Abstract
Muramyl peptides have been shown to exert several biological activities including potentiation of humoral and cell-mediated immunity and stimulation of natural resistance. The mode of action of muramyl peptides has not been elucidated fully and the immunological activities of some derivatives have been associated with toxic effects, including pyrogenicity and inflammatory reactions. Nevertheless, the impact of muramyl peptides on mitochondrial respiration has never been addressed. In this study, the in vitro effects of muramyl peptides on rat liver mitochondria were examined. Toxic muramyl peptides induced a significant decrease in respiratory control ratio versus non-toxic analogues. These results were confirmed by in vivo studies in mice and were extended to mitochondria isolated from spleens. Our data address, for the first time, the effect of muramyl peptides on mitochondrial bioenergetics. Further studies are required to reveal the mechanism of mitochondrial toxicity in relation to the damaging effects of toxic muramyl peptides.
Keywords: muramyl peptides, oxidative phosphorylation, respiratory control ratio
Introduction
Muramyl peptides are a family of immunomodulators with diverse biological effects. Their immunological activities include adjuvanticity [1], amelioration of non-specific resistance to viral and bacterial infections [2], enhancement of anti-tumour activity of macrophages [3], manipulation of cytokine release [4,5] and restoration of haematopoiesis [6]. Furthermore, some derivatives have been developed within the context of non-specific immune-based therapies and have already reached clinical stages of development [7]. The parent molecule of this family is muramyl dipeptide (MDP), which has been reported in 1974 by Ellouz et al. as the minimal adjuvant-active structure of bacterial peptidoglycan [8]. However, MDP administration into different hosts was associated with serious toxicity. Therefore, attempts have been made to generate analogues with desirable properties and reduced toxicities. Some derivatives maintained their toxic and immunological effects [e.g. MDP-lysine (MDP-Lys)], others showed reduced toxicity but retained certain biological activities [e.g. murabutide (MB) and muradimetide (MDM)], and some turned out to be totally inactive [e.g. MDP-D-alanyl-D-isoglutamine (MDP-DD)]. Some toxic effects of muramyl peptides include pyrogenicity, somnogenicity, uveitis [9–11], acute polyarthritis [12], autoimmune thyroiditis and increased serum amyloid A protein [13,14]. For instance, intracerebroventricular administration of 0·1 nmol of MDP or MDP-Lys was sufficient to induce somnogenicity in rabbits whereas MB, and to a lesser extent MDM, was not an effective inducer of slow-wave sleep [10]. Moreover, the minimal pyrogenic dose of MDP in rabbits is 25 µg/kg body weight following intravenous administration. MB and MDP-DD did not induce any febrile response at a concentration of 10 mg/kg when administered by the same route [9,15].
It was proposed that muramyl peptides molecules exert their biological activities following activation of the intracellular receptor nucleotide binding oligomerization domain 2 (NOD2). NOD2 is a cytoplasmic pattern recognition receptor that acts as a general sensor of bacterial peptidoglycan and was found recently to act as a receptor for MDP [16] and some of its analogues, including MB and MDM (Bahr & Girardin, unpublished observation). NOD2 activates the nuclear factor kappaB pathway after ubiquitination of cellular IκB kinase (IKKγ) resulting in the production of inflammatory mediators [17].
Despite a long-standing interest in the field of muramyl peptides, the implication of these molecules at the mitochondrial level has not yet been examined. The present study constitutes the first attempt to evaluate the effects of MDP and its derivatives on mitochondrial respiration. Our goal was to assess the potential toxic effect of three groups of muramyl peptides on mitochondrial respiration in vitro and in vivo. The first group included MDP and MDP-Lys, which are toxic and biologically active. The second was comprised of MB and MDM, which are relatively non-toxic but biologically active, and the third consisted of a non-active, non-toxic derivative, MDP-DD. Our data indicate that toxic muramyl peptides induce significant inhibition of succinate-linked respiratory control ratio (RCR) in vitro and in vivo. Conversely, the mitochondrial efficiency is not affected significantly by either non-toxic muramyl peptides or bacterial lipopolysaccharide (LPS), which constitutes a chemically different immunomodulator from muramyl peptides but exerts a high toxic effect in vivo[1]. These results shed light on mitochondria as a new target affected by MDP and its derivatives and reveal a new approach, distinct from the NOD2 signalling pathway, by which muramyl peptides could exert their toxic effect.
Material and methods
Animals
Experiments were performed on adult Sprague–Dawley rats weighing 250–300 g. Initially, rat livers were used because of the need to recover high concentrations of mitochondria that are necessary to perform multiple in vitro titrations. For the in vivo studies, experiments were performed on Balb/C mice weighing 40–50 g to limit the concentration of muramyl peptides needed for injection and to generate data comparable with most of the published work conducted on MDP. Animals were housed under standard conditions (12-h light/dark cycle, 22 ± 2°C). Mice were injected intraperitoneally with muramyl peptides or LPS, 2 h prior to mitochondria isolation. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Balamand and complied with the principles of laboratory animal care.
Chemicals and reagents
Muramyl peptides used in this work were kindly provided by ISTAC-SA (Lille, France) and were synthesized as described previously [18]. LPS, derived from Escherichia coli (0127:B8), was purchased from Sigma (Germany).
Isolation of mitochondria
Mitochondria from rat or mouse liver were prepared as described previously [19], with all steps carried out at 4°C. Tissues were homogenized using a glass Dounce homogenizer in isolation medium consisting of 250 mM sucrose, 5 mM Tris–HCl (pH 7·4) and 2 mM ethyleneglycol tetraacetic acid (EGTA). The homogenate was centrifuged at 1047 g for 3 min. The supernatant was centrifuged at 11 360 g for 10 min. Mitochondrial pellets were resuspended in the isolation medium and centrifuged at 11 360 g for 10 min. This step was repeated. Mitochondrial pellets were suspended in the isolation medium and protein concentration was determined by the Biuret method [20]. All results are expressed per mg mitochondrial protein.
Mitochondria from mice spleens were isolated similarly. The isolation medium was supplemented with 1% bovine serum albumin (BSA) and 0·1 mM phenylmethylsulphonyl fluoride (PMSF). The last two washes in the centrifugation step were carried out with the isolation medium without BSA.
Measurement of oxygen consumption
Measurements of oxygen consumption were performed using electrode sensitive to oxygen (Clark electrode; Rank Brothers Ltd, Cambridge, UK). Oxygen consumption rates were calculated assuming that the concentration of oxygen in the air-saturated incubation medium was 406 nmol/ml.
Mitochondria from liver (0·25 mg/ml) were incubated in standard assay medium (KHE) (3·5 ml) containing 120 mM KCl, 5 mM KH2PO4, 3 mM HEPES, 1 mM EGTA supplemented with 0·3% defatted BSA and 2 mM rotenone (pH 7·2, 37°C). Respiration was initiated with 2 mM succinate as substrate. Titration of state 3 was carried out in the presence of 200 mM adenosine diphosphate (ADP) and titration of state 4 was performed by adding 1 mg/ml oligomycin. Electrode linearity was checked by following the uncoupled respiration rate in the presence of 2 mM fluoro-carbonyl cyanide phenylhydrazone (FCCP) from 100% to 0% air saturation. RCR were calculated as state 3 divided by state 4 respiration rates. Mitochondria from spleen were incubated in 1·5 ml of standard assay medium at a concentration of 1·5 mg/ml.
Results
In vitro effect of muramyl peptides and LPS on liver mitochondrial oxygen consumption rates
At the biochemical level, several approaches are available for the determination of mitochondrial respiratory chain perturbations. In this study, the activities of the respiratory chain complexes have been examined polarographically as the oxygen consumption rate after addition of various substrates. The mitochondrial respiratory function is separated conventionally into different states. State 2 is the substrate-dependent respiratory state. State 3 is defined as the ADP-dependent oxygen consumption and reflects the mitochondrial respiration coupled to adenosine triphosphate (ATP) production; this is the phosphorylation state. State 4, the resting respiration or the non-phosphorylation state in the presence of oligimycin, is a measure of the oxygen consumed uncoupled from ATP synthesis. State 3/state 4, termed the RCR, is the ratio of the maximal rate of O2 consumption in the phosphorylation state to the basal rate in the non-phosphorylation state. This is used as an indicator of coupling between oxidative phosphorylation and the mitochondrial electron transport chain. By this criterion, well-coupled and high physical and functional mitochondrial integrity can be evaluated.
Figure 1a shows the effect of different MDP and MB concentrations on RCR of rat liver mitochondria in vitro. MDP induced 22% reduction in RCR at 10 µM and a maximum inhibition of 50% at 100 µM; higher concentrations up to 1000 µM did not induce any further inhibition (data not shown). MB concentrations up to 500 µM, as well as LPS at 50 and 100 µg/ml (data not shown), did not affect the RCR significantly. Figure 1b shows the time study of MDP, MB and LPS effects on RCR of rat liver mitochondria. MDP at 100 µM induced 50% decrease in RCR at 2 min and up to 3 h of incubation. Conversely, no significant changes were depicted in RCR of MB and LPS-treated mitochondria at any time-point evaluated. The RCR of control mitochondria was fairly constant at a value of between 5 and 6 during the entire study. Figure 2a and b shows the in vitro effects of MDP and MB within 2 min of incubation at 100 µM on the respiration rates indexes of rat liver mitochondria. The state 3 rate was depressed significantly by 25% with MDP, whereas state 4 respiration was stimulated by 75%. The state 2 and uncoupled respiration rates were unchanged (Fig. 2a). MB was without significant effect on the respiratory rates (Fig. 2b).
Fig. 1.
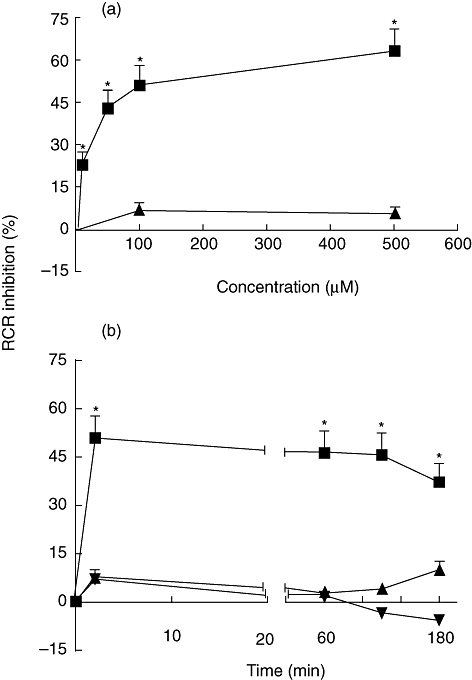
Effects of muramyl dipeptide (MDP), murabutide (MB) and lipopolysaccharide (LPS) on respiratory control ratio (RCR) in rat liver mitochondria in vitro. Oxygen consumption was measured (a) within 2 min in the presence of 1, 10, 50, 100 and 500 µM of MDP (▪) and 100 and 500 µM of MB (▴) and (b) at 100 µM of MDP (▪) and MB (▴) and at 50 µg/ml of LPS (▾) after 2, 60, 120 and 180 min of incubation. The decrease in RCR is presented as percentage of inhibition. Data are means ± standard error of the mean of three independent experiments each performed in triplicate. *P < 0·05.
Fig. 2.
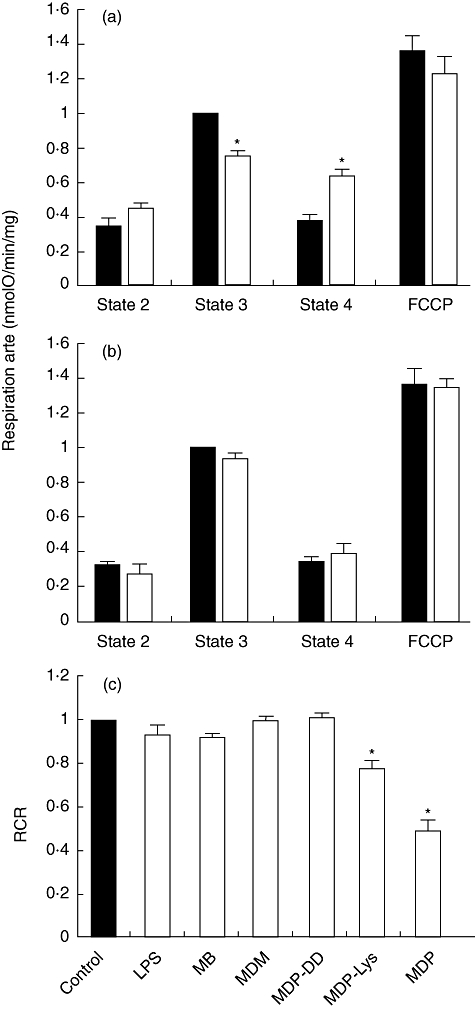
Effects of muramyl peptides and lipopolysaccharide (LPS) on respiration rates and respiratory control ratio (RCR) in rat liver mitochondria in vitro. Oxygen consumption was measured in the presence of (a) 100 µM muramyl dipeptide (MDP), (b) 100 µM murabutide (MB) and (c) 100 µM of muramyl peptides and 50 µg/ml of LPS after 2 min of incubation. Data were normalized (closed bars) to the state 3 rates as in (a) and (b) and to 1 as in (c) of control mitochondria. Data are means ± standard error of the mean of three independent experiments each performed in triplicate. *P < 0·05.
The conditions at which MDP exerted its maximum effects on the mitochondria were applied to examine the impact of the other derivatives. Figure 2c summarizes the effects of muramyl peptides and LPS on mitochondrial RCR at 100 µM and 50 µg/ml, respectively, after 2 min incubation. The RCR values of mitochondria treated with MDP-Lys decreased significantly by 23%. In contrast, the RCR remained almost unchanged after LPS, MB, MDM and MDP-DD treatment. The 21% decrease in state 3 induced by MDP-Lys justified the alterations in the RCR. No major changes were observed in state 4 (data not shown).
In vivo effect of muramyl peptides and LPS on liver mitochondrial oxygen consumption rates
It becomes clear from the preceding results that toxic MDP derivatives affect the performance of the mitochondria in vitro, but is this true in vivo? To answer this question, mice were injected intraperitoneally with 100 mg/kg of MDP, and RCR of liver mitochondria was measured at different time intervals. As shown in Fig. 3a, mitochondria from MDP-treated mice showed a maximum decrease in the RCR (about 27%) after 2 h of injection and returned to its basal level at 6 h. The in vivo effect of other MDP analogues on mitochondrial respiration was assessed at 100 mg/kg concentration after 2 h of injection and the results are presented in Fig. 3b. The RCR was depressed by 20% in MDP-Lys-treated mice. However, no significant differences in the RCR were observed between the control mice and those treated with LPS, MB, MDM and MDP-DD.
Fig. 3.
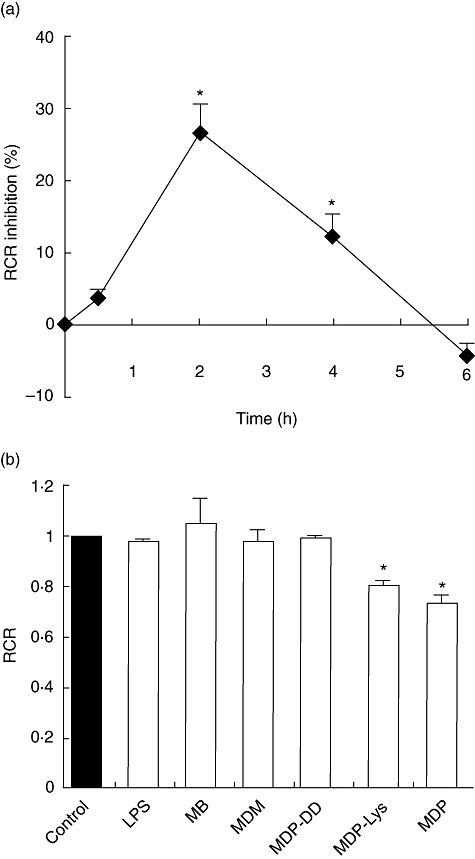
Effect of muramyl peptides and lipopolysaccharide (LPS) on the respiratory control ratio (RCR) in mice liver mitochondria in vivo. (a) Mice were injected intraperitoneally with 100 mg/kg of muramyl dipeptide (MDP) and oxygen consumption was measured after 0·5, 2, 4 and 6 h. The decrease in RCR is presented as percentage of inhibition. (b) Mitochondrial oxygen consumption was measured after 2 h of treatment with the studied compounds (100 mg/kg). The control mice were injected with saline and the data are normalized to 1. Data are means ± standard error of the mean of three independent experiments each performed in triplicate. *P < 0·05.
Table 1 shows that a 20% decrease in state 3 in vivo was responsible for the RCR drop in MDP-Lys-treated mice, whereas a 55% increase in state 4 triggered RCR reduction in the case of MDP.
Table 1.
Effects of lipopolysaccharide (LPS) and muramyl dipeptide (MDP) derivatives on respiration rates in mice liver mitochondria in vivo (nmolO/min/mg).
| Percentage of control | ||||
|---|---|---|---|---|
| State 2 | State 3 | State 4 | FCCP rate | |
| MDP | 109·7 ± 4·8 | 114·78 ± 16·2 | 155·09 ± 13·6* | 116·77 ± 6·3 |
| MDP-Lys | 92·34 ± 6·4 | 80·98 ± 1·9 (*) | 100 ± 0 | 101·92 ± 7·5 |
| MB | 144·44 ± 23·6 | 142·03 ± 25·7 | 132·14 ± 20·2 | 112·94 ± 18·4 |
| MDM | 125 ± 14·4 | 121·54 ± 8·2 | 124·04 ± 14·4 | 139·47 ± 9·8 |
| MDP-DD | 88·41 ± 0·4 | 108·68 ± 5·3 | 109·22 ± 4·8 | 99·06 ± 4 |
Liver mitochondria were isolated from mice after 2 h of intraperitoneal injection of MDP derivatives (100 mg/kg). Data are presented as percentage of control. Data are means ± standard error of the mean of three independent experiments each performed in triplicate.
P < 0·05. MB, murabutide; MDM, muradimetide; MDP-DD, N-acetyl-muramyl-D-alanyl-D-isoglutamine; MDP-Lys, N-acetyl-muramyl-L-alanyl-D-isoglutamine-L-lysine; FCCP, fluoro-carbonyl cyanide phenylhydrazone.
In vivo effect of muramyl peptides on spleen mitochondrial oxygen consumption rates
To check whether muramyl peptides have a general or tissue-specific toxic effect, we studied the effects of MDP and MB on mice spleen mitochondria. Similar to the effect observed in liver mitochondria, a 33% fall in RCR was noticed in MDP-treated mice; MB did not affect the mitochondrial performance, as shown in Fig. 4.
Fig. 4.
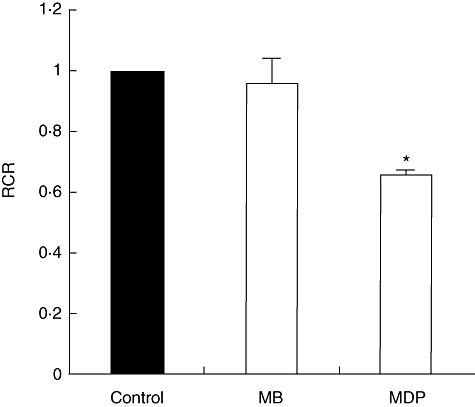
Effects of muramyl dipeptide (MDP) and murabutide (MB) on respiratory control ratio (RCR) in mice spleen mitochondria in vivo. Oxygen consumption was measured in mitochondria isolated from spleen mice after 2 h of intraperitoneal injection with 100 mg/kg of MDP or MB. Control mice were injected with saline and the data are normalized to 1. Data are means ± standard error of the mean of three independent experiments each performed in triplicate. *P < 0·05.
Discussion
Detailed and extensive experimental systems have been used in order to investigate the mode of action of muramyl peptides. Nevertheless, all studies have neglected one important aspect of potential toxic events, namely, those affecting mitochondrial function. Data in this study demonstrate the ability of toxic muramyl peptides to impair the mitochondrial bioenergetics system. MDP causes concentration-dependent inhibition of state 3 (phosphorylation state), increased state 4 (non-phosphorylation state) and decreased RCR of respiration in isolated rat liver mitochondria. MDP-Lys exerts similar effects, supporting the notion that these compounds have common toxic characteristics. In contrast, MB, MDM and MDP-DD have no effect on mitochondrial respiration. The toxic effect of MDP is also seen in in vivo studies which show that MDP decreases significantly the RCR of mouse liver mitochondria. Our results also show that MDP affect the RCR of spleen mitochondria similar to the liver mitochondria, indicating that MDP has a broad toxic effect on mitochondrial bioenergetics.
The lack of difference between the control and MDP for state 2 rates (substrate-dependent respiratory rate) with succinate suggests no effect induced by MDP on Complex II. A decrease in state 3 (phosphorylation state) indicates that toxic muramyl peptides act directly on the ATP synthase complex by disturbing the coupling between the F0 and F1 components, thereby depressing the phosphorylation efficiency of mitochondria. On the other hand, increased oxygen consumption during state 4 (non-phosphorylation state) with decreased RCR is an indication of increased basal proton leakage. This indicates that toxic muramyl peptides decrease the efficiency of ATP production. In this regard, the effect on state 4 is similar to an uncoupling effect, but all muramyl peptides do not possess any acid-based group which is typical of uncouplers (as FCCP). Moreover, this enhancement in state 4 rate cannot be explained as a detergent effect, as the FCCP respiration rate is not affected.
The inhibition of RCR by toxic derivatives in vitro reveals a direct structural effect on mitochondria. Thus, toxic muramyl peptides might be able to bind to mitochondria and induce defects, whereas non-toxic derivatives, regardless of their biological activity, do not. What strengthens this hypothesis is the time-independent effect of MDP and its toxic analogue in vitro. However, there is no common structural component among toxic analogues that is absent in non-toxic derivatives, which could help in predicting the link between structural modification and toxicity.
In order to understand the mechanism of action of muramyl peptides on mitochondria in vivo, it is necessary to address the way in which muramyl peptides reach the cytosol. hPEpT1, present in intestinal epithelial cells and macrophages, has been demonstrated to be an exclusive transporter, allowing MDP to pass the plasma membrane and reach the cytosol and activate NOD2 [21]. Despite the fact that liver cells contain NOD2 [22], the effect of MDP on mitochondria is not mediated through the NOD2 signalling pathway, as non-toxic but biologically active derivatives such as MB and MDM, known to activate NOD2 (Bahr & Girardin, unpublished observation), do not induce any impact on mitochondrial respiration. Furthermore, the observed effect could not be attributed to a degradation product of MDP because 90% of the molecule was reported to be excreted in urine, in an unchanged form, within 2 h after administration [23]. In addition, toxic derivatives do not exert the same effect on respiratory states indexes in in vitro and in vivo experiments. MDP and MDP-Lys decrease the rate of state 3 (phosphorylation state) in vitro, thus reducing the efficiency of mitochondrial oxidative phosphorylation by disturbing the ATP synthase complex. MDP stimulates the rate of state 4 (non-phosphorylation state) in vivo, thus increasing the energetic cost of mitochondrial ATP production. This increase in the basal proton leak activity of mitochondria from MDP-treated mice could be the result of activation or an induction of expression of a mitochondrial membrane protein (mitochondrial permeability transition pores, uncoupling proteins, adenine nucleotide transferase, etc.) that catalyzes a proton leak increasing the inefficiency of oxidative phosphorylation, thus reducing the generation of high energy bonds. Further studies are required to reveal the mode of action of MDP and its analogues on mitochondria.
The LPS does not show any significant effect on mitochondrial bioenergetics in vitro or in vivo within the time range studied. It has been demonstrated previously that LPS requires a period of 16 h to induce a significant impact on rat mitochondrial respiration in vivo[24]. Therefore, the mechanism of action of these two molecules in vivo is completely different, as LPS requires more time than MDP to induce mitochondrial inefficiency. Also, MDP induces a direct effect on mitochondrial respiration in vitro whereas LPS does not, indicating the inability of LPS to interact with the mitochondrial membrane proteins and/or lipids, and might explain the time-period required for the in vivo effect to become detectable.
Toxicological studies are required to ensure safe and appropriate administration of a certain molecule and to achieve a better understanding of its molecular pharmacology. It has been shown that parenteral administration of MDP induces an increase in tissue morphine levels in rats [25], neutrophil influx into the skin of rabbits [26], acute polyarthritis and oedemagenic activity in rats [14], autoimmune thyroiditis and amyloidosis in mice [13,14] and uveitis and decreased plasma metal iron levels in rabbits [11,27]. Moreover, administration of MDP via either the intravenous or the intracerebroventricular route induces elevation in body temperature in rabbits [9]. These biological effects are accompanied by the release of a battery of inflammatory cytokines [interleukin (IL)-1β, IL-6, IL-8, tumour necrosis factor-α]. Many of these inflammatory processes have been found to be minimal or even absent following administration of non-pyrogenic derivatives including MB [4]. Thus, in addition to inflammatory and autoimmune reactions, muramyl peptides with toxic profile are demonstrated, in this study, to alter the mitochondrial function. Moreover, it is of importance to note that the maximum in vivo effect of MDP and some of its derivatives on mitochondrial respiration was observed 2 h after administration, a time peak which has been reported for several of the toxicological effects of MDP in vivo[9,11,26].
Alterations in mitochondrial bioenergetics have been assessed previously upon treatment with several drugs. For example, 4-hydroxytamoxifen, a proposed chemotherapeutic agent for breast cancer treatment, has been found to affect the in vitro phosphorylation efficiency of mitochondria [28]. Furthermore, 5 days of dexamethasone treatment, a potent synthetic glucocorticoid, increases the contribution of the mitochondrial proton cycle to oxidative phosphorylation [29]. Such interactions of toxic compounds with mitochondrial function can result in severe impairment of the general metabolism, given that defective electron transport and oxidative phosphorylation can impair the function of physiological activities. A wide range of seemingly unrelated disorders, such as schizophrenia, dementia, Alzheimer's disease, migraine headaches, Parkinson's disease, diabetes and hepatitis C, have common underlying pathophysiological mechanisms, namely mitochondrial dysfunction and impaired ATP generation [30]. Therefore, some of the undesirable side effects of muramyl peptides could be the result of mitochondrial inefficiency in ATP synthesis. This implies that muramyl peptides-induced alterations in mitochondrial bioenergetics could contribute to the pathogenesis of toxic derivatives. Nevertheless, the implication of this effect in the overall toxicity of muramyl peptides in vivo still requires further investigation.
Acknowledgments
We would like to thank Michael Zakhem for technical assistance. This work is supported by grants from the university research council at the University of Balamand (UOBRC) and the National Council for Scientific Research (CNRS) in Lebanon.
References
- 1.Lederer E. Natural and synthetic immunomodulators derived from the mycobacterial cell wall. In: Bizzini B, Bonmassar E, editors. Advances in immunomodulation. Rome: Pythagora Press; 1988. pp. 9–36. [Google Scholar]
- 2.Chedid L, Audibert F, Lefrancier P, Choay JP, Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci USA. 1976;73:2472–5. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Reilly T, Zak O. Enhancement of the effectiveness of antimicrobial therapy by muramyl peptide immunomodulators. Clin Infect Dis. 1992;14:1100–9. doi: 10.1093/clinids/14.5.1100. [DOI] [PubMed] [Google Scholar]
- 4.Bahr GM, Darcissac E, Bevec D, Dukor P, Chedid L. Immunopharmacological activities and clinical development of muramyl peptides with particular emphasis on murabutide. Int J Immunopharmacol. 1995;17:117–31. doi: 10.1016/0192-0561(94)00094-5. [DOI] [PubMed] [Google Scholar]
- 5.Bahr GM, Chedid L. Immunological activities of muramyl peptides. Fed Proc. 1986;45:2541–44. [PubMed] [Google Scholar]
- 6.Galelli A, Chedid L. Modulation of myelopoiesis in vivo by synthetic adjuvant-active muramyl peptides: induction of colony-stimulating activity and stimulation of stem cell proliferation. Infect Immun. 1983;42:1081–5. doi: 10.1128/iai.42.3.1081-1085.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahr GM, De La Tribonniere X, Darcissac E, et al. Clinical and immunological effects of a 6 week immunotherapy cycle with murabutide in HIV-1 patients with unsuccessful long-term antiretroviral treatment. J Antimicrob Chemother. 2003;51:1377–88. doi: 10.1093/jac/dkg244. [DOI] [PubMed] [Google Scholar]
- 8.Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974;59:1317–25. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 9.Riveau G, Masek K, Parant M, Chedid L. Central pyrogenic activity of muramyl dipeptide. J Exp Med. 1980;152:869–77. doi: 10.1084/jem.152.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krueger JM, Walter J, Karnovsky M, et al. Muramyl peptides. Variation of somnogenic activity with structure. J Exp Med. 1984;159:68–76. doi: 10.1084/jem.159.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters RV, Terrell TG, Jones GH. Uveitis induction in the rabbit by muramyl dipeptides. Infect Immun. 1986;51:816–25. doi: 10.1128/iai.51.3.816-825.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohashi O, Kohashi Y, Shigematsu N, Ozawa A, Kotani S. Acute and chronic polyarthritis induced by an aqueous form of 6-0-acyl and N-acyl derivatives of N-acetylmuramyl-L-alanyl-o-isoglutamine in euthymic rats and athymic nude rats. Lab Invest. 1986;55:337–46. [PubMed] [Google Scholar]
- 13.Kong YC, Audibert F, Giraldo AA, Rose NR, Chedid L. Effects of natural or synthetic microbial adjuvants on induction of autoimmune thyroiditis. Infect Immun. 1985;49:40–5. doi: 10.1128/iai.49.1.40-45.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAdam KP, Foss NT, Garcia C, et al. Amyloidosis and the serum amyloid A protein response to muramyl dipeptide analogs and different mycobacterial species. Infect Immun. 1983;39:1147–54. doi: 10.1128/iai.39.3.1147-1154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chedid L, Parant M, Audibert F, et al. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982;35:417–24. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. J Biol Chem. 2003;278:5509–12. doi: 10.1074/jbc.C200673200. Implications for Crohn's disease. [DOI] [PubMed] [Google Scholar]
- 17.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–18. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 18.Lefrancier P, Choay JP, Derrien M, Lederman I. Synthesis of N-acetyl-muramyl-L-alanyl-D-isoglutamine, an adjuvant of the immune response, and of some n-acetyl-muramyl-peptide analogs. Int J Pept Protein Res. 1977;9:249–57. doi: 10.1111/j.1399-3011.1977.tb03488.x. [DOI] [PubMed] [Google Scholar]
- 19.Echtay KS, Roussel D, St-Pierre J, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–9. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 20.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–66. [PubMed] [Google Scholar]
- 21.Ismair MG, Vavricka SR, Kullak-Ublick GA, Fried M, Mengin-Lecreulx D, Girardin SE. hPepT1 selectively transports muramyl dipeptide but not Nod1-activating muramyl peptides. Can J Physiol Pharmacol. 2006;84:1313–19. doi: 10.1139/y06-076. [DOI] [PubMed] [Google Scholar]
- 22.Body-Malapel M, Dharancy S, Berrebi D, et al. NOD2: a potential role for regulating liver injury. Lab Invest. 2008;88:318–27. doi: 10.1038/labinvest.3700716. [DOI] [PubMed] [Google Scholar]
- 23.Parant M, Parant F, Chedid L, Yapo A, Petit JF, Lederer E. Fate of synthetic immunoadjuvant, muramyl dipeptide (14C-labelled) in the mouse. Int J Immunopharmacol. 1979;1:35–41. doi: 10.1016/0192-0561(79)90028-6. [DOI] [PubMed] [Google Scholar]
- 24.Kozlov AV, Staniek K, Haindl S, et al. Different effects of endotoxic shock on the respiratory function of liver and heart mitochondria in rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:543–9. doi: 10.1152/ajpgi.00331.2005. [DOI] [PubMed] [Google Scholar]
- 25.Horak P, Haberman F, Spector S. Endogenoumorphine and codeine in mice – effect of muramyl dipeptide. J Life Sci. 1993;52:255–60. doi: 10.1016/0024-3205(93)90011-q. [DOI] [PubMed] [Google Scholar]
- 26.Colditz IG, Cybulsky MI. Some characteristics of inflammation induced by muramyl dipeptide, endotoxin and concanavalin A. Inflammation. 1987;11:1–11. doi: 10.1007/BF00917767. [DOI] [PubMed] [Google Scholar]
- 27.Riveau G, Parant M, Damais C, Parant F, Chedid L. Dissociation between muramyl dipeptide-induced fever and changes in plasma metal levels. Am. J Physiol. 1986;250:C572–7. doi: 10.1152/ajpcell.1986.250.4.C572. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso CM, Moreno AJ, Almeida LM, Custódio JB. 4-Hydroxytamoxifen induces slight uncoupling of mitochondrial oxidative phosphorylation system in relation to the deleterious effects of tamoxifen. Toxicology. 2002;179:221–32. doi: 10.1016/s0300-483x(02)00392-x. [DOI] [PubMed] [Google Scholar]
- 29.Roussel D, Dumas JF, Simard G, Malthièry Y, Ritz P. Kinetics and control of oxidative phosphorylation in rat liver mitochondria after dexamethasone treatment. Biochem J. 2004;382:491–9. doi: 10.1042/BJ20040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]


