Abstract
Chitosan is a mucoadhesive polysaccharide that promotes the transmucosal absorption of peptides and proteins. At mucosal sites chitosan exhibits immunomodulatory activities and stimulates the release of regulatory cytokines. Herein we evaluated the effect of the co-administration of chitosan in the tolerance to type II collagen (CII) using an experimental model of arthritis. Rats were fed diluent (acetic acid), 1 mg CII, 1 mg chitosan or 1 mg CII + 1 mg chitosan during 5 days before immunization with CII in Freund's complete adjuvant. Systemic effects were evaluated in draining lymph nodes after antigenic challenge or during the clinical evolution of arthritis. Specific antibodies, proliferation against CII and the production of interferon (IFN)-γ and interleukin-10 were assessed. Clinical signs were observed 13–15 days after primary immunization. The CII : chitosan group presented the lowest incidence and developed moderate arthritis, with reduced levels of immunoglobulin (Ig)G2a anti-CII, a limited proliferation in draining lymph nodes and a lower release of IFN-γ after restimulation with CII. Our results demonstrate that chitosan enhances the tolerance to an articular antigen with a decrease in the inflammatory responses and, as a consequence, an improvement in clinical signs.
Keywords: antibodies, arthritis, chitosan, collagen, inflammation
Introduction
Oral tolerance is a key feature of the intestinal immunity, generating no responsiveness to ingested antigens [1,2]. The transport of antigens via afferent lymphatics into the draining mesenteric lymph nodes is obligatory for oral tolerance induction [3]. The antigen characteristics, administration protocol and primary contact with the immune system condition the induction of tolerance [1,2,4,5]. Moreover, agents that enhance anti-inflammatory cytokine profiles can improve the induction of oral tolerance. Chitosan is an abundant, natural linear polysaccharide obtained from deacetylation of chitin from crustaceans, insects and fungi [6]. Chitosan is a non-toxic, biodegradable and non-immunogenic agent used widely as biomaterial with an established safety profile in humans, as well as pharmaceutical excipient, weight loss supplement [6,7] and in a Food and Drug Administration-approved haemostatic dressing [8]. Due to its mucoadhesive properties, chitosan has also been explored as an adjuvant for mucosal vaccination [9]. The mechanism of chitosan enhancement is believed to involve both retention of antigens via mucoadhesion and opening of cell junctions for paracellular transport [10].
Rheumatoid arthritis (RA) is an autoimmune disease of synovial joints with a prevalence of 0·5–1% in the global population [11,12]. Although RA pathogenesis remains unknown, type II collagen (CII) is assumed to participate in the immune response [13]. The most common model of RA in rats and mice is CII-induced arthritis (CIA) that elicits both antibody and T cell responses to CII [14]. This joint-specific antigen has been tested frequently in oral tolerance-suppressing arthritis in animal models at 0·2–10 mg CII per day [15,16]. Results with RA patients have not been entirely successful [17] and the discrepancy could be due to differences in protocols, the strongly T helper type 1 (Th1)-biased mucosal T cell response in humans [18] or treatment with different drugs during CII clinical trials [19]. Also, it has been largely recognized that is harder to regulate an existing immune response than to prevent the induction prophylactically [20].
Previously, we have demonstrated that feeding rats a single dose of chitosan with 1 mg CII increases interleukin (IL)-10 release and IL-4 and transforming growth factor (TGF)-β mRNA expression at mucosal level [21]. The uptake of chitosan at inductive sites is mediated by CD11b/c+ OX62+ dendritic cells that keep an immature phenotype. As our findings demonstrated that chitosan acts by enhancing the Th2/Th3 microenvironment in the mucosa [22], we hypothesized that the activity of this mucoadhesive polysaccharide could improve the induction of tolerance to CII. To test this possibility we evaluated the effect of repetitive co-administration of CII : chitosan in the CIA model in rats. We found that the protocol alleviated the clinical signs of CIA, diminished immunoglobulin (Ig)G2a anti-CII levels and reduced both the proliferative response to CII and the release of interferon (IFN)-γ. Our findings demonstrate the ability of this polycationic polysaccharide to strengthen tolerance towards an articular protein dampening the inflammatory response during the development of CIA.
Materials and methods
Animals
Female Wistar rats (8–10 weeks old) were maintained at the Animal Resource Facilities, Department of Clinical Biochemistry, National University of Cordoba. Experimental procedures were approved by the Research Ethics Committee.
Preparation of CII and chitosan
Native bovine CII was extracted from the septum cartilage of 1-year-old animals [13,23] and dissolved in 0·1 M acetic acid at a final concentration of 9 mg/ml. Low molecular weight chitosan (average MW 80 kDa) 85% deacetylated (Sigma, St Louis, MO, USA) was prepared at a final concentration of 20 mg/ml in 0·1 M acetic acid, as described previously [24].
Induction of oral tolerance to CII and immunizations
Four groups of rats were fed 200 µl of either 0·1 M acetic acid (diluent group), 1 mg chitosan in 0·1 M acetic acid (Ch group), 1 mg CII in 0·1 M acetic acid (CII group) or 1 mg CII + 1 mg chitosan in 0·1 M acetic acid (CII : Ch group) every day for 5 successive days. For CIA induction, CII at 1 mg/ml in 0·1 M acetic acid was emulsified (at a 1:1 ratio) with Freund's complete adjuvant (CFA) at 4°C. Three days after the last feeding, rats were immunized with 1 ml of CFA containing 0·5 mg of CII in four sites at the base of the tail, as described by Trentham et al.[13]. To assess cellularity in lymph nodes, rats were immunized in the footpad with 50 µg CII in CFA in a total volume of 100 µl [25]. Seven days later, popliteal lymph nodes were harvested to determine the cell number and the antibody secreting cells (ASC) [26].
Evaluation of arthritis
Rats were examined daily for weight variation, disease onset and severity of joint inflammation. Each limb was assessed on a 0–4 scale, as follows: 0 = normal; 1 = mild inflammation: 2 = moderate arthritis; 3 = severe arthritis involving the entire paw; and 4 = severe arthritis with loss of joint movement [25,27]. For comparisons we calculated: the percentage of weight variation; the incidence as the percentage of animals with signs of arthritis; the clinical score as the sum of the score for each rat divided by the total number of rats in the group; and the mean number of arthritic limbs. Rats were killed 28 days after immunization.
Analysis of anti-CII antibodies
The frequency of ASC to CII was assessed by a cell enzyme-linked immunosorbent assay (cELISA) [28,29]. Briefly, 96-well plates were coated overnight at 4°C with 100 µg/well of CII in carbonate buffer pH 12, blocked with phosphate-buffered saline (PBS)/1% bovine serum albumin (BSA) for 1 h at 37°C and washed three times with RPMI-gentamicin. Popliteal lymph node cells (2 × 104–2 × 105) were added in 200 µl RPMI and incubated for 24 h at 37°C. After incubation, plates were washed three times with PBS/0·2% Tween-20 and incubated with 1:1000 horseradish peroxidase mouse anti-rat IgG for 24 h at 4°C. After thorough washing, o-phenylenediamine was added. The reaction was fixed with sulphuric acid and read in a Microplate Reader (BioRad, Hercules, CA, USA) at 490 nm. As controls we used lymph node cells from unimmunized rats. Results are expressed as optical density. Moreover, anti-CII IgG as well as the subclasses IgG1, IgG2a and IgG2b were determined by ELISA in serum samples [30].
Proliferative response and cytokine production
Mononuclear cells from draining lymph nodes and spleen from CII feed groups were cultured at 2 × 105 cells/well in quadruplicate in 200 µl of RPMI–10% fetal calf serum (FCS), 50 µg/ml gentamicin, 2 mM glutamine and 50 µM 2-mercaptoethanol with or without 40 µg/ml CII at 37°C–5% CO2. After 4 days cells were pulsed with [3H]-thymidine for the last 16 h [31]. Results are expressed as counts per minute with and without stimulus. In culture supernatants, IFN-γ release was measured by sandwich ELISA using reagents and protocols obtained from Pharmingen (San Diego, CA, USA) [21].
Flow cytometric analysis
To assess intracellular monocyte chemotactic protein-1 (MCP-1), IL-10 and IFN-γ production, 1 × 106 cells cultured for 48 h with 40 µg/ml CII were incubated with fluorescein isothiocyanate-labelled mouse anti-CD4, washed, fixed, permeabilized with 0·1% saponine and incubated with phycoerythrin-labelled anti-MCP-1, IL-10 or anti-IFN-γ (Pharmingen) for intracellular protein detection. Staining was performed at 4°C in RPMI–ethylenediamine tetraacetic acid–FCS [21,22,32]. Cells were analysed using a Cytoron Absolute (Ortho Diagnostic Systems, Raritan, NJ, USA). In all experiments we included isotype controls (Sigma).
Statistical analysis
Data are mean values ± standard deviation. Statistical significance and differences between groups were determined by Fisher's exact test, analysis of variance and Student–Newman–Keuls tests; P < 0·05 values were considered significant.
Results
Assessment of chitosan activity after the induction of tolerance
A feature of tolerance induced by fed antigens is reduced cell recruitment into the draining lymph nodes upon challenge with the relevant antigen [26]. To evaluate the systemic effect of the oral co-administration of chitosan and CII, rats fed previously either CII or CII : chitosan were immunized at the footpad with CII–CFA and the cell number was counted 7 days later. Groups feed diluent or chitosan were used as controls. As shown in Fig. 1a, a decreased cellular infiltration in popliteal lymph nodes was found in the CII and CII : chitosan groups, suggesting a comparable ability to induce tolerance to CII in both groups that received the articular antigen.
Fig. 1.
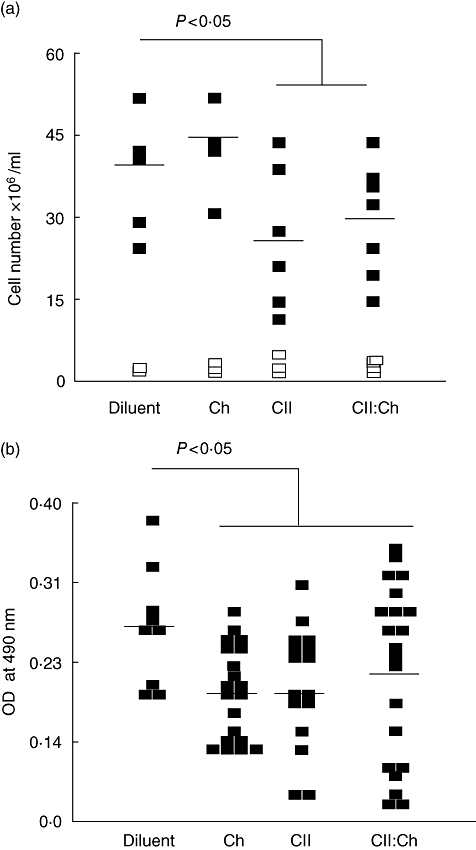
Evaluation of the tolerance induction after type II collagen (CII) : chitosan administration. Rats (n = 7–9) were fed diluent, chitosan, CII or CII : chitosan daily for 5 days. Forty h after the last feeding rats were immunized in the footpad with either 50 µg CII in complete Freund's adjuvant (CFA) or saline. Popliteal lymph node cells were obtained 7 days later to assess (a) the total number of living cells in lymph nodes; each rat is represented by two symbols: filled, for the antigen injected left foot, and unfilled, for the saline injected right foot. Lines represent the average value; (b) the number of antibody secreting cells (ASC) in draining lymph nodes upon challenge was evaluated by a cell enzyme-linked immunosorbent assay (CELISA). Each rat is represented by two to three dots. Lines represent the average value.
Using the same experimental protocol we measured the anti-CII ASC present in peripheral lymph nodes. As shown in Fig. 1b, a reduction in the number of anti-CII ASC was observed in groups fed CII either alone (CII) or co-administered with polysaccharide (CII : Ch). As the T cell co-operation of certain T cell subsets functions in CIA models to help antibody responses [33], the decrease in ASC anti-CII could be related to a poorly developed humoral response as part of the tolerance process when the articular antigen was administered. Interestingly, although chitosan itself was not able to dampen lymph node recruitment upon challenge (Fig. 1a), polysaccharide feeding reduced the number of anti-CII ASC suggesting that, after mucosal contact, the polysaccharide could modulate the T–B cell interaction. Compared with the diluent group, tolerization with either protein or polysaccharide produced a similar effect, possibly because orally tolerized T cells display an initial inability to provide adequate B cell help [34].
Effect of CII : chitosan administration in the development of arthritis
The systemic activity observed in CII- or CII : chitosan-fed groups prompted us to evaluate the effects of polysaccharide co-administration on the clinical signs of arthritis in the CIA model. As shown in Fig. 2a, a negative weight variation was observed following the onset (∼ day 14) in all groups; however, only CII : chitosan-fed rats exhibited a prompt recovery (P < 0·05). In terms of percentage of incidence, only treatment with CII : chitosan reduced this parameter significantly (Fig. 2b). No differences in the clinical score were observed between those rats that developed CIA independently of the oral treatment (Fig. 2c). However, compared with CII-fed rats, the CII : chitosan group showed the lowest number of affected limbs (Fig. 2d).
Fig. 2.
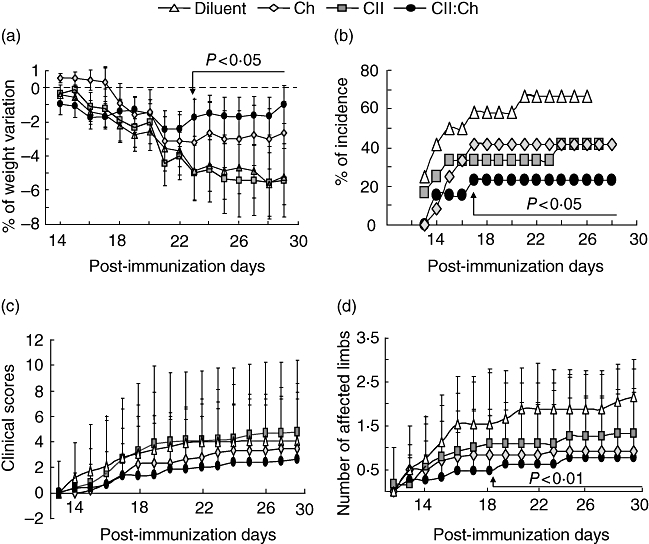
Improvement of clinical manifestations of arthritis in rats fed type type II collagen (CII) and chitosan. Rats (n = 12–14) were fed diluent (Δ), chitosan (♦), CII (▪) and CII : chitosan (♦), as described in Fig. 1, and immunized with 0·5 mg CII in complete Freund's adjuvant (CFA) 2 days later. Groups were examined daily for the onset of the disease and severity of joint inflammation during 28 days after primary immunization. The following parameters were evaluated: (a) the percentage of body weight variation starting the onset day (˜ day 14) until 3 weeks after primary immunization; (b) incidence as the percentage of arthritic rats of the total number studied in each group; (c) the average of the clinical score evaluated as described in Materials and methods; (d) mean number of arthritic limbs affected in each group. Arrows point to the day where the significance starts. For weight variation and affected limbs, P versus diluent and CII groups; for incidence, P versus diluent.
Serum levels of anti-CII IgG subtypes in tolerized rats
The pathogenic mechanisms of CIA involve the synergistic action of CII-specific T cells and anti-CII antibodies [33]. Among the three subtypes of IgG, IgG1 is reported to be associated with anti-inflammatory actions, whereas IgG2a is considered a mediator of inflammation that is increased in CIA [35]. Total levels of IgG anti-CII (Fig. 3a) as well as the isotype responses (Fig. 3b–d) were reduced significantly 28 days post-immunization in either group fed CII. Notably, the reduction of IgG2a and IgG2b was significantly greater in rats receiving CII : chitosan compared with those fed only CII. This reduction in the inflammatory humoral response against the articular antigen could explain, at least in part, the lower severity of CIA observed in the CII : chitosan group.
Fig. 3.
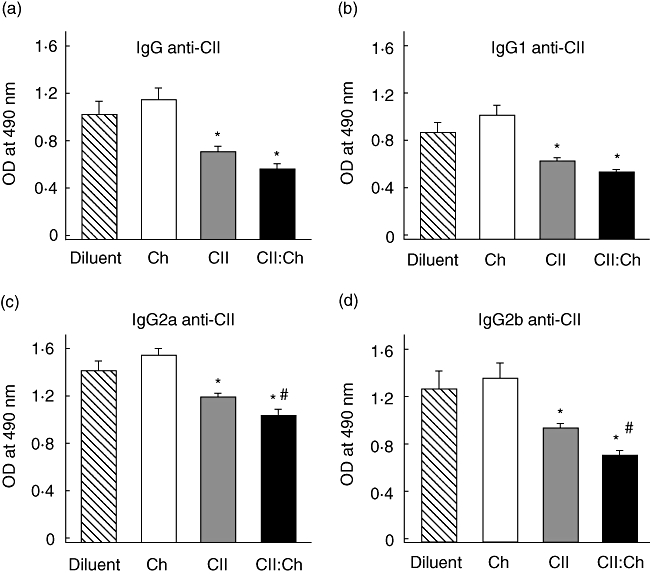
Serum levels of anti-type II collagen (CII) antibodies after CII : chitosan feeding. Rats (n = 7–9) were fed diluent, chitosan, CII and CII : chitosan and immunized as described in Fig. 2; 28 days after the primary immunization serum samples were obtained and the anti-CII response was evaluated by enzyme-linked immunosorbent assay at a 1:500 dilution; (a) total immunoglobulin (Ig)G anti-CII; (b) IgG1 anti-CII; (c) IgG2a anti-CII; (d) IgG2b anti-CII. Difference versus diluent *P < 0·05; versus CII #P < 0·05.
Proliferative response and production of IFN-γ, MCP-1 and IL-10 in spleen and draining lymph nodes of tolerized rats
We also studied the CII-stimulated proliferation of draining lymph node and spleen T cells from CII-fed groups. Twenty days after primary immunization with CII–CFA, the proliferative response was significantly lower in cells from the CII : chitosan group (Fig. 4a). We also found that the type 1 cytokine IFN-γ was reduced significantly in culture supernatants of lymph node cells in CII : chitosan-fed rats (Fig. 4b). In agreement, this group showed a lower percentage of CD4+ IFN-γ+, with no differences in spleen cells (Fig. 4c). Considering that MCP-1 is a necessary factor for the induction of high-dose oral tolerance and that its removal inhibits the immune deviation seen during this phenomenon [36], we assessed the percentage of CD4+MCP-1+ cells in the CII-treated groups. Only in lymph nodes was the percentage of MCP-1 cells significantly higher in rats fed the articular antigen compared with the diluent control (Fig. 4d), although no differences were observed between the CII-fed groups. Both in lymph node and spleen, the chitosan-fed group showed similar percentages than the CII and CII : chitosan groups (data not shown).
Fig. 4.
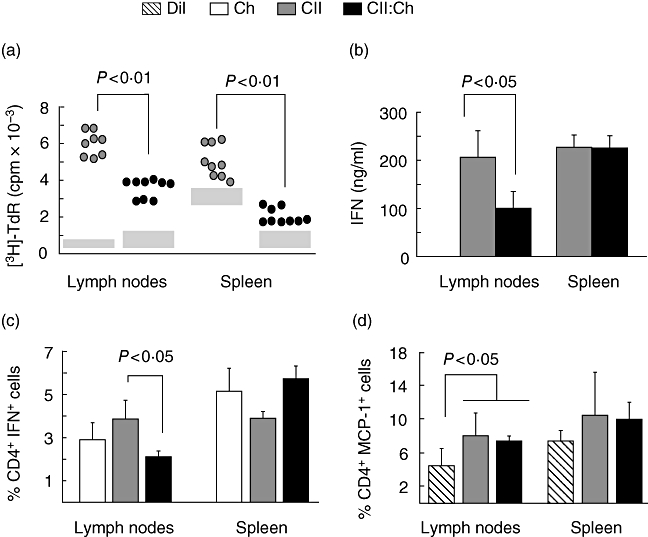
Evaluation of cellular response in draining lymph nodes and spleen of groups receiving either type II collagen (CII) or CII : chitosan. Mononuclear cells of draining lymph nodes and spleen (n = 7–9 rats per group) isolated 4 weeks after primary immunization were cultured for 72 h in the presence of 40 µg/ml CII. (a) Proliferative responses were assessed on the degree of [3H]-thymidine incorporation. Results are presented as counts per minute (cpm) of antigen-stimulated cells. Grey boxes represent the range of basal [3H]-thymidine incorporation; cpm values × 103 for chitosan group in lymph node 0·39 ± 0·102 (basal) and 5·24 ± 0·46 (CII-stimulated); in spleen 1·23 ± 0·24 (basal) and 4·41 ± 0·41 (CII-stimulated); (b) production of interferon (IFN)-γ by enzyme-linked immunosorbent assay in culture supernatants of mononuclear cells stimulated as described in (a); (c) percentage of CD4+ IFN-γ+ cells assessed by flow cytometry after stimulation in chitosan, CII- and CII : Ch-fed groups; (d) percentage of CD4+ monocyte chemotactic protein-1+ cells assessed by flow cytometry upon antigen stimulation in diluent, CII- and CII : Ch-fed groups.
We have demonstrated that oral administration of chitosan triggers the recruitment of immature dendritic cells [22], up-regulates IL-4 and TGF-β mRNA expression [21,22], reduces IL-2 levels and increases specific IL-10 release [21] at the inductive mucosal sites. Considering that most of these factors have been associated with the induction of regulatory subpopulations [21,22,30,37,38], we evaluated the occurrence of T cells with regulatory characteristics. Upon restimulation in vitro with the relevant antigen, both the diluent and chitosan groups showed similar percentages of CD4+IL-10+ cells in lymph nodes or spleen (Fig. 5 and data not shown). The percentage of CD4+IL-10+ T cells in lymph nodes of rats fed either CII or CII : chitosan was similar (P = not significant) and comparable to the value of diluent group. On the other hand, restimulation of splenocytes with CII induced a modest increase in CD4+IL-10+ cells in the CII and CII : chitosan groups compared with the diluent control (P < 0·05).
Fig. 5.
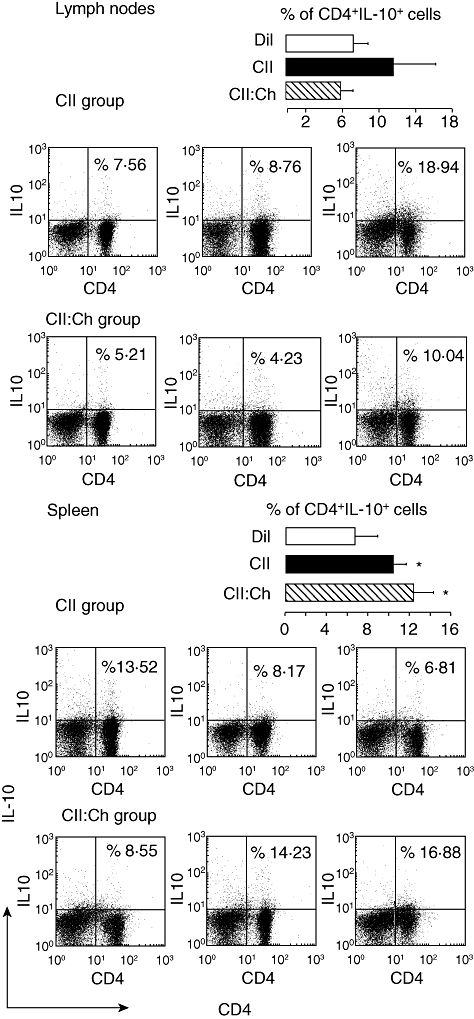
Frequency of CD4+ interleukin (IL)-10+ cells in draining lymph nodes and spleen after feeding type II collagen (CII) : chitosan. Rats (n = 7–9) were treated as described in Fig. 2; 28 days after primary immunization single-cell suspensions were prepared from lymph nodes and spleen, cultured for 48 h in the presence of 40 µg/ml CII and analysed for surface expression of CD4+ and intracellular IL-10. Data are representative dot-plots of three rats per group. Bars are the average of the percentage of CD4+ IL-10+ cells in lymph nodes and spleen in the CII and CII : chitosan groups. For comparative purposes, the percentage of CD4+ IL-10+ cells of diluent group in lymph nodes and spleen was included (*P < 0·05 versus diluent).
Discussion
Oral administration of low doses of antigen induces a form of tolerance that can be transferred by T cells and potentiated by several agents [35]. For instance, oral doses of CII suppress experimental arthritis in various animal models through the generation of regulatory cells that mediate bystander suppression [39]. In the present study, we demonstrate that the oral co-administration of chitosan with CII prevents the clinical signs of CIA, dampens the antibody response to CII and reduces the release and proportion of CD3+ IFN-γ+ T cells in lymph nodes.
Immune responses elicited by fed antigens differ from responses activated at other sites [2]. Typically, antigen feeding decreases delayed type hypersensitivity (DTH) responses and T proliferation after immunization with the same antigen [40]. In agreement, CII- and CII : chitosan-fed rats showed reduced lymph node cellularity upon CII challenge, which is a clear marker of the induction of systemic tolerance. Furthermore, both groups exhibited a lower proportion of ASC in draining lymph nodes. Interestingly, while the sustained contact of CII or CII : chitosan with the mucosal immune system generated specific tolerance, chitosan itself produced some effect on B cell function. We cannot explain this finding fully, although the result is in agreement with our previous reports showing that chitosan conditions, after sustained administration, an anti-inflammatory environment at the inductive mucosal sites [21,22]. Recently, novel and remarkable activities of polysaccharides on mucosal immunity have been described that include the settlement of the T cell repertoire and the release of IL-10 [41,42]. The significance of signals triggered by polysaccharides in immune homeostasis is associated with their ability to establish a proper Th1/Th2 balance in the host [41,42]. Although the molecular mechanism(s) of polysaccharide activity in mucosal immunity is characterized poorly, our previous data showing the release of IL-10 and the expression of IL-4 mRNA early upon chitosan feeding could help understanding of the reduction of serum IgG2a levels indicative of a Th1 response in the CII : chitosan group. In agreement, oral administration of proteoglycan isolated from Phellinus linteus results in the reduction of CIA in mice and is associated with decreased production of anti-CII IgG2a antibodies, reduced secretion of TNF-α and IFN-γ, as well as some enhanced secretion of IL-10 and TGF-β[43]. Moreover, purified polysaccharide from Klebsiella oxytoca administered orally at 125–250 mg/kg/day reduces the incidence and severity of CIA as well as serum levels of anti-CII IgG2a compared with untreated controls [44].
Chitosan's ability to modulate oral immunity is particular. At the same dose evaluated in this work, it reduces the enzymatic degradation of several proteins including CII [21,45], recruits immature dendritic cells at the inductive sites [22], promotes the expression of TGF-β and IL-4 mRNAs [21] and stimulates the release of IL-10 [21,22,46]. Compounds with modulatory properties might influence the tolerance induction in the mucosa [47]. For instance, the delivery of soluble peptides adsorbed to chitosan increases antigen release and maintains a sustained production of IL-10, facilitating tolerance induction [46]. Several inflammatory parameters tested herein were reduced significantly after CII : chitosan administration compared with rats receiving only CII, suggesting the stronger tolerogenic activity of the treatment. The intestine allows the development of tolerogenic and immunogenic responses [2,4,48–51]. Early after chitosan feeding, the number of immature dendritic cells increases and the percentage of IL-10+ cells rises in inductive sites at the same time that an articular antigen is presented to the immune system [22]. Could these events promote tolerance? Whether more immature dendritic cells are accessible, to pick up the relevant antigen in an IL-10-rich environment the probability of a tolerogenic dendritic–T cell interaction increases. The expansion of dendritic cells in vivo enhances the induction of oral tolerance [52]: mice treated with the haemopoietic growth factor Flt3 ligand to enlarge the number of these cells have more profound tolerance to fed antigens, with decreased DTH responses, suppressed antigen-specific proliferative reactivity and reduced specific total IgG, IgG1 and IgG2a serum levels [52]. Further, in Flt3 ligand-treated mice, most freshly isolated dendritic cells show an inactivated, resting phenotype [51,53]. In agreement, the uptake of chitosan is mediated by CD11 b/c+ OX62+ major histocompatibility complex (MHC) II+ cells that express constitutive levels of CD80 and CD86 molecules upon migrating to the mesenteric lymph nodes [22].
The T cell response generated in vivo depends upon the dose of the antigen, the route of delivery, the cytokine environment and the characteristics of antigen-presenting cells. Mechanisms of oral tolerance include anergy and/or active cellular suppression mediated by regulatory T cells. Although different T cell subsets participate in intestinal immunity, type 1 regulatory T (Tr1) cells are pivotal in oral tolerance. Antigen-specific Tr1 cells are induced by multiple feeding of exogenous proteins, suppressing multiple sclerosis and diabetes in animal models [54,55]. Greater proportions of IL-10-producing T cells are detected in Peyer's patches, mesenteric lymph nodes and spleen of tolerized mice in the CIA model [56]. While no single marker exists to track and purify Tr1 cells [38], their distinctive ability to produce IL-10 and TGF-β can be related to the down-regulated immune responses of naive and memory T cells [57]. A reduction in the frequency of IL-10+ CD4+ T cells in inflamed synovium and peripheral blood of patients with RA is contributing to the loss of tolerance [54,58]. In this study, both groups fed the articular antigen showed a similar percentage of CD4+ IL-10+ T cells in spleen that was higher than in control groups. In agreement, there is a greater induction of IL-10-producing CD4+CD25+ subsets among splenic T cells in mice fed CII before CIA induction occurs [37]. In a previous work we demonstrated that already 16 h after feeding, at the time where tolerance is being induced, restimulated splenocytes of the CII : chitosan group already release more IL-10 than CII-fed rats [21]. Perhaps, 28 days after CIA induction, the difference between groups associated with the polysaccharide administration is no longer evident. Another possibility is that most IL-10-producing cells have reached the affected joints where CII is accumulated from cartilage degradation and contribute to alleviate the inflammation, as suggested previously [37]. Frequently, oral tolerance has been addressed in transgenic mice over-expressing specific T cell clones [59]. To detect a significant increment in regulatory subsets in normal rats may be difficult. However, reduced proliferation and IFN-γ production suggest that upon CII : chitosan administration a stronger tolerogenic response was elicited, in agreement with the biological activity of chitosan at the mucosal inductive sites [21,22,46].
Our study illustrates the ability of a polysaccharide to improve tolerance to an articular antigen and to regulate the outcome of the inflammatory response in arthritic outcome. Understanding the properties of this immunomodulatory polysaccharide will contribute to the development of new strategies for oral tolerance to CII.
Acknowledgments
This study was supported by grants from CONICET, Agencia Nacional de Promoción Científica y Tecnológica (FONCYT) and Secretaría de Ciencia y Tecnología (SECyT) UNC. M. C. is a fellow from CONICET. C. P., I. D. B. and S. G. C. belong to the research staff of CONICET. We would like to thank Paula Icely for excellent technical assistance.
References
- 1.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–81. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 2.Weiner HL. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest. 2000;106:935–7. doi: 10.1172/JCI11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B, Goubier A, Joubert G, et al. Oral tolerance and regulation of mucosal immunity. Cell Mol Life Sci. 2005;62:1322–32. doi: 10.1007/s00018-005-5036-0. [DOI] [PubMed] [Google Scholar]
- 5.Husby S. Sensitization and tolerance. Curr Opin Allergy Clin Immunol. 2001;1:237–41. doi: 10.1097/01.all.0000011020.20431.84. [DOI] [PubMed] [Google Scholar]
- 6.Singla AK, Chawla M. Chitosan: some pharmaceutical and biological aspects – an update. J Pharm Pharmacol. 2001;53:1047–67. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- 7.Pittler MH, Ernst E. Dietary supplements for body-weight reduction: a systematic review. Am J Clin Nutr. 2004;79:529–36. doi: 10.1093/ajcn/79.4.529. [DOI] [PubMed] [Google Scholar]
- 8.Wedmore I, McManus JG, Pusateri AE, et al. A special report on the chitosan based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655–8. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 9.Read RC, Naylor SC, Potter CW. Effective nasal influenza vaccine delivery using chitosan. Vaccine. 2005;23:4367–74. doi: 10.1016/j.vaccine.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Illum L, Jabbal-Gill I, Hinchcliffe M, et al. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51:81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 11.Feldmann M. Pathogenesis of arthritis: recent research progress. Nat Immunol. 2001;2:771–3. doi: 10.1038/ni0901-771. [DOI] [PubMed] [Google Scholar]
- 12.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 13.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–68. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wooley PH, Luthra HS, Stuart JM, et al. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choy EH. Oral toleragens in rheumatoid arthritis. Curr Opin Invest Drugs. 2000;1:58–62. [PubMed] [Google Scholar]
- 16.Kalden JR, Sieper J. Oral collagen in the treatment of rheumatoid arthritis. Arthritis Rheum. 1998;41:191–4. doi: 10.1002/1529-0131(199802)41:2<191::AID-ART2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17.Barnett ML, Kremer JM, St Clair EW, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998;41:290–7. doi: 10.1002/1529-0131(199802)41:2<290::AID-ART13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald TT, Monteleone G. IL-12 and Th1 immune responses in human Peyer's patches. Trends Immunol. 2001;22:244–7. doi: 10.1016/s1471-4906(01)01892-0. [DOI] [PubMed] [Google Scholar]
- 19.Postlethwaite AE. Can we induce tolerance in rheumatoid arthritis? Curr Rheumatol Rep. 2001;3:64–9. doi: 10.1007/s11926-001-0052-z. [DOI] [PubMed] [Google Scholar]
- 20.Leishman AJ, Garside P, AMcI M. Induction of oral tolerance in the primed immune system: influence of antigen persistence and adjuvant form. Cell Immunol. 2000;202:71–8. doi: 10.1006/cimm.2000.1665. [DOI] [PubMed] [Google Scholar]
- 21.Porporatto C, Bianco ID, Cabanillas AM, et al. Early events associated to the oral co-administration of type II collagen and chitosan: induction of anti-inflammatory cytokines. Int Immunol. 2004;16:433–41. doi: 10.1093/intimm/dxh051. [DOI] [PubMed] [Google Scholar]
- 22.Porporatto C, Bianco ID, Correa SG. Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. J Leukoc Biol. 2005;78:62–9. doi: 10.1189/jlb.0904541. [DOI] [PubMed] [Google Scholar]
- 23.Ausar SF, Beltramo DM, Castagna LF, et al. Treatment of rheumatoid arthritis by oral administration of bovine tracheal type II collagen. Rheumatol Int. 2001;20:138–44. doi: 10.1007/s002960100099. [DOI] [PubMed] [Google Scholar]
- 24.Bianco ID, Balsinde J, Beltramo DM, et al. Chitosan-induced phospholipase A2 activation and arachidonic acid mobilization in P388D1 macrophages. FEBS Lett. 2000;466:292–4. doi: 10.1016/s0014-5793(00)01089-9. [DOI] [PubMed] [Google Scholar]
- 25.Campo GM, Avenoso A, Campo S. Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther. 2003;5:R122–31. doi: 10.1186/ar748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alpan O, Rudomen G, Matzinger P. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J Immunol. 2001;166:4843–52. doi: 10.4049/jimmunol.166.8.4843. [DOI] [PubMed] [Google Scholar]
- 27.Kim WU, Lee WK, Ryoo JW, et al. Suppression of collagen-induced arthritis by single administration of poly(lactic-co-glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance. Arthritis Rheum. 2002;46:1109–20. doi: 10.1002/art.10198. [DOI] [PubMed] [Google Scholar]
- 28.Baig SM. Anti-purified protein derivative cell-enzyme-linked immunosorbent assay, a sensitive method for early diagnosis of tuberculous meningitis. J Clin Microbiol. 1995;33:3040–1. doi: 10.1128/jcm.33.11.3040-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunow R, D'Apuzzo M, Wyss-Coray T, et al. A cell surface ELISA for the screening of monoclonal antibodies to antigens on viable cells in suspension. J Immunol Methods. 1994;171:93–102. doi: 10.1016/0022-1759(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 30.Brand DD, Myers LK, Whittington KB, et al. Detection of early changes in autoimmune T cell phenotype and function following intravenous administration of type II collagen in a TCR-transgenic model. J Immunol. 2002;168:490–8. doi: 10.4049/jimmunol.168.1.490. [DOI] [PubMed] [Google Scholar]
- 31.Porporatto C, Bianco ID, Riera CM, et al. Chitosan induces different L-arginine metabolic pathways in resting and inflammatory macrophages. Biochem Biophys Res Commun. 2003;304:266–72. doi: 10.1016/s0006-291x(03)00579-5. [DOI] [PubMed] [Google Scholar]
- 32.Borzi RM, Mazzetti I, Macor S, et al. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999;455:238–42. doi: 10.1016/s0014-5793(99)00886-8. [DOI] [PubMed] [Google Scholar]
- 33.Seki N, Sudo Y, Yoshioka T, et al. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J Immunol. 1988;140:1477–84. [PubMed] [Google Scholar]
- 34.Smith KM, McAskill F, Garside P. Orally tolerized T cells are only able to enter B cell follicles following challenge with antigen in adjuvant, but they remain unable to provide B cell help. J Immunol. 2002;168:4318–25. doi: 10.4049/jimmunol.168.9.4318. [DOI] [PubMed] [Google Scholar]
- 35.Thorbecke GJ, Schwarcz R, Leu J, et al. Modulation by cytokines of induction of oral tolerance to type II collagen. Arthritis Rheum. 1999;42:110–8. doi: 10.1002/1529-0131(199901)42:1<110::AID-ANR14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 36.DePaolo RW, Rollins BJ, Kuziel W, et al. CC Chemokine ligand 2 and its receptor regulate mucosal production of IL-12 and TGF-β in high dose oral tolerance. J Immunol. 2003;171:3560–7. doi: 10.4049/jimmunol.171.7.3560. [DOI] [PubMed] [Google Scholar]
- 37.Min SY, Hwang SY, Park KS, et al. Induction of IL-10-producing CD4+CD25+ T cells in animal model of collagen-induced arthritis by oral administration of type II collagen. Arthritis Res Ther. 2004;6:R213–9. doi: 10.1186/ar1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battaglia M, Gianfrani C, Gregori S, et al. IL-10-producing T regulatory type 1 cells and oral tolerance. Ann NY Acad Sci. 2004;1029:142–53. doi: 10.1196/annals.1309.031. [DOI] [PubMed] [Google Scholar]
- 39.Yoshino S, Quattrocchi E, Weiner HL. Suppression of antigen-induced arthritis in Lewis rats by oral administration of type II collagen. Arthritis Rheum. 1995;38:1092–6. doi: 10.1002/art.1780380811. [DOI] [PubMed] [Google Scholar]
- 40.Silverman GA, Peri BA, Fitch FW, et al. Enterically induced regulation of systemic immune responses. I. Lymphoproliferative responses in mice suppressed by ingested antigen. J Immunol. 1983;131:2651–5. [PubMed] [Google Scholar]
- 41.Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Perez B, Chung DR, Sharpe AH, et al. Modulation of surgical fibrosis by microbial zwitterionic polysaccharides. Proc Natl Acad Sci USA. 2005;102:16753–8. doi: 10.1073/pnas.0505688102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim GY, Kim SH, Hwang SY, et al. Oral administration of proteoglycan isolated from Phellinus linteus in the prevention and treatment of collagen-induced arthritis in mice. Biol Pharm Bull. 2003;26:823–31. doi: 10.1248/bpb.26.823. [DOI] [PubMed] [Google Scholar]
- 44.Sugihara R, Yoshimura M, Mori M, Kanayama N, Hikida M, Ohmori H. Prevention of collagen-induced arthritis in DBA/1 mice by oral administration of AZ-9, a bacterial polysaccharide from Klebsiella oxytoca. Immunopharmacology. 2000;49:325–33. doi: 10.1016/s0162-3109(00)00247-2. [DOI] [PubMed] [Google Scholar]
- 45.Taravel MN, Domard A. Collagen and its interactions with chitosan. III. Some biological and mechanical properties. Biomaterials. 1996;17:451–5. doi: 10.1016/0142-9612(96)89663-3. [DOI] [PubMed] [Google Scholar]
- 46.Hall G, Lund L, Lamb JR, et al. Kinetics and mode of peptide delivery via the respiratory mucosa determine the outcome of activation versus TH2 immunity in allergic inflammation of the airways. J Allergy Clin Immunol. 2002;110:883–90. doi: 10.1067/mai.2002.129800. [DOI] [PubMed] [Google Scholar]
- 47.Illum L, Farraj NF, Davis SS. Chitosan as a novel nasal delivery system for peptide drugs. Pharm Res. 1994;11:1186–9. doi: 10.1023/a:1018901302450. [DOI] [PubMed] [Google Scholar]
- 48.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayer L. Mucosal immunity. Pediatrics. 2003;111:1595–600. [PubMed] [Google Scholar]
- 50.Moser M. Dendritic cells in immunity and tolerance – do they display opposite functions? Immunity. 2003;19:5–8. doi: 10.1016/s1074-7613(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 51.Weiner HL. The mucosal milieu creates tolerogenic dendritic cells and T(R)1 and T(H)3 regulatory cells. Nat Immunol. 2001;2:671–2. doi: 10.1038/90604. [DOI] [PubMed] [Google Scholar]
- 52.Viney JL, Mowat AM, O'Malley JM, et al. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–25. [PubMed] [Google Scholar]
- 53.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J Immunol. 2001;166:4884–90. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Kuchroo VK, Inobe J, et al. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 55.Paimela L, Johansson-Stephansson EA, Koskimies S, et al. Depressed cutaneous cell-mediated immunity in early rheumatoid arthritis. Clin Exp Rheumatol. 1990;8:433–7. [PubMed] [Google Scholar]
- 56.Min SY, Park KS, Cho ML, et al. Antigen-induced, tolerogenic CD11c+,CD11b+ dendritic cells are abundant in Peyer's patches during the induction of oral tolerance to type II collagen and suppress experimental collagen-induced arthritis. Arthritis Rheum. 2006;54:887–98. doi: 10.1002/art.21647. [DOI] [PubMed] [Google Scholar]
- 57.Groux H, O'Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 58.Yudoh K, Matsuno H, Nakazawa F, et al. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 2000;43:617–27. doi: 10.1002/1529-0131(200003)43:3<617::AID-ANR19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 59.Gutgemann I, Fahrer AM, Altman JD, et al. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity. 1998;8:667–73. doi: 10.1016/s1074-7613(00)80571-3. [DOI] [PubMed] [Google Scholar]


