Abstract
To establish and persist within a host, Leishmania spp. parasites delay the onset of cell-mediated immunity by suppressing interleukin-12 (IL-12) production from host macrophages. Although it is established that Leishmania spp.-infected macrophages have impaired IL-12 production, the mechanisms that account for this suppression remain to be completely elucidated. Using a luciferase reporter assay assessing IL-12 transcription, we report here that Leishmania major, Leishmania donovani, and Leishmania chagasi inhibit IL-12 transcription in response to interferon-gamma, lipopolysaccharide, and CD40 ligand and that Leishmania spp. lipophosphoglycan, phosphoglycans, and major surface protein are not necessary for inhibition. In addition, all the Leishmania spp. strains and life-cycle stages tested inhibited IL-12 promoter activity. Our data further reveal that autocrine-acting host factors play no role in the inhibitory response and that phagocytosis signaling is necessary for inhibition of IL-12.
The hallmark of an intracellular pathogen is its ability to survive within the intracellular niche. These organisms must be resistant to, or able to evade, the host cell's microbiocidal mechanisms. This dilemma is particularly relevant to Leishmania spp. because these organisms primarily reside within vertebrate macrophages (MP). These host cells become activated for microbial destruction and cytokine secretion by exposure to immune modulators, such as interferon-gamma (IFN-γ) and CD40 ligand (CD40L) found on activated T-cells. One strategy Leishmania spp. parasites use to avoid host cell activation is to interfere with the signaling pathways that induce MP to become microbicidal (Gregory and Olivier, 2005); another is inhibiting or delaying the production of activating cytokines (Belkaid et al., 2000).
The most consistent dysfunction reported from Leishmania spp.-infected MP is the aberrant production of inflammatory cytokines, specifically an inhibition of interleukin-12 (IL-12) production (McDowell and Sacks, 1999). Being essential for T-helper 1 (Th1) cell differentiation, the inability of Leishmania spp.-infected MP to produce IL-12 allows Leishmania spp. to evade acquired resistance by postponing IL-12 production and the induction of IFN-γ, thereby allowing the establishment of the infection; both clinical and experimental studies indicate that the onset of Th1-mediated immunity and Leishmania spp. killing is indeed delayed (Melby, 1991). Inhibition of MP IL-12 production and resulting Th1 responses is not unique to Leishmania; viruses (Chehimi et al., 1994; Chougnet et al., 1996; Karp et al., 1996) and bacteria (Marth and Kelsall, 1997; Sutterwala et al., 1997; Matsunaga et al., 2003) also exploit this mechanism to avoid clearance.
The general nature of impaired IL-12 production in Leishmania spp.-infected MP has extended to every IL-12 agonist that has been tested. Leishmania spp.-infected MP are unable to produce IL-12 even in response to strong inflammatory stimuli, including microbial stimuli, e.g., lipopolysaccharide (LPS), Staphylococcus aureus (SAC), Toxoplasma gondii antigen, and mycobacteria (Carrera et al., 1996; Sartori et al., 1997; Belkaid et al., 1998; Weinheber et al., 1998; Piedrafita et al., 1999), and T-cell-dependent agonists, e.g., IFN-γ and CD40L (Carrera et al., 1996; Belkaid et al., 1998; Weinheber et al., 1998; Piedrafita et al., 1999). Furthermore, the inhibition observed in Leishmania spp.-infected MP is selective, that is, other proinflammatory cytokines and chemokines are not affected (Carrera et al., 1996).
Several studies have shown that Leishmania spp. infection interferes with IL-12 production in MP; however, conflicting data have been reported concerning the role of different Leishmania spp. life-cycle stages in the inhibitory process. Reiner et al. (1994) were the first to report that Leishmania major amastigotes stimulate, rather than inhibit, IL-12 production in murine bone marrow-derived MP (BMDM), an observation that was confirmed in the murine MP cell line J774 (Piedrafita et al., 1999). Conversely, Leishmania mexicana amastigotes inhibit IL-12 secretion in murine BMDM (Weinheber et al., 1998). Although L. major and Leishmania panamensis stationary-phase promastigotes induce small amounts of IL-12 secretion in human peripheral blood mononuclear cells (PBMC), these parasites, as well as Leishmania braziliensis, L. mexicana, and Leishmania guyanensis promastigotes, inhibit IL-12 release in response to SAC (Sartori et al., 1997). Furthermore, L. major and Leishmania donovani metacyclic promastigotes inhibit IL-12 production in murine MP in response to IFN-γ and LPS (Belkaid et al., 1998) and mycobacterial products (Carrera et al., 1996).
The fact that Leishmania spp.-conditioned medium is able to inhibit SAC-induced IL-12 production in human PBMC (Sartori et al., 1997) suggests that soluble parasite components mediate IL-12 inhibition. Specifically, both purified and synthetic phosphoglycans (PG) have been reported to inhibit IL-12 production in murine MP (Piedrafita et al., 1999). The leishmanial surface expresses other molecules that interact with the host cell, such as lipophosphoglycan (LPG), glycosylinositol phospholipids (Orlandi and Turco, 1987; McConville et al., 1995), and a surface protease (GP63, MSP) (Guha-Niyogi et al., 2001), that may mediate IL-12 inhibition.
IL-12p70 is a covalently linked heterodimer composed of 2 chains, p40 and p35, encoded by separate genes (Ma, AsteAmezaga et al., 1996). Whereas p40 transcripts are highly regulated and found only in cells producing biologically active IL-12, the p35-encoding gene is constitutively expressed in many cell types (Ma et al., 1995). Until recently, it was believed that expression of IL-12p40 alone was predictive of IL-12p70 production; however, it is now evident that IL-12p35 gene transcription can be tightly regulated (Hayes et al., 1995; Snijders et al., 1996). For most Leishmania species, the mechanism of IL-12 inhibition is mediated at the level of IL-12p40 mRNA accumulation (Carrera et al., 1996; Sartori et al., 1996, 1997; Piedrafita et al., 1999). However, steady-state IL-12p40 mRNA levels are not affected by L. mexicana infection (Weinheber et al., 1998), indicating that a posttranscriptional regulation mechanism may be occurring.
Although many studies indicate that Leishmania spp. infection inhibits MP production of IL-12, several discrepancies have been reported. The reason for the reported differences in the literature is unclear; explanations may lie in the differences between the cell types investigated, differences in the Leishmania species and life cycle stages used, the use of nonphysiological amounts of purified parasite molecules, or the utilization of different assays assessing either mRNA levels, protein production, or IL-12 secretion. In the present work, we examine several different parameters using a single assay to elucidate the parasite and host factors that mediate impaired IL-12p40 promoter activity in Leishmania spp.-infected MP. This assay consists of the RAW264.7 murine MP cell line, stably transfected with the human IL-12p40 promoter tagged to a firefly luciferase construct; this system was previously shown to recapitulate endogenous IL-12p40 production in terms of cell type specificity and stimulus responsiveness (Ma, Chow et al., 1996) and allows us to assess suppression of IL-12p40 promoter activity and thus implicate IL-12p40 transcription. Here, we demonstrate that inhibition of IL-12 in MP by Leishmania spp. infection extends to all Leishmania species and strains that were tested, does not require LPG, PG, or MSP, involves phagocytosis signaling, and likely requires cell contact.
MATERIALS AND METHODS
Cell lines and reagents
The murine macrophage cell line RAW264.7 stably transfected with human IL-12p40 promoter tagged to luciferase construct (RAWp40LUC) was previously generated (Ma, Chow et al., 1996). Cells were maintained in complete RPMI (10% fetal bovine serum [FBS], 1% penicillin/streptomycin, 1% l-glutamine) at 37 C and 5% CO2. Recombinant mouse (rm) IFN-γ purchased from Peprotech (Rocky Hill, New Jersey) was used at 1,000 U/ml. Escherichia coli LPS (Sigma Aldrich, St. Louis, Missouri) was used at 1 μg/ml. Recombinant mouse CD40L trimer was donated by Immunex (Seattle, Washington). Cytochalasin D (MP Biomedicals, Solon, Ohio) and phospholipase C (PLC) inhibitor U73122 (Calbiochem, San Diego, California) were used at a concentration of 5 μM.
Parasites
Infections were performed with L. major NIH Friedlin V1 strain (MHOM/IL/80/FN), isolated from a patient with localized cutaneous leishmaniasis in Israel; L. major substrain IR173 (MHOM/IR/-173), isolated from patients with localized cutaneous leishmaniasis in Iran; L. major LV39 (MRHO/SU/59/P), isolated from a gerbil reservoir in southern Russia; L. major NIH S strain (MHOM/SN/74/Seidman), isolated from a patient from Senegal, West Africa, with multiple subcutaneous nodules; L. donovani strain 9515 (MHOM/IN/95/9515), isolated from splenic aspirates of patients with visceral leishmaniasis in India; L. donovani strain Mongi (MHOM/IN/83/Mongi-142), isolated from bone marrow biopsies of patients with visceral leishmaniasis in India; L. donovani strain 1S isolated from a patient with visceral leishmaniasis in Sudan; L. donovani mutants LPG −/− (R2D2) and PG −/− (C3PO) generated by chemical mutagenesis of L. donovani (1S) (King and Turco, 1998); wild-type Leishmania chagasi was originally isolated from a patient in Brazil; and the attenuated L. chagasi strain (L5) was generated previously by extensive in vitro passage (Wilson et al., 1989). Infective-stage metacyclic promastigotes were isolated by ficoll gradient (Spath and Beverley, 2001) and opsonized in 3 ml of Hank's balanced salt solution media (0.15 mM CaCl2 and 1.0 mM MgCl2) containing 5% normal mouse serum at 37 C for 30 min. Procyclic promastigotes were obtained from 2-day-old parasite cultures. Lesion-derived amastigotes were isolated from footpads of BALB/c mice. All parasite strains were cultured at 26 C without CO2 in medium 199 containing 10% heat-inactivated FBS, 100 μg/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 40 mM HEPES, 0.1 mM adenine (in 50 mM HEPES), 5 mg/ml hemin (in 50% triethanolamine), and 1 mg/ml biotin. Parasites tested negative for mycoplasma (polymerase chain reaction [PCR] detection method) and tested below the detection limits for endotoxin (LAL assay; Endosafe, Charles River Laboratories, Charleston, South Carolina).
Parasite preparations
Parasite lysates were prepared by subjecting isolated metacyclics to alternating cycles of freezing (dry ice/90% ethanol bath) and thawing (37 C) for 5 min each. Parasites were heat killed at 56 C for 1 hr. All parasite preparations were examined by light microscopy to ensure complete lysis or death of parasites. Supernatants from parasite cultures were filter sterilized before use. Amicon ultracentrifugal filter devices (Millipore, Billerica, Massachusetts) were used to concentrate parasite supernatants.
Luciferase assay
RAWp40LUC cells were plated at 2.5 × 106 cells per well in 6-well plates, lower and upper chambers of the transwell system. Cells were primed with recombinant mouse IFN-γ (Peprotech) at 1,000 U/ml overnight followed by LPS (1 μg/ml) or recombinant mouse CD40L (Immunex, Seattle, Washington) at 0.5 μg/ml stimulation for 8 hr. Infections were carried out at multiplicity of infection of 5–10 parasites to 1 cell at the time of rm IFN-γ addition. One well was reserved for quantifying infection rates. After adherence to slides by cytospin centrifugation, cells were methanol fixed, stained with Diff-Quick, and visualized with light microscopy. Infection rates for all infections did not vary by more than 5% between the different infecting strains. Cells were lysed and luciferase activity determined according to instructions of the luciferase assay kit (Promega, Madison, Wisconsin) using LMax ll 384 (Molecular Devices, Sunnyvale, California). Triplicate samples were run and Student's t-test was used to determine statistical significance. Data were normalized to the mean luciferase units of the uninfected/unstimulated controls within a single assay and expressed as relative luciferase units. All cells were examined by Wright–Giemsa staining and light microscopy to ensure equal uptake of parasites, or, in the case of transwell system, to ensure that parasites did not cross the membrane from the upper chamber into the lower chamber.
Real-time quantitative reverse-transcription (RT)-PCR
Relative levels of IL-12p40 mRNA were determined by real-time PCR. mRNA levels were measured after 4 hr of infection. Total mRNA was prepared with the RNeasy Mini Kit (Qiagen). One microgram of total mRNA was reverse transcribed using random primers with Superscript lll Synthesis System for RT-PCR (Invitrogen, Carlsbad, California.) according to the manufacturer's instructions. For analysis of IL-12p40 and hypoxanthine–guanine phosphoribosyl transferase (HPRT) mRNA expression, real-time PCR was performed using SYBR green chemistry with the following primers: murine IL-12p40 forward: 5′-AAC CAT CTC CTG GTT TGC CA-3′; murine IL-12p40 reverse: 5′-CGG GAG TCC AGT CCA CCT C-3′; murine IL-10 forward: 5′-CAC AAA GCAG CCT TGC AGA A-3′; murine IL-10 reverse: 5′-CTG GCC CCT GCT GAT CCT-3′; tumor necrosis factor (TNF)-α forward: 5′-GAA ACA CAA GAT GCT GGG ACA GT-3′; TNF-α reverse: 5′-CAT TCG AGG CTC CAG TGA ATT C-3′; murine HPRT forward: 5′-CAA AGC CTA AGA TGA GCG CAA-3′; murine HPRT reverse: 5′-AGG CAG ATG GCC ACA GGA C-3′. Real-time PCR reactions were performed according to the manufacturer's recommendations with an ABI Prism 7500 sequence detection system (Perkin Elmer). The relative number of mRNA copies for p40 was determined by the following formula: number of copies = 2ΔΔct, where ΔΔct = Δct(experimental) − Δct(calibrator), Δct = ct(experimental) − ct(HPRT), ct = cycle at which there is a statistically significant increase in the emission intensity over the background, and Δct(calibrator) = mean Δct for the uninfected and unstimulated control. Student's t-test was utilized to determine statistical significance. A P-value of less than 0.05 was considered statistically significant.
RESULTS
Leishmania spp. infection inhibits CD40L-induced IL-12p40 promoter activity
To test if the Leishmania spp. infection blocks IL-12p40 promoter activity, we utilized a murine macrophage cell line (RAW264.7) stably expressing the 3.3-base pair region of the human IL-12p40 promoter fused to a luciferase reporter (RAWp40LUC) (Ma, Chow et al., 1996). Although BMMP exhibit inhibited IL-12p40 mRNA accumulation when infected with L. major and L. donovani in response to microbial stimuli (Carrera et al., 1996), inhibition of IL-12p40 induction by CD40L has yet to be evaluated during infections with these species and it is unclear whether the decreased levels of IL-12p40 mRNA observed in Leishmania spp.-infected MP is due to decreased mRNA stability or decreased transcriptional activation. To investigate inhibition of CD40L-induced IL-12p40 transcription, we infected RAWp40LUC cells with opsonized infectious-stage (metacyclic) Leishmania spp. parasites and then stimulated with purified CD40L trimer or LPS with and without IFN-γ stimulation. Similar to primary macrophages, L. major (Figs. 1, 2B) and L. donovani (Fig. 2A) infection had a significant inhibitory effect on LPS ± IFN-γ-induced IL-12p40 promoter activity in RAWp40LUC cells. Furthermore, both species were able to inhibit IL-12 promoter activity in response to CD40L ± IFN-γ stimulation (Fig. 1; data not shown), indicating that Leishmania spp. infection is able to block multiple signaling pathways, including nonmicrobial agonists. As previously reported (Carrera et al., 1996), IL-12p40 mRNA accumulation is inhibited by Leishmania spp. infection (Fig. 7); TNF-α mRNA was not affected and IL-10 mRNA expression was augmented (data not shown).
Figure 1.
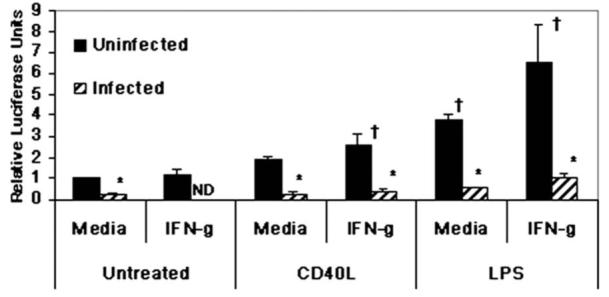
Leishmania infection inhibits IL-12p40 transcription in murine macrophage cell line. RAW264.7 cells stably transfected with human IL-12p40-LUC construct were infected with L. major strain V1 and subsequently treated with 3 μg/ml CD40L ± 100 U/ml IFN-γ or 1 μg/ml LPS ± 100 U/ml IFN-γ. Samples were run in triplicate and mean relative luciferase units ± standard deviation is reported. * Infected values were significantly different from uninfected, stimulated controls (P < 0.05). † Uninfected, stimulated values were different from uninfected, untreated controls (P < 0.05). One representative of 3 independent experiments is presented.
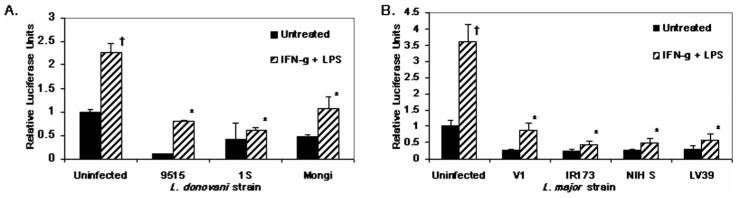
Figure 2.
Leishmania-induced IL-12p40 inhibition is independent of species and strain. RAW264.7 cells stably transfected with human IL-12p40-LUC construct were infected with different L. donovani (A) or L. major (B) substrains and treated with 100 U/ml IFN-γ plus 1 μg/ml LPS. Samples were run in triplicate and mean relative luciferase units ± standard deviation is reported. * Infected values were significantly different from uninfected, stimulated controls (P < 0.05). † Uninfected, stimulated values were different from uninfected, untreated controls (P < 0.05). In each case 1 representative of 2 independent experiments is shown.
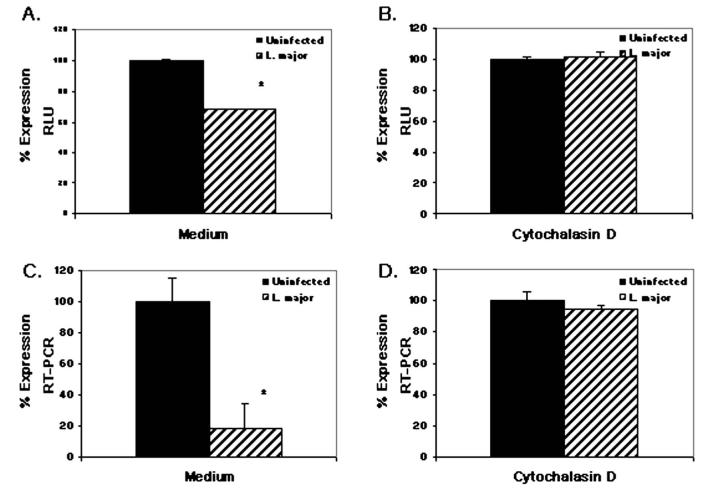
Figure 7.
Inhibition of IL-12p40Luc by Leishmania requires phagocytosis. RAW264.7 cells stably transfected with human IL-12p40-LUC construct were untreated (A, C) or pretreated with 1 μM cytochalasin D (B, D), then infected with L. major strain V1 and subsequently treated with 1 μg/ml LPS ± 100 U/ml IFN-γ. Samples were run in triplicate, treated with IFN-γ + LPS, and assessed for luciferase production (A, B) or endogenous levels of IL-12p40 by quantitative RT-PCR (C, D). Mean percentage expression ± standard deviation of is reported. * Infected values were significantly different from uninfected, stimulated controls (P < 0.05). One representative of 2 independent experiments is presented.
Leishmania spp.-induced inhibition of IL-12p40 promoter activity is independent of species, substrain, or life-cycle stage
It is apparent that different Leishmania substrains express structurally distinct surface molecules (Dobson, Mengeling et al., 2003) and that the clinical manifestations caused by these substrains also differ (Neva et al., 1979). To ensure that the Leishmania spp.-induced inhibition of IL-12 promoter activity was not just an anomaly due to the L. major and L. donovani strains utilized, we tested whether the inhibition was produced by infecting with other species-defined L. major and L. donovani isolates. In all cases, Leishmania spp. infection abrogated luciferase induction in response to IFN-γ + LPS (Fig. 2) and IFN-γ + CD40L (data not shown). It previously has been shown that L. major amastigotes induce IL-12 production in murine MP (Reiner et al., 1994; Piedrafita et al., 1999) and L. major log-phase (procyclic) promastigotes induce IL-12p40 in human PBMC (Sartori et al., 1996). In our studies, L. major metacyclic and log-phase promastigote, as well as amastigote infection, inhibited luciferase production (Fig. 3), indicating that all L. major life cycle stages can inhibit IL-12p40 production in murine MP. Furthermore, amastigotes were unable to induce transcription via the human IL-12p40 promoter in this system.
Figure 3.
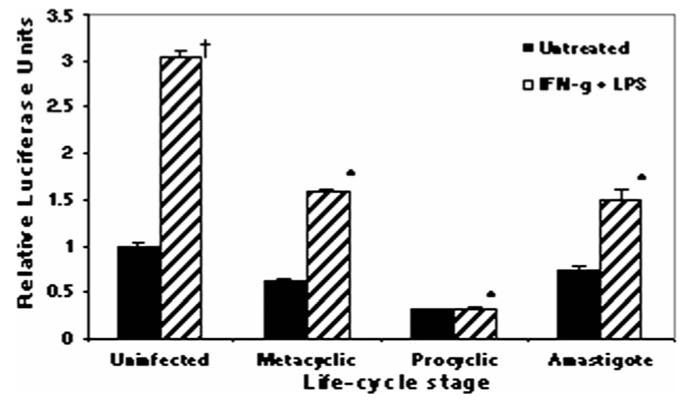
Inhibition of IL-12p40Luc by different Leishmania spp. life-cycle stages. RAW264.7 cells stably transfected with human IL-12p40-LUC construct were infected with L. major strain V1 life-cycle stages for 16 hr followed by 8 hr of LPS (1 μg/ml) stimulation. Life-cycle stages (met = metacyclic; log = log-phase promastigotes; am = amastigotes). Samples were run in triplicate and mean relative luciferase units ± standard deviation is reported. * Infected values were significantly different from uninfected, stimulated controls (P < 0.05). † Uninfected, stimulated values were different from uninfected, untreated controls (P < 0.05). One representative of 2 independent experiments is shown.
Leishmania spp. products inhibit IL-12p40 promoter activity
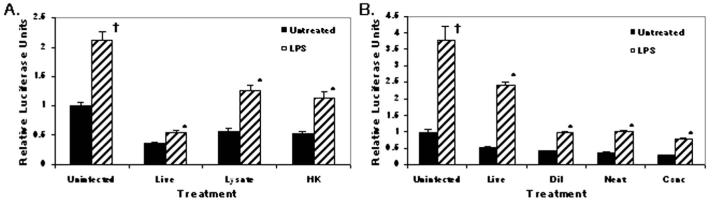
To determine if inhibition of IL-12p40 promoter activity required a live L. major infection, we infected RAWp40LUC cells with L. major metacyclic promastigotes and with parasites killed by heating for 1 hr at 65 C and assayed luciferase production after LPS stimulation (Fig. 4A). Our experiments revealed that live infection is not required for IL-12p40 inhibition. We further determined if L. major-derived products were able to mediate inhibition. Treatment of RAWp40LUC cells with either parasite lysate (Fig. 4A) or supernatants derived from promastigote cultures (Fig. 4B) abrogated LPS-induced luciferase expression, indicating that parasite products alone can inhibit IL-12 production.
Figure 4.
Inhibition of IL-12p40Luc by Leishmania spp. products. RAW264p7 cells stably transfected with human IL-12p40-LUC construct were infected with L. major strain V1 or treated with parasite products for 16 hr followed by 8 hr of LPS (1 μg/ml) stimulation. (A) Parasite products (Live = live metacyclic infection; Lysate = freeze–thaw lysates; HK = heat-killed metacyclics). (B) Secreted products (V1 = live metacyclic infection; Neat, Dil, Conc = cultured parasite supernantants either diluted or concentrated). Samples were run in triplicate and mean relative luciferase units ± standard deviation is reported. * Infected values were significantly different from uninfected, stimulated controls (P < 0.05). † Untreated, stimulated values were different from untreated, media controls (P < 0.05). In each case 1 of 3 independent experiments is shown.
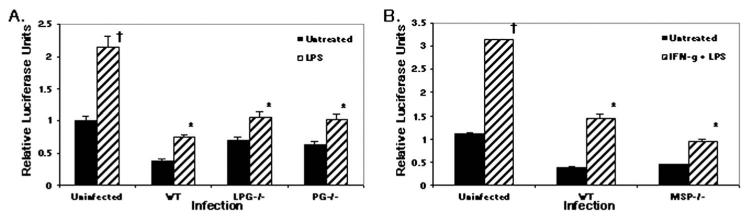
Purified PG from L. major promastigotes previously has been shown to inhibit IL-12p40 release in murine MP (Piedrafita et al., 1999). As the utilization of large amounts of purified or synthesized parasite molecules may not reflect the natural situation, we tested the role of Leishmania spp. PGs by utilizing a mutant deficient in the production of all PGs. This mutant, C3PO, is deficient for the guanosine 5′-diphospho-d-mannose transporter necessary for biosynthesis of the repeating units in all PG and, therefore, is lacking secreted PG, proteo-PG, and LPG (Descoteaux et al., 1995). Consistent with previous reports (Carrera et al., 1996; Sartori et al., 1997), the LPG-deficient L. donovani parasite, R2D2, is able to inhibit LPS-induced IL-12p40 promoter activity (Fig. 5A). Furthermore, PG-deficient metacyclic promastigotes also diminished luciferase production, indicating that the Gal(α1,4)Man(α1)-PO4 repeat motif present in all PG is not required for IL-12 inhibition.
Figure 5.
Inhibition of IL-12p40Luc by Leishmania is not dependent on LPG, PG, or GP63. RAW264.7 cells stably transfected with human IL-12p40-LUC construct were infected with Leishmania for 16 hr followed by 8 hr of LPS (1 μg/ml) stimulation. (A) Leishmania donovani wild-type, LPG-deficient, and PG-deficient mutants. (B) Leishmania mexicana wild-type and GP63-deficient mutant. Samples were run in triplicate and mean relative luciferase units ± standard deviation is reported. * Infected values were significantly different from uninfected, stimulated controls (P < 0.05). † Uninfected, stimulated values were different from uninfected, untreated controls (P < 0.05). One representative of 2 independent experiments is shown.
Being the most abundant surface glycoproteins on the Leishmania spp. surface, the MSPs are likely candidates for modulating host cell functions. To test the role of MSP in IL-12 inhibition, we infected RAWp40LUC cells with wild-type L. chagasi and an attenuated strain of L. chagasi that lacks MSP surface expression (Wilson et al., 1989) (Fig. 5B). Both attenuated and wild-type L. chagasi inhibited LPS-induced luciferase production, indicating that the most prominent Leishmania spp. molecules, LPG and MSP, are not necessary for inhibition of IL-12p40 promoter activity.
Autocrine regulation is not responsible for IL-12p40 inhibition
To investigate the role of autocrine or paracrine regulation of IL-12 production, we utilized a transwell system, whereby cells in the upper chambers could produce soluble mediators to affect luciferase production in cells in the lower chambers (Fig. 6). The upper chambers contained either uninfected or L. major-infected RAWp40LUC cells and the lower chambers contained RAWp40LUC cells either unstimulated or stimulated with IFN-γ + LPS. As a positive control for inhibition, we infected cells in the lower chamber with L. major parasites. Lower-chamber cells from wells containing uninfected cells in the upper chambers exhibited the typical pattern of luciferase production; that is, high amounts of luciferase from uninfected cells stimulated with IFN-γ and LPS and an inhibition of luciferase production from L. major-infected cells. The same pattern is found in lower-chamber cells from wells containing infected cells in the upper chambers, indicating that L. major infection is not inducing MP autocrine/paracrine regulation of IL-12 promoter activity.
Figure 6.
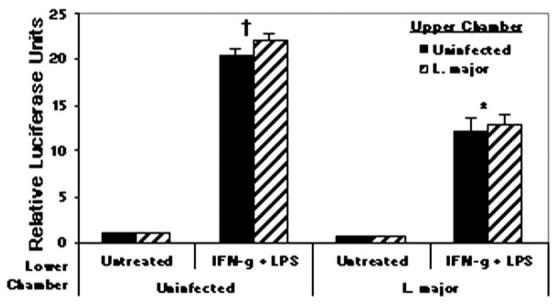
Inhibition of IL-12p40Luc by Leishmania does not involve autocrine MP factors; 1 × 106 RAW264.7 cells stably transfected with human IL-12p40-LUC construct were plated in lower wells and 1 × 106 were plated in upper wells of a transwell system. Cells were infected with L. major at a 5:1 ratio for 2 hr followed by IFN-γ (100 U/ml) stimulation for 16 hr and LPS (1 μg/ml) stimulation for 8 hr. Luciferase values were detected from cells in lower chambers. Samples were run in triplicate and mean relative luciferase units ± standard deviation is reported. * Infected values were significantly different from uninfected, stimulated controls (P < 0.05). † Uninfected, stimulated values were different from uninfected, untreated controls (P < 0.05). One representative of 3 independent experiments is shown.
Inhibitors of phagocytosis block Leishmania spp.-induced IL-12p40 inhibition
We investigated the role phagocytosis plays in Leishmania spp.-induced inhibition of IL-12 in response to IFN-γ and LPS stimulation by using 2 different phagocytosis inhibitors, cytochalasin D and the PLC inhibitor, U73122. Because both of these drugs do not block phagocytosis of Leishmania spp. infection completely in overnight infections, we shortened our assay to 4 hr; at this time point, both inhibitors blocked parasite uptake (data not shown). Both cytochalasin D and U73122 alone drastically inhibited luciferase expression and endogenous IL-12p40 mRNA production in response to IFN-γ + LPS stimulation; TNF-α and IL-10 production, however, was enhanced in the presence of inhibitors (data not shown), indicating that the phagocytosis inhibitors do not cause a general block in transcription. Although IL-12p40 promoter activity was downregulated by the phagocytosis inhibitors, in the presence of inhibitor we were still able to detect significant induction of luciferase in response to IFN-γ and LPS as compared with unstimulated cells (data not shown). In the presence of either cytochalasin D (Fig. 7) or U73122 (data not shown), L. major infection was unable to abrogate luciferase (Fig. 7A, B) or IL-12p40 mRNA (Fig. 7C, D) production, suggesting that phagocytosis may play a role in the inhibition of IL-12 production by Leishmania spp. infection.
DISCUSSION
Leishmaniasis is a chronic infection; even in the case of self-healing L. major infection, disease resolution takes several weeks to months. One mechanism Leishmania spp. parasites use to establish and persist in a host is the inhibition of IL-12 production and a delay in the ensuing cell-mediated response (Melby, 1991). MP are not the only cell type capable of producing of IL-12 and certainly it has been demonstrated that the in vivo source of IL-12 during Leishmania spp. infection is likely dendritic cells (Gorak et al., 1998; Marovich et al., 2000; McDowell et al., 2002). However, Leishmania spp.-infected dendritic cells require CD40L costimulation for IL-12 production (Marovich et al., 2000; McDowell et al., 2002), suggesting that during infection, dendritic cells initiate a protective response after interaction with antigen-specific T-cells in lymphoid tissue. For parasites that normally target MP for survival, the avoidance of IL-12 early during infection would serve as an adaptive strategy to establish an infection. Although it is known that Leishmania spp. parasites impair IL-12 production in host MP, defining the mechanisms by which these pathogens inhibit IL-12 has been difficult. In the present study, we demonstrate that IL-12 suppression by Leishmania spp. is transcriptionally mediated and requires cell signaling and cytoskeletal rearrangement associated with phagocytosis.
Using a luciferase reporter assay, we demonstrate that Leishmania spp.-mediated downregulation of IL-12p40 occurs at the level of transcription. Consistent with previous reports assessing steady-state IL-12p40 mRNA levels (Carrera et al., 1996), our data indicate that L. major, L. donovani, and L. chagasi infection suppresses IFN-γ- and LPS-induced IL-12p40 production and extend this finding to CD40L cross-linking.
Contrary to L. mexicana amastigote-infected MP, in which LPS- and CD40L-induced IL-12p40 mRNA levels are not altered, L. major and L. donovani infection inhibits stimuli-induced activation of the IL-12p40 promoter. One possible explanation, other than species differences, to explain this discrepancy is that the previous study investigated axenic amastigotes; our study used all life-cycle stages and utilized lesion-derived amastigotes. Amastigotes obtained from lesions are covered with immunoglobulin that binds Fc receptors (FcR) (Guy and Belosevic, 1993; Dominguez and Torano, 1999) and infectious-stage metacyclic promastigotes are readily coated with endogenous ligands (C3b and iC3b) for complement receptor 3 (CR3) (Guy and Belosevic, 1993). Axenic amastigotes, on the other hand, are cultured in vitro and, therefore, do not have any of these endogenous host proteins on their surface. It previously has been suggested that Leishmania spp.-induced suppression of IL-12 production is initiated by the binding of specific receptors on the host cell surface that send a negative signal (McDowell and Sacks, 1999). Activation of CR3, FcR, and scavenger receptor (SR) by antibodies or opsonized erythrocytes leads to the downregulation of LPS-induced IL-12 in murine MP (Sutterwala et al., 1997). Furthermore, FcR, CR3, and mannose receptor (MR) ligation of human phagocytes inhibits IL-12 production (Berger et al., 1997; Marth and Kelsall, 1997; Nigou et al., 2001), whereas the amastigotes used in our studies likely engaged the FcR–receptor interaction for promastigotes is less clear. Our studies indicate that opsonization of Leishmania spp. parasites with normal mouse serum is not necessary for inhibition (data not shown), suggesting that CR3 ligation is not mediating the interaction. However, MP are able to synthesize complement components (McPhaden and Whaley, 1993) that opsonize parasites even in serum-free conditions (Wozencraft et al., 1986) and parasite components have been suggested to bind CR3 directly (Russell and Wright, 1988; Talamas-Rohana et al., 1990; Kedzierski et al., 2004). Furthermore, the repetitive structure and glycan modifications associated with many Leishmania spp. cell-surface molecules suggest that these parasites also interact with MR and SR (Turco and Descoteaux, 1992), which have been shown to send a negative signal in other systems.
Interestingly, it previously has been reported that L. major lesion-derived amastigotes (Reiner et al., 1994; Piedrafita et al., 1999) and log-phase promastigotes (Sartori et al., 1997) stimulate IL-12p40 production in murine MP and human PBMC, respectively. We were unable to confirm this observation using the RAW 264.7 MP cell line, as procyclic promastigotes or amastigotes did not induce any luciferase expression in the presence or absence of LPS stimulation. The reason for these discrepancies are unclear; however, our data are in agreement with others that have shown that lesion-derived L. major amastigotes inhibit, at least indirectly, IFN-γ + LPS-induced IL-12 production (Kane and Mosser, 2001). Further, it is possible that the IL-12 detected in human PBMC infected with procyclic promastigotes may have been produced by cell types other than MP.
Here, we utilized a murine MP cell line stably expressing a human IL-12p40 promoter construct to assess the effect of Leishmania spp. infection on IL-12 production. Although this system certainly is not identical to human MP, it previously has been shown to mirror endogenous murine and human IL-12p40 production in terms of cell type specificity and stimulus responsiveness (Aste-Amezaga et al., 1998). We demonstrate that endogenous murine IL-12p40 mRNA is inhibited by L. major infection in this system. We cannot rule out, however, that the cell-surface receptors that engage Leishmania spp. or the signaling pathways activated by such engagement may differ between murine and human MP, thus altering the response in human cells. Although the effect of Leishmania spp. infection on human MP remains to be determined, supporting our conclusion is the observation that L. major promastigotes inhibit SAC-induced IL-12 production in PBMC (Sartori et al, 1996).
Although the predominant surface molecule of Leishmania spp. promastigotes, LPG, has been implicated in inhibiting macrophage signal transduction pathways (Descoteaux and Turco, 2002), this glycoconjugate is not required for IL-12 inhibition by Leishmania infection in murine MP (Carrera et al., 1996) or human PBMC (Sartori et al., 1997). High concentrations of PG, on the other hand, have been shown to inhibit LPS-induced IL-12p40 production (Piedrafita et al., 1999). Here, we utilized Leishmania spp. parasites that lack LPG or PG expression to test the necessity of these moieties in suppressing IL-12p40 responses. We confirmed previous observations (Carrera et al., 1996) that LPG is not necessary and discovered that in a natural context PG also is not essential for Leishmania spp.-induced inhibition. Either the physiological concentrations of PG that were presented in our experiments do not inhibit IL-12p40 promoter activity or Leishmania spp. parasites use redundant mechanisms to impair IL-12 production (or both).
The repeating PG domain is a characteristic feature of other molecules of the leishmania glycocalyx, including proteo-PG (Ilg, Overath et al., 1994; Ilg, Stierhof et al., 1994; Ilg et al., 1996), secreted PG, and acid phosphatase (Shakarian and Dwyer, 2000). The PG domain contains species and strain-specific substitutions (Tolson et al., 1989; McConville et al., 1990; Ilg et al., 1992) and developmentally regulated polymorphisms (Glaser et al., 1991; Sacks, 1992; Moody et al., 1993). Furthermore, it recently has become apparent that L. major strains differ in their PG structure (Dobson, Mengeling et al., 2003; Dobson, Scholtes et al., 2003). Our data indicate that these polymorphisms likely do not influence Leishmania spp.-induced inhibition because all of the species, strains, and life-cycle stages that we tested were able to block IL-12p40 promoter activity, implicating some nonpolymorphic parasite factor(s) or redundant mechanisms.
LPS-induced IL-12p40 promoter activity was inhibited by treatment of MP with heat-killed parasites, parasite lysates, or supernatants from in vitro L. major cultures, indicating that the factor(s) responsible were likely present on the cell surface and secreted. The most abundant surface glycoprotein, MSP, recently has been shown to be released into the extracellular medium (Ellis et al., 2002; McGwire et al., 2002; Yao et al., 2002). Our data using L. chagasi promastigotes that lack MSP surface expression (L. chagasi L5) indicate that MSPs are likely not involved in the inhibition of IL-12p40 production. However, the L5 strain is not a specific gene knockout and, therefore, trace amounts of MSP are possibly expressed. Glycosylphosphatidylinositol (GPI)-anchored molecules, including LPG, PG, and MSP, glycosylinositolphospholipids, and gp46, dominate the Leishmania spp. surface. Studies have demonstrated that GPI anchors from Leishmania spp. and other protozoan parasites are able to modulate MP signaling cascades (Tachado et al., 1999), suggesting that Leishmania spp. GPI anchors are responsible for inhibiting MP functions. Recent studies indicate that the lipid moiety of Leishmania spp. GPI anchors is not required for inhibiting MP activation (Zufferey et al., 2003); however, inhibition of IL-12 production was not assessed in these studies.
As uncontrolled pro-inflammatory cytokine production is potentially dangerous, negative feedback loops exist to down-modulate IL-12. Both IL-10 (Ma et al., 1998) and TGF-α (D'Andrea et al., 1995) are potent inhibitors of IL-12 production. In addition to these anti-inflammatory cytokines, prostaglandin-E2 downregulates this molecule (van der Pouw Kraan et al., 1995, 1996). One possible mechanism by which Leishmania spp. could regulate IL-12 production would be to activate infected MP to produce 1 or more of these downregulatory modulators. The fact that we observed that IL-10 is upregulated in MP following Leshmania spp. infection makes it particularly appealing to implicate IL-10 as the mechanism for downregulating IL-12 expression; however, it previously has been demonstrated that inhibition still occurs in the absence of IL-10 (Carrera et al., 1996; Weinheber et al., 1998). Using a transwell system, we show here that no autocrine- or paracrine-acting MP factors are involved in suppression of IL-12 by Leishmania spp. infection, lending support for a direct interaction of these parasites with host signaling machinery or the downregulatory receptor hypothesis.
The role of phagocytosis in IL-12 production and inhibition is unclear. Phagocytosis of inert polystyrene beads greater than 2 μm in diameter upregulates IL-12p40 mRNA in human monocytes (Fulton et al., 1996) and 3.1-μm latex beads induce IL-12p40 production in bone-marrow-derived MP, whereas 1.2-μm beads do not (Ladel et al., 1997). In a conflicting study, phagocytosis of 3.2-μm latex beads led to a 50% suppression of IFN-γ + CD40L-induced IL-12 production (Weinheber et al., 1998). However, phagocytosis is not required for IL-12 suppression by apoptotic cells (Kim et al., 2004). Leishmania spp. amastigotes are only 1–2 μm in size and still suppress MP IL-12 production, suggesting that at least for Leishmania spp., size is likely not a factor in the inhibitory process.
We utilized cytochalasin D to block actin polymerization and the PLC inhibitor U73122 to prevent phagocytosis of Leishmania spp. parasites to determine if only cell contact was required for IL-12 inhibition. In both cases, the inhibitors alone suppressed LPS-induced luciferase expression and endogenous IL-12 transcription, suggesting that contrary to previous reports (Poussin et al., 1998) LPS-dependent activation requires LPS internalization. The inhibition was selective, however, as TNF-α and IL-10 production were enhanced in the presence of the phagocytosis inhibitors. Regardless of this suppression, we were able to detect significant IL-12 mRNA and luciferase expression in response to IFN-γ + LPS stimulation over untreated controls. This upregulation was not inhibited by Leishmania spp. infection in the presence of either phagocytosis inhibitor. There are 2 possible explanations to explain this result. First, these data could indicate that phagocytosis is necessary to inhibit the signal that leads to IL-12 transcription. Consistent with this explanation is a kinetic analysis that revealed that 16 hr of infection was required for the inhibition of MP activation to be detected (Nandan and Reiner, 1995). In contrast, another study investigating the same responses indicates that inhibition actually occurs before the parasites have been internalized, suggesting that the suppression is mediated via cell-surface receptor binding (Blanchette et al., 1999). Alternatively, our results may indicate that phagocytosis per se is not necessary for inhibition but that the downregulatory signaling pathways require actin polymerization and PLC activation. Recent work has highlighted the role of the cytoskeleton in mediating cell-signaling events and suggests that endocytosis/phagocytosis machinery may play a role in signaling not directly related to the uptake of exogenous particles (Liu and Shapiro, 2003). It is likely that Leishmania spp. factors, perhaps redundant molecules, interact with downregulatory phagocytic receptors to mediate IL-12 inhibition. As it is extremely difficult to uncouple the signaling and phagocytosis associated with these receptor interactions, whether Leishmania spp. uptake is essential remains to be determined.
ACKNOWLEDGMENTS
We are grateful to Dr. Salvatore Turco (University of Kentucky) for the use of the LPG Leishmania donovani mutants and to Jesmin Ehlers for his help with the luciferase assays. We are also thankful to Dr. Jeff Schorey for his helpful suggestions regarding our work and thoughtful review of the manuscript. Our gratitude also goes to Dr. Mary Wilson for the generous gift of the L. chagasi strains and for her insightful suggestions regarding our manuscript. This work was supported by a Scientist Development grant from the American Heart Association (#0435333Z).
LITERATURE CITED
- Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. Journal of Immunology. 1998;164:5936–5944. [PubMed] [Google Scholar]
- Belkaid Y, Butcher B, Sacks DL. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: Selective impairment of IL-12 induction in Leishmania-infected cells. European Journal of Immunology. 1998;28:1389–1400. doi: 10.1002/(SICI)1521-4141(199804)28:04<1389::AID-IMMU1389>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. Journal of Immunology. 2000;165:969–977. doi: 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- Berger S, Chandra R, Ballo H, Hildenbrand R, Stutte HJ. Immune complexes are potent inhibitors of interleukin-12 secretion by human monocytes. European Journal of Immunology. 1997;27:2994–3000. doi: 10.1002/eji.1830271136. [DOI] [PubMed] [Google Scholar]
- Blanchette J, Racette N, Faure R, Siminovitch KA, Olivier M. Leishmania-induced increases in activation of macrophage SHP-1 tyrosine phosphatase are associated with impaired IFN-gamma-triggered JAK2 activation. European Journal of Immunology. 1999;29:3737–3744. doi: 10.1002/(SICI)1521-4141(199911)29:11<3737::AID-IMMU3737>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Carrera L, Gazzinelli RT, Badolato R, Hieny S, Muller W, Kuhn R, Sacks DL. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. Journal of Experimental Medicine. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehimi J, Starr SE, Frank I, D'Andrea A, Ma X, MacGregor RR, Sennelier J, Trinchieri G. Impaired interleukin 12 production in human immunodeficiency virus- infected patients. Journal of Experimental Medicine. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougnet C, Wynn TA, Clerici M, Landay AL, Kessler HA, Rusnak J, Melcher GP, Sher A, Sherarer GM. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. Journal of Infectious Diseases. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- D'Andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: Priming for IL-12 and tumor necrosis factor alpha production. Journal of Experimental Medicine. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux A, Luo Y, Turco SJ, Beverley SM. A specialized pathway affecting virulence glycoconjugates of Leishmania. Science. 1995;269:1869–1872. doi: 10.1126/science.7569927. [DOI] [PubMed] [Google Scholar]
- Descoteaux A, Turco SJ. Functional aspects of the Leishmania donovani lipophosphoglycan during macrophage infection. Microbes and Infection. 2002;4:975–981. doi: 10.1016/s1286-4579(02)01624-6. [DOI] [PubMed] [Google Scholar]
- Dobson DE, Mengeling BJ, Cilmi S, Hickerson S, Turco SJ, Beverley SM. Identification of genes encoding arabinosyltransferases (SCA) mediating developmental modifications of lipophosphoglycan (LPG) required for sand fly transmission of Leishmania major. Journal of Biological Chemistry. 2003;15:15. doi: 10.1074/jbc.M302728200. [DOI] [PubMed] [Google Scholar]
- Dobson DE, Scholtes LD, Valdez KE, Sullivan DR, Mengeling BJ, Cilmi S, Turco SJ, Beverley SM. Functional identification of galactosyltransferases (SCGs) required for species-specific modifications of the lipophosphoglycan adhesin controlling Leishmania major–sand fly interactions. Journal of Biological Chemistry. 2003;278:15523–15531. doi: 10.1074/jbc.M301568200. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Torano A. Immune adherence-mediated opsonophagocytosis: The mechanism of Leishmania infection. Journal of Experimental Medicine. 1999;189:25–35. doi: 10.1084/jem.189.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M, Sharma DK, Hilley JD, Coombs GH, Mottram JC. Processing and trafficking of Leishmania mexicana GP63. Analysis using GP18 mutants deficient in glycosylphosphatidylinositol protein anchoring. Journal of Biological Chemistry. 2002;277:27968–27974. doi: 10.1074/jbc.M202047200. [DOI] [PubMed] [Google Scholar]
- Fulton SA, Johnsen JM, Wolf SF, Sieburth DS, Boom WH. Interleukin- 12 production by human monocytes infected with Mycobacterium tuberculosis: Role of phagocytosis. Infection and Immunity. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser TA, Moody SF, Handman E, Bacic A, Spithill TW. An antigenically distinct lipophosphoglycan on amastigotes of Leishmania major. Molecular and Biochemical Parasitology. 1991;45:337–344. doi: 10.1016/0166-6851(91)90102-c. [DOI] [PubMed] [Google Scholar]
- Gorak PM, Engwerda CR, Kaye PM. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. European Journal of Immunology. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gregory DJ, Olivier M. Subversion of host cell signalling by the protozoan parasite Leishmania. Parasitology. 2005;130(Suppl):S27–S35. doi: 10.1017/S0031182005008139. [DOI] [PubMed] [Google Scholar]
- Guha-Niyogi A, Sullivan DR, Turco SJ. Glycoconjugate structures of parasitic protozoa. Glycobiology. 2001;11:45R–59R. doi: 10.1093/glycob/11.4.45r. [DOI] [PubMed] [Google Scholar]
- Guy RA, Belosevic M. Comparison of receptors required for entry of Leishmania major amastigotes into macrophages. Infection and Immunity. 1993;61:1553–1558. doi: 10.1128/iai.61.4.1553-1558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: Selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- Ilg T, Etges R, Overath P, McConville MJ, Thomas-Oates J, Thomas J, Homans SW, Ferguson MA. Structure of Leishmania mexicana lipophosphoglycan. Journal of Biological Chemistry. 1992;267:6834–6840. [PubMed] [Google Scholar]
- Ilg T, Overath P, Ferguson MA, Rutherford T, Campbell DG, McConville MJ. O- and N-glycosylation of the Leishmania mexicana-secreted acid phosphatase. Characterization of a new class of phosphoserine-linked glycans. Journal of Biological Chemistry. 1994;269:24073–24081. [PubMed] [Google Scholar]
- Ilg T, Stierhof YD, Craik D, Simpson R, Handman E, Bacic A. Purification and structural characterization of a filamentous, mucin-like proteophosphoglycan secreted by Leishmania parasites. Journal of Biological Chemistry. 1996;271:21583–21596. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]
- Ilg T, Stierhof YD, Stierhof YD, Wiese M, McConville MJ, Overath P. Characterization of phosphoglycan-containing secretory products of Leishmania. Parasitology. 1994;108:S63–S71. doi: 10.1017/s0031182000075739. [DOI] [PubMed] [Google Scholar]
- Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. Journal of Immunology. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- Karp CL, Wysocka M, Wahl LM, Ahearn JM, Cuomo PJ, Sherry B, Trinchieri G, Griffin DE. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- Kedzierski L, Montgomery J, Bullen D, Curtis J, Gardiner E, Jimenez-Ruiz A, Handman E. A leucine-rich repeat motif of Leishmania parasite surface antigen 2 binds to macrophages through the complement receptor 3. Journal of Immunology. 2004;172:4902–4906. doi: 10.4049/jimmunol.172.8.4902. [DOI] [PubMed] [Google Scholar]
- Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- King DL, Turco SJ. A ricin agglutinin-resistant clone of Leishmania donovani deficient in lipophosphoglycan. Molecular and Biochemical Parasitology. 1998;28:285–293. doi: 10.1016/0166-6851(88)90013-8. [DOI] [PubMed] [Google Scholar]
- Ladel CH, Szalay G, Riedel D, Kaufmann SH. Interleukin-12 secretion by Mycobacterium tuberculosis-infected macrophages. Infection and Immunity. 1997;65:1936–1938. doi: 10.1128/iai.65.5.1936-1938.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shapiro JI. Endocytosis and signal transduction: Basic science update. Biological Research and Nursing. 2003;5:117–128. doi: 10.1177/1099800403256860. [DOI] [PubMed] [Google Scholar]
- Ma X, Aste-Amezaga M, Trinchieri G. Regulation of interleukin-12 production. Annals of the New York Academy of Sciences. 1996;795:13–25. doi: 10.1111/j.1749-6632.1996.tb52651.x. [DOI] [PubMed] [Google Scholar]
- Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. Journal of Experimental Medicine. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, D'Andrea A, Kubin M, Aste-Amezaga M, Sartori A, Monteiro J, Showe L, Wysocka M, Trinchieri G. Production of interleukin-12. Research in Immmunology. 1995;146:432–438. doi: 10.1016/0923-2494(96)83012-4. [DOI] [PubMed] [Google Scholar]
- Ma X, Riemann H, Gri G, Trinchieri G. Positive and negative regulation of interleukin-12 gene expression. European Cytokine Network. 1998;9:54–64. [PubMed] [Google Scholar]
- Marovich MA, McDowell MA, Thomas EK, Nutman TB. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. Journal of Immunology. 2000;164:5858–5865. doi: 10.4049/jimmunol.164.11.5858. [DOI] [PubMed] [Google Scholar]
- Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. Journal of Experimental Medicine. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K, Yamaguchi H, Klein TW, Friedman H, Yamamoto Y. Legionella pneumophila suppresses macrophage interleukin-12 production by activating the p42/44 mitogen-activated protein kinase cascade. Infection and Immunity. 2003;71:6672–6675. doi: 10.1128/IAI.71.11.6672-6675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville MJ, Schnur LF, Jaffe C, Schneider P. Structure of Leishmania lipophosphoglycan: Inter- and intra-specific polymorphism in Old World species. Biochemical Journal. 1995;310:807–818. doi: 10.1042/bj3100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville MJ, Thomas-Oates JE, Ferguson MA, Homans SW. Structure of the lipophosphoglycan from Leishmania major. Journal of Biochemical Medicine. 1990;265:19611–19623. [PubMed] [Google Scholar]
- McDowell MA, Marovich M, Lira R, Braun M, Sacks D. Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infection and Immunity. 2002;70:3994–4001. doi: 10.1128/IAI.70.8.3994-4001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell MA, Sacks DL. Inhibition of host cell signal transduction by Leishmania: Observations relevant to the selective impairment of IL-12 responses. Current Opinions in Microbiology. 1999;2:438–443. doi: 10.1016/S1369-5274(99)80077-0. [DOI] [PubMed] [Google Scholar]
- McGwire BS, O'Connell WA, Chang KP, Engman DM. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis: Implications for parasite virulence. Journal of Biochemical Medicine. 2002;277:8802–8809. doi: 10.1074/jbc.M109072200. [DOI] [PubMed] [Google Scholar]
- McPhaden AR, Whaley K. Complement biosynthesis by mononuclear phagocytes. Immunology Research. 1993;12:213–232. doi: 10.1007/BF02918254. [DOI] [PubMed] [Google Scholar]
- Melby PC. Experimental leishmaniasis in humans: Review. Review in Infectious Diseases. 1991;13:1009–1017. doi: 10.1093/clinids/13.5.1009. [DOI] [PubMed] [Google Scholar]
- Moody SF, Handman E, McConville MJ, Bacic A. The structure of Leishmania major amastigote lipophosphoglycan. Journal of Biological Chemistry. 1993;268:18457–18466. [PubMed] [Google Scholar]
- Nandan D, Reiner NE. Attenuation of gamma interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovani: Selective inhibition of signaling through Janus kinases and Stat1. Infection and Immunity. 1995;63:4495–4500. doi: 10.1128/iai.63.11.4495-4500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neva FA, Wyler D, Nash T. Cutaneous leishmaniasis—A case with persistent organisms after treatment in presence of normal immune response. American Journal of Tropical Medicine and Hygiene. 1979;28:467–471. [PubMed] [Google Scholar]
- Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: Evidence for a negative signal delivered through the mannose receptor. Journal of Immunology. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- Orlandi PA, Jr., Turco SJ. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. Journal of Biological Chemistry. 1987;262:10384–10391. [PubMed] [Google Scholar]
- Piedrafita D, Proudfoot L, NIKOLAEV AV, Xu D, Sands W, Feng GJ, Thomas E, Brewer J, Ferguson MA, Alexander J, et al. Regulation of macrophage IL-12 synthesis by Leishmania phosphoglycans. European Journal of Immunology. 1999;29:235–244. doi: 10.1002/(SICI)1521-4141(199901)29:01<235::AID-IMMU235>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Poussin C, Foti M, Carpentier JL, Pugin J. CD14-dependent endotoxin internalization via a macropinocytic pathway. Journal of Biological Chemistry. 1998;273:20285–20291. doi: 10.1074/jbc.273.32.20285. [DOI] [PubMed] [Google Scholar]
- Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. Journal of Experimental Medicine. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DG, Wright SD. Complement receptor type 3 (CR3) binds to an Arg- Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. Journal of Experimental Medicine. 1988;168:279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks DL. The structure and function of the surface lipophosphoglycan on different developmental stages of Leishmania promastigotes. Infectious Agents and Disease. 1992;1:200–206. [PubMed] [Google Scholar]
- Sartori A, Oliveira MA, Scott P, Trinchieri G. Metacyclogenesis modulates the ability of Leishmania promastigotes to induce IL-12 production in human mononuclear cells. Journal of Immunology. 1997;159:2849–2857. [PubMed] [Google Scholar]
- Sartori A, Scott P, Trinchieri G. Leishmania major metacyclogenesis modulates ability to induce IL-12. Annals of the New York Academy of Sciences. 1996;795:400–402. doi: 10.1111/j.1749-6632.1996.tb52705.x. [DOI] [PubMed] [Google Scholar]
- Shakarian AM, Dwyer DM. Structurally conserved soluble acid phosphatases are synthesized and released by Leishmania major promastigotes. Experimental Parasitology. 2000;95:79–84. doi: 10.1006/expr.2000.4511. [DOI] [PubMed] [Google Scholar]
- Snijders A, Hilkens CM, Van Der Pouw Kraan TC, Engel M, Aarden LA, Kapsenberg ML. Regulation of bio-active IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. Journal of Immunology. 1996;156:1207–1212. [PubMed] [Google Scholar]
- Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Experimental Parasitology. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. Journal of Experimental Medicine. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachado SD, Mazhari-Tabrizi R, Schofield L. Specificity in signal transduction among glycosylphosphatidylinositols of Plasmodium falciparum, Trypanosoma brucei, Trypanosoma cruzi and Leishmania spp. Parasite Immunology. 1999;21:609–617. doi: 10.1046/j.1365-3024.1999.00268.x. [DOI] [PubMed] [Google Scholar]
- Talamas-Rohana P, Wright SD, Lennartz MR, Russell DG. Lipophosphoglycan from Leishmania mexicana promastigotes binds to members of the CR3, p150,95 and LFA-1 family of leukocyte integrins. Journal of Immunology. 1990;144:4817–4824. [PubMed] [Google Scholar]
- Tolson DL, Turco SJ, Beecroft RP, Pearson TW. The immunochemical structure and surface arrangement of Leishmania donovani lipophosphoglycan determined using monoclonal antibodies. Molecular and Biochemical Parasitology. 1989;35:109–118. doi: 10.1016/0166-6851(89)90113-8. [DOI] [PubMed] [Google Scholar]
- Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annual Reviews in Microbiology. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Van Der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. Journal of Experimental Medicine. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Pouw Kraan TC, Boeije C, Snijders A, Smeenk RJ, Wijdenes J, Aarden LA. Regulation of IL-12 production by human monocytes and the influence of prostaglandin E2. Annals of the New York Academy of Sciences. 1996;795:147–157. doi: 10.1111/j.1749-6632.1996.tb52663.x. [DOI] [PubMed] [Google Scholar]
- Weinheber N, Wolfram M, Harbecke D, Aebischer T. Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production. European Journal of Immunology. 1998;28:2467–2477. doi: 10.1002/(SICI)1521-4141(199808)28:08<2467::AID-IMMU2467>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Hardin KK, Donelson JE. Expression of the major surface glycoprotein of Leishmania donovani chagasi in virulent and attenuated promastigotes. Journal of Immunology. 1989;143:678–684. [PubMed] [Google Scholar]
- Wozencraft AO, Sayers G, Blackwell JM. Macrophage type 3 complement receptors mediate serum-independent binding of Leishmania donovani. Detection of macrophage-derived complement on the parasite surface by immunoelectron microscopy. Journal of Experimental Medicine. 1986;164:1332–1337. doi: 10.1084/jem.164.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Leidal KG, Brittingham A, Tarr DE, Donelson JE, Wilson ME. Biosynthesis of the major surface protease GP63 of Leishmania chagasi. Molecular and Biochemical Parasitology. 2002;121:119–128. doi: 10.1016/s0166-6851(02)00030-0. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, Almeida IC, Smith DF, Turco SJ, Ferguson MA, Beverley SM. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. Journal of Biological Chemistry. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200. [DOI] [PubMed] [Google Scholar]