Abstract
Objective
Although vigabatrin irreversibly constricts the visual field, it remains a potent therapy for infantile spasms and a third-line drug for refractory epilepsies. In albino animals, this drug induces a reduction in retinal cell function, retinal disorganisation and cone photoreceptor damage. The objective of this study was to investigate the light dependence of the vigabatrin-elicited retinal toxicity and to screen for molecules preventing this secondary effect of vigabatrin.
Methods
Rats and mice were treated daily with vigabatrin 40mg and 3mg, respectively. Retinal cell lesions were demonstrated by assessing cell function with electroretinogram measurements, and quantifying retinal disorganization, gliosis and cone cell densities.
Results
Vigabatrin-elicited retinal lesions were prevented by maintaining animals in darkness during treatment. Different mechanisms including taurine deficiency were reported to produce such phototoxicity; we therefore measured amino acid plasma levels in vigabatrin-treated animals. Taurine levels were 67% lower in vigabatrin-treated animals than in control animals. Taurine supplementation reduced all components of retinal lesions in both rats and mice. Among 6 vigabatrin-treated infants, the taurine plasma level was found to be below normal in three patients and undetectable in two patients.
Interpretation
These results indicate that vigabatrin generates a taurine deficiency responsible for its retinal phototoxicity. Future studies will investigate whether co-treatment with taurine and vigabatrin can limit epileptic seizures without inducing the constriction of the visual field. Patients on vigabatrin could gain immediate benefit from reduced light exposures and dietetic advice on taurine-rich foods.
Introduction
Epilepsy affects 1% of the world population and can be life threatening. Vigabatrin, (gamma-vinyl GABA, or VGB), was widely prescribed for the treatment of epilepsy1 because it reduces the occurrence of seizures by increasing the GABA concentration, the main inhibitory neurotransmitter of the central nervous system. VGB irreversibly inhibits GABA transaminase, the GABA degrading enzyme. Unfortunately, chronic administration of VGB was found to induce a bilateral constriction of the visual field in 10 to 40% of treated patients2–4. There have been several reports of altered visual acuity5, colour discrimination6 or contrast sensitivity6, 7 whereas other studies, such as the longitudinal cohort studies sponsored by the National Health Service of Scotland, have demonstrated preservation of central and colour vision in patients with bilateral constriction8. Consistent with these potential alterations in the cone pathway, photopic electroretinograms (ERG) and the flicker response are decreased in amplitude7, 9, 10. Despite these irreversible visual effects, VGB remains in infantile spasms the only alternative to adrenocorticotrophic hormone (ACTH) or steroid therapy1, 11–16. It is also prescribed as a third-line drug for other refractory epilepsies in Europe1. Furthermore, it is being evaluated for treatment of heroin, cocaine and methamphetamine addictions17, 18.
In rats, VGB accumulates in the retina at a concentration five-fold higher than in the brain19. Consequently, GABA concentrations increase five-fold higher in the retina and two-fold higher in the brain than those of control rats20, 21. VGB treatment disorganises the photoreceptor layer in the peripheral retina22–24, damages cone photoreceptors in broader areas23, 24 and reduces the photopic ERG and flicker responses23. The lesion area is marked by major glial reaction, and can be visualised by immunohistochemical staining for the glial fibrillary acidic protein (GFAP)23, a hallmark of retinal lesions. In vitro, VGB-elicited photoreceptor degeneration was reported to be light-dependent25. This is consistent with the selective VGB sensitivity of albino but not pigmented animals22.
To further assess the light dependence of VGB-elicited toxicity, we kept animals in darkness during chronic VGB treatment and examined the underlying mechanism of this phototoxicity.
Methods
Animal treatments
As described previously23, 24, Wistar rats Rj Wi IOPS Han or BALB/c mice were purchased from Janvier (Le Genest-St-Isle, France) at between six and seven weeks of age. VGB dissolved in 0.9% NaCl was administered at 40mg (125 mg/ml, 0.32ml) to rats by daily intraperitoneal injection for 45 or 65 days and at 3mg (60mg/ml, 0.05ml) to mice for 29 days. These daily doses (rats:200mg/kg; mice:150mg/kg) are in-line with those described for the treatment of epilepsy (adult patients: 1–6mg/kg; children:50–75mg/kg; or infants: 100–150 mg/kg)11, 26, 27. Taurine supplementation was given in drinking water at a concentration of 0.1M. Light intensity in the animal cages ranged between 125 and 130 lux for rats and between 70 and 85 lux for mice.
Electroretinogram (ERG)
Photopic ERGs were recorded after the last VGB injection, as described previously23. Anesthesia was induced by intraperitoneal injection (0.8 to 1.2 ml/kg) of a solution containing ketamine (40 mg/ml) and xylazine (4 mg/ml Rompum). Animals were light-adapted for 10 minutes with a background light of 25 cdm−2. Light flashes were then applied on this background light; the light intensity of the flash was 10 cdsm−2 for the mouse experiments and for the rats maintained in darkness (Fig. 1, Fig. 4) or 25 cdsm-2 for other experiments (Fig 2–Fig 3). Ten recordings were averaged with an interstimulus interval of 30s.
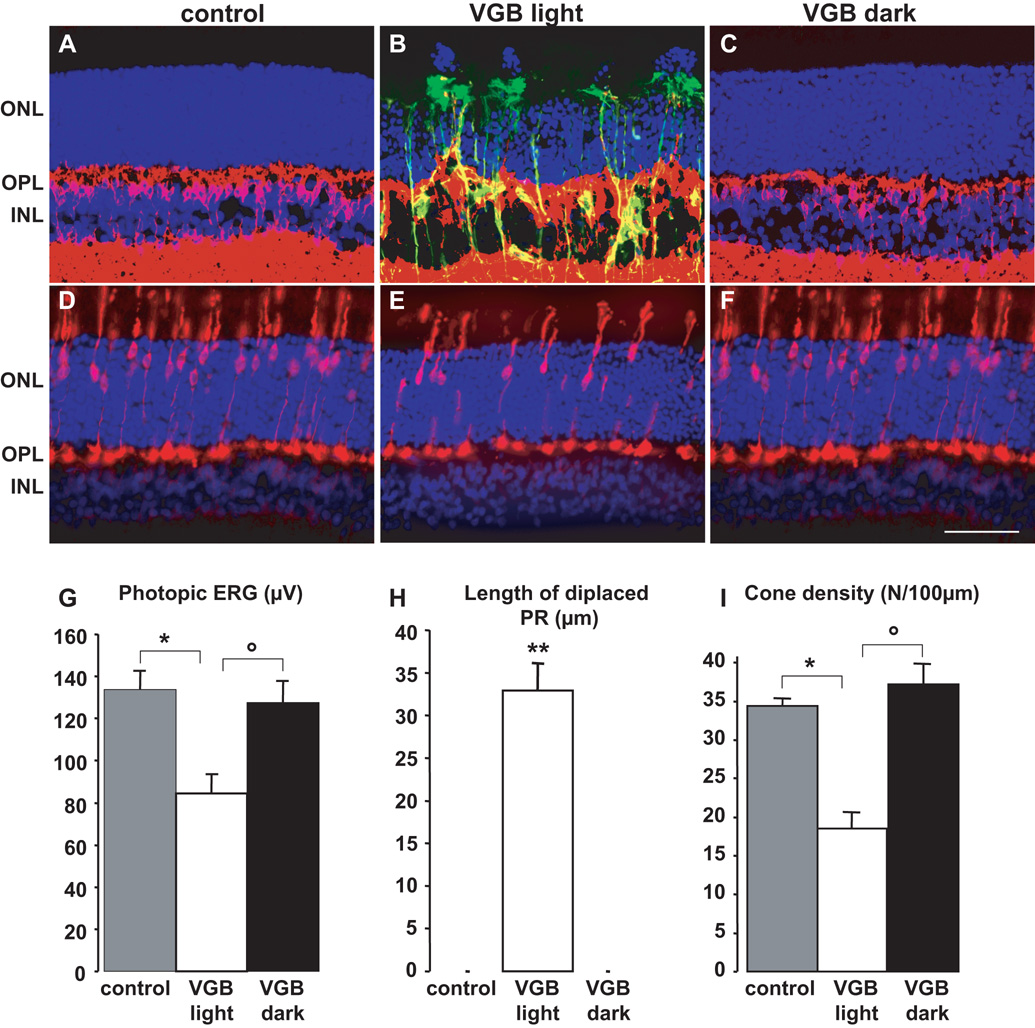
Figure 1. The in vivo retinal phototoxicity of vigabatrin.
(A–F) Retinal sections showing the lesions in a rat treated with VGB for 45 days in room light (B, E, VGB light) and absence of lesions in a VGB-treated rat maintained in darkness (C, F, VGB dark) and in a control animal (A, D). Sections were stained with the nuclear dye, DAPI, (blue in A–F) and immunolabelled for GFAP (green in A–C), Goα (red in A–C) and cone arrestin (red in D–F). Photoreceptor nuclei displaced above the outer nuclear layer (ONL), Goα-positive bipolar cell dendrites sprouting into the ONL and GFAP-positive processes extending vertically throughout the retina are only observed in rats treated with VGB in the 12h/12h light/dark cycle (B), not in control animals (A) or VGB-treated animals maintained in darkness (C). Similarly, fewer cone arrestin-positive photoreceptors and their inner/outer segments were observed in areas with normal retinal layering in VGB-treated rats exposed to a 12h/12h light/dark cycle (E), than were observed for control (D) or VGB-treated rats maintained in the dark (F). Quantification of photopic ERG amplitudes (G), lengths of retinal areas with displaced photoreceptor (PR) nuclei (H) and cone inner/outer segment density (I) in control rats (s.e.m., n=9), for VGB-treated animals either exposed to a 12h/12h light/dark cycle (VGB light, s.e.m., n= 10) or maintained in darkness (VGB dark, s.e.m., n= 10). The scale bar represents 50µm (IPL: inner plexiform layer).
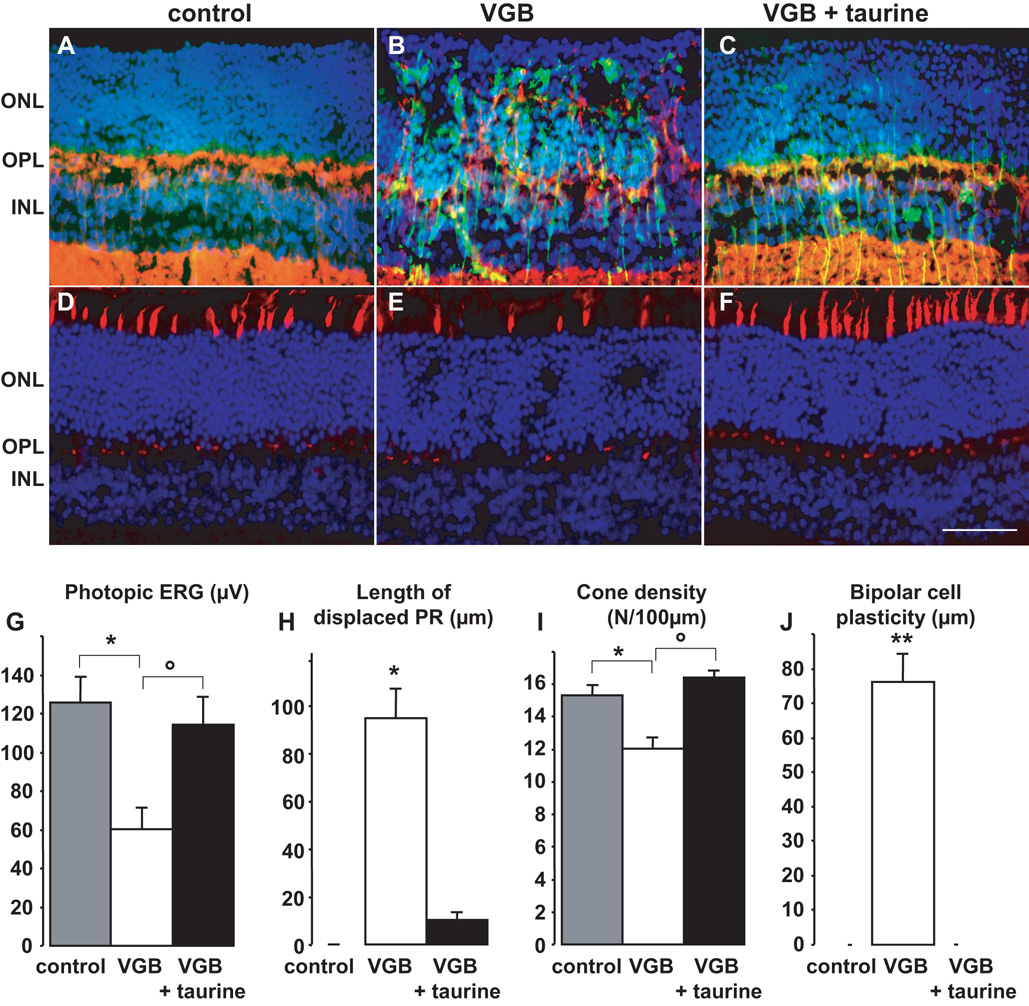
Figure 4. Partial prevention of vigabatrin-retinal toxicity in mice by taurine suppementation.
(A–F) Retinal sections showing that VGB-elicited retinal lesions are less extensive in a mouse with a taurine supplementation (C, F, VGB + taurine) than without (B, E, VGB), but still greater than in a control animal (A, D). These sections were immunolabelled with antibodies directed against Goα (red in A–C) and GFAP (green in A–C) and stained with a peanut lectin (PNA, red in D–F) and DAPI (blue in A–F). Photoreceptor nuclei displaced above the outer nuclear layer (ONL) were only observed in the VGB-treated mice (B), not in control animals (A) or VGB-treated mice receiving taurine supplementation. GFAP-positive processes extending vertically throughout the retina were observed in the VGB-treated animals (B), and increased GFAP staining was also observed in VGB-treated mice with taurine supplementation (C), compared to control animals (A). Similarly, there were fewer PNA-positive inner/outer segments of photoreceptors in VGB-treated mice (E) than in either control animals (D) or VGB-treated mice with taurine supplementation. Quantification of photopic ERG amplitude (G), length of retinal areas with displaced photoreceptor (PR) nuclei (H), density of cone inner/outer segments (I) and areas with bipolar cell sprouting into the ONL (J) in control mice (s.e.m., n=5), in VGB-treated animals with or without taurine supplementation (VGB, s.e.m., n= 7; VGB + taurine, s.e.m., n= 7). The scale bar represents 50µm (IPL: inner plexiform layer).
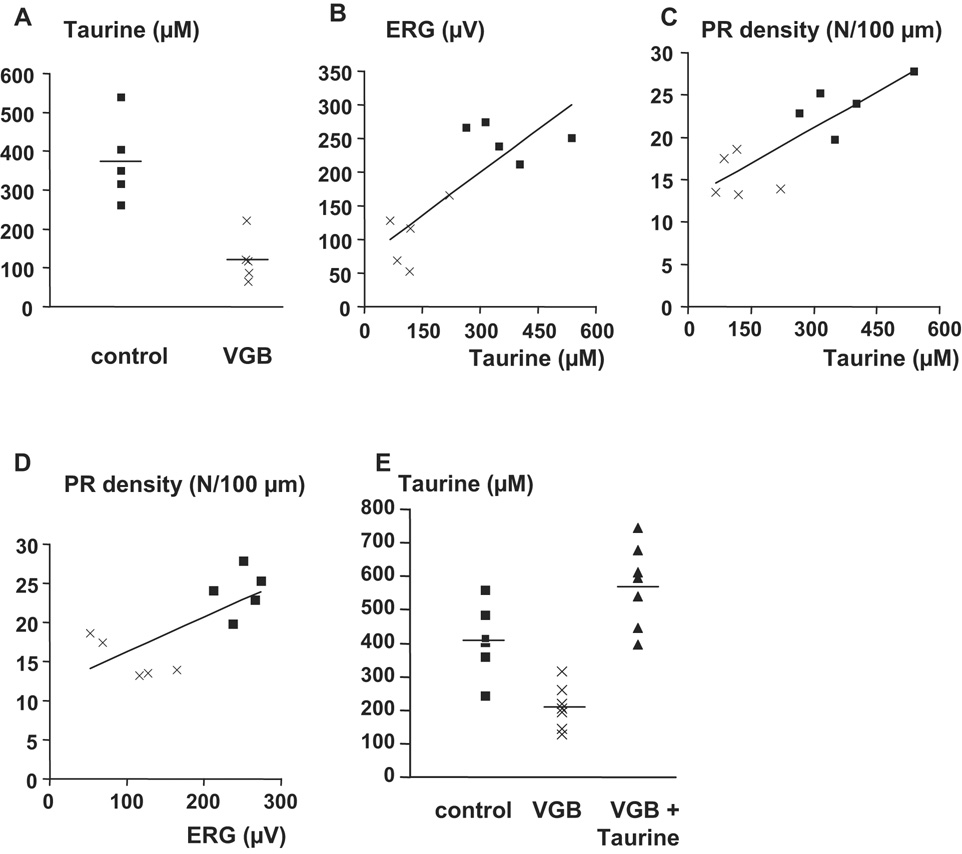
Figure 2. Correlation between taurine levels and the phototoxicity of vigabatrin.
A) Taurine plasma levels in control and treated animals in each group. B) Correlation between taurine plasma levels and the photopic ERG amplitude in VGB-treated control rats. The correlation factor between these two factors is equal to 0.769 (p=0.0093%, n=10). C) Correlation between the taurine plasma levels and the density of inner/outer cone photoreceptor segments. The correlation factor between these two parameters is equal to 0.818 (p=0.0038%, n=10). D) Correlation between the photopic ERG amplitudes and the density of inner/outer cone photoreceptor segments. The correlation factor between these two factors is equal to 0.703 (p=0.0023%, n=10). E) Recovery of taurine plasma levels by taurine supplementation in VGB-treated rats. In A and E, the horizontal line represents the mean value.
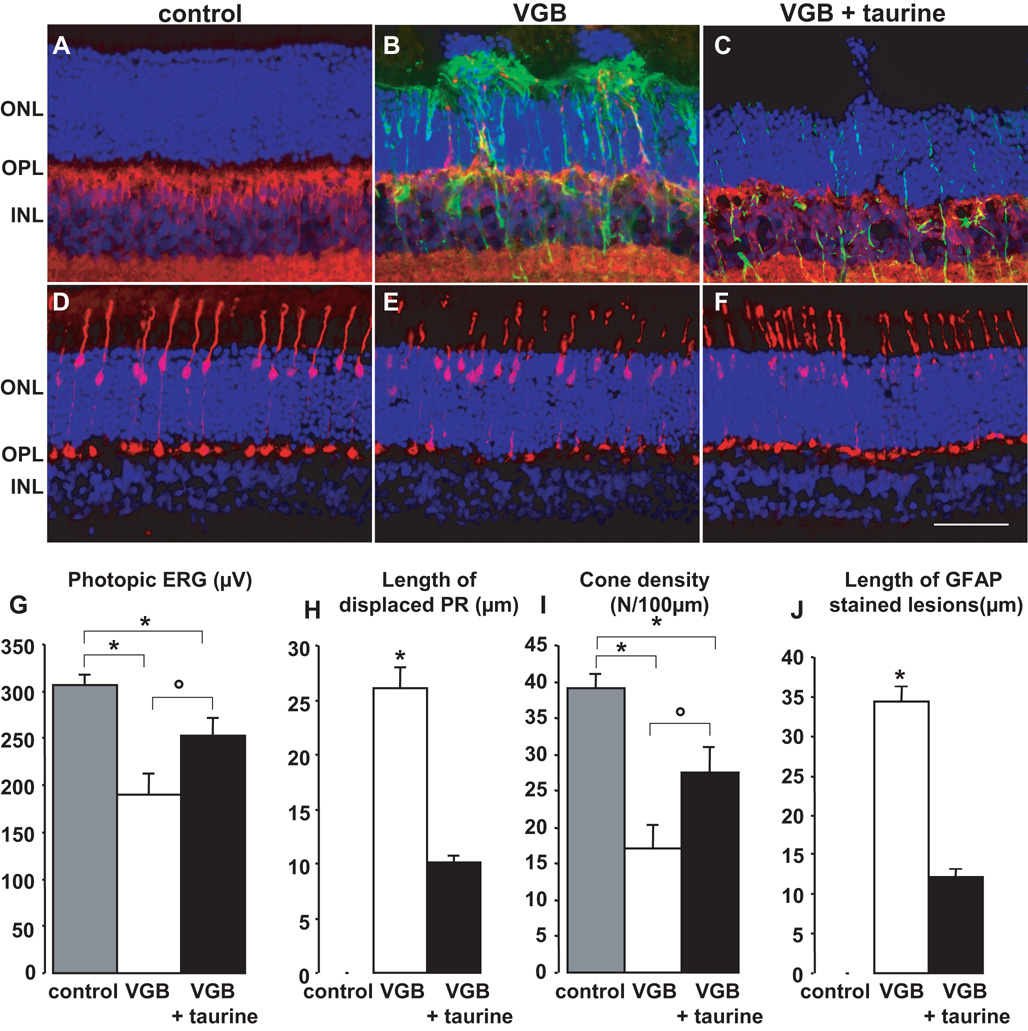
Figure 3. Partial prevention of vigabatrin-induced retinal toxicity in rats by taurine supplementation.
(A–F) Retinal sections showing that VGB-elicited retinal lesions are less extensive in a rat with taurine supplementation (C, F, VGB + taurine) than without (B, E, VGB), but still greater than in a control animal (A, D). These sections were stained with DAPI (blue in A–F) and immunolabelled with antibodies directed against Goα (red in A–C), GFAP (green in A–C) and cone arrestin (red in D–F). Photoreceptor nuclei displaced above the outer nuclear layer (ONL) are observed in both groups of VGB-treated rats treated with or without taurine supplementation (B, C), but not in control animals (A). GFAP-positive processes extending vertically throughout the retina were observed in VGB-treated rats (B), but not in control animals (A); higher levels of GFAP staining were also observed in VGB-treated rats receiving taurine supplementation (C) than those observed in control animals (A). Similarly, there were clearly fewer cone arrestin-positive photoreceptors in the VGB-treated rats receiving morning injections (E) than in control animals (D), with a smaller decrease in cone arrestin-positive photoreceptors in VGB-treated rats receiving evening injections (F). Quantification of photopic ERG amplitude (G), length of retinal areas with displaced photoreceptor (PR) nuclei (H), density of cone inner/outer segments (I) and areas with increased GFAP expression (J) in control rats (s.e.m., n=6), in the VGB-treated animals with or without taurine supplementation (VGB, n=7; VGB + taurine, n=7, s.e.m.). The scale bar represents 50µm (IPL: inner plexiform layer).
Histology
Eye cups were fixed overnight at 4°C in 4% (wt/vol) paraformaldehyde in phosphate buffered saline (PBS; 0.01M, pH 7.4). The tissue was cryoprotected in successive solutions of PBS containing 10%, 20% and 30% sucrose at 4°C, oriented along the dorso-ventral axis and embedded in OCT (Labonord, Villeneuve d’Ascq, France). Retinal sections (8–10µm thickness) were permeabilised for five minutes in PBS containing 0.1% Triton X-100 (Sigma, St. Louis, MO), rinsed, and incubated in PBS containing 1% bovine serum albumin (Eurobio, Les-Ulis, France), 0.1% Tween 20 (Sigma), and 0.1% sodium azide (Merck, Fontenay-Sous- Bois, France) for two hours at room temperature. The primary antibody added to the solution was incubated for two hours at room temperature. Polyclonal antibodies were directed against VGluT1 (1:2000, Chemicon), rabbit GFAP (1/400, Dako) and mouse cone arrestin (Luminaire junior, LUMIj, 1:20,000) 28. Monoclonal antibodies were directed against Goα (1:2000, Chemicon). Sections were rinsed and then incubated with the secondary antibody, goat anti-rabbit IgG or rabbit anti-mouse IgG conjugated to either Alexa TM594 or Alexa TM488 (1:500, Molecular Probes) for two hours. Inner/outer segments of cone photoreceptors were stained with a peanut lectin (PNA, Sigma) during 12 hours of incubation at 4°C. The dye, diamidiphenyl-indole (DAPI), was added during the final incubation period. Sections were rinsed, mounted with Fluorsave reagent (Calbiochem) and viewed with a Leica microscope (LEICA DM 5000B) equipped with a Ropper scientific camera (Photometrics cool SNAP TM FX).
For quantification, vertical sections along the dorso-ventral axis were selected at the optic nerve. Following DAPI nuclear staining, the lengths of disorganised retinal areas were measured; GFAP immunostaining was used for detection and quantification of retinal areas with reactive gliosis. The cone photoreceptor density was calculated following cone arrestin immunostaining (rats) or PNA labelling (mice) to visualise the inner/outer segments of cone photoreceptors; areas with disorganised retinal layering were excluded. Areas with sprouting bipolar cells were quantified following immunolabelling of these cells with the antibody directed against the protein Goα.
Amino-acid measurements
Blood samples were collected in haemolysis tubes containing heparin (14 IU/ml) and centrifuged (2200g, 15min). Plasma amino-acid analysis was performed by ion-exchange chromatography with nihydrin detection using a JEOL AMINOTAC analyser.
Statistical analysis
Statistical analysis of the results was performed by a one-way analysis of variance with the Student-Newman Keuls test (Sigmastat) for all measurements except for the quantification of the lesion length in the darkness experiments (Fig. 1H). In this case, the analysis was achieved with the Dunn’s test. A probability (p<0.05) was taken as a criterion for statistical significance
Results
Vigabatrin phototoxicity
To investigate potential VGB-induced retinal phototoxicity, we administered VGB to one group of rats for 45 days and kept them in darkness during the whole treatment period (n=10); another VGB-treated group was kept under 12h/12h light/dark cycles (n=10). Retinal cell function was assessed in these animals by examining photopic ERG. Photopic ERG amplitudes were significantly lower in VGB-treated animals maintained in the 12h/12h light/dark cycle than in the control group (n=9) (Fig. 1G, * p< 0.001), whereas there was no difference between the group maintained in constant darkness and control animals (Fig. 1G). The difference between the two VGB-treated groups was statistically significant (° p< 0.001). These findings suggest that dark rearing prevents loss in retinal function.
To further investigate the effect of dark rearing on the development of VGB-elicited lesions, the disorganisation of the photoreceptor layer was quantified by length on retinal sections of VGB-treated animals. The retinal outer nuclear layer (ONL) thickness was disrupted by rupture in the alignments of photoreceptor nuclei with some nuclei migrating toward the retinal pigment epithelium (Fig. 1B, H). The cumulative length of the disorganised areas varied from 345µm to 771µm with an average value of 503.7 ± 128.9 µm/section (Fig. 1H). No disorganised areas were detected in whole sections from rats maintained in darkness during VGB treatment (Fig. 1C, H) or in those from control animals (Figs 1A, H). Cone photoreceptors were labelled with a cone arrestin antibody and their outer/inner segments were quantified on retinal sections in areas with a normal layering (Fig. 1D–F). There were fewer cone segments in the VGB-treated animals maintained in room light (Fig. 1E) than in control animals (Fig. 1D) (Fig. 1I, * p< 0.001), but there was no difference between VGB-treated animals kept in darkness and controls (Fig. 1F). The difference between the two VGB-treated groups was statistically significant (Fig. 1I, °p< 0.001). Finally, retinal sections were immunolabelled with the glial fibrillary acidic protein (GFAP) to further demonstrate the absence of retinal degeneration in VGB-treated animals maintained in darkness. In control animals, GFAP immunolabelling was restricted to the Muller cell end-feet (Fig 1B), whereas it extended in Müller glial cells through the retina up to the outer limiting membrane of VGB-treated animals exposed to the 12h/12h light/dark cycle (Fig 1B). This increase in GFAP immunolabelling was observed in a large area of the dorsal retina. By contrast, GFAP immunostaining detected in VGB-treated animals maintained in darkness was similar to that of controls (Fig. 1C). Similarly, Goα-immunoreactive bipolar cell dendrites sprouted into the ONL in VGB-treated animals maintained in room light (Fig. 1B) but not in those maintained in darkness (Fig. 1C). These observations suggest that darkness prevents the development of VGB-elicited retinal lesions or protects against the VGB induction of retinal phototoxicity.
The phototoxicity of Vigabatrin relies on taurine deficiency
Light exposure potentiates photoreceptor degeneration in taurine-deficient conditions29, 30. Thus, we examined the potential role of taurine in VGB phototoxicity. Plasma amino-acid levels were measured in control animals and VGB-injected rats treated for 65 days. The differences in concentrations for most amino acids (threonine, serine, valine, citrulline, alanine, glycine, proline, glutamine, isoleucine, leucine, tyrosine, phenylalanine, arginine, histidine, lysine, and ornithine) were not statistically different (table 1) between the two groups of animals. By contrast, the plasma glutamic acid concentrations were much lower (by 56%) in VGB-treated animals than in control animals, consistent with previous reports31; this difference was statistically significant (control : 217.4±56.6 µM; VGB: 94.8±15.6 µM s.e.m., n=5). However, the greatest difference was observed for taurine. Taurine levels were by 67% lower in VGB-treated animals (122.2±26.6 µM) than in control animals (373.4±46.7 µM) (Fig. 2A, p<0.05, n=5, s.e.m.). A significant difference (22% reduction) was also observed for methionine. To determine if this decrease in taurine level is correlated to visual loss, both ERG amplitudes (r =0.769, p=0.0093%) and cone densities (r=0.818, p=0.0038%) were plotted against taurine levels in control animals and VGB-treated rats (Fig. 2B,C). Taurine levels were highly correlated to both ERG amplitudes and cone densities; these two factors were also correlated with each other (Fig. 2D, r =0.703, p=0.0023%). Thus, the low level of circulating taurine may directly contribute to retinal VGB toxicity in rats.
Table 1.
Amino acid levels in the plasma of albino rats treated with vigabatrin (VGB) or not treated (control). Measures are provided in µM (n=5, s.e.m.).
| Control | VGB | |
|---|---|---|
| Taurine | 373.4 ± 46.7 | 122.2 ± 26.6 ** |
| Threonine | 194.2 ± 30.8 | 130.6 ± 7.0 |
| Serine | 203.8 ± 28.7 | 169.4 ± 16.1 |
| Glutamic Acid | 217.4 ± 56.6 | 94.8 ± 15.6 ** |
| Valine | 167.4 ± 14.8 | 146 ± 8.9 |
| Citrulline | 54 ± 9.7 | 45.6 ± 4.2 |
| Alanine | 303.8 ± 32.3 | 270.4 ± 29.5 |
| Glycine | 190.6 ± 30.1 | 142.8 ± 17.6 |
| Proline | 155 ± 21.0 | 110.6 ± 20.1 |
| Glutamine | 375.6 ± 40.7 | 349 ± 13.4 |
| Methionine | 58 ± 4.7 | 45 ± 1.4 * |
| Isoleucine | 79.8 ± 8.0 | 68.2 ± 4.5 |
| Leucine | 152.6 ± 17.6 | 123.2 ± 11.5 |
| Tyrosine | 63.2 ± 6.8 | 51.6 ± 4.5 |
| Phenylalanine | 54.2 ± 6.9 | 42.8 ± 1.0 |
| Arginine | 130 ± 39.0 | 111.8 ± 15.1 |
| Histidine | 75.8 ± 5.4 | 63.2 ± 2.9 |
| Lysine | 307.2 ± 23.8 | 260.4 ± 32.3 |
| Ornithine | 78.6 ± 33.0 | 57.6 ± 10.6 |
Taurine is mainly obtained from nutrition; thus, we supplemented the drinking water of one group of VGB-treated rats with taurine (0.1M) (n=7) for 65 days (Fig 3). The plasma levels of taurine in these animals increased above those of control animals and above those of VGB-treated animals without taurine supplementation (Fig. 3K). The photopic ERG amplitude was greater in VGB-treated animals receiving taurine supplementation than in VGB-treated animals without taurine supplementation (n=7) (Fig. 3G, ° p<0.01), but remained lower than in control animals (n=5) (Fig. 3G, * p<0.05). Similarly, the cone segment density was greater in the VGB-treated group with taurine supplementation than in animals without supplementation (Fig. 3H, ° p<0.05), but remained lower than that of the control group (Fig. 3H, * p<0.001). Furthermore, the VGB-induced increase in GFAP immunostaining was less extensive in animals receiving taurine supplementation than in animals without supplementation (Fig. 3A–C, I, ° p<0.001). These findings show that VGB-induced retinal damage can be attenuated by taurine supplementation in rats.
To determine whether taurine can prevent VGB phototoxicity in another species, mice were administered VGB with (n=7) or without taurine supplementation (n=7) for 29 days (Fig 4). As in rats, VGB-treated mice had significantly lower photopic ERG amplitude than the control group (n=5) (Fig. 4G, * p<0.01). Taurine supplementation suppressed this decrease in photopic ERG amplitude such that the difference between these mice and the control group was not statistically different. The difference between the two VGB-treated groups with or without taurine supplementation was statistically significant (Fig. 4G, ° p<0.05). Quantification of the disorganisation of the ONL in the two VGB-treated groups showed that mice with taurine supplementation had smaller areas of retinal disorganisation than mice without supplementation (Fig. 4H, ° p<0.001). Quantification of cone outer and inner segments following lectin staining of their extracellular matrix indicated that VGB treatment resulted in lower densities (Fig. 4I, * p<0.001). Taurine supplementation prevented this decrease in cone density (Fig. 4I, ° p<0.001). Finally, the sprouting of bipolar cell dendrites, observed in VGB-treated mice, was completely suppressed by the taurine supplementation. These results indicate that taurine supplementation also reduces features of VGB toxicity in mice.
Taurine deficiency in infants receiving vigabatrin therapy
We then evaluated whether these findings have any clinical relevance in patients with epilepsy. Plasma taurine levels were reviewed retrospectively in patients presenting infantile spasms, who had received VGB treatment for at least six months in Necker Hospitals for the last 3 years. Plasma amino-acid levels are routinely measured in patients with infantile spasms as part of their etiological work-up, particularly when brain RMI is negative. Data were available in six patients (Table 2). Five patients had a taurine level below normal values for infants of a similar age; taurine was undetectable in two patients. For one of these two patients (patient 2), the taurine level was measured before starting a 15 month period of VGB therapy and this taurine level was normal. In patient 6, the taurine level was in the lower part of the normal range, but this measurement could have been overestimated by the delayed blood centrifugation. Thus, taurine levels seem to be decreased in VGB-treated epileptic infants.
Table 2.
Taurine plasma levels in VGB-treated patients. Note that in patient 1 to 5 that taurine levels are below the normal range at similar ages. For patient 2, the taurine plasma level was in the normal range before starting the VGB therapy and became undetectable after 15 months of treatment. In patients 5 and 6, the taurine level may have been artificially increased due to the delay in plasma preparation.
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Age at VGB onset | 6m | 9m | 7m | 3m | 3m | 6m | |
| Age at taurine measurement | 2.5y | 8.5m | 2y | 3y | 1.2y | 9m | 2.1y |
| Taurine level (µmoles/l) | und* | 75 | und* | 26 | 20 | 38 | 52 |
| Normal values (µmoles/l ± SD) at corresponding age | 70±22 | 81±35 | 70±22 | 70±22 | 76±36 | 81±35 | 70±22 |
| Delay between blood sampling and plasma preparation | <2h | <2h | <2h | <2h | <2h | 48h | 72h |
und: undetectable
SD: Standard Deviation
Discussion
We demonstrated here two related mechanisms underlying the retinal toxicity of VGB and thus provide several means to prevent its occurrence. First, we confirm the VGB phototoxicity and show that room light is sufficient to trigger the retinal damages. Indeed, retinal VGB toxicity has only been demonstrated in albino animals22; no lesions have been detected in pigmented rats even when treated for 105 days with 50mg/day (data not shown). Pigmentation is known to reduce the amount of light reaching photoreceptors such that, in conditions leading to pupil constriction, albino animals receive a 90-fold higher intensity on their retina than pigmented species32. Therefore, Izumi and coworkers25 suggested that light could be an important factor affecting retinal VGB toxicity. They tested this hypothesis in vitro and then in vivo using high-intensity illuminations (6,000 to 20,000 lux) for short periods (20–24 hours)25. In these acute conditions, photoreceptor degeneration was only observed in vitro25. In this study, we further demonstrated that normal room lighting can also induce retinal damage following chronic in vivo VGB administration.
The second uncovered mechanism is the induction of a taurine deficiency following the chronic VGB administration. Room light phototoxicity was previously reported in conditions of taurine deficiency29, 30, 33–35. We provide evidence that VGB-elicited phototoxicity could result from taurine deficiency by showing: 1) a decrease in taurine plasma level in VGBtreated animals, 2) a correlation between taurine plasma levels and the loss of visual functions, 3) the partial prevention of VGB-induced retinal lesions by taurine supplementation. The decrease in taurine plasma levels appears to be inconsistent with previous findings showing no change in taurine tissue levels after acute administration of VGB or following 17 days of chronic treatment36. However, the shorter administration period and the measurement of tissue rather than plasma levels in the previous study may account for this difference. VGB-induced lesions present additional similarities to retinal damage produced by taurine deficiency. The VGB-elicited lesions were more pronounced in the central superior region of the retina; similar lesions are described for taurine-deprived cats33. In both cases, retinal damage included disorganisation of the ONL with photoreceptor nuclei displaced towards the retinal pigment epithelium33. It remains unclear how vigabatrin lowers the taurine plasma level. The VGB-elicited increase in GABA could explain this observation because GABA is a known substrate and thus competitive inhibitor of the taurine transporter37. VGB itself could also have a direct action on the taurine uptake and release, this hypothesis would explain why VGB by contrast to GABA was found to induce a direct phototoxicity to photoreceptors in vitro25. The partial prevention of retinal lesions by taurine supplementation in VGB-treated animals could result from the inhibition of taurine transport at the hemato-retinal barriers and more specifically at the retinal pigment epithelium. However, we cannot exclude that other natural anti-oxidants are decreased by VGB treatment. Future studies will further investigate the cellular and molecular mechanisms involved in VGB induction of taurine deficiency and the correlated photoreceptor degeneration. They will also have to consider if a similar taurine deficiency is responsible for the visual disturbances generated by other antiepileptic drugs38.
Our initial observation of low taurine plasma levels in infants receiving VGB therapy is consistent with taurine deficiency and phototoxicity being the causes of VGB-induced visual loss in humans. Taurine deficiency triggers cone photoreceptor damage in monkeys fed a taurine-free diet34, 35. Similarly, in VGB-treated patients with epilepsy, cone photoreceptor damage might underlie reports of a decrease in visual acuity5, a loss of colour discrimination 6 and a reduction in the photopic ERG and the flicker response5, 7, 9, 39. Changes in electrooculogram (EOG) data are also consistent with altered cone photoreceptor/retinal pigment epithelium interactions that lead to cone damage. The absence of major changes in amplitude of the photopic ERG a-wave in VGB-treated epileptic patients5, 7, 9, 39 may appear inconsistent with potential cone damage in these patients. However, the primate photopic a-wave does not originate from cone photoreceptors, but originates mainly from their postsynaptic neurons40. Cone photoreceptor damage in VGB-treated epileptic patients would be highly consistent with our observations in VGB-treated animals. In VGB-treated infants, in vivo imaging techniques have also shown alterations of the retinal ganglion cell axon fibres and optic disc atrophy9. Future experiments will determine whether retinal ganglion cell degeneration is related to photoreceptor damage or a direct effect due to the expression of taurine transporters on retinal ganglion cells41. The taurine deficiency could also explain the variability in visual loss among VGB-treated patients because taurine is mainly obtained from our diet and may thus be highly dependent on individual diet. The first recommendation of the study would be to provide dietetic advices to patients under VGB therapy to verify their daily nutritional taurine intake.
Interestingly, taurine and its analogues exhibit anti-epileptic properties42. Our results indicate that taurine supplementation could eliminate or at least diminish the VGB-elicited visual field constriction in epileptic patients as it does in rodents. However, clinical trials are needed to determine whether taurine, as an adjunctive therapy, may prevent VGB retinal toxicity without preventing VGB efficacy on seizures. Taurine supplementation is very unlikely to perturb VGB therapy because the decrease in taurine level is not required for the drug to control seizures43, 44. Therefore, new clinical trials should focus on the effect of taurine supplementation on VGB therapy for infantile spasm or epilepsy1. However, in a first step, it would be important to verify that VGB-treated infants do drink baby milk formulae that contains taurine, as it is already included in such formulae commercialized by different companies in many countries.
Another important recommendation can be made, based on our findings, on reduced light exposure. Indeed, we demonstrated that VGB-elicited phototoxicity was prevented by maintaining animals in darkness during the treatment. Therefore, VGB-treated patients should limit their exposure to bright lights and wear sun glasses. Illumination should be avoided at night and during naps. This is particularly relevant for families of epileptic infants that are educated to observe and count seizures during sleep and thus maintain constant illumination during the night. Limiting light exposure is a simple and safe recommendation that could immediately and efficiently limit the extent of the VGB-induced retinal lesions.
This study provides a biological explanation for VGB-elicited retinal toxicity. It could lead to the use of a drug combination allowing epileptic patients to undergo VGB treatment again without having to choose between epileptic seizures and visual field constriction. Recommendations on reduced light exposure and nutrition could be rapidly implemented to limit visual field deterioration. However, we strongly discourage patients to take taurine supplementation without medical supervision because it may reduce the efficacy of their antiepileptic treatment.
Acknowledgments
We would like to thank Dr A. Malouvier, Dr J. Bursztyn, Dr D. Rabier, Dr Cougnard for their help and comments. This work was supported by INSERM, Universite Pierre et Marie Curie (Paris VI), the Fondation Ophtalmologique A. de Rothschild (Paris), Agence Nationale pour la Recherche (ANR: GABARET), the Fédération des Aveugles de France, the European Economic Community (EVI-GENORET-512036) and Ovation Pharmaceuticals (USA). QW received a fellowship from the city of Paris, FJ from the University of Tichcrine (Syria). Dr. Cheryl M. Craft is the Mary D. Allen Chair in Vision Research, Doheny Eye Institute (DEI) and acknowledges Mary D. Allen for her generous endowment and to NIH (EY015851, EY03040 [DEI Core]) and RPB for their support.
Footnotes
Conflicting interests: Patents were filed on the present results by INSERM U-592 (Institut de la Vision). INSERM U592 received financial support from the company Ovation Pharmaceuticals for the present work. Stephen Collins is an employee of Ovation pharmaceuticals, which has applied to the American Food and Drug Administration (FDA) for the use of vigabatrin in the USA for infantile spasms and refractory complex partial seizures in adults.
References
- 1.Ben-Menachem E, Dulac O, Chiron C. Vigabatrin. In: Engel Jerome, Jr, Pedley Timothy A., editors. Epilepsy: a comprehensive text book. Second edition. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 1683–1693. [Google Scholar]
- 2.Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: electroretinogram and ophthalmologic findings. Neurology. 1998;50:614–618. doi: 10.1212/wnl.50.3.614. [DOI] [PubMed] [Google Scholar]
- 3.Ruether K, Pung T, Kellner U, et al. Electrophysiologic evaluation of a patient with peripheral visual field contraction associated with vigabatrin. Archives of ophthalmology. 1998;116:817–819. [PubMed] [Google Scholar]
- 4.Eke T, Talbot JF, Lawden MC. Severe persistent visual field constriction associated with vigabatrin. BMJ (Clinical research ed. 1997;314:180–181. doi: 10.1136/bmj.314.7075.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson MA, Krauss GL, Miller NR, et al. Visual function loss from vigabatrin: effect of stopping the drug. Neurology. 2000;55:40–45. doi: 10.1212/wnl.55.1.40. [DOI] [PubMed] [Google Scholar]
- 6.Hilton EJ, Cubbidge RP, Hosking SL, et al. Patients treated with vigabatrin exhibit central visual function loss. Epilepsia. 2002;43:1351–1359. doi: 10.1046/j.1528-1157.2002.00502.x. [DOI] [PubMed] [Google Scholar]
- 7.Miller NR, Johnson MA, Paul SR, et al. Visual dysfunction in patients receiving vigabatrin: clinical and electrophysiologic findings. Neurology. 1999;53:2082–2087. doi: 10.1212/wnl.53.9.2082. [DOI] [PubMed] [Google Scholar]
- 8.McDonagh J, Stephen LJ, Dolan FM, et al. Peripheral retinal dysfunction in patients taking vigabatrin. Neurology. 2003;61:1690–1694. doi: 10.1212/01.wnl.0000098938.80082.25. [DOI] [PubMed] [Google Scholar]
- 9.Buncic JR, Westall CA, Panton CM, et al. Characteristic retinal atrophy with secondary "inverse" optic atrophy identifies vigabatrin toxicity in children. Ophthalmology. 2004;111:1935–1942. doi: 10.1016/j.ophtha.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westall CA, Logan WJ, Smith K, et al. The Hospital for Sick Children, Toronto, Longitudinal ERG study of children on vigabatrin. Doc Ophthalmol. 2002;104:133–149. doi: 10.1023/a:1014656626174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiron C, Dumas C, Jambaque I, et al. Randomized trial comparing vigabatrin and hydrocortisone in infantile spasms due to tuberous sclerosis. Epilepsy research. 1997;26:389–395. doi: 10.1016/s0920-1211(96)01006-6. [DOI] [PubMed] [Google Scholar]
- 12.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4:712–717. doi: 10.1016/S1474-4422(05)70199-X. [DOI] [PubMed] [Google Scholar]
- 13.Dulac O, Dalla Bernardina B, Chiron C. West syndrome. In: Engel Jerome, Jr, Pedley Timothy A., editors. Epilepsy: a comprehensive textbook. Second edition. Second edition. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 2329–2335. [Google Scholar]
- 14.Snead OC, 3rd, Benton JW, Myers GJ. ACTH and prednisone in childhood seizure disorders. Neurology. 1983;33:966–970. doi: 10.1212/wnl.33.8.966. [DOI] [PubMed] [Google Scholar]
- 15.Hrachovy RA, Frost JD, Jr, Glaze DG. High-dose, long-duration versus low-dose, short-duration corticotropin therapy for infantile spasms. The Journal of pediatrics. 1994;124:803–806. doi: 10.1016/s0022-3476(05)81379-4. [DOI] [PubMed] [Google Scholar]
- 16.Baram TZ, Mitchell WG, Tournay A, et al. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97:375–379. [PMC free article] [PubMed] [Google Scholar]
- 17.Gerasimov MR, Dewey SL. Gamma-vinyl gamma-aminobutyric acid attenuates the Gamma-vinyl gamma-aminobutyric acid attenuates the synergistic elevations of nucleus accumbens dopamine produced by a cocaine/heroin (speedball) challenge. Eur J Pharmacol. 1999;380:1–4. doi: 10.1016/s0014-2999(99)00526-9. [DOI] [PubMed] [Google Scholar]
- 18.Stromberg MF, Mackler SA, Volpicelli JR, et al. The effect of gamma-vinyl-GABA on the consumption of concurrently available oral cocaine and ethanol in the rat. Pharmacol Biochem Behav. 2001;68:291–299. doi: 10.1016/s0091-3057(00)00456-1. [DOI] [PubMed] [Google Scholar]
- 19.Sills GJ, Patsalos PN, Butler E, et al. Visual field constriction: accumulation of vigabatrin but not tiagabine in the retina. Neurology. 2001;57:196–200. doi: 10.1212/wnl.57.2.196. [DOI] [PubMed] [Google Scholar]
- 20.Neal MJ, Cunningham JR, Shah MA, Yazulla S. Immunocytochemical evidence that vigabatrin in rats causes GABA accumulation in glial cells of the retina. Neuroscience letters. 1989;98:29–32. doi: 10.1016/0304-3940(89)90368-6. [DOI] [PubMed] [Google Scholar]
- 21.Cubells JF, Blanchard JS, Makman MH. The effects of in vivo inactivation of GABA-transaminase and glutamic acid decarboxylase on levels of GABA in the rat retina. Brain research. 1987;419:208–215. doi: 10.1016/0006-8993(87)90585-3. [DOI] [PubMed] [Google Scholar]
- 22.Butler WH, Ford GP, Newberne JW. A study of the effects of vigabatrin on the central nervous system and retina of Sprague Dawley and Lister-Hooded rats. Toxicologic pathology. 1987;15:143–148. doi: 10.1177/019262338701500203. [DOI] [PubMed] [Google Scholar]
- 23.Duboc A, Hanoteau N, Simonutti M, et al. Vigabatrin, the GABA-transaminase inhibitor, damages cone photoreceptors in rats. Annals of neurology. 2004;55:695–705. doi: 10.1002/ana.20081. [DOI] [PubMed] [Google Scholar]
- 24.Wang QP, Jammoul F, Duboc A, et al. Treatment of epilepsy: the GABA-transaminase inhibitor, vigabatrin, induces neuronal plasticity in the mouse retina. Eur J Neurosci. 2008;27:2177–2187. doi: 10.1111/j.1460-9568.2008.06175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izumi Y, Ishikawa M, Benz AM, et al. Acute vigabatrin retinotoxicity in albino rats depends on light but not GABA. Epilepsia. 2004;45:1043–1048. doi: 10.1111/j.0013-9580.2004.01004.x. [DOI] [PubMed] [Google Scholar]
- 26.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364:1773–1778. doi: 10.1016/S0140-6736(04)17400-X. [DOI] [PubMed] [Google Scholar]
- 27.Aicardi J, Mumford JP, Dumas C, Wood S. Vigabatrin as initial therapy for infantile spasms: a European retrospective survey. Sabril IS Investigator and Peer Review Groups. Epilepsia. 1996;37:638–642. doi: 10.1111/j.1528-1157.1996.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Li A, Brown B, et al. Mouse cone arrestin expression pattern: light induced translocation in cone photoreceptors. Molecular vision. 2002;8:462–471. [PubMed] [Google Scholar]
- 29.Rapp LM, Thum LA, Anderson RE. Synergism between environmental lighting and taurine depletion in causing photoreceptor cell degeneration. Exp Eye Res. 1988;46:229–238. doi: 10.1016/s0014-4835(88)80080-0. [DOI] [PubMed] [Google Scholar]
- 30.Cocker SE, Lake N. Effects of dark maintenance on retinal biochemistry and function during taurine depletion in the adult rat. Visual neuroscience. 1989;3:33–38. doi: 10.1017/s0952523800012487. [DOI] [PubMed] [Google Scholar]
- 31.Bernasconi R, Klein M, Martin P, et al. Gamma-vinyl GABA: comparison of neurochemical and anticonvulsant effects in mice. J Neural Transm. 1988;72:213–233. doi: 10.1007/BF01243421. [DOI] [PubMed] [Google Scholar]
- 32.Lyubarsky AL, Daniele LL, Pugh EN., Jr From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vision research. 2004;44:3235–3251. doi: 10.1016/j.visres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Leon A, Levick WR, Sarossy MG. Lesion topography and new histological features in feline taurine deficiency retinopathy. Exp Eye Res. 1995;61:731–741. doi: 10.1016/s0014-4835(05)80024-7. [DOI] [PubMed] [Google Scholar]
- 34.Neuringer M, Sturman J. Visual acuity loss in rhesus monkey infants fed a taurine-free human infant formula. Journal of neuroscience research. 1987;18:597–601. doi: 10.1002/jnr.490180413. [DOI] [PubMed] [Google Scholar]
- 35.Imaki H, Moretz R, Wisniewski H, et al. Retinal degeneration in 3-month-old rhesus monkey infants fed a taurine-free human infant formula. Journal of neuroscience research. 1987;18:602–614. doi: 10.1002/jnr.490180414. [DOI] [PubMed] [Google Scholar]
- 36.Neal MJ, Shah MA. Development of tolerance to the effects of vigabatrin (gammavinyl-GABA) on GABA release from rat cerebral cortex, spinal cord and retina. Br J Pharmacol. 1990;100:324–328. doi: 10.1111/j.1476-5381.1990.tb15803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee NY, Kang YS. The brain-to-blood efflux transport of taurine and changes in the blood-brain barrier transport system by tumor necrosis factor-alpha. Brain research. 2004;1023:141–147. doi: 10.1016/j.brainres.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 38.Verrotti A, Manco R, Matricardi S, et al. Antiepileptic drugs and visual function. Pediatric neurology. 2007;36:353–360. doi: 10.1016/j.pediatrneurol.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Harding GF, Wild JM, Robertson KA, et al. Separating the retinal electrophysiologic effects of vigabatrin: treatment versus field loss. Neurology. 2000;55:347–352. doi: 10.1212/wnl.55.3.347. [DOI] [PubMed] [Google Scholar]
- 40.Bush RA, Sieving PA. A proximal retinal component in the primate photopic ERG a-wave. Investigative ophthalmology & visual science. 1994;35:635–645. [PubMed] [Google Scholar]
- 41.El-Sherbeny A, Naggar H, Miyauchi S, et al. Osmoregulation of taurine transporter function and expression in retinal pigment epithelial, ganglion, and muller cells. Investigative ophthalmology & visual science. 2004;45:694–701. doi: 10.1167/iovs.03-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta RC, Win T, Bittner S. Taurine analogues; a new class of therapeutics: retrospect and prospects. Current medicinal chemistry. 2005;12:2021–2039. doi: 10.2174/0929867054546582. [DOI] [PubMed] [Google Scholar]
- 43.Halonen T, Lehtinen M, Pitkanen A, et al. Inhibitory and excitatory amino acids in CSF of patients suffering from complex partial seizures during chronic treatment with gamma-vinyl GABA (vigabatrin) Epilepsy research. 1988;2:246–252. doi: 10.1016/0920-1211(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 44.Pitkanen A, Matilainen R, Ruutiainen T, et al. Effect of vigabatrin (gamma-vinyl GABA) on amino acid levels in CSF of epileptic patients. Journal of neurology neurosurgery, and psychiatry. 1988;51:1395–1400. doi: 10.1136/jnnp.51.11.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]