Abstract
Recent Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) and other clinical studies have reported that glucose control in patients with diabetes leads to a significant reduction of cardiovascular events and atherosclerosis, indicating that hyperglycemia plays an essential role in cardiovascular disease in diabetic patients. Although several mechanisms by which hyperglycemia promotes atherosclerosis have been proposed, it remains unclear how hyperglycemia promotes atherosclerosis by interaction with inflammatory cytokines. To test our hypothesis that hyperglycemia interplays with interferon gamma (IFNγ), a key factor involved in atherosclerosis, to up-regulate the expression of genes such as matrix metalloproteinases (MMPs) and cytokines that are involved in plaque destabilization, U937 macrophages cultured in medium containing either normal or high glucose were challenged with IFNγ and the expression of MMPs and cytokines were then quantified by real-time PCR and ELISA. Results showed that high glucose and IFNγ had a synergistic effect on the expression of MMP-1, MMP-9 and IL-1β. High glucose also enhanced IFNγ-induced priming effect on LPS-stimulated MMP-1 secretion. Furthermore, high glucose and IFNγ exert the synergistic effect on MMP-1 expression by enhancing STAT1 phosphorylation and STAT1 transcriptional activity. In summary, this study revealed a novel mechanism potentially involved in diabetes-promoted cardiovascular disease.
Keywords: Diabetes Mellitus, Glucose, Atherosclerosis, Interferon Gamma, Inflammation, Matrix Metalloproteinase
1. Introduction
The incidence of cardiovascular events in diabetic patients is significantly increased as compared to that in nondiabetic population (1). In recent years, a large population study called Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) trial has provided strong evidence to support an important role of hyperglycemia in cardiovascular disease (2). In this clinical trial, 1441 patients with type 1 diabetes were treated with either conventional or intensive therapy for a mean of 6.5 years between 1983 and 1993, and 94% of the patients were subsequently followed for a mean of 17 years until 2005. The results showed that the intensive glycemic control reduced the risk of any cardiovascular event by 42% (p=0.02) and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57% (p=0.02). Another clinical trial showed that reduction of postprandial hyperglycemia in type 2 diabetic patients is associated with regression of carotid intima-media thickness (3). Furthermore, a meta-analysis of 13 clinical trials suggests that chronic hyperglycemia is associated with an increased risk for cardiovascular disease in patients with diabetes (4). In addition to the clinical studies, numerous laboratory investigations have also indicated that high glucose plays an essential role in atherosclerosis (1). Among these investigations, the studies on advanced glycation endproducts (AGEs) have well demonstrated in vivo and in vitro that long-term exposure to high glucose leads to the formation of AGEs by non-enzymatic glycosylation and AGEs are atherogenic (5). All these clinical and laboratory studies clearly indicate that hyperglycemia contributes to atherosclerosis.
Atherosclerosis is an inflammatory disease (6). It has been well documented that pro-inflammatory cytokines play a crucial role in not only the development and progression of atherosclerosis, but also plaque destabilization, a state of atherosclerotic lesion prone to rupture (6, 7). In diabetic patients with poor glycemic control, it is expected that pro-inflammatory cytokines would interplay with hyperglycemia to promote atherosclerosis and plaque destabilization. However, the interaction between hyperglycemia and pro-inflammatory cytokines in atherosclerosis has not been well elucidated.
Among pro-inflammatory cytokines, interferon gamma (IFNγ), a cytokine mainly released by T lymphocytes, is considered a key factor involved in plaque destabilization (6, 7). It has been shown that IFNγ inhibits the expression of interstitial collagen by smooth muscle cells (SMCs) and stimulates the expression of matrix metalloproteinases (MMPs), a group of proteinases responsible for collagen and other matrix degradation and plaque destabilization (8), by mononuclear phagocytes (9,10). IFNγ also inhibits SMC proliferation and further reduces collagen production and weakens the fibrous cap (9). More importantly, IFNγ activates macrophages, the predominant type of cells in the rupture-prone shoulder regions and major source of MMP production in vulnerable plaques (7,8). Given the critical role of IFNγ in inflammation and plaque vulnerability, we hypothesized that hyperglycemia may act in concert with IFNγ to promote plaque destabilization by upregulating MMP and cytokine expression.
In the present study, we have tested our hypothesis in vitro and demonstrated a synergistic effect of high concentration of glucose (high glucose) and IFNγ on MMP-1, MMP-9 and IL-1β expression by U937 macrophages. We further demonstrated that high glucose and IFNγ exert the synergistic effect on MMP-1 expression by enhancing the signal transducing and activator of transcription (STAT)-1 signaling.
2. Materials and Methods
2.1. Cell Culture
U937 histiocytes (resident macrophages) (11) were purchased from American Type Culture Collection (Manassas, VA) and have been used in our previous studies to show the augmentation of lipopolysaccharide (LPS)-stimulated MMP and cytokine expression by high glucose (12–14). The cells were cultured in a 5% CO2 atmosphere in RPMI 1640 medium (GIBCO, Invitrogen Cop. Carlsbad, CA) containing 10% fetal calf serum, 1% MEM non-essential amino acid solution, 0.6 g/100 ml of HEPES, and 5 (normal glucose) or 25 mM (high glucose) of D-glucose. The medium was changed every 2–3 days. U937 cells were treated with IFNγ (Sigma, St. Louis, MO) after exposure to normal or high glucose for at least 2 weeks. The cell growth rate was determined using CyQUANT cell proliferation assay kit (Molecular Probes, Eugene, OR). Human monocytes were isolated, cultured and differentiated to macrophages as described previously (15). Human monocyte-derived macrophages were cultured in normal or high glucose-containing medium for 3 days before being treated with IFNγ.
2.2. Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from cells using the RNeasy minikit (Qiagen, Santa Clarita, CA). First-strand complementary DNA (cDNA) was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA) using 20 μl of reaction mixture containing 0.25 μg of total RNA, 4 μl of 5x iScript reaction mixture, and 1 μl of iScript reverse transcriptase. The complete reaction was cycled for 5 minutes at 25 °C, 30 minutes at 42 °C and 5 minutes at 85°C using a PTC-200 DNA Engine (MJ Research, Waltham, MA). The reverse transcription (RT) reaction mixture was then diluted 1:10 with nuclease-free water and used for PCR amplification of MMP and cytokine cDNA in the presence of the primers. The Beacon designer software (PREMIER Biosoft International, Palo Alto, CA) was used for primer designing. Primers for MMP-1 (5′ primer: CTGGGAAGCCATCACTTACCTTGC; 3′ primer: GTTTCTAGAGTCGCTGGGAAGCTG) were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). Real-time PCR was performed in duplicate using 25 μl of reaction mixture that contained 1.0 μl of RT mixture, 0.2 μM of both primers, and 12.5 μl of iQ™ SYBR Green Supermix (Bio-Rad; Hercules, CA) and run in the iCycler™ real-time detection system (Bio-Rad; Hercules, CA) with a two-step method. The hot-start enzyme was activated (95°C for 3 min) and cDNA was then amplified for 40 cycles consisting of denaturation at 95°C for 10 sec and annealing/extension at 53°C for 45 sec. A melt-curve was then performed (55°C for 1 min and then temperature was increased by 0.5°C every 10 sec) to detect the formation of primer-derived trimers and dimmers. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5′ primer: GAATTTGGCTACAGCAACAGGGTG; 3′ primer: TCTCTTCCTCTTGTGCTCTTGCTG) as a control was also subjected to real-time PCR. Data were analyzed with the iCycler iQ™ software. The average starting quantity (SQ) of fluorescence units was used for analysis. Quantification was calculated using the SQ of tested cDNA relative to that of GAPDH cDNA in the same sample.
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
MMPs and cytokines in conditioned medium were quantified using sandwich ELISA kits according to the protocol provided by the manufacturer (R&D System, Minneapolis, MN).
2.4. Treatment of Cells with the Inhibitors of Signaling Pathways
In the studies to determine the effects of the specific inhibitors of signaling pathways (Calbiochem/EMD Biosciences, Inc., San Diego, CA) on the stimulation of MMP-1 gene expression by IFNγ, U937 cells pre-exposed to normal or high glucose were treated with 100 units/ml of IFNγ in the absence or presence of 10 μM of AG490 (JAK-STAT pathway inhibitor) or bay117085 (NFκB pathway inhibitor) for 24 h. The concentrations of the inhibitors have been applied in the previous studies (13,14). After the treatment, MMP-1 in conditioned medium was quantified using ELISA.
2.5. Immunoblotting
U937 macrophages incubated in normal or high glucose-containing medium were treated with 100 units/ml of IFNγ for 0, 5, 10, 20, 30 and 60 min and cellular proteins were extracted after the treatment. Fifty μg of each sample was electrophoresed in a 10% polyacrylamide gel. After transferring proteins to a nitrocellulose membrane, total and phosphorylated STAT1 were immunoblotted with anti-total and anti-phosphorylated STAT1 primary antibodies (Cell Signaling Technology, Danvers, MA), respectively, and HRP-conjugated secondary antibody. STAT1 was detected by incubating the membrane with chemiluminescence reagent (NEN Life Science Products) for 1 min and exposing it to x-ray film for 30–60 seconds. The film was scanned by densitometry to quantify STAT1.
2.6. Extraction of Nuclear Proteins
Nuclear protein was extracted using NE-PER™ nuclear and cytoplasmic extraction reagents from Pierce (Rockfold, IL). The concentration of protein in the nuclear fractions was determined using a protein assay kit (Bio-Rad, Hercules, CA).
2.7. Transcription Factor Activity Assay
The STAT1 activity assay was performed using a TransBinding STAT1 assay kit (Panomics, Inc., Redwood, CA) according to the experimental protocol provide by the manufacturer. Briefly, 10 μl of nuclear extract was incubated with Binding Buffer Mix containing the biotinylated STAT1 probe. The STAT1/STAT1 probe complexes were then immobilized to the streptavidin-coated assay plate. The STAT1/STAT1 probe complexes were detected with STAT1 specific antibodies and the HRP-conjugated secondary antibody. The amount of STAT1 in all wells were determined as optic densities in a spectrophotometer and normalized to the protein concentrations. The STAT3 activity assay was performed using a STAT3 activity assay kit by following the experimental protocol provided by the manufacturer (Clontech Laboratories, Inc., Mountain View, CA).
2.8. Electrophoretic Mobility Shift Assay (EMSA)
U937 cells cultured in normal or high glucose-containing medium were treated without or with 100 units/ml of IFNγ for 2, 8 and 24 h. After the treatment, cells were harvested and nuclear protein was extracted as described above. Ten μg of nuclear proteins was used for the electrophoretic mobility shift assay to determine STAT1 DNA binding activity. DNA–protein binding reactions were performed at room temperature for 20 min in a buffer containing10 mM Trizma base (pH 7.9), 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 1 μg poly(dI-dC), 5% (v/v) glycerol, and approximately 0.3 pmole of STAT1 oligonucleotide (Promega, Madison, WI) labeled with DIG-ddUTP using terminal deoxynucleotidyl transferase (Roche Molecular Biochemicals, Indianapolis, ID). Protein–DNA complexes were resolved from protein-free DNA in 5% polyacrylamide gels at room temperature in 50 mM Tris, pH 8.3, 0.38 M Glycine, 2 mM EDTA, and electroblotted onto positively charged nylon membranes. The chemiluminescence detection of DIG-labeled probes was conducted by following the instruction provided by the Roche Molecular Biochemicals.
2.9. Statistic Analysis
Each experiment was performed in triplicate wells and data were presented as mean ± SD. Student t tests were performed to determine the statistical significance of cytokine expression among different experimental groups. A value of P< 0.05 was considered significant.
3. Results
3.1. Synergistic Effect of High Glucose and IFNγ on MMP-1 Expression and Secretion
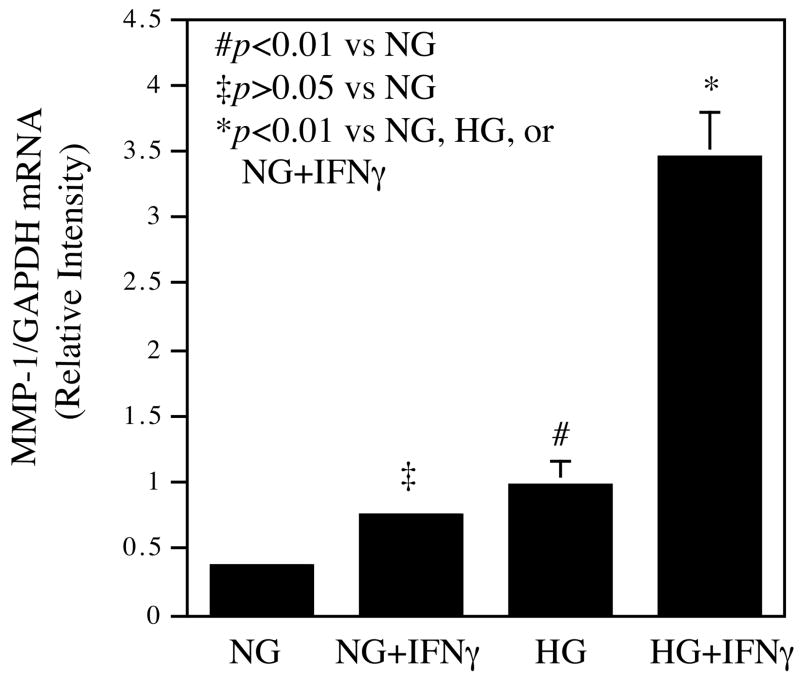
In our first experiment, U937 macrophages were challenged with IFNγ after being exposed to normal or high glucose in culture medium for at least 2 weeks. After the treatment, U937 cell growth rate was determined first and results showed that cell growth was not affected by 2 weeks culture in high glucose (data not shown). The cellular MMP-1 mRNA was then quantified by real-time PCR (Fig. 1A). Results showed that as compared to normal glucose, high glucose and IFNγ alone increased MMP-1 mRNA level by 1 and 1.5-fold, respectively, but the combination of high glucose and IFNγ resulted in a 3.5-fold increase in MMP-1 mRNA as compared with the combination of normal glucose and IFN. These results demonstrate that high glucose and IFNγ synergistically stimulate MMP-1 mRNA expression by U937 macrophages.
Figure 1.
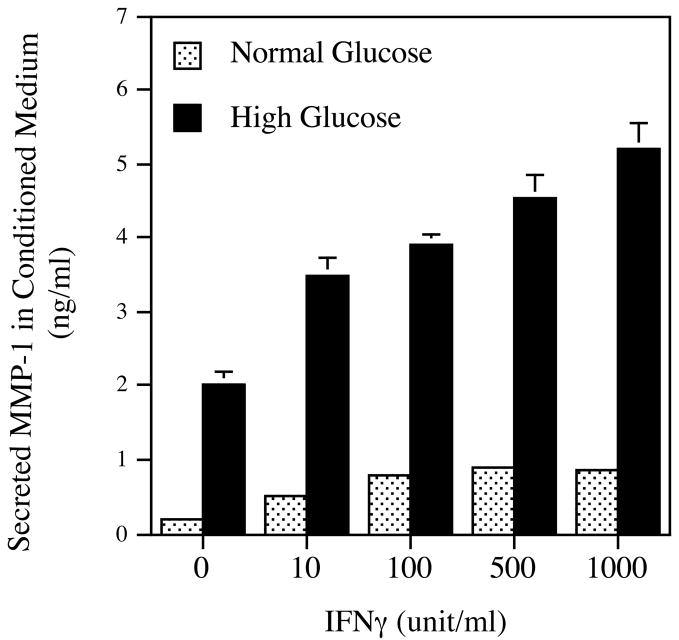
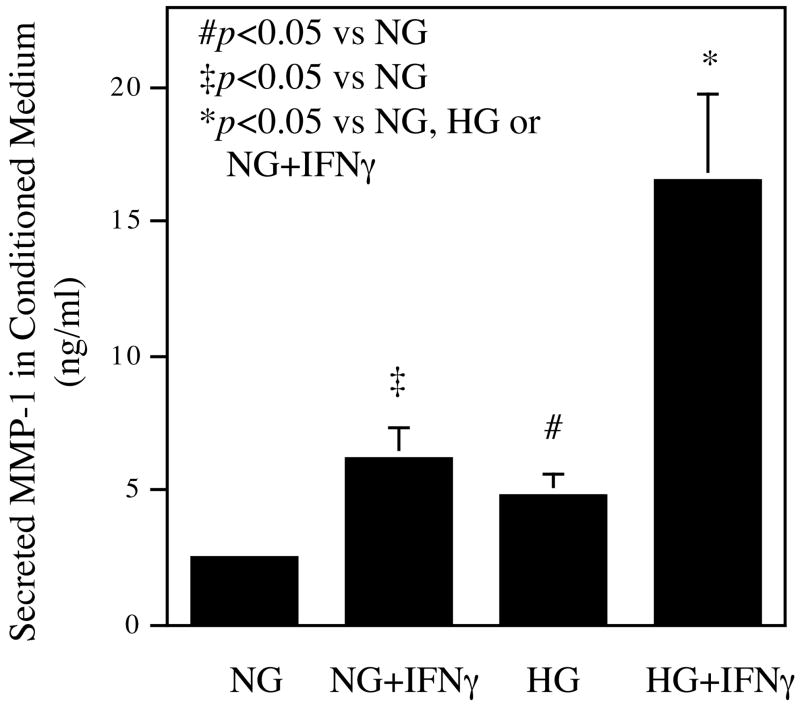
The synergistic effect of high glucose and IFNγ on MMP-1 mRNA expression and protein secretion. A. The real-time PCR analysis of MMP-1 expression by cells exposed to normal or high glucose and subsequent challenged with IFNγ. U937 cells cultured in normal or high glucose-containing medium were treated with 100 units/ml of IFNγ for 24 h. After the treatment, total RNA was isolated from the cells and used for real-time PCR to quantify MMP-1 and GAPDH mRNA expression as described in Methods. Relative MMP-1 mRNA expression was calculated by normalization to GAPDH mRNA. B. The concentration-dependent effect of IFNγ on MMP-1 secretion by U937 cells exposed to normal or high glucose. U937 cells cultured in normal or high glucose-containing medium were treated with different concentrations of IFNγ as indicated for 24 h and the conditioned medium was used for ELISA to quantify the amount of MMP-1 protein. C. The synergistic effect of high glucose and IFNγ on MMP-1 secretion by human monocyte-derived macrophages (HMDMs). HMDMs pre-exposed to normal or high glucose were treated with 100 units/ml of IFNγ for 24 h and MMP-1 in culture medium was quantified using ELISA. The Data presented are the representative of three independent experiments with the similar results. NG, normal glucose; HG, high glucose.
MMP-1 protein in the conditioned medium was also quantified. As shown in Fig. 1B, high glucose alone increased MMP-1 secretion while IFNγ stimulated MMP-1 secretion from both normal and high glucose-exposed cells in a concentration-dependent manner. Interestingly, the combination of high glucose and 100 units/ml of IFNγ led to a 3-fold increase in MMP-1 secretion over the amount of MMP-1 secreted by cells treated with the combination of normal glucose and 100 units/ml of IFNγ. Thus, the increase in MMP-1 protein (Fig. 1B) is similar to that in MMP-1 mRNA (Fig. 1A).
To determine if primary human peripheral monocytes or human monocyte-derived macrophages (HMDM) have similar responses as U937 cells to high glucose and IFNγ, we treated human monocytes or HMDM with normal or high glucose in the presence or absence of 100 units/ml of IFNγ. Results showed that for HMDM, either high glucose or IFNγ increased MMP-1 secretion, and high glucose plus IFNγ stimulated MMP-1 secretion by 2.6-fold as compared to normal glucose plus IFNγ (Fig. 1C). In contrast, no similar synergistic effect of high glucose and IFNγ was observed with human peripheral monocytes (data not shown).
3.2. The Effect of High Glucose on TIMP Secretion
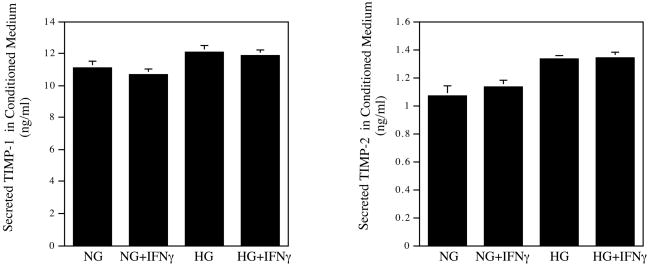
Tissue inhibitors of metalloproteinase (TIMP) control matrix degradation by inhibiting MMP activity (16). In this study, the effect of high glucose on TIMP-1 and -2 expression was also determined. Results showed that high glucose, IFNγ, or their combination had no significant effect on TIMP-1 and TIMP-2 secretion (Fig. 2A).
Figure 2.
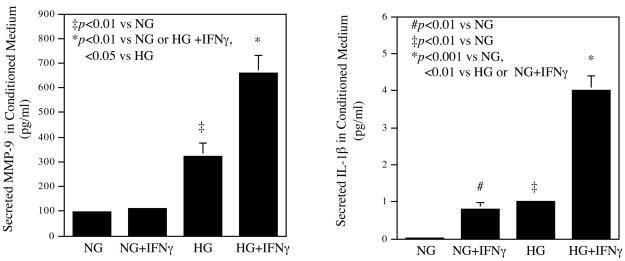
The effect of high glucose and 100 units/ml of IFNγ on TIMP-1, TIMP-2, MMP-9 and IL-1β secretion by U937 macrophages. The conditioned medium from the above studies was also used in ELISA to quantify TIMP-1 and TIMP-2 (A), MMP-9 and IL-1β (B) as described in Methods. The Data presented are the representative of three independent experiments with the similar results. NG, normal glucose; HG, high glucose.
3.3. Synergistic Effect of High Glucose and IFNγ on MMP-9 and IL-1β Secretion
In addition to MMP-1, high glucose and IFNγ also have a synergistic effect on MMP-9 and IL-1β secretion (Fig. 2B). Results showed that IFNγ did not stimulate MMP-9 secretion from cells pre-exposed to normal glucose, and high glucose alone increased MMP-9 secretion by 3-fold. Interestingly, the combination of high glucose and IFNγ stimulated MMP-9 secretion by 7.5-fold as compared with the combination of normal glucose and IFNγ (Fig. 2B). Results also showed that high glucose augmented IFNγ-stimulated IL-1β secretion by 4.5-fold (Fig. 2B). In contrast, no synergistic effects of high glucose and IFNγ on MMP-2, MMP-13, TNFα and IL-6 secretions were observed (data not shown), suggesting that the synergistic effects of high glucose and IFNγ on MMP-1, MMP-9 and IL-1β expression are specific.
3.4. Enhancement of Priming Effect of IFNγ by High Glucose on U937 Macrophages
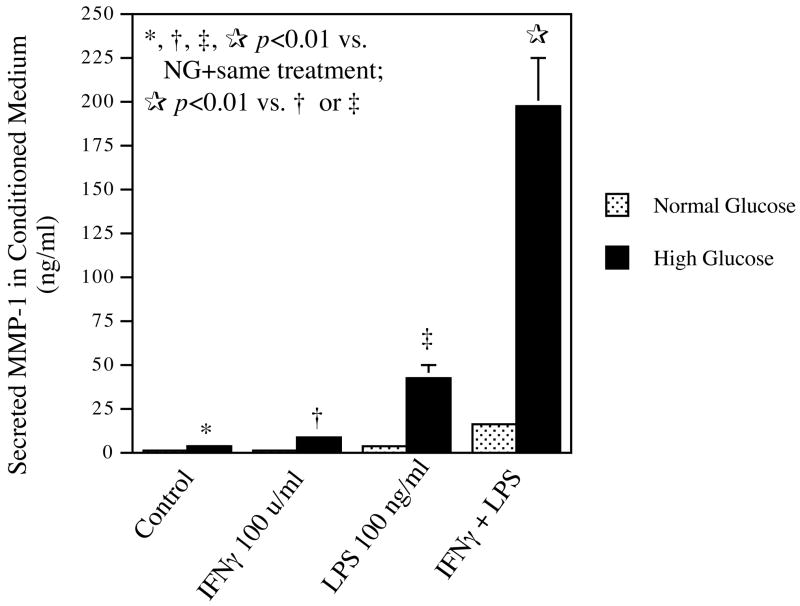
It is known that IFNγ activates macrophages (17). Activated macrophages are characterized by not only the increased expression of genes involved in inflammation, but also the enhanced responsiveness to inflammatory stimuli (18). For example, priming of macrophages with IFNγ leads to increased cellular responses to LPS (18). In this study, we determined if high glucose enhances the priming effect of IFNγ on macrophages. Results (Fig. 3) showed that high glucose augmented either IFNγ- or LPS-stimulated MMP-1 secretion as compared with normal glucose. Furthermore, results showed that IFNγ-primed cells cultured in high glucose-containing medium had a 13-fold increase in MMP-1 secretion in response to LPS as compared with IFNγ-primed cells cultured in normal glucose-containing medium (196.8 vs 15.3 ng/ml, p<0.001). Thus, these results showed that high glucose enhanced the priming effect of IFNγ on macrophages, leading to a marked increase in MMP-1 secretion in response to LPS.
Figure 3.
The effect of high glucose on MMP-1 secretion by IFNγ-primed U937 macrophages. U937 macrophages cultured in normal or high glucose-containing medium were primed with or without 100 units/ml of IFNγ for 24 h and then treated with or without 100 ng/ml of LPS for additional 24 h. After the treatment, MMP-1 in conditioned medium was quantified using ELISA. The data presented are the representative of two independent experiments with the similar results.
3.5. JAK-STAT Signaling Pathways Is Involved in IFNγ-stimulated MMP-1 Expression
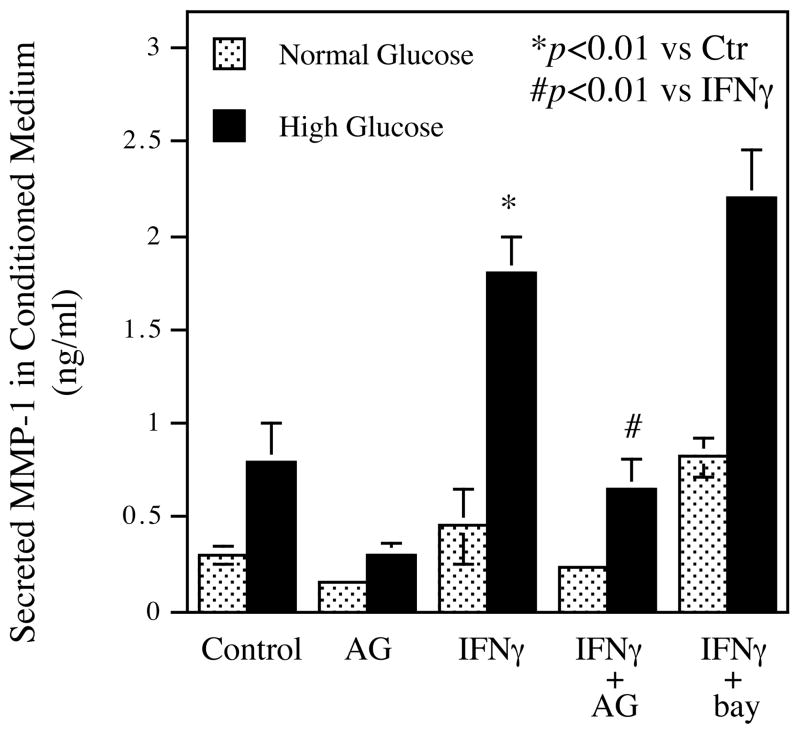
It is known that Janus tyrosine kinase (JAK)-signal transducing and activator of transcription (STAT) pathway is a major cascade mediating IFNγ-stimulated gene expression by macrophages (17). Thus, we determined if JAK-STAT pathway was involved in IFNγ-stimulated MMP-1 expression. U937 macrophages pre-exposed to normal or high glucose were challenged with or without IFNγ in the presence or absence of AG490, a specific inhibitor for JAK-STAT cascade. After the treatment, secreted MMP-1 was quantified. Results showed that AG490 not only inhibited high glucose-stimulated MMP-1 secretion, but also effectively attenuated IFNγ-stimulated MMP-1 secretion in either normal or high glucose-cultured cells (Fig. 4). AG490 also inhibited the synergistic effect of high glucose and IFNγ on MMP-9 and IL-1β secretion (data not shown). To show the specificity of JAK-STAT pathway in IFNγ-stimulated MMP-1 expression, U937 cells were treated with bay117085, an inhibitor for NFκB pathway that has been shown to be involved in LPS-stimulated MMP expression (19). Results showed that bay117085 did not inhibit IFNγ-stimulated MMP-1 secretion (Fig. 4).
Figure 4.
Inhibition of IFNγ-stimulated MMP-1 secretion by specific inhibitor of JAK-STAT pathway. U937 macrophages pre-exposed to normal or high glucose were treated with 100 units/ml of IFNγ for 24 h in the absence or presence of 10 μM of AG490 (JAK-STAT pathway specific inhibitor) or 10 μM of bay117085 (NFκB pathway specific inhibitor). After the treatment, MMP-1 in conditioned medium was quantified using ELISA. AG, AG490; bay, bay117085. The data presented are the representative of three independent experiments with the similar results.
3.6. Enhancement of IFNγ-triggered STAT1 Activation by High Glucose
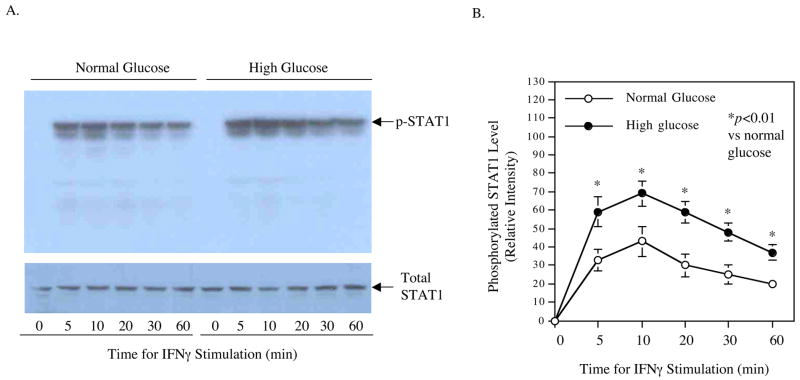
It is known that activation of JAK-STAT pathway in macrophages by IFNγ leads to increased STAT1 phosphorylation, and phosphorylated STAT1 enters the nucleus and binds to IFNγ-activated site (GAS) in the promoter regions of IFNγ-regulated genes for transcriptional activation (17). In this study, we first determined the effect of high glucose on IFNγ-induced phosphorylation of STAT1. Results from Western blot showed that IFNγ induced STAT1 phosphorylation in either normal glucose- or high glucose-exposed cells in a time-dependent fashion and the activation peaked at 10 min (Fig. 5). Moreover, high glucose led to a higher level of IFNγ-induced STAT1 phosphorylation than normal glucose (Fig. 5), suggesting that high glucose augments IFNγ-stimulated MMP-1 expression by enhancing STAT1 activation.
Figure 5.
Enhancement of IFNγ-induced STAT1 phosphorylation by high glucose. U937 macrophages cultured in normal or high glucose-containing medium were treated with 100 units/ml of IFNγ for different times as indicated. After the treatment, cells were lysed and aliquots of the lysate containing 50 μg protein for each sample were electrophoresed in a 10% polyacrylamide gel. The proteins were transferred to a nitrocellulose membrane that was subjected to immunoblotting using anti-phosphorylated STAT1 or anti-total STAT1 antibodies as described in Methods (A). The STAT1 bands on the film as shown in Fig. 5A were semi-quantified using densitometric scanning and the results were presented as relative intensity. The image presented in Fig. 5A is the representative of two independent experiments with the similar results. p-STAT1, phosphorylated STAT1.
We further determined if high glucose increases IFNγ-induced STAT1 transcriptional activity by performing both STAT1 DNA-binding activity assay and electrophoretic mobility shift assay (EMSA). The former is an ELISA-type quantitative method using anti-STAT1 antibody to determine the amount of STAT1 bound to STAT1 DNA probes while the latter is a semi-quantitative method using electrophoresis to show the shifting of the STAT1 protein-bound DNA probes from the free STAT1 DNA probes. Results from STAT1 DNA-binding activity assay showed that IFNγ stimulated STAT1 DNA-binding activity in either normal or high glucose-exposed cells in a time-dependent fashion, but the peak stimulations in high glucose-exposed cells were 11- and 5-fold of those in normal glucose-exposed cells at 2 and 8 h, respectively (Fig. 6A). When the same nuclear extracts were used for EMSA, the shifted bands were observed only in the samples from high glucose-exposed cells (Fig. 6B). It is possible that due to the low level of activated STAT1 in the nuclear extract isolated from normal glucose-exposed cells, the amount of the STAT1-DNA complexes was too little to be detected by EMSA.
Figure 6.
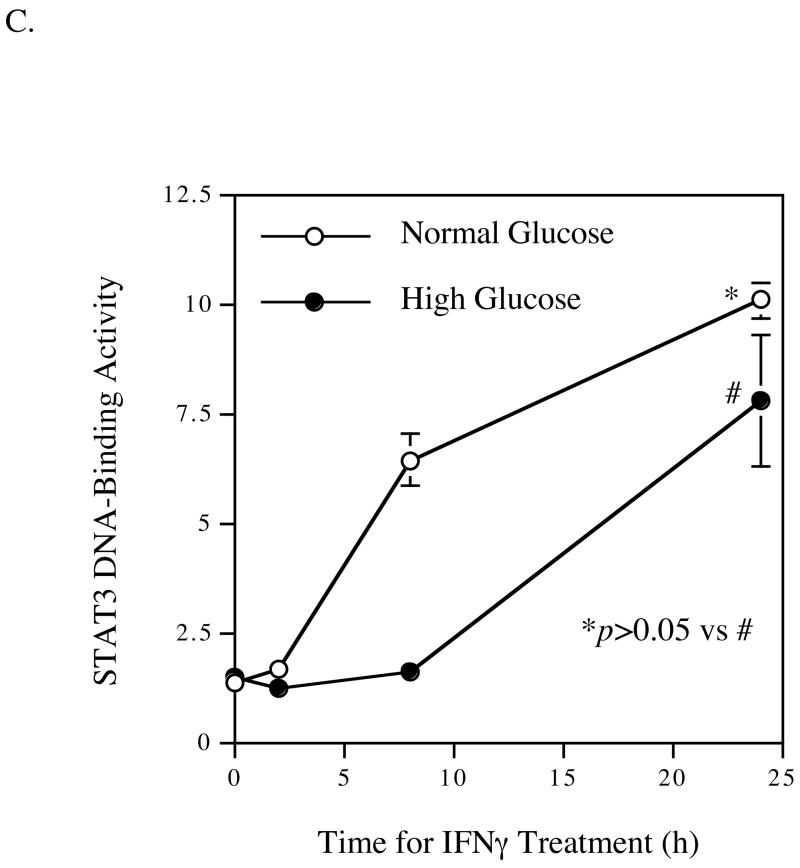
Enhancement of IFNγ-induced nuclear STAT1 DNA-binding activity by high glucose. U937 macrophages cultured in normal or high glucose-containing medium were treated with 100 units/ml of IFNγ for 2, 4, 8, or 24 h. After the treatment, nuclear protein was isolated from cells and subject to STAT1 (A) and STAT3 (C) DNA-binding activity assay, and STAT1 electrophoretic mobility shift assay (B) as described in Methods. The data presented are the representative of two independent experiments with the similar results.
The effect of high glucose and IFNγ on STAT3 DNA-binding activity was also examined. Results showed that while IFNγ stimulated STAT3 activity under either normal or high glucose condition at 24 hours, there was no significant difference of STAT3 DNA-binding activity between normal and high glucose-exposed cells (Fig. 6C), suggesting that STAT1 is specifically involved in the synergistic stimulation of MMP-1 expression by high glucose and IFNγ.
4. Discussion
Hyperglycemia is a chronic abnormality in diabetes. It is known that chronic exposure of proteins to high glucose leads to the formation of advanced glycation endproducts (AGEs) that are atherogenic (5). However, it remains unclear how chronic exposure to high glucose changes cellular responses to inflammatory stimuli. Since it is known that diabetes and diabetic complications are associated with inflammation (6, 7), we proposed that chronic exposure to high glucose might render macrophages more sensitive to inflammatory stimuli. To test this hypothesis, we exposed U937 macrophages to high glucose for more than 2 weeks and then challenged them with IFNγ, a pro-inflammatory cytokine (17). Our results showed that high glucose augmented IFNγ-stimulated MMP-1 expression compared with normal glucose. Thus, this finding in combination with our previous reports that high glucose augments lipopolysaccharide (LPS)-stimulated MMP-1 expression (12–14) clearly indicate that chronic exposure to high glucose enhances the responsiveness of macrophages to inflammatory stimuli.
Given the pivotal role of macrophages in inflammation, the clinical implication of these in vitro findings is that hyperglycemia in diabetes may promote inflammation by increasing macrophage susceptibility to inflammatory stimuli. This finding may partially explain why atherosclerosis as an inflammatory disease is more prevalent and severe in diabetic patients. As we described early, DCCT/EDIC studies have provided strong evidence to support a link between chronic hyperglycemia and cardiovascular disease (5) and the present study may have delineated a potential mechanism by which hyperglycemia increases cardiovascular events.
A large number of studies have shown an important role of MMPs in plaque rupture (7,19,20). Pathological studies have shown that the expression of several MMPs including MMP-1, MMP-9, MMP-2, MMP-13 and MMP-8 is significantly higher in vulnerable plaques than that in stable plaques (21). Clinical studies have consistently demonstrated that plasma levels of MMP-1, MMP-2 and MMP-9 are significantly elevated in patients with unstable angina or acute myocardial infarction (21). Furthermore, genetic studies have shown that the polymorphisms in the MMP gene promoters are highly related to the incidences of myocardial infarction (8). All these studies have well documented a crucial role of MMPs in plaque rupture and subsequent acute coronary syndrome. In the present study, we demonstrated that high glucose augments IFNγ-stimulated expression of MMP-1 and MMP-9, but had no effect on the expression of TIMP-1 and TIMP-2, two endogenous inhibitors of MMP activities, suggesting that hyperglycemia may increase matrix-degrading activity in atherosclerotic plaques and thus promotes plaque destabilization.
To understand how high glucose and IFNγ exert their synergistic effects on MMP-1 expression, we focused on JAK-STAT1 pathway since it is the major signaling cascade mediating IFNγ-regulated gene expression (17). We found that U937 macrophages pre-exposed to high glucose had a higher level of STAT1 phosphorylation in response to IFNγ than those pre-exposed to normal glucose. Furthermore, since phosphorylated STAT1 travels into nucleus to activate gene expression, the nuclear STAT1 DNA-binding activity is expected to be higher in cells exposed to high glucose than that in cells exposed to normal glucose. Indeed, the nuclear STAT1 DNA-binding activity stimulated by IFNγ was found to be remarkably higher in cells exposed to high glucose. With our earlier observation that JAK-STAT specific inhibitor AG490 effectively inhibits IFNγ-stimulated MMP-1 expression (Fig. 4), these results strongly indicate that high glucose and IFNγ synergistically upregulate MMP-1 expression by enhancing STAT1 transcriptional activity.
IFNγ is released predominantly by T lymphocytes, which are present along with macrophages in atherosclerotic plaques (17). T lymphocytes promote macrophage activation through the action of IFNγ that binds to IFNγ receptors and triggers the activation of JAK-STAT pathway. Studies have shown that priming of macrophages with IFNγ markedly enhances cellular responsiveness to inflammatory stimuli such as LPS (18), cytokines (21) and immune complexes (22). In the present study, we postulated that high glucose enhances the priming effect of IFNγ on macrophages. Strikingly, our results showed that U937 cells primed with IFNγ under the condition of high glucose had a 13-fold increase in MMP-1 secretion in response to LPS as compared with cells incubated under the condition of normal glucose. This finding indicates that hyperglycemia is capable of enhancing IFNγ-induced activation of macrophages that have increased cellular responses to inflammatory stimuli such as LPS, leading to an augmented gene expression.
In summary, we have demonstrated in this study that pre-exposure of U937 macrophages to high glucose enhanced IFNγ-stimulated MMP-1 expression via STAT1 activation. We have also shown that high glucose enhanced the priming effect of IFNγ on macrophages. Given that hyperglycemia is a key abnormality for diabetes, and diabetic patients have increased incidence of acute coronary syndromes, this study provides insight into the pathogenesis of acute coronary syndromes in diabetes.
Acknowledgments
This work was supported by a Merit Review Grant from Department of Veterans Affairs and NIH grant DE16353 (to Y.H.).
Footnotes
This study was presented at the American Heart Association Arteriosclerosis, Thrombosis, and Vascular Biology Annual Conference in Chicago, IL from April 19-21, 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pyorala K, Laakso M, Unsitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito K, Giugliano D, Nappo F, Marfella R. Campanian postprandial hyperglycemia study group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–9. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Internal Med. 2004;141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 5.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE. Circ Res. 2003;93:1159–69. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. Atherosclerosis-an inflammatory disease. New Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–50. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 8.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 9.Amento EP, Ehsani N, Palmer H, Libby P. Cytokine and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1223–30. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- 10.Zhou M, Zhang Y, Ardans JA, Wahl LM. Interferon-γ differentially regulates monocyte matrix metalloproteinase-1 and -9 through tumor necrosis factor-α and caspase 8. J Biol Chem. 2003;278:45406–13. doi: 10.1074/jbc.M309075200. [DOI] [PubMed] [Google Scholar]
- 11.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U937) Int J Cancer. 1976;17:565–77. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 12.Maldonado A, He L, Game BA, Nareika A, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. Pre-Exposure to High Glucose Augments Lipopolysaccharide-Stimulated Matrix Metalloproteinase-1 Expression by Human U937 Histiocytes. J Periodontal Res. 2004;39:415–23. doi: 10.1111/j.1600-0765.2004.00756.x. [DOI] [PubMed] [Google Scholar]
- 13.Nareika A, He L, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. Sodium lactate increases LPS-stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NFκB transcriptional activities. Am J Physiol (Endocrinology and Metabolism) 2005;289:E534–42. doi: 10.1152/ajpendo.00462.2004. [DOI] [PubMed] [Google Scholar]
- 14.Nareika A, Maldonado A, He L, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. High glucose-boosted inflammatory responses to lipopolysaccharide are suppressed by statin. J Periodontal Res. 2006;42:31–8. doi: 10.1111/j.1600-0765.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- 15.Danciger JS, Lutz M, Hama S, Cruz D, Castrillo A, Lazaro J, Phillips R, Premack B, Berliner J. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J Immunol Methods. 2004;288:123–134. doi: 10.1016/j.jim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Matrisian LM. The matrix-degrading metalloproteinases. BioEssays. 1992;14:455–63. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 17.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leuk Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 18.Yamada H, Odonnell MA, Matsumoto T, Luo Y. Interferon-γ upregulates toll-like receptor 4 and cooperates with lipopolysaccharide to produce macrophage-derived chemokine and interferon-γ inducible protein-10 in human bladder cancer cell line RT4. J Urology. 2005;174:1119–23. doi: 10.1097/01.ju.0000168619.25341.96. [DOI] [PubMed] [Google Scholar]
- 19.Faber BCG, Heeneman S, Daemen MJAP, Cleutjens KBJM. Genes potentially involved in plaque rupture. Curr Opin Lipidol. 2005;13:545–52. doi: 10.1097/00041433-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Galis ZS, Khatri JJ. Matrix metalloproteinase in vascular remodeling and atherosclerosis. The good, the bad, and the ugly. Circ Res. 2002;90:251–62. [PubMed] [Google Scholar]
- 21.Jones CB, Sane DC, Merrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndromes. Cardiovasc Res. 2003;59:812–23. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 22.Anderson F, Xu M, Game BA, Atchley D, Lopes-Virella MF, Huang Y. IFN-γ pre-treatment augments immune complex-induced matrix metalloproteinase-1 expression in U937 histiocytes. Clin Immunol. 2002;102:200–7. doi: 10.1006/clim.2001.5161. [DOI] [PubMed] [Google Scholar]