Abstract
The aryl hydrocarbon receptor (AhR) is known mainly as the mediator for the toxicity of certain xenobiotics. However, there is also much information to indicate that this transcription factor has important biological functions. Here we review the evidence that the AhR has a significant role in the regulation of hematopoietic stem cells (HSCs). Data to support this comes from studies with xenobiotic AhR ligands, phenotypic analyses of mice lacking AhR, examining the presence and regulation of the AhR within HSCs, knowledge of genes and signaling pathways regulated by the AhR, and investigations of hematopoietic disorders. Based on this information, we hypothesize that AhR expression is necessary for the proper maintenance of quiescence in HSCs, and that AhR down-regulation is essential for “escape” from quiescence and subsequent proliferation of these cells. This implicates the AhR as a negative regulator of hematopoiesis with a function of curbing excessive or unnecessary proliferation. This provides an important advantage by preventing the premature exhaustion of HSCs and sensitivity to genetic alterations, thus preserving HSC function and long-term multi-lineage generation over the lifespan of the organism. This also implicates a role of the AhR in aging processes. AhR dysregulation may result in the altered ability of HSCs to sense appropriate signals in the bone marrow microenvironment leading to hematopoietic disease. It is also reasonable to hypothesize that this protein has an important function in the regulation of other tissue stem cell populations. Suggestive evidence is consistent with a role in skin and neural stem cells.
Keywords: aryl hydrocarbon receptor, stem cells, hematopoietic stem cells, progenitor cells
1. Introduction
For the past 30 years, the aryl hydrocarbon receptor (AhR) has been largely known for its ability to mediate the toxicity of a wide variety of environmental pollutants including the dioxins and certain polychlorinated biphenyls. That this occurs through the ability of a ligand-activated AhR to bind to specific DNA enhancer sequences, known as AhR responsive elements (AhREs; also called dioxin responsive elements (DREs) or xenobiotic responsive elements (XREs)), to regulate a diverse set of genes has attracted the interest of biological scientists to study its mechanisms, function, and possible role in human disease. Despite much research, there are many critical questions that remain unresolved. Although the toxic and biological effects of dioxins in both animals and humans have been well characterized [1], and many biochemical effects and AhR-responsive genes have been identified [2], direct relationships between gene activation and functional consequences in specific cells and tissues have yet to be defined. The search for bona fide endogenous ligands that regulate AhR transcriptional activity under physiological conditions has had limited success, although several indoles, as well as related tryptophan derivatives and photoproducts, and leukotriene metabolites, have been shown to activate AhR-mediated transcription [3,4]. Finally, data showing that the AhR is conserved throughout evolution [5], and that mice lacking the AhR exhibit abnormal phenotypes [6], all strongly suggest that this protein has some important physiological function in the development and maintenance of mammalian tissues. Nevertheless, this function remains elusive.
Immune system toxicity and dysfunction are some of the most consistent features observed in all animal species following exposure to dioxins and related chemicals [7]. Mice lacking AhR or possessing a constitutively active AhR also show abnormalities in immune system development and function [8–11]. Together these data are consistent with the AhR having a significant role in the regulation of the immune system, possibly at multiple levels. Here, we review the evidence indicating that the AhR is a critical component in the regulation of hematopoietic stem (HSCs) and/or progenitor (HPCs) cells. In addition, we offer a testable hypothesis that the AhR is important for regulating the balance between HSC quiescence and proliferation through the ability to modulate critical genes that are important for these cells to sense signals in the bone marrow microenvironment. Given a possible role of the AhR in HSCs, we also consider available data suggesting that the AhR has similar functions in other tissue stem cell populations.
2. HSCs as models of stem cell biology
A common and defining characteristic of stem cells is the ability to supply tissues with progenitors that differentiate into mature lineages while maintaining pools, through self-renewal, to satisfy the demands during the lifetime of the organism. While there are intrinsic controls over these processes, both differentiation and self-renewal are also greatly influenced by the microenvironment in which those cells exist.
Stem cells responsible for hematopoiesis in the murine bone marrow were first described more than forty years ago. As a result, there is a great amount of information regarding HSCs and the process of blood and immune system cell formation. Today, it is possible to distinguish and isolate different populations within the bone marrow using phenotypic characterization based on flow cytometry and functional assays. The different assays developed to retrospectively study HSCs make them an excellent model to study stem cell biology [12]. The functionality of HSCs is determined by their ability to repopulate cell lineages following their injection into lethally irradiated animals, or to sustain repopulation of recipients after repetitive serial bone marrow transplantation. Since experimental isolation causes the HSCs to lose many of their properties, in most cases, only the progeny can be analyzed [13].
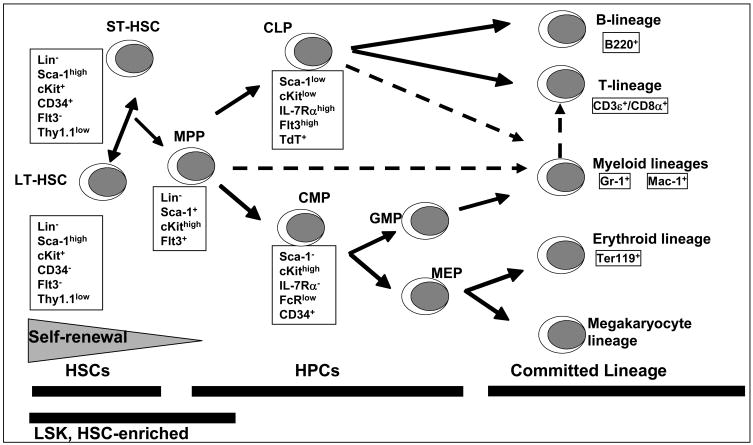
Through the process of multi-lineage differentiation, HSCs develop into progenitor cells, lineage committed cells and all the mature phenotypes of blood and immune tissues. It has been estimated that HSCs represent about 1 in 30,000 cells in bone marrow [14]. The Lineage negative, stem-cell antigen 1 (Sca-1) positive and c-kit (CD117) positive (LSK) population is a phenotype enriched for HSC in mice (Fig. 1). LSK cells lack the expression of surface proteins characteristic of lineage-committed cells (i.e. CD45R/B220, CD3ε, Gr-1, Ter-119, Mac-1/CD11b). Realizing that our understanding of the relationships between the expression of cell-surface markers and actual cell functions are likely oversimplified [12], the LSK population can be further phenotypically and functionally defined into both long-term (LT-HSC) and short-term (ST-HSC) repopulating HSCs as well as multipotent progenitor (MPP) populations that also have differential (decreasing) ability for self-renewal (Fig. 1).
Fig. 1.
Simplified classic schematic of hematopoiesis indicating the cell surface markers expressed at different stages of differentiation. Broken lines indicate the recently proposed revision to this classical model where a myeloid based model of hematopoiesis has been suggested [97].
Self-renewal and differentiation of HSCs are driven intrinsically by changes in gene expression AND extrinsically by molecules, e.g. cytokines and growth factors, present within the microenvironment. Stem cells are contained within physical and chemical environments known as microenvironments or niches that have been extensively described in the small intestine, the hair follicle, and the bone marrow for HSCs [15]. Depending on the stage of development of the organism, the location of hematopoietic niches may vary. In adult mammals, the preferential site of hematopoiesis is the bone marrow. Available data suggest the existence of both osteoblastic [16] and vascular [17] niches for HSCs. The osteoblastic niche is located at the endosteum of the bone marrow where the bone forming osteoblasts and/or other components of the microenvironment and HSCs are in close physical contact. However, the interactions of HSCs with the osteoblast niche do not appear to account for processes necessary for the mobilization and migration of HSC from and to the circulation and others sites of hematopoiesis such as liver and spleen. It has been shown that mobilized HSCs are associated with sinusoidal endothelium in the red pulp of the spleen as well as with the endosteum of bone [17]. Future research is needed to determine if there is a physiologically driven compartmentalization or if both niches are in such a close physical proximity that they act as a functional unit to support hematopoiesis.
3. Evidence that the AhR has a role in HSC regulation
Several different types of data support the postulate that the AhR has an important role in HSC regulation and function. These include results from studies with xenobiotic AhR ligands, phenotypic analyses of mice lacking AhR, examining the presence and regulation of the AhR within HSCs, knowledge of genes directly regulated by the AhR in conjunction with those known to regulate HSC function, and investigations of hematopoietic disorders.
3.1. Xenobiotic ligands affect HSC numbers and function
While examining the mechanism of TCDD-induced thymic atrophy, we previously observed that thymic seeding by progenitors isolated from bone marrow of TCDD-treated mice was substantially reduced. This was consistent with a significant reduction in mRNA for lymphoid-specific terminal deoxynucleotidyl transferase (TdT) and recombinase-activating gene 1 (RAG-1) in these cells [18,19]. That the effect was not specific for just the T-lymphoid precursor population was suggested by studies showing that numbers of cells in the immature B cell compartment also decreased following TCDD exposure [20]. However, the further differentiation of committed, but immature, B cells to more mature cells was not affected by the direct exposure of these cells to TCDD under culture conditions [21]. These studies suggested that either more immature progenitors or stem cells were directly affected by TCDD or that TCDD was targeting cell populations in the microenvironment in vivo that are, in part, responsible for directing B cell differentiation and which may not be active or present under culture conditions. To address the latter issue, wild-type and Ahr null-allele (KO) mice were used to produce radiation chimeric animals in which AhR was present or absent in hematopoietic or non-hematopoietic cell populations. These studies demonstrated that AhR presence in hematopoietic cells and NOT cells in the supporting bone marrow microenvironment is necessary for these effects to occur [22,23], suggesting that TCDD was acting directly on AhR present in HSCs and/or immature progenitor populations. Notably, a persistent several-fold increase in the number of HSC-enriched LSK cells was also observed following TCDD treatment [24], AND that this was also dependent on AhR presence in hematopoietic cells [22]. Further analysis indicated that TCDD treatment predominantly affected numbers of phenotypically-defined LT- and ST-HSCs but not MPPs, CLPs or CMPs contained within the LSK subset [25].
The studies summarized above suggested that an effect of TCDD on HSCs and/or very immature progenitor cells might be responsible for the observed effect on the lymphoid populations. A more detailed analysis of other lineage-committed cells and functional lineage-restricted progenitors suggested that in vivo TCDD-treatment causes a skewing of differentiation away from the lymphoid lineage in favor of granulocyte/macrophage myeloid lineage cells [25]. This was consistent with the finding that the number of functional CFU-preB progenitors was decreased following TCDD treatment [25]. A previous report indicated that cultured mobilized human progenitor CD34+ cells have a skewing towards myeloid cells in response to the polyaromatic hydrocarbon benzopyrene, another AhR ligand [26]. However, treatment of these cells with TCDD failed to produce this same response, suggesting that the effects were due to metabolites of benzo(a)pyrene and not solely due to AhR activation.
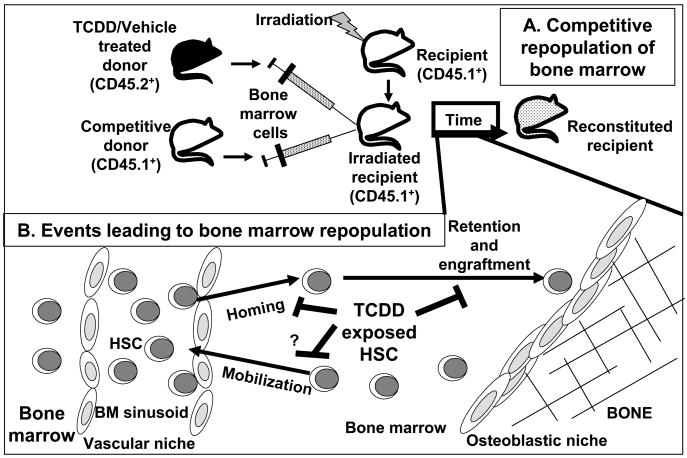
When the overall function of HSCs was evaluated in a competitive repopulation assay (Fig. 2), TCDD treatment to donor mice led to a substantially decreased short-term (6 wk) and long-term (40 wk) reconstitution activity of either LSK or LSK/CD34− cells [25]. These data were consistent with a previous report observing an almost total loss in the ability of LSK or LSK/CD34− cells from TCDD-treated mice to reconstitute white cells in peripheral blood of irradiated recipient mice [27]. They are also consistent with a recent finding from our lab that marrow taken from TCDD-treated mice exhibited a time-dependent decrease in HPP-CFC (unpublished observations). HPP-CFCs are among the most immature hematopoietic progenitors grown under culture conditions and their assessment has been used as a short-term assay of stem cell functional potential.
Fig. 2.
TCDD inhibits steps leading to repopulation of the bone marrow (BM) after transplantation. A, Competitive repopulation of recipients evaluates the ability of HSCs from TCDD- or vehicle-treated CD45.2+ donor mice to reconstitute the BM of irradiated recipient CD45.1+ animals in the presence of competitive donor (CD45.1+) HSCs. The percentages and phenotypic composition of CD45.2+ cells in the reconstituted recipient are analyzed. B, TCDD treatment alters HSCs to prevent successful repopulation of the BM. Repopulation requires HSC to migrate from the circulation through endothelial cells of the BM sinusoids into the BM. Once in the BM, HSC must be retained and engrafted or they will be mobilized back into circulation to find other niches to populate.
Together these data strongly suggest that TCDD may inhibit HSC functions through an ability to alter genes critical to the homing and/or trafficking of HSCs within the bone marrow microenvironment. Notably, it was reported that TCDD exposure down-regulates the expression of mRNAs for the G-protein-coupled receptor CXCR4 and it chemokine ligand CXCL12 (SDF-1), in MCF-7 breast cancer cells [28]. These molecules are critically important for HSC homing and movement within the marrow niche [29]. Since these molecules are also necessary for lymphoid cell development [30], we further postulate that TCDD-elicited alterations in this pathway may be responsible for the observed effects on B-cell development and decreased thymic seeding. Lacking sufficient signals for differentiation towards the lymphoid lineage, increased numbers of progenitors might then proceed along a default myeloid lineage [12,31], as we observed in response to TCDD. The possible significance of these pathways in HSCs exposed to TCDD still needs to be examined and verified. It should be pointed out that the whole-animal dosages (10–30 μg TCDD/kg) used in these investigations are somewhat higher than those observed to cause significant effects on other endpoints in the immune system [7]. However, at these dosages the bone marrow cell concentrations of TCDD are much less than 1 nM [19], indicating the high sensitivity of these cells to this chemical. Nevertheless, all of the above information is consistent with the hypothesis that dysregulation of the AhR by TCDD results in the inappropriate regulation of critical genes in HSCs that impairs their detection of signals within the marrow microenvironment to result in altered HSC function.
3.2. Characteristics of HSCs from AhR-KO mice
If TCDD exposure of HSCs results in altered HSC characteristics and function, one might suspect that lack of AhR would also affect HSCs. Young adult AhR-KO mice have increased numbers of LSK and LSK/CD34+ cells in bone marrow compared to either wild-type or heterozygote animals (unpublished observations). Consistent with this, but opposite to that observed in wild-type mice treated with TCDD [25], analysis of bone marrow from KO mice demonstrated increased numbers of HPP-CFCs (unpublished observations). LSK cells from young adult KO mice also have very high rates of cell division, determined by the in vivo incorporation of BrdU, compared to cells from wild-type animals. Surprisingly, the BrdU incorporation was found to be nearly identical to cells from wild-type animals treated with 5-FU that kills very rapidly dividing cells and stimulates HSCs into division. Treatment of KO mice with 5-FU did not further increase BrdU incorporation into LSK cells, suggesting that these cells are already dividing at a maximal rate as if under increased stimulatory conditions. Consistent with this, we observed a greater percentage of KO LSK cells to be in G1/S cell cycle phases compared to that from wild-type or heterozygote animals (unpublished observations). That progenitor cells from KO mice also have increased growth rates in culture compared to cells from wild-type mice (unpublished observations), supported the notion that high proliferation rates of KO cells in vivo is a property inherent to these cells and not due to a possible increased level of stimulatory signals present in vivo. Together these data further support a hypothesis that the AhR has a normal function in the regulation of HSCs, and more specifically as a regulator of the balance between quiescence and proliferation.
3.3. Presence and regulation of AhR in HSCs
Data from our lab and from other groups have shown the presence of both AhR protein and mRNA in phenotypically-defined HSCs [25,26,32]. Furthermore, TCDD treatment alters gene expression in hematopoietic precursors [32,33]. These data indicate the presence of a functional AhR protein and associated machinery necessary for an active AhR signaling pathway in HSCs. Of particular interest, however, are other data suggesting that the Ahr gene is actively regulated during different cell cycle phases in HSCs; cells actively cycling or stimulated to cycle following 5-FU treatment to animals have significantly less Ahr mRNA than HSCs in quiescence (G0) [34,35]. In addition, we have shown that treatment of mice with growth factors and cytokines (IL-6 + G-CSF or IL-11 + G-CSF) known to stimulate hematopoiesis resulted in a nearly 60% loss of Ahr mRNA expression in hematopoietic progenitors [25]. Data indicating that the Ahr is regulated in HSCs with cell cycle phases is also consistent with a number of studies suggesting that the AhR has a normal functional role in cell cycling [36].
That Ahr expression in HSCs may be regulated under conditions of quiescence/proliferation has several implications. HSC susceptibility to the actions of xenobiotic or endogenous ligands may depend on the marrow environment. Conditions in which HSCs are stimulated to proliferate may actually protect these cells from toxic AhR ligands. This also might be a protective mechanism against chemicals that can be metabolized to mutagenic intermediates by the cytochrome P450 isozymes regulated in part by the AhR. To test the former possibility, mice were exposed to 5-FU two days prior to treatment with a dose of TCDD previously found to produce significant effects on bone marrow cell populations. Under these conditions and up to 16 days after TCDD treatment, absolutely no effects of TCDD on bone marrow were observed compared to the vehicle-treated group also treated with 5-FU [25]. Thus, it appears that the down-regulation of Ahr in HSCs occurring following treatment with 5-FU renders these cells less susceptible to the effects of xenobiotic ligands such as TCDD.
3.4. Known molecular targets of the AhR signaling pathway support a role in HSC regulation
Under normal homeostatic conditions, HSCs exhibit a low metabolic rate as indicated by a high percentage (70–85%) of cells in quiescence (G0) [37]. As such, the entrance into and exit from cell cycle critically regulates HSC quiescence, proliferation and self-renewal. For example, in the absence of the G1 checkpoint regulator CDK inhibitor p21cip1/waf1, both the proliferation and numbers of HSCs are increased. However, self-renewal of HSCs deficient in p21 is blocked following transplantation, leading to hematopoietic failure [38]. In addition to regulating the expression of p21 and the CDK2 inhibitor p27kip1 [2,39,40], the AhR interacts with the tumor suppressor protein pRb to modulate its function as a G1 checkpoint regulator [36]. Other intrinsic genes and pathways known to regulate HSC characteristics and function include c-Myc, C/EBP, HES1, among others [41,42]. HES1 and c-Myc appear to be directly regulated by the AhR [2,43,44], and C/EBP expression is altered by AhR agonists in several cell types [45]. Finally, as indicated above, it is becoming appreciated that microenvironment-dependent signaling by soluble cytokines and contact with stromal cells in the bone marrow niche are both critical for maintaining the balance among HSC quiescence, division, differentiation and trafficking to other environments. In addition to altering the expression of CXCR4 and CXCL12 [28], AhR agonists are known to modulate the expression of a number of cell surface proteins and adhesion molecules [46,47] that are also important for the ability of HSCs to sense and respond to their environment. Clearly, it remains to be determined whether the AhR critically regulates these or other pathways that play a role HSC function. Nevertheless, these findings further implicate an important role of the AhR in HSC regulation.
3.5. Evidence suggests a relationship between AhR dysregulation and particular hematopoietic disorders
Increased incidence of leukemia and lymphoma has been reported in humans accidentally exposed to TCDD [48–52]. AhR ligands are major components of tobacco smoke [53], and smoking during pregnancy increases the risk of certain cancers including leukemia in the offspring [54,55]. A recent study found that the Ahr promoter is silenced by hypermethylation in human acute lymphoblastic leukemia cells [56]. Notably, the authors postulated that the AhR could be a cell-specific negative regulator of cell proliferation. AhR activation results in resistance to apoptosis in human lymphoma cell lines, and the pathogenesis of AhR-mediated lymphoma in animal models is associated with induced COX-2 by AhR ligands [57]. Finally, the AhR signaling pathway has been implicated in animal models of benzene-induced leukemia [58]. Together these studies suggest that AhR dysregulation may play an extremely important role in the etiology and/or progression of certain hematopoietic diseases.
4. Hypothesis and Implications
Together the available data are consistent with a compelling argument for a function of the AhR in HSC regulation. More specifically, the data suggests that the AhR has an important role in HSCs for regulating the balance between quiescence and proliferation. Consistent with this postulate are data indicating a role of the AhR in cell cycle [36], demonstrating hyperproliferation of HSCs from AhR-KO mice (Section 3.2), and showing differential regulation of Ahr expression in HSCs during cycles of quiescence and proliferation (Section 3.3). These data would further suggest that AhR expression is necessary for the proper maintenance of quiescence in HSCs, and that Ahr down-regulation allows HSCs to “escape” from quiescence and subsequently proliferate. This implicates the AhR acting specifically as a negative regulator of hematopoiesis with a function of curbing excessive or unnecessary proliferation of HSCs. Clearly, more work is needed to define these relationships. Furthermore, the specific genes and signaling pathways controlled by the AhR that may regulate HSC quiescence/proliferation need to be identified, although as indicated in section 3.4 there are several “likely suspects”. Notably, both CXCR4 and CXCL12 genes contain putative AhREs in the upstream promoter regions [28], and this signaling pathway is important in the maintenance of the quiescent HSC pool [59]. In addition, the AhR has been shown to regulate the expression of c-myc [44,60], and c-myc regulates the balance between HSC self-renewal and differentiation through the expression of adhesion molecules [61]. Ultimately, it appears likely that AhR may regulate critical genes within HSCs that allows them to differentially respond to signals in their microenvironment. This is consistent with a hypothesis for an overall function of proteins like the AhR within the PAS superfamily as “sensors of environmental and developmental signals” [62]. In this case, the presence and functional activity of the AhR may be to provide an important advantage to organisms by preventing the premature exhaustion of HSCs and sensitivity to genetic alterations, thus preserving HSC function and long-term multi-lineage generation.
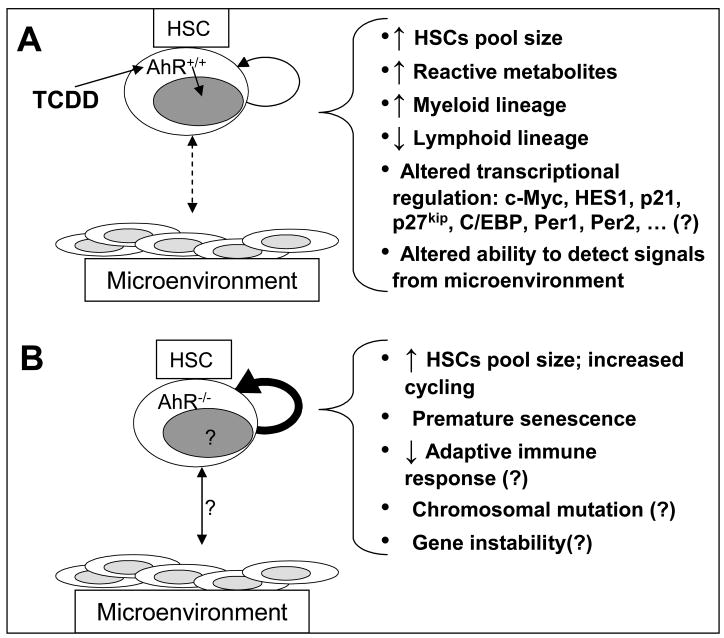
There are several important implications for a primary role of the AhR as a negative regulator of HSC proliferation. This may provide a plausible mechanism for reported relationships between dysregulation of AhR function and the etiology and/or progression of certain hematopoietic diseases such as leukemia (see 3.5). In the case of inadvertent exposure to xenobiotics such as the dioxins, the inappropriate and persistent AhR activation may result in HSCs dysfunction, as seen in mice exposed to TCDD, by altering the ability of HSCs to traffic and sense the appropriate signals within the bone marrow microenvironment (see Fig. 3). This may provide a permissive environment for altered hematopoietic development and the selection and expansion of tumor cell clones. Loss of AhR function, through an epigenetic control of AhR expression by, for example, hypermethylation of the Ahr gene [56], could have the same net effect through uncontrolled cell growth and proliferation (Fig. 3).
Fig. 3.
Simplified model of AhR regulation of HSCs. A, The AhR-ligand TCDD binds to AhR which translocates to the nucleus and mediates genetic/epigenetic regulation, HSC-niche interactions, and alterations of different end-points. B, In AhR−/− mice, the HSCs are constitutively in a higher cycling status that corresponds with a larger HSC pool size.
Notably, there is much evidence of a causal relationship between low to moderate cycling rates of HSCs and hematopoietic longevity [63,64]. Likewise, since adult stem cells are incapable of sustaining an indefinite line of generations, there is a general expansion of the HSC pool from youth to maturity, but this declines in old age. Many investigations have indicated genetic regulation of stem cell exhaustion in mice and a role of this exhaustion in the aging process [64–67]. In fact, there is an excellent negative correlation between the mean lifespan of various mouse strains and the replication rates of HSCs within these strains [64,68]. This is consistent with Hayflick’s proposal for a mitotic clock in which cells have an upper limit to replication, and once this is reached, proliferation decreases and the cells ultimately senesce [69]. Thus, a higher proliferation rate of stem cells throughout the lifetime of an organism decreases the time to reach this limit. However, this may also increase the rate at which potential fatal DNA damage accumulates. Not surprisingly then, there is also evidence to indicate that the increased proportion of actively cycling stem cells correlates with increased incidence of leukemia, lymphoma, and myelodysplastic syndrome in old age [66,67]. Importantly, a grouping of mouse strains correlated with differences in longevity [64] also corresponds to different Ahr polymorphisms present in these animals. For example, the C57Bl/6 and 129/Sv strains are characterized by low HSC cycling rates and long lifespan, and also possess a high affinity AhR (i.e. high affinity for TCDD). Other strains, DBA/2 and AKR, that are characterized by high HSC cycling rates and short lifespan possess a low affinity AhR form. The association between the AhR and longevity was actually made years earlier when it was found that there was a correlation between AhR expression levels and responsiveness with lifespan [70,71]. Whether these relationships are causal remains to be determined.
These relationships would also suggest that, due to a lifetime of high cycling rates, the HSCs in aging AhR-KO mice might undergo premature senescence. Notably, some lesions appear only with aging in the AhR-KO mice, and there has been a report of decreased lifespan in these animals [9]. In preliminary investigations, we have noted the following characteristics in aging AhR-KO animals: 1) Compared with young adult KO mice or old (10 month) wild-type C57Bl/6 mice (the parental strain), old KO mice have approximately 50 percent fewer LSK cells. 2) An analysis of bone marrow from one-year old KO mice showed significantly increased BFU-E, CFU-E, CFU-G, and CFU-M, but a profound decrease in CFU-preB progenitors. There is also splenic myeloid hyperplasia in aging KO mice [9]. It is known that as bone marrow senescence occurs in mice, there is a skewing of lineages toward myeloid at the expense of lymphoid populations [63,64,66,67,72]. 3) HSCs from old KO mice have decreased proliferation in culture, as determined by cell number and incorporation of 3H-thymidine, compared to cells from young adult KO animals or cells from old age-matched wild-type mice. All of these data are consistent with premature senescence occurring in the HSC/progenitor compartment during aging in KO mice, and further suggest a role of the AhR in the process of aging.
Data showing that HSCs from AhR-KO mice are hyperproliferative in vivo and ex vivo suggest that the AhR signaling pathway is important in the regulation of quiescence/proliferation in these cells since compensatory changes in KO-HSCs have not occurred to correct for AhR loss. In this sense, it will be important to determine what actually controls Ahr gene expression in HSCs, especially during loss of quiescence. The promoter region of the murine Ahr contains several GC and Sp1 sites. Other transcription factor sites include those for HNFs, DLX3, NKX3, PIT1, LHX3, STAT6, BRN3, TCF/Lef, AP-1, and E-box for a c-myc binding site, a CAGA-box that may be regulated by TGFβ, CRE, and potential binding sites for several hormone receptors [2,73,74]. Cytokines that operate through STAT-binding sites and those regulated by c-myc may be good candidates for factors regulating Ahr expression in bone marrow.
5. Possible role of the AhR in other stem cell populations
Given a likely role of the AhR in regulating HSCs, it is not unreasonable to hypothesize that this transcription factor may also have an important function in the regulation of other tissue stem cell populations. To date, however, data supporting or refuting such a hypothesis are very limited, simply because few studies have examined for a function of the AhR in any stem cells. There have been numerous studies examining the effects of TCDD on the differentiation of a variety of precursor cells [e.g. 75–78], and in most cases TCDD exposure affects differentiation and/or proliferation in an AhR-dependent manner. However, the type of effect observed is often very cell type-specific. For example, in some cases the AhR appears to act as a negative regulator of proliferation, while in other cases it affects proliferation in a positive manner [79,80]. This likely is dependent on the cellular context as well as the microenvironment in which these cells exist. TCDD has been shown to alter gene expression and characteristics of a number of stem or “stem-like” cells, including embryonic stem cells, liver stem cells, and human mesenchymal stem cells when exposure occurs under conditions in culture [81–84]. Although, these data suggest that the AhR is functional in these cells and that they may be sensitive targets for dysregulation by xenobiotic AhR ligands, it is difficult to know whether they might respond differently or at all under conditions in vivo within the particular microenvironment in which they are functional.
5.1. A possible role of the AhR in skin stem cells
A recent publication proposed that TCDD-elicited activation of the AhR in skin stem cells and a shift in differentiation of their progeny is the mechanism for the ability of this chemical to produce chloracne in both humans and animals [85]. They noted that the pathogenesis of the chloracne indicated alterations in several skin stem cell characteristics including 1) stimulation of stem cell into cycling, 2) increased stem cell self-renewal, and 3) and a skewing of differentiation towards the epidermal pathway at the expense of hair follicle and sebaceous gland cells. Some of these features are similar to what appears to occur in HSCs in response to TCDD. The authors also noted some, but not a complete, similarity between the skin phenotype induced by TCDD and that in transgenic mice overexpressing c-Myc [86]. Based on this, they further postulated a link between the c-myc and AhR signaling pathways in the regulation of skin stem cells.
5.2. Evidence for an important function of the AhR in neuronal development
There are many parallels between the regulation of neuroepithelial stem cells (NSCs) and HSCs during the processes of neurogenesis and hematopoiesis [87]. For example, the NSCs have the capacity to self-renew, and also are mutipotent as they give rise to the three major cell types in the central nervous system: neurons, astrocytes, and oligodendrocytes [88]. The generation of differentiated cells also appears to be dependent on the intermediated differentiation of lineage restricted progenitor cells. As with HSCs, NSC expansion and differentiation is governed by various transcription factors that regulate the expression of genes associated with the generation of neurons and glia. Recent studies have reported that there are common genes expressed in both HSCs and NSCs, suggesting that there may be a conserved group of molecules that govern stem cell behavior [89,90].
Previous reports have revealed that both Ahr and Arnt genes are expressed in the embryonic neuroepithelium [91,92]. We recently confirmed that NSC isolated from the developing forebrain express robust levels of AhR protein (unpublished observations). Moreover, granule neuron progenitors (GNPs) express transcriptionally active AhR during a critical period of neurogenesis [93]. Both in vivo and in vitro data support the contention that TCDD disrupts the balance between proliferation, differentiation, and apoptosis in GNPs during the early postnatal period, and that this ultimately results in diminished cell numbers in the cerebellum [93,94]. Other studies in C. elegans and Drosophila suggest that the AhR participates in normal nervous system development [95,96]. These observations suggest an ancestral role for the AhR family. Since the AhR is present and coordinately regulated with Arnt in the embryonic mouse neuroepithelium during prenatal neurogenesis [91,92], it is conceivable that AhR mediates similar developmental events in mammalian systems.
Studies in the AhR-KO mice are beginning to provide information regarding potential roles for the AhR in brain development [93]. Initial observations in KO mice indicate that cell number is diminished in the developing and adult cerebellum [94]. Moreover, GABAAα6 receptor expression, which is normally restricted to mature granule neurons, was markedly reduced [94], suggesting that AhR is important for development and/or maintenance of this neuronal population. More recently, we determined that NSC cell birth in the adult hippocampus was reduced by approximately 60% in KO mice (unpublished observations). Together, these studies are consistent with the argument that AhR participates in regulating neurogenesis through an important role in NSC and neuronal precursor cell regulation.
6. Summary: Significance and prospects for future work
Further defining a precise role of the AhR in HSCs or other tissue stem cells may lead to the identification of previously undefined functions of this transcription factor in particular human diseases. This could have important implications for the diagnosis and treatment of these diseases. Genetically- or epigenetically-defined AhR dysregulation may have a certain pattern of disease progression that may respond better to a focused treatment strategy. Given that the AhR is ligand activated also offers the opportunity to develop selective and therapeutic AhR modulators. Some of these might be useful for the maintenance of stem cells and their subsequent use in tissue regeneration, for example in the treatment of degenerative diseases. The exposure of human stem cells to a variety of AhR xenobiotic ligands, e.g. dioxins, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, and ligands in tobacco smoke, may also contribute to dysfunction of the AhR, and either directly or in conjunction with some other permissive condition, lead to abnormal development and disease. The implication that tissue stem cells may be targets for xenobiotic AhR ligands is itself a novel hypothesis that needs to be further assessed considering that 1) developing tissues, in particular, are extremely sensitive to these chemicals, 2) the precise cellular targets have not been clearly identified, and 3) AhR activation may result in increased levels of metabolizing enzymes in these stem cells [e.g. 26] and subsequent bioactivation of chemical carcinogens or other toxic chemicals. Finally, it should be emphasized again that the normal physiological function of the AhR is unknown and bona fide endogenous ligands have not been identified. Research on a possible role in stem cells will undoubtedly break new ground on the regulation of stem cells and, in particular, a role of the AhR in these cells.
Acknowledgments
This work was supported by National Institute of Health Grant ES04862, Training Grant ES07026, and Center Grant ES01247.
Abbreviations
- 5-FU
5-fluorouracil
- AhR
aryl hydrocarbon receptor
- AhRE
aryl hydrocarbon receptor response element
- BrdU
bromodeoxyuridine
- CFU-G
colony-forming unit granulocyte
- CFU-GM
CFU-granulocyte/megakaryocyte
- CFU-M
CFU-macrophage
- CLP
common lymphoid progenitor
- CMP
common myeloid progenitor
- GNP
granule neuron progenitor
- HPCs
hematopoietic progenitor cells
- HPP-CFC
high proliferative potential-colony forming cells
- HSCs
hematopoietic stem cells
- LSK
HSC-enriched lineage-negative, cKit-positive, Sca-1-positive cells
- LT-HSC
long-term repopulating HSCs
- MPP
mulipotent progenitors
- NSC
neuroepithelial stem cell
- ST-HSCs
short-term repopulating HSCs
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- 2.Gasiewicz TA, Henry EC, Collins LL. Expression and activity of aryl hydrocarbon receptors in development and cancer. Crit Rev Eukaryotic Gene Express. 2008;18:279–321. doi: 10.1615/critreveukargeneexpr.v18.i4.10. [DOI] [PubMed] [Google Scholar]
- 3.Yguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–16. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiaro CR, Morales JL, Prabhu KS, Perdew GH. Leukotriene A4 metabolites are endogenous ligands for the Ah receptor. Biochemistry. 2008;47:8445–55. doi: 10.1021/bi800712f. [DOI] [PubMed] [Google Scholar]
- 5.Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact. 2002;141:131–60. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 6.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor sans xenobiotics: endogenous function iin genetic model systems. Mol Pharmacol. 2007;72:487–98. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence BP, Kerkvliet NI. Immune modulation by TCDD and related polyhalogenated aromatic hydrocarbons. In: Leubke R, House RH, Kimber I, editors. Immunotoxicology and Immunopharmacology. Vol. 3. CRC Press; Boca Raton, FL: 2006. pp. 239–58. [Google Scholar]
- 8.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–26. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34:605–14. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 10.Brunnberg S, Andersson P, Lindstam M, Paulson I, Poellinger L, Hanberg A. The constitutively active Ah receptor (CA-AhR) mouse as a potential model for dioxin exposure – Effects in vital organs. Toxicology. 2006;224:191–201. doi: 10.1016/j.tox.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Shi LZ, Faith NG, Nakayama Y, Suresh M, Steinberg H, Czuprynski CJ. The aryl hydrocarbon receptor is required for optimal resistance to Listeria monocytogenes infection in mice. J Immunol. 2007;179:6952–62. doi: 10.4049/jimmunol.179.10.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132:631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cells assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 16.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Biochem Biophys Res Commun. 2005;195:301–9. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Fine JS, Gasiewicz TA, Fiore NC, Silverstone AE. Prothymocyte activity is reduced by perinatal 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. J Pharmacol Exp Therap. 1990;255:128–32. [PubMed] [Google Scholar]
- 19.Fine JS, Silverstone AE, Gasiewicz TA. Impairment of prothymocyte activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 1990;144:1169–76. [PubMed] [Google Scholar]
- 20.Thurmond TS, Gasiewicz TA. A single dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin produces a time- and dose-dependent alteration in the murine bone marrow B-lymphocyte maturation profile. Toxicol Sci. 2000;58:88–95. doi: 10.1093/toxsci/58.1.88. [DOI] [PubMed] [Google Scholar]
- 21.Wyman A, Lavin AL, Wilding GE, Gasiewicz TA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin does not directly alter the phenotype of maturing B cells in a murine co-culture system. Toxicol Appl Pharmacol. 2002;180:164–77. doi: 10.1006/taap.2002.9396. 2002. [DOI] [PubMed] [Google Scholar]
- 22.Staples JE, Murante FG, Fiore NC, Gasiewicz TA, Silverstone AE. Thymic alterations induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are strictly dependent on aryl hydrocarbon receptor activation in hemopoietic cells. J Immunol. 1998;160:3844–54. [PubMed] [Google Scholar]
- 23.Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA, Silverstone AE. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J Immunol. 2003;171:4582–91. doi: 10.4049/jimmunol.171.9.4582. [DOI] [PubMed] [Google Scholar]
- 24.Murante FG, Gasiewicz TA. Hemopoietic progenitor cells are sensitive targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J mice. Toxicol Sci. 2000;54:374–83. doi: 10.1093/toxsci/54.2.374. [DOI] [PubMed] [Google Scholar]
- 25.Singh KP, Wyman A, Casado FL, Garrett R, Gasiewicz TA. Treatment of mice with the aryl hydrocarbon receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn224. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Grevenynghe J, Bernard M, Langouet S, Le Berre C, Fest T. Human CD34-positive hematopoietic stem cells constitute targets for carcinogenic polyaromatic hydrocarbons. J Pharmacol Exp Ther. 2005;314:693–702. doi: 10.1124/jpet.105.084780. [DOI] [PubMed] [Google Scholar]
- 27.Sakai R, Kajiume T, Inoue H, Kanno R, Miyazaki M, Ninomiya Y, Kanno M. TCDD treatment eliminates the long-term reconstitution activity of hematopoietic stem cell. Toxicol Sci. 2003;72:84–91. doi: 10.1093/toxsci/kfg002. [DOI] [PubMed] [Google Scholar]
- 28.Hsu EL, Yoon D, Choi HH, Wang F, Taylor RT, Chen N, Zhang R, Hankinson O. A proposed mechanism for the protective effect of dioxin against breast cancer. Toxicol Sci. 2007;98:436–44. doi: 10.1093/toxsci/kfm125. [DOI] [PubMed] [Google Scholar]
- 29.Chute JP. Stem cell homing. Curr Opin Hematol. 2006;13:399–406. doi: 10.1097/01.moh.0000245698.62511.3d. [DOI] [PubMed] [Google Scholar]
- 30.Tokoyoda K, Egawa T, Sugiyama T, Choi B-I, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, Weissman IL. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–6. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 32.Frericks M, Meissner M, Esser C. Microarray analysis of the AHR system: Tissue-specific flexibility in signal and target genes. Toxicol Appl Pharmacol. 2007;220:320–32. doi: 10.1016/j.taap.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Garrett RW, Gasiewicz TA. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol Pharmacol. 2006;69:2076–83. doi: 10.1124/mol.105.021006. [DOI] [PubMed] [Google Scholar]
- 34.Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA, Goodell MA. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLOS Biol. 2004;2:1640–51. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noda S, Horiguchi K, Ichikawa H, Miyoshi H. Repopulating activity of ex vivo-expanded murine hematopoietic stem cell resides in the CD48-c-Kit+Sca-1+ lineage marker cell population. Stem Cells. 2008;26:646–55. doi: 10.1634/stemcells.2007-0623. [DOI] [PubMed] [Google Scholar]
- 36.Huang G, Elferink CJ. Multiple mechanisms are involved in Ah receptor-mediated cell cycle arrest. Mol Pharmacol. 2005;67:88–96. doi: 10.1124/mol.104.002410. [DOI] [PubMed] [Google Scholar]
- 37.Abkowitz JL, Golinelli D, Harrison DE, Guttorp P. In vivo kinetics of murine hemopoietic stem cells. Blood. 2000;96:3399–405. [PubMed] [Google Scholar]
- 38.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–8. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 39.Kolluri SK, Weiss C, Koff A, Gottlicher M. P27kip1 induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 1999;13:1742–53. doi: 10.1101/gad.13.13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes-Ellerbe S, Knudsen KE, Puga A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin blocks androgen-dependent cell proliferation in LNCaP cells through modulation of pRB phosphorylation. Mol Pharmacol. 2004;66:502–11. doi: 10.1124/mol.104.000356. [DOI] [PubMed] [Google Scholar]
- 41.Bruno L, Hoffman R, McBlane F, Brown J, Gupta R, Joshi C, Pearson S, Siedl T, Heyworth C, Enver T. Molecular signatures of self-renewal, differentiation, and lineage choice in multipotential hemopoietic progenitor cells in vitro. Mol Cell Biol. 2004;24:741–56. doi: 10.1128/MCB.24.2.741-756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terskikh AV, Miyamoto T, Chang C, Diatchenko L, Weissman IL. Gene expression analysis of purified hematopoietic stem cells and committed progenitors. Blood. 2003;102:94–101. doi: 10.1182/blood-2002-08-2509. [DOI] [PubMed] [Google Scholar]
- 43.Thomsen JS, Keitz S, Strom A, Gustafsson J-A. HES-1, a novel target gene for the aryl hydrocarbon receptor. Mol Pharmacol. 2004;65:165–71. doi: 10.1124/mol.65.1.165. [DOI] [PubMed] [Google Scholar]
- 44.Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- 45.Vogel CFA, Sciullo E, Park S, Liedtke C, Trautwein C, Matsumura F. Dioxin increases C/EBPb transcription by activating cAMP/protein kinase A. J Biol Chem. 2004;279:8886–94. doi: 10.1074/jbc.M310190200. [DOI] [PubMed] [Google Scholar]
- 46.Niermann T, Schmutz S, Erne P, Resink T. Aryl hydrocarbon receptor ligands repress T-cadherin expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 2003;300:943–49. doi: 10.1016/s0006-291x(02)02970-4. [DOI] [PubMed] [Google Scholar]
- 47.Adachi J, Mori Y, Matsui S, Matsuda T. Comparison of gene expression patterns between 2,3,7,8-tetrachlorodibenzo-p-dioxin and a natural arylhydrocarbon receptor ligand, indirubin. Toxicol Sci. 2004;80:161–9. doi: 10.1093/toxsci/kfh129. [DOI] [PubMed] [Google Scholar]
- 48.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Polychlorinated Dibenzo-Para-Dioxins and Polychlorinated Dibenzofurans. IARC Monogr Eval Carcinog Risks Hum. 1997;69:1–631. [PMC free article] [PubMed] [Google Scholar]
- 49.Bertazzi PA, Pesatori AC, Bernucci I, Landt MT, Consonni D. Dioxin exposure and human leukemias and lymphomas. Lessons from the Seveso accident and studies on industrial workers. Leukemia. 1999;1(13 Suppl):S72–4. doi: 10.1038/sj.leu.2401290. [DOI] [PubMed] [Google Scholar]
- 50.Zheng T, Blair A, Zhang Y, Weisenburger DD, Zahm A. Occupation and risk of non-Hodgkin’s lymphoma and chronic lymphocyic leukemia. J Occup Environ Med. 2002;44:469–74. doi: 10.1097/00043764-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Fingerhut MA, Halperin WE, Marlow DA. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. N Engl J Med. 1991;324:212–18. doi: 10.1056/NEJM199101243240402. [DOI] [PubMed] [Google Scholar]
- 52.Hooiveld M, Heederik DJ, Kogevinas M, Boffetta P, Needham LL, Patterson DG, Jr, Dueno-de-Mesquita HB. Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenols, and contaminants. Am J Epidemiol. 1998;147:891–901. doi: 10.1093/oxfordjournals.aje.a009543. [DOI] [PubMed] [Google Scholar]
- 53.Gebremichael A, Tullis K, Denison MS, Cheek JM, Pinkerton KE. Ah-receptor-dependent modulation of gene expression by aged and diluted sidestream cigarette smoke. Toxicol Appl Pharmacol. 1996;141:76–83. doi: 10.1006/taap.1996.0262. [DOI] [PubMed] [Google Scholar]
- 54.Filippini G, Farinotti M, Ferrarini M. Active and passive smoking during pregnancy and risk of central nervous system tumors in children. Paediatr Perinat Epidemiol. 2000;14:78–84. doi: 10.1046/j.1365-3016.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 55.Magnani C, Pastore G, Luzzatto L, Terracini B. Perental occupation and other environmental factors in the etiology of leukemias and non-Hodkin’s lymphoma in childhood: A case-control study. Tumori. 1990;76:413–19. doi: 10.1177/030089169007600501. [DOI] [PubMed] [Google Scholar]
- 56.Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, Ballestar E, Fraga MF, Ropero S, Esteller M, Fernandez-Salguero PM. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis. 2006;27:1099–104. doi: 10.1093/carcin/bgi344. [DOI] [PubMed] [Google Scholar]
- 57.Vogel CFA, Li W, Sciullo E, Newman J, Hammock B, Reader JR, Tuscano J, Matsumura F. Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am J Pathol. 2007;171:1538–48. doi: 10.2353/ajpath.2007.070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon BI, Hirabayashi K, Kawasaki Y, Kodama Y, Kaneko T, Kanno J, Kim DY, Fujii-Kuriyama Y, Inoue T. Aryl hydrocarbon recepor mediates benzene-induced hematotoxicity. Toxicol Sci. 2002;70:150–6. doi: 10.1093/toxsci/70.1.150. [DOI] [PubMed] [Google Scholar]
- 59.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–83. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X, Liu D, Murray TJ, Mitchell GC, Hesterman EV, Karchner SI, Merson RR, Hahn ME, Sherr DH. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene. 2005;24:7869–81. doi: 10.1038/sj.onc.1208938. [DOI] [PubMed] [Google Scholar]
- 61.Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, Pasche AC, Knabenhans C, MacDonald HR, Trumpp A. c-Myc controls the balance between HSC self-renewal and differentiation. Genes and Dev. 2004;18:2747–63. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–61. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 63.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–80. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Haan G, Nijhof W, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cell during aging: correlation between lifespan and cycling activity. Blood. 1997;89:1543–50. [PubMed] [Google Scholar]
- 65.Yuan R, Astle CM, Chen J, Harrison DE. Genetic regulation of hematopoietic stem cell exhaustion during development and growth. Exp Hematol. 2005;33:243–50. doi: 10.1016/j.exphem.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Van Zant G, Liang Y. The role of stem cell in aging. Exp Hematol. 2003;31:659–72. doi: 10.1016/s0301-472x(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 67.Bell DR, Van Zant G. Stem cells, aging and cancer: inevitabilities and outcomes. Oncogene. 2004;23:7290–96. doi: 10.1038/sj.onc.1207949. [DOI] [PubMed] [Google Scholar]
- 68.De Haan G, Van Zant G. Genetic analysis of hemopoietic cell cycling in mice suggests its involvement in organismal life span. FASEB J. 1999;13:707–13. doi: 10.1096/fasebj.13.6.707. [DOI] [PubMed] [Google Scholar]
- 69.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 70.Nebert DW, Brown DD, Towne DW, Eisen HJ. Association of fertility, fitness and longevity with the murine Ah locus among (C57BL/6N) (C3H/HeN) recombinant inbred strains. Biol Reprod. 1984;30:363–73. doi: 10.1095/biolreprod30.2.363. [DOI] [PubMed] [Google Scholar]
- 71.Spindler SR, Koizumi A, Walford RL, Mote PL. P1-450 and P3-450 gene expression and maximum life span in mice. Mut Res. 1989;219:89–94. doi: 10.1016/0921-8734(89)90018-0. [DOI] [PubMed] [Google Scholar]
- 72.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nature Med. 1996;2:1011–16. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 73.Harper PA, Riddick DS, Okey AB. Regulating the regulator: factors that control levels and activity of the aryl hydrocarbon receptor. Biochem Pharmacol. 2006;72:267–79. doi: 10.1016/j.bcp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt JV, Carer LA, Bradfield CA. Molecular characterization of the murine Ahr gene. Organization, promoter analysis, and chromosomal assignment. J Biol Chem. 1993;268:22203–9. [PubMed] [Google Scholar]
- 75.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 76.Lee JA, Hwang JA, Sung HN, Jeon CH, Gill BC, Youn HJ, Park JH. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modulates functional differentiation of mouse bone marrow-derived dendritic cells. Downregulation of RelB by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 2007;173:31–40. doi: 10.1016/j.toxlet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Ryan EP, Holz JD, Mulcahey M, Sheu TJ, Gasiewicz TA, Puzas JE. Environmental toxicants may modulate osteoblast differentiation by a mechanism involving the aryl hydrocarbon receptor. J Bone Miner Res. 2007;22:1571–80. doi: 10.1359/jbmr.070615. [DOI] [PubMed] [Google Scholar]
- 78.Diry M, Tomkiewicz C, Koehle C, Coumoul X, Bock KW, Transy C. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 2006;25:5570–4. doi: 10.1038/sj.onc.1209553. [DOI] [PubMed] [Google Scholar]
- 79.Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581:3608–15. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 80.Bock KW, Kohle C. Ah receptor: Dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol. 2006;72:393–404. doi: 10.1016/j.bcp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 81.Neri T, Merico V, Garagna S, Redi CA, Zuccotti M. Expression of phase I and phase II genes in mouse embryonic stem cells cultured in the presence of 2,3,7,8-tetrachlorodibenzo-para-dioxin. Biochim Biophys Acta. 2008;1780:826–36. doi: 10.1016/j.bbagen.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Li W, Vogel CF, Fijiyoshi P, Matsumuraa F. Development of a human adipocyte model derived from human mesenchymal stem cells (hMSC) as a tool for toxicological studies on the action of TCDD. Biol Chem. 2008;389:169–77. doi: 10.1515/BC.2008.015. [DOI] [PubMed] [Google Scholar]
- 83.Weiss C, Faust D, Schreck I, Ruff A, Farwerck T, Melenberg A, Schneider S, Oesch-Bartlomowicz B, Zatloukalova J, Vondracek J, Oesch F, Dietrich C. TCDD deregulates contact inhibition in rat liver oval cells via Ah receptor, JunD and cyclin A. Oncogene. 2008;27:2198–207. doi: 10.1038/sj.onc.1210859. [DOI] [PubMed] [Google Scholar]
- 84.Umannova L, Zatloukalova J, Machala M, Krcmar P, Majkova Z, Hennig B, Kozubik A, Vondracek J. Tumor necrosis factor-alpha modules effects of aryl hydrocarbon receptor ligands on cell proliferation and expression of cytochrome P450 enzymes in rat liver “stem-like” cells. Toxicol Sci. 2007;99:79–89. doi: 10.1093/toxsci/kfm149. [DOI] [PubMed] [Google Scholar]
- 85.Panteleyev AA, Bicker DR. Dioxin-induced chloracne – reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp Dermatol. 2006;15:705–30. doi: 10.1111/j.1600-0625.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 86.Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–68. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 87.Quesenberry PJ, Hulspas R, Joly M, Benoit B, Engstrom C, Rielly J, Savarese T, Pang L, Recht L, Ross A, Stein G, Stewart M. Correlates between hematopoiesis and neuropoiesis: neural stem cells. J Neurotrauma. 1999;16:661–6. doi: 10.1089/neu.1999.16.661. [DOI] [PubMed] [Google Scholar]
- 88.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 89.Easterday MC, Dougherty JD, Jackson RL, Ou J, Nakano I, Paucar AA, Roobini B, Dianati M, Irvin DK, Weissman IL, Terskikh AV, Geschwind DH, Kornblum HI. Neural progenitor genes. Germinal zone expression and analysis of genetic overlap in stem cell populations. Dev Biol. 2003;264:309–22. doi: 10.1016/j.ydbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 90.Terskikh AV, Easterday MC, Li L, Hood L, Kornblum HI, Geschwind DH, Weissman IL. From hematopoiesis to neuropoiesis: evidence of overlapping genetic programs. Proc Natl Acad Sci USA. 2001;98:7934–9. doi: 10.1073/pnas.131200898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abbott BD, Birnbaum LS, Perdew GH. Developmental expression of two members of a new class of transcription factors: I Expression of aryl hydrocarbon receptor in C57BL/6N mouse embryo. Dev Dyn. 1995;204:133–43. doi: 10.1002/aja.1002040204. [DOI] [PubMed] [Google Scholar]
- 92.Abbott BD, Probst MR. Developmental expression of two members of a new class of transcription factors: II Expression of aryl hydrocarbon receptor nuclear translocator in the C57BL/6N mouse embryo. Dev Dyn. 1995;204:144–55. doi: 10.1002/aja.1002040205. [DOI] [PubMed] [Google Scholar]
- 93.Williamson MA, Gasiewicz TA, Opanashuk LA. Aryl hydrocarbon receptor expression and activity in cerebellar granule neuroblasts: implications for development and dioxin neurotoxicity. Toxicol Sci. 2005;83:340–8. doi: 10.1093/toxsci/kfi031. [DOI] [PubMed] [Google Scholar]
- 94.Collins LL, Williamson MA, Thompson BD, Dever DP, Gasiewicz TA, Opanashuk LA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure disrupts granule neuron precursor maturation in the developing mouse cerebellum. Toxicol Sci. 2008;103:125–36. doi: 10.1093/toxsci/kfn017. [DOI] [PubMed] [Google Scholar]
- 95.Kim MD, Jan LY, Jan YN. The bHLH-PAS protein Spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev. 2006;20:2806–19. doi: 10.1101/gad.1459706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qin H, Powell-Coffman JA. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev Biol. 2004;270:64–75. doi: 10.1016/j.ydbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Wada H, Masuda K, Satoh R, Kukugawa K, Ikawa T, Katsura Y, Kawamoto H. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–72. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]