Abstract
Background
Given the wealth of data in the literature on schizophrenia endophenotypes, it is useful to have one source to reference their frequency data. We reviewed the literature on disease-liability associated variants in structural and functional magnetic resonance images (MRI), sensory processing measures, neuromotor abilities, neuropsychological measures, and physical characteristics in schizophrenia patients (SCZ), their first-degree relatives (REL), and healthy controls (HC). The purpose of this review was to provide a summary of the existing data on the most extensively published endophenotypes for schizophrenia.
Methods
We searched PubMed and MedLine for all studies on schizophrenia endophenotypes comparing SCZ to HC and/or REL to HC groups. Percent abnormal values, generally defined as > 2 SD from the mean (in the direction of abnormality) and/or associated effect sizes (Cohen’s d) were calculated foreach study.
Results
Combined, the articles reported an average 39.4% (SD=20.7%; range=2.2-100%) of abnormal values in SCZ, 28.1% (SD=16.6%; range=1.6-67.0%) abnormal values in REL, and 10.2% (SD=6.7%; range=0.0-34.6%) in HC groups.
Conclusions
These findings are reviewed in the context of emerging hypotheses on schizophrenia endophenotypes, as well as a discussion of clustering trends among the various intermediate phenotypes. In addition, programs for future research are discussed, as instantiated in a few recent large-scale studies on multiple endophenotypes across patients, relatives, and healthy controls.
Keywords: schizophrenia, endophenotypes, event-related potential, magnetic resonance imaging, neuromotor, physical anomalies, relatives
Introduction
Schizophrenia is an inherited, likely complex genetic disorder that “runs in families” and the single best predictor for developing the illness is having an affected first-degree relative (Waddington et al., 2007). However, most affected individuals lack a family history, leaving open the question of how risk is acquired in such cases. Therefore, it is important, while studying prevalence rates for endophenotypes in patients and first-degree relatives, to also be aware of prevalence rates within the general population.
Because the pathophysiology of schizophrenia remains unknown, there are presently no laboratory tests or biological markers (biomarkers) related to the central etiopathology of the illness. Biomarkers are objectively measured characteristics that are “indicators of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention (Atkinson, 2001).” They are disease-specific indicators of the presence or severity of the biological process directly linked to the clinical manifestations and outcome of a particular disorder (Ritsner & Gottesman, 2009). For example, hemoglobin A1c (HbA1c or glycosylated hemoglobin) is a minor component of hemoglobin which binds glucose and whose levels are proportional to average recent blood glucose concentrations. HbA1c is thus a useful indicator of adequacy of blood glucose control in patients with type II diabetes, as well as being related to the pathophysiology of this disorder of carbohydrate metabolism and in detecting an important disease feature, i.e. pathologically elevated blood glucose.
In contrast, Endophenotypes, or “intermediate phenotypes,” are best considered as quantifiable biological variations or deficits that are types of stable trait markers or indicators of presumed inherited vulnerability or liability to a disease (Ritsner & Gottesman, 2009). Because the pathophysiology of schizophrenia remains obscure, and thus biomarkers are lacking, genetic research into the disorder has generally focused on the clinical phenomenology of this complex and likely multi-determined, multi-path, inherited disorder as the relevant phenotype. Endophenotypes are associated with the illness, state-independent, co-segregate within families and are found in some unaffected relatives of individuals with the disorder (because they represent vulnerability for the disorder, not the disorder itself), although at a higher prevalence than in the general population (Gottesman & Gould, 2005). They are not visible to the naked eye and are assessed by experimental, laboratory-based methods rather than by clinical observation. Because schizophrenia is likely to fall into the category of common, multi-genetic disorders (analogous to hypertension or type II diabetes; Pearlson and Folley, 2008a,b) endophenotype strategies are increasingly employed by researchers, based on the presumption that endophenotypes have more straightforward inheritance patterns and are coded for by smaller numbers of genes than are complex, heterogeneous phenomenological entities such as Diagnostic and Statistical Manual-IV-TR (DSM-IV-TR; American Psychiatric Association, 2000) diagnostic categories. Seen in this light, the endophenotype is “intermediate” between a clinical entity and the associated disease vulnerability genes. The hope is that by employing endophenotypes, the search for the etiopathology, including genetic determinants, of schizophrenia is made more straightforward (Chan and Gottesman, 2008; Pearlson and Folley, 2008a,b).
Although new mutations, deletions, or copy number variants may account for some cases (Walsh et al., 2008), other affected individuals are believed to acquire their liability for the disorder through inheritance of several common single nucleotide polymorphism (SNP) based variants, likely acting multiplicatively (Gangestad and Yeo, 2006). At a genetic level, collections of smaller numbers of SNPs may manifest as endophenotypic abnormalities (Campbell et al., 2006).
Population geneticists often assume the Hardy-Weinberg equilibrium (Hardy, 1908) when predicting genetic outcomes in subsequent populations, stating that genes and their phenotypes remain constant barring changes. However, changes including mutations (particularly caused by duplications), selection, migration, and other consequences of population and individual mating choices can cause disorders to be introduced and propagated by these forces, disrupting the Hardy-Weinberg equilibrium. Complex inherited disorders are examples of such disequilibrium including schizophrenia (Sullivan et al., 2003), bipolar disorder (Smoller and Finn, 2003), multiple sclerosis (Oksenberg and Barcellos, 2005), and type II diabetes (Permutt, et al., 2005), which affect multiple loci and have been related to population drift. Thus, through multiplicative and additive models, these relatively prevalent disorders can persist in the population. This complicates the known inheritance of these disorders, but it also makes it possible to observe these multiple loci pooling in certain individuals, which are likely to be more affected by the clinical phenotypes.
It is thus likely not uncommon for healthy individuals in the general population to possess one or a few schizophrenia-associated endophenotypes, although actual prevalence rates are poorly documented. Theoretically, these endophenotypes could be neutral or even beneficial singly, if not combined with other intermediate phenotypes (Keller and Miller, 2006, Pearlson and Folley, 2008a,b). Like the hypothesized “thrifty genes” associated with type II diabetes, they may confer selective advantage under particular circumstances (Neel, 1962). These multiple genetic loci (polygenes) of small relative effect are likely additive or epistatic (interactive) with regard to cumulative schizophrenia risk; only in combination are they deleterious and likely then often in conjunction with environmental events.
There is a wealth of data in the literature on disease-related endophenotypes in schizophrenia patients (SCZ) and their first-degree relatives (REL), yet very few reviews of prevalence rates within all three categories [SCZ, REL, & healthy controls (HC)], despite the theoretical importance of such information. Heinrichs (2001) provides a thorough review of endophenotypes, but concentrates mainly on SCZ and REL, with little data on HC. Recent articles, such as the “Just the Facts” series in this journal (Tandon et al., 2008 a, b; Keshavan et al. 2008), have brought to light the importance of evaluating endophenotypes for schizophrenia in order to assess research progress in this area thus far. As a prelude to further study of the co-occurrence of multiple schizophrenia biomarkers in a large, representative community sample, including all three categories, we surveyed the existing literature on the most widely published endophenotypes (Heinrichs, 2001) in order to continue our examination of the prevalence of endophenotypic abnormalities in the general population (Pearlson and Folley, 2008, a,b). Articles that compared SCZ to HC or REL to HC (or both) within six different groups of endophenotypes (structural and functional brain abnormalities, sensory processing measures, neuromotor abnormalities, neuropsychological measures, physiologic abnormalities and minor physical anomalies) were included in this review. A conservative definition of abnormality was utilized in this review based on a model of statistical infrequency. As such, depending on available data, percent of abnormal findings, generally defined as greater than two standard deviations (SD) from the mean (in the direction of abnormality) and/or effect sizes (Cohen’s d; Cohen, 1988) were extracted from each article. Under our summaries of each endophenotype, the total number of articles reviewed is reported; however, not all articles reviewed reported data on SCZ, REL, and HC samples. The number of articles reported within tables for each endophenotype reflect the number of articles that report unique data contributing to the calculations of each summary statistic, which may be different that the overall total within each endophenotype.
The purpose of this review was to assess the frequency of these established endophenotypes in all three categories, in order to provide a source of reference, as well as a beginning point for discussion on prevalence rates within SCZ, REL, and HC.
Structural and Functional Brain Abnormalities
Ventricular Volume
A Medline search was completed with the search terms of “schizophrenia” combined with “ventricular volume or lateral ventricles.” Studies that assessed volumetric measurements in cubic centimeters or milliliters of the right and left lateral ventricles in SCZ, REL, and HC were included. Effect sizes were calculated based on measurements of absolute volume rather than ventricle-brain ratios since they provided greater discrimination between groups. A total of 13 articles met our criteria and reported data on both left and right lateral ventricle volumes (Buchsbaum et al., 1997; Chua et al., 2007; Degreef et al., 1992; DeLisi et al., 2004; DeLisi et al., 2006; Dickey et al., 2000; Kelsoe et al., 1988; Lawrie et al., 1999b; Marsh et al., 1997; McDonald et al., 2006; Shenton et al., 1991; Suddath et al., 1989; Whitworth et al., 2005). Effect sizes are summarized in Table 1.
Table 1.
Mean effect size and 95% CI for structural and functional brain abnormalities.
| Endophenotype: Ventricular Volume | |||
|---|---|---|---|
| Effect Size | 95% CI | N | |
| Left Lateral Ventricle | |||
| SCZ | 0.7 | 0.48-0.92 | 12 |
| REL | 0.4 | -1.21-1.96 | 2 |
| HC | N/A | 0 | 0 |
| Right Lateral Ventricle | |||
| SCZ | 0.7 | 0.42-0.97 | 12 |
| REL | 0.3 | -0.30-1.0 | 2 |
| HC | N/A | 0 | 0 |
| Endophenotype: planum Temporale Volume | |||
| Left Planum Temporale | |||
| SCZ | 0.5 | 0.19-0.80 | 18 |
| REL | 0.1 | N/A | 1 |
| HC | N/A | 0 | 0 |
| Right Planum Tenmporale | |||
| SCZ | 0.3 | 0.16-0.50 | 19 |
| REL | 0.2 | N/A | 1 |
| HC | N/A | 0 | 0 |
| Endophenotype: Superior Temporal Gyrus | |||
| Left Superior Temporal Gyrus | |||
| SCZ | 0.6 | 0.38-0.80 | 17 |
| REL | 0.3 | N/A | 1 |
| HC | N/A | 0 | 0 |
| Right Superior Temporal Gyrus | |||
| SCZ | 0.5 | 0.24-0.70 | 16 |
| REL | 0.2 | N/A | 1 |
| HC | N/A | 0 | 0 |
| Endophenotype: fMRI Activation During 2-Back Task | |||
| SCZ | 0.9 | 0.19-1.67 | 4 |
| REL | N/A | N/A | 0 |
| HC | N/A | 0 | 0 |
N = number of studies included; N/A = not applicable; unable to calculate based on available published data
Planum Temporale Volume or Surface Area
A Medline search with the search terms of “schizophrenia” combined with “planum temporale” was conducted. Studies that assessed volumetric measurements in cubic centimeters or millimeters, as well as surface area in centimeters squared, in the regions of the right and left planum temporale were included. A total of 20 articles met our criteria (19 left: Barta et al., 1997; Crespo-Facorro et al., 2004; DeLisi et al., 1994; Dickey et al., 2002; Falkai et al., 1995; Frangou et al., 1997; Hirayasu et al., 2000; Kulynych et al., 1995; Kulynych et al., 1996; Kwon et al., 1999; McCarley et al., 2002; Meisenzahl et al., 2002; Petty et al., 1995; Rossi et al., 1994; Rossi et al., 1992; Shapleske et al., 2001; Sumich et al., 2002; Takahashi, Suzuki, Zhou, Tanino, Hagino, Niu et al., 2006; Yamasue et al., 2004; and 20 right: Barta et al., 1997; Crespo-Facorro et al., 2004; DeLisi et al., 1994; Dickey et al., 2002; Falkai et al., 1995; Frangou et al., 1997; Goldstein et al., 2002; Hirayasu et al., 2000; Kulynych et al., 1995; Kulynych et al., 1996; Kwon et al., 1999; McCarley et al., 2002; Meisenzahl et al., 2002; Petty et al., 1995; Rossi et al., 1994; Rossi et al., 1992; Shapleske et al., 2001; Sumich et al., 2002; Takahashi, Suzuki, Zhou, Tanino, Hagino, Niu et al., 2006; Yamasue et al., 2004)) and effect sizes were calculated for each (see Table 1).
Superior Temporal Gyrus Volume
A Medline search with the terms of “schizophrenia” combined with “superior temporal gyrus” was completed. Studies that assessed volumetric measurements in cubic centimeters and milliliters of the right and left superior temporal gyrus in SCZ, REL, and HC were included. Effect size calculations were completed on a total of 17 articles that reported data on both left and right superior temporal gyrus (Anderson et al., 2002; Barta et al., 1990; Bryant et al., 1999; DeLisi and Hoff, 2005; DeLisi et al., 1994; Dickey et al., 1999; Frangou et al., 1997; Holinger et al., 1999; Kim et al., 2003; Kulynych et al., 1996; Marsh et al., 1997; McCarley et al., 1993; Meisenzahl et al., 2004; Onitsuka et al., 2004; Rajarethinam et al., 2000; Takahashi, Suzuki, Zhou, Tanino, Hagino, Kawasaki et al., 2006; Vita et al., 1995). Effect sizes are summarized in Table 1.
fMRI Activation during 2-back Task
Functional magnetic resonance imaging (fMRI) BOLD signal activation during performance of the 2-back task was examined. A total of 4 articles reported data on activation in the dorsolateral prefrontal cortex (Callicott et al., 2000; Jansma et al., 2004; Meisenzahl et al., 2006; Thermenos et al., 2005) and effect sizes were calculated based on differences in activation between groups. Effect sizes are summarized in Table 1.
Sensory Processing and Event-Related Potential Measures
Prepulse Inhibition
All studies that assessed sensory gating deficits measured by prepulse inhibition in SCZ, REL, and HC were included. A Medline search with the terms “schizophrenia” combined with “prepulse inhibition” was completed. Due to multiple variations in paradigm conditions, we specified two from which effect sizes were calculated: interstimulus interval and sound intensity. We chose an interstimulus interval of 60-120 milliseconds between the prepulse and pulse stimuli and sound intensities ranging from 40-90 decibels. The selection of these criteria increased group differences, resulting in higher effect sizes. In total, 15 records were selected that fit our selection criteria (Braff et al., 2001; Braff et al., 1999; K. Cadenhead et al., 1996; Cadenhead et al., 1993; Cadenhead et al., 2000; Dawson et al., 2000; Hong et al., 2007; Kumari et al., 2000; Kumari et al., 1999; Mackeprang et al., 2002; McDowd et al., 1993; Oranje et al., 2002; Parwani et al., 2000; Perry et al., 2002; Weike et al., 2000). Percent abnormal calculations and effect sizes are summarized in Table 2.
Table 2.
Mean percent abnormal, effect size, and 95% CI for sensory processing and event-related potential measures.
| Endophenotype: Pre-Pulse Inhibition | |||||
|---|---|---|---|---|---|
| %Abnormal | N | Effect Size | 95% CI | N | |
| SCZ | 38.0 | 2 | 0.8 | 0.07-0.09 | 14 |
| REL | 47.0 | 1 | 0.8 | N/A | 1 |
| HC | 21.8 | 2 | N/A | N/A | 0 |
| Endophenotype: P50 | |||||
| SCZ | 69.6 | 8 | 1.5 | 0.99-1.98 | 22 |
| REL | 59.4 | 2 | 1.7 | -0.12-3.52 | 5 |
| HC | 15.5 | 6 | N/A | N/A | 0 |
| Endophenotype: P300 | |||||
| SCZ | 45.8 | 7 | 0.8 | 0.46-1.10 | 12 |
| REL | 18.5 | 3 | 0.7 | -0.16-1.64 | 4 |
| HC | 6.5 | 6 | N/A | N/A | 0 |
| Endophenotype: N400 | |||||
| SCZ | 45.7 | 2 | 0.8 | 0.60-0.91 | 6 |
| REL | N/A | 0 | N/A | N/A | 0 |
| HC | 0.0 | 1 | N/A | N/A | 0 |
N = number of studies included; N/A = not applicable; unable to calculate based on available published data
P50
A Medline search with the terms of “schizophrenia” combined with “P50” was completed. All studies that assessed sensory gating deficits measured by the P50 ratio (amplitude of the testing stimulus/amplitude of the conditioning stimulus) in SCZ, REL, and HC were included. We chose the P50 ratio rather than the P50 suppression ratio due to greater discrimination between groups, resulting in greater effect sizes. In total, 21 records were selected that fit our criteria (Adler et al., 1985; Adler et al., 1990; Boutros et al., 1999; Clementz et al., 1997; Cullum et al., 1993; de Wilde et al., 2007; Freedman et al., 1987; Freedman R, 1996; Hong et al., 2007; Jin et al., 1997; Johannesen et al., 2005; Kathmann and Engel, 1990; Louchart-de la Chapelle et al., 2005; Myles-Worsley, 2002; Nagamoto et al., 1989; Olincy et al., 2000; Price et al., 2006; M. C. Waldo et al., 1988; Merilyne C. Waldo et al., 1991; Ward et al., 1996; Yee et al., 1998). Percent abnormal calculations and effect sizes are presented in Table 2.
P300
For studies that report prevalence rates of abnormal P300 response, a Medline search was performed using the terms “schizophrenia” and “P300” or “P3”. Two methods for determining individuals with abnormal P300s were used. In some studies, the prevalence rates were determined by individual subject data presented in scatter plots, where abnormality was defined as the portion of the distribution beyond 2 standard deviations of the HC mean. In addition, we calculated effect sizes from the reported peak latency. Though schizophrenia patients presented event-related potentials with diminished amplitudes as well as increased latencies, we chose to examine only peak latencies due to the temporal precision of electroencephalography. All of the studies reviewed used the auditory oddball task with 1000 Hz and 1500 Hz tones. In total, 12 studies were selected that fit our criteria (Blackwood et al., 1991; Bobes et al., 1996; Bramon et al., 2005; Faux et al., 1990; Ford et al., 1999; Frangou et al., 1997; Mathalon et al., 2000; McCarley et al., 1993; Price et al., 2006; Saitoh et al., 1984; Salisbury et al., 1996; Souza et al., 1995). Percent abnormal calculations and effect sizes are summarized and presented in Table 2.
N400
For the N400, a Medline search was performed with the terms “schizophrenia” and “N400”. The prevalence rates and effect sizes were determined similarly to P300. A potential confound for this endophenotype is the variability in tasks used to elicit the N400. Mostly, studies utilized an incongruent sentence completion task with visual presentation. However, some used an auditory presentation, or a different task, such as the semantic priming task or semantic matching task. In total, 8 studies fit our selection criteria (Adams et al., 1993; Bobes et al., 1996; Condray et al., 1999; Grillon et al., 1991; Koyama et al., 1991; Nestor et al., 1997; Niznikiewicz et al., 2002; Niznikiewicz et al., 1999). Percent abnormal calculations and effect sizes are presented in Table 2.
Neuromotor Abnormalities
Smooth Pursuit Eye Movement
For abnormal smooth pursuit eye movements, a Medline search was completed using the terms “schizophrenia” combined with “smooth pursuit eye movement”. Typically referred to as eye tracking dysfunction (ETD), prevalence rates were determined primarily from qualitative observations. However, some studies reported prevalence rates based on quantitative measures, including the natural log of signal-to-noise ratio (ln S/N), or the root mean square error (RMSE). In addition, we calculated effect sizes from the reported frequency of catch-up saccades (Table 3). This measure was chosen because increased numbers of catch-up saccades are a fundamental characteristic of the smooth pursuit eye movement impairments in schizophrenia. Most of the studies reviewed used a 0.4 Hz pendulum to examine smooth pursuit. However, some studies varied the pendulum frequency, and other studies used a sine wave to examine smooth pursuit. Most of the studied used electro-oculography (EOG) to record eye movement, others used infrared reflectometry (IR), or both. A total of 26 articles fulfilled our selection criteria (Acker and Toone, 1978; Allen et al., 1990; Altman et al., 1990; Amador et al., 1991; Blackwood et al., 1991; Boudet et al., 2005; Clementz et al., 1992; Holahan and O’Driscoll, 2005; Holzman et al., 1973; Iacono et al., 1992; Jones and Pivik, 1985; Keefe et al., 1989; Kinney et al., 1998; Levin et al., 1981; Levin et al., 1988; Levy et al., 1992; Levy et al., 2000; Louchart-de la Chapelle et al., 2005; Matthysse et al., 1986; Ross et al., 1998; Saletu et al., 1986; Scarone et al., 1987; Sereno and Holzman, 1995; Siever et al., 1990; Smeraldi et al., 1987; Thaker et al., 1996). Effect sizes and percent abnormal calculations are presented in Table 3.
Table 3.
Mean percent abnormal, effect size, and 95% CI for neuromotor abnormalities.
| Endophenotype: Smooth Pursuit Eye Movement | |||||
|---|---|---|---|---|---|
| %Abnormal | N | Effect Size | CI 95% | N | |
| SCZ | 47.8 | 22 | 1.0 | 0.74-1.28 | 24 |
| REL | 18.2 | 6 | 0.5 | 0.76-0.95 | 7 |
| HC | 8.9 | 24 | N/A | N/A | 0 |
| Endophenotype: Saccadic Eye Movement | |||||
| SCZ | 44.8 | 8 | 1.1 | 0.71-1.49 | 15 |
| REL | 22.9 | 4 | 0.6 | 0.25-0.85 | 9 |
| HC | 7.7 | 12 | N/A | N/A | 0 |
| Endophenotype: Handedness | |||||
| Non-Right | |||||
| SCZ | 23.9 | 3 | N/A | N/A | 0 |
| HC | 11.8 | 3 | N/A | N/A | 0 |
| Left | |||||
| SCZ | 8.2 | 11 | N/A | N/A | 0 |
| REL | 5.7 | 3 | N/A | N/A | 0 |
| HC | 6.2 | 10 | N/A | N/A | 0 |
| Mixed | |||||
| SCZ | 32.5 | 10 | N/A | N/A | 0 |
| REL | 29.2 | 2 | N/A | N/A | 0 |
| HC | 14.1 | 10 | N/A | N/A | 0 |
| Endophenotype: Neuromotor Deviations | |||||
| SCZ | 80.8 | 3 | 1.6 | 1.27-1.89 | 12 |
| REL | N/A | 0 | 1.6 | 0.30-2.82 | 5 |
| HC | 9.8 | 3 | N/A | N/A | 0 |
N = number of studies included; N/A = not applicable; unable to calculate based on available published data
Saccadic Eye Movement
For the saccadic eye movement biomarker, a Medline search was completed using the search terms “schizophrenia” combined with “saccadic eye movement”. Data were reviewed similarly to those for smooth pursuit eye movement, except that in addition to using varied pendulum frequency and sine wave, some studies used step-ramp or constant velocity to examine saccadic eye movement. We calculated effect sizes from the reported percent accuracy on the antisaccade task. This measure was chosen because it is independent of the recording apparatus, is easily quantified, and remains consistent across studies. A total of 19 articles fulfilled our selection criteria (Amador et al., 1991; Blackwood et al., 1991; Boudet et al., 2005; Clementz et al., 1992; Clementz, McDowell et al., 1994; Crawford et al., 1998; Ettinger et al., 2006; Fukushima et al., 1988; Holahan and O’Driscoll, 2005; Levin et al., 1988; Levy et al., 2000; Louchart-de la Chapelle et al., 2005; Maccabe et al., 2005; Matthysse et al., 1986; McDowell et al., 1999; O’Driscoll et al., 1998; Price et al., 2006; Sereno and Holzman, 1995; Thaker et al., 2000). Percent abnormal calculations and effect sizes are summarized in Table 3.
Handedness
One of the oldest markers for schizophrenia, mixed- or left-handedness has been purported to be correlated with the development of the disease. Initially, the literature focused on left and non-right handedness. More recently, there has been an interest in mixed-handedness. In order to present a complete review, we included all studies that assessed non-right-, left-, or mixed-handedness in SCZ, REL, and HC. We performed a Medline search with the terms “schizophrenia” combined with “handedness and (left or mixed or dextral or sinistral or non-right)”. Articles were included if they presented a clear description of their handedness assessment. The majority of articles used self-report scales (e.g. Annett Hand Preference, Edinburgh Handedness Inventory) or hand performance tests (e.g. Hand Preference Demonstration Test). While the scoring of handedness varied in strictness between articles, each article presented frequencies for abnormal (all versions of non-right) handedness, which allowed for the comparison of percent abnormal between diagnostic groups between studies. A total of 16 articles fulfilled our selection criteria, with some articles reporting data for multiple handedness categories (3 non-right: O’Callaghan et al., 1995; Sperling et al., 1999; Yan et al., 1985; and 11 left: Clementz, Iacono et al., 1994; Dragovic and Hammond, 2005; Egan, Hyde et al., 2001; Green, Satz, Smith et al., 1989; Lawrie et al., 1999a; Malesu et al., 1996; Nelson et al., 1993; Reilly et al., 2001; Shapleske et al., 2001; Taylor and Abrams, 1984; Upadhyay et al., 2004; and 10 mixed: Dragovic and Hammond, 2005; Egan, Hyde et al., 2001; Giotakos, 2001; Green, Satz, Smith et al., 1989; Gureje, 1988; Malesu et al., 1996; Nelson et al., 1993; Reilly et al., 2001; Taylor and Abrams, 1984; Upadhyay et al., 2004). Percent abnormal calculations are presented in Table 3.
Neuromotor Deviations
For neuromotor deviations, we included all studies that assessed neurological deficits in SCZ, REL, and HC. We performed a Medline search with the search terms “schizophrenia” combined with “neuromotor or neurol* sign, hard sign, or soft sign or NSS or psychomotor”. With the heterogeneity of the neurological assessments used in the literature, any article that provided a general measure of hard signs, soft signs, or global neurological deficit through standardized scales or clinical examination was included in the review. When more than one measure was available, the global deficit was chosen, followed by the soft signs, and lastly the hard signs. A total of 12 articles fulfilled our criteria (Arango et al., 1999; Buchanan and Heinrichs, 1989; Chen et al., 2000; Egan, Hyde et al., 2001; Gourion et al., 2004; Ismail et al., 1998b; Manschreck et al., 1981; Rossi et al., 1990; Sachdev et al., 1999; Taylor and Abrams, 1984; Walker and Green, 1982; Woods et al., 1986). Percent abnormal calculations and effect sizes are presented in Table 3.
Neuropsychological Measures
Wisconsin Card Sorting Task
A Medline search with the terms “Wisconsin Card Sorting Task” combined with “schizophrenia” was conducted. Articles reporting number of categories achieved and perseverative errors in SCZ, REL, and HC were included. Effect sizes were calculated and reported on a total of 41 articles (38 categories achieved: Altshuler et al., 2004; Battaglia et al., 1994; Braff et al., 1991; Cadenhead et al., 1999; Condray et al., 1999; Dieci et al., 1997; Drakeford et al., 2006; Egan, Goldberg et al., 2001; Franke et al., 1992; Glahn et al., 2000; Gold et al., 1997; Goldberg et al., 1998; Gooding et al., 1999; Gooding and Tallent, 2002; Gooding et al., 2001; Haut et al., 1996; Hoff et al., 1992; Hoff et al., 1998; Josman and Katz, 2006; Keefe et al., 1994; Keri et al., 2001; Laurent et al., 2000; Laurent et al., 2001; Merrin et al., 2006; Morrens et al., 2006; Perry and Braff, 1998; Rybakowski and Borkowska, 2002; Seidman et al., 1991; Shum et al., 2004; Snitz et al., 1999; Stratta et al., 2003; Stratta, Daneluzzo, Mattei et al., 1997; Stratta, Daneluzzo, Prosperini et al., 1997; Suhr, 1997; Sullivan et al., 1993; Tallent and Gooding, 1999; Toomey et al., 1998; Wolf et al., 2002; and 30 perseverative errors: Altshuler et al., 2004; Battaglia et al., 1994; Condray et al., 1999; Dieci et al., 1997; Drakeford et al., 2006; Egan, Goldberg et al., 2001; Glahn et al., 2000; Gold et al., 1997; Gooding et al., 1999; Gooding and Tallent, 2002; Gooding et al., 2001; Haut et al., 1996; Josman and Katz, 2006; Keefe et al., 1994; Keri et al., 2001; Laurent et al., 2000; Laurent et al., 2001; Merrin et al., 2006; Rybakowski and Borkowska, 2002; Scarone et al., 1993; Shum et al., 2004; Snitz et al., 1999; Stratta, Daneluzzo, Mattei et al., 1997; Stratta, Daneluzzo, Prosperini et al., 1997; Suhr, 1997; Sullivan et al., 1993; Szoke et al., 2006; Tallent and Gooding, 1999; Wolf et al., 2002; Zanello et al., 2006), with some articles reporting data on both types of scores. Effect sizes are summarized in Table 4.
Table 4.
Mean percent abnormal, effect size, and 95% CI for neuropsychological measures.
| Endophenotype: Wisconsin Card Sorting Task | |||||
|---|---|---|---|---|---|
| %Abnormal | N | Effect Size | CI 95% | N | |
| Categories Achieved | |||||
| SCZ | N/A | 0 | 1.15 | 0.97-1.31 | 30 |
| REL | N/A | 0 | 0.38 | 0.15-0.60 | 10 |
| HC | N/A | 0 | N/A | N/A | 0 |
| Perseverative Errors | |||||
| SCZ | N/A | 0 | 0.95 | 0.75-1.10 | 24 |
| REL | N/A | 0 | 0.37 | 0.20-0.55 | 10 |
| HC | N/A | 0 | N/A | N/A | 0 |
| Endophenotype: Continuous Performance Task | |||||
| Numbers | |||||
| SCZ | 28.6 | 1 | 1.19 | 0.64-1.75 | 6 |
| REL | 26.6 | 1 | 0.22 | -0.07-0.51 | 3 |
| HC | 11.4 | 1 | N/A | N/A | 0 |
| Shapes | |||||
| SCZ | 30.4 | 1 | 1.50 | 0.90-2.18 | 6 |
| REL | 27.9 | 1 | 0.50 | 0.20-0.84 | 3 |
| HC | 13.9 | 1 | N/A | N/A | 0 |
| Endophenotype: Delayed Response Task | |||||
| SCZ | N/A | 0 | 1.26 | 0.85-1.67 | 8 |
| HC | N/A | 0 | N/A | N/A | 0 |
N = number of studies included; N/A = not applicable; unable to calculate based on available published data
Continuous Performance Task
For the Continuous Performance Test, we included studies that only used the Identical Pairs (CPT-IP) version (B. Cornblatt et al., 1988) to assess performance on the numbers and shapes conditions in SCZ, REL, and HC. Effect sizes were calculated for one of the five major performance indices, d’. A Medline search with the search terms “continuous performance task” and “identical pairs version” and “schizophrenia” was conducted. Effect sizes are reported for a total of 6 articles (Cornblatt et al., 1989; Cosway et al., 2002; Franke et al., 1992; Laurent et al., 1999; Obiols et al., 1992; Roitman et al., 1997) across both categories, with percent abnormal performance reported for one. Percent abnormal calculations and effect sizes are presented in Table 4.
Visuospatial Delayed Response
To examine working memory, we included studies that used a visuospatial delayed response task. A Medline search with the terms “delayed response task” and “schizophrenia” and “visuospatial working memory” and “schizophrenia”. Effect sizes were reported for a total of 8 articles (Coleman et al., 2002; Fleming et al., 1997; Gooding and Tallent, 2004; Lencz et al., 2003; Minor and Park, 1999; Park, 1997; Stratta et al., 1999; Stratta et al., 2001), with all studies only looking at SCZ vs. HC. Effect sizes are presented in Table 4.
Physiologic Abnormalities
Niacin Flushing
A small amount of research has addressed the prevalence of presumed prostaglandin deficiency in schizophrenia with deficient niacin flushing as a potential test for disease liability. For this biomarker, we included all relevant studies on oral or topical niacin administration that compared SCZ, REL, and HC. A Medline search with the term “schizophrenia” combined with “niacin or flush” was completed. After excluding articles that did not present percent abnormal scores (absolute measures of the flush response were not reported in a comparable way), 6 (2 oral: Hudson et al., 1999; Hudson et al., 1997; and 4 topical: Lin et al., 2007; Puri et al., 2001; Puri et al., 2002; Ward et al., 1998) articles fulfilled our criteria. The percent abnormal calculations and effect sizes are presented in Table 5.
Table 5.
Mean percent abnormal, effect size, and 95% CI for physiological abnormalities.
| Endophenotype: Niacin Flush | |||||
|---|---|---|---|---|---|
| %Abnormal | N | Effect Size | 95% CI | N | |
| Oral Administration | |||||
| SCZ | 43.2 | 2 | 0.6 | 0.64-0.64 | 2 |
| HC | 1.7 | 2 | N/A | N/A | 0 |
| Topical Administration | |||||
| SCZ | 60.7 | 4 | 0.7 | 0.26-1.17 | 5 |
| REL | 28.0 | 2 | N/A | N/A | 0 |
| HC | 24.4 | 5 | N/A | N/A | 0 |
N = number of studies included; N/A = not applicable; unable to calculate based on available published data
Minor Physical Anomalies
Dysmorphology
A well-documented correlate for schizophrenia, minor physical anomalies (MPA) are theorized to represent evidence of a genetic variant and/or prenatal insult, resulting in abnormal physical development, that may serve as a marker for schizophrenia, and that may additionally be a proxy for disturbed neurodevelopment. For dysmorphology, a Medline search with the search terms “schizophrenia” combined with “MPA or physical anomalies or dysmorphology” was completed. Two measures were subsequently chosen that best represented the literature. Waldrop or modified Waldrop scores greater than or equal to 3 were regularly cited as the distinction of abnormal physical anomaly. Minor physical anomaly scores that were not rated through the Waldrop scale were considered abnormal when ≥ 6. The majority of the literature supplied data on at least one of these measures and allowed for the broadest assessment of prevalence. A total of 11 articles (5 Waldrop: Green et al., 1994; Green, Satz, Gaier et al., 1989; Griffiths et al., 1998; Ismail et al., 1998a; Lohr and Flynn, 1993; and 6 non-Waldrop: Gourion et al., 2004; Gualtieri et al., 1982; Ismail et al., 1998a; Lohr and Flynn, 1993; Sivkov and Akabaliev, 2004; Trixler et al., 2001) fulfilled our selection criteria. Effect sizes and percent abnormal calculations are presented in Table 6.
Table 6.
Mean percent abnormal, effect size, and 95% CI for minor physical anomalies.
| Endophenotype: Dysmorphology | |||||
|---|---|---|---|---|---|
| %Abnormal | N | Effect Size | 95% CI | N | |
| Waldrop > 3 | |||||
| SCZ | 22.8 | 4 | 0.7 | 0.35-1.06 | 5 |
| REL | 5.0 | 3 | 0.2 | -0.27-0.58 | 4 |
| HC | 5.3 | 5 | N/A | N/A | 0 |
| MPA > 6 | |||||
| SCZ | 34.9 | 6 | 1.0 | 0.73-1.28 | 6 |
| REL | 49.0 | 2 | 0.9 | -2.05-3.92 | 2 |
| HC | 5.1 | 6 | N/A | N/A | 0 |
N = number of studies included; N/A = not applicable; unable to calculate based on available published data
Summary
Summary statistics (mean and standard deviations) across all endophenotypes reviewed are presented in Table 7. Median effect size and 95% CI for each endophenotype are presented in Figure 1. Also, see Figure 2 for summary of percent abnormals for each category (with the exception of “structural and functional MRI” due to not having percent abnormals available for this category).
Table 7.
Mean percent abnormal, effect size, and 95% CI for all endophenotypes examined.
| %Abnormal | N | Effect Size | 95% CI | N | |
|---|---|---|---|---|---|
| SCZ | 39.4 | 11 | 0.9 | 0.85-1.03 | 15 |
| REL | 28.1 | 8 | 0.6 | 0.44-0.77 | 11 |
| HC | 10.2 | 11 | N/A | N/A | 0 |
N = number of endophenotypes included; N/A = not applicable; unable to calculate based on available published data
Figure 1.
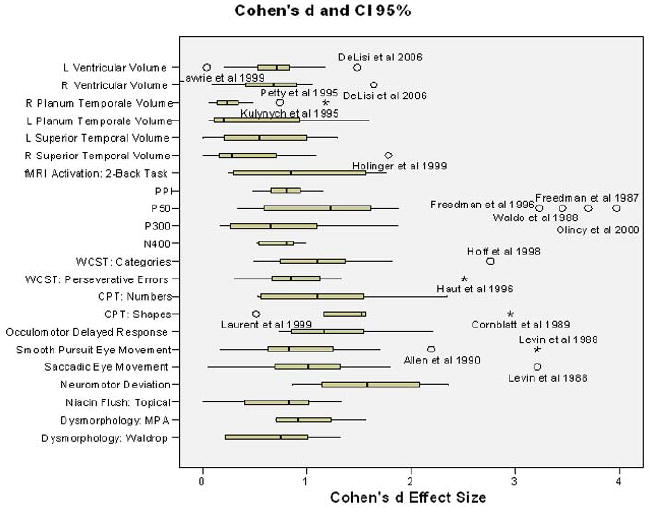
Median effect sizes for each endophenotype are plotted with 95% CI. Outliers (denoted by “o”) and extreme cases (denoted by “*”) are labeled by author and year. Note: Outliers were included in main effects summarization tables.
Figure 2.
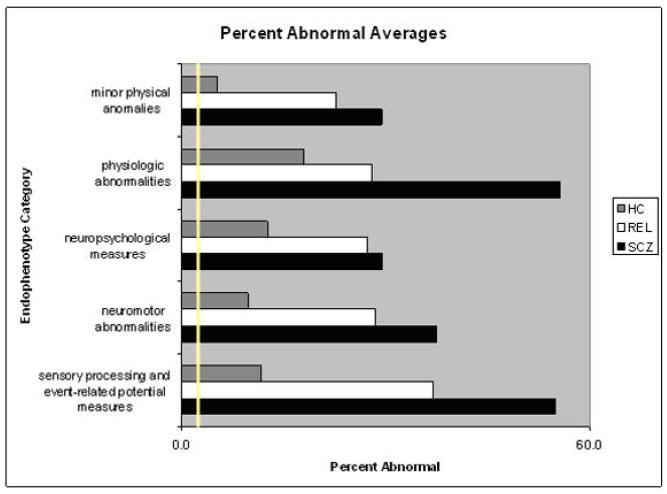
Average percent abnormal for each endophenotype category. The yellow line represents the expected percent abnormal (2.5%). Note: MRI results are not included in this graph, as there were no percent abnormal values for this category.
Discussion
To examine the prevalence of known endophenotypes for schizophrenia in SCZ, REL, and HC, we reviewed the literature to assess the percent of participants in each category with endophenotypic abnormalities. The intention of this review was to provide a summary of prevalence data for endophenotypes, as well as to begin a discussion on these prevalence rates, especially those of the general population who present with endophenotypes at a rate higher than would be expected based on a statistically normal bell curve (10.2 %, with a range of 0 to 34.6%, compared to the expected 2.5%; Keller and Miller, 2006; Pearlson and Folley, 2008, a,b).
Schizophrenia is a complex genetic disorder, where disease risk for a significant but unknown proportion of individuals is likely epistatic and contributed to by many alleles, individually adding little risk (Waddington et al., 2007), although recent reports also stress that in some cases multiple, individually rare mutations altering genes in neurodevelopmental pathways may also contribute to schizophrenia risk (Walsh et al., 2008). Endophenotypes are presumed to have a simpler genetic structure and to be “closer to the relevant genes” than the overt clinical disorder. When viewed within this context, the results support a hypothesis that random mating of healthy individuals in the general population who have single or small numbers of endophenotypic abnormalities may be more likely to produce offspring with greater numbers of abnormal endophenotypes, thus increasing the risk of their developing schizophrenia (Pearlson and Folley, 2008a,b). At this time, what also remains unknown is how the relevant genes operate under this model (for example how to explain increasing risk in specific terms of genetic recombination/inheritance). In part, this reflects multiple unanswered questions regarding relationships between mechanisms operating in the genetic and in the endophenotype domains.
Because studies examining multiple endophenotypes in the same individuals are only very recent, clustering patterns within patients, relatives, and healthy controls currently remain obscure. Understanding the genetic architecture of such related endophenotypes is likely to prove extremely important in better comprehending what constitutes biological risk for schizophrenia (Ritsner & Gottesman, 2009).
It is important to emphasize that one inevitable limitation of a study such as ours, compared to true population genetic studies, is that we gathered information from multiple, previously published but unconnected studies examining individual endophenotypic abnormalities gathered in separate populations. To compare rates of such abnormalities on a “level playing field” and in a maximally informative manner, data would need to be gathered within a single large study, ideally with multiple assessments in the same individuals.
This highlights the importance of large-scale studies of multiple endophenotypes within and between categories of psychosis, such as Consortium on the Genetics of Schizophrenia (COGS; Calkins et al., 2007) and The Bipolar Schizophrenia Network on Intermediate Phenotypes (B-SNIP [http://www.b-snip.org/]; Thaker, 2008). These recent studies have used such approaches across multiple geographic sites using the same equipment and assessment methods with extensive cross-validation and cross-training. An additional advantage of some of these studies is that they address the question of whether endophenotypic risk is specific to schizophrenia or generalizes to psychosis across various conditions, including bipolar disorder.
A major limitation of this review was the relatively modest number of studies that reported percent of abnormal findings within each group, or event sufficient data to allow for a post-hoc calculation of percent abnormal or effect sizes in healthy controls. We propose, based on the findings from this review, that the inclusion of such data in future research on schizophrenia endophenotypes would be beneficial in terms of providing the basis for discussion on the points raised in this paper, as well as allowing for a standardized comparison of findings across the literature as a whole. This issue may be related to our choice of effect size statistic. We chose to use Cohen’s d in the context of a two-group comparison (analogous to a t-test) between SCZ and HC. Although Hedge’s g is often used when sample sizes are different, we did not find dramatic differences between sample sizes of SCZ and HC in the studies that we had investigated, with the one exception of pre-pulse inhibition (F=11.07, p=0.003).
Another limitation, again not addressable in a review article such as this, is the possibility that that the definition of “healthy controls” (i.e., non-relative vs. non-relative with no other psychiatric illness) varied from study to study. In addition, there is a possibility that healthy controls, usually volunteers, were not stringently screened for psychiatric illnesses or family psychiatry psychiatric history, leading to a potential confound in terms of the prevalence of these endophenotypes in the general population. In fact, these “healthy controls” may not be fair representatives of the general population, as discussed elsewhere (Raz et al., 1988; Schwartz and Link, 1989; Shtasel et al., 1991); Smith et al., 1988). In the studies that we reviewed, only a little over half used a structured clinical interview (e.g. SCID-IV-TR), about a third used self-report of no psychiatric history, and the rest either did not screen their healthy controls or did not report their exclusion methods. In addition, the studies we reviewed seldom performed toxicology screens for alcohol or drugs, or at least did not include this information in their methods section.
This issue also relates to the “file drawer” problem when using published studies; it is important to address the general caveat to our findings, as with any systematic review of published data, may inherently misrepresent true population-wide statistics as negative or equivocal findings tend to be under-reported in the literature (Rosenthal, 1979).
A counterargument to the significance of the larger than expected number of HC with endophenotypes is the risk of assuming normality in endophenotypic traits (i.e., that these traits follow a Gaussian bell curve). Unless data are provided to allow a determination of whether or not a trait is normally distributed, normality is assumed. Some traits have been explored for normal distribution trends with different results depending on the measure. Structural brain volume (Lange et al., 1997), including all of the areas that were examined in this study except left superior temporal gyrus, and dysmorphology (Ward et al., 1998), for instance, have been shown to be normally distributed, while some of the neuromotor traits (e.g. smooth pursuit and saccadic eye movement) have not (O’Driscoll et al., 1998), and still others have not yet been explored.
Despite the limitations of this review, these findings provide a basis for future explorations of the prevalence of schizophrenia endophenotpyes in SCZ, REL, and HC. Especially notable is that these biological markers seem to be present in a larger percentage of individuals than would be expected based on our assumptions of statistical normality, and perhaps have an evolutionary role that has yet to be fully uncovered.
Acknowledgments
The authors do not wish to make any acknowledgements.
Role of Funding Source Funding for this study was provided by the following NIMH grants to GDP: R01 MH077945, R37 MH43775 (MERIT Award) and MH074797.
Footnotes
Conflict of Interest None of the authors have a conflict of interest to report.
Contributors Author Pearlson designed the study. Authors Allen and Griss managed the literature search and analysis of the literature, as well as the statistical analysis. Author Allen wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acker W, Toone B. Attention, eye tracking and schizophrenia. British Journal of Social and Clinical Psychology. 1978;17(2):173–181. doi: 10.1111/j.2044-8260.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Adams J, Faux SF, Nestor PG, Shenton M, Marcy B, Smith S, et al. Erp abnormalities during semantic processing in schizophrenia. Schizophrenia Research. 1993;10(3):247–257. doi: 10.1016/0920-9964(93)90059-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler LE, Waldo MC, Freedman R. Neurophysiologic studies of sensory gating in schizophrenia: Comparison of auditory and visual responses. Biological Psychiatry. 1985;20(12):1284–1296. doi: 10.1016/0006-3223(85)90113-1. [DOI] [PubMed] [Google Scholar]
- Adler LE, Waldo MC, Tatcher A, Cawthra E, Baker N, Freedman R. Lack of relationship of auditory gating defects to negative symptoms in schizophrenia. Schizophrenia Research. 1990;3(2):131–138. doi: 10.1016/0920-9964(90)90046-a. [DOI] [PubMed] [Google Scholar]
- Allen JS, Matsunaga K, Hacisalihzade S, Stark L. Smooth pursuit eye movements of normal and schizophrenic subjects tracking an unpredictable target. Biological Psychiatry. 1990;28(8):705–720. doi: 10.1016/0006-3223(90)90457-d. [DOI] [PubMed] [Google Scholar]
- Altman E, Hedeker D, Davis JM, Comaty JE, Jobe TH, Levy DL. Neuropsychological test deficits are associated with smooth pursuit eye movement impairment in affective disorders but not in schizophrenia. International Journal of Clinical Neuropsychology. 1990;12:49–59. [Google Scholar]
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar i disorder or schizophrenia and normal control subjects. Biological Psychiatry. 2004;56(8):560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Amador XF, Sackeim HA, Mukherjee S, Halperin R, Neeley P, Maclin E, et al. Specificity of smooth pursuit eye movement and visual fixation abnormalities in schizophrenia. Comparison to mania and normal controls. Schizophrenia Research. 1991;5(2):135–144. doi: 10.1016/0920-9964(91)90040-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Anderson JE, Wible CG, McCarley RW, Jakab M, Kasai K, Shenton ME. An mri study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophrenia Research. 2002;58(23):123–134. doi: 10.1016/s0920-9964(01)00372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango C, Bartko JJ, Gold JM, Buchanan RW. Prediction of neuropsychological performance by neurological signs in schizophrenia. American Journal of Psychiatry. 1999;156(9):1349–1357. doi: 10.1176/ajp.156.9.1349. [DOI] [PubMed] [Google Scholar]
- Atkinson AEA. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharm and Therapeutics. 2001;89:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Brill LB, 2nd, Royall R, McGilchrist IK, Pulver AE, et al. Planum temporale asymmetry reversal in schizophrenia: Replication and relationship to gray matter abnormalities. American Journal of Psychiatry. 1997;154(5):661–667. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. American Journal of Psychiatry. 1990;147(11):1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Abbruzzese M, Ferri S, Scarone S, Bellodi L, Smeraldi E. An assessment of the wisconsin card sorting test as an indicator of liability to schizophrenia. Schizophrenia Research. 1994;14(1):39–45. doi: 10.1016/0920-9964(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, St Clair DM, Muir WJ, Duffy JC. Auditory p300 and eye tracking dysfunction in schizophrenic pedigrees. Archives of General Psychiatry. 1991;48(10):899–909. doi: 10.1001/archpsyc.1991.01810340031004. [DOI] [PubMed] [Google Scholar]
- Bobes MA, Lei ZX, Ibanez S, Yi H, Valdes-Sosa M. Semantic matching of pictures in schizophrenia: A cross-cultural erp study. Biological Psychiatry. 1996;40(3):189–202. doi: 10.1016/0006-3223(95)00352-5. [DOI] [PubMed] [Google Scholar]
- Boudet C, Bocca ML, Chabot B, Delamillieure P, Brazo P, Denise P, et al. Are eye movement abnormalities indicators of genetic vulnerability to schizophrenia? Eur Psychiatry. 2005;20(4):339–345. doi: 10.1016/j.eurpsy.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Belger A, Campbell D, D’Souza C, Krystal J. Comparison of four components of sensory gating in schizophrenia and normal subjects: A preliminary report. Psychiatry Research. 1999;88(2):119–130. doi: 10.1016/s0165-1781(99)00074-8. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacologia. 2001;156(23):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, et al. The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous wisconsin card sorting test results. Archives of General Psychiatry. 1991;48(10):891–898. doi: 10.1001/archpsyc.1991.01810340023003. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. American Journal of Psychiatry. 1999;156(4):596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Bramon E, McDonald C, Croft RJ, Landau S, Filbey F, Gruzelier JH, et al. Is the p300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. NeuroImage. 2005;27(4):960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Bryant NL, Buchanan RW, Vladar K, Breier A, Rothman M. Gender differences in temporal lobe structures of patients with schizophrenia: A volumetric mri study. American Journal of Psychiatry. 1999;156(4):603–609. doi: 10.1176/ajp.156.4.603. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Heinrichs DW. The neurological evaluation scale (nes): A structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Research. 1989;27(3):335–350. doi: 10.1016/0165-1781(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Yang S, Hazlett E, Siegel BV, Jr, Germans M, Haznedar M, et al. Ventricular volume and asymmetry in schizotypal personality disorder and schizophrenia assessed with magnetic resonance imaging. Schizophrenia Research. 1997;27(1):45–53. doi: 10.1016/S0920-9964(97)00087-X. [DOI] [PubMed] [Google Scholar]
- Cadenhead K, Kumar C, Braff D. Clinical and experimental characteristics of “hypothetically psychosis prone” college students. Journal of Psychiatric Research. 1996;30(5):331–340. doi: 10.1016/0022-3956(96)00020-9. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. American Journal of Psychiatry. 1993;150(12):1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, Geyer MA, Braff DL. Sensory gating deficits assessed by the p50 event-related potential in subjects with schizotypal personality disorder. American Journal of Psychiatry. 2000;157(1):55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Perry W, Shafer K, Braff DL. Cognitive functions in schizotypal personality disorder. Schizophrenia Research. 1999;37(2):123–132. doi: 10.1016/s0920-9964(98)00147-9. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. The Consortium on the Genetics of Endophenotypes in Schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr Bull. 2007;33(1):33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cerebral Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Campbell T, Osipova D, Kähkönenb S. Finland’s galapagos: Founder effect, drift, and isolation in the inheritance of susceptibility alleles. Behav Brain Sci. 2006;29:409–410. [Google Scholar]
- Chan RC, Gottesman II. Neurological soft signs as candidate endophenotypes for schizophrenia: A shooting star or a Northern star? Neurosci Biobehav Rev. 2008;32(5):951–957. doi: 10.1016/j.neubiorev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Chen YL, Chen YH, Mak FL. Soft neurological signs in schizophrenic patients and their nonpsychotic siblings. Journal of Nervous and Mental Disease. 2000;188(2):84–89. doi: 10.1097/00005053-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Chua SE, Cheung C, Cheung V, Tsang JT, Chen EY, Wong JC, et al. Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophrenia Research. 2007;89(13):12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff D. P50 suppression among schizophrenia and normal comparison subjects: A methodological analysis. Biological Psychiatry. 1997;41:1035–1044. doi: 10.1016/S0006-3223(96)00208-9. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Grove WM, Iacono WG, Sweeney JA. Smooth-pursuit eye movement dysfunction and liability for schizophrenia: Implications for genetic modeling. Journal of Abnormal Psychology. 1992;101(1):117–129. doi: 10.1037//0021-843x.101.1.117. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Iacono WG, Beiser M. Handedness in first-episode psychotic patients and their first-degree biological relatives. Journal of Abnormal Psychology. 1994;103(2):400–403. [PubMed] [Google Scholar]
- Clementz BA, McDowell JE, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. Journal of Abnormal Psychology. 1994;103(2):277–287. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Coleman MJ, Cook S, Matthysse S, Barnard J, Lo Y, Levy DL, et al. Spatial and object working memory impairments in schizophrenia patients: A bayesian item-response theory analysis. J Abnorm Psychol. 2002;111(3):425–435. doi: 10.1037//0021-843x.111.3.425. [DOI] [PubMed] [Google Scholar]
- Condray R, Steinhauer SR, Cohen JD, van Kammen DP, Kasparek A. Modulation of language processing in schizophrenia: Effects of context and haloperidol on the event-related potential. Biological Psychiatry. 1999;45(10):1336–1355. doi: 10.1016/s0006-3223(98)00317-5. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Risch N, Faris G, Friedman D, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version (cpt-ip): I. New findings about sustained attention in normal families. Psychiatry Research. 1988;26(2):223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: Ii. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Research. 1989;29(1):65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Morris J, et al. Sustained attention in young people at high risk for schizophrenia. Psychological Medicine. 2002;32(2):277–286. doi: 10.1017/s0033291701005050. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW. Saccadic eye movements in families multiply affected with schizophrenia: The maudsley family study. American Journal of Psychiatry. 1998;155(12):1703–1710. doi: 10.1176/ajp.155.12.1703. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim JJ, Chemerinski E, Magnotta V, Andreasen NC, Nopoulos P. Morphometry of the superior temporal plane in schizophrenia: Relationship to clinical correlates. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16(3):284–294. doi: 10.1176/jnp.16.3.284. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, et al. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophrenia Research. 1993;10(2):131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Hazlett EA, Nuechterlein KH, Filion DL. On the clinical and cognitive meaning of impaired sensorimotor gating in schizophrenia. Psychiatry Research. 2000;96(3):187–197. doi: 10.1016/s0165-1781(00)00208-0. [DOI] [PubMed] [Google Scholar]
- de Wilde OM, Bour LJ, Dingemans PM, Koelman JHTM, Linszen DH. Failure to find p50 suppression deficits in young first-episode patients with schizophrenia and clinically unaffected siblings. Schizophr Bull. 2007:sbm001. doi: 10.1093/schbul/sbm001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degreef G, Ashtari M, Bogerts B, Bilder RM, Jody DN, Alvir JM, et al. Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Archives of General Psychiatry. 1992;49(7):531–537. doi: 10.1001/archpsyc.1992.01820070025004. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Hoff AL. Failure to find progressive temporal lobe volume decreases 10 years subsequent to a first episode of schizophrenia. Psychiatry Research. 2005;138(3):265–268. doi: 10.1016/j.pscychresns.2005.02.005. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Hoff AL, Neale C, Kushner M. Asymmetries in the superior temporal lobe in male and female first-episode schizophrenic patients: Measures of the planum temporale and superior temporal gyrus by mri. Schizophrenia Research. 1994;12(1):19–28. doi: 10.1016/0920-9964(94)90080-9. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Research. 2004;130(1):57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Szulc KU, Bertisch H, Majcher M, Brown K, Bappal A, et al. Early detection of schizophrenia by diffusion weighted imaging. Psychiatry Research. 2006;148(1):61–66. doi: 10.1016/j.pscychresns.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Frumin M, Niznikiewicz MA, Hirayasu Y, et al. Smaller left heschl’s gyrus volume in patients with schizotypal personality disorder. American Journal of Psychiatry. 2002;159(9):1521–1527. doi: 10.1176/appi.ajp.159.9.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, et al. Schizotypal personality disorder and mri abnormalities of temporal lobe gray matter. Biological Psychiatry. 1999;45(11):1393–1402. doi: 10.1016/s0006-3223(99)00030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, Shenton ME, Hirayasu Y, Fischer I, Voglmaier MM, Niznikiewicz MA, et al. Large csf volume not attributable to ventricular volume in schizotypal personality disorder. American Journal of Psychiatry. 2000;157(1):48–54. doi: 10.1176/ajp.157.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieci M, Vita A, Silenzi C, Caputo A, Comazzi M, Ferrari L, et al. Non-selective impairment of wisconsin card sorting test performance in patients with schizophrenia. Schizophrenia Research. 1997;25(1):33–42. doi: 10.1016/S0920-9964(96)00125-9. [DOI] [PubMed] [Google Scholar]
- Dragovic M, Hammond G. Handedness in schizophrenia: A quantitative review of evidence. Acta Psychiatrica Scandinavica. 2005;111(6):410–419. doi: 10.1111/j.1600-0447.2005.00519.x. [DOI] [PubMed] [Google Scholar]
- Drakeford JL, Edelstyn NM, Oyebode F, Srivastava S, Calthorpe WR, Mukherjee T. Auditory recognition memory, conscious recollection, and executive function in patients with schizophrenia. Psychopathology. 2006;39(4):199–208. doi: 10.1159/000093524. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biological Psychiatry. 2001;50(2):98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Egan MF, Hyde TM, Bonomo JB, Mattay VS, Bigelow LB, Goldberg TE, et al. Relative risk of neurological signs in siblings of patients with schizophrenia. American Journal of Psychiatry. 2001;158(11):1827–1834. doi: 10.1176/appi.ajp.158.11.1827. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Picchioni M, Hall MH, Schulze K, Toulopoulou T, Landau S, et al. Antisaccade performance in monozygotic twins discordant for schizophrenia: The maudsley twin study. American Journal of Psychiatry. 2006;163(3):543–545. doi: 10.1176/appi.ajp.163.3.543. [DOI] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Schneider T, Greve B, Pfeiffer U, Pilz K, et al. Disturbed planum temporale asymmetry in schizophrenia. A quantitative postmortem study. Schizophrenia Research. 1995;14(2):161–176. doi: 10.1016/0920-9964(94)00035-7. [DOI] [PubMed] [Google Scholar]
- Faux SF, Shenton ME, McCarley RW, Nestor PG, Marcy B, Ludwig A. Preservation of p300 event-related potential topographic asymmetries in schizophrenia with use of either linked-ear or nose reference sites. Electroencephalography and Clinical Neurophysiology. 1990;75(5):378–391. doi: 10.1016/0013-4694(90)90083-v. [DOI] [PubMed] [Google Scholar]
- Fleming K, Goldberg TE, Binks S, Randolph C, Gold JM, Weinberger DR. Visuospatial working memory in patients with schizophrenia. Biol Psychiatry. 1997;41(1):43–49. doi: 10.1016/s0006-3223(96)00263-6. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Marsh L, Faustman WO, Harris D, Hoff AL, et al. P300 amplitude is related to clinical state in severely and moderately ill patients with schizophrenia. Biological Psychiatry. 1999;46(1):94–101. doi: 10.1016/s0006-3223(98)00290-x. [DOI] [PubMed] [Google Scholar]
- Frangou S, Sharma T, Sigmudsson T, Barta P, Pearlson G, Murray RM. The maudsley family study. 4. Normal planum temporale asymmetry in familial schizophrenia. A volumetric mri study. British Journal of Psychiatry. 1997;170:328–333. doi: 10.1192/bjp.170.4.328. [DOI] [PubMed] [Google Scholar]
- Franke P, Maier W, Hain C, Klingler T. Wisconsin card sorting test: An indicator of vulnerability to schizophrenia? Schizophrenia Research. 1992;6(3):243–249. doi: 10.1016/0920-9964(92)90007-r. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, et al. Neurobiological studies of sensory gating in schizophrenia. Schizophrenia Bulletin. 1987;13(4):669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- Freedman R, A L, Myles-Worsley M, Nagamato HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M. Inhibiotry gating of an evoked response to repeated auditory stimuli in schizophrenic and dnormal subjects. Human recordings, computer simulation, and an animal model. Archives of General Psychiatry. 1996;53(12):1114–1121. doi: 10.1001/archpsyc.1996.01830120052009. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M. Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biological Psychiatry. 1988;23(7):670–677. doi: 10.1016/0006-3223(88)90050-9. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Yeo RA. Mutations, developmental instability, and the red queen. Behavioral and Brain Sciences. 2006;29(04):412–413. [Google Scholar]
- Giotakos O. Narrow and broad definition of mixed-handedness in male psychiatric patients. Perceptual and Motor Skills. 2001;93(3):631–638. doi: 10.2466/pms.2001.93.3.631. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Cannon TD, Gur RE, Ragland JD, Gur RC. Working memory constrains abstraction in schizophrenia. Biological Psychiatry. 2000;47(1):34–42. doi: 10.1016/s0006-3223(99)00187-0. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and wisconsin card sorting test performance in schizophrenia. Archives of General Psychiatry. 1997;54(2):159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Aloia MS, Gourovitch ML, Missar D, Pickar D, Weinberger DR. Cognitive substrates of thought disorder, i: The semantic system. American Journal of Psychiatry. 1998;155(12):1671–1676. doi: 10.1176/ajp.155.12.1671. comment. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Archives of General Psychiatry. 2002;59(2):154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Kwapil TR, Tallent KA. Wisconsin card sorting test deficits in schizotypic individuals. Schizophrenia Research. 1999;40(3):201–209. doi: 10.1016/s0920-9964(99)00124-3. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA. Spatial working memory performance in patients with schizoaffective psychosis versus schizophrenia: A tale of two disorders? Schizophrenia Research. 2002;53(3):209–218. doi: 10.1016/s0920-9964(01)00258-4. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA. Nonverbal working memory deficits in schizophrenia patients: Evidence of a supramodal executive processing deficit. Schizophr Res. 2004;68(23):189–201. doi: 10.1016/j.schres.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Hegyi JV. Cognitive slippage in schizotypic individuals. Journal of Nervous and Mental Disease. 2001;189:750–756. doi: 10.1097/00005053-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Gourion D, Goldberger C, Bourdel M-C, Jean Bayle F, Lôo H, Krebs M-O. Minor physical anomalies in patients with schizophrenia and their parents: Prevalence and pattern of craniofacial abnormalities. Psychiatry Research. 2004;125(1):21–28. doi: 10.1016/j.psychres.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Gourion D, Goldberger C, Olie JP, Loo H, Krebs MO. Neurological and morphological anomalies and the genetic liability to schizophrenia: A composite phenotype. Schizophrenia Research. 2004;67(1):23–31. doi: 10.1016/s0920-9964(03)00099-9. [DOI] [PubMed] [Google Scholar]
- Green MF, Satz P, Christenson C. Minor physical anomalies in schizophrenia patients, bipolar patients, and their siblings. Schizophrenia Bulletin. 1994;20(3):433–440. doi: 10.1093/schbul/20.3.433. [DOI] [PubMed] [Google Scholar]
- Green MF, Satz P, Gaier DJ, Ganzell S, Kharabi F. Minor physical anomalies in schizophrenia. Schizophrenia Bulletin. 1989;15(1):91–99. doi: 10.1093/schbul/15.1.91. [DOI] [PubMed] [Google Scholar]
- Green MF, Satz P, Smith C, Nelson L. Is there atypical handedness in schizophrenia? Journal of Abnormal Psychology. 1989;98(1):57–61. doi: 10.1037//0021-843x.98.1.57. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Sigmundsson T, Takei N, Frangou S, Birkett PB, Sharma T, et al. Minor physical anomalies in familial and sporadic schizophrenia: The maudsley family study. Journal of Neurology, Neurosurgery and Psychiatry. 1998;64(1):56–60. doi: 10.1136/jnnp.64.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Glazer WM. N400 and semantic categorization in schizophrenia. Biol Psychiatry. 1991;29(5):467–480. doi: 10.1016/0006-3223(91)90269-r. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Adams A, Shen CD, Loiselle D. Minor physical anomalies in alcoholic and schizophrenic adults and hyperactive and autistic children. American Journal of Psychiatry. 1982;139(5):640–643. doi: 10.1176/ajp.139.5.640. [DOI] [PubMed] [Google Scholar]
- Gureje O. Sensorimotor laterality in schizophrenia: Which features transcend cultural influences? Acta Psychiatrica Scandinavica. 1988;77(2):188–193. doi: 10.1111/j.1600-0447.1988.tb05099.x. [DOI] [PubMed] [Google Scholar]
- Hardy GH. Mendelian proportions in a mixed population. Science. 1908;28:49–50. doi: 10.1126/science.28.706.49. [DOI] [PubMed] [Google Scholar]
- Haut MW, Cahill J, Cutlip WD, Stevenson JM, Makela EH, Bloomfield SM. On the nature of wisconsin card sorting test performance in schizophrenia. Psychiatry Research. 1996;65(1):15–22. doi: 10.1016/0165-1781(96)02940-x. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. In search of madness: Schizophrenia and neuroscience. New York: Oxford University Press; 2001. [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and heschl gyrus volume reduction in schizophrenia: A magnetic resonance imaging study of first-episode patients. Archives of General Psychiatry. 2000;57(7):692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE. Neuropsychological functioning of first-episode schizophreniform patients. American Journal of Psychiatry. 1992;149(7):898–903. doi: 10.1176/ajp.149.7.898. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Wieneke M, Faustman WO, Horon R, Sakuma M, Blankfeld H, et al. Sex differences in neuropsychological functioning of first-episode and chronically ill schizophrenic patients. American Journal of Psychiatry. 1998;155(10):1437–1439. doi: 10.1176/ajp.155.10.1437. [DOI] [PubMed] [Google Scholar]
- Holahan AL, O’Driscoll GA. Antisaccade and smooth pursuit performance in positive- and negative-symptom schizotypy. Schizophrenia Research. 2005;76(1):43–54. doi: 10.1016/j.schres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Holinger DP, Shenton ME, Wible CG, Donnino R, Kikinis R, Jolesz FA, et al. Superior temporal gyrus volume abnormalities and thought disorder in left-handed schizophrenic men. American Journal of Psychiatry. 1999;156(11):1730–1735. doi: 10.1176/ajp.156.11.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman PS, Proctor LR, Hughes DW. Eye-tracking patterns in schizophrenia. Science. 1973;181(95):179–181. doi: 10.1126/science.181.4095.179. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Wonodi I, Adami H, Buchanan RW, Thaker GK. Independent domains of inhibitory gating in schizophrenia and the effect of stimulus interval. Am J Psychiatry. 2007;164(1):61–65. doi: 10.1176/ajp.2007.164.1.61. [DOI] [PubMed] [Google Scholar]
- Hudson C, Gotowiec A, Seeman M, Warsh J, Ross BM. Clinical subtyping reveals significant differences in calcium-dependent phospholipase a2 activity in schizophrenia. Biological Psychiatry. 1999;46(3):401–405. doi: 10.1016/s0006-3223(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Hudson CJ, Lin A, Cogan S, Cashman F, Warsh JJ. The niacin challenge test: Clinical manifestation of altered transmembrane signal transduction in schizophrenia? Biological Psychiatry. 1997;41(5):507–513. doi: 10.1016/s0006-3223(96)00112-6. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Moreau M, Beiser M, Fleming JA, Lin TY. Smooth-pursuit eye tracking in first-episode psychotic patients and their relatives. Journal of Abnormal Psychology. 1992;101(1):104–116. [PubMed] [Google Scholar]
- Ismail B, Cantor-Graae E, McNeil TF. Minor physical anomalies in schizophrenic patients and their siblings. American Journal of Psychiatry. 1998a;155(12):1695–1702. doi: 10.1176/ajp.155.12.1695. [DOI] [PubMed] [Google Scholar]
- Ismail B, Cantor-Graae E, McNeil TF. Neurological abnormalities in schizophrenic patients and their siblings. American Journal of Psychiatry. 1998b;155(1):84–89. doi: 10.1176/ajp.155.1.84. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: A parametric fmri study. Schizophrenia Research. 2004;68(23):159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- Jin Y, Potkin SG, Patterson JV, Sandman CA, Hetrick WP, Bunney WE., Jr Effects of p50 temporal variability on sensory gating in schizophrenia. Psychiatry Research. 1997;70(2):71–81. doi: 10.1016/s0165-1781(97)03091-6. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of p50 erp amplitude and suppression in schizophrenia. Schizophrenia Research. 2005;78(23):269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Jones AM, Pivik RT. Vestibular activation, smooth pursuit tracking, and psychosis. Psychiatry Research. 1985;14(4):291–308. doi: 10.1016/0165-1781(85)90097-6. [DOI] [PubMed] [Google Scholar]
- Josman N, Katz N. Relationships of categorization on tests and daily tasks in patients with schizophrenia, post-stroke patients and healthy controls. Psychiatry Research. 2006;141(1):15–28. doi: 10.1016/j.psychres.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Kathmann N, Engel RR. Sensory gating in normals and schizophrenics: A failure to find strong p50 suppression in normals. Biological Psychiatry. 1990;27(11):1216–1226. doi: 10.1016/0006-3223(90)90419-3. comment. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Siever LJ, Mohs RC, Peterson AE, Mahon TR, Bergman RL, et al. Eye tracking, schizophrenic symptoms, and schizotypal personality disorder. European Archives of Psychiatry and Neurological Sciences. 1989;239(1):39–42. doi: 10.1007/BF01739742. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Silverman JM, Roitman SE, Harvey PD, Duncan MA, Alroy D, et al. Performance of nonpsychotic relatives of schizophrenic patients on cognitive tests. Psychiatry Research. 1994;53(1):1–12. doi: 10.1016/0165-1781(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Keller MC, Miller G. Resolving the paradox of common, harmful, heritable mental disorders: Which evolutionary genetic models work best? Behavioral and Brain Sciences. 2006;20:385–452. doi: 10.1017/S0140525X06009095. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR, Jr, Cadet JL, Pickar D, Weinberger DR. Quantitative neuroanatomy in schizophrenia. A controlled magnetic resonance imaging study. Archives of General Psychiatry. 1988;45(6):533–541. doi: 10.1001/archpsyc.1988.01800300029003. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: A neurocognitive approach. Psychological Medicine. 2001;31(5):915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, “just the facts”: What we know in 2008: Part 3: Neurobiology. Schizophrenia Research. 2008;106(23):89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Crespo-Facorro B, Andreasen NC, O’Leary DS, Magnotta V, Nopoulos P. Morphology of the lateral superior temporal gyrus in neuroleptic naive patients with schizophrenia: Relationship to symptoms. Schizophrenia Research. 2003;60:173–181. doi: 10.1016/s0920-9964(02)00299-2. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Levy DL, Yurgelun-Todd DA, Tramer SJ, Holzman PS. Inverse relationship of perinatal complications and eye tracking dysfunction in relatives of patients with schizophrenia: Evidence for a two-factor model. American Journal of Psychiatry. 1998;155(7):976–978. doi: 10.1176/ajp.155.7.976. [DOI] [PubMed] [Google Scholar]
- Koyama S, Nageishi Y, Shimokochi M, Hokama H, Miyazato Y, Miyatani M, et al. The n400 component of event-related potentials in schizophrenic patients: A preliminary study. Electroencephalography and Clinical Neurophysiology. 1991;78(2):124–132. doi: 10.1016/0013-4694(91)90112-h. [DOI] [PubMed] [Google Scholar]
- Kulynych JJ, Vladar K, Fantie BD, Jones DW, Weinberger DR. Normal asymmetry of the planum temporale in patients with schizophrenia. Three-dimensional cortical morphometry with mri. British Journal of Psychiatry. 1995;166(6):742–749. doi: 10.1192/bjp.166.6.742. [DOI] [PubMed] [Google Scholar]
- Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Superior temporal gyrus volume in schizophrenia: A study using mri morphometry assisted by surface rendering. American Journal of Psychiatry. 1996;153(1):50–56. doi: 10.1176/ajp.153.9.A50. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Mathew VM, Sharma T. Prepulse inhibition of the startle response in men with schizophrenia: Effects of age of onset of illness, symptoms, and medication. Archives of General Psychiatry. 2000;57(6):609–614. doi: 10.1001/archpsyc.57.6.609. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. American Journal of Psychiatry. 1999;156(7):1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, et al. Left planum temporale volume reduction in schizophrenia. Archives of General Psychiatry. 1999;56(2):142–148. doi: 10.1001/archpsyc.56.2.142. [DOI] [PubMed] [Google Scholar]
- Lange N, Giedd JN, Castellanos FX, Vaituzis AC, Rapoport JL. Variability of human brain structure: Ages 4-20 years. Psychiatry Research: Neuroimaging. 1997;74:1–12. doi: 10.1016/s0925-4927(96)03054-5. [DOI] [PubMed] [Google Scholar]
- Laurent A, Biloa-Tang M, Bougerol T, Duly D, Anchisi AM, Bosson JL, et al. Executive/attentional performance and measures of schizotypy in patients with schizophrenia and in their nonpsychotic first-degree relatives. Schizophrenia Research. 2000;46(23):269–283. doi: 10.1016/s0920-9964(99)00232-7. [DOI] [PubMed] [Google Scholar]
- Laurent A, Duly D, Murry P, Foussard N, Boccara S, Mingat F, et al. Wcst performance and schizotypal features in the first-degree relatives of patients with schizophrenia. Psychiatry Research. 2001;104(2):133–144. doi: 10.1016/s0165-1781(01)00306-7. [DOI] [PubMed] [Google Scholar]
- Laurent A, Saoud M, Bougerol T, d’Amato T, Anchisi AM, Biloa-Tang M, et al. Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Research. 1999;89(3):147–159. doi: 10.1016/s0165-1781(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, et al. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999a;353:30–33. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, et al. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999b;353(9146):30–33. doi: 10.1016/S0140-6736(98)06244-8. comment. [DOI] [PubMed] [Google Scholar]
- Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, et al. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Arch Gen Psychiatry. 2003;60(3):238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- Levin S, Holzman PS, Rothenberg SJ, Lipton RB. Saccadic eye movements in psychotic patients. Psychiatry Research. 1981;5(1):47–58. doi: 10.1016/0165-1781(81)90060-3. [DOI] [PubMed] [Google Scholar]
- Levin S, Luebke A, Zee DS, Hain TC, Robinson DA, Holzman PS. Smooth pursuit eye movements in schizophrenics: Quantitative measurements with the search-coil technique. Journal of Psychiatric Research. 1988;22(3):195–206. doi: 10.1016/0022-3956(88)90005-2. [DOI] [PubMed] [Google Scholar]
- Levy DL, Bogerts B, Degreef G, Dorogusker B, Waternaux C, Ashtari M, et al. Normal eye tracking is associated with abnormal morphology of medial temporal lobe structures in schizophrenia. Schizophrenia Research. 1992;8(1):1–10. doi: 10.1016/0920-9964(92)90055-a. [DOI] [PubMed] [Google Scholar]
- Levy DL, Lajonchere CM, Dorogusker B, Min D, Lee S, Tartaglini A, et al. Quantitative characterization of eye tracking dysfunction in schizophrenia. Schizophrenia Research. 2000;42(3):171–185. doi: 10.1016/s0920-9964(99)00122-x. [DOI] [PubMed] [Google Scholar]
- Lin S, Liu C, Chang S, Hwu H, Liu SK, Hwang TJ, et al. Familial aggregation in skin flush response to niacin patch among schizophrenic patients and their nonpsychotic relatives. Schizophr Bull. 2007;33(1):174–182. doi: 10.1093/schbul/sbl038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JB, Flynn K. Minor physical anomalies in schizophrenia and mood disorders. Schizophrenia Bulletin. 1993;19(3):551–556. doi: 10.1093/schbul/19.3.551. [DOI] [PubMed] [Google Scholar]
- Louchart-de la Chapelle S, Nkam I, Houy E, Belmont A, Menard J, Roussignol A, et al. A concordance study of three electrophysiological measures in schizophrenia. Am J Psychiatry. 2005;162(3):466–474. doi: 10.1176/appi.ajp.162.3.466. [DOI] [PubMed] [Google Scholar]
- Maccabe JH, Simon H, Zanelli JW, Walwyn R, McDonald CD, Murray RM. Saccadic distractibility is elevated in schizophrenia patients, but not in their unaffected relatives. Psychological Medicine. 2005;35(12):1727–1736. doi: 10.1017/S0033291705005738. [DOI] [PubMed] [Google Scholar]
- Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naive schizophrenic patients. Biological Psychiatry. 2002;52(9):863–873. doi: 10.1016/s0006-3223(02)01409-9. [DOI] [PubMed] [Google Scholar]
- Malesu RR, Cannon M, Jones PB, McKenzie K, Gilvarry K, Rifkin L, et al. Mixed-handedness in patients with functional psychosis. British Journal of Psychiatry. 1996;168(2):234–236. doi: 10.1192/bjp.168.2.234. [DOI] [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Rucklos ME, Vereen DR, Jr, Ader DN. Deficient motor synchrony in schizophrenia. Journal of Abnormal Psychology. 1981;90(4):321–328. doi: 10.1037//0021-843x.90.4.321. [DOI] [PubMed] [Google Scholar]
- Marsh L, Harris D, Lim KO, Beal M, Hoff AL, Minn K, et al. Structural magnetic resonance imaging abnormalities in men with severe chronic schizophrenia and an early age at clinical onset. Archives of General Psychiatry. 1997;54(12):1104–1112. doi: 10.1001/archpsyc.1997.01830240060009. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Rosenbloom M, Pfefferbaum A. P300 reduction and prolongation with illness duration in schizophrenia. Biological Psychiatry. 2000;47(5):413–427. doi: 10.1016/s0006-3223(99)00151-1. [DOI] [PubMed] [Google Scholar]
- Matthysse S, Holzman PS, Lange K. The genetic transmission of schizophrenia: Application of mendelian latent structure analysis to eye tracking dysfunctions in schizophrenia and affective disorder. Journal of Psychiatric Research. 1986;20(1):57–67. doi: 10.1016/0022-3956(86)90023-3. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal p300 amplitude in first-episode schizophrenia. Archives of General Psychiatry. 2002;59(4):321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton ME, O’Donnell BF, Faux SF, Kikinis R, Nestor PG, et al. Auditory p300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Archives of General Psychiatry. 1993;50(3):190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, et al. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. American Journal of Psychiatry. 2006;163(3):478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- McDowd JM, Filion DL, Harris MJ, Braff DL. Sensory gating and inhibitory function in late-life schizophrenia. Schizophrenia Bulletin. 1993;19(4):733–746. doi: 10.1093/schbul/19.4.733. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Myles-Worsley M, Coon H. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. J Abnorm Psychol. 1999;36:138–141. doi: 10.1017/s0048577299980836. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Frodl T, Muller D, Schmitt G, Gallinat J, Zetzsche T, et al. Superior temporal gyrus and p300 in schizophrenia: A combined erp/structural magnetic resonance imaging investigation. Journal of Psychiatric Research. 2004;38(2):153–162. doi: 10.1016/s0022-3956(03)00078-5. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Scheuerecker J, Zipse M, Ufer S, Wiesmann M, Frodl T, et al. Effects of treatment with the atypical neuroleptic quetiapine on working memory function: A functional mri follow-up investigation. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:522–531. doi: 10.1007/s00406-006-0687-x. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Zetzsche T, Preuss U, Frodl T, Leinsinger G, Moller HJ. Does the definition of borders of the planum temporale influence the results in schizophrenia? American Journal of Psychiatry. 2002;159(7):1198–1200. doi: 10.1176/appi.ajp.159.7.1198. [DOI] [PubMed] [Google Scholar]
- Merrin EL, Floyd TC, Deicken RF, Lane PA. The wisconsin card sort test and p300 responses to novel auditory stimuli in schizophrenic patients. International Journal of Psychophysiology. 2006;60(3):330–348. doi: 10.1016/j.ijpsycho.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Minor K, Park S. Spatial working memory: Absence of gender differences in schizophrenia patients and healthy control subjects. Biol Psychiatry. 1999;46(7):1003–1005. doi: 10.1016/s0006-3223(99)00149-3. [DOI] [PubMed] [Google Scholar]
- Morrens M, Hulstijn W, Lewi PJ, De Hert M, Sabbe BG. Stereotypy in schizophrenia. Schizophrenia Research. 2006;84(23):397–404. doi: 10.1016/j.schres.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M. P50 sensory gating in multiplex schizophrenia families from a pacific island isolate. American Journal of Psychiatry. 2002;159(12):2007–2012. doi: 10.1176/appi.ajp.159.12.2007. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Freedman R. Sensory gating in schizophrenics and normal controls: Effects of changing stimulation interval. Biological Psychiatry. 1989;25(5):549–561. doi: 10.1016/0006-3223(89)90215-1. [DOI] [PubMed] [Google Scholar]
- Neel J. Diabetes mellitus: A “thrifty” genotype rendered detrimental by “progress”. American Journal of Human Genetics. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- Nelson LD, Satz P, Green M, Cicchetti D. Re-examining handedness in schizophrenia: Now you see it--now you don’t. Journal of Clinical and Experimental Neuropsychology. 1993;15(2):149–158. doi: 10.1080/01688639308402553. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kimble MO, O’Donnell BF, Smith L, Niznikiewicz M, Shenton ME, et al. Aberrant semantic activation in schizophrenia: A neurophysiological study. American Journal of Psychiatry. 1997;154(5):640–646. doi: 10.1176/ajp.154.5.640. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, Shenton ME, Voglmaier M, Nestor PG, Dickey CC, Frumin M, et al. Semantic dysfunction in women with schizotypal personality disorder. American Journal of Psychiatry. 2002;159(10):1767–1774. doi: 10.1176/appi.ajp.159.10.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niznikiewicz MA, Voglmaier M, Shenton ME, Seidman LJ, Dickey CC, Rhoads R, et al. Electrophysiological correlates of language processing in schizotypal personality disorder. American Journal of Psychiatry. 1999;156(7):1052–1058. doi: 10.1176/ajp.156.7.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiols JE, Clos M, Corbero E, Garcia-Domingo M, de Trincheria I, Domenech E. Sustained attention deficit in young schizophrenic and schizotypic men. Psychological Reports. 1992;71(3 Pt 2):1131–1136. doi: 10.2466/pr0.1992.71.3f.1131. [DOI] [PubMed] [Google Scholar]
- O’Callaghan E, Buckley P, Madigan C, Redmond O, Stack JP, Kinsella A, et al. The relationship of minor physical anomalies and other putative indices of developmental disturbance in schizophrenia to abnormalities of cerebral structure on magnetic resonance imaging. Biological Psychiatry. 1995;38(8):516–524. doi: 10.1016/0006-3223(94)00381-C. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Lenzenweger MF, Holzman PS. Antisaccades and smooth pursuit eye tracking and schizotypy. Archives of General Psychiatry. 1998;55(9):837–843. doi: 10.1001/archpsyc.55.9.837. [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Barcellos LF. Multiple sclerosis genetics: Leaving no stone unturned. Genes and Immunity. 2005;6:375–387. doi: 10.1038/sj.gene.6364237. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Harris JG, Young DA, McAndrews MA, Cawthra E, et al. The p50 auditory event-evoked potential in adult attention-deficit disorder: Comparison with schizophrenia. Biological Psychiatry. 2000;47(11):969–977. doi: 10.1016/s0006-3223(00)00239-0. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: An mri study. American Journal of Psychiatry. 2004;161(9):1603–1611. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje B, Van Oel CJ, Gispen-De Wied CC, Verbaten MN, Kahn RS. Effects of typical and atypical antipsychotics on the prepulse inhibition of the startle reflex in patients with schizophrenia. Journal of Clinical Psychopharmacology. 2002;22(4):359–365. doi: 10.1097/00004714-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Park S. Association of an oculomotor delayed response task and the wisconsin card sort test in schizophrenic patients. Int J Psychophysiol. 1997;27(2):147–151. doi: 10.1016/s0167-8760(97)00045-7. [DOI] [PubMed] [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biological Psychiatry. 2000;47(7):662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Folley BS. Schizophrenia, psychiatric genetics, and darwinian psychiatry: An evolutionary framework. Schizophr Bull. 2008a doi: 10.1093/schbul/sbm130. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Folley BS. Endophenotypes, dimensions, risks: is psychosis analogous to common inherited medical illnesses? Clin EEG Neurosci. 2008b;39(2):73–77. doi: 10.1177/155005940803900210. [DOI] [PubMed] [Google Scholar]
- Permutt MA, Wasson J, Cox N. Genetic epidemiology of diabetes. Journal of Clinical Investigation. 2005;115(6):1431–1439. doi: 10.1172/JCI24758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Braff DL. A multimethod approach to assessing perseverations in schizophrenia patients. Schizophrenia Research. 1998;33(12):69–77. doi: 10.1016/s0920-9964(98)00061-9. [DOI] [PubMed] [Google Scholar]
- Perry W, Feifel D, Minassian A, Bhattacharjie I, Braff DL. Information processing deficits in acutely psychotic schizophrenia patients medicated and unmedicated at the time of admission. American Journal of Psychiatry. 2002;159(8):1375–1381. doi: 10.1176/appi.ajp.159.8.1375. [DOI] [PubMed] [Google Scholar]
- Petty RG, Barta PE, Pearlson GD, McGilchrist IK, Lewis RW, Tien AY, et al. Reversal of asymmetry of the planum temporale in schizophrenia. American Journal of Psychiatry. 1995;152(5):715–721. doi: 10.1176/ajp.152.5.715. [DOI] [PubMed] [Google Scholar]
- Price GW, Michie PT, Johnston J, Innes-Brown H, Kent A, Clissa P, et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the western australian family study of schizophrenia. Biological Psychiatry. 2006;60(1):1–10. doi: 10.1016/j.biopsych.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Puri BK, Easton T, Das I, Kidane L, Richardson AJ. The niacin skin flush test in schizophrenia: A replication study. International Journal of Clinical Practice. 2001;55(6):368–370. [PubMed] [Google Scholar]
- Puri BK, Hirsch SR, Easton T, Richardson AJ. A volumetric biochemical niacin flush-based index that noninvasively detects fatty acid deficiency in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26(1):49–52. doi: 10.1016/s0278-5846(01)00220-2. [DOI] [PubMed] [Google Scholar]
- Rajarethinam RP, DeQuardo JR, Nalepa R, Tandon R. Superior temporal gyrus in schizophrenia: A volumetric magnetic resonance imaging study. Schizophrenia Research. 2000;41(2):303–312. doi: 10.1016/s0920-9964(99)00083-3. [DOI] [PubMed] [Google Scholar]
- Raz S, Raz N, Bigler ED. Ventriculomegaly in schizophrenia: Is the choice of controls important? Psychiatry Res. 1988;24(1):71–77. doi: 10.1016/0165-1781(88)90142-4. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Murphy PT, Byrne M, Larkin C, Gill M, O’Callaghan E, et al. Dermatoglyphic fluctuating asymmetry and atypical handedness in schizophrenia. Schizophrenia Research. 2001;50(3):159–168. doi: 10.1016/s0920-9964(00)00044-x. [DOI] [PubMed] [Google Scholar]
- Ritsner MS, editor. Neuropsychiatric Biomarkers, Endophenotypes and Genes: Promises, Advances and Challenges. Springer; 2009. in press. [Google Scholar]
- Roitman SE, Cornblatt BA, Bergman A, Obuchowski M, Mitropoulou V, Keefe RS, et al. Attentional functioning in schizotypal personality disorder. American Journal of Psychiatry. 1997;154(5):655–660. doi: 10.1176/ajp.154.5.655. erratum appears in am j psychiatry 1997 aug;154(8):1180. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null results. Psychological Bulletin. 1979;86:638–641. [Google Scholar]
- Ross RG, Olincy A, Harris JG, Radant A, Adler LE, Freedman R. Anticipatory saccades during smooth pursuit eye movements and familial transmission of schizophrenia. Biological Psychiatry. 1998;44(8):690–697. doi: 10.1016/s0006-3223(98)00052-3. [DOI] [PubMed] [Google Scholar]
- Rossi A, De Cataldo S, Di Michele V, Manna V, Ceccoli S, Stratta P, et al. Neurological soft signs in schizophrenia. British Journal of Psychiatry. 1990;157:735–739. doi: 10.1192/bjp.157.5.735. [DOI] [PubMed] [Google Scholar]
- Rossi A, Serio A, Stratta P, Petruzzi C, Schiazza G, Mancini F, et al. Planum temporale asymmetry and thought disorder in schizophrenia. Schizophrenia Research. 1994;12(1):1–7. doi: 10.1016/0920-9964(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Rossi A, Stratta P, Mattei P, Cupillari M, Bozzao A, Gallucci M, et al. Planum temporale in schizophrenia: A magnetic resonance study. Schizophrenia Research. 1992;7(1):19–22. doi: 10.1016/0920-9964(92)90069-h. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A. Eye movement and neuropsychological studies in first-degree relatives of schizophrenic patients. Schizophrenia Research. 2002;54(12):105–110. doi: 10.1016/s0920-9964(01)00357-7. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Brodaty H, Rose N, Cathcart S. Schizophrenia with onset after age 50 years. 2: Neurological, neuropsychological and mri investigation. British Journal of Psychiatry. 1999;175:416–421. doi: 10.1192/bjp.175.5.416. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Niwa S, Hiramatsu K, Kameyama T, Rymar K, Itoh K. Abnormalities in late positive components of event-related potentials may reflect a genetic predisposition to schizophrenia. Biological Psychiatry. 1984;19(3):293–303. [PubMed] [Google Scholar]
- Saletu B, Kufferle B, Grunberger J, Anderer P. Quantitative eeg, spem, and psychometric studies in schizophrenics before and during differential neuroleptic therapy. Pharmacopsychiatry. 1986;19(6):434–437. doi: 10.1055/s-2007-1017283. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Voglmaier MM, Seidman LJ, McCarley RW. Topographic abnormalities of p3 in schizotypal personality disorder. Biological Psychiatry. 1996;40(3):165–172. doi: 10.1016/0006-3223(95)00373-8. [DOI] [PubMed] [Google Scholar]
- Scarone S, Abbruzzese M, Gambini O. The wisconsin card sorting test discriminates schizophrenic patients and their siblings. Schizophrenia Research. 1993;10(2):103–107. doi: 10.1016/0920-9964(93)90044-j. [DOI] [PubMed] [Google Scholar]
- Scarone S, Gambini O, Hafele E, Bellodi L, Smeraldi E. Neurofunctional assessment of schizophrenia: A preliminary investigation of the presence of eye-tracking (spems) and quality extinction test (qet) abnormalities in a sample of schizophrenic patients. Biological Psychology. 1987;24(3):253–259. doi: 10.1016/0301-0511(87)90006-8. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Link BG. The ‘well control’ artefact in case/control studies of specific psychiatric disorders. Psychol Med. 1989;19(3):737–742. doi: 10.1017/s0033291700024338. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Talbot NL, Kalinowski AG, McCarley RW, Faraone SV, Kremen WS, et al. Neuropsychological probes of fronto-limbic system dysfunction in schizophrenia. Olfactory identification and wisconsin card sorting performance. Schizophrenia Research. 1991;6(1):55–65. doi: 10.1016/0920-9964(91)90021-i. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Holzman PS. Antisaccades and smooth pursuit eye movements in schizophrenia. Biological Psychiatry. 1995;37(6):394–401. doi: 10.1016/0006-3223(94)00127-O. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Simmons A, David AS, Woodruff PW. Are auditory hallucinations the consequence of abnormal cerebral lateralization? A morphometric mri study of the sylvian fissure and planum temporale.[erratum appears in biol psychiatry 2001 sep 1;50(5):394] Biological Psychiatry. 2001;49(8):685–693. doi: 10.1016/s0006-3223(00)01006-4. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, McCarley RW, Metcalf D, Tieman J, Jolesz FA. Application of automated mri volumetric measurement techniques to the ventricular system in schizophrenics and normal controls. Schizophrenia Research. 1991;5(2):103–113. doi: 10.1016/0920-9964(91)90037-r. [DOI] [PubMed] [Google Scholar]
- Shtasel DL, Gur RE, Mosley PD, Richards J, Taleff MM, Heimberg C, Gallacher F, Gur RC. Volunteers for biomedical research. Recruitment and screening of normal controls. Archives of General Psychiatry. 1991;48(11):1022–5. doi: 10.1001/archpsyc.1991.01810350062010. [DOI] [PubMed] [Google Scholar]
- Shum D, Ungvari GS, Tang WK, Leung JP. Performance of schizophrenia patients on time-, event-, and activity-based prospective memory tasks. Schizophrenia Bulletin. 2004;30(4):693–701. doi: 10.1093/oxfordjournals.schbul.a007123. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Keefe R, Bernstein DP, Coccaro EF, Klar HM, Zemishlany Z, et al. Eye tracking impairment in clinically identified patients with schizotypal personality disorder. American Journal of Psychiatry. 1990;147(6):740–745. doi: 10.1176/ajp.147.6.740. comment. [DOI] [PubMed] [Google Scholar]
- Sivkov ST, Akabaliev VH. Discriminating value of total minor physical anomaly score on the waldrop physical anomaly scale between schizophrenia patients and normal control subjects. Schizophr Bull. 2004;30(2):361–366. doi: 10.1093/oxfordjournals.schbul.a007085. [DOI] [PubMed] [Google Scholar]
- Smeraldi E, Gambini O, Bellodi L, Sacchetti E, Vita A, di Rosa M, et al. Combined measure of smooth pursuit eye movements and ventricle-brain ratio in schizophrenic disorders. Psychiatry Research. 1987;21(4):293–301. doi: 10.1016/0165-1781(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Smith GN, Iacono WG, Moreau M, Tallman K, Beiser M, Flak B. Choice of comparison group and findings of computerised tomography in schizophrenia. Br J Psychiatry. 1988;153:667–674. doi: 10.1192/bjp.153.5.667. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. American Journal of Medical Genetics. 2003;123C(1):48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Curtis CE, Zald DH, Katsanis J, Iacono WG. Neuropsychological and oculomotor correlates of spatial working memory performance in schizophrenia patients and controls. Schizophrenia Research. 1999;38(1):37–50. doi: 10.1016/s0920-9964(98)00178-9. [DOI] [PubMed] [Google Scholar]
- Souza VB, Muir WJ, Walker MT, Glabus MF, Roxborough HM, Sharp CW, et al. Auditory p300 event-related potentials and neuropsychological performance in schizophrenia and bipolar affective disorder. Biological Psychiatry. 1995;37(5):300–310. doi: 10.1016/0006-3223(94)00131-L. [DOI] [PubMed] [Google Scholar]
- Sperling W, Martus P, Barocka A. Non-right-handedness and obstetrical complications in paranoid hallucinatory schizophrenics. Psychopathology. 1999;32(5):267–276. doi: 10.1159/000029099. [DOI] [PubMed] [Google Scholar]
- Stratta P, Arduini L, Daneluzzo E, Rinaldi O, di Genova A, Rossi A. Relationship of good and poor wisconsin card sorting test performance to illness duration in schizophrenia: A cross-sectional analysis. Psychiatry Research. 2003;121(3):219–227. doi: 10.1016/s0165-1781(03)00256-7. [DOI] [PubMed] [Google Scholar]
- Stratta P, Daneluzzo E, Mattei P, Bustini M, Casacchia M, Rossi A. No deficit in wisconsin card sorting test performance of schizophrenic patients’ first-degree relatives. Schizophrenia Research. 1997;26(23):147–151. doi: 10.1016/s0920-9964(97)00047-9. [DOI] [PubMed] [Google Scholar]
- Stratta P, Daneluzzo E, Prosperini P, Bustini M, Marinangeli MG, Rossi A. Spatial working memory assessment by a visual-manual delayed response task: A controlled study in schizophrenia. Neurosci Lett. 1999;275(1):9–12. doi: 10.1016/s0304-3940(99)00726-0. [DOI] [PubMed] [Google Scholar]
- Stratta P, Daneluzzo E, Prosperini P, Bustini M, Mattei P, Rossi A. Is wisconsin card sorting test performance related to ‘working memory’ capacity? Schizophrenia Research. 1997;27(1):11–19. doi: 10.1016/S0920-9964(97)00090-X. [DOI] [PubMed] [Google Scholar]
- Stratta P, Prosperini P, Daneluzzo E, Bustini M, Rossi A. Educational level and age influence spatial working memory and wisconsin card sorting test performance differently: A controlled study in schizophrenic patients. Psychiatry Res. 2001;102(1):39–48. doi: 10.1016/s0165-1781(01)00230-x. [DOI] [PubMed] [Google Scholar]
- Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR, Jr, Weinberger DR. Temporal lobe pathology in schizophrenia: A quantitative magnetic resonance imaging study. American Journal of Psychiatry. 1989;146(4):464–472. doi: 10.1176/ajp.146.4.464. [DOI] [PubMed] [Google Scholar]
- Suhr JA. Executive functioning deficits in hypothetically psychosis-prone college students. Schizophrenia Research. 1997;27(1):29–35. doi: 10.1016/s0920-9964(97)00072-8. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. Factors of the wisconsin card sorting test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Research. 1993;46(2):175–199. doi: 10.1016/0165-1781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendlre KS, Neale MC. Schizophrenia as a complex trait—evidence from a meta-analysis of twin studies. Archives General Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Sumich A, Chitnis XA, Fannon DG, O’Ceallaigh S, Doku VC, Falrowicz A, et al. Temporal lobe abnormalities in first-episode psychosis. American Journal of Psychiatry. 2002;159(7):1232–1235. doi: 10.1176/appi.ajp.159.7.1232. [DOI] [PubMed] [Google Scholar]
- Szoke A, Schurhoff F, Golmard JL, Alter C, Roy I, Meary A, et al. Familial resemblance for executive functions in families of schizophrenic and bipolar patients. Psychiatry Research. 2006;144(23):131–138. doi: 10.1016/j.psychres.2005.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Zhou SY, Tanino R, Hagino H, Kawasaki Y, et al. Morphologic alterations of the parcellated superior temporal gyrus in schizophrenia spectrum. Schizophrenia Research. 2006;83(23):131–143. doi: 10.1016/j.schres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Zhou SY, Tanino R, Hagino H, Niu L, et al. Temporal lobe gray matter in schizophrenia spectrum: A volumetric mri study of the fusiform gyrus, parahippocampal gyrus, and middle and inferior temporal gyri. Schizophrenia Research. 2006;87(13):116–126. doi: 10.1016/j.schres.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Tallent KA, Gooding DC. Working memory and wisconsin card sorting test performance in schizotypic individuals: A replication and extension. Psychiatry Research. 1999;89(3):161–170. doi: 10.1016/s0165-1781(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: What we know in 2008: Part 1: Overview. Schizophrenia Research. 2008a;100(13):4–19. doi: 10.1016/j.schres.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophrenia Research. 2008b;102(13):1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Taylor MA, Abrams R. Cognitive impairment in schizophrenia. American Journal of Psychiatry. 1984;141(2):196–201. doi: 10.1176/ajp.141.2.196. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Cassady S, Adami H, Moran M, Ross DE. Eye movements in spectrum personality disorders: Comparison of community subjects and relatives of schizophrenic patients. American Journal of Psychiatry. 1996;153(3):362–368. doi: 10.1176/ajp.153.3.362. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Cassady SL, Adami HM, Medoff DR, Sherr J. Saccadic eye movement abnormalities in relatives of patients with schizophrenia. Schizophrenia Research. 2000;45(3):235–244. doi: 10.1016/s0920-9964(99)00193-0. [DOI] [PubMed] [Google Scholar]
- Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34(4):760–73. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos HW, Goldstein JM, Buka SL, Poldrack RA, Koch JK, Tsuang MT, et al. The effect of working memory performance on functional mri in schizophrenia. Schiz Res. 2005;74(23):179–194. doi: 10.1016/j.schres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Toomey R, Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Tsuang MT. Association of neuropsychological vulnerability markers in relatives of schizophrenic patients. Schizophrenia Research. 1998;31(23):89–98. doi: 10.1016/s0920-9964(98)00025-5. [DOI] [PubMed] [Google Scholar]
- Trixler M, Tenyi T, Csabi G, Szabo R. Minor physical anomalies in schizophrenia and bipolar affective disorder. Schizophrenia Research. 2001;52(3):195–201. doi: 10.1016/s0920-9964(00)00182-1. [DOI] [PubMed] [Google Scholar]
- Upadhyay N, Mandal MK, Asthana AK, Sharma HO. Hand preference in patients with allergy, juvenile cancer, and schizophrenia. Laterality. 2004;9(3):325–337. doi: 10.1080/13576500342000194. [DOI] [PubMed] [Google Scholar]
- Vita A, Dieci M, Giobbio GM, Caputo A, Ghiringhelli L, Comazzi M, et al. Language and thought disorder in schizophrenia: Brain morphological correlates. Schizophrenia Research. 1995;15:243–251. doi: 10.1016/0920-9964(94)00050-i. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Corvin AP, Donohoe G, O’Tuathaigh CMP, Mitchell KJ, Gill M. Functional genomics and schizophrenia: Endophenotypes and mutant models. Psychiatric Clinics of North America. 2007;30(3):365–399. doi: 10.1016/j.psc.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Waldo MC, Adler LE, Freedman R. Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophrenia Research. 1988;1(1):19–24. doi: 10.1016/0920-9964(88)90035-7. [DOI] [PubMed] [Google Scholar]
- Waldo MC, Carey G, Myles-Worsley M, Cawthra E, Adler LE, Nagamoto HT, et al. Codistribution of a sensory gating deficit and schizophrenia in multi-affected families. Psychiatry Research. 1991;39(3):257–268. doi: 10.1016/0165-1781(91)90092-4. [DOI] [PubMed] [Google Scholar]
- Walker E, Green M. Soft signs of neurological dysfunction in schizophrenia: An investigation of lateral performance. Biological Psychiatry. 1982;17(3):381–386. [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Ward PB, Hoffer LD, Liebert BJ, Catts SV, O’Donnell M, Adler LE. Replication of a p50 auditory gating deficit in australian patients with schizophrenia. Psychiatry Research. 1996;64(2):121–135. doi: 10.1016/0165-1781(96)02876-4. [DOI] [PubMed] [Google Scholar]
- Ward PE, Sutherland J, Glen EM, Glen AI. Niacin skin flush in schizophrenia: A preliminary report. Schizophrenia Research. 1998;29(3):269–274. doi: 10.1016/s0920-9964(97)00100-x. [DOI] [PubMed] [Google Scholar]
- Ward RE, Jamison PL, Farkas LG. Craniofacial variability index: A simple measure of normal and abnormal variation in the head and face. American Journal of Medical Genetics. 1998;80(3):232–240. doi: 10.1002/(sici)1096-8628(19981116)80:3<232::aid-ajmg11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Weike AI, Bauer U, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biological Psychiatry. 2000;47(1):61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- Whitworth AB, Kemmler G, Honeder M, Kremser C, Felber S, Hausmann A, et al. Longitudinal volumetric mri study in first- and multiple-episode male schizophrenia patients. Psychiatry Research. 2005;140(3):225–237. doi: 10.1016/j.pscychresns.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Wolf LE, Cornblatt BA, Roberts SA, Shapiro BM, Erlenmeyer-Kimling L. Wisconsin card sorting deficits in the offspring of schizophrenics in the new york high-risk project. Schizophrenia Research. 2002;57(23):173. doi: 10.1016/s0920-9964(01)00301-2. [DOI] [PubMed] [Google Scholar]
- Woods BT, Kinney DK, Yurgelun-Todd D. Neurologic abnormalities in schizophrenic patients and their families. I. Comparison of schizophrenic, bipolar, and substance abuse patients and normal controls. Archives of General Psychiatry. 1986;43(7):657–663. doi: 10.1001/archpsyc.1986.01800070043006. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Yamada H, Yumoto M, Kamio S, Kudo N, Uetsuki M, et al. Abnormal association between reduced magnetic mismatch field to speech sounds and smaller left planum temporale volume in schizophrenia. Neuroimage. 2004;22(2):720–727. doi: 10.1016/j.neuroimage.2004.01.042. [DOI] [PubMed] [Google Scholar]
- Yan SM, Flor-Henry P, Chen DY, Li TG, Qi SG, Ma ZX. Imbalance of hemispheric functions in the major psychoses: A study of handedness in the people’s republic of china. Biological Psychiatry. 1985;20(8):906–917. doi: 10.1016/0006-3223(85)90216-1. [DOI] [PubMed] [Google Scholar]
- Yee CM, Nuechterlein KH, Morris SE, White PM. P50 suppression in recent-onset schizophrenia: Clinical correlates and risperidone effects. Journal of Abnormal Psychology. 1998;107(4):691–698. doi: 10.1037//0021-843x.107.4.691. [DOI] [PubMed] [Google Scholar]
- Zanello A, Perrig L, Huguelet P. Cognitive functions related to interpersonal problem-solving skills in schizophrenic patients compared with healthy subjects. Psychiatry Research. 2006;142(1):67–78. doi: 10.1016/j.psychres.2003.07.009. [DOI] [PubMed] [Google Scholar]


