Abstract
Using QCT, we made a longitudinal, population-based assessment of rates of bone loss over life at the distal radius, distal tibia, and lumbar spine. Cortical bone loss began in perimenopause in women and later in life in men. In contrast, trabecular bone loss began in young adulthood in both sexes.
Introduction
Although conventional wisdom holds that bone loss begins at menopause in women and later in life in men, this has not been examined longitudinally in population-based studies using precise technology capable of distinguishing cortical and trabecular bone.
Materials and Methods
In an age- and sex-stratified population sample (n = 553), we measured volumetric BMD (vBMD) of trabecular and cortical bone by QCT annually for up to 3 yr at the distal radius (DR) and distal tibia (DT) (n = 552) and trabecular vBMD at baseline and 3 yr at the lumbar spine (LS) (n = 474).
Results
Substantial cortical bone loss began in middle life in women but began mainly after age 75 in men. In contrast, substantial trabecular bone loss began in young adult women and men at all three skeletal sites and continued throughout life with acceleration during perimenopause in women. Women experienced 37% and men experienced 42% of their total lifetime trabecular bone loss before age 50 compared with 6% and 15%, respectively, for cortical bone. Median rates of change in trabecular bone (%/yr) were −0.40, −0.24, and −1.61 in young adult women and −0.38, −0.40, and −0.84 in young adult men at the DR, DT, and LS, respectively (all p < 0.001). The early trabecular bone loss did not consistently correlate with putative causal factors, except for a trend with IGF-related variables at DT in women. However, in postmenopausal women and, to a lesser extent, in older men, higher rates of cortical and trabecular bone loss were associated with lower levels of biologically-active sex steroids and with higher levels of follicle-stimulating hormone and bone turnover markers.
Conclusions
The late onset of cortical bone loss is temporally associated with sex steroid deficiency. However, the early-onset, substantial trabecular bone loss in both sexes during sex steroid sufficiency is unexplained and indicates that current paradigms on the pathogenesis of osteoporosis are incomplete.
Key words: osteoporosis, menopause, aging, BMD
INTRODUCTION
Osteoporosis is one of the most important diseases affecting the aging population.(1) It has been generally believed that the bone loss leading to osteoporosis begins at menopause in women (caused principally by sex steroid deficiency) and later in life in men (caused by age-related factors).(2–4) This is consistent with most cross-sectional observational studies using DXA at either central or peripheral scanning sites.(5) However, in an early population-based, longitudinal study in women, Riggs et al.(6) found that the rate of bone loss from the predominantly trabecular bone of the lumbar spine as assessed by DXA was similar before and after 50 yr of age, whereas bone loss from the predominantly cortical bone of the peripheral skeleton, assessed by single-energy photon absorptiometry, did not begin until midlife. In a longitudinal study in women using DXA at multiple scanning sites, Slemenda et al.(7) failed to find bone loss until after menopause, except for a small amount of loss at the proximal femur in the 5 yr before menopause. However, the possibility of early trabecular bone loss was not excluded by that study because DXA measures total bone, and the proximal femur and radius consist of predominantly cortical bone; even in the vertebrae, the cortical rim is made up of a substantial amount of its total mass.(8) More recently, Nordstrom et al.(9) conducted an 8-yr longitudinal study of BMD assessed by DXA in young adult men, 17–25 yr of age. They found that, after achieving peak bone mass at age 19, there was a progressive decrease in BMD of ∼1.5%/yr from the hip, but peak BMD was maintained for measurements at the lumbar spine and total body. Finally, Aaron et al.(10) showed that trabecular bone volume decreased linearly with age in both sexes in iliac biopsy samples from cadavers of subjects 20–90 yr of age, but this study was limited by its cross-sectional design and sampled a trabecular site not typically associated with osteoporotic fractures. These results raise the possibility of an earlier onset of trabecular bone loss than was evident from DXA measurements.
It is important to resolve when bone loss begins because current strategies for prevention of osteoporosis assume negligible bone loss before midlife and because the documentation of early trabecular bone loss could elucidate a new and important mechanism(s) in the causation of osteoporosis in both sexes. This resolution now is possible because cortical and trabecular volumetric BMD (vBMD) can be assessed independently with QCT. In a recent cross-sectional study using central and peripheral QCT at multiple scanning sites, we found that decreases in vBMD of trabecular bone began in young adulthood in both women and men, whereas decreases in vBMD of cortical bone did not begin until middle life.(11) An early loss of trabecular bone at the lumbar spine has also been suggested by other cross-sectional studies using QCT.(12,13) However, rates of bone loss estimated from cross-sectional studies may be biased by secular changes in skeletal size and other confounders. Longitudinal studies of the central and peripheral skeleton using QCT are therefore required to establish whether young adults do, in fact, have substantial trabecular bone loss. We addressed this important issue by making longitudinal measurements of trabecular vBMD at the lumbar spine (LS) and of trabecular and cortical vBMD at the distal radius (DR) and distal tibia (DT) in a relatively large, population-based sample of men and women.
MATERIALS AND METHODS
Study subjects
We studied an age-stratified, random sample (n = 700) of the adult population of Rochester, MN, 21–97 yr of age, as described previously.(11) This community is highly characteristic of United States residents, except that minority populations are underrepresented. The sample included 375 women and 325 men. Reflecting the ethnic composition of the community, 96% of the men and 99% of the women were white. Thirty-two percent of the men and 29% of the women were obese (>30% of the ideal weight for their height). Age-groups were defined at the time of the last visit. QCT measurements were made at baseline and then annually at the DR and DT and at baseline and again at 3 yr at the LS. Blood samples for hormone measurements were obtained only at baseline. One hundred nineteen subjects (114 women and 5 men) were excluded from this analysis because they were being treated with drugs (estrogens, selective-estrogen receptor modulator [SERMs], or bisphosphonates) known to affect rates of bone loss. After these exclusions, there remained 553 unique subjects. Of those, 552 (243 women and 309 men) had repeat measurements at peripheral sites for at least 1 and up to 3 yr, and 474 subjects (214 women and 260 men) who had 3 yr of follow-up including repeat spiral QCT measurements of the LS at 3 yr.
Bone densitometry
The methods of central and peripheral QCT have been previously described in detail.(11) All baseline LS CT measurements were made with a four-channel multidetector-row scanner (LightSpeed QX/i; General Electric Medical Systems, Waukesha, WI, USA). Patients were positioned over a quantitative CT calibration phantom (Mindways Software, Austin, TX, USA) to allow accurate conversion of measured CT numbers into BMD. We analyzed a single slice obtained at the midportion of the L1, L2, and L3 vertebrae. Slice width was 2.5 mm, and the in-plane voxel size was 0.74 mm. The trabecular vBMD of the LS was measured in each slice across the entire vertebral body excluding the cortical rim. To validate our image processing algorithm, we made 10 scans of the European spine phantom (ESP; QRM, Möhrendorf, Germany), which is composed of hydroxyapatite. The correlation between BMD results determined by our algorithm and that of the ESP was r = 0.998.
During the study interval, a conversion was made from the MindWays Model 2 Liquid Calibration Phantom to the MindWays Model 3 Solid Calibration Phantom. Conversion accuracy was confirmed using the ESP. For the 3-yr repeat measurements, 65 subjects were restudied on the original machine, whereas 409 subjects were crossed-over to an eight-channel system (LightSpeed Ultra; General Electric Medical Systems). The X-ray tube and filtration, detector material and dimensions, and scanner geometry were identical between these systems, and we maintained the image width and acquisition mode for both systems. Throughout the study period, the Mindways (Mindways Software) quality assurance procedure was performed regularly to maintain CT system calibration of both machines. The ESP was scanned daily for 10 days with the Mindways Model 2 liquid calibration phantom in the QX/I scanner and with the Mindways Model 3 solid calibration phantom in the Ultra scanner. All scans were performed using the table positioning and energy specifications of the patient protocol, and all images analyzed using the patient measurement program with the appropriate calibration algorithm and historical quality assurance information for each scanner and phantom. This analysis revealed a systematic difference between the responses of the two systems that could be corrected by a linear transformation. This correction was applied to all patient images collected on the QX/i scanner. Statistical corrections for small differences in machine characteristics, drifts over time, and uniformity of CT numbers across the field of measurement were made based on monthly scans of quality assurance reference phantoms. Both machines were maintained according to manufacturer specifications, state and federal regulations, and our more stringent in-house quality assurance requirements.
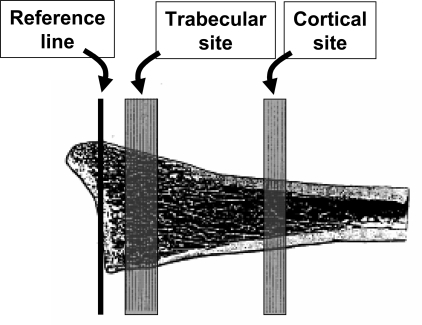
Peripheral QCT measurements were made using the Densican 1000 instrument (Scanco, Bassersdorf, Switzerland). This method has been previously described in detail.(11,14,15) From a digital image (scout view) of the lower forearm or lower leg, the joint space is visualized and a reference point is set electronically at the intersection of the joint space with the radius–ulnar junction for the forearm and the tibial–fibular junction for the distal leg. From this line, an automated program selects a more distal and a more proximal scanning site at both the distal radius and distal tibia. For the DR, the trabecular site (the more distal of the two scanning sites) and the cortical site (the more proximal scanning site) were located 7–20 and 48–55.5 mm, respectively, from the reference line in the joint space (see Fig. 1 for the relationship of these scanning sites to skeletal anatomy). For the DT, the more distal trabecular site was located 20–33.5 mm and the more proximal cortical site was located 63–70.5 mm from the reference line. Ten consecutive slices were obtained at the trabecular sites and six consecutive slices at the cortical sites. For repeat measurements, the manufacturer's software program compared the slices by shape and contour to the baseline scan to obtain the closest possible match.(11,14,15) Trabecular vBMD were obtained at the central 50% of bone at the trabecular sites. Cortical vBMD was determined at the cortical sites, after the cortex was identified by a surface detection program and the outer 10% was excluded to avoid volume averaging artifacts.
FIG. 1.
Illustration of the location of the reference line in the joint space and the trabecular and cortical scanning areas at the distal radius. An analogous process was used for the distal tibia.
Biochemical analyses
Fasting serum samples were obtained at the time of the baseline visit (and during the first 7 days of the menstrual cycle for premenopausal women). Sex steroids were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS, API 5000; Applied Biosystems-MDS Sciex, Foster City, CA, USA). Testosterone (T) was prepared by acetonitrile precipitation and high-throughput liquid chromatography (HTLC) extraction. This was followed by analysis on the LC-MS/MS equipped with a heated nebulizer ion source. For the 17β-estradiol (17β-E2) measurements, an organic extraction of serum was performed using methylene chloride to remove water-soluble conjugates and concentrate the specimen. After derivatization with dansyl chloride, the specimen was processed by high-pressure liquid chromatography (HPLC) and passed into the LC-MS/MS. Deuterated 17β-E2-d5, and d3-testosterone served as internal standards. Values as low as 1 ng/dl for T and 1.25 pg/ml for E2 were detectable by this method. For T values of 8, 4, 2, and 1 ng/dl, the respective CVs were 7.5%, 2.2%, 6.3%, and 28.8%. For E2 values of 10, 5, 2.5, and 1.25 pg/ml, the respective CVs were 2.3%, 6.1%, 14.5%, and 13.4%. The non–sex hormone-binding globulin, biologically active (Bio) fraction was measured as previously described(16); interassay CVs for each were each <12%. Follicle-stimulating hormone (FSH) was measured using a sequential two-step immunoenzymatic (sandwich) assay (Beckman Coulter, Fullerton, CA, USA; interassay CV < 10%). Serum IGF-I and IGF-II were measured by two-site radioimmunometric assays after separation from its binding proteins with a simple organic solvent extraction (Diagnostic Systems Laboratories, Webster, TX, USA; interassay CV, 6%). Serum IGF binding protein-3 (IGFBP-3) was also measured by double antibody immunoassay (Diagnostic Systems Laboratories; interassay CV, 16%). We did not obtain sex steroid or serum FSH measurements on the 44 premenopausal women who were receiving contraceptive hormones. Bone turnover was assessed by serum aminoterminal propeptide of type I collagen (P1NP), an index of bone formation, and by serum C-telopeptide of type I collagen (CTX), an index of bone resorption. Serum PINP was measured by RIA (DiaSorin, Stillwater, MN, USA) with an interassay CV of <9%. Serum CTX was measured by a one-step ELISA (interassay CV < 8%; Osteometer BioTech, Herlev, Denmark).
Statistical analysis
The Wilcoxon signed-rank test was used to test whether the rates of bone loss were different from zero, and the Wilcoxon rank-sum test was used to compare the younger and older age groups. The relationships between the rates of change measurements and serum levels of reproductive hormones and growth factors were studied using the Spearman correlation. A generalized additive model with a cubic B-spline was used to explore the data in the figures. Those segments of the sex-specific age regression with 95% CIs that did not overlap with zero had significant rates of change in vBMD.
RESULTS
For assessing the effect of aging on rates of change in vBMD, these values are plotted against the age of the subject at the time of the last bone densitometry exam. Table 1 provides the rates of cortical and trabecular bone loss at the DR and DT and rates of trabecular bone loss at the LS for in premenopausal and in postmenopausal women. Results are similar in women if plotted before and after age 50 (data not shown). Table 2 provides analogous information for adult men before and after age 50 yr. Table 3 shows the median and interquartile range for these rates of change by decade in women and in men.
Table 1.
Median and Interquartile Range (IQR) for Rates of Change of Cortical and Trabecular vBMD for Woman During Premenopause and Postmenopause at Different Scanning Sites
|
Premenopausal women (n = 103) |
Postmenopausal women (n = 141) |
|||
| Median %/yr (IQR) | p | Median %/yr (IQR) | p | |
| Distal radius | ||||
| Trabecular | −0.40 (−0.98, −0.02) | <0.001 | −0.55 (−1.27, −0.23) | <0.001 |
| Cortical | −0.04 (−0.21, 0.15)* | 0.408 | −0.48 (−0.89, −0.10) | <0.001 |
| Distal tibia | ||||
| Trabecular | −0.24 (−0.70, 0.12) | <0.001 | −0.24 (−0.95, 0.05) | <0.001 |
| Cortical | 0.00 (−0.20, 0.18)* | 0.891 | −0.36 (−0.68, −0.06) | <0.001 |
| Lumbar spine | ||||
| N | 91 | 123 | ||
| Trabecular | −1.61 (−2.47, −0.69)* | <0.001 | −2.60 (−4.42, −1.55) | <0.001 |
See the Materials and Methods section for details of scanning sites, p values from signed rank tests are reported for difference from zero. Rank sum p value (*p < 0.001) indicates that values in premenopausal and postmenopausal women are significantly different.
Table 2.
Median and Interquartile range (IQR) for Rates of Change of Cortical and Trabecular vBMD for Men From 21 to 49 and ≥50 yr of Age at Different Scanning Sites
|
21-49 yr (n = 108) |
50+ yr (n = 201) |
|||
| Median %/yr (IQR) | p | Median %/yr (IQR) | p | |
| Distal radius | ||||
| Trabecular | −0.38 (−0.99, −0.09) | <0.001 | −0.38 (−0.81, −0.13) | <0.001 |
| Cortical | −0.07 (−0.28, 0.09)* | 0.001 | −0.32 (−0.52, −0.11) | <0.001 |
| Distal tibia | ||||
| Trabecular | −0.40 (−1.09, −0.08)* | <0.001 | −0.17 (−0.39, 0.00) | <0.001 |
| Cortical | −0.08 (−0.28, 0.09) | <0.001 | −0.15 (−0.40, 0.03) | <0.001 |
| Lumbar spine | ||||
| N | 86 | 174 | ||
| Trabecular | −0.84 (−1.96, 0.04)* | <0.001 | −1.85 (−3.34, −0.81) | <0.001 |
See the Materials and Methods section for details of scanning sites, p values from signed rank tests are reported for difference from zero. Rank sum p value (*p < 0.001) indicates that men <50 and ≥50 yr of age are significantly different.
Table 3.
Median (Interquartile Range) for Rates of Change (%/yr) at Various Scanning Sites by Decades as a Function of Age and Sex
| Age (yr) | N |
Distal radius |
Distal tibia |
N | Lumbar spine (trabecular) | ||
| Trabecular | Cortical | Trabecular | Cortical | ||||
| Women | |||||||
| 20–29 | 15 | −1.01 (−2.43, −0.16)* | 0.16 (−0.05, 0.55) | −0.76 (−1.77, −0.05)† | 0.25 (0.05, 0.45)* | 9 | −1.75 (−2.28, −1.42)† |
| 30–39 | 33 | −0.14 (−0.57, 0.15)† | 0.06 (−0.13, 0.27) | −0.52 (−1.01, 0.03)† | −0.02 (−0.18, 0.21) | 28 | −1.16 (−2.46, −0.03)* |
| 40–49 | 49 | −0.34 (−0.65, 0.00)* | −0.08 (−0.26, 0.06)* | −0.08 (−0.41, 0.26) | 0.02 (−0.20, 0.11) | 49 | −1.60 (−2.14, −1.03)* |
| 50–59 | 46 | −0.99 (−1.35, −0.45)* | −0.42 (−0.80, −0.11)* | −0.11 (−0.50, 0.16) | −0.41 (−0.66, −0.09)* | 43 | −2.97 (−4.58, −2.00)* |
| 60–69 | 34 | −0.65 (−1.26, −0.40)* | −0.58 (−1.15, −0.22)* | −0.15 (−0.75, 0.13)† | −0.31 (−0.71, −0.21)* | 30 | −2.50 (−3.44, −1.79)* |
| 70–79 | 31 | −0.37 (−0.81, −0.03)† | −0.25 (−0.66, −0.01)* | −0.30 (−0.71, −0.00)* | −0.32 (−0.58, −0.12)* | 30 | −2.20 (−3.79, −1.17)* |
| 80+ | 35 | −0.35 (−1.47, 0.15)† | −0.57 (−1.59, −0.18)* | −0.76 (−1.96, −0.08)* | −0.50 (−1.38, −0.04)* | 24 | −2.64 (−3.98, −1.55)* |
| Men | |||||||
| 20–29 | 8 | 0.18 (−0.23, 0.35) | −0.09 (−0.37, 0.21) | −0.02 (−0.26, 0.31) | 0.04 (−0.14, 0.18) | 6 | −0.98 (−2.13, 1.72) |
| 30–39 | 50 | −0.25 (−0.72, −0.05)* | −0.03 (−0.12, 0.12) | −0.68 (−1.48, −0.10)* | −0.05 (−0.23, 0.10) | 39 | −0.79 (−1.96, 0.31)* |
| 40–49 | 50 | −0.46 (−1.27, −0.23)* | −0.12 (−0.36, 0.01)* | −0.34 (−0.82, −0.12)* | −0.18 (−0.31, 0.02)* | 41 | −0.89 (−1.95, −0.43)* |
| 50–59 | 55 | −0.38 (−0.84, −0.30)* | −0.22 (−0.40, −0.04)* | −0.25 (−0.49, −0.03)* | −0.17 (−0.39, −0.01)* | 50 | −1.72 (−2.82, −0.35)* |
| 60–69 | 46 | −0.47 (−0.92, 0.00)* | −0.23 (−0.40, −0.07)* | −0.22 (−0.39, 0.00)* | −0.10 (−0.31, 0.07)* | 42 | −1.78 (−3.26, −0.75)* |
| 70–79 | 51 | −0.32 (−0.57, −0.04)* | −0.26 (−0.53, −0.00)* | −0.13 (−0.35, 0.03)* | −0.03 (−0.24, 0.14) | 47 | −1.77 (−3.97, −1.03)* |
| 80+ | 49 | −0.51 (−0.93, −0.19)* | −0.59 (−0.98, −0.35)* | −0.14 (−0.38, 0.00)* | −0.39 (−0.86, −0.02)* | 35 | −2.46 (−5.35, −1.67)* |
N, number of subjects in each sex- and age-specific decade.
For significance of difference from zero: *p < 0.005 and †p < 0.05.
In premenopausal women, there was highly significant trabecular bone loss at all three scanning sites. However, the rate of loss was 4- to 6-fold higher at the LS than at the peripheral sites. For postmenopausal women, rates of trabecular bone loss were similar to that of premenopausal women at the peripheral scanning sites but were ∼60% higher at LS. There was no significant cortical bone loss in the premenopausal women at the DR or DT site. Cortical bone loss was substantially higher in postmenopausal women at both the DR and DT.
Men also had highly significant rates of trabecular bone loss before age 50 yr. These rates of loss were similar to or larger than those in men ≥50 yr at the peripheral scanning sites, but at the LS, the rates of loss were >2-fold greater in the older men. For cortical bone, men had low, but significant, rates of bone loss before age 50. The cortical bone loss increased 2- to 4-fold after age 50, and this increase began in the fifth decade, especially at the DT. The amount of trabecular bone loss before 50 yr of age (mean of the three skeletal sites) accounted for ∼37% of lifetime bone loss in women and for ∼42% of it in men. In contrast, the amount of cortical bone lost before 50 yr of age represented only 6% in women and 15% in men.
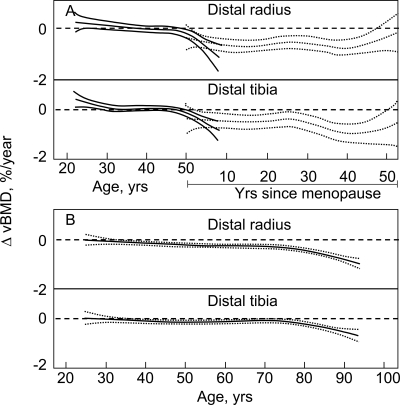
The patterns of bone loss are shown in Figs. 2–4, in which sex- and age-specific rates of change are plotted as smoothing splines with 95% CIs. Figure 2 plots the age-specific changes for cortical bone at the DR and DT scanning sites in women (Fig. 2A) and in men (Fig. 2B). In postmenopausal women, they are plotted as a function of years after menopause, although the pattern was very similar when plotted as a function of age (data not shown). In premenopausal women, there was minimal cortical bone loss at either the DR or DT scanning sites until the perimenopausal interval as indicated by the inclusion of zero in the 95% CIs over that range (Fig. 2A) and by the lack of significance until the fifth decade in the decade-by-decade comparison (Table 3). An exception was the moderate cortical bone gain in the third decade at the DT scanning site. Statistically significant bone loss occurred thereafter, with a relatively constant subsequent rate of loss. In young adult men, there was a low, but significant, rate of cortical bone loss before age. This loss continued at a relatively constant rate until ∼75 yr of age, after which the cortical bone loss underwent a progressive acceleration (Fig. 2B; Table 3).
FIG. 2.
Age-specific changes in vBMD at cortical scanning sites at DR and DT in (A) women and (B) men. Data are shown with a smoothing spline and the 95% CI. Premenopausal women (solid lines) are plotted against age in years, whereas postmenopausal women (broken lines) are plotted against years since menopause. Flaring of confidence limits at beginning and end of regression plots is a statistical artifact caused by smaller numbers of subjects at these ages.
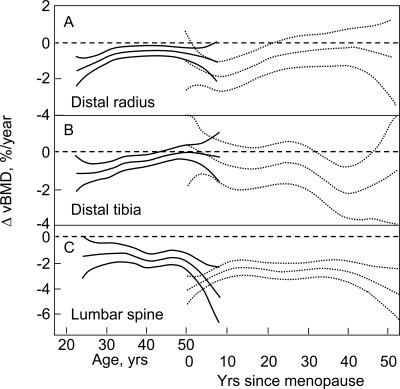
FIG. 4.
Age-specific changes in vBMD at trabecular scanning sites in men at the (A) DR, (B) DT, and (C) LS. Data are shown with a smoothing spline and the 95% CI.
Figure 3 plots the corresponding changes for trabecular vBMD with age and menopausal status at the DR, DT, and LS in women, and Fig. 4 plots these changes in men. In women, trabecular bone loss appeared to begin in the third decade and was continuous over life, with an apparent acceleration during the perimenopausal interval, especially at the LS. Of interest, at the DR and DT, but not at the LS, the rate of trabecular bone loss was maximal in the third decade and declined until midlife, when it again accelerated. In elderly women, the trabecular bone loss waned at the DR and DT, but not at the LS, where the rate of trabecular bone loss remained relatively constant until late in life when there was a suggestion of acceleration. Before 50 yr of age, in men, but not in women, trabecular bone loss at the DR and DT accelerated and then decelerated (spooning), with a maximal loss rate occurring around 35–40 yr of age. After 50 yr of age in men, significant trabecular bone loss continued at both sites but at a relatively low rate. In contrast, the rate of trabecular bone loss in men at LS was apparent in young adulthood, and the rate increased progressively over life, with a further acceleration after 70 yr of age.
FIG. 3.
Age-specific changes in vBMD at trabecular scanning sites in women at the (A) DR, (B) DT, and (C) LS. Data are shown with a smoothing spline and the 95% CI. Premenopausal women (solid lines) are plotted against age in years, whereas postmenopausal women (broken lines) are plotted against years since menopause.
Table 4 gives the correlations (unadjusted and age-adjusted) between rates of change in vBMD and serum levels of biologically active sex steroids, FSH, IGF-I, IGF-II, IGFBP-3, PINP, and CTX in premenopausal and postmenopausal women. Table 5 gives analogous values for adult men, before and after 50 yr of age. Positive correlation coefficients indicate that higher rates of bone loss (i.e., more negative rate of change in vBMD) correspond with lower values for the independent variable. Conversely, negative correlation coefficients indicate that higher rates of bone loss correspond with higher values for the independent variable. Thus, in postmenopausal women, the correlation coefficient for decreased Bio-E2 and increased bone loss would have a positive sign and that for increased serum levels of biomarkers for bone turnover and increased bone loss would have a negative sign. For premenopausal women, there were no significant correlations with sex steroid levels, although serum FSH correlated negatively with trabecular bone loss at the LS. However, there were positive correlations between serum IGF-I, IGFBP3, and the IGF-I/IGFBP3 ratio and cortical bone loss at the DT and with serum IGF-I and cortical bone loss at the DR. There were also significant or near significant correlations of these IGF-related indices and trabecular bone loss at the DT, but interestingly, these were negative correlations. For postmenopausal women, there were multiple significant correlations of Bio-E, Bio-T, and FSH, with both cortical and trabecular bone loss at all scanning sites. There was also a negative correlation between biochemical markers for bone turnover and rates of bone loss in the postmenopausal women and a few such correlations in the premenopausal women.
Table 4.
Spearman Correlations (Unadjusted/Age–Adjusted) in Women Between Rates of Change of Cortical and Trabecular vBMD (%/yr) at Selected Scanning Sites and Serum Levels of Hormones and Bone Turnover Markers
| Women | N |
Site |
|||||
|
Distal radius |
Distal tibia |
Lumbar spine (trabecular) | |||||
| Trabecular | Cortical | Trabecular | Cortical | ||||
| PreM | Bio-E2* | 59 | 0.07/0.13 | −0.04/−0.01 | 0.06/0.02 | −0.04/0.06 | 0.14/0.13 |
| Bio-T | 102 | 0.16/0.16 | −0.04/−0.02 | 0.13/0.13 | 0.07/0.08 | 0.18/0.18 | |
| FSH* | 59 | −0.24/−0.14 | −0.11/−0.04 | 0.06/−0.05 | −0.25/−0.04 | −0.31†/−0.22 | |
| IGF-I | 102 | −0.14/−0.18 | 0.21†/0.01 | −0.23†/−0.06 | 0.32‡/0.13 | 0.19/0.09 | |
| IGFBP-3 | 102 | −0.07/−0.08 | 0.18/0.04 | −0.19/−0.07 | 0.28‡/0.13 | 0.09/−0.01 | |
| IGF-I/IGFBP-3 ratio | 102 | −0.14/−0.17 | 0.17/−0.01 | −0.21†/−0.06 | 0.25†/0.07 | 0.16/0.07 | |
| IGF-II | 102 | 0.06/0.06 | −0.02/−0.05 | 0.06/0.09 | 0.05/0.02 | 0.08/0.03 | |
| CTX (ng/ml) | 103 | −0.20†/−0.21† | 0.07/−0.05 | −0.07/0.04 | 0.16/0.05 | −0.06/−0.12 | |
| P1NP (μg/liter) | 103 | −0.16/−0.17 | 0.08/−0.01 | −0.09/−0.01 | 0.20†/0.11 | 0.03/−0.00 | |
| PostM | Bio-E2 | 137 | 0.24‡/0.36§ | 0.20†/0.21† | 0.45§/0.36§ | 0.29§/0.28‡ | 0.03/0.09 |
| Bio-T | 139 | 0.14/0.18† | 0.14/0.13 | 0.36§/0.32§ | 0.16/0.15 | 0.11/0.12 | |
| FSH | 133 | −0.25‡/−0.31§ | −0.24‡/−0.23‡ | −0.20†/−0.15 | −0.15/−0.14 | −0.21†/−0.23† | |
| IGF-I | 141 | −0.16/−0.11 | 0.10/0.09 | 0.18†/0.11 | 0.08/0.06 | −0.15/−0.13 | |
| IGFBP-3 | 141 | −0.12/−0.09 | 0.05/0.04 | 0.20†/0.15 | 0.15/0.14 | −0.14/−0.13 | |
| IGF-I/IGFBP-3 ratio | 141 | −0.12/−0.08 | 0.10/0.09 | 0.10/0.04 | 0.04/0.02 | −0.14/−0.12 | |
| IGF-II | 141 | −0.06/−0.04 | −0.06/−0.06 | 0.09/0.06 | −0.04/−0.05 | −0.19†/−0.18 | |
| CTX (ng/ml) | 141 | −0.26‡/−0.27‡ | −0.20†/−0.20† | −0.24‡/−0.25‡ | −0.07/−0.07 | −0.08/−0.08 | |
| P1NP (μg/liter) | 141 | −0.34§/−0.31§ | −0.08/−0.09 | −0.13/−0.21† | −0.01/−0.03 | 0.00/0.03 | |
N, number of subjects with data for each serum level of reproductive hormones or other variables.
Women in the sample are divided by menopausal status: PreM, premenopausal; PostM, postmenopausal.
* Premenopausal women on contraceptive hormones did not have Bio-E2 or FSH measured.
For significance of correlation: † p < 0.05; ‡ p < 0.01; and § p < 0.001.
Table 5.
Spearman Correlations (Unadjusted/Age–Adjusted) in Men Between Rates of Change of Cortical (cort) and Trabecular (trab) vBMD (%/yr) at Selected Scanning Sites and Serum Levels of Hormones and Bone Turnover Markers
| Men | N |
Site |
|||||
|
Distal radius |
Distal tibia |
Lumbar spine (trabecular) | |||||
| Trabecular | Cortical | Trabecular | Cortical | ||||
| 20–49 yr | Bio-E2 | 108 | 0.14/0.06 | 0.17/0.11 | 0.09/0.10 | 0.34*/0.32* | 0.04/0.01 |
| Bio-T | 108 | 0.04/−0.09 | 0.07/−0.03 | 0.00/0.01 | 0.39*/0.37* | 0.05/0.02 | |
| FSH | 108 | −0.07/0.02 | −0.08/−0.01 | −0.06/−0.07 | −0.03/0.00 | −0.07/−0.05 | |
| IGF-I | 108 | 0.11/0.04 | 0.11/0.06 | −0.05/−0.04 | 0.24†/0.22† | −0.13/−0.15 | |
| IGFBP-3 | 108 | 0.21†/0.14 | 0.07/0.00 | 0.05/0.05 | 0.17/0.14 | 0.05/0.03 | |
| IGF-I/IGFBP-3 ratio | 108 | 0.01/−0.02 | 0.08/0.07 | −0.08/−0.08 | 0.18/0.17 | −0.17/−0.17 | |
| IGF-II | 108 | −0.13/−0.16 | −0.02/−0.03 | −0.07/−0.06 | 0.14/0.13 | 0.02/0.02 | |
| CTX (ng/ml) | 108 | −0.01/−0.06 | −0.00/−0.04 | −0.13/−0.13 | 0.19†/0.17 | −0.06/−0.08 | |
| P1NP (μg/liter) | 108 | 0.11/0.04 | −0.04/−0.10 | −0.04/−0.04 | 0.33*/0.31‡ | 0.11/0.09 | |
| 50+ yr | Bio-E2 | 199 | −0.01/0.00 | 0.23‡/0.10 | 0.05/0.11 | 0.06/0.03 | 0.28*/0.19† |
| Bio-T | 199 | −0.08/−0.09 | 0.33*/0.19‡ | −0.11/−0.05 | 0.10/0.07 | 0.15/−0.00 | |
| FSH | 199 | −0.01/−0.02 | −0.21‡/−0.11 | −0.07−0.11 | −0.07/−0.05 | −0.13/−0.04 | |
| IGF-I | 201 | −0.06/−0.06 | 0.11/−0.02 | −0.10/−0.06 | −0.05/−0.08 | 0.11/0.01 | |
| IGFBP-3 | 201 | −0.07/−0.08 | 0.15†/0.02 | −0.02/0.03 | 0.02/−0.02 | 0.13/0.02 | |
| IGF-I/IGFBP-3 ratio | 201 | −0.04/−0.04 | 0.04/−0.04 | −0.13/−0.11 | −0.08/−0.10 | 0.01/−0.05 | |
| IGF-II | 201 | −0.03/−0.03 | 0.02/−0.06 | −0.04/−0.01 | −0.05/−0.07 | 0.21‡/0.16† | |
| CTX (ng/ml) | 201 | −0.19‡/−0.19‡ | −0.14†/−0.16† | −0.16†/−0.15† | −0.12/−0.12 | −0.10/−0.12 | |
| P1NP (μg/liter) | 200 | −0.16†/−0.16† | −0.14/−0.20‡ | −0.11/−0.10 | −0.08/−0.10 | −0.09/−0.13 | |
N, number of subjects with data for each serum level of reproductive hormones or other variables.
For significance of correlation: * p < 0.001; † p < 0.05; and ‡ p < 0.01.
For men <50 yr of age, however, there were several positive correlations between biologically active sex steroids, IGF-I and bone turnover markers, and rate of change in cortical bone at the DT, but no correlations with rate of change in trabecular bone at this site. Other than this, there was only one low level correlation that reached significance in the young adult men. In the men who were ≥50 yr of age, there were significant positive correlations between biologically active sex steroids and cortical bone loss at DR and with trabecular bone loss at LS. Bone turnover markers correlated negatively with cortical and trabecular bone loss at DR and with trabecular loss at DT. Other significant correlations were a negative one between FSH and cortical bone loss at DR and a positive one between IGF-II and trabecular bone loss at LS. For both sexes, most correlations of variables with skeletal rates of change remained significant after adjusting for age.
DISCUSSION
The prospective design of our study and the high precision of the instrumentation used in it have allowed us to establish the age of onset and relative rates of cortical and trabecular bone loss in adult women and men with considerable reliability. In women, we found that substantial cortical bone loss did not begin until midlife in association with menopause, consistent with current belief on the key role of estrogen deficiency.(3) Our finding of significant correlations between cortical bone loss and serum levels of biologically active sex steroids in aging women suggests that sex steroid deficiency plays an important role in cortical bone loss well into the latter decades of life, again consistent with current belief.(17,18)
The pattern of bone loss in men differed from that in women. A small amount of cortical bone loss began in young adulthood and continued at a relatively constant rate until accelerating late in life. Several population-based studies using DXA have showed that the decrease of BMD in elderly men is associated with decreases in sex steroids, especially Bio-E,(16,19,20) and Khosla et al.(21,22) further showed that more and more elderly men in the seventh and eighth decade will have in serum Bio-E2 levels that fall below the threshold that accelerates bone loss. This is consistent with our finding of an acceleration of cortical bone loss around 75 yr of age and of significant correlations with serum Bio-E and Bio-T levels at the DR, although not at the DT in older men. In the young adult men, the inverse correlations between cortical bone loss at the DT with sex steroids and with IGF-I are anomalous and could be related to remodeling at this weight-bearing site.
The most important finding in our study was the demonstration that trabecular bone loss begins in young adulthood in both sexes and continues unabated throughout life. In women, the apparent attenuation of trabecular bone loss at the DR scanning site late in life may be caused by depletion of trabecular bone there. In premenopausal women, trabecular bone loss was most rapid in the third decade at the DR and DT, but not at the LS, scanning sites. The reason for this pattern is unclear, although it could be related a redistribution of trabecular bone as part of the completion of pubertal growth pattern at the end of the long bones. The acceleration of trabecular bone loss at all three scanning sites in the perimenopause is consistent with the well-accepted causal role of estrogen deficiency in inducing trabecular bone loss.(23)
Young adult men also underwent early trabecular bone loss, beginning at least as early as the fourth decade. Of interest, there was an acceleration of the trabecular bone loss at the LS around 65 yr of age, when serum levels of bioavailable sex steroid levels in this cohort begin to decrease.(22) We also observed a “spooning” pattern of transient acceleration of trabecular bone loss in young adult men at the DR and DT sites. This finding is consistent with the previous observation by Khosla et al.,(24) who used high-resolution peripheral QCT (HR-pQCT) to show changes in trabecular microarchitecture in young adult men but not women. These investigators further suggested that thick trabeculae were being converted into more numerous thin trabeculae and found that this process was associated with declining serum IGF-I levels.
The significant correlations between Bio-E and Bio-T and the rates of trabecular bone loss in the postmenopausal women are consistent with previous studies showing a causal role for sex steroid deficiency.(23) The failure to show these correlations in elderly men was probably the result of their lesser degree of sex steroid deficiency. In the postmenopausal women, serum FSH correlated inversely with rates of trabecular bone loss at all three scanning sites and with cortical bone loss at DR. In a 4-yr longitudinal transmenopausal study, serum FSH correlated inversely with the LS and hip BMD as assessed by DXA.(25) Moreover, recent studies in rodents suggest that FSH may directly increase osteoclastic activity and bone loss independently of estrogen deficiency,(26) although this was not confirmed in another study.(27) Thus, whether the correlations between serum FSH and bone loss that we have observed reflect only the integral effects of decreased serum sex steroids, a direct role of serum FSH in the causation of the bone loss, or both, requires further study.
Whereas sex steroid deficiency is strongly implicated in the causation of cortical and trabecular bone loss in older women and men, the mechanism(s) responsible for the trabecular bone loss in young adult women and men is unclear. It is not caused by sex steroid deficiency such as occurs in postmenopausal bone loss and in some aging men because Bio-E2 and Bio-T remained at “normal” values until midlife as assessed by the highly accurate method of liquid chromatography-tandem mass spectrometry and failed to correlate with the trabecular bone loss.
A potential causal factor is a decrease in one or more of the components of the IGF-regulatory system. During puberty in both sexes, the IGF regulatory system and sex steroids act coordinately to facilitate attainment of peak bone mass, and in young adults, they act tonically to maintain bone mass.(3,28,29) In this study, we found that between the ages of 20 and 50 yr, serum IGF-I decreased by 56% in women and by 24% in men, whereas serum IGFBP3, a surrogate for integral growth hormone (GH) secretion,(30) decreased by 10% in women and by 15% in men. Serum levels of IGF-II, another component of the IGF regulatory system, did not change significantly during young adulthood. Moreover, we found several significant correlations between the rate of skeletal changes at the DT and the circulating components of the IGF regulatory system in the premenopausal women. Interestingly, however, the correlation coefficients were positive for changes in cortical bone but were negative for changes in trabecular bone. This may relate to the rate of trabecular bone loss being highest in the third decade in the premenopausal women and decreasing progressively until the perimenopause. There was also a trend for similar findings at the DR, although only the correlation between IGF-I and changes in cortical bone was significant. In young adult men, the only significant correlations were positive ones between cortical bone changes at DT and serum IGF-I and between trabecular bone changes at DR and IGFBP-3. Although the coefficients for all of these correlations ranged only between 0.21 and 0.32, this may reflect the technical difficulties in assessing true rates of change in vBMD and the 24-h integrated levels of the hormones. Finally, we cannot exclude the possibility that decreases in paracrine production of IGF-I or IGF-II by osteoblasts(30–33) could play a contributing role in the early trabecular bone loss.
Our study has several limitations. First, the measurement of rates of loss at the LS is less reliable than those at the peripheral measurement sites. This was because of the poorer precision of central CT measurements, to having only baseline and 3-yr rather than annual measurements, and to the necessity of crossing-over the majority of subjects in the cohort to a second CT scanner for the 3-yr scans. In contrast, the peripheral QCT measurements were highly precise and were made annually on a single instrument. Thus, because the early trabecular bone loss in the young women and men was clearly detectable at both of the peripheral scanning sites, rather than just at the LS scanning site, this does not seem to be a scanning artifact. Second, single energy QCT is affected by changes in the ratio of red to yellow marrow.(34) However, it seems unlikely that there would be significant change in marrow content over a 3-yr time interval. Moreover, this would not be an issue for the DR and DT measurement sites because bone marrow is yellow there from young adulthood onward,(35) and these sites clearly showed early trabecular bone loss. Finally, although we used state-of-the-art measurements including liquid chromatography-tandem mass spectrometry mass spectrometry for sex steroid measurements, serum hormone levels are subject to wide physiological changes and can be affected by multiple variables. Thus, we found the correlation coefficients with circulating hormones to be relatively low, even for sex steroids and bone loss in the postmenopausal women.
In summary, the preservation of cortical bone until midlife in women and until even later in men is consistent with current paradigms on the role of sex steroid deficiency and perhaps of other age-related factors in causing age-related bone loss. However, our longitudinal, population-based studies provide strong evidence for substantial trabecular bone loss in both sexes during young adult life. This early bone loss accounts for between one third and one half of total trabecular bone loss over life in both sexes. Indeed, if the process responsible for the bone loss in young adults continues beyond midlife, which seems possible, it would account for even larger proportions. Either way, it undoubtedly makes a major contribution to the pathogenesis of fragility fractures in elderly women and men. Moreover, the onset of trabecular bone loss soon after the completion of pubertal growth in both sexes highlights a major gap in the current conceptual framework for the pathogenesis of age-related osteoporosis. Identifying the endogenous or exogenous factors that may accelerate or ameliorate this seemingly inexorable loss of trabecular bone in young adulthood could lead to the development of prevention measures in young adults that result in reduced occurrence of fragility fractures late in life.
ACKNOWLEDGMENTS
The authors thank Margaret Holets for making the pQCT measurements; Lisa McDaniel, RN, and Louise McCready, RN, for assistance in recruitment and management of the study subjects; James Peterson for assistance with graphics; and Sara J Achenbach for help with the statistical analysis and data management. We also thank Ravinder Singh, PhD, for developing the tandem mass spectroscopy method used in this study for measuring sex steroid levels. This work was supported by NIH Grants R01AR027065 and 1UL1RR024150.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD, USA: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004. The frequency of bone disease; pp. 68–87. [Google Scholar]
- 2.Raisz LG. Local and systemic factors in the pathogenesis of osteoporosis. N Engl J Med. 1988;318:818–828. doi: 10.1056/NEJM198803313181305. [DOI] [PubMed] [Google Scholar]
- 3.Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 4.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 5.Blake GM, Wahner HW, Fogelman I, editors. 2nd ed. London, UK: Martin Dunitz; 1999. The evaluation of osteoporosis dual energy X-ray absorptiometry and ultrasound in clinical practice. [Google Scholar]
- 6.Riggs BL, Wahner HW, Melton LJ, III, Richelson LS, Judd HL, Offord KP. Rates of bone loss in the appendicular and axial skeletons of women: Evidence of substantial vertebral bone loss before menopause. J Clin Invest. 1986;77:1487–1491. doi: 10.1172/JCI112462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slemenda C, Longcope C, Peacock M, Hui S, Johnston CC. Sex steroids, bone mass and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest. 1996;97:14–21. doi: 10.1172/JCI118382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eastell R, Mosekilde L, Hodgson SF, Riggs BL. Proportion of human vertebral body bone that is cancellous. J Bone Miner Res. 1990;5:1237–1241. doi: 10.1002/jbmr.5650051208. [DOI] [PubMed] [Google Scholar]
- 9.Nordstrom P, Neovius M, Nordstrom A. Early and rapid bone mineral density loss of the proximal femur in men. J Clin Endocrinol Metab. 2007;92:1902–1908. doi: 10.1210/jc.2006-2613. [DOI] [PubMed] [Google Scholar]
- 10.Aaron JE, Makins NB, Sagreiya K. The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop. 1987;215:260–271. [PubMed] [Google Scholar]
- 11.Riggs BL, Melton LJI, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 12.Kalender WA, Felsenberg D, Louis O, Lopez P, Klotz E, Osteaux M, Fraga J. Reference values for trabecular and cortical vertebral bone density in single and dual-energy quantitative computed tomography. Eur J Radiol. 1989;9:75–80. [PubMed] [Google Scholar]
- 13.Meier DE, Orwoll ES, Jones JM. Marked disparity between trabecular and cortical bone loss with age in healthy men. Ann Intern Med. 1984;101:605–612. doi: 10.7326/0003-4819-101-5-605. [DOI] [PubMed] [Google Scholar]
- 14.Ruegsegger P, Durand EP, Dambacher MA. Differential effects of aging and disease on trabecular and compact bone density of the radius. Bone. 1991;12:99–105. doi: 10.1016/8756-3282(91)90007-6. [DOI] [PubMed] [Google Scholar]
- 15.Dambacher MA, Neff M, Kissling R, Qin L. Highly precise peripheral quantitative computed tomography for the evaluation of bone density, loss of bone density and structures. Drugs Aging. 1998;12(Suppl 1):15–24. doi: 10.2165/00002512-199812001-00003. [DOI] [PubMed] [Google Scholar]
- 16.Khosla S, Melton LJI, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B. Endogenous hormones and the risk of hip and vertebral fractures among older women. N Engl J Med. 1998;339:733–738. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 18.Riggs BL, Khosla S, Melton LJI. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 19.Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston C. Sex steroids and bone mass in older men: Positive associations with serum estrogens and negative associations with androgens. J Clin Invest. 1997;100:1755–1759. doi: 10.1172/JCI119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: The Rancho Bernardo study. J Bone Miner Res. 1997;12:1833–1843. doi: 10.1359/jbmr.1997.12.11.1833. [DOI] [PubMed] [Google Scholar]
- 21.Khosla S, Melton LJ, Atkinson EJ, O'Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 22.Khosla S, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, Oberg AL, Rouleau PA, Riggs BL. Relationship of volumetric BMD and structural parameters at different skeletal sites to sex steroid levels in men. J Bone Miner Res. 2005;20:730–740. doi: 10.1359/JBMR.041228. [DOI] [PubMed] [Google Scholar]
- 23.Genant HK, Cann CE, Ettinger B, Gordan GS. Quantitative computed tomography of vertebral spongiosa: A sensitive method for detecting early bone loss after oophorectomy. Ann Intern Med. 1982;97:699–705. doi: 10.7326/0003-4819-97-5-699. [DOI] [PubMed] [Google Scholar]
- 24.Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ., III Effects of sex and age on bone microstructure at the ultradistal radius: A population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21:124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sowers M-FR, Jannausch M, McConnell D, Little RD, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 26.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu L-L, Yaroslavskiy BB, Zhou H, Zallone AZ, Sairam MR, Kumar TR, Bo W, Braun JJ, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Tiwari-Pandey R, Samadfam R, Yang Y, Miao D, Karaplis AC, Sairam MR, Goltzman D. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone (FSH) Endocrinology. 2007;148:2613–2621. doi: 10.1210/en.2006-1404. [DOI] [PubMed] [Google Scholar]
- 28.Ohlsson C, Bengtsson B-A, Isaksson OGP, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 29.Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. (Review) Endocr Rev. 2006;27:101–140. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- 30.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: Biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 31.Mohan S, Jennings JC, Linkhart TA, Baylink DJ. Primary structure of human skeletal growth factor: Homology with human insulin-like growth factor-II. Biochim Biophys Acta. 1988;966:44–55. doi: 10.1016/0304-4165(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 32.Kanzaki S, Baxter R, Knutsen R, Baylink D, Mohan S. Evidence that human bone cells in culture secrete insulin-like growth factor (IGF)-II and IGF binding protein-3 but not acid-labile subunit both under basal and regulated conditions. J Bone Miner Res. 1995;10:854–858. doi: 10.1002/jbmr.5650100605. [DOI] [PubMed] [Google Scholar]
- 33.Kassem M, Okazaki R, Harris SA, Spelsberg TC, Conover CA, Riggs BL. Estrogen effects on insulin-like growth factor gene expression in a human osteoblastic cell line with high levels of estrogen receptor. Calcif Tissue Int. 1998;62:60–66. doi: 10.1007/s002239900395. [DOI] [PubMed] [Google Scholar]
- 34.Genant HK, Boyd D. Quantitative bone mineral analysis using dual energy computed tomography. Invest Radiol. 1977;12:545–551. doi: 10.1097/00004424-197711000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Kricun ME. Red-yellow marrow conversion: Its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14:10–19. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]