SYNOPSIS
The clinical and pathologic features of Eosinophilic Esophagitis (EE) include extensive tissue remodeling. Increasing evidence supports a key role for the eosinophil in multiple aspects of the esophageal remodeling and fibrosis seen in this allergic disease, including epithelial hyperplasia, subepithelial fibrosis, smooth muscle hyperplasia, and angiogenesis. These structural changes contribute to the endoscopic findings of esophageal thickening, luminal narrowing, furrowing, transient and fixed rings (trachealization) and stricture, as well as the clinical features of dysmotility, dysphagia and food impactions in pediatric and adult EE. This chapter reviews the clinical implications of esophageal remodeling and fibrosis in EE and discusses the possible pathogenic mechanisms inducing and regulating these responses. We focus specifically on eosinophil and cytokine interactions with the esophageal epithelium, vascular endothelium, resident fibroblasts, and smooth muscle. Current and potential therapeutic interventions are discussed that may impact the development or resolution of chronic esophageal remodeling and fibrosis in EE.
Keywords: eosinophilic esophagitis, eosinophils, inflammation, remodeling, fibrosis, angiogenesis, transforming growth factor β
INTRODUCTION
Eosinophilic esophagitis (referred to herein as EE) is a disease of increasing prevalence and/or detection.13,78,94,98 Its pathogenesis relies in part on an allergic immune reaction that can involve both IgE and T cell mediated hypersensitivity to inhaled aeroallergens and ingested food allergens. The clinical manifestations of EE include vomiting, abdominal pain, regurgitation, heartburn, and failure to thrive, especially in young children. In adolescents and adults, the symptoms can progress to odynophagia and dysphagia that can be associated with the clinical complications of food impactions with or without concurrent esophageal strictures.59,68,95 EE appears to be a chronic disease with persistent dysphagia when left untreated in adults.90 In children, the disease remits with therapies, including systemic or topical esophageal corticosteroids, and elimination or elemental diets, but recurs in the majority of patients when the therapeutic intervention is removed.6
The histopathologic changes that occur in EE traverse the depths of the esophageal wall.28,89 The mucosa becomes infiltrated with eosinophils, mast cells, and T cells, and active proliferation of the epithelium leads to the histologic finding of basal zone hyperplasia.32,67 The submucosal lamina propria also becomes infiltrated by inflammatory cells and demonstrates increased collagen deposition that provides an extracellular matrix for the capture of cells and their cytokine, interleukin, and chemokine4,14,64,93 products. The muscularis mucosa, as well as the circular and longitudinal muscle layers have reported abnormalities with hypertrophy and dysfunction.28 In combination, the multiple facets of esophageal remodeling and subepithelial fibrosis that occur during the instigation and propagation of EE could explain many of the clinical symptoms. In this chapter, we review the known aspects of esophageal remodeling, the basic molecular mechanisms utilized by eosinophils to promote tissue remodeling and fibrosis, and the clinical correlates of esophageal remodeling in EE.
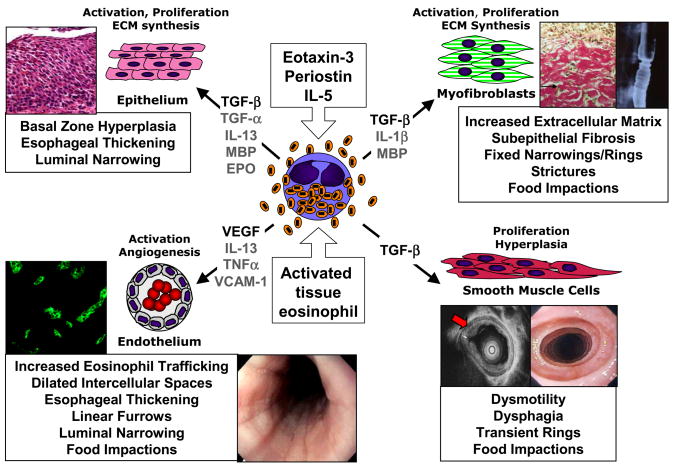
Based on the paradigms of other eosinophil-mediated diseases, the role of tissue remodeling in the pathogenesis and clinical manifestations of EE are beginning to be investigated (Figure 1). Tissue remodeling in response to Th2 and eosinophil-associated diseases was first characterized in the hypereosinophilic syndrome (HES) and asthma.48,54,58,96 In HES, eosinophil activation and degranulation causes target organ fibrosis. Significant patient mortality and morbidity is related to the development of endomyocardial fibrosis and subsequent cardiac failure associated with eosinophilic infiltration.87,96 Another Th2 and eosinophil-associated disease, asthma, is characterized by tissue remodeling consisting of airway epithelial cell transformation to mucous production, smooth muscle hyperplasia and hypertrophy, subepithelial fibrosis, and angiogenesis.54 These histologic and structural changes cause clinical disease manifestations of bronchial hyperreactivity, airway edema and mucous plugging with subsequent airway lumen narrowing. In a subset of patients, this airways obstruction becomes irreversible. While the role of the eosinophil in HES is clear, its role in asthma is beginning to be resolved.48,49
Figure 1. Eosinophil induction of esophageal remodeling and fibrosis in EE: relationships to endoscopic and histologic pathologies.
Eosinophil activation during recruitment to the esophagus occurs in response to eotaxin-3, periostin, IL-5 and interactions with vascular endothelium, epithelium and fibroblasts, leading to their expression of fibrogenic factors such as TGF-β. Eosinophil-expressed TGF-β and granule proteins (MBP, EPO) induce epithelial basal zone hyperplasia, contributing to esophageal thickening and luminal narrowing. Eosinophil-derived TGF-β induces fibroblast activation, with transdifferentiation to myofibroblasts and consequent over-production of ECM leading to subepithelial fibrosis, fixed narrowings/rings, strictures and food impactions. Alternatively, TGF-β expressed by eosinophils or MBP/EPO damaged epithelium itself may induce epithelial to mesenchymal (myofibroblast) transition (EMT) contributing to subepithelial fibrosis. Eosinophil-expressed TGF-β may induce smooth muscle cell hypertrophy/hyperplasia leading to thickening of the esophageal muscularis propria, contributing to dysmotility, dysphagia, transient rings and non-stricture food impactions. Eosinophil expression of VEGF likely supports increased angiogenic responses of vascular endothelium with VCAM-1 activation by IL-13 and TNF-α, contributing to increased eosinophil trafficking, dilated intercellular spaces, esophageal thickening, furrowing, luminal narrowing and non-stricture food impactions.
Murine models of asthma have demonstrated a significant contribution of the eosinophil to disease pathogenesis. Double transgenic mice with airway eotaxin-2 and systemic IL-5 have severe asthma and collagen deposition that is significantly diminished if the animals lack eosinophils.58,70 The best human correlate to absent eosinophils is treatment with anti-IL-5 antibodies. Through the reduction in IL-5, the major eosinophilopoietic stimulus is lost. As compared to control patients, asthmatics treated with a humanized, monoclonal anti-IL-5 antibody demonstrate decreased levels of extracellular matrix proteins such as lumican, tenascin, and pro-collagen III.25 Studies on the role of tissue remodeling in eosinophilic gastrointestinal diseases is beginning to evolve and are delineated below.
CLINICAL IMPLICATIONS OF TISSUE REMODELING
Epithelial Changes in Relation to Fibrosis
The non-keratinized squamous epithelial cells of the esophagus are important mediators of inflammation in EE. When in a Th2 milieu, the esophageal epithelium becomes an immunologically active tissue that expresses chemotactic factors for eosinophils, including eotaxin-3 and periostin.7,10 In addition, both an aeroallergen driven murine model of EE and human biopsy specimens demonstrate the induction of mucin genes such as muc-5 in the epithelium.65
The Th2 cytokine, interleukin-13 (IL-13) is pivotal for airway remodeling. Studies in murine asthma models over-expressing airway epithelial IL-13 demonstrate robust tissue fibrosis and airway mucous production.31,103 IL-13 also appears to be an important inflammatory mediator in EE with IL-13 mRNA levels induced 16-fold in epithelial biopsies from pediatric EE patients as compared with normal controls.8 Esophageal epithelial cells increase their production of eotaxin-3 in a STAT-6 dependent manner when cultured with IL-13, providing a potential positive feedback loop for eosinophil recruitment.8,64 The increased bulk of epithelial cells in EE patients, reflected in the histologic finding of basal zone hyperplasia and the endoscopic finding of epithelial mucosal thickening with luminal narrowing, may further enhance eosinophil recruitment and, hence, eosinophil-mediated esophageal remodeling and fibrosis (Figure 1). Although the eosinophil can be a significant cellular source of IL-13 in other disease states,86 the source of IL-13 in EE remains to be clearly identified.
Clinical Implications of Smooth Muscle Hyperplasia
Dysphagia, in part a reflection of esophageal dysmotility, is a cardinal and distinguishing clinical feature in both pediatric and adult EE.5,52,73,78 Pediatric studies59 have shown that EE patients complain of dysphagia at significantly higher rates than their normal, allergic, or gastroesophageal reflux disease (GERD) counterparts.1,3,5 Whereas young children with EE have vomiting, heartburn, and poor growth, older children and adults often complain of persistent or recurrent dysphagia.69,90 In one adult series, 10% of patients who complained of solid food dysphagia, met histologic criteria (defined as >20 eosinophils per hpf) for EE, and adults with EE are 2.6 times more likely to complain of dysphagia.79
Studies of esophageal dysmotility in EE have occurred exclusively in adult patients. To date, 61 adult patients have had published motility studies.60 Of these patients, 60% had abnormal motility, mainly categorized as spastic or hypercontractile. 60,83 More recently, Hariprasad and colleagues have reported that the longitudinal muscle contractions in EE patients are abnormal while circular muscle contractions are normal, leading to the dissociation of coordinated muscle contraction.39 The eosinophil, with granule products such as major basic protein-1 (MBP-1) that are known to alter smooth muscle contractility through inhibition of M2 muscarinic receptors,50 may contribute to the motor dysfunction of the esophagus, and topical steroid therapy with resultant resolution of eosinophilia is associated with the resolution of esophageal dysmotility.60
Muscular hypertrophy and hyperplasia may exacerbate the contractile abnormalities seen in EE. Although analyses of the smooth muscle in EE are limited to date, endoscopic ultrasound studies in pediatric EE patients have demonstrated thickening through the entire esophagus wall, including the mucosa and submucosa as well as the muscularis propria.28 Esophageal involvement in eosinophilic gastroenteritis is also reportedly associated with smooth muscle hypertrophy and infiltration of the muscularis propria with eosinophils.89 Lastly, an EE model using inhaled Aspergillus demonstrates an increase in muscularis mucosa thickness.65 Taken together, these data demonstrate that aeroallergen driven EE can cause muscle hypertrophy/hyperplasia with eosinophilic inflammation. Resultant discordant hypercontractility, likely driven in part by eosinophil granule products such as MBP-1, could explain the endoscopic finding of transient concentric rings and the clinical symptom of food impaction without strictures (Figure 1).
Pathogenic Mechanisms of Eosinophil – Smooth Muscle Interactions
Evidence for the pathophysiologic participation of eosinophils in smooth muscle hypertrophy and hyperplasia, contraction and hyperreactivity to cholinergic agents comes from in vitro, animal model, and human studies of eosinophil-derived TGF-β and the eosinophil granule cationic proteins, particularly MBP-1, on airway bronchial smooth muscle in asthma. In chronic murine allergic asthma models, eosinophil-deficient mice show significant decreases in airway smooth muscle hyperplasia in association with decreased numbers of TGF-β positive cells, primarily eosinophils, and myofibroblasts in the airways.15,47,70 Likewise, treatment of mild to moderate asthmatics with anti-IL-5 antibody (Mepolizumab™) significantly decreases the numbers of TGF-β positive cells, primarily eosinophils, in the airways, with concomitant decreases in airways remodeling in terms of the deposition of extracellular matrix proteins and numbers of myofibroblasts.25,54
A connection between eosinophils and smooth muscle contractility was initially demonstrated by the ability of eosinophil granule cationic proteins such as MBP-1 to directly induce airway smooth muscle contraction, bronchoconstriction and airways hyperreactivity in rat, 19,20 guinea pig21 and primate38 asthma models. The mechanism by which eosinophils increase (airway) smooth muscle contractility and hyperresponsiveness to cholinergic stimulation was initially highlighted by studies demonstrating that MBP-1 is a potent antagonist of inhibitory M2 muscarinic receptors.51 Studies in the Guinea pig asthma model followed showing that parasympathetic neurons in the airways secrete eotaxin, which recruits eosinophils to the nerves, resulting in eosinophil secretion of MBP and inhibition of M2 muscarinic receptors, leading to airways hyperreactivity.50 Importantly, pre-treatment of allergen-challenged Guinea pigs with a neutralizing antibody to eosinophil MBP was shown to prevent airway hyperresponsiveness by protecting neuronal M2 muscarinic receptors.24 As well, hyperreactivity to histamine in this model was vagally mediated and dependent on MBP.16 Loss of M2 receptor function leads to increased acetylcholine release from cholinergic nerves, providing a mechanism for the vagally-mediated airway hyperreactivity seen in this model.17 Studies also showed that eosinophils localize to the airway nerves of sensitized animals after antigen challenge, and that inhibiting this localization with an antibody to IL-523 or the eosinophil adhesion molecule VLA-4,29,101 or with an eotaxin receptor (CCR3) antagonist,30 prevents airway hyperreactivity subsequent to the loss of inhibitory M2 muscarinic receptor function. Although this mechanism remains to be confirmed in human asthma, loss of function of lung neuronal M2 muscarinic receptors may also occur, and neurons in human airways have been shown to secrete eotaxin30 and to be infiltrated by eosinophils in fatal human asthma.18 The rapid reappearance of both eosinophils and concentric rings (trachealization) in the esophagus of some EE patients within 2–3 days of re-introducing an offending food allergen into their diet is entirely consistent with eosinophil-mediated effects on smooth muscle and/or neurons through these types of mechanisms.37 Whether eosinophils contribute to the discordant hypercontractility of esophageal smooth muscle directly in EE, or whether eosinophil-neuronal cell interactions contribute to the endoscopic finding of trachealization and clinical symptoms of food impaction in the absence of strictures remains to be determined.
Clinical Implications of Fibrosis
Fibrosis is defined histologically by increased collagen content of the subepithelial tissue. Although the exact collagen subtypes that are elevated in esophageal remodeling in EE remain to be elucidated, both adult and pediatric patients have increased subepithelial fibrosis.4,14,95 Fibrosis likely contributes to multiple clinical aspects of EE, including dysphagia symptoms, disease chronicity, and stricture formation.
Among pediatric EE patients with increased subepithelial fibrosis, 42% complain of dysphagia, often with concurrent food impaction.14 Pediatric EE patients with long-standing or stricture-associated disease have increased subepithelial collagen deposition as compared with their normal or gastroesophageal reflux disease counterparts. It is likely that both TGFβ and the eosinophil play important roles in the mechanism of fibrosis in EE since pediatric EE patients have increased numbers of TGFβ1 producing cells and increased activation of the TGFβ signaling pathway as reflected by the increased numbers of cells expressing the nuclear phosphorylated Smad2/3 complex.4 The eosinophil is one cellular source of TGFβ in EE patients and animals that lack eosinophils have diminished subepithelial fibrosis in response to aeroallergen challenge.4,65
Interleukin-5 (IL-5) is a master regulator of eosinophilopoesis, trafficking, survival, and activation. Biopsies from both adult and pediatric EE patients demonstrate elevated IL-5 levels91 and murine allergen driven EE requires IL-5.65 IL-5, together with the eotaxins, activates eosinophils to release their inflammatory products. IL-5 deficient EE mice lack esophageal subepithelial fibrosis65 and patients with both asthma and atopic dermatitis have decreased tissue remodeling of the airways and skin, respectively, when treated with a humanized monoclonal antibody that blocks IL-5.25,76 Adult EE patients treated with anti-IL-5 (Mepolizumab™) have been reported to have decreased dysphagia and improvement in EE associated strictures following therapy in a small open-label study,88 suggesting the role of eosinophils and/or IL-5 in the pathogenesis of human esophageal narrowing. In contrast, a recent placebo-controlled study of anti-IL-5 in adult EE patients showed ~55% decreases in esophageal eosinophils without improvements in clinical disease.92 It is possible that, as suggested by studies with topical corticosteroids and food elimination or elemental diets, esophageal eosinophils may need to be reduced to near normal levels (i.e. essentially no eosinophils) to reverse clinical symptoms, and anti-IL-5 alone may therefore not be sufficient to induce significant clinical and pathologic remissions. Current clinical trials ongoing in EE should contribute to our understanding of the role of IL-5 and the eosinophil in esophageal remodeling and fibrosis.
Pathogenic Mechanisms of Eosinophil-mediated Fibrosis
In addition to EE,28,85 eosinophils are considered a major effector cell of tissue fibrosis35 in a variety of eosinophil-associated allergic diseases and hypereosinophilic syndromes including asthma,53,63 eosinophil myalgia syndrome,100 eosinophilic endomyocardial fibrosis,87 idiopathic pulmonary fibrosis,34 scleroderma,100 and eosinophilic esophagitis. Eosinophils are implicated in fibrogenesis through these clinical disease associations, their elaboration of fibrogenic growth factors such as TGF-β,33,72 PDGF-BB,71, IL-1β36 and secretion of their granule cationic proteins, particularly major basic protein (MBP)84 and eosinophil peroxidase (EPO).74 The association of degranulating eosinophils and deposition of their granule cationic proteins in tissues with pathological fibrosis is a recurrent finding in a broad group of eosinophilic illnesses including EE.59 Eosinophils have been identified as the major TGF-β producing cell in the lungs of asthmatics63 and in the esophagus in pediatric EE.4.
Both human and animal model studies provide compelling evidence for eosinophils as effectors of tissue remodeling and fibrosis. Reduction in bronchial eosinophils induced by treatment of asthmatics with anti-IL-5 antibody (Mepolizumab™) decreases the expression of ECM proteins in the reticular basement membrane,25 and anti-IL-5 similarly decreases both eosinophils and deposition of ECM proteins in allergen-induced late-phase skin reactions in atopic subjects.76 Direct evidence for eosinophil induction of remodeling and fibrosis comes from studies in eosinophil-deficient mice, demonstrating their essential role in the development of airway remodeling, including mucus (goblet) cell metaplasia, smooth muscle cell hyperplasia, and subepithelial fibrosis.15,47,58
Multiple growth factors and cytokines expressed by eosinophils56 are implicated in tissue remodeling and fibrosis. TGF-β, the most widely studied and potently fibrogenic, regulates the expression of the pro-fibrogenic cytokine IL-6, the myofibroblast marker α-smooth muscle actin (α-SMA), and other ECM proteins such as the collagens. TGF-β expression is correlated with bronchial airway fibrosis and asthma severity,62, and its over-expression in the lung in rodent animal models induces pulmonary fibrosis.33
Eosinophil-fibroblast interactions have been implicated in the generation of subepithelial fibrosis and airway remodeling characteristic of human asthma in murine allergic asthma models.42,43 However, mechanistic assessments of eosinophil-fibroblast interactions that may lead to fibrosis are still limited. Ackerman and colleagues reported that eosinophil granule MBP synergizes with TGF-β or IL-1β primed lung fibroblasts to induce significant increases in gene transcription and secretion of the IL-6 family of inflammatory and fibrogenic cytokines, including IL-6 and IL-11.84 TGF-β induced fibroblast secretion of IL-6 is implicated in the overproduction of collagens, tissue inhibitor of metalloproteinases (TIMPs), and glycosaminoglycans in fibrogenesis.22,99 Eosinophil-lung fibroblast co-culture in the presence of IL-5 induces fibroblasts to transdifferentiate into myofibroblasts with increased expression of α-SMA and ECM proteins.77 Eosinophils may indirectly impact fibroblast phenotype and fibrogenesis through activation of the epithelial-mesenchymal trophic unit,75 e.g. through secretion of MBP and EPO (Figure 1).74 Alternatively, eosinophils may induce fibrogenesis through TGF-β induction of the epithelial to mesenchymal transition (EMT) as shown to occur in the kidney102 and lung.55
Subepithelial fibrosis, a component of airway remodeling in asthma pathogenesis, is initiated by insults that include Th2-mediated allergic responses. Eosinophilic inflammation is thought to drive the differentiation of airway fibroblasts to myofibroblasts as characterized by the expression of myofibroblast-specific markers such as α-SMA, the deposition of ECM proteins such as collagens, fibronectin, and other ECM constituents such as tenascin and lumican.25,75 Eosinophils recruited to the lung in asthma likely interact with fibroblasts beneath the reticular basement membrane, become activated to release their fibrogenic growth factors such as TGF-β, driving fibroblasts to differentiate into myofibroblasts, which then deposit pathologic amounts of collagens and other ECM proteins contributing to airway subepithelial fibrosis.54 A report showing correlations between pulmonary expression of eotaxin-1, expression of eotaxin-1 receptor (CCR3), TGF- and pulmonary fibrosis in a bleomycin mouse model β1 support this general mechanism.46
Studies of eosinophil-mediated tissue remodeling and fibrosis in EE are still limited to date, principally due to the difficulties inherent in obtaining sufficient biopsies containing esophageal lamina propria below the stiffened hyperplastic epithelium. For this reason, evidence for progressive remodeling and fibrosis of the esophagus has been derived principally from endoscopic and radiologic features of the disease.95 However, the recent study by Aceves et al demonstrated that pediatric EE esophageal biopsies showed increased levels of subepithelial fibrosis and increased expression of TGF-β1 by eosinophils and its signaling molecule phospho-SMAD2/3 compared with gastroesophageal reflux disease and normal controls.4 Beyond this report, the mechanisms regulating esophageal remodeling and fibrosis in chronic EE have not been systematically studied to define the changes in epithelial cell and fibroblast phenotype, the role of eosinophil-fibroblast interactions, or the contribution of EMT to this process.
Finally, recent genome-wide expression profiling studies of EE esophageal tissue that identified increased expression of eotaxin-3 as the principal mediator of eosinophil recruitment,11 interestingly did not identify many genes known to participate in tissue remodeling and fibrosis, perhaps because the biopsy specimens analyzed were sufficiently superficial to include mainly hyperplastic epithelium and not sub-epithelial fibrotic tissue. However, one of the identified genes, periostin, expressed predominantly in collagen-rich fibrous connective tissues subject to mechanical stresses and in wound healing, has been reported to participate in the development of subepithelial fibrosis in bronchial asthma downstream of the IL-4 and IL-13 signals.97 Primary esophageal fibroblasts have recently been shown to release periostin when cultured with IL-13 and TGFβ.7 Periostin, found mainly in the vascular papillae (projections of subepithelial lamina propria into the epithelium) in the esophagus, could contribute to eosinophil trafficking by increasing eosinophil adhesion to fibronectin.7 Therefore, eosinophil-derived TGFβ, through induction of fibroblast periostin expression might provide an amplification loop for eosinophilic inflammation and its consequent induction of the remodeling and fibrosis characteristic of EE.
Clinical Implications of Angiogenesis
Angiogenesis, the formation of new blood vessels, has a number of implications in inflammatory diseases such as EE. Increased vasculature increases the density of conduits for inflammatory cell trafficking, thus propagating inflammation. In addition, interleukins and histamine can modulate vascular permeability and lead to enhanced tissue edema. One of the histologic feature of EE is dilated intercellular spaces,82 which may be a reflection of increased tissue edema and, ultimately, increased mucosal and submucosal thickness when compared to control patients.27,66 Linear furrowing, caused by a thickened esophagus folding on itself, could be an endoscopic finding related to esophageal edema. Ultimately, an edematous esophagus will decrease luminal size and predispose to clinical complications such as food impaction.
Pediatric EE patients have an increased vascular density as compared to their age-matched counterparts with either a normal esophagus or esophagitis associated with gastroesophageal reflux disease.4 Blood vessels from EE patients also demonstrate an activated endothelial phenotype with increased expression of vascular cell adhesion molecule-1 (VCAM-1).4 VCAM-1 interactions with its the cognate ligand very late activation-4 molecule (VLA-4) allows leukocytes, particularly eosinophils, to selectively traffic to Th2 activated tissues. Interestingly, IL-13 and TNFα in induce vascular endothelial VCAM-1 expression, and both are present at elevated levels in the esophageal mucosa in patients EE.8,93
Recent EE animal model studies demonstrate that IL-13 also increases vessel density in EE in a manner dependent upon IL-13 interaction with IL-13Rα2. 104 Mice with increased expression of airway Clara cell CC-10-driven IL-13 have increased esophageal vascular density and increased esophageal circumference,104 consistent with the linear furrowing and luminal narrowing seen in the human disease; this IL-13 effect is dependent on an intact IL-13Rα.104 Since pediatric EE patients demonstrate an elevated expression of IL-13 mRNA,8 IL-13 is also implicated in the generation of new blood vessels in the human disease. Animal models have also shown that the induction of EE via IL-13 is dependent on both IL-5 and Signal Transducer Activation Transcription-6 (STAT-6),8,64 and thus therapy with either IL-5 or IL-13 blocking agents9,88 may remediate not only esophageal fibrosis, but also the increased angiogenesis associated with EE.4
Pathogenic Mechanisms of Eosinophil-Induced Angiogenesis
Eosinophils express a number of angiogenic factors, not the least of which is vascular endothelial growth factor (VEGF),40 and they are therefore implicated in the increased angiogenesis seen in many eosinophil-associated allergic inflammatory diseases such as asthma.2,12,81 The expression of VEGF, basic fibroblast growth factor (bFGF), angiogenin and the VEGF receptors (flt-1 and flk-1) is increased asthmatic airways, and the numbers of cells including eosinophils expressing these factors and receptors is correlated with measurements of increased lung vascularity.41,44 As well, increased expression of VEGF has been demonstrated in the induced sputum of asthmatic children, supporting the concept that it participates in the pathophysiology of augmented angiogenesis seen bronchial asthma.45
Eosinophils, through expression of VEGF, have been shown experimentally by Puxeddu, Levi-Schaffer and colleagues to induce new vessel formation chick embryo models.81 Blood eosinophil extracts were found to induce rat aortic endothelial cell proliferation in vitro, rat aorta sprouting ex vivo, and angiogenesis in the chick embryo chorioallantoic membrane in vivo.80 These pro-angiogenic effects were mediated principally by eosinophil-expressed VEGF, since neutralizing antibodies to VEGF could significantly inhibit them.80 As well, intact eosinophils were found to induce VEGF mRNA expression and increased VEGF receptor density expression on endothelial cells, to enhance endothelial cell proliferation and to augment angiogenic responses in aorta rings and chorioallantoic membranes.80 Thus, eosinophils are capable of inducing angiogenesis, in part by their secretion of pre-formed VEGF.81 Whether eosinophils, through their expression of angiogenic factors such as VEGF (Figure 1), contribute to increased vascularization and activated VCAM-1 positive vascular endothelium in the esophagus, as shown by one of us in children with EE,4 remains to be determined.
Therapeutics and Tissue Remodeling
Currently, there are no large clinical trials that demonstrate the efficacy of EE therapies on reducing tissue remodeling and fibrosis. One case report demonstrates that inhaled budesonide in a patient with stricture associated EE and concurrent asthma was associated with decreased subepithelial fibrosis following 2 months of therapy.61 Our current observations demonstrate that a subset of children with EE will have reversal of deep tissue remodeling following topical esophageal corticosteroid therapy (Aceves and Broide, unpublished). Successful EE therapy with swallowed fluticasone results in the normalization of an EE-specific transcriptome, including IL-13.8 As such, topical corticosteroids could diminish esophageal remodeling by their effects on the esophageal levels of interleukins such as IL-13. As noted above, anti-IL-5 therapy has also been reported to result in the improvement of EE-associated strictures/esophageal narrowing and improvement of basal zone hyperplasia and eosinophilic inflammation in an open-label trial in a small number of patients.88 In contrast, a second placebo-controlled study in adults with severe EE concluded that anti-IL-5 therapy, though effective in reducing the numbers of esophageal eosinophils on average by ~55% (but not less than 10 eosinophils/hpf), showed little efficacy in reducing patient’s clinical symptom scores.92 Whether this is sufficient to result in histologic remission of the remodeling and fibrosis of the subepithelial tissue in EE remains to be determined. Of note, this finding is reminiscent of the first studies of anti-IL-5 therapy in asthmatic subjects, in which reductions of pulmonary tissue eosinophils by ~55% did not significantly impact pulmonary function or airway hyperreactivity,26,57 but did significantly reduce the deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics.25 These results suggest that an additional therapy, e.g. one targeting eosinophil recruitment through antagonism of eotaxin-3 or its receptor on eosinophils (CCR3), may need to be combined with anti-IL-5 to achieve a therapeutic reduction in eosinophils with complete endoscopic and histologic remission of esophageal remodeling in EE.
CONCLUSIONS AND FUTURE DIRECTIONS
The central role of the eosinophil in esophageal remodeling and subepithelial fibrosis, and the relationships of this remodeling to the clinical signs, symptoms and pathogenesis of EE, are now beginning to be defined at the cellular and molecular levels, but clearly warrant further study. However, the natural history of EE, the time frame from disease onset to the development of epithelial hyperplasia, thickening of the muscularis propria and esophageal wall, and subepithelial deposition of collagens and other ECM constituents that contribute to esophageal remodeling and fibrosis in EE are still not well defined. Also unclear are the relationships of esophageal remodeling to disease severity and duration, and to what extent esophageal remodeling and fibrosis are reversible with treatments that significantly reduce tissue eosinophils in the esophagus. Better understanding of the mechanisms by which eosinophils promote tissue remodeling and fibrogenesis in the esophagus should contribute to the development of novel therapeutic approaches for blocking eosinophil recruitment to the esophagus and/or reversing the debilitating consequences of esophageal remodeling in EE, and the tissue remodeling and fibrosis seen in many other eosinophil-associated allergic diseases and hypereosinophilic syndromes.
Footnotes
This work was supported by grants from the NIH – R21AI079925 (SJA), an American Gastroenterological Association (AGA) Translational Research Award (SJA), an American Partnership for Eosinophilic Diseases (APFED) HOPE Research Award (SSA), an American Academy of Allergy, Asthma, Immunology Junior Women in Allergy grant (SSA), and a gift from Campaign Urging Research on Eosinophilic Diseases (CURED) (SJA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Aceves SS, Arii B, Dohil M, et al. Prospective analysis of an abdominal symptom scoring system tool’s efficacy in the clinical distinction of pediatric eosinophilic esophagitis from gastroesophageal reflux disease. J Allergy Clin Immunol. 2008;121:S70. [Google Scholar]
- 2.Aceves SS, Broide DH. Airway Fibrosis and Angiogenesis due to Eosinophil Trafficking in Chronic Asthma. Curr Mol Med. 2008;8:350. doi: 10.2174/156652408785161023. [DOI] [PubMed] [Google Scholar]
- 3.Aceves SS, Furuta GT, Spechler SJ. Integrated approach to treatment of children and adults with eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:195. doi: 10.1016/j.giec.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Aceves SS, Newbury RO, Dohil R, et al. Distinguishing eosinophilic esophagitis in pediatric patients: clinical, endoscopic, and histologic features of an emerging disorder. J Clin Gastroenterol. 2007;41:252. doi: 10.1097/01.mcg.0000212639.52359.f1. [DOI] [PubMed] [Google Scholar]
- 6.Assa’ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119:731. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard C, Mingler MK, McBride M, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard C, Mishra A, Saito-Akei H, et al. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by anti-human-interleukin-13 antibody (CAT-354) Clin Exp Allergy. 2005;35:1096. doi: 10.1111/j.1365-2222.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol. 2008;121:560. doi: 10.1016/j.jaci.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chehade M, Sampson HA. Epidemiology and etiology of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:33. doi: 10.1016/j.giec.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Chehade M, Sampson HA, Morotti RA, et al. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 15.Cho JY, Miller M, Baek KJ, et al. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costello RW, Evans CM, Yost BL, et al. Antigen-induced hyperreactivity to histamine: role of the vagus nerves and eosinophils. Am J Physiol. 1999;276:L709. doi: 10.1152/ajplung.1999.276.5.L709. [DOI] [PubMed] [Google Scholar]
- 17.Costello RW, Jacoby DB, Gleich GJ, et al. Eosinophils and airway nerves in asthma. Histol Histopathol. 2000;15:861. doi: 10.14670/HH-15.861. [DOI] [PubMed] [Google Scholar]
- 18.Costello RW, Schofield BH, Kephart GM, et al. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol. 1997;273:L93. doi: 10.1152/ajplung.1997.273.1.L93. [DOI] [PubMed] [Google Scholar]
- 19.Coyle AJ, Ackerman SJ, Burch R, et al. Human eosinophil-granule major basic protein and synthetic polycations induce airway hyperresponsiveness in vivo dependent on bradykinin generation. J Clin Invest. 1995;95:1735. doi: 10.1172/JCI117850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coyle AJ, Ackerman SJ, Irvin CG. Cationic proteins induce airway hyperresponsiveness dependent on charge interactions. Am Rev Respir Dis. 1993;147:896. doi: 10.1164/ajrccm/147.4.896. [DOI] [PubMed] [Google Scholar]
- 21.Desai SN, Van G, Robson J, et al. Human eosinophil major basic protein augments bronchoconstriction induced by intravenous agonists in guinea pigs. Agents Actions. 1993;39 doi: 10.1007/BF01972744. Spec No. C132. [DOI] [PubMed] [Google Scholar]
- 22.Eickelberg O, Pansky A, Mussmann R, et al. Transforming growth factor-beta1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J Biol Chem. 1999;274:12933. doi: 10.1074/jbc.274.18.12933. [DOI] [PubMed] [Google Scholar]
- 23.Elbon CL, Jacoby DB, Fryer AD. Pretreatment with an antibody to interleukin-5 prevents loss of pulmonary M2 muscarinic receptor function in antigen-challenged guinea pigs. Am J Respir Cell Mol Biol. 1995;12:320. doi: 10.1165/ajrcmb.12.3.7873198. [DOI] [PubMed] [Google Scholar]
- 24.Evans CM, Fryer AD, Jacoby DB, et al. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest. 1997;100:2254. doi: 10.1172/JCI119763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flood-Page PT, Menzies-Gow AN, Kay AB, et al. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 27.Fox VL. Eosinophilic esophagitis: endoscopic findings. Gastrointest Endosc Clin N Am. 2008;18:45. doi: 10.1016/j.giec.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Fox VL, Nurko S, Teitelbaum JE, et al. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointest Endosc. 2003;57:30. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 29.Fryer AD, Costello RW, Yost BL, et al. Antibody to VLA-4, but not to L-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J Clin Invest. 1997;99:2036. doi: 10.1172/JCI119372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fryer AD, Stein LH, Nie Z, et al. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest. 2006;116:228. doi: 10.1172/JCI25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulkerson PC, Fischetti CA, Rothenberg ME. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. Am J Pathol. 2006;169:2117. doi: 10.2353/ajpath.2006.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Gauldie J, Sime PJ, Xing Z, et al. Transforming growth factor-beta gene transfer to the lung induces myofibroblast presence and pulmonary fibrosis. Curr Top Pathol. 1999;93:35. doi: 10.1007/978-3-642-58456-5_5. [DOI] [PubMed] [Google Scholar]
- 34.Gharaee-Kermani M, Phan SH. Molecular mechanisms of and possible treatment strategies for idiopathic pulmonary fibrosis. Curr Pharm Des. 2005;11:3943. doi: 10.2174/138161205774580561. [DOI] [PubMed] [Google Scholar]
- 35.Gharaee-Kermani M, Phan SH. The role of eosinophils in pulmonary fibrosis (Review) Int J Mol Med. 1998;1:43. [PubMed] [Google Scholar]
- 36.Gomes I, Mathur SK, Espenshade BM, et al. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005;116:796. doi: 10.1016/j.jaci.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 37.Gonsalves N, Yang G-Y, Doerfler B, et al. A prospective clinical trial of six food elimination diet and reintroduction of causative agents in adults with eosinophilic esophagitis. Gastroenterology. 2008;134:A104. [Google Scholar]
- 38.Gundel RH, Letts LG, Gleich GJ. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest. 1991;87:1470. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hariprasad R, Korsapati Dohil R, et al. Normal circular muscle but dysfunctional longitudinal muscle in eosinophilic esophagitis. Gastroenterology. 2008;134A [Google Scholar]
- 40.Horiuchi T, Weller PF. Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol. 1997;17:70. doi: 10.1165/ajrcmb.17.1.2796. [DOI] [PubMed] [Google Scholar]
- 41.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001;107:1034. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- 42.Hoshino M, Nakamura Y, Sim J, et al. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998;102:783. doi: 10.1016/s0091-6749(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 43.Hoshino M, Nakamura Y, Sim JJ. Expression of growth factors and remodelling of the airway wall in bronchial asthma. Thorax. 1998;53:21. doi: 10.1136/thx.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107:295. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 45.Hossny E, El-Awady H, Bakr S, et al. Vascular endothelial growth factor overexpression in induced sputum of children with bronchial asthma. Pediatr Allergy Immunol. 2008 doi: 10.1111/j.1399-3038.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 46.Huaux F, Gharaee-Kermani M, Liu T, et al. Role of Eotaxin-1 (CCL11) and CC chemokine receptor 3 (CCR3) in bleomycin-induced lung injury and fibrosis. Am J Pathol. 2005;167:1485. doi: 10.1016/S0002-9440(10)61235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsen EA, Ochkur SI, Lee NA, et al. Eosinophils and asthma. Curr Allergy Asthma Rep. 2007;7:18. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- 49.Jacobsen EA, Ochkur SI, Pero RS, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacoby DB, Costello RM, Fryer AD. Eosinophil recruitment to the airway nerves. J Allergy Clin Immunol. 2001;107:211. doi: 10.1067/mai.2001.112940. [DOI] [PubMed] [Google Scholar]
- 51.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993;91:1314. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kapel RC, Miller JK, Torres C, et al. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316. doi: 10.1053/j.gastro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med. 2005;11:148. doi: 10.1016/j.molmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25:477. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- 57.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 58.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 59.Liacouras CA, Bonis P, Putnam PE, et al. Summary of the first international gastrointestinal research sypmosium. J Pediatr Gastroenterol Nutr. 2007;45:370. doi: 10.1097/MPG.0b013e318142b4f8. [DOI] [PubMed] [Google Scholar]
- 60.Lucendo AJ, Pascual-Turrion JM, Navarro M, et al. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39:765. doi: 10.1055/s-2007-966738. [DOI] [PubMed] [Google Scholar]
- 61.Maples KM, Henderson SC, Graham M, et al. Treatment of eosinophilic esophagitis with inhaled budesonide in a 7-year-old boy with concomitant persistent asthma: resolution of esophageal submucosal fibrosis and eosinophilic infiltration. Ann Allergy Asthma Immunol. 2007;99:572. doi: 10.1016/S1081-1206(10)60390-0. [DOI] [PubMed] [Google Scholar]
- 62.Minshall EM, Cameron L, Lavigne F, et al. Eotaxin mRNA and protein expression in chronic sinusitis and allergen- induced nasal responses in seasonal allergic rhinitis. Am J Respir Cell Mol Biol. 1997;17:683. doi: 10.1165/ajrcmb.17.6.2865. [DOI] [PubMed] [Google Scholar]
- 63.Minshall EM, Leung DY, Martin RJ, et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 64.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Mishra A, Wang M, Pemmaraju VR, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mueller S, Aigner T, Neureiter D, et al. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: a retrospective and comparative study on pathological biopsy. J Clin Pathol. 2006;59:1175. doi: 10.1136/jcp.2005.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noel RJ, Putnam PE, Collins MH, et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2:568. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- 68.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 69.Noel RJ, Tipnis NA. Eosinophilic esophagitis -- a mimic of GERD. Int J Pediatr Otorhinolaryngol. 2006;70:1147. doi: 10.1016/j.ijporl.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 70.Ochkur SI, Jacobsen EA, Protheroe CA, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 71.Ohno I, Nitta Y, Yamauchi K, et al. Eosinophils as a potential source of platelet-derived growth factor B-chain (PDGF-B) in nasal polyposis and bronchial asthma. Am J Respir Cell Mol Biol. 1995;13:639. doi: 10.1165/ajrcmb.13.6.7576701. [DOI] [PubMed] [Google Scholar]
- 72.Ohno I, Nitta Y, Yamauchi K, et al. Transforming growth factor beta 1 (TGF beta 1) gene expression by eosinophils in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1996;15:404. doi: 10.1165/ajrcmb.15.3.8810646. [DOI] [PubMed] [Google Scholar]
- 73.Parfitt JR, Gregor JC, Suskin NG, et al. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19:90. doi: 10.1038/modpathol.3800498. [DOI] [PubMed] [Google Scholar]
- 74.Pegorier S, Wagner LA, Gleich GJ, et al. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol. 2006;177:4861. doi: 10.4049/jimmunol.177.7.4861. [DOI] [PubMed] [Google Scholar]
- 75.Phipps S, Benyahia F, Ou TT, et al. Acute allergen-induced airway remodeling in atopic asthma. Am J Respir Cell Mol Biol. 2004;31:626. doi: 10.1165/rcmb.2004-0193OC. [DOI] [PubMed] [Google Scholar]
- 76.Phipps S, Flood-Page P, Menzies-Gow A, et al. Intravenous anti-IL-5 monoclonal antibody reduces eosinophils and tenascin deposition in allergen-challenged human atopic skin. J Invest Dermatol. 2004;122:1406. doi: 10.1111/j.0022-202X.2004.22619.x. [DOI] [PubMed] [Google Scholar]
- 77.Phipps S, Ying S, Wangoo A, et al. The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002;169:4604. doi: 10.4049/jimmunol.169.8.4604. [DOI] [PubMed] [Google Scholar]
- 78.Potter JW, Saeian K, Staff D, et al. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc. 2004;59:355. doi: 10.1016/s0016-5107(03)02713-5. [DOI] [PubMed] [Google Scholar]
- 79.Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol. 2007;102:2627. doi: 10.1111/j.1572-0241.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 80.Puxeddu I, Alian A, Piliponsky AM, et al. Human peripheral blood eosinophils induce angiogenesis. Int J Biochem Cell Biol. 2005;37:628. doi: 10.1016/j.biocel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Puxeddu I, Ribatti D, Crivellato E, et al. Mast cells and eosinophils: a novel link between inflammation and angiogenesis in allergic diseases. J Allergy Clin Immunol. 2005;116:531. doi: 10.1016/j.jaci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Ravelli AM, Villanacci V, Ruzzenenti N, et al. Dilated intercellular spaces: a major morphological feature of esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:510. doi: 10.1097/01.mpg.0000215312.78664.b9. [DOI] [PubMed] [Google Scholar]
- 83.Remedios M, Campbell C, Jones DM, et al. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3. doi: 10.1016/j.gie.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 84.Rochester CL, Ackerman SJ, Zheng T, et al. Eosinophil-fibroblast interactions. Granule major basic protein interacts with IL-1 and transforming growth factor-beta in the stimulation of lung fibroblast IL-6-type cytokine production. J Immunol. 1996;156:4449. [PubMed] [Google Scholar]
- 85.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID) J Allergy Clin Immunol. 2004;113:11. doi: 10.1016/j.jaci.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 86.Schmid-Grendelmeier P, Altznauer F, Fischer B, et al. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002;169:1021. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- 87.Spry CJ. The pathogenesis of endomyocardial fibrosis: the role of the eosinophil. Springer Semin Immunopathol. 1989;11:471. doi: 10.1007/BF00201883. [DOI] [PubMed] [Google Scholar]
- 88.Stein ML, Collins MH, Villanueva JM, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 89.Stevoff C, Rao S, Parsons W, et al. EUS and histopathologic correlates in eosinophilic esophagitis. Gastrointest Endosc. 2001;54:373. doi: 10.1067/mge.2001.116569. [DOI] [PubMed] [Google Scholar]
- 90.Straumann A. The natural history and complications of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:99. doi: 10.1016/j.giec.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 91.Straumann A, Bauer M, Fischer B, et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 92.Straumann A, Conus S, Kita H, et al. Mepolizumab, a humanized antibody to IL-5, for severe eosinophilic esophagitis in adults: A randomized, placebo-controlled double-blind trial. J Allergy Clin Immunol. 2008;121:S44. [Google Scholar]
- 93.Straumann A, Kristl J, Conus S, et al. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm Bowel Dis. 2005;11:720. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- 94.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115:418. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 95.Straumann A, Spichtin HP, Grize L, et al. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125:1660. doi: 10.1053/j.gastro.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 96.Tai PC, Ackerman SJ, Spry CJ, et al. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet. 1987;1:643. doi: 10.1016/s0140-6736(87)90412-0. [DOI] [PubMed] [Google Scholar]
- 97.Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 98.Vanderheyden AD, Petras RE, DeYoung BR, et al. Emerging eosinophilic (allergic) esophagitis: increased incidence or increased recognition? Arch Pathol Lab Med. 2007;131:777. doi: 10.5858/2007-131-777-EEAEII. [DOI] [PubMed] [Google Scholar]
- 99.Varga J, Jimenez SA. Modulation of collagen gene expression: its relation to fibrosis in systemic sclerosis and other disorders. Ann Int Med. 1995;122:60. doi: 10.7326/0003-4819-122-1-199501010-00010. [DOI] [PubMed] [Google Scholar]
- 100.Varga J, Kahari VM. Eosinophilia-myalgia syndrome, eosinophilic fasciitis, and related fibrosing disorders. Curr Opin Rheumatol. 1997;9:562. doi: 10.1097/00002281-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 101.Yost BL, Gleich GJ, Jacoby DB, et al. The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L627. doi: 10.1152/ajplung.00377.2004. [DOI] [PubMed] [Google Scholar]
- 102.Zeisberg M, Kalluri R. Fibroblasts emerge via epithelial-mesenchymal transition in chronic kidney fibrosis. Front Biosci. 2008;13:6991. doi: 10.2741/3204. [DOI] [PubMed] [Google Scholar]
- 103.Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zuo L, Mingler M, Blanchard C, et al. IL-13 transgene induced experimental eosinophilic esophagitis is associated with increased esophageal circumference and extensive angiogenesis. J Allergy Clin Immunol. 2008;121:S72. [Google Scholar]