Abstract
Background & Aims
The immuno-inhibitory receptor Programmed Death-1 (PD-1) is upregulated on dysfunctional virus-specific CD8 T-cells during chronic viral infections and blockade of PD-1:PD-ligand (PD-L) interactions can restore their function. As hepatitis C virus (HCV) persists in the liver with immune-mediated disease pathogenesis, we examined the role of PD1/PD-L pathway in antigen-specific CD8 T-cell dysfunction in the liver and blood of HCV-infected patients.
Methods
PD-1 expression and function of circulating CD8 T-cells specific for HCV, EBV and Flu were examined ex vivo and following antigenic stimulation in vitro in patients with acute, chronic and resolved HCV infection using class I tetramers and flow cytometry. Intrahepatic CD8 T-cells were examined from liver explants of chronically HCV-infected transplant recipients.
Results
Intrahepatic HCV-specific CD8 T-cells from chronically HCV-infected patients were highly PD-1-positive, profoundly dysfunctional and unexpectedly refractory to PD-1:PD-L blockade, contrasting from circulating PD-1-intermediate HCV-specific CD8 T-cells with responsiveness to PD-1:PD-L blockade. This intrahepatic functional impairment was HCV-specific and directly associated with the level of PD-1 expression. Highly PD-1-positive intrahepatic CD8 T-cells were more phenotypically exhausted with increased cytotoxic T-lymphocyte antigen 4 (CTLA-4) and reduced CD28 and CD127 expression, suggesting that active antigen-specific stimulation in the liver induces a profound functional exhaustion not reversible by PD-1:PD-L blockade alone.
Conclusion
HCV-specific CD8 T-cell dysfunction and responsiveness to PD-1:PD-L blockade are defined by their PD-1 expression and compartmentalization. These findings provide new and clinically relevant insight to differential antigen-specific CD8 T-cell exhaustion and their functional restoration.
Keywords: Cellular immunity, immune exhaustion, immune tolerance, immune regulation, effector T-cell dysfunction, co-stimulation, PD-1, immunotherapy, viral pathogenesis, intrahepatic T-cell response, vaccine
INTRODUCTION
Programmed Death-1 (PD-1) is an inhibitory co-stimulatory receptor with a key role in peripheral tolerance and immune regulation1,2. Binding of PD-1 to its ligands PD-L1 and PD-L2 activates a critical immunosuppressive pathway. Sustained PD-1 expression in antigen-specific CD8 T-cells is associated with impaired effector function in acute and chronic viral infections, suggesting that it is a marker of T-cell exhaustion3. Importantly, PD-1:PD-L blockade by anti-PD-L1 can augment virus-specific effector CD8 T-cell function and suppress viral replication in mice chronically infected with lymphocytic choriomeningitis virus (LCMV)4. In HIV-infected patients, PD-1 expression on virus-specific T-cells is associated with clinical and virological outcomes while anti-PD-L1 enhances HIV-specific T-cell function in vitro3,5,6. Similarly, virus-specific CD8 T-cells from patients infected with hepatitis B virus (HBV) or hepatitis C virus (HCV) exhibit improved proliferation and cytokine production in vitro with anti-PD-L17–11. These findings raise the hope that modulation of PD-1/PD-L interactions may provide therapeutic benefit by rejuvenating exhausted T-cells3.
However, PD-1:PD-L interactions also provide an important negative signal that limits inflammatory responses. In this respect, PD-1:PD-L blockade might have serious inflammatory consequences, particularly when vital organs such as the liver are involved. For example, severe hepatitis (albeit transient) occurred in adenovirus-infected PD-1-null mice12 and PD-1:PD-L1 blockade precipitated autoimmune insulitis in nonobese diabetic mice13. As CD8 T-cells play an important role in HCV pathogenesis and HCV replicates primarily in the liver14–16, PD-1:PD-L signaling in antiviral CD8 T-cells in the liver might be pivotal for immune-mediated control of HCV and the disease.
In this study, we confirm that PD-1 expression in HCV-specific CD8 T-cells directly correlates with their functional impairment and that PD-1:PD-L blockade can restore the effector function of peripheral HCV-specific CD8 T-cells. However, in the liver, HCV-specific (but not influenza-specific) CD8 T-cells were highly PD-1+, profoundly dysfunctional and poorly responsive to PD-1:PD-L blockade, suggesting a differential level of HCV-specific CD8 T-cell exhaustion in HCV-infected patients that is defined by their compartmentalization and PD-1 expression.
MATERIALS & METHODS
Study subjects
Patients were recruited at the Philadelphia Veterans Affairs Medical Center (PVAMC) and the Hospital of the University of Pennsylvania with informed consent approved by the Institutional Review Boards. They included 10 patients with acute hepatitis C (Group A) diagnosed by acute serum alanine amino-transferase (sALT) elevation with HCV-seroconversion and/or viremic fluctuations greater than 10-fold without prior liver disease17; 27 patients with chronic hepatitis C (Group C) including 16 cirrhotic patients undergoing liver transplantation; 8 HCV-seropositive but RNA-negative resolvers (Group R) without prior antiviral therapy, and 12 healthy HCV-seronegative controls (Group H). Patients with acute hepatitis C were examined within 24 weeks of clinical presentation (median 4.5 weeks, range 1–24) with sALT elevation (median 281 IU/ml, range 40–1517) and viremia. Spontaneous HCV clearance occurred in 1 subject whereas 6 began antiviral therapy with sustained virological response in one thus far. HCV viremia was quantified by quantitative RT-PCR by Roche COBAS or TaqMan assays (Roche Diagnostics, Indianapolis, IN). The patient characteristics are shown in Table 1.
Table 1.
Patient Groups
| Patient Groups | Sex (M:F) | HLAA2+ | Genotype 1 | Age (y) | HCV RNA (IU/ml) | ALT (IU/ml) | Albumin (g/dl) | Bilirubin (mg/dl) | Platelets (×1000/mm 3) |
|---|---|---|---|---|---|---|---|---|---|
| Median values | |||||||||
| A. Acute (n=10) | 7:3 | 7/10 | 7/10 | 35 | 4,000,000 | 281 | 4.4 | 1.8 | 224 |
| C. Chronic | |||||||||
| Stable (n=11) | 11:0 | 11/11 | 9/11 | 55 | 850,000 | 36 | 4.3 | 0.6 | 244 |
| Transplanted (n=16) | 14:2 | 8/16 | 16/16 | 53 | 415,500 | 53 | 2.4 | 2.8 | 93 |
| R. Resolved (n=8) | 8:0 | 8/8 | - | 55 | 0 | 23 | 4.5 | 0.6 | 232 |
| H. Healthy controls (n=12) | 9:3 | 4/12 | - | 50 | 0 | 28 | 4.4 | 0.6 | 250 |
Fluorescent antibodies and reagents
All monoclonal antibodies (mAbs) were purchased from BD Bioscience (San Jose, CA) except for anti-CD27, anti-CD28 and anti-granzyme B from eBioscience (San Diego, CA). Dead cells were excluded with 7-AAD. PD-1 expression in all subjects was examined using FITC-labeled anti-human CD279 (PD-1) (clone M1H4, BD Bioscience). Comparison with PE-labeled anti-human CD279 (PD-1) clone EH12 from the Dana Farber Institute5, 8 showed that anti-CD279-FITC (clone M1H4) used in our study detects highly PD-1+ cells (including tetramer+ cells) whereas PE-labeled EH12 mAb also identifies PD-1-intermediate cells (Supplementary Fig. 1A/B). Anti-PD-L1 and anti-PD-L2 mAbs from the Dana Farber were used for functional blocking as previously described8,18.
Peptides and HLA class I tetramers
Fluorochrome-labeled peptide-HLA-A2 tetramers were used as previously described19,20. They included HCV NS3 1073 (CINGVCWTV), NS3 1406 (KLVALGINAV) and NS5B 2594 (ALYDVVSKL); Flu matrix (GILGFVFTL) and EBV BMLF1 (GLCTLVAML)21–23. Overlapping 15mers were synthesized as previously described17,24.
Isolation of Peripheral Blood Mononuclear Cells (PBMC) and Liver Infiltrating Lymphocytes (LIL)
PBMC were isolated by Ficoll-Histopaque (Sigma Chemical Co., St Louis, MO) density centrifugation24,25. LIL were isolated in a protocol modified from Heydtmann et al26. Briefly, explant liver tissue was processed within 24 hours of explant (usually 1–3 hours). Tissue was diced into 5mm3 pieces and incubated at 37°C for 30 min with 1mg/ml collagenase (Type 1a; Roche Molecular) and 1µg/ml DNase (Sigma Aldrich). T-cell marker expression including PD-1 was maintained after 30 minutes of collagenase digestion (data not shown). Digested liver samples were washed in RPMI, mechanically dissociated by the Seward Stomacher 400 Lab Blender (Brinkman Instruments, Westbury, NY) for 5 minutes and passed through a 70µm nylon mesh filter before Ficoll-Histopaque density centrifugation.
Immunophenotyping and functional analysis by flow cytometry
Cells were stained by fluorescent antibodies per manufacturer's instructions; events were acquired with a FACSCalibur or FACSCanto (Becton Dickinson, San Jose, CA) and analyzed with FlowJo (Tree Star Inc., San Carlos, CA)17, PD-1 positivity was determined by an isotype control-defined cutoff (99.5%). MFI analysis was restricted to samples acquired by FACSCanto. Antigen-specific CD107a mobilization and IFNγ expression was quantified by adding APC-conjugated tetramers and anti-CD107a-FITC before peptide stimulation (10µg/ml) in the presence of brefeldin A (10 µg/ml) for five hours before surface staining, permeabilization and intracellular IFNγ staining.
In vitro expansion with and without anti-PD-L1
PBL and LIL (2×106 cells/ml) from HLA-A2+ subjects were stimulated with antigenic peptides (10µg/ml) in complete media with rIL2 (100 IU/ml) with and without 10µg/ml anti-PD-L1 before analysis on day 7.
Carboxy fluorescein succinidyl ester (CFSE) proliferation assay
Lymphocytes were labeled with 5µM CFSE (Molecular Probes, Eugene, OR) as previously described27 and cultured for 7 days with 100 IU/ml rIL-2 and antigenic peptides (10µg/ml) before FACS analysis. In selected assays, anti-PD-L1 and/or anti-PD-L2 were added at 10µg/ml.
IFNγ ELISpot assay
IFNγ ELISpot assay was performed with 200,000 PBL or LIL per well in triplicates stimulated by overlapping HCV-derived 15-mers (2µM)17,24 with and without anti-PD-L1 and/or anti-PD-L2 at 10µg/ml. After 45 hours, plates were developed and IFNγ spot-forming units (SFUs) counted by an ELISpot reader (Hitech Instruments, Media, PA)17,24. Antigen-specific IFNγ+ T-cells were quantified by subtracting the mean IFNγ SFU in negative control wells from the mean SFU in antigen-stimulated wells and expressed as IFNγ SFU/106 cells.
Statistics
Clinical and immunological parameters were compared by the non-parametric Mann-Whitney U, paired t-test and Kruskal-Wallis test. Frequency differences were compared by Fisher's Exact or the Chi-square test as appropriate. Correlations were tested for significance by the Spearman rank correlation test. P-values below 0.05 were considered significant.
RESULTS
PD-1 expression is increased in circulating HCV-specific CD8 T-cells in patients with acute or chronic hepatitis C
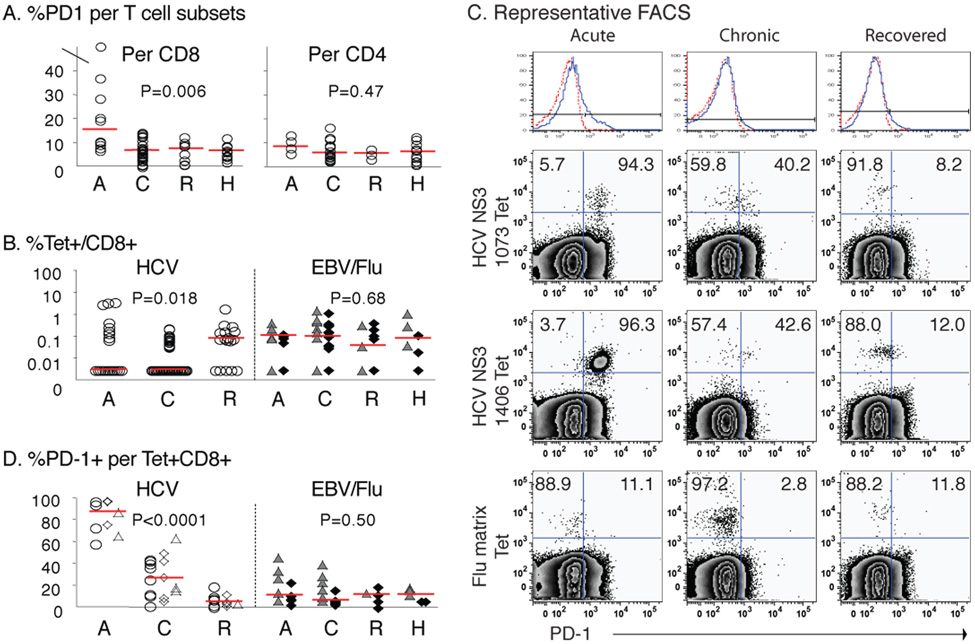
We began by examining the level of PD-1 expression in T-cell subsets from patients with acute (A), chronic (C) and resolved (R) HCV infection as well as healthy controls (H) (Table 1). As shown in Fig. 1A, PD-1 expression was increased in CD8 (but not CD4) T-cells from patients with acute hepatitis C compared to those with chronic or resolved HCV infection (p=0.006). As for antigen-specific CD8 T-cells, CD8 T-cells specific for HCV, EBV and influenza virus (Flu) epitopes expressed variable levels of PD-1 (Fig. 1B/C). As shown in Fig. 1C/D, PD-1 expression was substantially greater in HCV-specific CD8 T-cells from patients with acute than chronic HCV infection (A87.4% vs C26.7%, p<0.0001). PD-1 expression was also greater in HCV-specific than Flu- or EBV-specific CD8 T-cells from patients with acute (median 87.4% vs 11.1%; p<0.0001) and chronic HCV infection (median 26.7% vs 6.5%; p=0.0086) (Fig. 1D). By contrast, HCV-specific CD8 T-cells from the resolvers only expressed low level of PD-1 similar to total, EBV-specific and Flu-specific CD8 T-cells. These results confirm the preferential PD-1 expression reported in HCV-specific CD8 T-cells in our patients with acute and chronic but not resolved HCV infection7–10.
Figure 1. Increased PD-1 expression in circulating HCV-specific CD8 T-cells from viremic patients with acute and chronic but not resolved hepatitis C.
(A) %PD-1 expression in CD8 and CD4 T-cells in 10 acute (A), 27 chronic (C) and 8 resolved (R) patients with HCV infection, and 12 healthy HCV-seronegative (H) controls. Median %PD-1+/CD8 T-cells: A15.4% vs. C6.8% vs. R7.5% vs. H6.6% (p=0.006). Median %PD-1+/CD4 T-cells: A8.5% vs. C5.8% vs. R5.7% vs. H6.4% (p=0.47). (B) %Tetramer+ CD8 T-cells specific for HCV (circle), EBV (triangle), and Flu (diamond) in 7 Acute, 19 Chronic, 8 Recovered and 3 Healthy patients. Median %HCV-specific (A0.00% vs. C0.00% vs. R0.08%, p=0.018); Median %EBV or Flu-specific (A0.11% vs. C0.10% vs. R0.04% vs. H0.08, p=0.68). (C) Representative PD-1 stainings for peripheral HCV- and Flu-specific tetramer+ CD8 T-cells from acute, chronic and recovered patients. Top panel shows the PD-1 cutoff strategy with isotype control (dotted red line). (D) %PD-1+ per tetramer+ CD8 T-cells (circle, NS3 1073; diamond, NS3 1406; triangle, NS5B 2594), EBV (filled triangle) and Flu (filled diamond) in 7 acute, 19 chronic, 8 resolved and 3 healthy individuals. Median %PD-1+: HCV-specific CD8 T-cells (A87.4% vs. C26.7% vs. R5.1%, p<0.0001); non-HCV-specific CD8 T-cells (A11.1% vs. C6.5% vs. R11.8% vs. H11.8%, p=0.50). Red horizontal bars indicate the medians. P-values were determined by the Kruskal-Wallis test.
Ex vivo PD-1 expression is inversely correlated with HCV-specific CD8 T-cell expansion and effector function in vitro
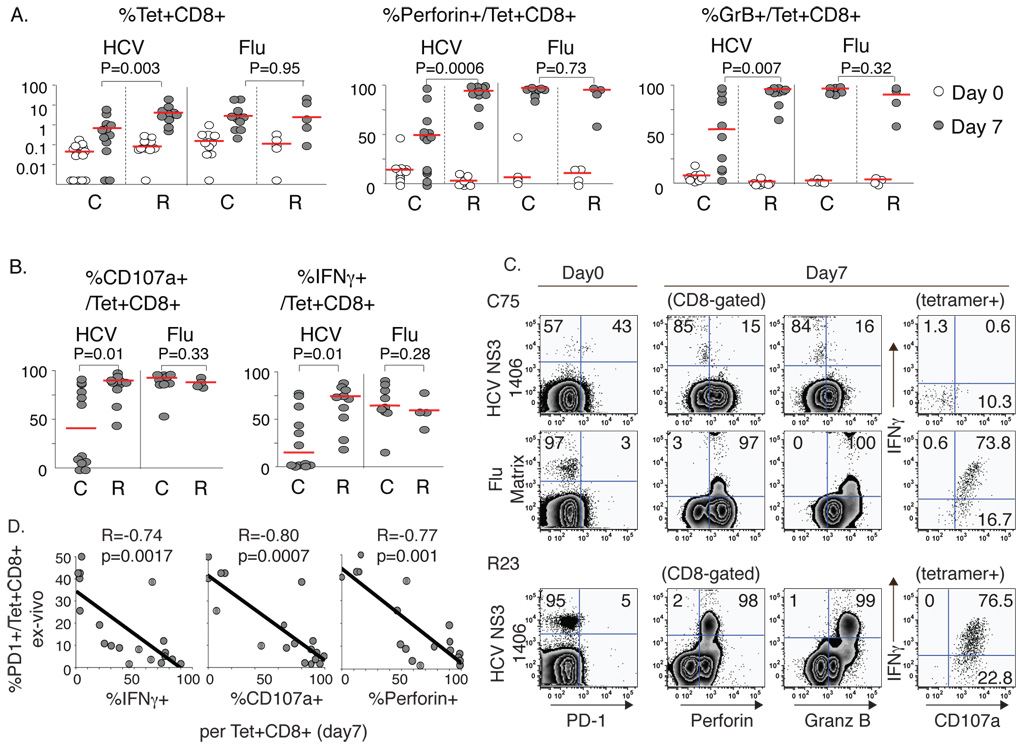
We next asked if the differential PD-1 expression ex vivo in HCV-specific CD8 T-cells correlated with antigen-specific effector function following 7 days of antigenic stimulation with rIL2 in vitro. Recall responses to the HLA A2-restricted Flu matrix epitope were examined for comparison. As shown in Fig. 2A, HCV-specific and Flu-specific CD8 T-cells from both chronic and resolved subjects were deficient in perforin and granzyme B expression ex vivo, although perforin was more readily detected in HCV-specific CD8 T-cells from chronic than resolved subjects. Following antigenic stimulation, HCV-specific CD8 T-cells from chronic HCV patients were less able to expand, upregulate perforin and granzyme B expression (Fig. 2A/C) or express IFNγ and mobilize CD107a (LAMP-1) (Fig. 2B/C) compared to the resolvers. This defect was HCV-specific because Flu-specific CD8 T-cells from chronic patients expanded and expressed both perforin and granzyme B efficiently following antigenic stimulation. Furthermore, neither antigen-specific CD8 T-cell expansion nor functional changes were observed in cells cultured with rIL2 alone (data not shown). Importantly, these in vitro effector functions correlated tightly with PD-1 expression ex vivo (Fig. 2D), indicating that PD-1 expression ex vivo is a marker of poor antigen-specific CD8 T-cell effector function in vitro. However, the perforin or granzyme B expression ex vivo did not correlate with PD-1 expression ex vivo (data not shown).
Figure 2. HCV-specific CD8 T-cells in patients with chronic HCV infection display impaired antigen-specific expansion and effector function in vitro.
(A) HCV- and Flu-specific tetramer+ CD8 T-cell frequency and expression of perforin and granzyme B on day 0 (empty circle) and day 7 of culture (filled circle) with peptides (10µg/ml) and 100IU/ml rIL2 for 15 chronic (C) and 6 recovered (R) patients. %Tet+CD8+ (median C0.69% vs. R4.16% on day 7; p=0.003). %Perforin+/Tet+CD8+ (median C49.1% vs R92.3% on day 7, p=0.0006). %Granzyme B+/Tet+CD8+ (median C55.2% vs. R96.2% on day 7; p=0.007). Red horizontal bars indicate the median. (B) %CD107a+ and %IFNγ+ in HCV- and Flu-specific CD8 T-cells on day 7. HCV-specific CD8 T-cells: median %CD107a+ (C40.7% vs. R89.8%, p=0.01); median %IFNγ+ (C14.7% vs. R74.7%, p=0.01). (C) Representative FACS plots comparing HCV-specific and Flu-specific tetramer+ CD8 T cell expansion and effector function in chronic (C75) and resolved (R23) patients on day 0 and after 7 days of antigenic stimulation. Events are gated on CD8+ cells except for the far right intracellular staining gating on tetramer+ CD8 T-cells (D) Inverse correlation between %PD-1 expression ex vivo and antigen-specific IFNγ, CD107a and perforin expression on day 7 by HCV-specific CD8 T-cells. P-values were determined by Mann-Whitney U or Spearman Rank Correlation test).
PD-1 expression is markedly increased in HCV-specific CD8 T-cells in the liver compared to peripheral blood
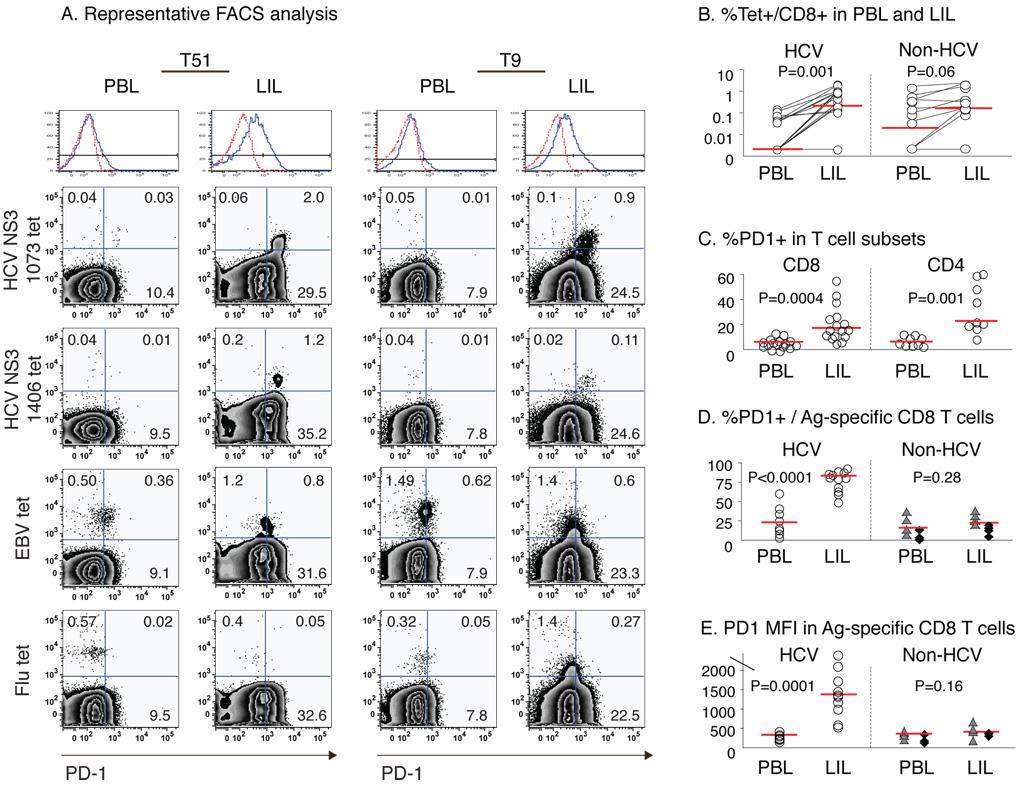
As liver is the primary site of HCV replication and disease pathogenesis, PD-1 expression and function of HCV-specific CD8 T-cells were examined in explanted liver and peripheral blood from HCV-infected liver transplant recipients. Fig. 3A shows the representative PD-1 staining characteristics for HCV-, EBV- and Flu-specific CD8 T-cells in the liver and blood from 2 HLA A2+ patients (T51, T9). Compared to blood, HCV-specific CD8 T-cells were enriched in the liver by more than ten-fold (Fig. 3A/B). While both CD8 and CD4 T-cell subsets displayed increased PD-1 expression in the liver compared to peripheral blood (Fig. 3C), intrahepatic HCV-specific CD8 T-cells displayed even greater PD-1 expression compared to peripheral HCV-specific CD8 T-cells based on the percentage and mean fluorescence intensity (MFI) (Fig. 3D/E). Interestingly, Flu- and EBV-specific CD8 T-cells were also detected in the liver of HCV-infected patients (median 0.16% Flu/EBV tetramer+ vs 0.21% HCV tetramer+ CD8 T-cells), although displaying similar PD-1 expression in both the liver and blood. Thus, while CD8 T-cells specific for HCV, Flu and EBV were detected in the liver, PD-1 expression was preferentially increased only on HCV-specific CD8 T-cells.
Figure 3. PD-1 expression is increased in HCV-specific CD8 T-cells in the liver compared to peripheral blood.
(A) Representative FACS plots for PBL and LIL from HLA-A2+ HCV-infected liver transplant recipients (T51 and T9) gating on CD8 T-cells. The top histogram shows the PD-1 cutoff strategy with isotype control (dotted red line). (B) Frequency of tetramer+CD8 T-cells specific for HCV (NS3 1073, NS3 1406, NS5B 2594), EBV and Flu epitopes in 8 HLA A2+ patients. Median values are indicated by red horizontal line. Median %HCV-specific CD8 T-cells: PBL 0.00% vs LIL 0.21%, p=0.001. Median %Flu or EBV-specific CD8 T-cells: PBL 0.02% vs. LIL 0.16%, p=0.06. (C) %PD-1 expression in CD8 and CD4 T-cells in 16 HCV-infected patients: Median %PD-1+/CD8 (PBL 6.1% vs. LIL 17.1%; p=0.0004); Median %PD-1+/CD4 (PBL 5.4% vs. LIL 21.9%; p=0.001). (D) %PD-1 expression and (E) PD-1 MFI on CD8 T-cells specific for HCV and non-HCV epitopes (EBV: triangle; Flu: diamond) from 8 HLA-A2+ HCV-infected patients. HCV-specific CD8 T cells: median %PD-1+ (PBL 23.0% vs. LIL 83.3%, p<0.0001); median PD-1 MFI (PBL 281 vs. LIL 1375, p=0.0001). Non-HCV-specific CD8 T-cells: median %PD-1+ (PBL 16.1% vs LIL 22.5%, p=0.28); median PD-1 MFI (PBL 356 vs LIL 406, p=0.16). P-values were determined by paired t-test or Mann-Whitney U.
PD-1+ CD8 T-cells in the liver display a highly activated and more exhausted phenotype than circulating PD-1+ CD8 T-cells
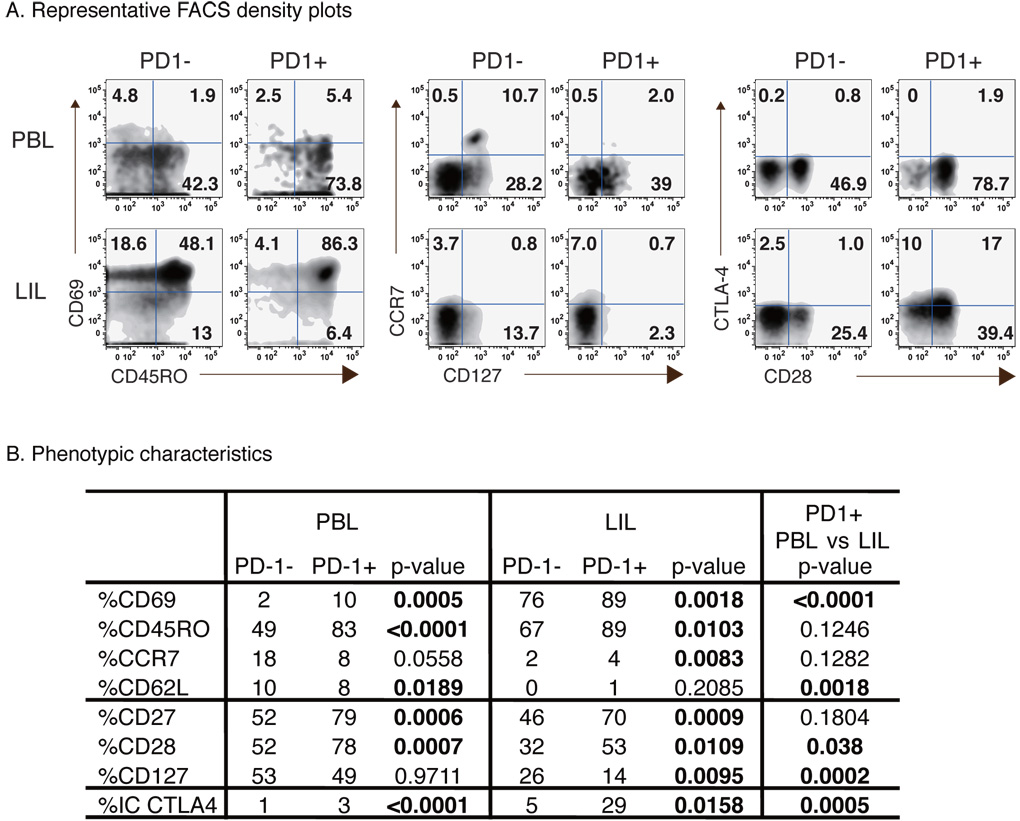
Further phenotypic analysis of PD-1+ CD8 T-cells in peripheral blood and liver of HCV-infected patients (Fig. 4A/B) showed that circulating PD-1+CD8 T-cells displayed increased expression of CD69 (p=0.0005), CD45RO (p<0.0001), CD27 (p=0.0006), CD28 (p=0.0007) and intracellular (IC) CTLA-4 (p<0.0001), but not CD127, CCR7 or CD62L compared to PD-1- CD8 T-cells, consistent with an activated memory phenotype5. In the liver, CD8 T-cells were mostly positive for CD69 and CD45RO but negative for CD62L and CCR7 regardless of PD-1 status. Similar to the peripheral compartment, intrahepatic PD-1+CD8 T-cells expressed increased CD69 (p=0.0018) and CD45RO (p=0.0103) expression than PD-1-cells. Importantly, intrahepatic PD-1+ CD8 T-cells expressed less CD28 (53% vs 78%; p=0.038) and CD127 (14% vs 49%; p=0.0002), but more CTLA-4 (29% vs 3%; p=0.0005), than peripheral PD-1+ CD8 T-cells. Thus, intrahepatic PD-1+ CD8 T-cells displayed a highly activated but also more exhausted phenotype than circulating PD-1+ CD8 T-cells, a finding relevant for intrahepatic HCV-specific CD8 T-cells which were highly PD-1-positive (although not directly examined for these markers).
Figure 4. Phenotypic characteristics of PD-1+ CD8 T-cells in peripheral blood and liver of patients with chronic HCV infection.
(A) Representative FACS density plots comparing phenotypic markers for PD-1- and PD-1+ CD8 T-cells in PBL and LIL from an HCV-infected patient. Numbers in each quadrant reflect percentage of gated PD-1- and PD-1+ CD8 T-cells. (B) Phenotype of PD-1+ and PD-1− CD8 T-cells in PBL (n=24) and LIL (n=14) shown as median percentages. P-values were calculated by Mann-Whitney U.
Highly PD-1-positive intrahepatic HCV-specific CD8 T-cells display a profound functional impairment
We then compared the antigen-specific expansion and effector function of HCV-specific CD8 T-cells from the liver and blood. Despite their enrichment in the liver, intrahepatic HCV-specific CD8 T-cells expanded poorly in vitro compared to peripheral HCV-specific CD8 T-cells from the same patients (Fig. 5A, gray bars). As shown in Fig. 5B, HCV-specific CD8 T-cells failed to expand from the LIL (1% ex vivo to 1% on day 7), contrasting with over a 10-fold expansion from PBL (day 0: 0.06%; day 7: 0.76%) following antigenic stimulation in vitro. Moreover, expanded intrahepatic HCV-specific CD8 T-cells expressed very little perforin, unlike expanded peripheral HCV-specific CD8 T-cells (median 9% vs 54%) (Fig. 5A/B). This difference was not specific to the liver compartment, because liver-derived Flu-specific CD8 T-cells expanded efficiently (6–9 fold) with high levels of perforin expression (72–100%). The poor effector function of intrahepatic HCV-specific CD8 T-cells extended to CD107a mobilization and IFNγ expression (Fig. 5B).
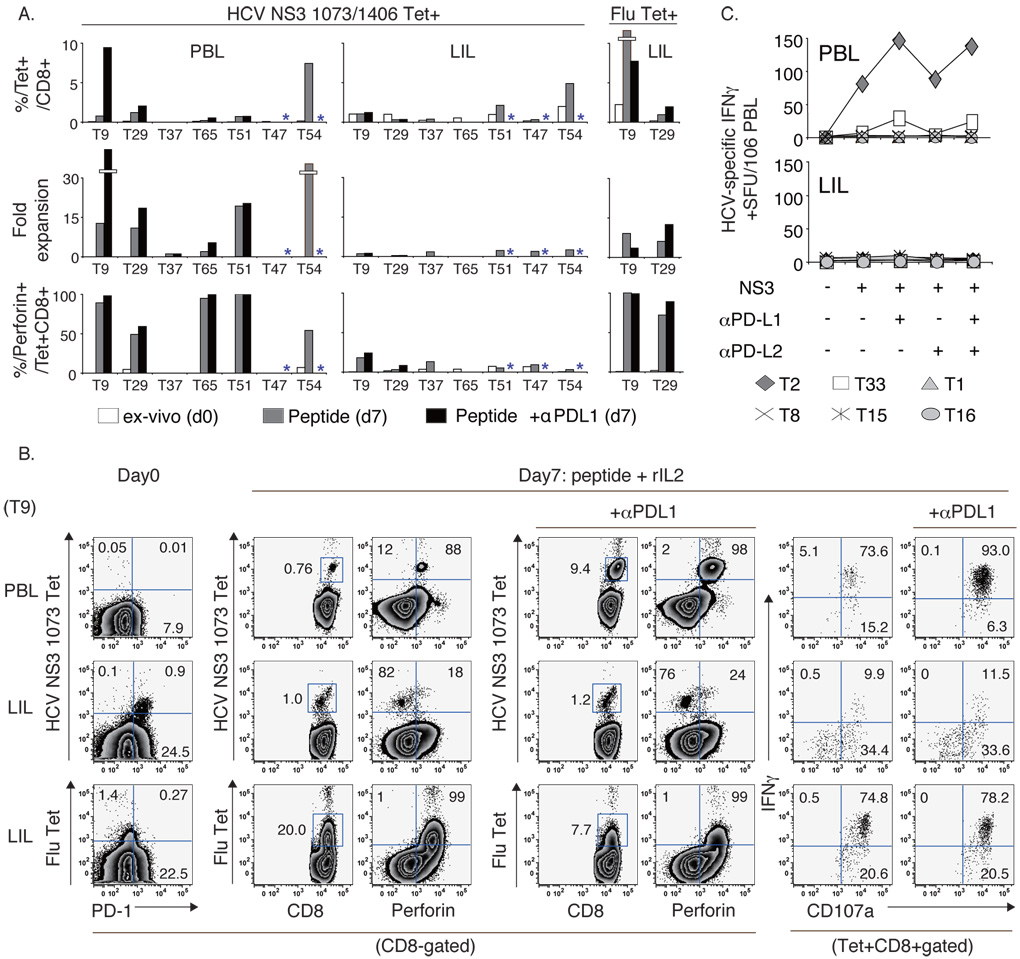
Figure 5. Impaired expansion and effector function unresponsive to PD1:PD-L blockade in highly PD1+ HCV-specific CD8 T-cells in the liver of HCV-infected patients.
(A) The frequency, fold expansion and perforin expression of HCV-specific and Flu-specific tetramer+ CD8 T-cells on day 0 (white bars) and on day 7 following antigenic stimulation without (gray bars) or with anti-PD-L1 (black bars) *concurrent blockade not done. All subjects were studied with NS3 1073 tetramer except T47 and T51 who displayed a dominant NS3 1406 response. (B) FACS plots showing HCV-specific and Flu-specific expansion and effector function in PBL and LIL from patient T9. (C) HCV-specific T-cell IFNγ response by IFNγ ELISpot in 6 HLA-A2-negative HCV-infected patients following stimulation with overlapping NS3 peptides +/− PD-1:PD-L blockade.
PD-1:PD-Ligand blockade does not restore antigen-specific function to highly PD-1+ HCV-specific CD8 T-cells from the liver of patients with chronic HCV infection
The effect of anti-PD-L1 on HCV-specific CD8 T-cells was examined in 5 HLA A2+ transplant recipients with chronic HCV infection, including 4 subjects (T9, T29, T37, and T65) also examined both in peripheral and liver compartments. As shown in Fig. 5A/B (black bars), expansion of peripheral HCV-specific CD8 T-cells was enhanced by over 50% by anti-PD-L1 in T9 (12.4 fold), T29 (1.7 fold), and T65 (2.8 fold) but not in T37 or T51. In T54, anti-PD-L1 also increased HCV-specific CD8 T-cell expansion by 2-fold from PBL in a separate CFSE-dilution assay (data not shown). Thus, anti-PD-L1 enhanced peripheral HCV-specific CD8 T-cell proliferation in 4/6 patients. By contrast, HCV-specific CD8 T-cells from the liver failed to proliferate in the presence of anti-PD-L1, even in patients who showed significant augmentations upon PD-1:PD-L1 blockade in peripheral blood. PD-1:PD-L1 blockade did not augment perforin, IFNγ or CD107a expression in liver-derived HCV-specific CD8 T-cells. Analysis of the total T-cell IFNγ response to the entire HCV NS3 region using overlapping 15mer peptides in IFNγ ELISpot assay showed that PD-1:PD-L blockade enhanced HCV-specific IFNγ response in 2/6 patients (T2 and T33), but only in peripheral blood (Fig. 5C). Of note, anti-PD-L2 had little effect on the HCV-specific T-cell response, either alone or with anti-PD-L1. Combining both tetramer-based and IFNγ ELISpot assay results, PD-1:PD-L1 blockade augmented HCV-specific CD8 T-cell response from peripheral blood in 6/12 patients compared to 0/10 from the liver (50% vs 0%; p=0.010). Thus, highly PD-1+ intrahepatic HCV-specific CD8 T-cells from HCV-infected patients were profoundly impaired without functional restoration through PD-1:PD-L blockade.
Highly PD-1-positive peripheral HCV-specific CD8 T-cells during acute hepatitis C are also functionally impaired and refractory to PD-1/PD-L blockade
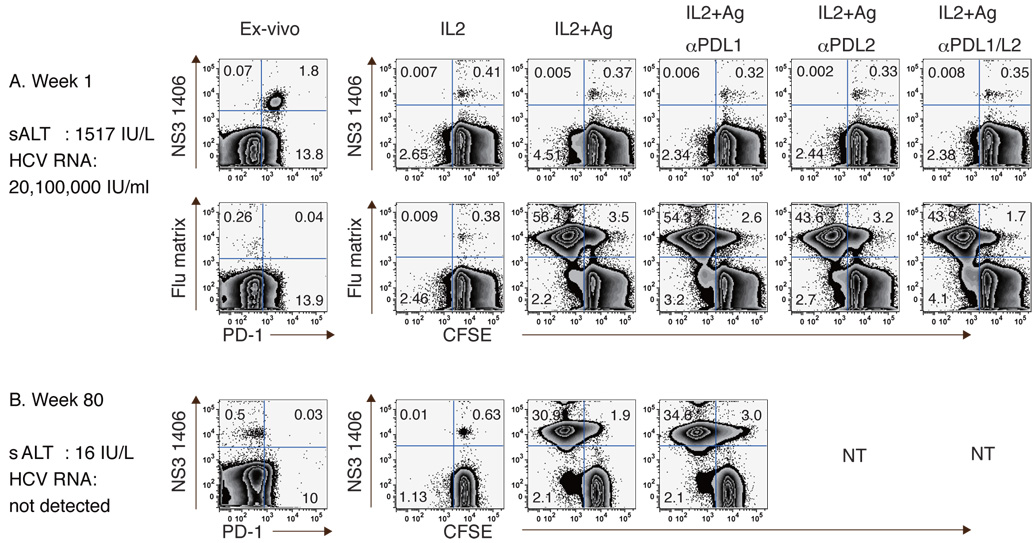
Since HCV-specific CD8 T-cells are also highly PD-1+ during acute infection, the effect of PD-1:PD-L blockade on circulating CD8 T-cells was examined in patient A29 with acute hepatitis C. As shown in Fig. 6A, HCV NS3 1406-specific CD8 T-cells were readily detectable (1.87%) and almost entirely PD-1+ during acute infection with high sALT activity and viremia. Notably, antigenic stimulation resulted in minimal HCV-specific CD8 T-cell expansion regardless of PD-1:PD-L blockade. By contrast, Flu-specific CD8 T-cells displayed low PD1 expression (13% PD-1+, MFI 282) and expanded vigorously following antigenic stimulation regardless of PD-1:PD-L blockade. Following HCV clearance (week 80), however, HCV-specific CD8 T-cells regained their effector function with reduced PD-1 expression (Fig. 6B), suggesting that HCV-specific CD8 T-cell dysfunction in acute HCV infection can be reversed by viral clearance and reduced PD-1 expression.
Figure 6. PD-1:PD-L blockade does not enhance expansion of highly PD-1+ HCV-specific CD8 T-cells in acute HCV infection.
Expansion of HCV- and Flu-specific CD8 T-cells in an acute hepatitis C patient during (A) the acute (week 1) and (B) resolved phase (week 80). CFSE-labeled PBMCs were stimulated with peptides (10µg/ml) and rIL2 (100IU/ml) with/without anti-PD-L1 and/or anti-PD-L2 for 7 days.
The level of PD-1 expression correlates inversely with functional restoration of HCV-specific CD8 T-cells by PD-1:PD-L1 blockade
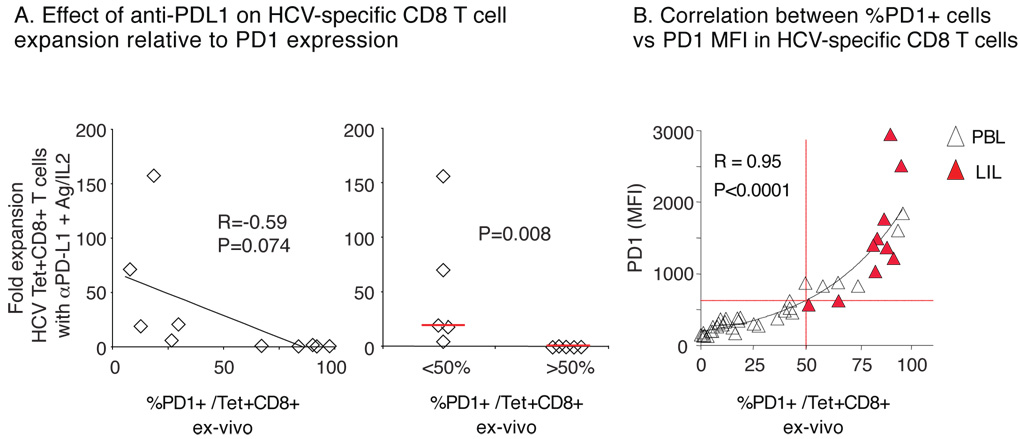
Notably, the level of PD-1 expression on HCV-specific CD8 T-cells correlated inversely with their antigen-specific expansion in the presence of PD-1:PD-L1 blockade (Fig. 7A, left graph). For example, antigen-specific CD8 T-cell expanded poorly when displaying PD-1 expression above 50% (Fig. 7A, right graph). Comparison of HCV-specific CD8 T-cells from acute, chronic and resolved subjects (Fig. 7B) showed that %PD-1 correlated tightly with PD-1 MFI (R=0.95). In fact, PD-1 expression above 50% (corresponding to MFI 609 by extrapolation) occurred only in HCV-specific CD8 T-cells from the liver (red triangles) of chronic HCV patients or peripheral blood of acutely HCV-infected patients. Collectively, these results suggest that high levels of PD-1 expression on HCV-specific CD8 T-cells represent a profound functional exhaustion refractory to PD-1:PD-L blockade that is particularly prominent in HCV-infected infected liver or during acute hepatitis C, perhaps reflecting active antigenic exposure. These findings provide new insights into HCV-specific CD8 T-cell dysfunction with therapeutic relevance.
Figure 7. Inverse relationship between PD-1 expression and HCV-specific CD8 T-cell expansion with anti-PD-L1 blockade.
(A) Comparison of HCV tetramer+ CD8 T-cell expansion in vitro after 7 days of antigenic stimulation with anti-PD-L1 and ex vivo PD-1 expression directly (left) and in subgroups by %PD-1 cutoff of 50% (right). (B) Correlation between the frequency and MFI for PD-1 expression on HCV-specific CD8 T-cells with an exponential trendline (32 PBL, unfilled triangles; 10 LIL, red filled triangles).
DISCUSSION
HCV persists with impaired antigen-specific CD8 T-cell function and progressive immune-mediated liver disease28–30. Since PD-1 expression has been linked with virus-specific effector T-cell dysfunction in chronic viral infections3,5–11,31, we examined the extent to which PD-1 signaling might contribute to immune regulation in HCV-infected patients, particularly within the liver. As expected, PD-1 expression in circulating HCV-specific CD8 T-cells was increased in HCV-infected patients in association with their effector dysfunction and PD-1:PD-L blockade could enhance HCV-specific CD8 T-cell function in some cases. However, HCV-specific CD8 T-cells from the liver of HCV-infected patients displayed uniformly high levels of PD-1 expression with profound functional impairment that was refractory to PD-1:PD-L blockade. A similar lack of response to PD-1:PD-L blockade was also observed for highly PD-1+ HCV-specific CD8 T-cells in acute evolving hepatitis C, suggesting that the level of PD-1 expression and the compartmentalization may define the extent of functional exhaustion in HCV-specific CD8 T-cells and their potential for PD-1:PD-L blockade-mediated restoration.
Although HCV-specific CD8 T-cells were generally impaired in HCV persistence, their level of dysfunction varied considerably between patients in association with PD-1 expression. Within an individual patient, there were further differences in the function and PD-1 expression of HCV-specific CD8 T-cells between the liver and peripheral blood. To our knowledge, simultaneous examination of peripheral and intrahepatic HCV-specific as well as Flu and EBV-specific CD8 T-cell phenotype and function has not been reported previously. In this study, we examined 7 HLA-A2+ and 6 HLA-A2- patients to show a preferential PD-1 upregulation and dysfunction of HCV-specific (but not Flu or EBV-specific) CD8 T-cells in the liver compared to peripheral blood. While the liver is believed to be a tolerizing immunological 'graveyard' in which activated antigen-specific T-cells become trapped, inactivated and even deleted32,33, these compartmental differences were specific to HCV since intrahepatic Flu-specific CD8 T-cells were functionally intact without high PD-1 expression. Since PD-1 is also upregulated in activated T-cells, we hypothesize that HCV-specific CD8 T-cells become highly PD-1+ and functionally impaired as they encounter their cognate antigens in the HCV-infected liver, thus limiting both liver inflammation and virus control. In peripheral blood, HCV-specific CD8 T-cells may retain moderate PD-1 expression and partial function due to limited exposure to HCV-expressing cells. Marked PD-1-upregulation with a functionally 'stunned phenotype21 was also seen in circulating HCV-specific CD8 T-cells in acute hepatitis C, consistent with active antigenic exposure in vivo, although these functional defects did not persist after viral clearance nor necessarily predict virological outcome34. Thus, the level of PD-1 expression defined a hierarchy in HCV-specific CD8 T-cell function perhaps based on the extent of active antigenic exposure in different compartments in vivo.
Surprisingly, highly PD-1+ HCV-specific CD8 T-cells were poorly responsive to PD-1:PD-L blockade. For example, while PD-1:PD-L1 blockade could augment the expansion and function of peripheral HCV-specific CD8 T-cells with intermediate PD-1 expression, no enhancement was observed for highly PD-1+ intrahepatic HCV-specific CD8 T-cells in any of the patients. This was not due to inadequate antigen presentation in the LILs since Flu-specific CD8 T-cells from the liver expanded readily upon antigenic stimulation. Furthermore, the addition of CD3-depleted PBL as antigen presenting cells (APC) did not promote intrahepatic HCV-specific CD8 T-cell expansion regardless of PD-1:PD-L blockade (Supplementary Figure 2). Based on increased CTLA-4 with reduced CD28 and CD127 expression in PD-1+ CD8 T-cells in the liver, we speculate that antigenic stimulation in the liver induces further negative costimulatory signals such as CTLA-4 while downregulating positive receptors like CD28 and CD127, collectively resulting in a terminally exhausted state resistant to PD-1:PD-L blockade. If this is correct, PD-1:PD-L blockade may enhance the expansion and effector function of antigen-specific CD8 T-cells with intermediate PD-1 expression (e.g. peripheral blood), but not the terminally exhausted, highly PD-1+ CD8 T-cells (e.g. liver) with the activation of additional negative pathways. Along these lines, increased CTLA-4 expression was recently reported in HIV-specific CD4 (although not CD8) T-cells with a pathogenetic significance35.
There were notable differences between our findings and previous reports. For example, PD-1+CD8 T-cells from HCV-infected livers retained considerable CD28 expression in our study (53%), similar to a recent report (65%)10. However, it differs from HIV-specific CD8 T-cells which are highly PD-1+ but with much lower CD28 expression (11%). Interestingly, HIV-specific CD8 T-cells displayed efficient functional responses to PD-1:PD-L blockade despite poor CD28 expression5. In this respect, the phenotypic and functional characteristics of exhausted virus-specific CD8 T-cells may differ between HCV and HIV infection. Furthermore, anti-PD-L2 did not enhance the HCV-specific CD8 T-cell IFNγ response in our study, unlike previous studies5,10. While this difference could reflect a shorter antigenic stimulation in our IFNγ ELISpot assay (2 days compared to 6–7 days in previous studies)5,10, HCV-specific IFNγ response in PBL was augmented in 2/6 patients by anti-PD-L1 but not at all by anti-PD-L2, suggesting a greater responsiveness to anti-PD-L1. Of note, our PD-1 detection strategy targeted cells with high (rather than intermediate) PD-1 expression, differing from previous studies5,8,10. It would be interesting to explore the potential difference between PD-1 intermediate and high cells in future studies. Nevertheless, %PD-1 positivity in our study associated significantly with antigen-specific effector CD8 T-cell function and correlated tightly with the PD1 MFI. Finally, PD-1:PD-L blockade failed to enhance intrahepatic HCV-specific CD8 T-cell function in 10/10 patients in our study, contrasting with a recent study reporting augmentation in 3 patients10. This poor functional response in the liver was HCV-specific, since Flu-specific CD8 T-cells from the same liver were highly functional. Importantly, the functional responses to PD-1:PD-L blockade correlated inversely with PD-1 expression, suggesting that PD-1:PD-L1 blockade may target cells without high PD-1 expression.
These findings have important implications for therapeutic approaches using PD-1:PD-L blockade. In HCV, PD-1:PD-L blockade may not immediately restore function to HCV-specific CD8 T-cells in the liver, thus limiting the likelihood of acute fulminant hepatitis. This is an important safety consideration. Alternatively, if rejuvenated peripheral HCV-specific CD8 T-cells (with intermediate PD-1 expression) subsequently home to the liver, they might induce active liver inflammation or become rapidly impaired upon encountering HCV-infected hepatocytes. In this respect, concurrent virus suppression by antiviral therapy could reduce PD-1 expression while reducing HCV-expressing hepatocytes, thus promoting better immune induction while limiting liver damage during PD-1:PD-L blockade. Furthermore, PD-1 expression in HCV-specific CD8 T-cells may help predict responsiveness to therapeutic PD-1:PD-L blockade. While our in-vitro findings require further validation in-vivo, these findings in HCV may also be relevant for other conditions in which T-cells are differentially activated or exhausted based on compartmentalization and antigenic exposure.
In conclusion, highly PD-1+ HCV-specific CD8 T-cells in the liver of HCV-infected patients displayed a profound functional impairment that was refractory to PD-1:PD-L blockade, suggesting that the level of PD-1 expression and tissue compartmentalization define the functional capacity of virus-specific CD8 T-cells in chronic HCV infection and their potential for responsiveness to PD-1:PD-L blockade.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the members of the Liver Transplant Team at the Hospital of the University of Pennsylvania for the liver sample procurement, Mary E. Valiga RN for patient recruitment, and Sutharsan Ganesan and Diana Lim for technical assistance. We also thank all our study subjects.
Grant Support: This study was supported by NIH grants AI47519, AA12849 and AGA Elsevier Research Initiative Award in addition to Philadelphia VA Medical Research, the NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases P30DK50306 and its Molecular Biology and Cell Culture Core Facilities, and the NIH Public Health Service Research Grant M01-RR00040. DAP is a Medical Research Council (UK) Senior Clinical Fellow. Funded in part by a grant from the Foundation for the NIH through the Grand Challenges in Global Health Initiative and NIH AI56299 (G.F.). Support for the Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource of the University of Pennsylvania was funded through NIH P30 CA016520 from the National Cancer Institute.
Abbreviations
- APC
allophycocyanin
- CFSE
carboxyfluorescein succinimidyl ester
- CTLA-4
cytotoxic T-lymphocyte-associated antigen-4
- FITC
fluorescein isothiocyanate
- Flu
influenza virus
- IFN
interferon
- LCMV
lymphocytic choriomeningitis virus
- LIL
liver infiltrating lymphocytes
- MFI
mean fluorescence intensity
- NOD
nonobese diabetic
- PD-1
Programmed Death 1
- PD-L
PD-ligand
- PE
phycoerythrin
- PFA
paraformaldehyde
- SFU
spot-forming unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
GF has a patent licensing arrangement for antibodies blocking the PD-1/PD-ligand pathways.
REFERENCES
- 1.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 2.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman GJ, Wherry EJ, Ahmed R, et al. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 5.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 6.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 7.Urbani S, Amadei B, Tola D, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radziewicz H, Ibegbu CC, Fernandez ML, et al. Liver infiltrating lymphocytes in chronic human HCV infection display an exhausted phenotype with high PD-1 and low CD127 expression. J Virol. 2006;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penna A, Pilli M, Zerbini A, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 10.Golden-Mason L, Palmer B, Klarquist J, et al. Upregulation of PD-1 Expression on Circulating and Intrahepatic Hepatitis C Virus-Specific CD8+ T Cells Associated with Reversible Immune Dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwai Y, Terawaki S, Ikegawa M, et al. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grakoui A, Shoukry NH, Woollard DJ, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 16.Rehermann B, Chang K-M, McHutchison JG, et al. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J.Virol. 1996;70:7092–7102. doi: 10.1128/jvi.70.10.7092-7102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan DE, Sugimoto K, Newton K, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 18.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson SL, Wooldridge L, Tafuro S, et al. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J Biol Chem. 2003;278:24285–24293. doi: 10.1074/jbc.M300633200. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto K, Kaplan DE, Ikeda F, et al. Strain-specific T-cell suppression and protective immunity in patients with chronic hepatitis C virus infection. J Virol. 2005;79:6976–6983. doi: 10.1128/JVI.79.11.6976-6983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang KM, Rehermann B, McHutchison JG, et al. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376–2385. doi: 10.1172/JCI119778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He XS, Rehermann B, Lopez-Labrador FX, et al. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci U S A. 1999;96:5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto K, Ikeda F, Stadanlick J, et al. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex-vivo in persistent HCV infection. Hepatology. 2003;38:1437–1448. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan DE, Sugimoto K, Ikeda F, et al. T-cell response relative to genotype and ethnicity during antiviral therapy for chronic hepatitis C. Hepatology. 2005;41:1365–1375. doi: 10.1002/hep.20706. [DOI] [PubMed] [Google Scholar]
- 26.Heydtmann M, Hardie D, Shields PL, et al. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J Immunol. 2006;177:729–738. doi: 10.4049/jimmunol.177.1.729. [DOI] [PubMed] [Google Scholar]
- 27.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 28.Chang KM. Immunopathogenesis of hepatitis C virus infection. Clin Liver Dis. 2003;7:89–105. doi: 10.1016/s1089-3261(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 29.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 30.Liang TJ, Rehermann B, Seeff LB, et al. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 31.Maier H, Isogawa M, Freeman GJ, et al. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007;178:2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 32.Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J Immunol. 1999;163:3202–3210. [PubMed] [Google Scholar]
- 33.Crispe IN, Dao T, Klugewitz K, et al. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 34.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, et al. High PD-1 expression on HCV-specific CD8+ and CD4+ T cells during acute Hepatitis C irrespective of clinical outcome. J Virol. 2007 doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4(+) T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.