Abstract
Cardiac fibroblasts produce and degrade extracellular matrix and are critical in regulating cardiac remodeling and hypertrophy. Fibroblasts are activated by factors such as transforming growth factor β and inhibited by agents that elevate 3′,5′-cyclic adenosine monophosphate (cAMP) levels. cAMP signal generation and response is known to be compartmentalized in many cell types in part through the colocalization of receptors and specific adenylyl cyclase isoforms in lipid rafts and caveolae. The present study sought to define the localization of key G protein-coupled receptors with adenylyl cyclase type 6 (AC6) in lipid rafts of rat cardiac fibroblasts and to determine if this colocalization was functionally relevant. We found that cardiac fibroblasts produce cAMP in response to agonists for β-adrenergic (isoproterenol), prostaglandin EP2 (butaprost), adenosine (adenosine-5′-N-ethylcarboxamide, NECA), and prostacyclin (beraprost) receptors. Overexpression of AC6 increased cAMP production stimulated by isoproterenol and beraprost but not by butaprost or NECA. A key function of fibroblasts is the production of collagen. Isoproterenol- and beraprost-mediated inhibition of collagen synthesis was also enhanced by AC6 overexpression, while inhibition by butaprost and NECA were unaltered. Lipid raft fractions from cardiac fibroblasts contain the preponderance of β-adrenergic receptors and AC6 but exclude EP2 receptors. While we could not determine the localization of native prostacyclin receptors, we were able to determine that epitope-tagged prostanoid IP receptors (IPR) expressed in COS7 cells did localize, in part, in lipid raft fractions. These findings indicate that IP receptors are expressed in lipid rafts and can activate raft-localized AC isoforms. AC6 is completely compartmentized in lipid raft domains where it is activated solely by coresident G protein-coupled receptors to regulate cardiac fibroblast function.
Keywords: Collagen, Fibrosis, Caveolae, PGI2, PGE2
Introduction
Cardiac remodeling, a consequence of certain cardiovascular diseases such as heart failure and hypertension, occurs via structural rearrangement of components of myocardial wall. Such changes include cardiomyocyte hypertrophy, cardiac fibroblast proliferation, and increased deposition of fibrillar collagen. These responses eventually lead to cardiac fibrosis and a decline in cardiac function (Eghbali and Weber 1990). The role of cardiac fibroblasts in these processes is of considerable interest. In particular, strategies to limit activation of cardiac fibroblasts could form the basis of new approaches for treating heart failure (Weber et al. 1994).
G protein-coupled receptors (GPCRs) signal via heterotrimeric G-proteins to regulate effector molecules and generate second messengers that activate downstream signaling. β-Adrenergic receptors (βAR) are the most highly studied of GPCRs and they, like receptors for various prostanoids and other hormones, couple to Gs to stimulate adenylyl cyclase (AC) activity and generation of the second messenger 3′,5′-cyclic adenosine monophosphate (cAMP). Cardiac fibroblasts express β2AR and various isoforms of AC, and activity of this pathway elevates cAMP levels and inhibits fibrogenic activity of these cells (Gustafsson and Brunton 2000; Ostrom et al. 2003; Swaney et al. 2005). The antifibrotic action of cAMP appears to be largely due to its opposition of various aspects of transforming growth factor β signaling, including Smad and non-Smad pathways (Liu et al. 2006). Cardiac fibroblasts produce prostacyclin (PGI2) as their main eicosanoid product, and this factor is known to reduce cell proliferation and collagen production (Yu et al. 1997). Because PGI2 receptors (IPR) couple to Gs and the stimulation of AC, it is presumed that its effects on cardiac fibroblast function are mediated through the cAMP pathway.
IPR are expressed in the vasculature (smooth muscle and endothelial cells, lymphocytes, megakaryocytes, and platelets), kidney, and dorsal root ganglia (Narumiya et al. 1999; Bundey and Insel 2006). PGI2 is the endogenous agonist of IPR and is a product of the cyclooxygenase pathway, particularly that of cyclooxygenase 2 (McAdam et al. 1999). Synthetic analogs of PGI2, including iloprost, cicaprost, and beraprost, are useful for the treatment of pulmonary hypertension and Raynaud’s phenomenon and are being examined for their clinical usefulness in a variety of other clinical conditions (Rubin et al. 1982; Block and Sequeira 2001; Nasrallah and Hebert 2005).
βAR are efficiently coupled to the activation of AC6 because of a high degree of colocalization of these two signaling components in plasma membrane lipid rafts or caveolae (Schwencke et al. 1999; Rybin et al. 2000; Ostrom et al. 2001; Ostrom, et al. 2002). Other GPCRs, such as prostanoid EP2R and EP4R, are unable to couple to AC6 because of the exclusion of these receptors from lipid rafts (Ostrom et al. 2001). Thus, colocalization of GPCR and components of their downstream signal transduction cascade appears to be a key determinant of signaling efficiency. However, it is less clear whether such colocalization in lipid rafts or caveolae has an impact on the regulation of cellular function.
Caveolae are 50–100-Å membrane invaginations enriched in the protein caveolin (Anderson 1998). Caveolae are considered a subset of lipid rafts in that both membrane regions are enriched in sphingolipids and cholesterol and are distinct lipid environments of plasma membrane that attract and retain certain proteins to create distinct signaling domains (Shaul et al. 1996; Razani et al. 2002; Foster et al. 2003; Pike 2003). We and others have found that only certain GPCR and particular AC isoforms are expressed in lipid rafts and caveolae (Ostrom et al. 2003; Ostrom and Insel 2004). These and other similar findings have challenged the notion that membrane-associated signaling proteins are randomly distributed in the plasma membrane and interact via random collision coupling (Steinberg and Brunton 2001; Razani et al. 2002). It is now readily accepted that many GPCR and their signaling partners are restricted in their diffusion in the membrane by lipid rafts or other domains and perhaps even prearranged in signaling complexes (Ostrom et al. 2000; Steinberg and Brunton 2001). Such a model more easily explains how cross-talk can occur between signaling molecules of different signaling pathways and serves to explain the compartmentation of signaling observed through various approaches (Brunton et al. 1981; Fagan et al. 2000; Davare et al. 2001; Razani et al. 2002; Shaul 2002).
The present study was designed to examine the role of lipid rafts/caveolar domains in GPCR–Gs–AC signaling and to assess if compartmentation of cAMP signaling has relevance to the regulation of cardiac fibroblast function. We overexpressed a lipid raft localized isoform of AC, AC6, and found that this selectively enhanced βAR signaling and did not increase the second-messenger generation by prostanoid EP receptors. We did observe that AC6 overexpression increases response to beraprost, an agonist of IPRs. Beyond just selective alteration of cAMP production, AC6 overexpression also enhanced the ability of βAR and IPR to reduce collagen synthesis and myofibroblast differentiation but did not alter similar responses mediated by prostanoid EP2 or adenosine receptors. Using COS7 cells that express epitope-tagged IPR, we determined that IPR are expressed, in part, in lipid rafts. Thus, we conclude that PGI2 may be an important regulator of cardiac remodeling and that the observed compartmentation of GPCR/AC signaling in lipid rafts can have physiologically relevant effects.
Materials and methods
Materials
Adenovirus expressing the murine AC6 complementary deoxyribonucleic acid (cDNA) was generated as described previously (Gao et al. 1998). Primary antibodies for AC isoforms and secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Beraprost, butaprost, and primary antibodies for EP2R and IPR were obtained from Cayman Chemical. Forskolin was obtained from Calbiochem. Radiolabeled chemicals were obtained from Perkin Elmer Life Sciences. All other chemicals and reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Preparation and culture of adult rat cardiac fibroblasts
Cardiac fibroblasts were prepared from adult male 250–300 g Sprague–Dawley rat hearts. After rapid excision of the hearts, the ventricles were isolated, minced, pooled, and placed in a collagenase/pancreatin digestion solution. After sequential digestions, the fibroblasts were pelleted and resuspended in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with penicillin, streptomycin, fungizone, and 10% fetal bovine serum (FBS, Atlanta Biologicals). After a 30-min period of attachment to uncoated culture plates, cells that were weakly attached or unattached were rinsed free and discarded. After 2–3 days, confluent cultures were amplified by trypsinization and seeding onto new dishes. For signaling assays, only early passage (≤3) cells grown to 80–90% confluency were used. The purity of these cultures was greater than 95% cardiac fibroblasts as determined by positive staining for vimentin and negative staining for smooth muscle actin and von Willebrand factor, as previously described (Ostrom et al. 2003). All animals were treated according to the principles of laboratory animal care (NIH publication no. 85-23, revised 1985) and applicable US law.
Measurement of cAMP accumulation
Cells were washed three times with serum and NaHCO3-free DMEM supplemented with 20 mM hydroxyethyl piperazineethanesulfonic acid (HEPES), pH 7.4 (DMEH) and equilibrated for 30 min. The assay for cAMP accumulation was performed by incubation with drugs of interest and 0.2 mM isobutylmethylxanthine, a PDE inhibitor, for 10 min. To terminate reactions, the assay medium was aspirated, and 200 µL lysis buffer (GE Healthcare) was added. cAMP content of the extract was quantified using the Biotrak EIA Kit (GE Healthcare). Data were normalized to the amount of protein per sample, as determined using a dye-binding protein assay (Bio-Rad). In other studies, cAMP accumulation was measure using [3H]adenine labeling as described previously (Ostrom et al. 2000). Briefly, cells were labeled with 0.5 Ci/well [3H]adenine in the growth medium overnight to allow incorporation into intracellular adenosine triphosphate (ATP) pools. The growth medium was removed, and cells were washed extensively and equilibrated for 30 min at 37°C in serum-free DMEH. Cells were then incubated for 10 min in with 200 µM isobutylmethylxanthine and various drugs of interest. Reactions were terminated by aspiration of the medium and addition of 7.5% trichloroacetic acid. [3H]cAMP and [3H]ATP were separated from the supernatant fraction using a chromatography method modified from Salomon et al. and quantified by liquid scintillation counting. Data are expressed as the percent conversion of [3H]ATP into [3H]cAMP.
Assays of collagen synthesis
Cells were plated as described above and synchronized by incubation in serum-free DMEM for 2 h followed by serum deprivation in Modified Eagle’s Medium (MEM) containing 0.25% FBS for 24 h. MEM was then supplemented with 2.5% FBS (except for unstimulated conditions, where 0.25% FBS was used) for 24 h along with 0.5 mCi of [3H]proline/well and drugs of interest. Iso was solubilized in 2 mM ascorbic acid to prevent degradative oxidation. The medium was removed, and the cells were washed with ice-cold phosphate-buffered saline (PBS) and then incubated with 7.5% tricarboxylic acid (TCA) for 1 h at 4°C. TCA-precipitated counts were determined by liquid scintillation counting.
Membrane fractionation
Cells were fractionated using a detergent-free method adapted from Song et al. (1996) and described previously (Ostrom et al. 2001). Briefly, cells were homogenized in 500 mM sodium carbonate plus mammalian protease inhibitor cocktail (Sigma cat# P-8340) with 20 strokes in a Dounce homogenizer, three 10-s bursts in a tissue grinder, then three 20-s bursts with an ultrasound cell disruptor. The homogenate was brought to 45% sucrose by addition of 90% sucrose in 25 mM 2-N-morpholinoethanesulfonic acid, 150 mM NaCl, pH 6.5, and loaded in an ultracentrifuge tube. A discontinuous sucrose gradient was layered on top of the sample by placing 2 ml 35% sucrose then 1 ml 5% sucrose. The gradient was centrifuged at 44,000 rpm on a SW55Ti rotor (Beckman Instruments) for 16–20 h at 4°C. Fractions were collected in ten 0.5-ml aliquots from the top of the gradient. Equal volumes of fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Millipore) by electroblotting. Membranes were blocked in PBS–Tween with 5% nonfat dry milk and incubated with primary antibody (see “Materials and methods”) overnight at 4°C. Bound primary antibodies were visualized using an appropriate secondary antibody with conjugated horseradish peroxidase (Santa Cruz Biotechnology) and electrochemiluminescence reagent (Pierce). The amount of protein per sample was determined using a dye-binding protein assay (Bio-Rad).
Immunoisolation of caveolae and measurement of AC activity
Immunoisolation of caveolin-rich membranes were performed as described previously (Ostrom et al. 2001). Briefly, cardiac fibroblasts were plated into 15-cm plates, grown to about 90% confluence, then starved with 0% FBS medium for 24 h. In some cases, cells were then treated with an adenovirus expressing AC6 for 24 h. Cells were scraped and homogenized in a modified lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM ethylene glycol tetraacetic acid [EGTA], 2 mM dithiothreitol [DTT], 0.5% Igepal CA-630, plus mammalian protease inhibitor cocktail). Homogenate was precleared by inclubating with 50 µl protein A–agarose for 30 min at 4°C then incubated with caveolin-1 monoclonal antibody (Pharmingen) for 1 h. Antibody complexes were then precipitated after incubation with protein A–agarose and resuspended in membrane buffer (30 mM Na-HEPES, 5 mM MgCl2, 2 mM DTT, pH 7.5). AC activity was conducted by adding 30 µl sample (immunoprecipitate [IP] or supernatant) into tubes containing assay buffer (30 mM Na-HEPES, 100 mM NaCl, 1 mM EGTA, 10 mM MgCl2, 1 mM isobutylmethylxanthine, 1 mM ATP, 10 mM phos-phocreatine, 5 µM guanosine triphosphate, 60 U/ml creatine phosphokinase, and 0.1% bovine serum albumin, pH 7.5) and drugs of interest. Mixture was incubated for 15 min at 30°C, and reactions were stopped by boiling for 5 min. cAMP content of each tube was assayed by using the Biotrak EIA kit (GE Healthcare). Total protein concentration was determined using a dye-binding protein assay (Bio-Rad), and immunoblot analysis was used to ensure the majority of caveolin-1 was precipitated.
Immunofluorescent confocal microscopy
Intracellular localization of HA-IPR was visualized in COS7 cells. COS7 cells (1 × 105 cells) were plated onto poly-l-lysine-coated glass coverslips and cultured for 24 h. Cells were then transfected with 0.4 µg of HA-IPR (in pcDNA 3.1) cDNA using Lipofectamine (Invitrogen). Cells were then washed with PBS (12.1 mM Na2HPO4, 4 mM KH2PO4, and 130 mM NaCl, pH 7.4) and fixed using 4% paraformaldehyde (1 h, 20°C). After fixation, cells were washed further in PBS and permeabilized (1 h, 20°C) with PBS containing 0.2% Triton X-100 and 1% goat serum (blocking solution) before being incubated (12 h, 20%C) with anti-HA mAb (1:500 dilution). After further washes in PBS (3 × 10 min), cells were incubated (1 h, 20°C) with Alexa Fluor 488 donkey anti-goat IgG in blocking solution. Coverslips were mounted in Slowfade® light antifade (Molecular Probes) according to the manufacturer’s procedures, and the cells were visualized on a Zeiss Axiovert LSM510 confocal microscope, using a ×63 oil immersion objective and a slice depth of 1 µm.
Data analysis and statistics
Data are presented as the mean ± SEM and in some cases as representative images of at least three separate experiments. Statistical comparisons (t tests and one-way analysis of variance) and graphics were performed using GraphPad Prism 4.0 (GraphPad Software).
Results
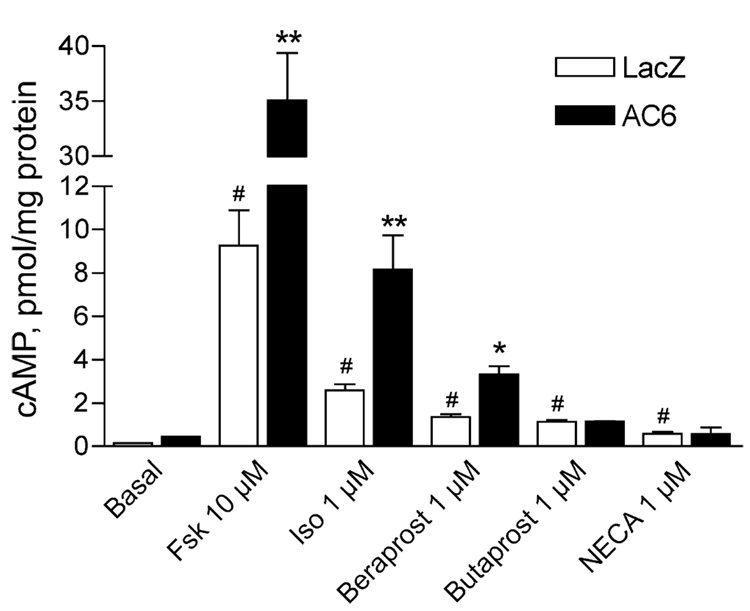
We previously found that overexpression of AC6 enhances cAMP responses by receptors colocalized in lipid rafts or caveolae without effecting responses by GPCRs in nonraft domains (Ostrom et al. 2000; Ostrom et al. 2001; Ostrom et al. 2002). Thus, we assayed cAMP production in response to various receptor-specific agonists in cells expressing LacZ (control) or AC6 to infer the localization of these receptors. Adenoviruses were used to express the desired gene, and cAMP accumulation was measured 18 h later. AC6 overexpression enhanced forskolin-, isoproterenol-(βAR-selective agonist), and beraprost (IPR-selective agonist)-mediated increases in cAMP production without a significant effect on basal cAMP accumulation (Fig. 1). AC6 enhanced the Emax of each of these drugs without significantly altering their EC50 (Table 1). Overexpression of AC6 did not alter response to butaprost (EP2R-selective agonist) or adenosine-′-N-ethylcarboxamide (NECA, adenosine receptor agonist) despite the fact that these drugs stimulated cAMP production to levels significantly greater than basal. Thus, βAR and IPR are likely expressed in lipid rafts and caveolae of adult rat cardiac fibroblasts along with AC6, while prostanoid EP2R and adenosine receptors are likely excluded from these domains.
Fig. 1.
cAMP production stimulated by certain GPCR is enhanced by overexpression of AC6. cAMP production was measured in cells incubated with either LacZ- or AC6-expressing recombinant adenovirus for 24 h. Cells were incubated with the indicated drugs along with a phosphodiesterase inhibitor for 10 min at 37°C before cells were lysed and cAMP content assayed by EIA (see “Materials and methods”). Each bar represents the mean±SEM of at least three experiments. Asterisk denotes p<0.05, double asterisk denotes p<0.01 as compared to the same condition in LacZ cells, and number sign denotes p<0.05 as compared to basal by ANOVA
Table 1.
EC50 values and maximal responses (Emax, pmol cAMP/mg protein) to various Gs-coupled receptor agonists for cAMP production in LacZ- (control) and AC6-overexpressing cells
| Drug | LacZ (control) |
AC6 |
||
|---|---|---|---|---|
| −log (EC50) | Emax | −log (EC50) | Emax | |
| Forskolin | 4.65±0.16 | 10.21±1.19a | 4.41 ±0.30 | 37.68±3.79a |
| Isoproterenol | 7.44±0.29 | 3.49±0.53 | 7.62±0.04 | 8.63±0.21 |
| Beraprost | 6.72±0.09 | 1.87±0.14 | 6.18±0.07 | 8.90±0.30 |
| Butaprost | 7.31±0.14 | 1.79±0.22 | 7.29±0.08 | 1.81±0.04 |
| NECA | 6.19±0.24 | 1.10±0.05 | 6.21±0.19 | 1.13±0.11 |
Adenoviruses were used to overexpress each gene product. Values are presented as mean±SEM of at least three experiments. Basal levels of cAMP production were not significantly different (LacZ-expressing cells=0.24±0.09 pmol/mg protein, AC6-overexpressing cells=0.45±0.1 pmol/mg protein).
Emax fixed to observed maximum response to obtain fit of the data by nonlinear regression
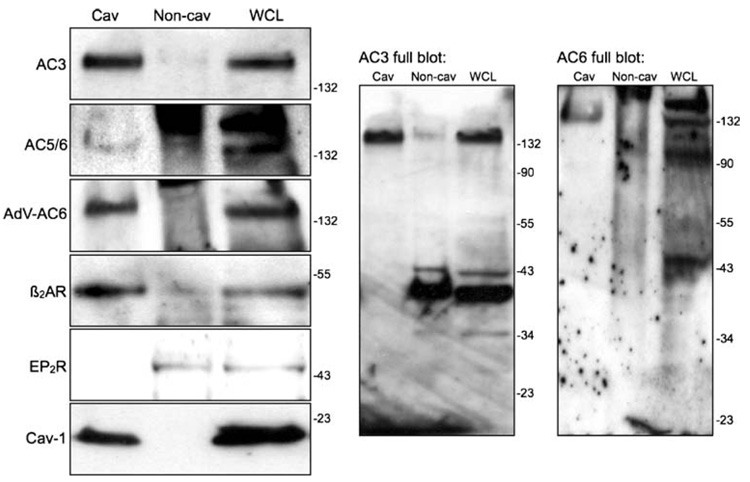
To directly assess the localization of these natively expressed GPCRs in cardiac fibroblasts, we isolated caveolin-rich lipid raft fractions using a detergent-free method followed by sucrose density centrifugation. We previously detected expression of AC2, AC3, AC4, AC5/6, and AC7 in adult rat cardiac fibroblasts, with AC3 being the most readily detectible isoform (Ostrom et al. 2003). Immunoblot analysis confirmed the expression of predominantly AC3 in lipid raft fractions along with faint bands corresponding to AC5/6 (Fig. 2). Several bands were observed on immunoblots for β2AR, AC3, and AC5/6 (Fig. 2, right panels). The primary antibodies for β2AR and the various AC isoforms available from Santa Cruz Biotechnology often yield many nonspecific bands and smears the size of which vary by cell type. However, the lipid raft fractionation procedure can yield samples that, when heavily loaded on SDS-PAGE gels and probed with antibodies diluted by a 1:500 ratio, produce interpretable immunoblots. We identified the appropriate bands in these blots by overexpressing AC6 using an adenovirus (Fig. 2, left panels) or by transiently transfecting β2AR or AC3 in COS7 cells (data not shown). The buoyant, lipid raft fractions contained the vast majority of caveolin-1 immunoreactivity (bottom panel) but excluded markers for Golgi (mannosidase II) or clathrin-coated pits (β-adaptin, not shown). Adenoviral treatment of cells to overexpress AC6 led to distinct AC5/6 immunoreactive bands in the lipid raft fractions. We also detected expression of β2AR in lipid raft fractions, consistent with previous reports from other cell types (Ostrom et al. 2000; Rybin et al. 2000; Ostrom et al. 2002). By contrast, EP2R immunoreactivity was completely excluded from the lipid raft factions. We were unable to detect the endogenous expression of either IPR or adenosine receptors using commercially available antibodies.
Fig. 2.
Localization of GPCRs and ACs in lipid raft fractions from rat cardiac fibroblasts. Expression of caveolin-1, AC3, AC5/6, β2AR, and EP2R was assessed by SDS-PAGE and immunoblot analysis of buoyant (cav) and “heavy” (non-cav) membrane fractions and whole-cell lysates (WCL) from cardiac fibroblasts isolated using a nondetergent method followed by sucrose density centrifugation. Fractions from cells overexpressing AC6 (using a recombinant adenovirus) were also analyzed for AC5/6 immunoreactivity. Cav samples were pooled from the fractions at the 5–35% sucrose interface while the non-cav samples were pooled from the fractions in the 45% sucrose layer. Equal volumes of each fraction were loaded to represent the relative proportions of protein as they exist in intact cells. Right panels show full-length immunoblots probed with AC3 and AC5/6 antibodies. Images are representative of at least three experiments
To confirm the localization of endogenous GPCRs and AC6 with respect to lipid rafts and caveolae of cardiac fibroblasts, we used a different approach to isolate these domains and assayed for agonist-stimulated activity of AC. Immunoisolation of caveolae from detergent-solubilized cardiac fibroblasts was achieved using antibodies to caveolin-1 (see “Materials and methods”). This modified immunoprecipitation of caveolin-1 trapped the bulk of the caveolin-1 and AC5/6 immunoreactivity (Fig. 3, top panels) along with the majority of cholesterol (not shown). AC activity assays were then measured in caveolin-1 IPs or the supernatants from cells expressing either LacZ (control) or AC6. AC6 overexpression enhanced the AC activity in the IPs stimulated by forskolin, isoproterenol, and beraprost (Fig. 3 and Table 2). By contrast, AC activity stimulated by butaprost was not higher in caveolin-1 IPs but was slightly elevated in the supernatants. These data indicate that immunoisolated caveolae contain functional units of AC6 coupled to βAR and IPR but not to EP2R, consistent with our observations shown in Fig. 2. These data also imply that a small amount of AC6 may couple to EP2R in the supernatants left behind from the immunoisolation of caveolae.
Fig. 3.
βAR and IPR but not EP2R stimulate AC activity in immunoisolated caveolae. Caveolin-1 antibodies were used to immunoprecipitate caveolar fractions isolated by Triton-X100 insolubility. Precipitates and supernatants were assayed for AC activity as described in “Materials and methods.” Caveolin-1 immunoprecipitates contained the bulk of caveolin-1 and AC5/6 immunoreactivity while the supernatants contained very little caveolin-1 and less AC5/6 (top panels). Forskolin (Fsk), isoproterenol (Iso), and beraprost-stimulated AC activities in the IPs were enhanced by AC6 overexpression while butaprost-stimulated AC activity was unaffected (bottom panel). Data are expressed as the fold increase induced by AC6 overexpression (raw data is shown in Table 2). Asterisk denotes p<0.05, and double asterisk denotes p<0.01 as compared to 1 (no increase over control) by ANOVA
Table 2.
Adenylyl cyclase activity stimulated by various drugs in immunoisolated caveolar membranes isolated from LacZ- (control) and AC6-overexpressing rat cardiac fibroblasts
| Drug | LacZ (control) |
AC6 |
||
|---|---|---|---|---|
| IP | Sup | IP | Sup | |
| Basal (no drug) | 0.62±0.08 | 0.29±0.11 | 0.70±0.12 | 0.27±0.10 |
| Forskolin (10 µM) | 1.43±0.25* | 0.57±0.18* | 5.18±0.36*,** | 1.03±0.29*,** |
| Isoproterenol (1 µM) | 0.94±0.12* | 0.42±0.09* | 2.98±0.41*,** | 0.49±0.08* |
| Beraprost (1 µM) | 0.86±0.13* | 0.34±0.10 | 1.76±0.20*,** | 0.30±0.12 |
| Butaprost (1 µM) | 0.59±0.17 | 0.40±0.06 | 0.64±0.05 | 0.56±0.07** |
Adenoviruses were used to overexpress each gene product. Values are pmol cAMP min−1 mg protein−1 (mean±SEM of at least three experiments). Transformed version of this data is shown in Fig. 3.
p<0.05 as compared to basal by ANOVA.
p<0.05 as compared to LacZ cells by ANOVA
To determine if IP receptors localize in lipid raft fractions of mammalian cells, we transiently transfected the human IPR gene fused with an HA epitope into COS7 cells and assessed (1) cAMP production in response to beraprost and (2) the localization of the epitope in lipid raft fractions and intact cells. Mock transfected COS7 cells did not produce cAMP in response to beraprost, indicating that these cells do not express IPR (data not shown). Transfection of HA-IPR into control COS-7 cells led to detectable cAMP production in response to beraprost (−log EC50=7.42±0.11 and Emax=3.6-fold over basal, Fig. 4a). Over-expression of AC6 in IPR-expressing COS-7 cells led to an increase in Emax with no shift in EC50 (−log EC50=7.40±0.16 and Emax=8.9-fold over basal). AC6-overexpressing COS7 cells responded to a submaximal concentration of forskolin (1 µM) with a 12.8±0.57-fold increase over basal cAMP production. Thus, the expression of HA-IPR in COS7 cells appears to be a reasonable model for studying the localization and function of IPR in cardiac fibroblasts.
Fig. 4.
HA-IPR are detected in lipid raft fractions and intracellular stores in COS-7 cells. a cAMP production was measured using [3H]adenine labeling in COS-7 cells transiently transfected with an HA-tagged IPR construct. Responses to forskolin or various concentrations of beraprost were compared in cells treated with recombinant adenovirus expressing either LacZ (control) or AC6. Each bar or point represents the mean±SEM of at least three experiments. Asterisk denotes p<0.05, double asterisk denotes p<0.01 as compared to the same condition in LacZ cells, and number sign denotes p<0.05 as compared to basal by ANOVA. b COS7 cells transiently transfected with HA-IPR were fractionated using a nondetergent method and analyzed by SDS-PAGE and immunoblotting (left panels) or fixed and visualized by immunofluorescent microscopy (right panels). Much HA-IPR is detected in intracellular stores and “heavy” fractions, but a portion is detected in the plasma membrane and buoyant lipid raft fractions coexpressing caveolin-1. Images are representative of at least three experiments
To directly assess the localization of IPR in COS7 cells, we transfected the HA-IPR construct and performed immunofluorecent microscopy and isolated lipid raft fractions using the same method as above (Fig. 2). Immunofluorescence microscopy of untreated cells shows that much of the HA epitope is expressed in intercellular domains with less fluorescence at the plasma membrane of most cells (Fig. 4b, right panel). These observations are consistent with a majority of the HA-IPR protein being retained in the endoplasmic reticulum or Golgi and lower levels of the receptor reaching the plasma membrane. Detergent-free isolation of lipid raft fractions followed by sucrose density centrifugation show that a majority of the HA-IPR immunoreactivity appears in heavy fractions, consistent with our microscopic findings. However, a distinct portion of HA-IPR immunoreactivity is detectible in caveolin-rich, lipid raft fractions (Fig. 4b, left panels). To determine if agonist activation alters the subcellular localization of IPR, we treated cells with 1 µM beraprost for 10 min before fixing cells for microscopy or lysing for lipid raft fractionation. Beraprost treatment did not significantly alter the distribution of HA immunofluorescence (Fig. 4b, bottom). Agonist treatment did cause a shift of HA immunoreactivity from fractions 8–9 to fractions 6–7 on the sucrose density gradients but did not alter the proportion of immunoreactivity observed in the buoyant lipid raft fractions (3–4). Therefore, IPR appear to be expressed, in part, in lipid rafts, and agonist activation does not significantly increase or decrease the proportion of this receptor in lipid rafts. Previous studies indicate that IPR do not undergo densensitization (Hasse et al. 2003). While our observations imply that IPR also do not significantly translocate into or out of lipid rafts, more studies are needed to confirm if these receptors translocate or internalize in other ways.
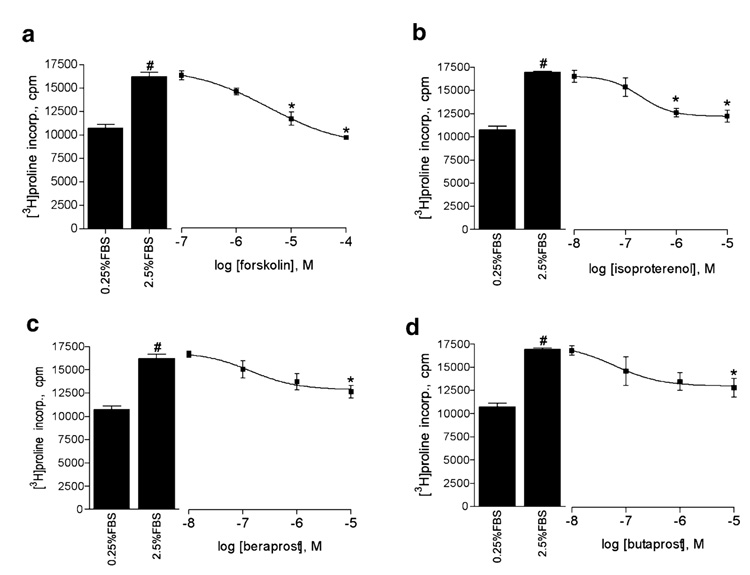
We sought to extend our observations of GPCR–AC6 compartmentation to a functional response in cardiac fibroblasts. Previous studies indicate that cAMP-elevating agents and AC6 overexpression can inhibit collagen production and myofibroblast differentiation of cardiac and other fibroblasts (Liu et al. 2004; Swaney et al. 2005; Liu et al. 2006). Thus, we assessed proline incorporation as a measure of total collagen synthesis. Forskolin, isoproterenol, beraprost, and butaprost each inhibited proline incorporation stimulated by 2.5% FBS in a concentration-dependent manner (Fig. 5). Beraprost and butaprost only induced significant levels of inhibition at the highest concentrations used (10 µM), while forskolin and isoproterenol caused inhibition at lower concentrations. NEC A also significantly (p<0.05 by paired t test) inhibited serum-stimulated proline incorporation at higher concentrations (NECA 16±3.2% inhibition). Therefore, each of the agents that elevated cAMP production (Fig. 1) was able to inhibit proline incorporation.
Fig. 5.
cAMP-elevating agents inhibit collagen synthesis in rat cardiac fibroblasts. [3H]Proline incorporation stimulated by 2.5% FBS was measured in the absence or presence of increasing concentrations of forskolin (a), isoproterenol (b), beraprost (c), or butaprost (d). Cells were serum deprived for 24 h and then treated with the indicated drug for 20 min before addition of 2.5% FBS for another 48 h (see “Materials and methods”). Each bar or point represents the mean±SEM of at least three experiments. Asterisk denotes p<0.05 as compared to 2.5% FBS, and number sign denotes p<0.05 as compared to 0.25% FBS (basal) by ANOVA
We then compared the percent inhibition of proline incorporation by a maximal concentration of each drug in LacZ (control) and AC6-overexpressing cells. AC6 over-expression reduced FBS-stimulated proline incorporation and enhanced the responses to forskolin, isoproterenol, and beraprost (Fig. 6a). By contrast, the inhibition of proline incorporations by either butaprost or NECA was unaltered in AC6-overexpressing cells. Viewing this same data transformed as percent inhibition (where the level of proline incorporation stimulated by 2.5% FBS represents 0% inhibition and the basal level of incorporation is 100% inhibition) makes clear that the level of inhibition that stimulated forskolin, isoproterenol, and beraprost is enhanced by AC6 overexpression (Fig. 6b). The percent inhibition by forskolin in AC6-overexpressing cells was greater than 100%, indicating that this condition reduced levels of proline incorporation to levels below that of basal (0% serum). Thus, while EP2R and adenosine receptors may be capable of stimulating cAMP production and regulating fibroblast function, these receptors do not signal via the lipid raft-localized AC6 to stimulate cAMP production or inhibit collagen synthesis. Only receptors colocalized with AC6 (βAR and IPR) appear to utilize the overexpressed AC to enhance their regulation of cell function.
Fig. 6.
AC6 overexpression enhances the inhibition of collagen synthesis mediated by βAR and IPR but not EP2R or adenosine receptors. [3H]Proline incorporation stimulated by 2.5% FBS was measured in the absence or presence of forskolin, isoproterenol, beraprost, butaprost, or NECA. Cells were serum deprived for 24 h and then treated with the indicated drug for 20 min before addition of 2.5% FBS for another 48 h (see “Materials and methods”). Data are expressed as the total incorporated proline counts (a) or as the percent inhibition of the FBS-stimulated proline incorporation (b, forskolin inhibits greater than 100% in the AC6 conditions because it induces a level of incorporation that is less than the 0% FBS condition). Each bar represents the mean±SEM of at least three experiments. Asterisk denotes p<0.05 as compared to the LacZ condition, and number sign denotes p<0.05 as compared to 2.5% FBS (0% inhibition) by ANOVA
Discussion
The rapidity of GPCR signal transduction defies the relative low abundance of GPCR and the multiple other proteins (G proteins, ACs, etc.) involved in the signaling mechanism. Moreover, most mammalian cells express 20–30 different GPCRs that couple to just five different classes of G proteins. This equates to at least four or five different GPCRs in a single cell sharing the same signal transduction pathway. It would be overly simplistic to propose that each of these GPCRs, some of which might respond to neuro-transmitters, some to circulating hormones, and others to local peptide signals, have identical effects on cell function. For these reasons, a more complex model that incorporates some degree of compartmentation of GPCR signaling is more satisfying from a teleological perspective.
In adult rat cardiac fibroblasts, we find that AC6 overexpression selectively enhances both proximal signaling events and downstream alterations in cell function by βAR and IPR over other receptors coupled to the same pathway. Specifically, AC6 overexpression increased the maximal cAMP production in response to isoproterenol (βAR) and beraprost (IPR) but did not alter responses to butaprost (EP2R) or NECA (adenosine receptors). These results are consistent with our observations in other cell types (Ostrom et al. 2000; Ostrom et al. 2001; Ostrom et al. 2002). However, we also find that this same pattern of enhanced effects extends to a key regulatory action of the cAMP pathway in cardiac fibroblasts: the inhibition of collagen production. Our studies of lipid raft fractions from cardiac fibroblasts and COS-7 cells expressing HA-IPR, along with immunoisolated caveolar membranes from cardiac fibroblasts, demonstrate that AC6 is colocalized with βAR and IPR but not with EP2R. These results show that GPCR–AC signaling is highly compartmentized in cardiac fibroblasts and that responses mediated by specific receptors can be enhanced by overexpression of a particular AC isoform.
Consistent with the present findings, Swaney et al. (2005) previously reported that AC6 overexpression enhances forskolin-mediated inhibition of myofibroblast transformation and collagen synthesis. These investigators also reported that AC6 overexpression uncovered an inhibition of cardiac fibroblast function by adrenomedullin. Thus, one would speculate that adrenomedullin/calcitonin gene-related peptide receptors are expressed at low levels in lipid rafts of cardiac fibroblasts such that when the colocalized AC6 is overexpressed, the receptors are able to stimulate cAMP to levels sufficient for inhibiting cell function.
Our findings that PGI2 can regulate cardiac fibroblast collagen production are not novel. Yu et al. (1997) described that cardiac fibroblasts from Wistar–Kyoto and spontaneously hypertensive rats produce and respond to PGI2. It is interesting to note that these investigators found that cells isolated from spontaneously hypertensive rats produced less PGI2 and, thus, proposed that this lower eicosenoid production could account for the increased fibrosis of hypertensive rats. Increasing expression of AC6 in cardiac fibroblasts could lead to an abrogation of this defect in hypertensive rats by inducing a larger response to the decreased level of IPR activation. Studies to directly test this idea have yet to be performed.
Our studies of epitope-tagged IPR expressed in COS7 cells directly demonstrate that IPR is expressed, at least in part, in caveolae and/or lipid rafts. We detected a small proportion of HA-IPR in lipid raft fractions from COS-7 cells but also determined that a large majority of the receptor expression under these conditions is not plasma membrane localized. Instead, the exogenously expressed receptor appears to be expressed in intracellular stores, perhaps because of high levels of expression. Despite this limitation, our data regarding signaling and function of IPR in cardiac fibroblasts are consistent with the hypothesis that these receptors are localized in lipid rafts. First, responses to beraprost are enhanced by expression of AC6, which is localized completely in lipid raft fractions. Second, beraprost was able to stimulate AC activity in immunoisolated caveolae but not in the supernatants left behind from these isolations (which did contain butaprost-mediated stimulation of AC activity). Taken together, these data indicate that natively expressed IPR reside largely in lipid rafts. Consistent with this idea, Bundey and Insel (2006) recently describe that in human umbilical vein endothelial cells, AC6 overexpression preferentially enhances PGI2-stimulated responses. They measured both cAMP production in response to beraprost and beraprost-mediated inhibition of thrombin-stimulated endothelial cell barrier function. Thus, the data imply that native IPR are also expressed largely in lipid raft domains of endothelial cells where these receptors can couple efficiently to AC6.
The fact that agonists for nonraft GPCRs can increase cAMP production and inhibit collagen production seemingly implies that cAMP action is not highly compartmentized in cardiac fibroblasts. While this may be true, it may also be a factor of the long-term stimulation of cAMP levels that is required for observing effects on collagen gene expression. Studies that have directly demonstrated compartmentation of cAMP signaling in other cells have focused on rapid responses to AC activation (Davare et al. 2001). Thus, cardiac fibroblasts may still have discreet compartments of cAMP generation when stimulation times are transient but that these compartmentizing mechanisms are overcome by hour-long activation. Alternatively, different cAMP compartments may exist for certain functions, but these different pools converge to regulate other consequences, such as the regulation of collagen gene expression. These ideas need further exploration using new, innovative research tools.
Our findings indicate that GPCR/AC signaling is compartmentized in lipid rafts of cardiac fibroblasts such that certain receptors couple selectively to AC6. We and others have shown that AC isoforms display discrete isoform-specific localization with respect to lipid rafts (Smith et al. 2002; Ostrom et al. 2003). AC3, AC5/6, and AC8 are characteristically localized in lipid rafts, while other isoforms are excluded from these domains. Thus, it would be expected that nonraft GPCR would couple selectively to nonraft isoforms of AC. While this idea has not been directly tested, it is intriguing to note that Gros et al. (2006) overexpressed AC1, AC2 AC5, and AC6 in vascular smooth muscle cells and found that different cellular responses (such as cell proliferation and cytoskeletal reorganization) were selectively enhanced by different ACs. Such results imply that different AC isoforms participate in discreet signaling complexes that can contain different partners to achieve heterogeneity of signaling properties (Cooper 2003).
In conclusion, our findings further the idea that GPCR exist in functional compartments such that receptors for very diverse extracellular signals can elicit heterogeneous cellular responses despite their utilizing redundant signal transduction mechanisms. GPCRs that increase cAMP production in cardiac fibroblasts can inhibit activation of these cells, and AC overexpression can enhance these effects if there is colocalization between the receptor and the AC. These results provide the impetus to test if therapies that enhance signaling via the AC/cAMP/PKA pathway, such as overexpression of AC, can limit the development of cardiac fibrosis in various models of cardiovascular disease.
Acknowledgments
The authors would like to thank Ms. Fengying Li for her technical assistance. This work was supported by grant number HL071781 from the National Institutes of Health (RSO).
Abbreviations
- AC
Adenylyl cyclase
- βAR
β-Adrenergic receptor
- cAMP
3′,5′-Cyclic adenosine monophosphate
- Fsk
Forskolin
- Iso
Isoproterenol PKA
- PGE2
Prostaglandin E2
- IPR
Prostanoid IP receptor
Contributor Information
Xiaoqiu Liu, Department of Pharmacology, University of Tennessee Health Science Center, 874 Union Ave., Crowe 115, Memphis, TN 38163, USA.
Muthusamy Thangavel, Department of Pharmacology, University of Tennessee Health Science Center, 874 Union Ave., Crowe 115, Memphis, TN 38163, USA.
Shu Qiang Sun, Department of Pharmacology, University of Tennessee Health Science Center, 874 Union Ave., Crowe 115, Memphis, TN 38163, USA.
Joseph Kaminsky, Department of Pharmacology, University of Tennessee Health Science Center, 874 Union Ave., Crowe 115, Memphis, TN 38163, USA.
Penden Mahautmr, Department of Pharmacology, University of Tennessee Health Science Center, 874 Union Ave., Crowe 115, Memphis, TN 38163, USA.
Jeremiah Stitham, Department of Pharmacology and Toxicology, Dartmouth Medical School, Hanover, NH 03755, USA.
John Hwa, Department of Pharmacology and Toxicology, Dartmouth Medical School, Hanover, NH 03755, USA.
Rennolds S. Ostrom, Email: rostrom@utmem.edu, Vascular Biology Center of Excellence, University of Tennessee Health Science Center, Memphis, TN 38163, USA; Department of Pharmacology, University of Tennessee Health Science Center, 874 Union Ave., Crowe 115, Memphis, TN 38163, USA.
References
- Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001;357(9273):2042–2048. doi: 10.1016/S0140-6736(00)05118-7. [DOI] [PubMed] [Google Scholar]
- Brunton LL, Hayes JS, et al. Functional compartmentation of cyclic AMP and protein kinase in heart. Adv Cycl Nucleotide Res. 1981;14:391–397. [PubMed] [Google Scholar]
- Bundey RA, Insel PA. Adenylyl cyclase 6 overexpression decreases the permeability of endothelial monolayers via preferential enhancement of prostacyclin receptor function. Mol Pharmacol. 2006;70(5):1700–1707. doi: 10.1124/mol.106.028035. [DOI] [PubMed] [Google Scholar]
- Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375(Pt 3):517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, et al. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293(5527):98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem. 1990;96(1):1–14. doi: 10.1007/BF00228448. [DOI] [PubMed] [Google Scholar]
- Fagan KA, Smith KE, et al. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J Biol Chem. 2000;275(34):26530–26537. doi: 10.1074/jbc.M001369200. [DOI] [PubMed] [Google Scholar]
- Foster LJ, De Hoog CL, et al. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100(10):5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Ping P, et al. Increased expression of adenylyl cyclase type VI proportionately increases beta-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes. Proc Natl Acad Sci USA. 1998;95(3):1038–1043. doi: 10.1073/pnas.95.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros R, Ding Q, et al. Adenylyl cyclase isoform-selective regulation of vascular smooth muscle proliferation and cytoskeletal reorganization. Circ Res. 2006;99(8):845–852. doi: 10.1161/01.RES.0000245189.21703.c0. [DOI] [PubMed] [Google Scholar]
- Gustafsson AB, Brunton LL. Beta -adrenergic stimulation of rat cardiac fibroblasts enhances induction of nitric-oxide synthase by interleukin-1beta via message stabilization. Mol Pharmacol. 2000;58(6):1470–1478. doi: 10.1124/mol.58.6.1470. [DOI] [PubMed] [Google Scholar]
- Hasse A, Nilius SM, et al. Long-term-desensitization of prostacyclin receptors is independent of the C-terminal tail. Biochem Pharmacol. 2003;65(12):1991–1995. doi: 10.1016/s0006-2952(03)00184-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Ostrom RS, et al. cAMP-elevating agents and adenylyl cyclase overexpression promote an antifibrotic phenotype in pulmonary fibroblasts. Am J Physiol Cell Physiol. 2004;286(5):C1089–C1099. doi: 10.1152/ajpcell.00461.2003. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun SQ, et al. cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006;70(6):1992–2003. doi: 10.1124/mol.106.028951. [DOI] [PubMed] [Google Scholar]
- McAdam BF, Catella-Lawson F, et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA. 1999;96(1):272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, et al. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79(4):1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nasrallah R, Hebert RL. Prostacyclin signaling in the kidney: implications for health and disease. Am J Physiol Renal Physiol. 2005;289(2):F235–F246. doi: 10.1152/ajprenal.00454.2004. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: Implications for molecular pharmacology. Br J Pharmacol. 2004;143(2):235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, et al. Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem. 2000;275(16):11735–11739. doi: 10.1074/jbc.275.16.11735. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Post SR, et al. Stoichiometry and compartmentation in G Protein-coupled receptor signaling: implications for therapeutic interventions involving Gs. J Pharmacol Exp Ther. 2000;294(2):407–412. [PubMed] [Google Scholar]
- Ostrom RS, Violin JD, et al. Selective enhancement of beta-adrenergic receptor signaling by overexpression of adenylyl cyclase type 6: colocalization of receptor and adenylyl cyclase in caveolae of cardiac myocytes. Mol Pharmacol. 2000;57(5):1075–1079. [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, et al. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem. 2001;276(45):42063–42069. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Liu X, et al. Localization of adenylyl cyclase isoforms and G protein-coupled receptors in vascular smooth muscle cells: expression in caveolin-rich and noncaveolin domains. Mol Pharmacol. 2002;62(5):983–992. doi: 10.1124/mol.62.5.983. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Naugle JE, et al. Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq–Gs cross-talk regulates collagen production in cardiac fibroblasts. J Biol Chem. 2003;278(27):24461–24468. doi: 10.1074/jbc.M212659200. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44(4):655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- Razani B, Woodman SE, et al. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54(3):431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Groves BM, et al. Prostacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertension. Circulation. 1982;66(2):334–338. doi: 10.1161/01.cir.66.2.334. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Xu X, et al. Differential targeting of beta-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275(52):41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- Schwencke C, Yamamoto M, et al. Compartmentation of cyclic adenosine 3′,5′-monophosphate signaling in caveolae. Mol Endocrinol. 1999;13(7):1061–1070. doi: 10.1210/mend.13.7.0304. [DOI] [PubMed] [Google Scholar]
- Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Smart EJ, et al. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271(11):6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Smith KE, Gu C, et al. Residence of adenylyl cyclase type 8 in caveolae is necessary but not sufficient for regulation by capacitative Ca2+ entry. J Biol Chem. 2002;277(8):6025–6031. doi: 10.1074/jbc.M109615200. [DOI] [PubMed] [Google Scholar]
- Song SK, Li S, et al. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae micro-domains. J Biol Chem. 1996;271(16):9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Steinberg SF, Brunton LL. Compartmentation of g protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- Swaney JS, Roth DM, et al. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA. 2005;102(2):437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KT, Sun Y, et al. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26(3):279–292. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- Yu H, Gallagher AM, et al. Prostacyclin release by rat cardiac fibroblasts: inhibition of collagen expression. Hypertension. 1997;30(5):1047–1053. doi: 10.1161/01.hyp.30.5.1047. [DOI] [PubMed] [Google Scholar]