Abstract
Allyl acetate (AAC), allyl alcohol (AAL), and acrolein (ACR) are used in the manufacture of detergents, plastics, pharmaceuticals, and chemicals and as agricultural agents. A metabolic relationship exists between these chemicals in which allyl acetate is metabolized to allyl alcohol and subsequently to the highly reactive,α,β-unsaturated aldehyde, acrolein. Due to the weaker reactivity of the protoxicants, allyl acetate and allyl alcohol, relative to acrolien we hypothesized the protoxicants would attain greater systemic exposure and therefore deliver higher doses of acrolein to the internal organs. By extension, the higher systemic exposure to acrolein we hypothesized should lead to more internal organ toxicity in the allyl acetate and allyl alcohol treated animals relative to those treated with acrolein. To address our hypothesis we compared the range of toxicities produced by all three chemicals in male and female Fischer 344/N rats and B6C3F1 mice exposed 5 days a week for 3 months by gavage in 0.5% methylcellulose. Rats (10/group) were dosed with 0 to 100 mg/kg allyl acetate, 0 to 25 mg/kg allyl alcohol, or 0 to 10 mg/kg acrolein. Mice (10/group) were dosed with 0 to 125 mg/kg allyl acetate, 0 to 50 mg/kg allyl alcohol, or 0 to 20 mg/kg acrolein. The highest dose of allyl acetate and acrolein decreased survival in both mice and rats. The primary target organ for the toxicity of all three chemicals in both species and sexes was the forestomach; squamous epithelial hyperplasia was observed following exposure to each chemical. In both species the highest allyl acetate dose group exhibited forestomach epithelium necrosis and hemorrhage and the highest dose of acrolein led to glandular stomach hemorrhage. Liver histopathology was the most apparent with allyl acetate, was also observed with allyl alcohol, but was not observed with acrolein. All chemicals had effects on the hematopoietic system with allyl acetate having the most pronounced effect. When dosed at quantities limited by toxicity, allyl acetate and allyl alcohol produce higher levels of urinary mercapturic acids than the minimally toxic dose of acrolein. This observation is likely due to biotransformation of allyl acetate and ally alcohol to acrolein that occurs after absorption and suggests that these chemicals are protoxicants that increase systemic exposure of acrolein. Increased systemic exposure to acrolein is likely responsible for the differences in hepatic toxicological profile observed with these chemicals.
Keywords: allyl acetate, allyl alcohol, acrolein, prechronic, gavage
1. Introduction
Allyl acetate is an important intermediate in the synthesis of many industrial chemicals and has several industrial applications and can be found in consumer products hair conditioners and detergents. Allyl alcohol is an industrial chemical used in synthesis of glycerol and acrolein (Liao et al 1969) and in pesticide formulations (Scorecard 2005). Human dietary exposure to allyl alcohol, as well as several allyl esters, has occurred through their use as food additives (1997). Acrolein is a naturally occurring chemical found in food and is also used as an industrial chemical and herbicide(Ghilarducci & Tjeerdema). Although the toxicology of acrolein has been studied extensively(Kehrer & Biswal 2000), less is known about allyl acetate and allyl alcohol.
Metabolism studies of allyl acetate and allyl alcohol strongly suggest these chemicals are protoxicants that are metabolized to acrolein, a highly reactive,α,β-unsaturated aldehyde. Upon oral administration allyl acetate is rapidly reduced by carboxyl esterases in the stomach, liver and blood to yield allyl alcohol and acetic acid. Allyl alcohol is then further oxidized to acrolein by alcohol dehydrogenase. Acrolein can subsequently be detoxified by aldehyde dehydrogenase to acrylic acid or be conjugated to glutathione. The glutathione adducts can potentially be converted by cytochromes P450 to the hard electrophile and known mutagen/carcinogen, glycidaldehyde (Barros et al 1994; Feron et al 1991). Alternatively, degradation products of glutathione adducts, S-(3-hydroxypropyl) mercapturic acid and S-(2-carboxyethyl) mercapturic acid, are found in the urine of rats administered acrolein, allyl alcohol, allyl chloride, allyl amine, or allyl bromide (Kaye 1973; Sanduja et al 1989). In support of this proposed mechanism of toxicity, pharmacological inhibition of carboxylesterase or alcohol dehydrogenase was shown to attenuate allyl ester mediated hepatotoxicity (Silver & Murphy 1978). Similar studies with allyl alcohol demonstrated that inhibition of alcohol dehydrogenase leads to a reduction in hepatotoxicity (Reid, 1972). Furthermore, mice hypomorphic for alcohol dehydrogenase are resistant to allyl alcohol induced liver injury (Belinsky et al 1985). Inhibition of aldehyde dehydrogenase enhances the toxicity of allyl alcohol suggesting that not only does the production acrolein affect toxicity, but its metabolic breakdown also plays a role in liver injury (Rikans 1987). Collectively, the data from previous studies strongly support the role of acrolein as the primary reactive species and toxicant following allyl acetate or allyl alcohol exposure.
Conflicting results have been obtained in studies assessing the carcinogenic potential of acrolein. Increased papillomas of the urinary bladder were observed in an initiation/promotion study in male F344 rats that involved 6 weeks of acrolein intraperiteneal injection followed by 28-week uracil (Cohen et al 1992). In another study increased incidences of adrenal cortical adenomas in F344 rats administered acrolein in drinking water (Lijinsky & Reuber 1987). In a more recent study, male and female Sprague-Dawley rats were given doses of 0.05, 0.50, or 2.4 mg/kg acrolein in water by gavage daily for 24 months (Parent et al 1992). Although survival of the 2.4 mg/kg animals was reduced compared to vehicle controls, no chemical related increases in the incidences of neoplastic or nonneoplastic lesions were observed in this study. The carcinogenic potential of acrolein has also been examined in CD-1 mice (Parent et al 1991). Groups of 70 males and female mice received doses of 0, 0.5, or 2.0 mg/kg acrolein in distilled water daily by gavage for 2 years, and additional groups of 75 males or females received 4.5 mg/kg. Survival and mean body weights of the 4.5 mg/kg males were reduced, but survival and mean body weights of all groups of females were similar to those of the vehicle controls. Complete histopathology conducted on the 4.5 mg/kg animals and vehicle controls revealed no carcinogenic response associated with acrolein administration. Neither allyl acetate nor allyl alcohol has been evaluated for carcinogenic potential in a traditional bioassay.
Allyl acetate is mutagenic in Salmonella typhimurium strains TA 1535 and TA100 in the absence of activation, but not in the presence of activation (Dean et al 1985; Irwin 2006). Allyl alcohol was mutagenic in V79 cells, but there are no reports of bacterial mutagenicity (Smith et al 1990), however it was not mutagenic to four strains of Salmonella typhimurium with or without activation (Irwin 2006). Acrolein is a mutagen in bacteria and V79 cells and forms DNA adducts in human fibroblasts (Foiles et al 1989; Irwin 2006; Smith et al 1990; Wilson et al 1991). In the absence of S9 activation, acrolein has been demonstrated to be a clastogen in cultured CHO cells (Irwin 2006). Recently published cell culture studies have demonstrated preferential formation of acrolein-DNA adducts at lung cancer mutational hotspots in the p53 tumor suppressor gene in normal human bronchial epithelial cells and lung fibroblasts (Feng et al 2006). Because of the high production volume and widespread use of these compounds, the potential for occupational and consumer exposure, and the lack of adequate toxicity and carcinogenicity data, allyl alcohol and allyl acetate were selected for prechronic studies. Because allyl acetate and allyl alcohol are metabolized to acrolein, which is significantly more acutely toxic than either parent compound, similar toxicities between the three compounds would suggest that the effects are a byproduct of metabolism to acrolein. If this is the case, carcinogenicity studies of allyl acetate and allyl alcohol may not be necessary, because acrolein was not carcinogenic following oral exposure (Parent et al 1991; Parent et al 1992); however, substantially different toxicities would point to the need for additional testing. To address this question, a comparative toxicology study of allyl acetate, allyl alcohol, and acrolein was conducted in the same animal strains and at the same laboratory and the data are presented here. For a detailed breakdown of the study the reader is directed to the NTP Toxicity Report, TOX 48 (Irwin 2006).
2. Materials and Methods
2.1 Chemicals
Technical grade allyl acetate (CAS No. 591-87-7; lot 0425EF), allyl alcohol (CAS No. 107-18-6; lot 00501TF) and acrolein (CAS No. 591-87-1; lot 11163AG) were obtained in single lots from Aldrich Chemical Company (Milwaukee, WI). Based on the analyses performed by the manufacturer, allyl acetate, allyl alcohol and acrolein were a purity of approximately 93.3%, 98.8% and 98.8%, respectively. All preadministration dose analyses indicated concentrations were within 10% of the target concentration, except in one case in which a dose of allyl acetate was administered at 11% below the target dose.
2.2 Animals
All animals were obtained from Taconic Laboratory Animals and Services (Germantown, NY). F344/N rats and B6C3F1 mice were shipped at approximately 5 weeks of age, were quarantined for 10–14 days, and were approximately 6 to 7-weeks-old at the start of the study. Animals were randomly assigned to dose groups by sex and body weight. There were no statistically significant differences between group mean body weights prior to initiation of these studies. Rats and female mice were housed 5/cage. Male mice were individually housed. NIH-2000 pelleted diet (Zeigler Bothers, Inc, Gardeners, PA) and tap water were available ad libitum. The animal room temperatures were approximately 70 ± 3°F and humidity was 50 ± 15%. Fluorescent lights were on for 12 h/day, and there were a minimum of 10 room air changes per hour.
2.3 Experimental Design
In the core toxicology study, groups of 10 animals of each species and sex were given allyl acetate, allyl alcohol or acrolein by gavage, once a day, 5 days/week, for 14 weeks. Doses for each chemical were selected following review of previous studies and are shown in Table 1. For a more detailed discussion of the dose selection process the reader is referred to the NTP toxicity report, NTP TOX 48 (Irwin 2006). All dosing volumes were 5 mL/kg for rats and 10 mL/kg for mice in 0.5% methylcellulose. Animals were checked twice each day for signs for moribundity or morbidity, and were examined once weekly for clinical signs of toxicity. The individual animal’s body weights were recorded once weekly, and the most recent weight was used to determine the dosing volume. At study termination, survivors were weighed, anesthetized with carbon dioxide, and were bled for clinical pathology studies prior to euthanasia and necropsy. Animals received at least two consecutive dose administrations, with the last dose administered approximately 30 min prior to bleeding. Blood was collected into microcollection tubes (Sarstedt, Inc., Newton, NC) containing potassium-EDTA for hematology studies and into serum separator tubes to obtain samples for clinical chemistry. Clinical chemistry parameters included urea nitrogen, creatinine, total protein, albumin, alanine aminotransferase, alkaline phosphatase, creatine kinase, sorbitol dehydrogenase, and total bile salts. Hematological analyses included hematocrit; hemoglobin concentration erythrocyte, reticulocyte and platelet cell counts; mean cell volume; mean cell hemoglobin concentration; and total leukocyte counts and differentials.
Table 1.
Survival and Mean Body Weight in Rats
| Survivala | Final body weightb | ||||||
|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (AAC, AAL, ACR) |
Allyl Acetate |
Allyl Alcohol |
Acrolein | Allyl acetate |
Allyl Alcohol |
Acrolein | |
| Male | |||||||
| 0 | 10/10 | 10/10 | 10/10 | 340 ± 5 | 338 ± 6 | 337 ± 6 | |
| 6, 1.5, 0.75 | 10/10 | 10/10 | 10/10 | 326 ± 6 | 331 ± 5 | 336 ± 4 | |
| 12, 3, 1.25 | 10/10 | 10/10 | 10/10 | 317 ± 5* | 322 ± 5 | 336 ± 6 | |
| 25, 6, 2.5 | 10/10 | 10/10 | 8/10 | 331 ± 4 | 335 ± 6 | 348 ± 6 | |
| 50, 12, 5 | 10/10 | 10/10 | 8/10 | 319 ± 7* | 337 ± 6 | 331 ± 8 | |
| 100, 25, 10 | 0/10 | 10/10 | 2/10 | ----- | 334 ± 7 | 263 ± 10** | |
| Female | |||||||
| 0 | 10/10 | 10/10 | 10/10 | 193 ± 3 | 201 ± 4 | 195 ± 4 | |
| 6, 1.5, 0.75 | 10/10 | 10/10 | 10/10 | 186 ± 2 | 196 ± 3 | 196 ± 2 | |
| 12, 3, 1.25 | 10/10 | 9/10 | 9/10 | 191 ± 4 | 195 ± 5 | 198 ± 3 | |
| 25, 6, 2.5 | 10/10 | 10/10 | 8/10 | 190 ± 3 | 204 ± 4 | 190 ± 3 | |
| 50, 12, 5 | 10/10 | 10/10 | 9/10 | 187 ± 4 | 203 ± 4 | 188 ± 3 | |
| 100, 25, 10 | 0/10 | 10/10 | 2/10 | ----- | 204 ± 3 | 176 ± 7* | |
Number of animals surviving at 14 weeks/number initially in group
Weights in grams are given as mean ± standard error
Significantly different (P ≤ 0.05) from the vehicle control group by Dunnett’s test
P ≤ 0.01
In the clinical pathology study, groups of 10 male and 10 female rats were administered allyl acetate, allyl alcohol or acrolein in identical fashion to the core study rats. Blood was collected from the retroorbital sinus of these rats on days 4 and 23 and subjected to hematological and clinical chemistry analysis as described above.
All animals, including early death animals, received a necropsy. During each necropsy, all tissues were examined in situ for gross lesions. At study termination, selected organ weights (spleen, liver, thymus, heart, lung, right testis and kidney) were determined. All collected tissues were preserved in 10% neutral buffered formalin. Tissue examined microscopically can be found in (Irwin 2006).
2.4 S-(3-hydroxypropyl) mercapturic acid
Urine was collected from all core study rats and mice after the first dose and again after 45 doses. Approximately 15 to 30 minutes after dosing, animals were placed in metabolism cages for a 24-hour period where feed and water were available ad libitum. Urine collection tubes were kept on ice throughout the 24 hrs collection period and were frozen at −20° C after conclusion of the collection period. Samples were analyzed for S-(3-hydroxypropyl) mercapturic acid levels using a liquid chromatography-mass spectroscopy method (Irwin 2006).
2.5 Statistical Analyses
Body weight and organ weight data were analyzed using the parametric comparison (Williams 1971; 1972) or Dunnett procedures (Dunnett 1955). Clinical pathology data and S-(3-hydroxypropyl) mercapturic acid concentrations were analyzed using the nonparametric comparative procedures of Shirley (Shirley 1977) or Dunn (Dunn 1964). The Fisher exact test, a procedure based on the overall proportion of affected animals, was used to analyze histopathology findings (Gart et al 1979). In all tables (unless indicated otherwise), values are expressed as group mean and SE. Significant differences from control are indicated by asterisks (*p ≤0.05, **p ≤0.01).
3. Results
3.1 F344/N Rats
3.1.1 In life observations
3.1.1.1 Allyl acetate
Survival of male and female rats that received 100 mg/kg was decreased by the last week of the study (Table 1). Final mean body weights and mean body weight gains of male rats administered 12 or 50 mg/kg and mean body weight gains of 6 mg/kg males were significantly less than those of the vehicle controls. The final mean body weights and mean body weight gains of the female rats were similar to those of the vehicle control group (Table 1). Clinical findings included pallor and eye or nasal discharge in males and females and ruffled fur lethargy, diarrhea and thinness in males in the 100 mg/kg groups.
3.1.1.2 Allyl alcohol
All rats survived to the end of the study except one female in the 6 mg/kg group that was removed on day 57 in a moribund condition (Table 1). The final mean body weights of male and female rats were similar to those of the vehicle controls (Table 1). No clinical findings were observed in the dosed male or female rats.
3.1.1.3 Acrolein
Eight males and eight females in the 10 mg/kg groups died by week 9 of the study. Two males in the 2.5 mg/kg group and 5 mg/kg groups and one or two females in the 1.25, 2.5 and 5.0 mg/kg groups also died early. Survival data is shown in Table 1. Final mean body weights of the male and female rats in the 10 mg/kg groups were significantly less than those of the vehicle controls (Table 1). Clinical findings included abnormal breathing, eye and nasal discharge, ruffled fur, and thinness in the males and females in the 10 mg/kg groups; two females in this group were also lethargic.
3.1.2 Pathology
3.1.2.1 Allyl acetate
The absolute and relative liver weights of the 50 mg/kg females were significantly greater than those of the vehicle controls.
Males administered 12 mg/kg or greater and females in the 25 and 50 mg/kg groups had significantly increased incidences of squamous epithelial hyperplasia in the forestomach compared to those of the vehicle controls (Table 2). The incidences of epithelial necrosis, hemorrhage, and inflammation of the forestomach in the 100 mg/kg males and females were significantly greater than those in the vehicle controls. Incidences of hemorrhage, inflammation, and epithelial necrosis were also increased in the large and small intestines of male rats in the 100 mg/kg group.
Table 2.
Forestomach Squamous Epithelial Hyperplasia in Rats
| Dose (mg/kg) (AAC, AAL, ACR) |
Allyl Acetate |
Allyl Alcohol |
Acrolein | |
|---|---|---|---|---|
| Male | ||||
| 0 | 0/10 | 0/10 | 0/10 | |
| 6, 1.5, 0.75 | 2/10 (1.0) | 0/10 | 0/10 | |
| 12, 3, 1.25 | 6/10**(1.3) | 0/10 | 0/10 | |
| 25, 6, 2.5 | 5/10*(1.8) | 5/10*(1.0) | 3/10(1.0) | |
| 50, 12, 5 | 10/10**(2.0) | 7/10**(1.0) | 8/10**(1.0) | |
| 100, 25, 10 | 4/10*(1.8) | 6/10**(1.0) | 9/10**(1.6) | |
| Female | ||||
| 0 | 0/10 | 0/10 | 0/10 | |
| 6, 1.5, 0.75 | 1/10(1.0) | 0/10 | 0/10 | |
| 12, 3, 1.25 | 3/10(1.3) | 1/10(1.0) | 3/10(1.0) | |
| 25, 6, 2.5 | 9/10**(1.2) | 4/10*(1.0) | 5/10*(1.0) | |
| 50, 12, 5 | 7/10**(1.3) | 9/10**(1.0) | 8/10**(1.0) | |
| 100, 25, 10 | 1/10(2.0) | 8/10**(1.0) | 9/10**(1.6) | |
Significantly different (P ≤ 0.05) from the vehicle control group by Fischer exact test
P ≤ 0.01
The incidences of periportal hepatocyte hypertrophy in the liver in males and females in the 25, 50 and 100 mg/kg groups were significantly greater than those in the vehicle controls (Table 3). Males and females in the 50 and 100 mg/kg groups generally had increased incidences of bile duct hyperplasia, hemorrhage, hepatocyte necrosis, periportal hepatocyte hydropic degeneration, mineralization, mitotic alteration, hemosiderin pigmentation, portal fibrosis and granulomatous inflammation when compared to vehicle controls. Females in the 25 mg/kg group also had significantly increased incidence of hemosidern pigmentation.
Table 3.
Periportal Hepatocyte Hypertrophy in Rats
| Dose (mg/kg) (AAC, AAL, ACR) |
Allyl Acetate |
Allyl Alcohol |
Acrolein | |
|---|---|---|---|---|
| Male | ||||
| 0 | 0/10 | 0/10 | 0/10 | |
| 6, 1.5, 0.75 | 0/10 | 0/10 | 0/10 | |
| 12, 3, 1.25 | 0/10 | 0/10 | 0/10 | |
| 25, 6, 2.5 | 5/10*(1.0) | 0/10 | 0/10 | |
| 50, 12, 5 | 8/10**(1.1) | 0/10 | 0/10 | |
| 100, 25, 10 | 9/10*(2.6) | 1/10(1.0) | 0/10 | |
| Female | ||||
| 0 | 0/10 | 0/10 | 0/10 | |
| 6, 1.5, 0.75 | 0/10 | 0/10 | 0/10 | |
| 12, 3, 1.25 | 0/10 | 0/10 | 0/10 | |
| 25, 6, 2.5 | 7/10**(1.1) | 0/10 | 0/10 | |
| 50, 12, 5 | 10/10**(2.9) | 0/10 | 0/10 | |
| 100, 25, 10 | 6/10(3.0) | 8/10**(1.1) | 0/10 | |
Significantly different (P ≤ 0.05) from the vehicle control group by Fischer exact test
P ≤ 0.01
Incidences of hyperplasia in the bone marrow, hemorrhage in the mediastinal lymph node, lymphoid depletion, in the mandibular lymph node, hemorrhage and lymphoid depletion in the mesenteric lymph node, lymphoid follicular cell depletion and hematopoietic cell proliferation of the red pulp in the spleen, and hemorrhage and thymocyte necrosis in the thymus in the 100 mg/kg males were significantly increased relative to the vehicle controls. Incidences of hyperplasia in bone marrow, lymphoid depletion in the mandibular lymph node, lymphoid follicular cell depletion and hematopoietic cell proliferation of the red pulp in the spleen, and hemorrhage and thymocyte necrosis in the thymus in 100 mg/kg females were significantly increased relative to those in the vehicle controls
3.1.2.2 Allyl alcohol
The absolute liver weights in the 25 mg/kg males were significantly greater than those of the vehicle controls. The relative liver weights in 6, 12 and 25 mg/kg males were significantly greater than those of the vehicle controls. No treatment related gross lesions were observed in the male or female rats administered allyl alcohol. The incidences of squamous cell hyperplasia in the forestomach of the males and females in the 6, 12, and 25 mg/kg groups were significantly greater than those in the vehicle controls (Table 2). The incidences of bile duct hyperplasia and periportal hepatocyte hypertrophy in the liver of the 25 mg/kg females were significantly greater than those in the vehicle controls. One male in the 25 mg/kg group also had bile duct hyperplasia and periportal hepatocyte hypertrophy (Table 4). One female in the 25 mg/kg group had hepatocyte necrosis.
Table 4.
Survival and Mean Body Weight in Mice
| Survivala | Final body weightb | ||||||
|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (AAC, AAL, ACR) |
Allyl Acetate |
Allyl Alcohol |
Acrolein | Allyl Acetate |
Allyl Alcohol |
Acrolein | |
| Male | |||||||
| 0 | 9/10 | 10/10 | 10/10 | 36.3 ± 1.1 | 40.9 ± 1.1 | 35.6 ± 1.2 | |
| 8, 3, 1.25 | 7/10 | 10/10 | 9/10 | 38.1 ± 0.8 | 39.5 ± 1.2 | 36.1 ± 1.0 | |
| 16, 6, 2.5 | 10/10 | 10/10 | 10/10 | 36.6 ± 0.8 | 40.3 ± 0.9 | 37.7 ± 1.0 | |
| 32, 12, 5 | 9/10 | 10/10 | 9/10 | 36.8 ± 0.8 | 39.4 ± 1.0 | 36.5 ± 0.5 | |
| 62.5, 25, 10 | 2/10 | 10/10 | 9/10 | 36.2 ± 0.9 | 39.1 ± 1.0 | 34.3 ± 1.0 | |
| 125, 50, 20 | 0/10 | 10/10 | 0/10 | ----- | 37.9 ± 0.5 | ----- | |
| Female | |||||||
| 0 | 9/10 | 10/10 | 9/10 | 30.7 ± 0.6 | 31.7 ± 1.2 | 29.8 ± 0.8 | |
| 8, 3, 1.25 | 8/10 | 10/10 | 10/10 | 31.9 ± 0.4 | 33.0 ± 1.3 | 33.6 ± 1.6 | |
| 16, 6, 2.5 | 6/10 | 10/10 | 10/10 | 32.7 ± 1.7 | 34.8 ± 1.5 | 31.5 ± 1.3 | |
| 32, 12, 5 | 7/10 | 10/10 | 9/10 | 31.3 ± 1.1 | 33.9 ± 1.0 | 27.1 ± 0.6 | |
| 62.5, 25, 10 | 6/10 | 10/10 | 8/10 | 29.7 ± 0.9 | 32.5 ± 1.3 | 28.5 ± 0.6 | |
| 125, 50, 20 | 0/10 | 9/10 | 0/10 | ----- | 31.4 ± 1.1 | ----- | |
Number of animals surviving at 14 weeks/number initially in group
Weights in grams are given as mean ± standard error
3.1.2.3 Acrolein
The absolute and relative liver weights of the 5 and 10 mg/kg females were significantly greater than those of the vehicle controls. The absolute and relative thymus weights of the 10 mg/kg females and the absolute heart weights of 5 and 10 mg/kg females were significantly less than those of the vehicle controls.
Gross lesions related to acrolein treatment were observed in the forestomach and glandular stomach of the male and female rats, primarily in the 10 mg/kg groups and consisted of red or white discoloration. Microscopically, males in the 5 and 10 mg/kg groups and females in the 2.5, 5 and 10 mg/kg groups had increased incidences of squamous epithelial hyperplasia in the forestomach relative to those of the vehicle controls (Table 3). Incidences of hemorrhage in the glandular stomach of males and females in the 10 mg/kg groups were significantly greater than those in the vehicle controls.
3.1.3 Hematology and Clinical Chemistry
3.1.3.1 Allyl acetate
On day 4, hematocrit values, hemoglobin concentrations, and erythrocyte counts were decreased in surviving 100 mg/kg males and females, and the decreases are consistent with mild to marked anemia. The decrease in the eythron was accompanied by the increases in reticulocyte and nucleated erythrocyte counts. Mean cell volume and mean hemoglobin in 100 mg/kg females were increased, and these increases are consistent with the increased numbers of circulating immature erythocytes on day 4; also mean cell hemoglobin concentration was increased. Platelet counts were markedly reduced in 100 mg/kg males (by approximately 77%) and females (by approximately 91%). There was also a mild platelet count decrease in the 50 mg/kg females; 50 mg/kg males were unaffected. Neutrophilia, evidenced by increased segmented neutrophil counts, occurred in the 50 and 100 mg/kg males and females. On day 4, there was evidence of hepatocellular injury, as demonstrated by and increases in serum alanine aminotransferase and sorbitol dehydrogenase activities and bile acid concentrations in 50 (approximately 1.5-fold to 4-fold increase) and 100 mg/kg (nine fold or greater increase) males and females. Albumin and, consequently, total protein concentrations were significantly decreased and urea nitrogen concentrations were increased in the 100 mg/kg males and females
On day 23 and or at week 14, there were minimal decreases in the mean cell volumes and mean hemoglobin values and increases in platelet counts in the 50 mg/kg males and females; the mean cell hemoglobin concentration was minimally decreased at each time point in the these females. The biochemical evidence of hepatocellular injury that occurred in the 50 mg/kg males and females at day 4 was also observed on day 23 and, to lesser extent at week 14. The increase in segmented neutrophil counts that occurred in the 50 mg/kg males and females on day 4 resolved with time, and by week 14 occurred only in males.
3.1.3.2 Allyl alcohol
There were minimal decreases in the mean cell volume and increases in platelet counts in the 25 mg/kg males. There were no other changes in hematology data that indicated a treatment related effect. At week 14 alkaline phosphatase activities were decreased and bile acid concentrations were increased in the 12 mg and 25 mg/kg males and females.
3.1.3.3 Acrolein
On day 4, the hematocrit values, hemoglobin concentrations, and erythrocyte counts were generally significantly increased in the 10 mg/kg males and females. Also on day 4 platelets were notably increase in the 2.5, 5 and 10 mg/kg males and females and remained elevated at day 23 and week 14. In the 2.5, 5.0 and 10 mg/kg males and females alkaline phosphatase levels were increased while albumin levels were decreased at all three time points. Increases in total serum protein closely paralleled the observed increases in albumin.
3.2 B6C3F1 mice
3.2.1 In life observations
3.2.1.1. Allyl acetate
All males and females in the 125 mg/kg group died during the first week of the study (Table 4). Final mean body weight gains of males and females were similar to those of the vehicle controls (Table 4). Clinical findings included lethargy, abnormal breathing, thinness, and ruffled fur among the mice that died early.
3.2.1.2 Allyl alcohol
All animals with the exception of one female survived to the end of the study; all other animals survived to the end of the study (Table 4). Final mean body weights of the dosed male and female mice were similar to those of the vehicle controls; the mean body weight gains in all other male and female dosed groups were similar to those of the vehicle control groups (Table 4). No clinical findings were evident in the dosed animals.
3.2.1.3 Acrolein
All males and females administered 20 mg/kg died during the first week of the study (Table 4). All other early deaths, except one male and one female administered 10 mg/kg, appeared to be unrelated to chemical administration. Final mean body weights and mean body weight gains of all the dosed groups were to those of the vehicle controls (Table 4). No clinical findings were evident in the dosed animals.
3.2.2 Pathology
3.2.2.1 Allyl acetate
Gross lesions related to allyl acetate treatment included red discoloration in the forestomach and granular, mottled livers in the male and female mice and red foci in the glandular stomach of a male mouse. Microscopically, males in the 32 and 62.5 mg/kg groups and females in the 16, 32, and 62.5 mg/kg groups had significantly increased incidences of squamous epithelial hyperplasia in the forestomach compared to those of the vehicle controls (Table 5). The incidence of hemorrhage of the forestomach in the 125 mg/kg males was significantly greater than that in the vehicle controls. Males in the 62.5 group and males and females in 125 mg/kg group had significantly increased incidences of hemorrhage in the glandular stomachs compared to vehicle controls. In general, males in the 62.5 and 125 mg/kg groups had increased incidences of fibrosis, hepatocyte necrosis, and mineralization in the liver. The incidences of portal cytoplasmic vacuolization of the 62.5 mg/kg males and females were significantly greater than those in the vehicle control groups as was the incidence of hepatocyte necrosis in the 125 mg/kg females (Table 6).
Table 5.
Forestomach Squamous Epithelial Hyperplasia in Mice
| Dose (mg/kg) (AAC, AAL, ACR) |
Allyl Acetate |
Allyl Alcohol |
Acrolein | |
|---|---|---|---|---|
| Male | ||||
| 0 | 0/10 | 0/10 | 0/10 | |
| 8, 3, 1.25 | 0/10 | 1/10(1.0) | 2/10(1.0) | |
| 16, 6, 2.5 | 0/10 | 3/10(1.0) | 6/10**(2.0) | |
| 32, 12, 5 | 4/10*(1.5) | 9/10*8(1.1) | 7/10**(1.1) | |
| 62.5, 25, 10 | 10/10**(2.8) | 10/10**(2.0) | 10/10**(2.0) | |
| 125, 50, 20 | 1/10*(3.0) | 10/10**(1.0) | 0/10 | |
| Female | ||||
| 0 | 0/10 | 0/10 | 0/10 | |
| 8, 3, 1.25 | 0/10 | 0/10 | 0/10 | |
| 16, 6, 2.5 | 4/10*(1.5) | 0/10 | 4/10*(1.0) | |
| 32, 12, 5 | 5/10*(1.2) | 8/10**(1.1) | 7/10**(1.1) | |
| 62.5, 25, 10 | 8/10**(1.4) | 10/10**(2.0) | 8/10**(1.3) | |
| 125, 50, 20 | 1/10(2.0) | 9/10**(2.0) | 2/10(1.5) | |
Significantly different (P ≤ 0.05) from the vehicle control group by Fischer exact test
P ≤ 0.01
Table 6.
Cytoplasmic vacuolization of hepatocytes in mice
| Dose (mg/kg) (AAC, AAL, ACR) |
Allyl Acetate |
Allyl Alcohol |
Acrolein | |
|---|---|---|---|---|
| Male | ||||
| 0 | 0/10 | 0/10 | 0/10 | |
| 8, 3, 1.25 | 0/10 | 0/10 | 0/10 | |
| 16, 6, 2.5 | 0/10 | 0/10 | 0/10 | |
| 32, 12, 5 | 0/10 | 0/10 | 0/10 | |
| 62.5, 25, 10 | 6/10**(1.0) | 2/10(1.0) | 0/10 | |
| 125, 50, 20 | 0/10 | 10/10**(1.0) | 0/10 | |
| Female | ||||
| 0 | 0/10 | 1/10(1.0) | 0/10 | |
| 8, 3, 1.25 | 0/10 | 1/10(1.0) | 0/10 | |
| 16, 6, 2.5 | 0/10 | 1/10(1.0) | 0/10 | |
| 32, 12, 5 | 3/10(1.0) | 5/10(1.0) | 0/10 | |
| 62.5, 25, 10 | 4/10*(1.3) | 8/10**(1.0) | 0/10 | |
| 125, 50, 20 | 0/10 | 9/10(1.2) | 0/10 | |
Significantly different (P ≤ 0.05) from the vehicle control group by Fischer exact test
P ≤ 0.01
3.2.2.2 Allyl alcohol
No treatment-related gross lesions were observed. Microscopically, males and females in the 12, 25, and 50 mg/kg groups had significantly increased incidences of squamous epithelial hyperplasia in the forestomach (Table 5). Incidences of portal cytoplasmic vacuolization in the liver were significantly increased in the 50 mg/kg males and females and 25 mg/kg females (Table 6). One male and one female in the 50 mg/kg groups had hemosiderin pigmentation; one 50 mg/kg female also had granulomatous inflammation and hepatocyte necrosis.
3.2.2.3 Acrolein
The absolute liver weights of the 10 mg/kg males were significantly increased. Gross lesions related to acrolein treatment included red or white discoloration in the forestomach and glandular stomach of the female mice in the 20 mg/kg group. Microscopically, males and females in the 2.5, 5 and 10 mg/kg groups had significant increased incidences of squamous epithelial hyperplasia in the forestomach (Table 5). Incidences of hemorrhage in the glandular stomach in the 20 mg/kg males and females significantly exceeded those of vehicle controls. The incidences of epithelial necrosis and chronic active inflammation in the glandular stomach in the 20 mg/kg females were significantly increased.
3.2.3 Hematology and Clinical Chemistry
In allyl acetate treated mice there were no biologically significant differences in hematology parameters between dose groups. Minimal increases in platelet counts occurred in the 50 mg/kg allyl alcohol treated males and in all dosed females; the increases in females were not dose related. At week 14, in acrolein treated mice, there were minimal increases in hematocrit values, hemoglobin concentrations and erythrocyte counts in the 10 mg/kg males and in the 2.5, 5.0 and 10.0 mg/kg females; in females the increases were considered to be dose related. Platelets were significantly increased in the 10 mg/kg acrolein treated males.
3.3 Urinary S-(3-Hydroxypropyl) Mercapturic Acid
The concentrations of S-(3-hydroxypropyl) mercapturic acid in the urine of male and female rats after one or 45 doses of allyl acetate or allyl alcohol increased linearly with the dose (Table 7). In the rats dosed with acrolein, the concentrations increased nonlinearly with dose at the first time point and linearly with the dose at the second time point (except 10 mg/kg).
Table 7.
Urinary S-(3-hydroxypropyl) mercapturic acid concentrationa in F344 rats following administration of 1st and 45th dose (n=10/dose group)
| Dose (mg/kg) (AAC, AAL, ACR) |
Allyl Acetate | Allyl Alcohol | Acrolein | |
|---|---|---|---|---|
| Males | ||||
| 0 | 1st | ----b | ---- b | --- b |
| 6, 1.5, 0.75 | 1st | 17.66±1.10** | 13.42±0.63** | 13.82±0.94** |
| 12, 3, 1.25 | 1st | 51.83±5.28**! | 22.33±0.96** | 18.41±0.80** |
| 25, 6, 2.5 | 1st | 126.09±8.08** | 54.17±3.23**! | 32.64±1.82** |
| 50, 12, 5 | 1st | 355.10±15.76**# | 103.25±6.00** | 60.65±3.39** |
| 100, 25, 10 | 1st | ---- c | 239.20±18.73**# | 63.85±9.45** |
| 0 | 45th | ---- b | ---- b | ---- b |
| 6, 1.5, 0.75 | 45th | 57.25±2.74** | 19.59±1.10** | 18.56±1.61** |
| 12, 3, 1.25 | 45th | 104.43±8.34**! | 37.38±2.68** | 24.07±1.48** |
| 25, 6, 2.5 | 45th | 260.00±18.61** | 77.74±4.81**! | 42.80±3.02**e |
| 50, 12, 5 | 45th | 479.70±33.78**# | 140.10±17.59**d | 69.61±3.51** |
| 100, 25, 10 | 45th | ---- c | 354.40±29.12**# | 44.30±18.50**f |
| Females | ||||
| 0 | 1st | ---- b | ---- b | --- b |
| 6, 1.5, 0.75 | 1st | 18.63±1.91** | 13.08±0.75** | 12.60±1.22**d |
| 12, 3, 1.25 | 1st | 63.87±3.77**! | 21.03±0.82** | 17.08±1.39** |
| 25, 6, 2.5 | 1st | 147.94±17.42** | 44.47±2.39**! | 31.81±2.46** |
| 50, 12, 5 | 1st | 335.20±33.13**# | 100.22±7.07** | 52.17±3.68** |
| 100, 25, 10 | 1st | ---- c | 249.13±24.90**# | 73.92±8.15** |
| 0 | 45th | ---- b | ---- b | ---- b |
| 6, 1.5, 0.75 | 45th | 33.33±2.56** | 16.91±1.14** | 11.09±0.55** |
| 12, 3, 1.25 | 45th | 73.60±4.17**! | 36.83±2.14** | 19.34±1.82**d |
| 25, 6, 2.5 | 45th | 162.92±17.88** | 93.58±7.12**! | 26.56±2.90**e |
| 50, 12, 5 | 45th | 380.40±36.09**# | 183.40±18.49** | 49.22±7.15**d |
| 100, 25, 10 | 45th | ---- c | 453.80±26.66**# | 98.20g |
Significantly different (P≤0.01) from vehicle control group by Shirley’s test
Significantly different (P≤0.01) from 5.0 mg/kg acrolein (approximate equimolar dose comparison) by unpaired t-test
Significantly different (P≤0.01) from 5.0 mg/kg acrolein (MTD) by unpaired t-test
Data are presented as µg/mL of urine (mean ± standard deviation)
Below the limit of detection (1.30 µg/mL)
No data available due to 100% mortality
n=9
n=8
n=2
n=1
In male and female mice, the concentration of S-(3-hydroxypropyl) mercapturic acid in the urine after one or 45 doses of allyl acetate or allyl alcohol increased linearly with the dose (Table 8). In mice dosed with acrolein, the concentrations increased nonlinearly with the dose at the first time point and linearly with dose at the second time point (except at 10 mg/kg).
Table 8.
Urinary S-(3-hydroxypropyl) mercapturic acid concentrationa in B6C3F1 mice following administration of 1st and 45th dose (n=2/dose group)
| Dose (mg/kg) (AAC, AAL, ACR) |
Allyl Acetate | Allyl Alcohol | Acrolein | |
|---|---|---|---|---|
| Males | ||||
| 0 | 1st | 11.24±2.86 | 7.72±0.35 | 12.15±1.65 |
| 8, 3, 1.25 | 1st | 29.55±5.15 | 63.80±12.70 | 28.00±5.70 |
| 16, 6, 2.5 | 1st | 84.25±0.55 | 58.35±11.35 | 39.00±5.70 |
| 32, 12, 5 | 1st | 88.65±7.05 | 113.85±54.15* | 65.00±10.90* |
| 62.5, 25, 10 | 1st | 108.20±27.80 | 253.00±7.00* | 68.20±3.10* |
| 125, 50, 20 | 1st | ----c | 407.00±174.00*# | ---- c |
| 0 | 45th | 17.25±3.95 | 15.05±1.05 | 13.45±0.85 |
| 8, 3, 1.25 | 45th | 98.05±19.95 | 41.35±5.95 | 25.10±7.90 |
| 16, 6, 2.5 | 45th | 171.00±9.00 | 63.70±13.00* | 36..90±2.40* |
| 32, 12, 5 | 45th | 232.00±93.00# | 118.50±12.50* | 62.20±5.50* |
| 62.5, 25, 10 | 45th | 157.50±33.50 | 161.50±31.50* | 100.55±7.45* |
| 125, 50, 20 | 45th | ---- c | 328.50±17.50*# | ---- c |
| Females | ||||
| 0 | 1st | ---- b | ---- b | --- b |
| 8, 3, 1.25 | 1st | 16.55±1.95** | 22.50±0.70** | 22.70±3.70** |
| 16, 6, 2.5 | 1st | 26.75±1.45** | 35.75±4.25** | 32.10±0.80** |
| 32, 12, 5 | 1st | 63.00±0.00** | 48.95±0.65** | 34.35±16.05** |
| 62.5, 25, 10 | 1st | 145.00±15.00** | 158.00±32.00** | 35.55±7.95** |
| 125, 50, 20 | 1st | ---- | 229.00±47.00**# | ---- |
| 0 | 45th | ---- b | ---- b | ---- b |
| 8, 3, 1.25 | 45th | 26.65±3.15** | 17.50±1.20** | 12.30±1.00** |
| 16, 6, 2.5 | 45th | 46.00±14.00** | 35.60±3.10** | 33.85±0.85** |
| 32, 12, 5 | 45th | 57.60±2.10** | 69.75±8.25** | 42.45±4.65** |
| 62.5, 25, 10 | 45th | 172.50±0.50** | 118.00±1.00** | 73.15±11.85** |
| 125, 50, 20 | 45th | ---- c | 252.50±1.50** | ---- c |
Significantly different (P≤0.05) from vehicle control group by Shirley’s test
P≤0.01
Data are presented as µg/mL of urine (mean ± standard deviation)
Below the limit of detection (1.30 µg/mL)
No data available due to 100% mortality
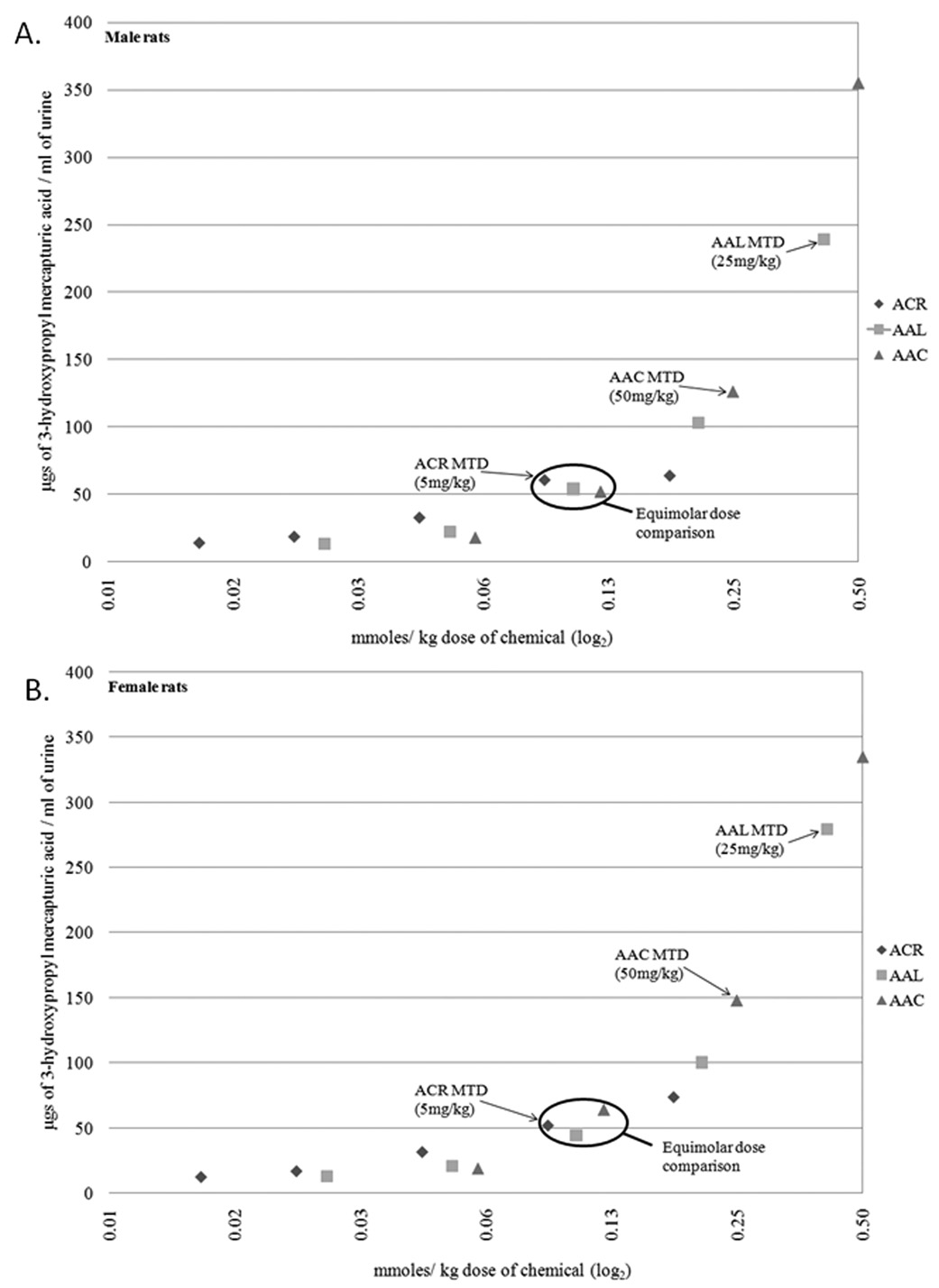
When near equimolar doses of the chemicals were administered to male rats there were slight, yet significant differences in urinary mercapturic acid production. In male rats doses of 12 mg/kg of allyl acetate, 6 mg/kg allyl alcohol and 5 mg/kg acrolein yielded 63.87 ±3.77, 44.47±2.39 and 52.17±3.68 µg/ml of mercapturic acid conjugate, respectively (Table 7, Figure 1a). A similar pattern was observed in female rats (Table 7, Figure 1b). However, when the levels of urinary mecrapturic acids in the groups representing the minimally toxic dose (MTD; as determined by terminal weight and survival) for each chemical were compared dramatically higher levels were observed in the allyl alcohol and allyl acetate treated rats relative to those treated with acrolein. For example, in male rats doses of 50 mg/kg of allyl acetate, 25 mg/kg allyl alcohol and 2.5 mg/kg acrolein yielded 355.10±15.76, 239.20±18.73 and 63.85±9.45 µg/ml of mercapturic acid conjugate, respectively (Table 7, Figure 1a). Similar comparisons were not performed in the mouse metabolism studies due to reduced numbers of animals, however trends that parallel those observed in the rat are clearly present in the mice.
Figure 1.
S-(3-hydroxypropyl) mercapturic acid production per molar dose of allyl acetate, allyl alcohol or acrolein. Near equimolar dose comparisons that are noted in table 7 are indicated. MTD doses used for comparisons noted in table 7 are indicated.
4. Discussion
Via enzymatic metabolism, allyl esters are metabolized to allyl alcohol, which is then converted to the highly toxic, α,β-unsaturated aldehyde, acrolein. Acrolein can be further oxidized to acrylic acid, conjugated to glutathione, or react with cellular macromolecules to cause toxicity. Because of the metabolic relationship between allyl acetate, allyl alcohol and acrolein a comparative 90-day toxicity study was performed to allow simultaneous comparison of all three compounds in the same strain of rats and mice. Oral dosing was chosen to mimic human ingestion of these and related compounds.
In the present studies, acrolein was clearly the most toxic of the three compounds in rats, causing reduced survival in the 2.5, 5, and 10 mg/kg groups and reduced body weight in the 10 mg/kg groups. Over the same dose range, allyl acetate administration caused only a marginal reduction in body weight and allyl alcohol administration had no effect on survival or body weights of rats. Marginal increases in absolute or relative liver weights occurred in all three rat studies, mostly higher dose concentrations; however, no pattern of treatment-related changes was apparent in other organs.
Acrolein was also the most toxic of the three compounds in mice, causing reduced survival in the 20 mg/kg groups. Neither the allyl alcohol nor allyl acetate caused reduced body weights or survival over the same dose range. Absolute and relative liver weights of the male mice and relative liver weights of female mice were increased in the groups that received 10 mg/kg acrolein, and relative liver weights were increased in groups that received 50 mg/kg allyl alcohol.
The major toxic response in both mice and rats for all three compounds occurred in the forestomach. Exposure to allyl alcohol was associated with only a mild response characterized by squamous epithelial hyperplasia. In rats, the severity was minimal in all dose groups, but in mice the severity was mild in the 25 and 50 mg/kg allyl alcohol groups. A mild response also occurred in rats and mice administered 6 or 8 mg/kg of allyl acetate, respectively; however, the incidences and severities of forestomach lesions increased with increasing dose. In the allyl acetate studies, epithelial necrosis, hemorrhage and chronic active inflammation in the forestomach of 100 mg/kg rats and 62.5 mg/kg mice likely contributed to the moribund condition in these animals. Epithelial hyperplasia of the forestomach was present in all groups of male mice exposed to acrolein and in females that received at least 2.5 mg/kg. Epithelial necrosis and/or hemorrhage also occurred in the glandular stomach of mice exposed to 20 mg/kg acrolein and contributed to the reduced survival in those groups. The primary toxicity observations are largely in agreement with previous studies that demonstrate gastric and hepatic toxicities with repeat doses greater than 2.5 mg/kg in rats and 4.5 mg/kg in mice. (Parent et al 1991; Parent et al 1992).
Toxicity to the liver also occurred following exposure to allyl acetate and in mice and female rats administered allyl alcohol. Treatment with 25 mg/kg allyl alcohol, the highest dose evaluated, significantly increased the incidences of bile duct hyperplasia and periportal hypertrophy in female rats, but not in males. In the groups of mice administered allyl alcohol, females were somewhat more responsive than males, and increased incidences of portal cytoplasmic vacuolization occurred in 12 mg/kg or greater females; this lesion was first observed at 25 mg/kg in male mice. Rikans and Moore reported a sex difference in allyl alcohol hepatoxicity in rats that appeared to be correlated with the greater alcohol dehydrogenase activity in female rats than in male rats (Rikans & Moore 1987). As the male rats aged, the alcohol dehydrogenase activity in the liver increased, and their sensitivity to allyl alcohol hepatoxicity also increased, although neither the alcohol dehydrogenase activity nor hepatotoxic response in older males became equal to that observed in the young or older females.
In contrast to allyl alcohol, the hepatotoxic response to allyl acetate did not differ between males and females. Lesions similar to those that occurred in animals administered allyl alcohol also occurred in male and female rats administered 25 mg/kg allyl acetate; at higher doses the toxic response was more significant and included hepatocellular necrosis and hypertrophy, among other toxic lesions. A similar response occurred in male and female mice administered 62.5 mg/kg or greater. Recent metanalysis of NTP data suggests both hepatocellular necrosis and hypertrophy in 90-day studies are predictive of hepatocarcinogenic potential (Allen et al 2004). The increased alanine aminotransferase and sorbitol dehydrogenase activities in the serum of the rats administered allyl acetate but not in rats administered allyl alcohol were consistent with microscopic findings in the liver.
The periportal hepatotoxicity associated with allyl alcohol exposure is well documented (Badr 1991), and, based on a number of observations is likely due to the biotransformation of allyl alcohol to acrolein. However, acrolein administered at doses used in the present studies was not hepatotoxic in either rats or mice. After oral administration, acrolein is eliminated primarily in the urine as a glutathione conjugate or oxidized acrylic acid, which in turn is rapidly metabolized to carbon dioxide by the propionic acid pathway (Parent et al 1996). S-(3-hydroxypropyl) mercapturic acid, the major metabolite of acrolein, was present in the urine of all groups of rats and mice exposed to allyl acetate or allyl alcohol, demonstrating the formation and detoxification of acrolein in vivo in these animals. However, Parent et al. showed that acrolein also reacts with food in the intestinal tract (Parent et al 1996). This is not surprising because acrolein is a relatively soft electrophile and therefore reacts preferentially with soft nucleophiles such as sulfylhydryl groups on proteins and peptides (Carlson 1990). Therefore, although the local concentration of acrolein in the gut may have been sufficient to produce forestomach lesions, reaction with contents of the gastrointestinal tract must have reduced the systemic bioavailability to levels low enough to permit effective detoxification in the liver without causing a hepatotoxic response. Because neither allyl acetate nor allyl alcohol is as reactive as acrolein, their bioavailability would not have been reduced in the same way. Allyl acetate and alcohol are substantially less electrophic than acrolein and, consequently, their tissue distribution will be relatively broad.
Polymorphic metabolism of allyl esters and their metabolites, allyl alcohol and acrolein, caused by genetic variation is likely to affect the outcome of exposure to these agents in humans and should therefore be considered in any risk assessment done on these chemicals. The 2 major forms of carboxylesterase, CES1 and CES2, perform xenobiotic ester hydrolysis. Depending on the acyl group either isoform can play a significant role in ester hydrolysis (Imai 2006). Although CES1 seems to exhibit little functional polymorphism a number of nucleotide changes have been identified that have yet to characterized (Marsh et al 2004). Unlike CES1, genetic variants of consequence have been identified in CES2 that exhibit compromised activity towards the anticancer agent irinotecan, p-nitrophenol acetate and 4-methylumbelliferyl acetate (Marsh et al 2004). An extensive number of studies have documented the polymorphic metabolism of alcohols by the pyrazole sensitive class I alcohol dehydrogenase enzymes that are responsible for ally alcohol metabolism (Crabb et al 2004). In particular, allelic variants in ADH1B and ADH1C have been associated with phenotypic differences in ethanol metabolism in a large portion of the human population and, although not currently documented, these polymorphic enzymes are likely to impact the metabolism of allyl alcohol to acrolein (Crabb et al 2004). Allyl alcohol toxicity is reduced by cyanamide- and disulfuram-sensitive aldehyde dehydrogenases from both cytosol and mitochondria (Rikans 1987). These enzymes likely correspond to ALDH1 and ALDH2. A hypomorphic allele of ALDH2, ALDH2*2, has been extensively studied in relationship to acetylaldehyde metabolism and is anticipated to play a role in the detoxification of acrolein. Conjugation of acrolein with glutathione, the primary pathway of detoxification, is largely a nonezymatic process therefore polymorphisms in enzymes that are involved in the maintenance of glutathione levels (as opposed to glutathione conjugating enzymes) would be expected to have an effect on acrolein toxicity. GCLM encodes a modifier subunit of glutamate-cysteine ligase (GCL), the rate limiting, mutlimeric, enzyme in glutathione synthesis. Polymorphism in this gene causes decreased expression that is associated with the manifestation of vascular disease and schizophrenia (Nakamura et al 2002; Tosic et al 2006). GCLC encodes the catalytic subunit of the GCL complex. A trinucleotide repeat polymorphism in GCLC was associated with decreased expression of glutamate cysteine ligase activity and risk of schizophrenia (Gysin et al 2007). In the human population there is a significant degree of documented functional polymorphism in the allyl ester metabolic pathway that has the potential to influence outcomes of exposure to allyl esters, allyl alcohol and acrolein.
Acrolein has been evaluated for carcinogenic potential in gavage studies in both rats and mice. In the rat study, doses 0, 0.05, 0.5, or 2.5 mg/kg were used (Parent et al 1992). In the present rat study, 2.5 mg/kg caused some alteration in clinical chemistry parameters and epithelial hyperplasia in the forestomach of males and females. Moreover, the incidences of forestomach hyperplasia increased substantially, especially in males, at the next highest dose concentration, 5 mg/kg. Therefore, the results of the present rat study indicate that the 2.5 mg/kg used in the Parent et al. rat study was an adequate high dose (Parent et al 1992). In the present mouse study the incidences of forestomach hyperplasia were significantly increased in 2.5 and 5 mg/kg males and females; therefore, the 4.5 mg/kg used in the Parent et al. study was an adequate high dose (Parent et al 1991). Acrolein was not carcinogenic in the Parent et al. rat and mouse studies (Parent et al 1991; Parent et al 1992); therefore, it is a strong possibility that allyl alcohol and allyl acetate would also be negative in properly conducted carcinogenicity studies. However, the prospect remains that allyl acetate and allyl alcohol due to their greater gastrointestinal tolerability are capable of delivering higher systemic doses of acrolein through post-absorption metabolism; doses that would not be achievable through administration of acrolein itself. In tissues that may be more sensitive to mutagenesis (Bedard et al 2005) this higher rate of delivery may lead to carcinogenesis. Notably, another allyl ester, allyl isovalerate, the toxicity of which is correlated with its hydrolysis to allyl alcohol and subsequent conversion to acrolein (Butterworth et al 1975), was tested by the National Toxicology Program and was shown to be a carcinogen in male rats and female mice (1983). Because of the high reactivity of acrolein the previous carcinogenicity studies (Parent et al 1991; Parent et al 1992) are of limited use in assessing the risk from acrolein exposure away from the point of contact that is due to post-absorbtion metabolic breakdown of protoxicants such as the allyl esters. Exposure to acrolein that is manifest due to post-absorption metabolic breakdown of allyl esters may pose a significant carcinogenic hazard to internal organs and tissues; an observation supported by carcinogenesis studies involving intraperitoneal injection of acrolein (Cohen et al 1992). Furthermore, although it is arguable that the soft electrophile, acrolein, is carcinogenic in vivo through a direct mutagenic mechanism it certainly plausible that cytochrome P450 dependent formation of the epoxide glycidaldehyde (a much harder electrophile and powerful mutagen) from the glutathione adducts of acrolien would lead to direct DNA damage (Barros et al 1994; Feron et al 1991). Due to higher internal dose levels of acrolein glutathione adducts that are achievable with allyl acetate and allyl alcohol there is notably greater risk of exposing the internal organs to glycidaldehyde. The plausibility of such a mechanism of genotoxicity is supported by studies that demonstrate the mutagenicity and DNA reactivity of acrylamide is limited by its ability to form its epoxide metabolite, glycidamide (Besaratinia & Pfeifer 2004). For these reasons caution should be exercised in when determining carcinogenic risk of compounds that yield acrolein through metabolic breakdown.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to report for the authors
Contributor Information
Scott S. Auerbach, Email: auerbachs@niehs.nih.gov.
Gregory S. Travlos, Email: travlos@niehs.nih.gov.
Richard D. Irwin, Email: irwin@niehs.nih.gov.
References
- Allen DG, Pearse G, Haseman JK, Maronpot RR. Prediction of rodent carcinogenesis: an evaluation of prechronic liver lesions as forecasters of liver tumors in NTP carcinogenicity studies. Toxicol Pathol. 2004;32:393–401. doi: 10.1080/01926230490440934. [DOI] [PubMed] [Google Scholar]
- Badr MZ. Effects of the anti-AIDS drug dideoxyinosine on hepatic glycolysis in the perfused rat liver: role of intracellular calcium stores. Biochem Pharmacol. 1991;41:146–148. doi: 10.1016/0006-2952(91)90024-y. [DOI] [PubMed] [Google Scholar]
- Barros AR, Sierra LM, Comendador MA. Acrolein genotoxicity in Drosophila melanogaster. III. Effects of metabolism modification. Mutation Research/Genetic Toxicology. 1994;321:119–126. doi: 10.1016/0165-1218(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Bedard LL, Alessi M, Davey S, Massey TE. Susceptibility to aflatoxin B1-induced carcinogenesis correlates with tissue-specific differences in DNA repair activity in mouse and in rat. Cancer Res. 2005;65:1265–1270. doi: 10.1158/0008-5472.CAN-04-3373. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Bradford BU, Forman DT, Glassman EB, Felder MR, Thurman RG. Hepatotoxicity due to allyl alcohol in deermice depends on alcohol dehydrogenase. Hepatology. 1985;5:1179–1182. doi: 10.1002/hep.1840050619. [DOI] [PubMed] [Google Scholar]
- Besaratinia A, Pfeifer GP. Genotoxicity of Acrylamide and Glycidamide. J. Natl. Cancer Inst. 2004;96:1023–1029. doi: 10.1093/jnci/djh186. [DOI] [PubMed] [Google Scholar]
- Butterworth KR, Carpanini FM, Gaunt IF, Grasso P, Lloyd AG. Proceedings: A new approach to the evaluation of the safety of flavouring esters. British journal of pharmacology. 1975;54:268P. [PMC free article] [PubMed] [Google Scholar]
- Carlson RM. Assessment of the propensity for covalent binding of electrophiles to biological substrates. Environ Health Perspect. 1990;87:227–232. doi: 10.1289/ehp.9087227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Garland EM, St John M, Okamura T, Smith RA. Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res. 1992;52:3577–3581. [PubMed] [Google Scholar]
- Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. The Proceedings of the Nutrition Society. 2004;63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- Dean BJ, Brooks TM, Hodson-Walker G, Hutson DH. Genetic toxicology testing of 41 industrial chemicals. Mutat Res. 1985;153:57–77. doi: 10.1016/0165-1110(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6:241–252. [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. Journal of the American Statistical Association. 1955;50:1096–1121. [Google Scholar]
- Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron VJ, Til HP, de Vrijer F, Woutersen RA, Cassee FR, van Bladeren PJ. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat Res. 1991;259:363–385. doi: 10.1016/0165-1218(91)90128-9. [DOI] [PubMed] [Google Scholar]
- Foiles PG, Akerkar SA, Chung FL. Application of an immunoassay for cyclic acrolein deoxyguanosine adducts to assess their formation in DNA of Salmonella typhimurium under conditions of mutation induction by acrolein. Carcinogenesis. 1989;10:87–90. doi: 10.1093/carcin/10.1.87. [DOI] [PubMed] [Google Scholar]
- Gart JJ, Chu KC, Tarone RE. Statistical issues in interpretation of chronic bioassay tests for carcinogenicity. J Natl Cancer Inst. 1979;62:957–974. [PubMed] [Google Scholar]
- Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T. Human carboxylesterase isozymes: catalytic properties and rational drug design. Drug metabolism and pharmacokinetics. 2006;21:173–185. doi: 10.2133/dmpk.21.173. [DOI] [PubMed] [Google Scholar]
- Irwin RD. NTP Technical Report on the comparative toxicity studies of allyl acetate (CAS No. 591-87-7), allyl alcohol (CAS No. 107-18-6) and acrolein (CAS No. 107-02-8) administered by gavage to F344/N rats and B6C3F1 mice. Toxicity report series. 2006:1–73. A1-H10. [PubMed]
- Kaye CM. Biosynthesis of mercapturic acids from allyl alcohol, allyl esters and acrolein. Biochem J. 1973;134:1093–1101. doi: 10.1042/bj1341093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Liao H-p, Smith LA, Jr., Tuemmler WB. United States. 1969 [Google Scholar]
- Lijinsky W, Reuber MD. Chronic carcinogenesis studies of acrolein and related compounds. Toxicol Ind Health. 1987;3:337–345. doi: 10.1177/074823378700300306. [DOI] [PubMed] [Google Scholar]
- Marsh S, Xiao M, Yu J, Ahluwalia R, Minton M, et al. Pharmacogenomic assessment of carboxylesterases 1 and 2. Genomics. 2004;84:661–668. doi: 10.1016/j.ygeno.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kugiyama K, Sugiyama S, Miyamoto S, Koide S, et al. Polymorphism in the 5'-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation. 2002;105:2968–2973. doi: 10.1161/01.cir.0000019739.66514.1e. [DOI] [PubMed] [Google Scholar]
- NTP. Natl Toxicol Program Tech Rep Ser. 1983. Carcinogenesis Studies of Allyl Isovalerate (CAS No. 2835-39-4) in F344/N Rats and B6C3F1 Mice (Gavage Studies) pp. 1–185. [PubMed] [Google Scholar]
- Parent RA, Caravello HE, Long JE. Oncogenicity study of acrolein in mice. J. Am. Coll. Toxicology. 1991;10:647–659. [Google Scholar]
- Parent RA, Caravello HE, Long JE. Two-year toxicity and carcinogenicity study of acrolein in rats. J Appl Toxicol. 1992;12:131–139. doi: 10.1002/jat.2550120210. [DOI] [PubMed] [Google Scholar]
- Parent RA, Caravello HE, Sharp DE. Metabolism and distribution of [2,3-14C]acrolein in Sprague-Dawley rats. J Appl Toxicol. 1996;16:449–457. doi: 10.1002/(SICI)1099-1263(199609)16:5<449::AID-JAT369>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Rikans LE. The oxidation of acrolein by rat liver aldehyde dehydrogenases. Relation to allyl alcohol hepatotoxicity. Drug Metab Dispos. 1987;15:356–362. [PubMed] [Google Scholar]
- Rikans LE, Moore DR. Effect of age and sex on allyl alcohol hepatotoxicity in rats: role of liver alcohol and aldehyde dehydrogenase activities. J Pharmacol Exp Ther. 1987;243:20–26. [PubMed] [Google Scholar]
- Sanduja R, Ansari GA, Boor PJ. 3-Hydroxypropylmercapturic acid: a biologic marker of exposure to allylic and related compounds. J Appl Toxicol. 1989;9:235–238. doi: 10.1002/jat.2550090406. [DOI] [PubMed] [Google Scholar]
- Scorecard. Chemical Profile for allyl alcohol Green Media Toolshed. 2005 [Google Scholar]
- Shirley E. A non-parametric equivalent of Williams' test for contrasting increasing dose levels of a treatment. Biometrics. 1977;33:386–389. [PubMed] [Google Scholar]
- Silver EH, Murphy SD. Effect of carboxylesterase inhibitors on the acute hepatotoxicity of esters of allyl alcohol. Toxicol Appl Pharmacol. 1978;45:377–389. doi: 10.1016/0041-008x(78)90102-3. [DOI] [PubMed] [Google Scholar]
- Smith RA, Cohen SM, Lawson TA. Acrolein mutagenicity in the V79 assay. Carcinogenesis. 1990;11:497–498. doi: 10.1093/carcin/11.3.497. [DOI] [PubMed] [Google Scholar]
- Tosic M, Ott J, Barral S, Bovet P, Deppen P, et al. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. American journal of human genetics. 2006;79:586–592. doi: 10.1086/507566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Evaluation of certain food additives and contaminants. Forty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organ Tech Rep Ser. 1997;868:i–viii. 1–69. [PubMed]
- Williams DA. A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics. 1971;27:103–117. [PubMed] [Google Scholar]
- Williams DA. The comparison of several dose levels with a zero dose control. Biometrics. 1972;28:519–531. [PubMed] [Google Scholar]
- Wilson VL, Foiles PG, Chung FL, Povey AC, Frank AA, Harris CC. Detection of acrolein and crotonaldehyde DNA adducts in cultured human cells and canine peripheral blood lymphocytes by 32P-postlabeling and nucleotide chromatography. Carcinogenesis. 1991;12:1483–1490. doi: 10.1093/carcin/12.8.1483. [DOI] [PubMed] [Google Scholar]