Abstract
The regenerating gene (Reg) was originally isolated from regenerating rat pancreatic islets and revealed recently to constitute a multi-gene family in humans. REG Iα protein is known to be overexpressed not only in various human inflammatory diseases but also in various experimental models of inflammation in animal tissues. However, its involvement in pathophysiology of the minor salivary gland (MSG) is not clear. We investigated REG Iα expression in the MSG of patients with primary Sjögren's syndrome (SS) and assessed its role in ductal epithelial cell proliferation in such tissues. Lip biopsy specimens were obtained from 40 patients with primary SS and examined using immunohistochemistry for REG Iα protein, Ki67 and single-strand DNA (ssDNA). The relationships among clinicopathological factors and expression of REG Iα protein, Ki67 and ssDNA in the MSG were then analysed. REG Iα protein was expressed rarely in ductal epithelial cells of the normal MSG but was apparently overexpressed in those of patients with SS. The labelling indices for both Ki67 and ssDNA in the ductal cells of the MSGs were significantly higher in SS patients than in controls. Moreover, these labelling indices were significantly higher in REG Iα-positive than in negative SS patients. REG Iα protein may play a role in the regeneration of ductal epithelial cells in the MSGs of patients with SS.
Keywords: apoptosis, minor salivary gland, proliferation, REG Iα protein, Sjögren's syndrome
Introduction
The regenerating gene (Reg) was isolated originally from a complementary DNA library derived from regenerating rat pancreatic islets, and its human homologue was named REG Iα[1]. REG Iα protein is expressed not only in the pancreas but also the gastrointestinal tract [2–4]. Moreover, it is interesting that REG Iα protein is involved in the pathophysiology of gastritis [5] or pancreatitis [6] and is suggested to play a role in tissue regeneration in those diseases [1,7,8]. The salivary gland is a component of the digestive system and its tissue structure, comprising acinar and ductal cells, is similar to that of the exocrine pancreas. Therefore, we hypothesized that REG Iα protein may play some role in the salivary gland under inflammatory conditions.
Sjögren's syndrome (SS) is a systemic autoimmune disease characterized predominantly by dryness of the eyes and mouth because of tissue injury resulting from lymphocyte infiltration into the lacrimal and minor salivary glands (MSGs) respectively [9]. In this context, it is tempting to speculate that some molecules play roles in remodelling of the MSG in patients with SS. In the present study, we investigated the expression of REG Iα in the MSG of patients with SS and analysed its histopathological significance. Moreover, we assessed the role of REG Iα protein in ductal cell proliferation in the MSG of patients with SS.
Materials and methods
Patients, tissue samples and histology
Forty patients with primary SS (four males, 36 females; mean age 50·3 years, range 17–74 years) who were diagnosed according to the Revised Japanese Criteria for SS [10,11] at Dokkyo University School of Medicine between 1996 and 2002 were enrolled. Samples of labial salivary gland tissue were obtained by biopsy and surgery from patients with primary SS, and also from 10 controls (five males, five females; mean age 28·0 years, range 4–50 years) who were treated for mucocele. The non-inflamed region far from the cystic wall in the mucocele tissue was investigated as a non-inflamed control. Tissue specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. Multiple haematoxylin and eosin-stained sections of each sample were examined histologically. To assess the severity of periductal inflammation, the focus score was expressed as the number of focus points consisting of ≥ 50 periductal infiltrating lymphoid cells in a 4-mm [2] MSG biopsy sample [10,11]. The severity of periductal inflammation was graded as: slight, focus score < 1; evident, focus score ≥ 1. This work was conducted with the approval of the Dokkyo University Surgical Pathology Committee, and informed consent was obtained from all patients.
Anti-REG Iα antibody
The monoclonal antibody for human REG Iα protein was generated, as reported previously, against human REG Iα protein corresponding to positions 23–166 of the deduced human REG Iα protein [2]. The specificity of the antibody was proven by not only Western blot analysis [2] but also immunohistochemistry [6]. Regarding the controls in immunohistochemistry, the sections of pancreas were used as positive control, and anti-REG Iα antibody was not applied to the negative control.
Immunohistochemistry
Immunohistochemical staining for REG Iα, Ki67 and single-strand DNA (ssDNA) was performed with a LSAB-2 kit (Dako, Marseille, France) as described previously [3]. In brief, 4-µm-thick sections were placed on slides, deparaffinized and dehydrated. They were then placed in 0·01 mol/l citrate buffer (pH 6·0) and treated by microwave heating (MI-77, Azumaya, Tokyo, Japan; 400 W, 95°C) for 10 and 40 min to facilitate antigen retrieval for REG Iα and Ki 67 respectively, whereas they received no treatment with microwave heating for the immunostaining of ssDNA. Then, the sections were followed by pretreatment with 0·3% H2O2 in methanol for 20 min at room temperature to quench endogenous peroxidase activity. The sections were incubated with 1% bovine serum albumin in phosphate-buffered saline (PBS) for 30 min, and then with anti-REG Iα (dilution 1:100), anti-Ki 67 (Dako Japan; dilution 1:50) and anti-ssDNA (Dako Japan; dilution 1:50) for 1 h. Thereafter, the sections were incubated with biotinylated secondary antibody for 15 min, washed with PBS and treated with peroxidase-conjugated streptavidin for 20 min. Finally, the sections were incubated in 3,3′-diaminobenzidine tetrahydrochloride with 0·05% H2O2 for 3 min and then counterstained with Mayer's haematoxylin.
Evaluation of REG Iα expression
The REG Iα immunoreactivity was observed in the ductal cells but not in other cells in the MSG tissues. All ducts (at least 25) were observed in each tissue sample, and a specimen was considered positive for REG Iα protein if ≥ 20% of the ductal cells were stained positively; otherwise, the specimens were considered negative.
Evaluation of cell proliferation and apoptosis of ductal cells in MSGs
Ki67 and ssDNA were used as markers for measures of cell proliferation [12] and apoptosis [13] respectively. We evaluated the expression of Ki67 and ssDNA in the ducts of the MSG where REG Iα immunoreactivity was localized. All ducts (at least 25) were observed in each tissue section, and the labelling index of both Ki67 and ssDNA was calculated as the percentage of positive cells.
Statistical analysis
Statview 5.0J statistical software (Abacus Concepts Inc., Berkeley, CA, USA) was used for all analyses. χ2 analyses were performed to investigate the relationship between REG Iα expression and severity of periductal lymphoid cell infiltration. All values were expressed as the mean ± standard error of the mean and the significance of differences between two groups was assessed using the Mann–Whitney U-test. Differences at P < 0·05 were considered to be significant.
Results
Expression of REG Iα and Ki67 in MSGs of patients with SS
The REG Iα expression was observed rarely in the ductal epithelial cells of normal MSGs. We found no REG Iα expression in either acinar or interstitial cells in normal MSGs (Fig. 1a). In addition, we hardly observed REG Iα-positive cells in the cystic lesion of the resected mucocele (data not shown). In patients with SS, REG Iα expression was observed in the MSG ductal cells, but not in acinar or interstitial cells (Fig. 1b). Twenty-six (65·0%) of the 40 SS samples were positive for REG Iα protein expression, whereas all 10 control MSGs examined were negative, suggesting that the ductal cells in the MSGs of SS patients showed ectopic overexpression of REG Iα (P < 0·001).
Fig. 1.
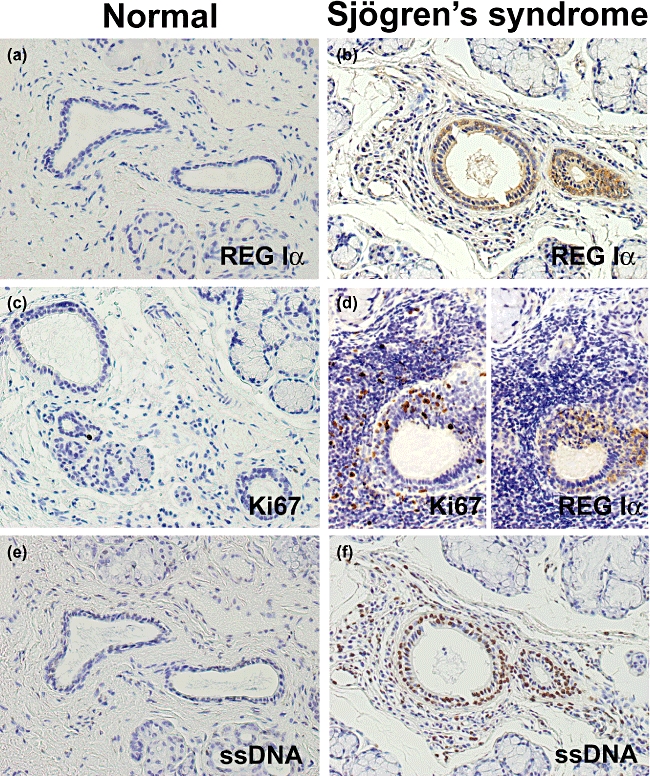
Immunostaining of REG Iα, Ki67 and single-strand DNA (ssDNA) in minor salivary gland (MSG) tissues. REG Iα expression is (a) negative in any types of cell in normal MSG but (b) positive in the ductal epithelial cells in inflamed MSG from Sjögren's syndrome (SS) patients. (c) Ki67 immunoreactivity is scattered in the normal MSG. (d) Ki67 immunoreactivity is observed in a considerable number of inflammatory cells and REG Iα-positive ductal cells in inflamed MSG from SS patients. (e) Hardly any signal for ssDNA in the ductal cells of the normal MSG. (f) Considerable signal for ssDNA in the ductal cells of SS patients.
Immunoreactivity for Ki67 was scattered in the normal MSG (Fig. 1c). However, Ki67 immunoreactivity was observed in a considerable number of inflammatory cells and ductal cells in the MSGs of patients with SS (Fig. 1d). The Ki67 labelling index of ductal cells in the MSG was significantly higher in SS patients than in controls (1·5 ± 0·3 versus 0·4 ± 0·1; Fig. 2, P = 0·033).
Fig. 2.
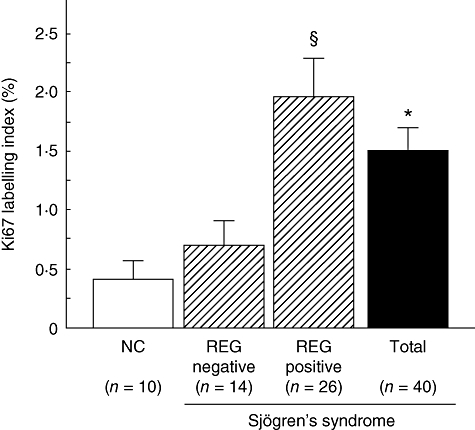
Comparison of Ki67-labelling index of ductal epithelial cells in minor salivary gland (MSG) between REG Iα-positive and negative Sjögren's syndrome (SS) patients. All ducts (at least 25) were observed in each tissue section, and the labelling index of Ki67 was calculated as the percentage of positive cells. The vertical lines represent mean ± standard error of the mean. *Significantly greater than in normal control (NC) (P < 0·05). §Significantly greater than in the REG Iα-negative group with SS (P < 0·05); n.s.: normal control.
Hardly any signal for ssDNA was observed in the ductal cells of the normal MSG (Fig. 1e), but considerable signal expression was evident in the ductal cells of SS patients (Fig. 1f). The ssDNA labelling index of ductal cells was significantly higher in SS patients than in controls (1·0 ± 0·4 versus 13·5 ± 3·0; Fig. 3, P = 0·047).
Fig. 3.
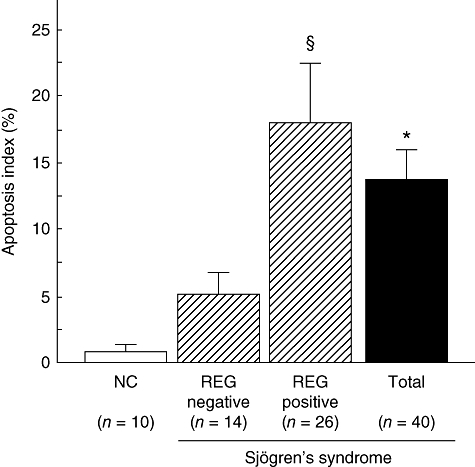
Comparison of ssDNA-labelling index of ductal epithelial cells in minor salivary gland (MSG) between REG Iα-positive and negative Sjögren's syndrome (SS) patients. All ducts (at least 25) were observed in each tissue section, and the labelling index of ssDNA was calculated as the percentage of positive cells. The vertical lines represent mean ± standard error of the mean. *Significantly greater than in normal control (NC) (P < 0·05). §Significantly greater than in the REG Iα-negative group with SS (P < 0·05); n.s.: normal control.
None of the parameters – age, gender or the severity of periductal inflammation – was related significantly to REG Iα expression, Ki67 labelling index or ssDNA labelling index in the ductal cells in patients with SS.
Relationship between REG Iα expression and Ki67 or ssDNA labelling index in MSGs of patients with SS
We next investigated the relationship between REG Iα expression and Ki67 or ssDNA labelling index in patients with SS. As shown in Figs 2 and 3, the Ki67 and ssDNA labelling indices were both significantly higher in the REG Iα-positive group than in the negative group respectively.
Discussion
In the present study, we demonstrated for the first time that REG Iα protein is expressed in a very few ductal cells, but not in acinar or interstitial cells, in the normal MSG. This finding was unexpected, because REG Iα protein is expressed not only in ductal but also in acinar cells in the normal pancreas. However, although the MSG is histologically similar to the exocrine pancreas, its development differs from that of the pancreas [14]. In fact, unlike the pancreas, the salivary glands lack a distinct endocrine system but possess myoepithelial cells [15]. Moreover, it is noteworthy that the expression level of REG Iα in the normal pancreas is very high [2], whereas it is extremely low in the normal MSG. Thus, these findings suggest that tissue-specific differences in development and/or the regulatory mechanism of gene expression may affect the level of the gene product or the cell specificity of REG Iα protein expression.
The most important finding of this study was that REG Iα protein was overexpressed in the ductal cells of MSGs from patients with SS. Although the aetiology of SS is still unclear, it is thought to be an autoimmune disease characterized by marked ductal cell destruction with inflammatory cell infiltration [9]. Therefore, it is an interesting question as to whether REG Iα overexpression is associated directly with the immune disorder in patients with SS. We and others have reported previously that REG Iα is overexpressed not only in various human inflammatory diseases such as gastritis [5], pancreatitis [6] and colitis [4], but also in various experimental models of inflammation in animal tissues [7,8]. Thus, it is most likely that inflammation, regardless of whether or not it is autoimmune-associated, is a key event triggering REG Iα overexpression in many tissues. We have suggested that a variety of major proinflammatory cytokines, such as interleukin (IL)-6, IL-8, tumour necrosis factor-α and interferon-γ, are responsible for REG Iα expression in pancreatic and gastric tissues [16–18]. Although we could not determine the level of proinflammatory cytokines in our biopsy samples, both infiltrated inflammatory cells and ductal epithelial cells are known to produce these proinflammatory cytokines in the MSG of SS patients [9,19,20]. Accordingly, as in the case in gastric and pancreatic tissues, cytokines may be associated closely with REG Iα overexpression in MSG ductal cells of SS patients. However, in the present study we found no correlation between the severity of periductal inflammation and the positivity of REG Iα expression in the ductal cells. This may be explained by the fact that the threshold of REG Iα expression by inflammation is low and therefore weak inflammation may be enough to induce REG Iα expression in the MSG tissues.
The role of REG Iα protein in the pathophysiology of patients with SS is a matter of interest. From the viewpoint of histopathology, ductal epithelial cells are being injured continuously by inflammation, and subsequently regenerated [9,21]. Indeed, in the present study we demonstrated that both proliferation and apoptosis of ductal cells were occurring actively in the MSGs of SS patients. Interestingly, we also found significant REG Iα overexpression in MSGs where both proliferation and apoptosis were occurring actively in ductal epithelial cells. On the other hand, we have shown previously that REG Iα protein promotes cell proliferation and confers cell resistance to apoptosis in vitro[16,22], and furthermore that REG Iα protein is overexpressed during healing of the gastric epithelium [7,8]. Taken together, the data suggest that REG Iα protein may be involved in the regeneration of ductal tissues by exerting a cell-proliferative and/or anti-apoptotic effect, although it is unknown whether REG Iα protein is enough to prevent the ductal cells from apoptosis induced by strong inflammatory stimuli. On the other hand, it is noteworthy that REG Iα expression was not observed in the mucocele lesion with inflammatory cell infiltration. However, this is not surprising because the cystic lesion in mucocele tissue lacks the components of epithelial cell in which REG Iα should be expressed. Thus, REG Iα protein plays a role specifically in the ductal epithelial cells under inflammatory condition.
In summary, we have shown that REG Iα protein is overexpressed in the ductal epithelial cells of MSGs in SS patients, and that its expression is associated with the proliferation of these cells. These data suggest that REG Iα protein may play a role in the regeneration of MSG ductal epithelial cells in SS.
Acknowledgments
The authors thank Chiaki Matsuyama, Ayako Shimizu, Takako Ono, Midori Katayama, Atsuko Kikuchi and Sachiko Miyahara (Department of Surgical and Molecular Pathology, Dokkyo University School of Medicine, Tochigi, Japan) for their excellent technical and secretarial assistance. We are grateful to Dr Hiroshi Okamoto in Tohoku University Graduate School of Medicine, Sendai, Japan, for providing anti-REG Iα antibody. This work was supported in part by Grants-in-aid for Scientific Research 20590747 from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Terazono K, Yamamoto H, Takasawa S, et al. A novel gene activated in regenerating islets. J Biol Chem. 1988;263:2111–4. [PubMed] [Google Scholar]
- 2.Watanabe T, Yonekura H, Terazono K, Yamamoto H, Okamoto H. Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues. J Biol Chem. 1990;265:7432–9. [PubMed] [Google Scholar]
- 3.Fukui H, Fujii S, Takeda J, et al. Expression of Reg Iα in human gastric cancers. Digestion. 2004;69:177–84. doi: 10.1159/000078762. [DOI] [PubMed] [Google Scholar]
- 4.Sekikawa A, Fukui H, Fujii S, et al. Possible role of REG Iα protein in ulcerative colitis and colitic cancer. Gut. 2005;54:1437–44. doi: 10.1136/gut.2004.053587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukui H, Franceschi F, Penland RL, et al. Effects of Helicobacter pylori infection on the link between regenerating gene expression and serum gastrin levels in Mongolian gerbils. Lab Invest. 2003;83:1777–86. doi: 10.1097/01.lab.0000106501.56339.ce. [DOI] [PubMed] [Google Scholar]
- 6.Satomura Y, Sawabu N, Ohta H, et al. The immunohistochemical evaluation of PSP/reg-protein in normal and diseased human pancreatic tissues. Int J Pancreatol. 1993;13:59–67. doi: 10.1007/BF02795200. [DOI] [PubMed] [Google Scholar]
- 7.Kawanami C, Fukui H, Kinoshita Y, et al. Regenerating gene expression in normal gastric mucosa and indomethacin-induced mucosal lesions of the rat. J Gastroenterol. 1997;32:12–8. doi: 10.1007/BF01213290. [DOI] [PubMed] [Google Scholar]
- 8.Asahara M, Mushiake S, Shimada S, et al. Reg gene expression is increased in rat gastric enterochromaffin-like cells following water immersion stress. Gastroenterology. 1996;111:45–55. doi: 10.1053/gast.1996.v111.pm8698224. [DOI] [PubMed] [Google Scholar]
- 9.Tapinos NI, Polihronis M, Tzioufas AG, Moutsopoulos HM. Sjögren's syndrome. Autoimmune epithelitis. Adv Exp Med Biol. 1999;455:127–34. [PubMed] [Google Scholar]
- 10.Fujibayashi T, Sugai S, Miyasaka N, et al. Revised diagnostic criteria for Sjögren's syndrome. In: Fujibayashi T, editor. Annual report of the Ministry of Health and Welfare. Tokyo: The Ministry of Health and Welfare, Japan; 1999. pp. 135–8. [Google Scholar]
- 11.Gotoh S, Watanabe Y, Fujibayashi T. Validity of stimulated whole saliva collection as a sialometric evaluation for diagnosing Sjögren's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:299–302. doi: 10.1016/j.tripleo.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes J, Li L, Schlueter C, et al. Immunohistochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138:867–73. [PMC free article] [PubMed] [Google Scholar]
- 13.Kawarada Y, Miura N, Sugiyama T. Antibody against single-stranded DNA useful for detecting apoptotic cells recognizes hexadeoxynucleotides with various base sequences. J Biochem. 1998;123:492–8. doi: 10.1093/oxfordjournals.jbchem.a021963. [DOI] [PubMed] [Google Scholar]
- 14.Goodman AS, Stern IB. Morphologic development of the human fetal labial salivary glands. J Dent Res. 1972;51:990–9. doi: 10.1177/00220345720510044401. [DOI] [PubMed] [Google Scholar]
- 15.Kadoya Y, Yamashina S. Salivary gland morphogenesis and basement membranes. Anat Sci Int. 2005;80:71–9. doi: 10.1111/j.1447-073x.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 16.Sekikawa A, Fukui H, Fujii S, et al. REG Iα protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology. 2005;128:642–53. doi: 10.1053/j.gastro.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Yoshino N, Ishihara S, Rumi MA, et al. Interleukin-8 regulates expression of Reg protein in Helicobacter pylori-infected gastric mucosa. Am J Gastroenterol. 2005;100:2157–66. doi: 10.1111/j.1572-0241.2005.41915.x. [DOI] [PubMed] [Google Scholar]
- 18.Dusetti NJ, Mallo GV, Ortiz EM, Keim V, Dagorn JC, Iovanna JL. Induction of lithostathine/reg mRNA expression by serum from rats with acute pancreatitis and cytokines in pancreatic acinar AR-42J cells. Arch Biochem Biophys. 1996;330:129–32. doi: 10.1006/abbi.1996.0234. [DOI] [PubMed] [Google Scholar]
- 19.Cauli A, Yanni G, Pitzalis C, Challacombe S, Panayi GS. Cytokine and adhesion molecule expression in the minor salivary glands of patients with Sjögren's syndrome and chronic sialoadenitis. Ann Rheum Dis. 1995;54:209–15. doi: 10.1136/ard.54.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox PC, Grisius MM, Bermudez DK, Sun D. Cytokine mRNA expression in labial salivary glands and cytokine secretion in parotid saliva in Sjögren's syndrome. Adv Exp Med Biol. 1998;438:909–15. doi: 10.1007/978-1-4615-5359-5_129. [DOI] [PubMed] [Google Scholar]
- 21.Polihronis M, Tapinos NI, Theocharis SE, Economou A, Kittas C, Moutsopoulos HM. Modes of epithelial cell death and repair in Sjögren's syndrome (SS) Clin Exp Immunol. 1998;114:485–90. doi: 10.1046/j.1365-2249.1998.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukui H, Kinoshita Y, Maekawa T, et al. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology. 1998;115:1483–93. doi: 10.1016/s0016-5085(98)70027-7. [DOI] [PubMed] [Google Scholar]


