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Perkin–Elmer). Samples were run on a 377 DNA sequencer (Perkin–Elmer).
Flow Cytometry.
Peripheral blood mononuclear cells from the patient and a control donor were analyzed by using triple color immunofluorescence. Monoclonal antibodies were biotin-conjugated anti-CD4 (leu3, Becton Dickinson) or anti-CD8 (Becton Dickinson), phycoerythrin-conjugated anti-CD27 (Immunotech, Marseilles, France), fluorescein-conjugated anti-CD28 (Immunotech), and either fluorescein- or phycoerythrin-conjugated anti-CD45RO and anti-CD45RA (Immunotech). Biotin was revealed with TRIC-streptavidin (Caltag, South San Francisco, CA). Cells were analyzed on a flow cytometer (fluorescence-activated cell sorter, FACScan, Becton Dickinson) with cellquest software after gating on CD4+ or CD8+ cells.
Fetal Thymic Organ Culture (FTOC).
Fetal thymuses were carefully excised from fetuses on indicated days of gestation. Cell suspensions were prepared from 15 to 20 thymuses. The FTOC assay was performed essentially as described (17, 18). Thymic lobes were isolated from day-14 fetuses and irradiated at a dose of 3,000 rads by using a cesium γ-irradiator. For limiting dilution analysis, day-12 fetal thymocytes were suspended in Opti-MEM (GIBCO/BRL) supplemented with 10% (vol/vol) FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5 × 10−5 M β2-ME. Various numbers of cells (15, 25, and 150) were mixed with 12 individual irradiated lobes for each dilution and cultured in hanging drops. Lobes were transferred onto filters (0.8 μm, Millipore) at day 2 of culture. After 2 weeks, individual lobes were analyzed for the presence of αβ-TCR positive cells by flow cytometry, and for each point, the percentage of negative lobes was determined. The frequency of T cell progenitor was calculated from the results of two independent experiments, based on Poisson probability distribution. In the same experiments, individual lobes reconstituted with one or five T cell progenitors were randomly selected for further T cell repertoire analyses.
Results and Discussion
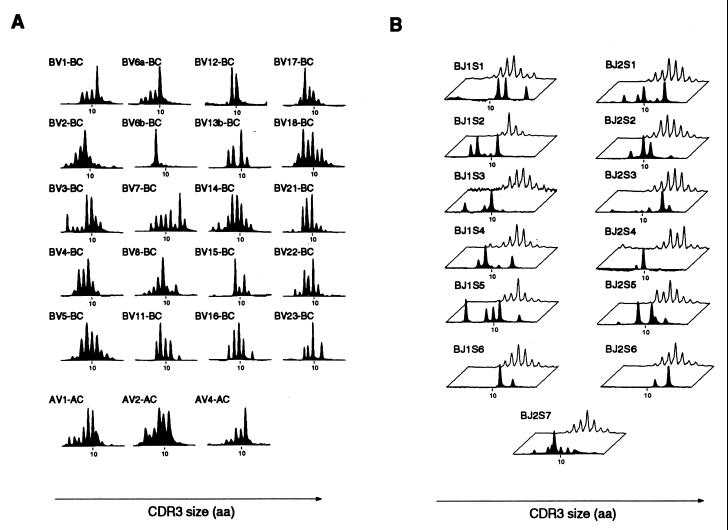
To quantitate the number of distinct T cell clones generated from the revertant progenitor in the aforementioned patient, we first analyzed CDR3 size distributions of the patient's peripheral T cells. This experiment was performed when the patient was 3 years old, the first time point of the present study. This approach provides a global picture of TCRβ and TCRα diversity by revealing the number of distinct CDR3 lengths associated with a given BV or TCR-AV (AV) segment (13). This analysis was performed for almost all BV and three AV segments and is shown in Fig. 1A. Interestingly, numerous TCRβ rearrangements were observed: all BV segments were used, and for each of them, several CDR3 lengths were detected, although with a limited number of CDR3 lengths for some of them (as in BV6b, BV12, or BV15). The three AV profiles performed yielded similar results (Fig. 1A). We then increased the level of resolution of the analysis by examining, for five BV segments (BV1, BV2, BV3, BV6b, and BV7), the CDR3β profiles of T cells sharing all possible BV-BJ combinations. As an example, the various BV7-BJ profiles are shown in Fig. 1B. When compared with a healthy control, it can be seen that fewer distinct CDR3β sizes are detected in the patient's profiles. This observation is likely to reflect the limited complexity of the patient's T cell repertoire. About 50 peaks were detected in BV7-BJs profiles, indicating that at least 50 distinct TCRβ chains bearing the BV7 segment had been generated. Similar numbers were obtained with the other BV segments (43.2 ± 9.4). Four BV-BJ combinations were randomly selected for further cloning and sequencing of TCRβ rearrangements. A limited number of distinct TCRβ rearrangements (3–8) were detected, confirming the oligoclonality of the T cell population bearing a given BV-BJ combination (data not shown). On average, each peak corresponded to 1.6 distinct TCRβ sequences (SD = 0.8; n = 15 peaks analyzed). By counting the average number of distinct rearrangements sharing a BV and a BJ segment and multiplying by the number of BV-BJ combinations, we calculated that at least 1,000–2,000 distinct TCRβ chains had been generated from the revertant progenitor. This number may be an underestimation, because it is possible that some T cell clones of very low frequency were not detected by this approach. Because the patient's T cell pool contained 109 lymphocytes and was mostly comprised of 1,000 TCRβ clonotypes, an average of 106 T cells shared the same TCRβ sequence. This huge number of cells per clone could be explained by the empty space in the T cell compartment, which may have allowed each rearranged clone to expand considerably (about 20 cell divisions) in the periphery. It is likely that the actual number of distinct αβ-TCRs is higher than the TCRβ diversity. Indeed, after TCRβ gene rearrangement, thymocytes continue dividing before undergoing TCRα rearrangement (19, 20). In addition, studies have shown that, in healthy humans, a given TCRβ chain is generally associated with more than 25 distinct TCRα chains (21). If the same is true in the patient studied, more than 25,000 distinct T cell clones would have arisen from the revertant progenitor. However, this diversity remains at least 1,000 times lower than that usually observed in healthy individuals (21).
Figure 1.
Numerous distinct T cell clones have been generated from a single T cell progenitor. (A) cDNA prepared from the peripheral blood mononuclear cells of the patient (at 3 years of age) was amplified in multiple PCRs primed by BV- and BC-specific primers. PCR products were then subjected to run-off reactions by using a nested fluorescent primer specific for the BC segment. Labeled DNA copies were analyzed on a sequencing gel in an automated DNA sequencer. The size and the intensity of each band were recorded and analyzed with the immunoscope software. CDR3 size distribution analysis of the patient's peripheral T cells was performed for almost all BV segments and for three AV segments. (B) Altered CDR3β distribution in all BV-BJ profiles analyzed from the patient. For several BV segments, BV-BC PCR products obtained from either a healthy donor or the patient were subjected to run-off reactions by using nested fluorescent primers specific for one of the BJ segments. CDR3β size distribution profiles of the patient (black profiles) are compared with those obtained from the healthy donor (white profiles).
The stability of this repertoire was examined over a period of 2 years. Representative CDR3β and CDR3α profiles obtained from the patient at the ages of 3 and 5 years are compared in Fig. 2. In most of the profiles, dominant peaks (indicated by arrows in Fig. 2) are conserved over time, indicative of the persistence of some of the most abundant clones. This persistence was confirmed by sequencing the TCRβ rearrangements bearing a given BV-BJ combination at various time points (BV1-BJ2S5 bearing rearrangements are shown in Table 1). Variations were, however, detected in several profiles (for example, see the BV18 profile), suggesting evolution of clonotype frequencies. Finally, the estimation of TCR diversity at the ages of 3 and 5 yielded a similar number (Fig. 2, Table 1, and data not shown). In summary, although changes in the relative contribution to the repertoire of some individual T cell clones occurred, no obvious gain or loss of TCR diversity was observed during this 2-year period.
Figure 2.
Relative stability of the patient's T cell repertoire over time. CDR3β size distribution analyses were performed from the patient's peripheral blood mononuclear cells at various time points. The figure shows the comparison of the profiles obtained at the age of 3 (black profiles) and 5 (white profiles) years. Arrows indicate the persistence of some expanded populations.
Table 1.
TCRβ rearrangements bearing the BV1-BJ2S5 gene segments
| CDR3β sequences (nucleotides) | CDR3β sequences (amino acids) | Age 3* | Age 5 |
|---|---|---|---|
| AGC GTA GCT ACG GCG GGT GGG GCC CAG | SVATAGGAQ | 7/23 | 13/22 |
| AGC ACT ACT AGC GGG GTC CAA GAG ACC CAG | STTSGVQETQ | 7/23 | 3/22 |
| AGC GTA GGC GGG TTC CAA GAG ACC CAG | SVGGFQETQ | 3/23 | 2/22 |
| AGC GTA CCA GGG AAA GAG ACC CAG | SVPGKETQ | 2/23 | 0/22 |
| AGC GTA TCA CTA GCG GGG GGG CAA GAG ACC CAG | SVSLAGGQETQ | 2/23 | 0/22 |
| AGC GTA CAT ACT AGC GAT CGG GAG ACC CAG | SVHTSDRETQ | 1/23 | 0/22 |
| AGC CCA CTC GGG GCA CTA GAG GGA GAG ACC CAG | SPLGALEGETQ | 1/23 | 0/22 |
| AAG ACG GGG GCC CAG AGA GAG ACC CAG | KTGAQRETQ | 0/23 | 2/22 |
| AGC GAG ACA GGG TCC GAG ACC CAG | SETGSETQ | 0/23 | 1/22 |
| AGC GTA GGA GAG TCC GGG GAG ACC CAG | SVGESGETQ | 0/23 | 1/22 |
*Sequence occurrence/total number of sequence.
The patient's T cell counts were slightly below normal ranges during this 2-year period (Table 2). Phenotypic examination of the T cell subsets was unremarkable, except that CD8 represented the major T cell population. In addition, naive circulating T cells expressing the CD45RA+CD27+ phenotype were almost undetectable, both in CD4 and CD8 populations (Fig. 3). This finding likely reflects the absence of a renewal of the T lymphocyte population from functional lymphoid progenitors. Among the CD8 cells, only 2% had the CD45RA+CD45RO− phenotype (data not shown). A large subset (50–65%) was found to express both CD45 isoforms but neither CD27 nor CD28 (Fig. 3). These cells had down-regulated CD62L and up-regulated CD95 (data not shown). Based on previous observations (22), this population contains both memory- and effector-type cells generated through antigen-dependent differentiation and proliferation processes. Both the absence of naive circulating T cells and the stability of TCR repertoire over time overall suggest that one wave of thymopoiesis had occurred from the revertant precursor, followed by the persistence and expansion of T cell clones.
Table 2.
Immunological investigations of the 6-year-old patient
| Cell type or stimulation | Patient | Normal range or control value |
|---|---|---|
| Blood lymphocyte subsets, cells per μl* | ||
| T lymphocytes (CD3+) | 825 | 1,200–2,500 |
| B lymphocytes (CD19+) | 165 | 100–600 |
| Natural killer cells (CD56+) | <15 | 100–500 |
| T cell subsets, %* | ||
| CD4+ | 16 | 60–80 |
| CD8+ | 58 | 20–40 |
| TCRαβ | 88 | 90–100 |
| TCRγδ | 12 | 0–10 |
| CD132(γc) | 98 | 95–100 |
| T cell proliferation, cpm × 10-3†‡ | ||
| None, day 4 | 0.3 | 0.4 ± 0.2 |
| Phytohemagglutinin | 5.0 | 34.0 ± 15.0 |
| OKT3 | 5.0 | 35.5 ± 13.2 |
| None, day 6 | 0.3 | 0.3 ± 0.1 |
| Tetanus toxoid | 0.2 | 23.2 ± 9.7 |
| Purified protein derivative | 4.5 | 24.0 ± 8.1 |
| Allogeneic cells | 2.3 | 34.9 ± 11.1 |
Absolute numbers of T, B, and natural killer cells and percentages of the various T cell subsets were determined by flow cytometric analyses following standard procedures.
Proliferation assays were performed as described (10) during a period of 4 days for phytohemagglutinin and OKT3 and of 6 days for antigens and allogeneic cells stimulations.
Values in the right column are means ± SD.
Figure 3.
Phenotypic characterization of the patient's T cell subsets. Peripheral blood mononuclear cells from the patient and from the control donor were triple stained with the indicated antibodies. Profiles are gated on either CD4 or CD8 positive cells.
In vitro, functional analysis detected a T cell response against mitogens and allogeneic cells, although at a lower level than that observed with control cells (Table 2). The patient's T cell population was also reactive to purified protein derivative antigen, whereas reactivity to tetanus toxoid was apparently lost. In vivo, T cells seemed generally functional, because no opportunistic infection occurred in this child. He developed varicella without complication. B cell functions were altogether low, and the patient required intravenous Ig substitution. Remarkably, despite some immune impairment, the good clinical outcome of the patient during his first 6 years of life indicates that the T lymphocyte repertoire generated from a single T cell precursor in vivo is grossly able to cope with a large number of foreign antigens. Degeneracy of TCR specificities may participate to the broad functional response indicated by this reduced number of clones (23).
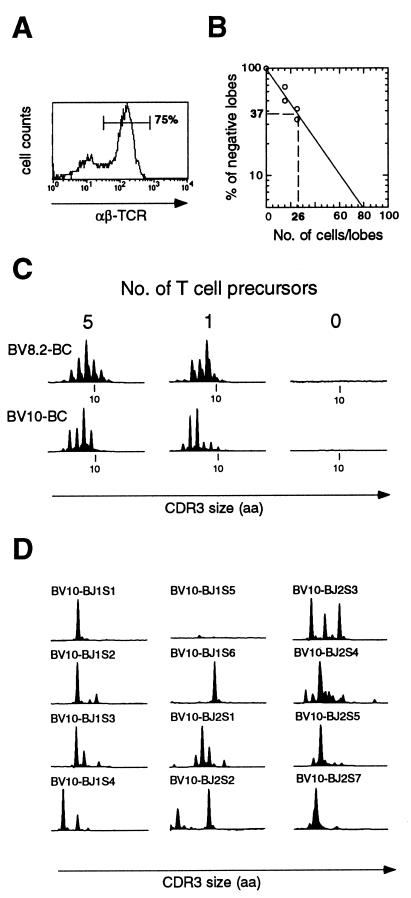
Our results predict that a T cell precursor can undergo at least 10 cell divisions before rearranging the TCRβ locus. To extend this observation, we designed an in vitro assay to estimate the TCR diversity generated by a single murine T cell progenitor by using a limiting dilution assay combined with FTOC. To this end, various numbers (15, 25, and 150 cells) of fetal thymocytes from day-12 embryos were seeded into irradiated day-14 thymuses and maintained in culture. After 2 weeks, individual thymic lobes were analyzed for reconstitution by fluorescence-activated cell sorter analysis. Reconstituted lobes contained from 20,000 to 50,000 cells. Surface expression of αβ-TCR was systematically detected on the majority of cells derived from reconstituted lobes (Fig. 4A). No cellularity was observed in nonreconstituted lobes. For each number of fetal thymocytes seeded, the percentage of reconstituted lobes was calculated. The frequency of T cell precursors among day-12 fetal thymocytes was then determined by a limiting dilution analysis and estimated to be 1 in 26 cells (Fig. 4B). Accordingly, an inoculum of 15 thymocytes contains on average less than one T cell progenitor. The reconstituted lobes were used to analyze the TCR diversity generated by one or five T cell precursors (corresponding to inoculum of 15 or 150 fetal thymocytes, respectively). For that purpose, the CDR3β size distributions of the generated T cells were analyzed. Fig. 4C shows BV8.2 and BV10 profiles obtained from representative FTOCs seeded with the equivalent of one or five T cell precursors (Fig. 4C, Left and Center). As a negative control, the same procedure was applied to a nonreconstituted thymus (Fig. 4C, Right). In reconstituted lobes, the detected CDR3β sizes corresponded to in-frame rearrangements, and multiple TCRβ chains have been generated as shown by the distinct peaks systematically visible on each of the BV-BC combination analyzed (Fig. 4C). The number of TCRβ chains bearing a given BV segment was estimated by analyzing the corresponding BV-BJ profiles. A representative BV10 repertoire, corresponding to a lobe reconstituted from a single precursor, is shown in Fig. 4D. Because the number of peaks obtained reflects the minimal number of distinct TCRβ chains, it can be deduced from this analysis that at least 30 distinct TCRβ chains bearing the BV10 segment were generated in this lobe. Similar numbers were obtained when analyzing other BV-BJ combinations in several individual lobes (not shown). We calculated that a minimum of 500 distinct TCRs had been generated from a single T cell progenitor. It may be difficult to compare the diversity observed in this assay to that observed in the patient. Some differences could exist between human and murine T cell development. In addition, thymocytes were analyzed in the mouse model, whereas peripheral memory T cells were studied in the patient. Both studies, however, indicate that T cell precursors can undergo at least 9–10 cell divisions before rearranging the TCRβ locus, providing a somewhat diversified T cell repertoire.
Figure 4.
TCR diversity generated in vitro by a single murine T cell progenitor. Various number of fetal thymocytes (15, 25, or 150) were seeded in individual γ-irradiated lobes. After 2 weeks of FTOC, lobes were examined by flow cytometry for the presence of αβ-TCR positive cells. (A) Representative fluorescence-activated cell sorter profile of cells from an individual reconstituted thymic lobe. The majority of the cells expressed αβ-TCR. No cellularity was observed in nonreconstituted (negative) lobes. For each point, the percentage of negative lobes was calculated. (B) Estimation of T cell precursor frequency. According to the Poisson probability distribution, the inoculum that produces 37% negative recipients is expected to contain a single T cell progenitor. This frequency was estimated to be 1 in 26 cells. Therefore, lobes seeded with 15 cells, when reconstituted, contain the progeny of a single T cell precursor. T cell repertoire analyses were performed on these individual lobes. (C) Analysis of the T cell repertoire in lobes reconstituted with one or five T cell precursors. cDNAs prepared from individual thymic lobes were submitted to PCR by using BV10- (or BV8.2-) and BC-specific primers. Representative CDR3β size distribution profiles corresponding to an unreconstituted lobe or a lobe reconstituted with one or five T cell progenitors are shown. (D) Analysis of BV10-BJ rearrangements in lobes reconstituted with a single T cell precursor.
In several immune diseases such as severe combined immune deficiencies, T lymphocyte differentiation is blocked as the result of various genetic defects. The observation that a partially diversified and functional TCR repertoire can be generated from a single T cell precursor indicates that a strong positive pressure for survival, proliferation, and differentiation of the T lymphocytes exists in vivo. In these conditions, despite the low efficiency of retrovirally mediated gene transfer into human hematopoietic stem cells, T cell deficiencies could be overcome by the gene therapy-mediated rescuing of the very few existing transduced stem cells or cycling common lymphoid progenitors. The absence of self-renewal of the latter compartment might not be an obstacle, because the generated T cells are long lived as shown in this study.
Acknowledgments
We thank Drs. A. Cumano and M. Mempel for comments on the manuscript. This work was supported by grants from l'Institut National de la Santé et de la Recherche Médicale and l'Assistance Publique Hôpitaux de Paris. P.B. is supported by the Délegation Générale de l'Armement.
Abbreviations
- TCR
T cell receptor
- BV
TCR-BV
- BC
TCR-BC
- BJ
TCR-BJ
- AV
TCR-AV
- CDR3
complementary determining region 3
- FTOC
fetal thymic organ culture
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Davis M M, Bjorkman P J. Nature (London) 1988;334:395–401. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 2.Malek T R, Porter B O, He Y W. Immunol Today. 1999;20:71–76. doi: 10.1016/s0167-5699(98)01391-7. [DOI] [PubMed] [Google Scholar]
- 3.Alt F W, Oltz E M, Young F, Gorman J, Taccioli G, Chen J. Immunol Today. 1992;13:306–314. doi: 10.1016/0167-5699(92)90043-7. [DOI] [PubMed] [Google Scholar]
- 4.Sant'Angelo D B, Lucas B, Waterbury P G, Cohen B, Brabb T, Goverman J, Germain R N, Janeway C A., Jr Immunity. 1998;9:179–186. doi: 10.1016/s1074-7613(00)80600-7. [DOI] [PubMed] [Google Scholar]
- 5.Viret C, Wong S, Janeway C. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 6.Rosen F S, Cooper M D, Wedgwood R J. N Engl J Med. 1995;333:431–440. doi: 10.1056/NEJM199508173330707. [DOI] [PubMed] [Google Scholar]
- 7.Fischer A, Cavazzana-Calvo M, De Saint Basile G, DeVillartay J P, Di Santo J P, Hivroz C, Rieux-Laucat F, Le Deist F. Annu Rev Immunol. 1997;15:93–124. doi: 10.1146/annurev.immunol.15.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Fischer A, Malissen B. Science. 1998;280:237–243. doi: 10.1126/science.280.5361.237. [DOI] [PubMed] [Google Scholar]
- 9.Kondo M, Akashi K, Domen J, Sugamura K, Weissman I L. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 10.Stephan V, Wahn V, Le Deist F, Dirksen U, Broker B, Muller-Fleckenstein I, Horneff G, Schroten H, Fischer A, de Saint Basile G. N Engl J Med. 1996;335:1563–1567. doi: 10.1056/NEJM199611213352104. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Yi H, Rosenblatt H M, Filipovich A H, Adelstein S, Modi W S, McBride O W, Leonard W J. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 12.Puck J M. Immunol Today. 1996;17:507–511. doi: 10.1016/0167-5699(96)30062-5. [DOI] [PubMed] [Google Scholar]
- 13.Pannetier C, Cochet M, Darche S, Casrouge A, Zöller M, Kourilsky P. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pannetier C, Even J, Kourilsky P. Immunol Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 15.Pannetier C, Levraud J-P, Lim A, Even J, Kourilsky P. In: The Antigen T Cell Receptor: Selected Protocols and Applications. Oksenberg J R, editor. Austin, TX: Landes; 1997. pp. 287–325. [Google Scholar]
- 16.Bousso P, Levraud J P, Kourilsky P, Abastado J P. J Exp Med. 1999;189:1591–1600. doi: 10.1084/jem.189.10.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkinson E J, Franchi L L, Kingston R, Owen J J T. Eur J Immunol. 1982;12:583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 18.Ema H, Douagi I, Cumano A, Kourilsky P. Eur J Immunol. 1998;28:1563–1569. doi: 10.1002/(SICI)1521-4141(199805)28:05<1563::AID-IMMU1563>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Dudley E C, Petrie H T, Shah L M, Owen M J, Hayday A C. Immunity. 1994;1:83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 20.von Boehmer H, Fehling H J. Annu Rev Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- 21.Arstila T P, Casrouge A, Baron V, Even J, Kanellopolous J, Kourilsky P. Science. 1999;29:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 22.Hamann D, Baars P A, Rep M H, Hooibrink B, Kerkhof-Garde S R, Klein M R, van Lier R A. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason D. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]