Abstract
Fucosylated oligosaccharides and glycoconjugates have been implicated in several biological events, including the cell-cell adhesion processes which mediate inflammation. Alpha-L-fucosidase (ALF) is an exoglycosidase which is involved in the hydrolytic degradation of α-L-fucose from glycoconjugates. In this study we investigated the potential role of ALF in regulation of leukocyte migration. Measurement of trans-endothelial migration in response to CCL5 demonstrated that pre-treatment of monocytic cells with ALF reduced migration (p=0.0004) to a greater extent than treatment of the endothelial monolayer (p=0.0374). Treatment with ALF significantly reduced the adhesion of monocytic cells to immobilized P-selectin.Fc. A murine model of experimental autoimmune uveitis was then used to show that treatment of splenic cells with ALF produced an 8.6-fold decrease in rolling and a 3.2-fold decrease in cell migration across the retinal vasculature. Further in vitro studies demonstrated that treatment of monocytes with the chemokines CCL3 or CCL5 increased the level of mRNA encoding ALF; this was accompanied by the detection of significant increases in both the 51kDa and 56kDa components of ALF by Western blotting. Treatment of monocytic cells with ALF for 2h significantly reduced the cell-surface expression of CD31, with a further decrease in expression observed after 5h (p=0.002). Thus, CD31 and fucosylated ligands of P-selectin seem to be the candidates through which ALF mediates its effect in vitro. These data identify a previously unrecognized immunoregulatory role for ALF in late stages of inflammation.
Keywords: Chemokine, α-L-Fucosidase, extravastion, gene regulation, inflammation
Introduction
Fucose can be linked through α(1,3), α(1,4) and α(1,6) bonds to N-acetylglucosamine and through α(1,2) to galactose in a range of glycoproteins and glycolipids. Fucosylated oligosaccharides and glycoconjugates play roles in several biological events including the cell-to-cell adhesion processes which mediate inflammation, embryonic development, tumorigenesis/metastasis and antigen recognition. The important role played by fucose residues has led to an increasing interest in α-L-Fucosidase (α-L-Fucoside fucohydrolase; ALF; EC3.2.1.51), an exoglycosidase involved in the hydrolytic degradation of fucose-containing components of glycoproteins 1, glycolipids and oligosaccharides 2.
Around 10-20% of total ALF activity occurs on the surface of a range of human cell types, including haemopoietic, epithelial and mesenchymal cells 1. Mammalian α-L-fucosidases are multimeric, containing glycoprotein subunits of about 53kDa; they have a maximal activity between pH4 and 6.5. At least five major isoforms of the ALF enzyme have been identified 3. There is a considerable degree of structural heterogeneity, both tissue-specific and within tissues. This has been attributed to variations in the sialic acid content as well as the two different alleles and polymorphisms within the FUC1 gene. The serum ALF activity in normal individuals is reported to be 381U/ml ±10.86 4.
The ALF enzyme is associated with a variety of biological functions. The significance of this enzyme in human catabolism is implied by the genetic neurovisceral storage disease, fucosidosis, which results from an absence or gross deficiency of ALF; this allows accumulation of fucoglycoconjugates which result in mental and motor retardation 5,6. A syndrome previously known as leukocyte adhesion deficiency II is now recognised as a generalized metabolic disease caused by severe hypofucosylation of glycoconjugates including selectin ligands 7. Further to this, alterations in levels of ALF have been reported in the plasma and/or serum of patients with endometrial, hepatocellular and colorectal cancer 8,4,9.
Several molecules involved in leukocyte transmigration, including selectin ligands, proteoglycans, integrins and CD31, are glycosylated and contain fucosylated moieties. L-selectin dependent interactions mediate lymphocyte tethering and rolling prior to chemokine-dependent cell activation and transmigration across high endothelial venules (HEV). On lymphocytes, L-selectin is a carbohydrate-binding protein that binds to ligands on HEV; the function of these ligands is dependent on the expression of carbohydrate 6-sulfo sialyl Lewis X (sialyl-(2-3)-galactopyranosyl-(1-4)-Nacetyl glucosamine-((1-3)-fucopyranosyl) groups termed sLeX or CD15s. The pivotal function of fucosylation of L-selectin ligands has been established by genetic studies in mice. Mice lacking fucosyltransferase VII (FucT-VII) or both FucT-VII and FucT-IV show a reduction of 80% or more than 95% respectively in lymphocyte homing to lymph nodes 10.
P and E selectins are expressed on activated endothelial cells and cooperatively mediate leukocyte rolling. They are membrane bound C-type lectins that bind to cell-surface glycoconjugate ligands. P-selectin glycoprotein ligand-1 (PSGL-1), a mucin expressed on leukocytes, is the best characterized selectin ligand. The receptor function of PSGL-1 depends on two post-translational events. One consists of the generation of O-linked glycan and includes α(1,3) fucosylation and α(2,3)sialylation (such as sLewX). The other involves sulphation of tyrosine residues. In vivo, PSGL-1 mediates leukocytes tethering to and rolling on P-selectin and supports tethering to E-selectin11,12. Fucosylated proteoglycans also play a key role in CD11d/CD18 mediated adhesive interactions and the migration of polymorphonuclear leukocytes across the intestinal epithelium 13. Additionally, systemic treatment with fucoidin, a sulphated polysaccharide containing L-fucose and L-fucose-4-sulphate, interferes with selectin-receptor function and inhibits leukocyte rolling 14-16.
It is well established that the responsiveness of migratory inflammatory cells to chemokines is governed by the expression of chemokine receptors but the processes involved in the resolution of the inflammatory response are poorly defined. It is believed that chemokines are normally presented to intravascular leukocytes in the form of complexes bound to endothelial cell-surface glycosaminoglycans (GAG). Significantly it has been shown that the concentration of ALF and soluble GAGs increases in Grave’s disease 17, suggesting the potential for cleavage of pro-inflammatory chemokine complexes and selectin ligands from the endothelial cell surface.
Based on these observations we hypothesised that ALF plays an important regulatory role in the later stages of inflammation. A series of experiments was designed to examine the role of ALF in leukocyte migration both in vitro and in vivo. Trans-endothelial chemotaxis assays were used to determine the functional significance of ALF in regulating leukocyte trafficking in vitro. A murine experimental autoimmune uveoretinitis (EAU) model was then used to assess the role of ALF in leukocyte rolling and tissue infiltration in vivo.
Materials and Methods
Cell Culture
THP-1 monocytic cells were supplied by ECACC (Cambs., UK) and expanded in RPMI 1640 medium with 10% FCS, 100U/ml penicillin, 100μg/ml streptomycin and 1μg/ml folic acid (all from Sigma, Dorset, UK). EAhy926, a human aortic endothelial cell hybridoma created by fusion of human umbilical vein endothelial cells with human lung carcinoma derived cell line A549 18 were grown in DMEM medium (Sigma) supplemented with FCS (10%), 2mM glutamine and penicillin and streptomycin as above.
Transendothelial Chemotaxis Assay
EAhy926 cells (5×104) cells were seeded onto 3μM pore Falcon Transwell filters (Fahrenheit UK) and allowed to grow to confluency; the endothelial monolayers were then treated for 1 h at 37°C with either no ALF, an optimal 0.08U/ml ALF (Sigma) or 0.08U/ml of ALF which had been denatured by heating to 100°C for 5 minutes.
THP-1 cells were assayed for their ability to migrate through a 3μM pore diameter filter towards the basal compartment containing either no CCL5 or 100ng/ml CCL5 (Peprotech, London, UK). THP-1 cells were stimulated for 16hrs using 300U/ml IFNγ then 5×105 cells were added to each well and incubated for 2hrs at 37°C with 5% CO2. For some experiments, monocytic THP-1 cells and EAhy926 cells were also treated with 0.08U/ml ALF for 1 hour. Following the removal of excess cells from both the upper and lower chambers, the upper surface of each filter was carefully wiped with a clean swab. The filters were then fixed in 100% cold methanol for 1hr. After fixation the filters were stained with Gill’s haematoxylin (Sigma, Poole, Dorset UK) for 30min, followed by a 5 min wash in Scott’s tap water (166mM MgSO4, 24mM NaHCO3,). Sequential washing and dehydration using increasing concentrations of ethanol was then performed before mounting with DPX mounting media. Migrant cells were counted using high power microscopy (x400) in four random fields on each filter.
Cell surface staining with fucose-specific lectin
THP-1 cells (200,000/ml) were washed twice with PBS containing 5% FCS. Cells were incubated with biotin-conjugated fucose specific lectin from Tetragonolobus purpureas (50 μg/ml; Sigma) in HBSS buffer containing 1mM CaCl2, 1mM MgCl2 and 1% FBS for 1h at 37 C. Cells were subsequently washed twice with HBSS buffer and resuspended in 50 μl HBSS buffer containing Streptavidin-FITC conjugated antibody (BD Pharmingen) for 30 min at 4C. Following washing the stained cells were analyzed by flow cytometry (FACSort; Becton Dickinson).
Cells (100,000/ml) resuspended in RPMI containing 0.1%BSA and 2% HEPES were treated with ALF (0.5 U/ml-0.04U/ml) for different time periods (1-24h). These cells were then stained with the fucose binding lectin as described above. Trypan blue exclusion was assessed to ensure cell viability. Controls included cells stained with secondary antibody only. The stained cells were analyzed by flow cytometry (FACSort; Becton Dickinson); data analysis was performed using Lysis II software (Becton Dickinson).
Solid phase adhesion assays
Flat-profile 96-well ELISA plates (Linbro; MP Biomedicals, Cambridge, UK) were coated with goat anti-human IgG Fc antibody (Sigma) at 1μg/well in PBS at 4°C for 18 h. Excess antibody was removed by washing twice with PBS and human P-selectin.Fc fusion protein (1μg/well; R&D Systems) was added in HBSS buffer (Sigma) for a further 18 h at 4°C. Excess protein was washed twice with HBSS buffer and nonspecific binding was blocked for 2 h at 37°C with HBSS containing 2% FBS.
THP-1 cells (100,000/ml) resuspended in RPMI containing 0.1%BSA and 2% HEPES were treated with ALF (0.08U/ml) (8 h), untreated cells were incubated in the same medium for 8 h. Viability of treated cells was 95%; equal number of viable cells from control and treated group were used in subsequent experiment. THP-1 cells (2×106/ml) were labelled with 2′,7′-bis(2-carboxyethyl)-5-(6)-carboxyfluorescein tetrakis (acetoxymethyl) ester (BCECF-AM; Sigma) at 20μg/ml for 15 min at 37°C. After washing by centrifugation, the cells were resuspended in HBSS, 5×105 cells added to each assay well and the plates centrifuged at 60g for 2 min at 37°C.
Following incubation of the assay plates at 37°C for 1h, the non-adherent cells were removed by mechanical oscillation and gentle washing with warm HBSS and the remaining, adherent T cells were lysed by the addition of 2% Triton X-100 (Sigma). The quantity of released fluorochrome was measured using a plate-format fluorimeter (Fluostar Optima; BMG Labtech, Aylesbury, UK).
In vivo assay of leukocyte extravasation
Induction of EAU
Female B10R.III mice, 12-20 weeks old, (Medical Research Facility, University of Aberdeen) were treated humanely according to the Animals (scientific procedures) Act (UK). EAU was induced with a subcutaneous 50μl injection into each thigh of 25 μg peptide 161-180 (SGIPYIISYLHPGNTILHVD; purity >85%; Sigma Genosys, Cambridge, UK) of the human interphotoreceptor retinoid-binding protein (IRBP) emulsified 1:1 with CFA (CFA, H37Ra; Difco Laboratories, Detroit, MI) 19,20. Animals were observed using a slit-lamp for clinical evaluation (grading) of the eye at day 11 post-immunization.
Leukocyte preparation
A single cell suspension of splenocytes was prepared 11 days after sensitisation for EAU. Erythrocytes were lysed with 0.75% (w/v) NH4Cl in 17mM Tris buffer pH 7.2 and the remaining leukocytes were washed twice in RPMI1640 medium. The cells were then re-suspended in 10ml medium supplemented with 0.1% (w/v) BSA at 1×106 cells/ml and some samples were incubated for at 37°C with 0.08U/ml ALF per 5×105 cells for optimal time. The cells were then pelleted, re-suspended in 5 ml RPMI 1640 plus BSA 0.1% and incubated with 40 μg/ml calcein-AM (C-AM, Molecular Probes Europe BV, Leiden, The Netherlands) at 37 °C for 30 minutes with gentle mixing. Cells were spun down and re-suspended in 150 μl medium
Tracking cells with scanning laser ophthalmoscopy (SLO)
Eleven days after sensitisation mice of equivalent EAU severity were anaesthetized and fitted with contact lenses as described 21. 100 μl of 0.05% (v/v) sodium fluorescein (Sigma-Aldrich Co. Ltd., Poole, UK) was injected via the tail vein, followed by 1×107 calcein-AM -labelled cells in 150 μl medium. For each eye, three regions of interest containing one to three veins/venules were observed by SLO and images were recorded for at least 15 minutes after cell injection 21. Video analysis was carried out off-line as described elsewhere21. Rolling leukocytes and those not interacting with the endothelium were counted in each venule 22-24. The rolling efficiency was calculated as the percentage of labelled rolling cells among the total number of labelled cells that entered a venule. The sticking efficiency was determined as the percentage of labelled cells becoming firmly adherent for at least 20 seconds compared to the total number of labelled cells that rolled within the venule during the same time interval.
Whole retinal mounts for confocal microscopy
Fifty minutes after the injection of labelled resting cells and SLO, the anaesthetised mice were injected via the tail vein with 100 μl Evans Blue solution (2% (w/v) in PBS, Sigma-Aldrich). After 10 minutes, animals were killed by terminal anaesthesia and the eyes were immersed in 2% (w/v) paraformaldehyde for 1 hour. The retinas were removed, washed twice in PBS for 15 min, spread on clean glass slides and mounted with the vitreous side upwards 25. The mounts were examined using a confocal laser scanning microscope, LSM 510 (Carl Zeiss, Gottingen, Germany).
Northern Hybridisation Analysis
Total RNA from control and stimulated cells was extracted using RNAzol B (Ambion, Cambs., UK) according to the manufacturer’s protocol. Poly (A)+ RNA was purified using Oligotex mRNA kits (Qiagen, Crawley, UK) as recommended by the manufacturer. RNA samples were electrophoresed in formaldehyde-containing, denaturing agarose gels and blotted onto Hybond XL nitrocellulose (Amersham Pharmacia, Buckinghamshire, UK). 25μg of total RNA was loaded onto the gel. Probes were prepared using 25ng of PCR product as template and [α32p] dCTP (Amersham Pharmacia Bucks UK) as label. Excess nucleotides were removed from the probes by purification using sephadex G-50 nick columns (Amersham Pharmacia, Bucks. UK). Blots were prehybridized for 4 hours at 50°C in high SDS Church buffer (7% SDS, 50% formamide, 25% 20x SSC, 5% 1M Sodium Phosphate (pH7), 10% 50x Denhardt’s solution, 1% 10x N-lauroyl sarcosine (Sigma)) containing 100μg/ml salmon sperm DNA for blocking. Hybridization was performed for 16 hours at 50°C and detection was performed by X Ray film exposure.
Preparation of Cell Lysates
THP-1 cells were washed in sterile 0.01% PBS then serum starved for 24 hours. The cells were washed and supplemented with BSA 2mg/ml or BSA 2mg/ml and 100ng/ml CCL5 or CCL3 (Peprotech, London, UK). Cells were incubated at 37°C for 24 hours or in subsequent experiments for 0, 2, 4, 16, or 24 hours respectively. Following incubation cells were resuspended in lysis buffer (dH2O, 150mM NaCl, 1% NP-40, 50mM Tris, 0.2mM NaVO4, 1mM DTT and 25μg/ml each of aprotinin, leupeptin and pepstatin). Lysates were incubated at 4°C with agitation for 30 minutes followed by centrifugation at 12,000g for two minutes. An equal volume of glycerol (Sigma, Poole, Dorset UK) was added to each sample. Protein estimation was performed on samples using the BCA Protein Assay Kit (Pierce, Illinois, USA).
SDS-PAGE and Western Blot Analysis of α-L-Fucosidase using THP-1 Cells
An optimum concentration of protein (20μg) was loaded onto 10% SDS-PAGE and transferred to a Hybond P nitrocellulose membrane (Amersham Pharmacia, Bucks, UK). Western blot analysis was performed using 5% non-fat milk in TBS with 0.5% tween for blocking. The membrane was incubated with primary antibody Mab72 specific for ALF (University of Iowa, USA) at 4°C overnight at a concentration of 1:50. This was followed by incubation with goat anti-rat HRP conjugated antibody (Santa Cruz, CA, USA) for 1 hour at room temperature with shaking. Reactions were developed using Pierce ECL Chemiluminescence Substrate kit (Pierce, Illinois, USA).
Flow cytometric detection of cell-surfaceα-L-Fucosidase and CD31
To quantify the membrane bound fraction of ALF, flow cytometry was performed using polyclonal rabbit anti-ALF, kindly provided by Dr. María Páez de la Cadena, Universidad de Vigo, Spain 1. THP-1 cells were labelled with an optimal concentration of primary antibody at 4 °C for 30 min, washed, and counterstained with a fluorescein isothiocyanate-conjugated goat anti-rabbit Ig (Sigma) for a further 20 min. CD31 was labelled using a murine monoclonal antibody (clone JC/70a; Abcam, UK) for 30 min before washing and counterstaining with an appropriate fluorochrome-conjugated goat anti-mouse IgG reagent (Sigma). Antibody specificity was controlled with pre-immune rabbit serum or an isotype-matched, irrelevant monoclonal antibody (Dako). In both cases the stained cells were analyzed by flow cytometry (FACSort; Becton Dickinson); data analysis was performed using Lysis II software (Becton Dickinson).
Results
The role of α-L-fucosidase in cellular migration
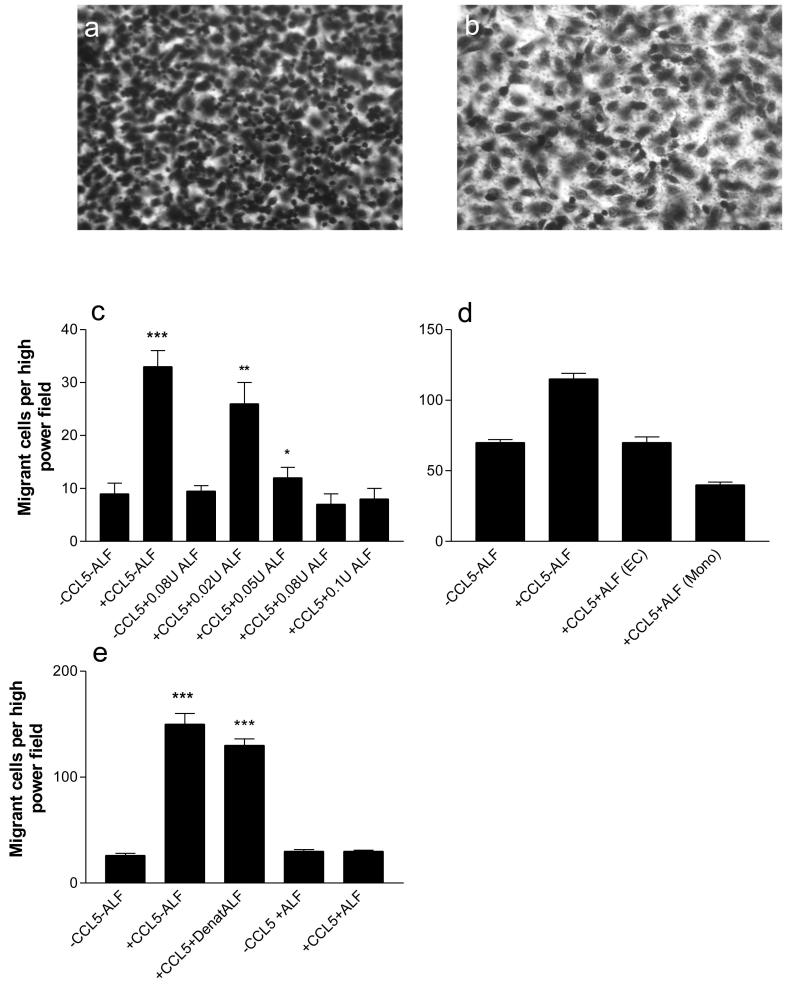
In order to determine whether ALF has the potential to modify the extravasation process, a series of chemotaxis experiments was performed. In these experiments, a monolayer of the endothelial fusion cell line EAhy926 was grown on the upper surface of the membrane which contained 3μm pores. Figure 1 shows a visual representation of cell morphology following treatment with ALF. Panel 1a shows the normal chemotactic response towards 100ng/ml CCL5 in the lower chamber; treatment of the monocytic cells with 0.08U/ml ALF markedly decreased migration (1b) but no decrease in cell viability was observed. These findings suggest that the cell morphology following treatment with ALF remains generally unchanged whilst migration is reduced to basal levels.
Figure 1. Transendothelial Chemotaxis Assay.
A monolayer of the endothelial fusion cell line EAhy926 was grown on the upper surface of the membrane which contained 3μm pores. THP-1 cells were assayed for their ability to migrate. Following migration for 2 hours, the filters were fixed in methanol and stained with Gill’s haematoxylin. Migrant cells were counted using high power microscopy (x400) in four random fields on each filter. a) THP-1 cells migrating (as small densely stained cells) in response to CCL5 (100ng/ml). b) THP-1 cells treated with 0.08U/ml ALF and allowed to migrate in response to 100ng/ml CCL5. Results are representative of three similar experiments. c) Dose response to determine the optimal concentration of α-L-fucosidase. THP-1 cells were treated for 120 minutes with 0.02 -0.1U/ml of α-L-fucosidase, and then added to the upper compartment of the chemotaxis chamber to assess migration through a monolayer of EAhy926 endothelial cells in presence or absence of 100ng/ml of CCL5. d) THP-1 cells or EAhy926 were treated for 1 hour with an optimal 0.08U/ml human α-L-fucosidase. Enzyme treated cells were then rinsed and THP-1 cells were added to the upper compartment of the chemotaxis chamber system. e) The endothelial monolayers were treated with 0.08U/ml ALF, no ALF, or 0.08U/ml ALF which had been denatured by heating to 100°C for 5 minutes. THP-1 cells were assayed for their ability to migrate through a 3μM pore diameter filter towards the basal compartment containing either no CCL5 or an optimal 100ng/ml CCL5. ALF = α-L-fucosidase, EC = endothelial cell monolayer, mono = migratory monocytic cell line. Bars show mean values ± SEM; ***, p<0.001; **, p<0.01; *, p<0.05.
Dose response assays using 0 to 0.1U/ml of ALF revealed that the prior incubation of THPs with 0.08U/ml produced a maximal inhibition of the transmonolayer migration of monocytic THP-1 cells (Figure 1c). The addition of 100ng/ml CCL5 and 0.08U/ml of α-L-fucosidase decreased migration to background levels.
To examine whether both the THP-1 migratory cells and the endothelial monolayers were sensitive to treatment with the acid hydrolase, a parallel experiment was carried out in which either the model endothelium or the migratory monocytic cells were treated individually or together with 0.08U/ml of ALF. As with previous experiments, an optimal 100ng/ml CCL5 was added to the lower chamber during the chemotaxis assay. The results demonstrated that compared to the controls, migration was markedly inhibited in all cases where ALF was added, (Fig 1d). Furthermore, compared to the basal migration levels, pre-treatment of the monocytic cells with ALF produced a greater decrease in cell migration (p=0.0004) than pre-treatment of the endothelial monolayer (p=0.0374). Furthermore, combined pre-treatment of monocytes and endothelial cells seem to have an additive effect on transmigration. The decrease in migration compared to individual treatment of monocytes and endothelial cells was significant (p=0.0255 and p=0.0195 respectively). Controls for basal migration in ALF pre-treated monocytes and endothelial cells in absence of CCL5 were not significantly different from untreated cells (data not shown).
To control for the possibility that the migratory process was affected by agents present in the ALF suspension buffer, further chemotaxis experiments were performed. Figure 1e demonstrates that compared to samples containing CCL5 alone, the additional presence of denatured ALF did not significantly decrease cell migration (p>0.4).
Cell surface staining with fucose-specific lectin following α-L-fucosidase treatment of THP-1 cells
Labeling experiments were performed to examine the effect of fucosidase treatment on binding of fucose specific lectin (Tetragonolobus purpureas) to THP-1 cells. Suspension of THP-1 cells was treated with fucosidase (0.08U/ml) for 1, 2, 8 and 16 h respectively, followed by binding with lectin. Untreated cells strongly labelled with the lectin (Figure 2). In contrast, ALF treatment for 8 and 16h demonstrated a significant reduction in labelling of THP-1. However, incubation for 1 and 2h with 0.08U/ml of ALF did not show significant reduction in binding of lectin (data not shown). The enzyme-treated cells were 95% and 85% viable following 8 and 16 h incubation based on trypan blue exclusion assay
Figure 2. Cell surface staining with fucose-specific lectin following α-L-fucosidase treatment of THP-1.
Representative flow cytometric analysis showing binding of Tetragonolobus purpureas lectin to α-L-Fucose structures. THP-1 cells were treated with fucosidase (0.08 U/ml) for 8 and 16h respectively. Following this cells were incubated with lectin (50ug/ml) for 1h at 37, followed by staining with FITC conjugated antibody. Black histogram indicates untreated cells whereas dark grey and pale grey are cells treated with ALF for 8and 16h respectively. Representative of three independent experiments.
Effect of α-L-fucosidase treatment on adhesion of THP-1 cells to P-selectin
In order to determine whether the decrease in transmigration following ALF treatment is due to the effect of enzyme on selectin ligands, we carried out adhesion experiments for ALF treated THP-1 cells to P-selectin.Fc. For these experiments THP-1 cells were treated with 0.08U/ml of ALF followed by labelling with BCECF. As control, parallel experiment was performed in which cells were untreated (Figure 3). ALF treatment significantly reduced THP-1 cell adhesion to P-selectin.Fc (p= 0.0004).
Figure 3. Adhesion of THP-1 cells to P-selectin.Fc following treatment with α-L-fucosidase.
Analysis of the adhesion of untreated THP-1 cells or following treatment with ALF (0.08 U/ml) to P-selectin.Fc. Control consist of wells with goat anti-human IgG-Fc antibody with labelled cells only. 500,000 cells were added per well and the experiment shows mean data from 3 assay well. Results are representative of two independent experiments.
Effect of α-L-fucosidase treatment on the trafficking of splenocytes in vivo in the retinal vasculature
In order to examine the functional consequences of α-L-fucosidase treatment under shear flow an in vivo study was carried out using a single cell splenocyte suspension from mice immunized (11 days post immunization) with IRBP peptide to induce EAU. Cells were divided into two groups and one group was treated with ALF. In order to ensure the cleavage of fucose residues present on the surface of splenocytes, the cells were stained with a biotinylated fucose-specific lectin (Tetragonolobus purpureas agglutinin) following treatment with ALF to validate removal from the cell surface (data not shown).
Results from SLO (Figure 4a) revealed that the treatment of splenocytes with ALF had a highly significant effect on both the rolling and retinal infiltration of cells. Following treatment with ALF (0.08U/5×105 cells) an 8.6 fold decrease (p<0.0001) in rolling compared to untreated cells was observed (Figure 4b). Furthermore the ability of the splenocytes to migrate through retinal vasculature to penetrate the retinal tissue was also inhibited following treatment of migratory cells with the fucosidase, resulting in a 3.5 fold decrease in infiltration (4c; p=0.0119).
Figure 4. Effect of α-L-fucosidase on rolling of cells on retinal vasculature.
A) Representative single frame from video analysis of labelled splenocytes passing through retinal venules. B) Analysis of the proportion of rolling cells which were defined by visible interaction with the vessel wall and reduced velocity compared with non-rolling cells. Rolling and non-interacting splenocytes were counted in each venule. The percentage of rolling cells among the total number of labelled splenocytes that entered a venule was calculated for untreated and ALF-treated splenocytes. Three eyes were used for each test with rolling and adherence being measured in at least three venules; the results are presented as mean values ± SEM. C) Analysis of retinal infiltration by labelled splenocytes. Following cell infusion (50 minutes), 100μl of 2% (w/v) Evans blue (Sigma) was injected via the tail vein and allowed to bind for 10 min. The eyes were harvested and fixed in 2% (w/v) paraformaldehyde (Agar Scientific Ltd., Cambridge, UK) for 1 h. Untreated and ALF-treated samples were observed using a confocal scanning laser imaging system fitted with krypton/argon lasers (Zeiss LSM510; Carl Zeiss, Gottingen, Germany). Three eyes were used for each test and results presented as mean values ± SEM.
Examining the regulation of the α-L-fucosidase following stimulation with chemokine
To determine whether ALF plays a natural role in regulation of inflammation, the human monocytic cell line THP-1 was stimulated for various time periods with a prototypical inflammatory chemokine CCL5 at 100ng/ml.
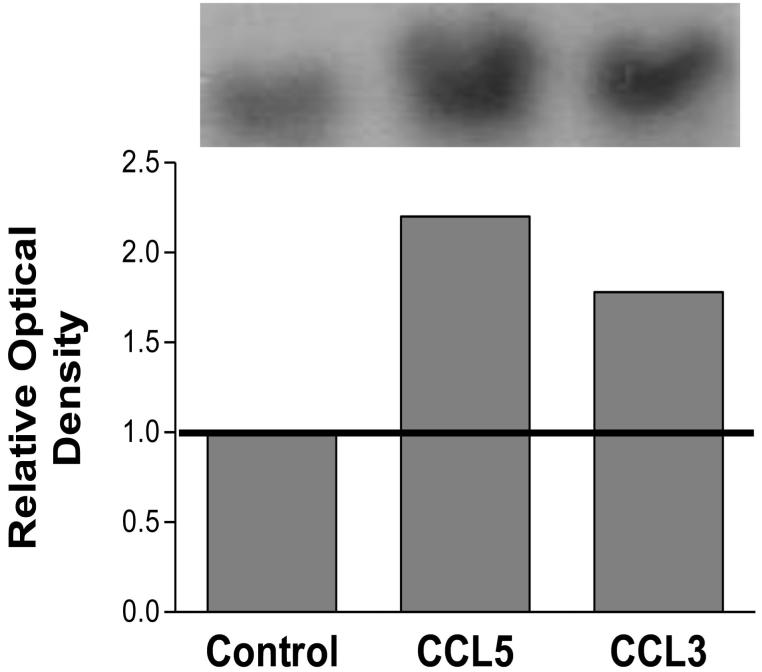
Northern blot analysis was performed to examine regulation of the gene encoding ALF. Figure 5 shows the densitometric analysis of the Northern blots and demonstrates that there was a 2.2 fold increase in the expression of ALF following 24-hour simulation with CCL5 and a 1.8 fold increase in expression following 24-hour stimulation with CCL3. The Northern membranes were also subsequently probed for GAP-DH, to ensure equal loading (data not shown).
Figure 5. Northern blot analysis using the human monocytic cell line THP-1 to examine regulation of α-L-fucosidase following stimulation with 100ng/ml CCL5 or CCL3 for 24 hours.
RNA samples were electrophoresed in formaldehyde denaturing agarose gels and blotted onto nitrocellulose; 25μg total RNA was loaded onto each gel. Probes were prepared using DNA amplified from differentially expressed bands using 25ng of the PCR product as template with [α32P] dCTP. The results are representative of three separate experiments
To further examine the regulation of ALF at the protein level, Western blot analysis was performed; this revealed two bands at 56kDa and 51kDa, which showed increased expression after 4 hours (not shown) and 24 hours (Figure 6). Densitometric analysis (normalised to an α-tubulin loading control) was performed which yielded values for optical density; this showed that after 24-hour stimulation with CCL5, the 51kDa and 56 kDa bands were upregulated 2.4- and 3.2-fold respectively.
Figure 6. Western Blot and flow cytometric analysis of α-L-fucosidase using THP-1.
A) An optimum concentration of 20μg of protein was loaded onto 10% SDS-PAGE and transferred to a nitrocellulose membrane. Western blot analysis was performed using 5% non-fat milk in TBS with 0.5% Tween-20 for blocking. The membrane was incubated with primary antibody Mab72 α-L-fucosidase at a concentration of 1:50. This was followed by incubation with goat anti-rat HRP conjugated antibody. Lane 1) cells treated with BSA 2mg/ml for 24 h; lane 2) cells treated with BSA 2mg/ml and 100ng/ml CCL5 for 24h. The results are representative of three separate experiments.
ALF is usually found as a soluble component of the lysosomes and antiserum. However, it has also been reported that it can exist associated with plasma membrane (10-20% of the total cellular fucosidase activity) in a range of cell types including monocytes 1. Therefore, to determine whether stimulation with chemokines altered the distribution of membrane bound and cytoplasmic ALF a flow cytometric analysis was performed. Although ALF was detected on the cell surface, no significant change in cell surface expression was observed following stimulation with CCL5 for 30 min, 4h or 24h (data not shown).
Modification of cell-surface CD31 by treatment with α-L-fucosidase
The process of transendothelial migration involves a series of adhesion molecules together with their respective receptors; ALF is known to degrade fucosylated moieties. As the fucosylated molecule CD31 (PECAM-1) is central to the process of leukocyte migration, immunofluorescence flow cytometry was used to measure the surface expression of CD31 on monocytic THP-1 and EaHY.926 cells up to 24 hours after the addition of 0.08U/ml of ALF. CD31 expression was examined at each timepoint using flow cytometric analysis.
EAhy926 cells demonstrated no significant difference in CD31 expression following ALF treatment for 2h however, at 5h there was a 7% increase in cell surface expression (p=0.0001). The expression of CD31 declined dramatically between 5 and 16h and gradually increased by 24h such that there was no significant difference between 0 and 24 h. Following treatment with ALF, THP-1 cells demonstrated decreased expression of CD31 between 0 and 5 h (p=0.002), but antibody binding then gradually increased (Figure 7). The level of CD31 expression was significantly lower at 24h compared to 0h with p value of 0.006 .
Figure 7. Examination of CD31 antibody binding site following α-L-fucosidase treatment of THP-1 and EAhy926 cells.
THP-1 and EAhy926 cells were incubated with 0.08U/ml of ALF (0- 24h). Using THP-1 and EAhy926 cells labelled with an appropriate isotype control, a positivity marker was set at 3%. Cell populations which were gated beyond this threshold were defined as positive for the expression of CD31. Eahy926 is denoted by ▲ and THP-1 by ●.
Discussion
α-L-Fucosidase is a ubiquitous acid hydrolase which is normally found as a soluble component of lysosomes and blood plasma, but up to 20% of ALF activity is associated with the cell-surface 1. This enzyme can process fucosylated residues which normally play a role in stabilising intercellular adhesion, potentially regulating cell trafficking in health and disease. Furthermore, ALF from some sources has also been shown to have unique property to perform the synthesis of complex oligosaccharides by transglycosylation26. This might provide a valuable approach for synthesis of fucose containing oligosaccharides as α-glycosynthases are difficult to obtain. The current study was designed to assess the possibility that ALF can modulate inflammation by reducing the interaction between fucoslyated adhesion molecules which normally support leukocyte extravasation.
The ability of the monocytic cell line, THP-1 to migrate through an endothelial cell monolayer in response to the chemokine CCL5 was tested in a model system. Dose response studies showed that the presence of 0.08U/ml of ALF inhibited leukocyte migration to a background level. A comparison of treatment of either the endothelial cells or the monocytes showed that both cell types express migration-critical residues which were inhibited by ALF. However, the slightly greater inhibition produced by treatment of the monocytes suggests that fucosylated ligands may play a dominant role on these cells. Furthermore, combined pre-treatment of endothelial and THP-1 cells had an additive effect on inhibition of transmigration.
Using flow cytometry, we identified a significant decrease in the binding of fucose-specific lectin following ALF treatment. Interestingly, treatment of both the monocytic and endothelial cells for 1h with 0.8U/ml of ALF was sufficient to reduce cell migration to a basal level. However, a significant reduction in the binding of the lectin was only evident after treatment for 8 and 16h. It is difficult to compare the results with previous observations due to variability in the source of enzyme and lectins/antibodies used 27.
P-selectin glycoprotein ligand-1 (PSGL-1) originally identified as a ligand for P-selectin, can bind all three selectins and has a proadhesive function in many inflammatory settings 28. L-selectin is constitutively expressed by most leukocytes whereas the other members of selectin family, P and E selectin are expressed by activated endothelium. Acting in cooperation with L-selectin, P-selectin mediates the initial interaction between circulating leukocytes and endothelial cells that produces characteristic rolling of leukocytes on endothelium. In order to assess the contribution of fucose residues on selectin binding, solid-phase adhesion experiments were carried out. The current study was consistent with previous observations that fucosylation of appropriate carbohydrate determinants is critical for selectin ligand generation 13. Furthermore, treatment of THP-1 cells with ALF resulted in 53% decrease in the adhesion of THP-1 cells to P-selectin.Fc.
To further examine the role of ALF in vivo we used an experimental autoimmune uveoretinitis (EAU) model, induced by immunization of mice with retinal antigens 20. The blood-retina barrier is breached in EAU, allowing both T lymphocytes and monocytes to move into the retina 29. The pre-treatment of splenocytes from sensitised animals with ALF resulted in a profound decrease in the ability of these cells to roll along the surface of the retinal endothelium and also reduced the number of cells which undergo diapedesis and penetrate the retinal tissues.
Homophilic interactions between CD31 (PECAM-1) on the surface of inflammatory leukocytes and CD31 within the junctions between endothelial cells allows the passage of leukocytes across confluent endothelium 30, with antibody blockade of CD31 reducing transendothelial migration by up to 90% 31. In order to identify a further potential mechanism by which ALF can mediate its affect, a series of studies was performed to measure the potential of this enzyme to degrade CD31.
Investigation of the nature of the N-linked glycans in Chinese hamster ovary cell-expressed CD31 has already revealed the presence of sialylated bi-, tri- and tetra-antennary compounds, of which 62% are fucosylated at the core GlcNAc residue 32. The current study demonstrated CD31 expression by both endothelial and monocytic cell lines. ALF does have an effect on CD31 expression on EAhy926 endothelial cells at several of the time points which were observed, however after 24 hour exposure the CD31 levels return to the basal level. THP-1 cells treated under the same conditions show a decline in CD31 expression between 0-5 hours. The difference in the level of CD31 expression between the two cell types might be attributed to the location of CD31 molecule. It is possible that the cleavage of fucose residues, contained within the CD31 molecules, within the junction of an endothelial monolayer, would be more difficult to access than those which are expressed on cell surface of migratory cells. Significantly, the potential of a monoclonal CD31 antibody to label these cells was reduced by pre-treatment of the cells with ALF, suggesting degradation of a fucose-containing epitope which may be critical for transendothelial migration.
The possibility of regulation of ALF expression at both the mRNA and protein levels was investigated. Interestingly, following treatment with CCL5 (100ng/ml) the gene product which coded for ALF was upregulated by more than two-fold. A similar increase in expression (1.8 fold) was produced by treatment with 100ng/ml CCL3. To examine the protein expression of ALF, THP-1 cells were stimulated with 100ng/ml CCL5 for 4 and 24 hours; ALF bands were identified at 51kDa and 56kDa by Western blotting, which is consistent with previous reports5, 1, 3. Treatment with CCL5 increased protein expression after 4 hours (data not shown), with stimulation for 24 hours increasing expression of both products by between two- and three-fold.
In conclusion, this study shows that the acid hydrolase, α-L-fucosidase is potentially upregulated by chemokines in the later stages of inflammation. The function of this enzyme includes cleavage of fucosylated residues from adhesion molecules such as sLeX and CD31, which play an important role in intravascular leukocyte rolling and subsequent extravasation. The potential for inflammatory chemokines to upregulate the expression of ALF is consistent with activation of a natural regulatory loop resulting in a gradual diminution of the potential of blood-borne leukocytes to penetrate the endothelium at sites of existing inflammation.
Acknowledgments
This work was supported by grants from Northern Counties Kidney Research Fund and the Wellcome Trust
REFERENCES
- 1.Cordero OJ, et al. Cell surface human alpha-L-fucosidase. Eur J Biochem. 2001;268:3321–31. doi: 10.1046/j.1432-1327.2001.02237.x. [DOI] [PubMed] [Google Scholar]
- 2.Fukushima H, de Wet JR, O’Brien JS. Molecular cloning of a cDNA for human alpha-L-fucosidase. Proc Natl Acad Sci U S A. 1985;82:1262–5. doi: 10.1073/pnas.82.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson SW, Piesecki S, Wang RF, Damjanov I, Alhadeff JA. Analysis of purified human liver alpha-L-fucosidase by western-blotting with lectins and polyclonal and monoclonal antibodies. Biochem J. 1992;282(Pt 3):829–34. doi: 10.1042/bj2820829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Aleem H, et al. Serum alpha L-fucosidase enzyme activity in ovarian and other female genital tract tumors. Int J Gynaecol Obstet. 1996;55:273–9. doi: 10.1016/s0020-7292(96)02770-1. [DOI] [PubMed] [Google Scholar]
- 5.Aviles M, et al. Immunocytochemical localization and biochemical characterization of a novel plasma membrane-associated, neutral pH optimum alpha-L-fucosidase from rat testis and epididymal spermatozoa. Biochem J. 1996;318(Pt 3):821–31. doi: 10.1042/bj3180821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landing BH, et al. Fucosidosis: clinical, pathologic, and biochemical studies of five patients. Adv Exp Med Biol. 1976;68:147–65. doi: 10.1007/978-1-4684-7735-1_10. [DOI] [PubMed] [Google Scholar]
- 7.Hirschberg CB. Golgi nucleotide sugar transport and leukocyte adhesion deficiency II. J Clin Invest. 2001;108:3–6. doi: 10.1172/JCI13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayude D, et al. Value of the serum alpha-L-fucosidase activity in the diagnosis of colorectal cancer. Oncology. 2000;59:310–6. doi: 10.1159/000012188. [DOI] [PubMed] [Google Scholar]
- 9.Giardina MG, et al. Serum alpha-L-fucosidase activity and early detection of hepatocellular carcinoma: a prospective study of patients with cirrhosis. Cancer. 1998;83:2468–74. [PubMed] [Google Scholar]
- 10.Homeister JW, et al. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–26. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 11.Xia L, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–50. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, et al. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190:1769–82. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J Immunol. 2002;169:5270–8. doi: 10.4049/jimmunol.169.9.5270. [DOI] [PubMed] [Google Scholar]
- 14.Granert C, Raud J, Xie X, Lindquist L, Lindbom L. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J Clin Invest. 1994;93:929–36. doi: 10.1172/JCI117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linnemann G, et al. The effects of inhibiting leukocyte migration with fucoidin in a rat peritonitis model. Intensive Care Med. 2000;26:1540–6. doi: 10.1007/s001340000642. [DOI] [PubMed] [Google Scholar]
- 16.Giblin PA, Hwang ST, Katsumoto TR, Rosen SD. Ligation of L-selectin on T lymphocytes activates beta1 integrins and promotes adhesion to fibronectin. J Immunol. 1997;159:3498–507. [PubMed] [Google Scholar]
- 17.Komosinska-Vassev K, et al. Graves’ disease-associated changes in the serum lysosomal glycosidases activity and the glycosaminoglycan content. Clin Chim Acta. 2003;331:97–102. doi: 10.1016/s0009-8981(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 18.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–7. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver PB, Chan CC, Wiggert B, Caspi RR. The requirement for pertussis to induce EAU is strain-dependent: B10.RIII, but not B10.A mice, develop EAU and Th1 responses to IRBP without pertussis treatment. Invest Ophthalmol Vis Sci. 1999;40:2898–905. [PubMed] [Google Scholar]
- 20.Jiang HR, et al. IL-18 not required for IRBP peptide-induced EAU: studies in gene-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:177–82. [PubMed] [Google Scholar]
- 21.Xu H, et al. Improved leukocyte tracking in mouse retinal and choroidal circulation. Exp Eye Res. 2002;74:403–10. doi: 10.1006/exer.2001.1134. [DOI] [PubMed] [Google Scholar]
- 22.Vajkoczy P, Laschinger M, Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–65. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley K, Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res. 1991;69:1034–41. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, et al. Recruitment of IFN-gamma-producing (Th1-like) cells into the inflamed retina in vivo is preferentially regulated by P-selectin glycoprotein ligand 1:P/E-selectin interactions. J Immunol. 2004;172:3215–24. doi: 10.4049/jimmunol.172.5.3215. [DOI] [PubMed] [Google Scholar]
- 25.Chan-Ling T. Glial, vascular, and neuronal cytogenesis in whole-mounted cat retina. Microsc Res Tech. 1997;36:1–16. doi: 10.1002/(SICI)1097-0029(19970101)36:1<1::AID-JEMT1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Berteau O, et al. Alpha-L-fucosidases: exoglycosidases with unusual transglycosylation properties. Biochemistry. 2004;43:7881–91. doi: 10.1021/bi036066z. [DOI] [PubMed] [Google Scholar]
- 27.Yuan K, et al. Alterations in human breast cancer adhesion-motility in response to changes in cell surface glycoproteins displaying alpha-L-fucose moieties. Int J Oncol. 2008;32:797–807. [PMC free article] [PubMed] [Google Scholar]
- 28.Veerman KM, et al. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol. 2007;8:532–9. doi: 10.1038/ni1456. [DOI] [PubMed] [Google Scholar]
- 29.Crane IJ, et al. Involvement of CCR5 in the passage of Th1-type cells across the blood-retina barrier in experimental autoimmune uveitis. J Leukoc Biol. 2005 doi: 10.1189/jlb.0305130. [DOI] [PubMed] [Google Scholar]
- 30.Liao F, et al. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med. 1995;182:1337–43. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–60. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton JP, Hunter AP, Simmons DL, Buckley CD, Harvey DJ. CD31 (PECAM-1) exists as a dimer and is heavily N-glycosylated. Biochem Biophys Res Commun. 1999;261:283–91. doi: 10.1006/bbrc.1999.1018. [DOI] [PubMed] [Google Scholar]