Abstract
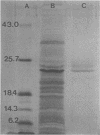
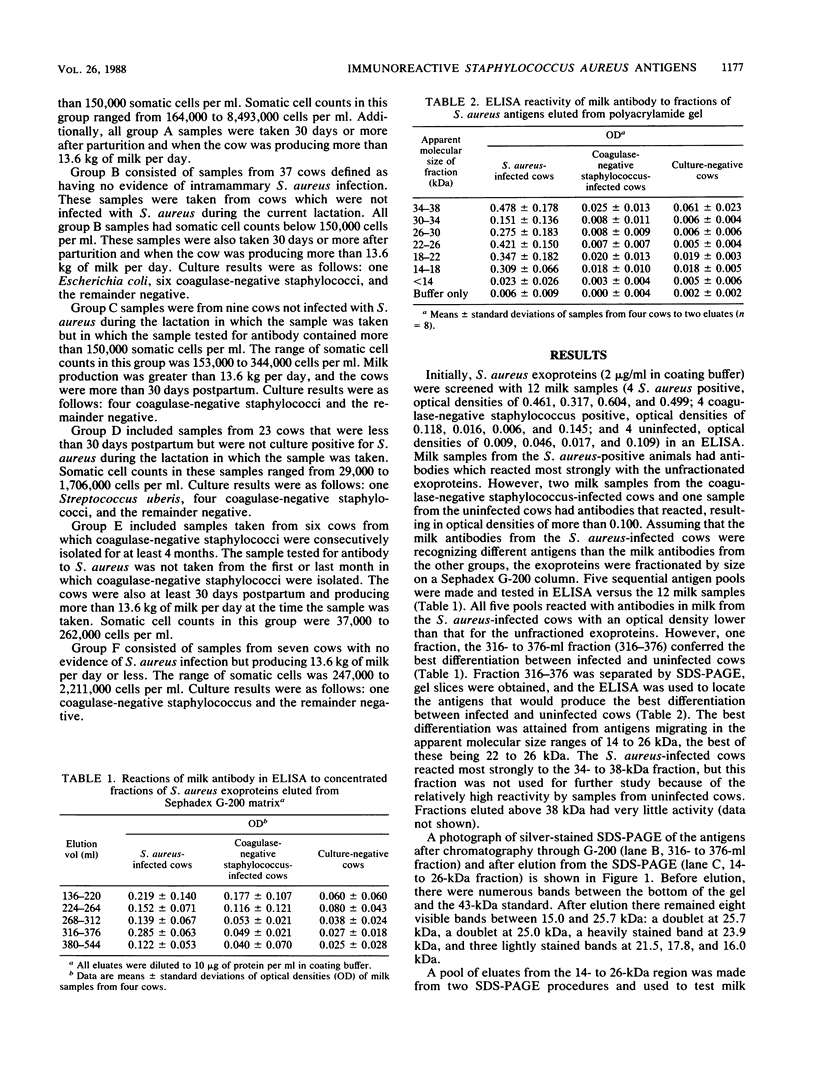
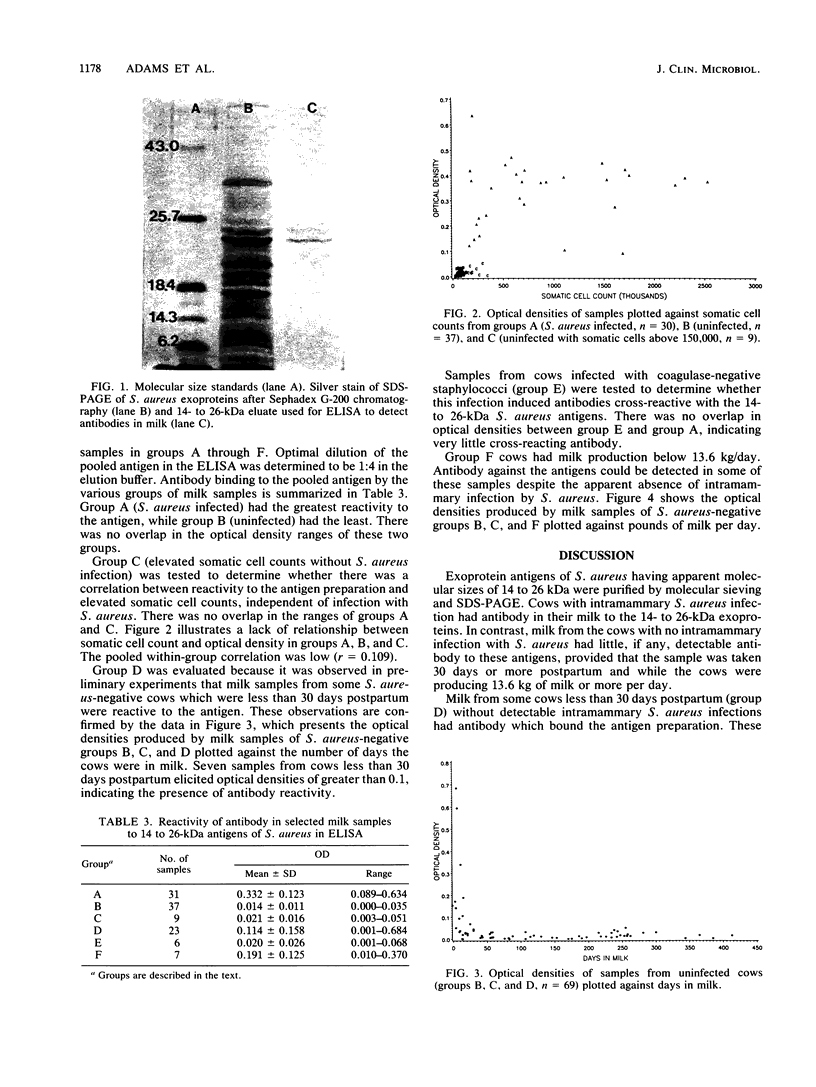
A 14- to 26-kilodalton fraction of Staphylococcus aureus exoproteins isolated by molecular sieve chromatography and electroelution from polyacrylamide gels was shown to specifically react with antibodies in milk of naturally infected dairy cows. Silver staining of the antigen preparation electrophoresed in polyacrylamide gels showed the strongest reactivity in the 24- to 26-kilodalton region with lesser staining at lower apparent molecular sizes. An enzyme-linked immunosorbent assay was developed to differentiate infected from uninfected cows for diagnostic purposes. Samples from S. aureus-infected cows reacted in the assay, and samples from uninfected cows did not. There was no correlation between numbers of somatic cells in the samples and reactivity to the antigens. Samples from cows infected with coagulase-negative staphylococci did not react with the antigens. It was found, however, that some samples from uninfected cows that were recently postpartum or producing low amounts of milk contained antibodies which bound the antigens. This was believed to be due to transport from blood to the mammary gland of antibodies which were generated by previous intramammary infections or infections at other sites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bramley A. J., Dodd F. H. Reviews of the progress of dairy science: mastitis control--progress and prospects. J Dairy Res. 1984 Aug;51(3):481–512. doi: 10.1017/s0022029900023797. [DOI] [PubMed] [Google Scholar]
- Brandon M. R., Watson D. L., Lascelles A. K. The mechanism of transfer of immunoglobulin into mammary secretion of cows. Aust J Exp Biol Med Sci. 1971 Dec;49(6):613–623. doi: 10.1038/icb.1971.67. [DOI] [PubMed] [Google Scholar]
- Bushnell R. B. Herd health approach to mastitis control and milk quality. J Am Vet Med Assoc. 1980 Apr 15;176(8):746–750. [PubMed] [Google Scholar]
- Caffin J. P., Poutrel B., Rainard P. Physiological and pathological factors influencing bovine immunoglobulin G1 concentration in milk. J Dairy Sci. 1983 Oct;66(10):2161–2166. doi: 10.3168/jds.S0022-0302(83)82063-3. [DOI] [PubMed] [Google Scholar]
- Christensson B., Espersen F., Hedström S. A., Kronvall G. Solid-phase radioimmunoassay of immunoglobulin G antibodies to Staphylococcus aureus peptidoglycan in patients with staphylococcal infections. Acta Pathol Microbiol Immunol Scand B. 1983 Dec;91(6):401–406. doi: 10.1111/j.1699-0463.1983.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Sonnenfeld E. M., Cost K., Thaniyavarn S. Anti-poly(glycerolphosphate) in human sera. J Clin Microbiol. 1982 Jun;15(6):1169–1171. doi: 10.1128/jcm.15.6.1169-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodger W. J., Ferguson G. Benefits and costs of a control program for an epizootic of Staphylococcus aureus mastitis. J Am Vet Med Assoc. 1987 May 15;190(10):1284–1287. [PubMed] [Google Scholar]
- Granström M., Julander I. G., Hedström S. A., Möllby R. Enzyme-linked immunosorbent assay for antibodies against teichoic acid in patients with staphylococcal infections. J Clin Microbiol. 1983 Apr;17(4):640–646. doi: 10.1128/jcm.17.4.640-646.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudding R. An agar diffusion method for the determination of antibodies against Staphylococcus aureus deoxyribonuclease. Acta Vet Scand. 1977;18(4):480–493. doi: 10.1186/BF03548411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASMANIS J., SPENCER G. R. Antibodies for hemolysins of Micrococcus pyogenes in whey and serum of dairy cattle. Am J Vet Res. 1954 Oct;15(57):517–519. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Live I., Ranu R. S. Serological activity of protein A of Staphylococcus aureus: the precipitinogen as an antigen for determining antibodies by the passive hemagglutination test. J Bacteriol. 1968 Jul;96(1):14–23. doi: 10.1128/jb.96.1.14-23.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler D. A., Norcross N. L. Use of enzyme-linked immunosorbent assay to measure bovine milk and serum antibodies to alpha toxin, beta toxin, and capsular antigens of Staphylococcus aureus. Vet Immunol Immunopathol. 1987 Feb;14(2):145–156. doi: 10.1016/0165-2427(87)90049-3. [DOI] [PubMed] [Google Scholar]
- Mathison B. A., Kelley K. W., Davis W. C. Quantitation of bovine immunoglobulin G2 antibodies binding Staphylococcus aureus, using a murine monoclonal antibody. Am J Vet Res. 1984 Dec;45(12):2518–2524. [PubMed] [Google Scholar]
- McDonald J. S. Streptococcal and staphylococcal mastitis. Vet Clin North Am Large Anim Pract. 1984 Jul;6(2):269–285. doi: 10.1016/s0196-9846(17)30022-8. [DOI] [PubMed] [Google Scholar]
- Nickerson S. C. Immune mechanisms of the bovine udder: an overview. J Am Vet Med Assoc. 1985 Jul 1;187(1):41–45. [PubMed] [Google Scholar]
- Opdebeeck J. P., Norcross N. L. Antibodies in bovine serum and lacteal secretions to capsular antigens of Staphylococcus aureus. Am J Vet Res. 1985 Jul;46(7):1561–1564. [PubMed] [Google Scholar]
- Opdebeeck J., Norcross N. L. Enzyme immunoassay for detection of antibodies specific for staphylococcal alpha haemolysin in bovine milk. Res Vet Sci. 1981 Jan;30(1):83–86. [PubMed] [Google Scholar]
- SPENCER G. R., STABENFELDT G. H., FLUHARTY D. M. The use of a growth-agglutination test for the detection of antibodies to Staphylococcus aureus in cattle. Am J Vet Res. 1963 Jan;24:83–87. [PubMed] [Google Scholar]
- Sheagren J. N. Staphylococcus aureus. The persistent pathogen (first of two parts). N Engl J Med. 1984 May 24;310(21):1368–1373. doi: 10.1056/NEJM198405243102107. [DOI] [PubMed] [Google Scholar]
- Sheagren J. N. Staphylococcus aureus. The persistent pathogen (second of two parts). N Engl J Med. 1984 May 31;310(22):1437–1442. doi: 10.1056/NEJM198405313102206. [DOI] [PubMed] [Google Scholar]
- WOODIN A. M. Fractionation of a leucocidin from Staphylococcus aureus. Biochem J. 1959 Oct;73:225–237. doi: 10.1042/bj0730225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. L., Brandon M. R., Lascelles A. K. Concentrations of immunoglobulin in mammary secretion of ruminants during involution with particular reference to selective transfer of IgG. Aust J Exp Biol Med Sci. 1972 Aug;50(4):535–539. doi: 10.1038/icb.1972.46. [DOI] [PubMed] [Google Scholar]
- Watson D. L., Davies H. I. Immunophysiological activity of supramammary lymph nodes of the ewe during the very early phase of staphylococcal mastitis. Res Vet Sci. 1985 Jul;39(1):52–58. [PubMed] [Google Scholar]
- West T. E., Burdash N. M., Boehm A. M., West M. E. Evaluation of a commercial counterimmunoelectrophoresis kit for detection of Staphylococcus aureus teichoic acid antibodies. J Clin Microbiol. 1983 Apr;17(4):567–570. doi: 10.1128/jcm.17.4.567-570.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]