Abstract
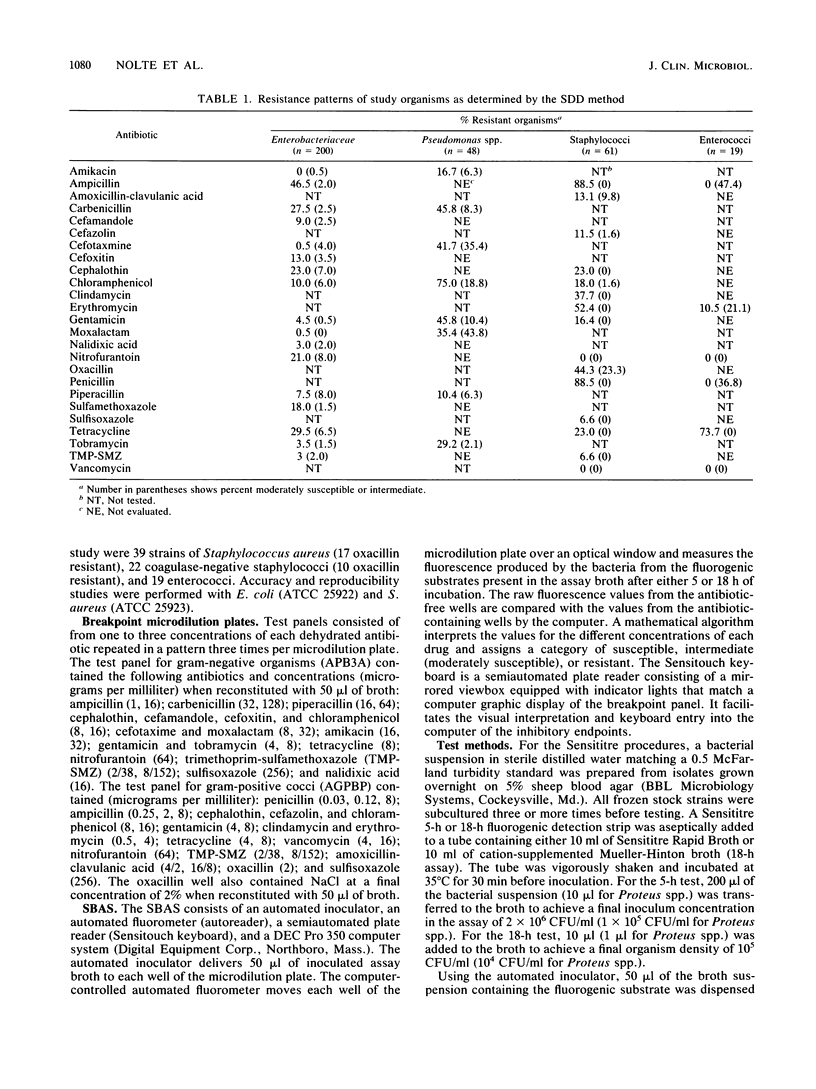
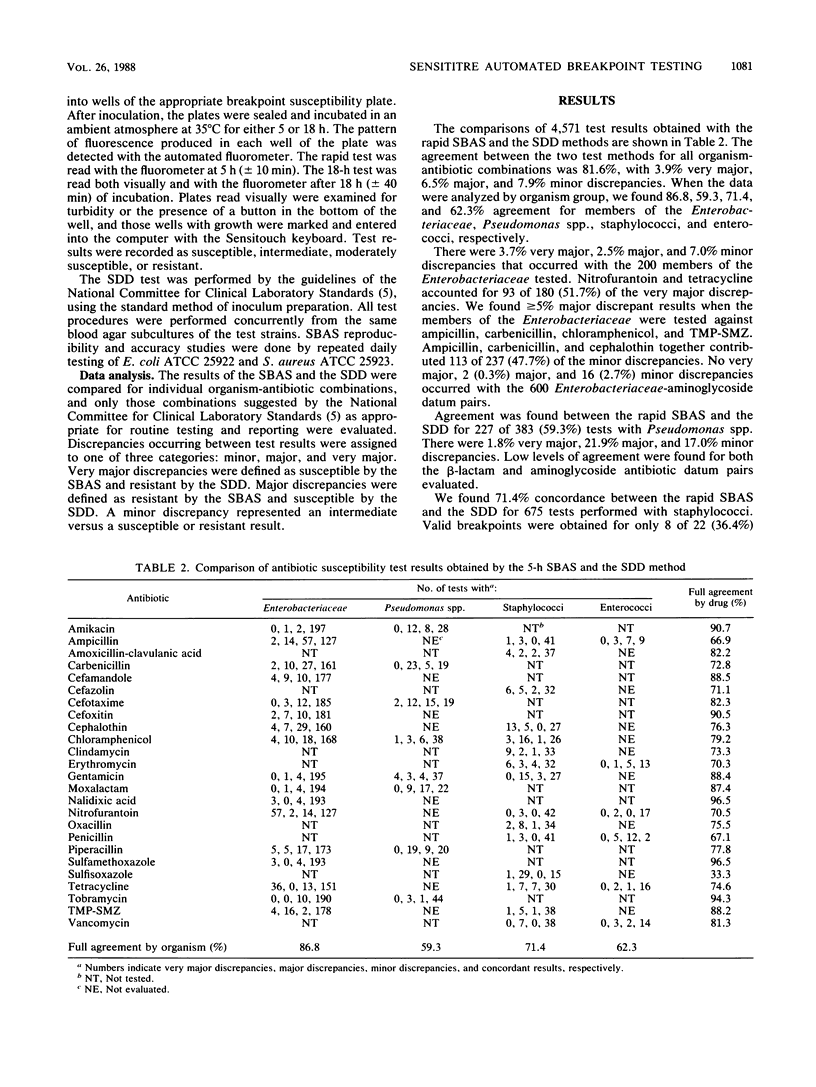
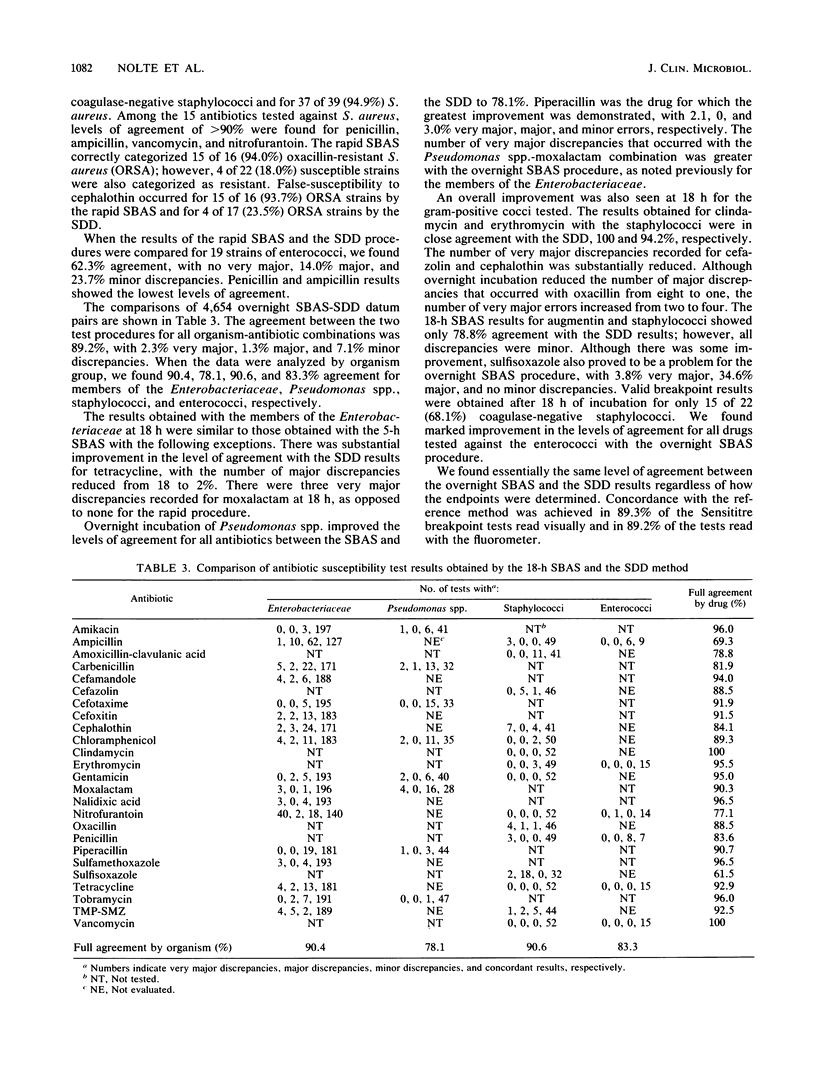
The Sensititre Breakpoint Autoreader system (SBAS) is a broth microdilution method with one to three concentrations of each antibiotic and innovative fluorescence technology to define inhibitory endpoints. We tested 248 gram-negative bacilli and 80 gram-positive cocci using both the rapid (5 h) and overnight (18 h) SBAS procedures. Inhibitory endpoints were also determined by visual inspection of the microdilution trays after 18 h of incubation. SBAS results were compared with those obtained by a standardized disk diffusion (SDD) procedure. Agreement between the rapid SBAS and SDD results for all antibiotic-organism combinations was found in 3,730 of 4,571 (81.6%) tests, with 3.9% very major, 6.5% major, and 7.9% minor discrepancies noted. Data analysis by organism group revealed 86.8, 57.3, 71.4, and 62.3% agreement for members of the family Enterobacteriaceae, Pseudomonas spp., staphylococci, and enterococci, respectively. The results of the overnight SBAS and SDD agreed in 4,154 of 4,654 (89.2%) tests, with 2.3% very major, 1.3% major, and 7.1% minor discrepancies recorded. Concordance was noted in 90.4, 78.1, 90.6, and 83.3% of the comparisons for the members of the Enterobacteriaceae, Pseudomonas spp., staphylococci, and enterococci, respectively. The inhibitory endpoints determined with the Autoreader were as reliable as those determined by visual inspection after 18 h of incubation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUER A. W., SHERRIS J. C. THE DETERMINATION OF SULFONAMIDE SUSCEPTIBILITY OF BACTERIA. Chemotherapy. 1964;9(1):1–19. doi: 10.1159/000220337. [DOI] [PubMed] [Google Scholar]
- Doern G. V., Dascal A., Keville M. Susceptibility testing with the sensititer breakpoint broth microdilution system. Diagn Microbiol Infect Dis. 1985 May;3(3):185–191. doi: 10.1016/0732-8893(85)90030-6. [DOI] [PubMed] [Google Scholar]
- Doern G. V., Staneck J. L., Needham C., Tubert T. Sensititre autoreader for same-day breakpoint broth microdilution susceptibility testing of members of the family Enterobacteriaceae. J Clin Microbiol. 1987 Aug;25(8):1481–1485. doi: 10.1128/jcm.25.8.1481-1485.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. L., Freedy P. K. Concurrent comparability of automated systems and commercially prepared microdilution trays for susceptibility testing. J Clin Microbiol. 1983 May;17(5):878–886. doi: 10.1128/jcm.17.5.878-886.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck J. L., Allen S. D., Harris E. E., Tilton R. C. Automated reading of MIC microdilution trays containing fluorogenic enzyme substrates with the Sensititre Autoreader. J Clin Microbiol. 1985 Aug;22(2):187–191. doi: 10.1128/jcm.22.2.187-191.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck J. L., Allen S. D., Harris E. E., Tilton R. C. Rapid MIC testing with the sensititre autoreader. J Clin Microbiol. 1988 Jan;26(1):1–7. doi: 10.1128/jcm.26.1.1-7.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd Antimicrobial susceptibility of enterobacteriaceae and nonfermenting gram-negative bacilli. Mayo Clin Proc. 1969 Nov;44(11):811–824. [PubMed] [Google Scholar]



