Abstract
Purpose
Lack of reliable biomarkers limits accurate prediction of PSA biochemical recurrence (disease progression) in prostate cancer (CaP). The two inflammatory chemokines, Osteopontin (OPN) and interleukin-8 (IL-8) are associated with tumor angiogenesis and metastasis. We investigated whether OPN and IL-8 expression in CaP correlates with disease progression.
Experimental Design
Archival prostatectomy specimens (n = 103) were obtained from patients with minimum 72 month follow-up. OPN and IL-8 expression was evaluated by immunohistochemistry and graded for intensity and the area. Association of OPN and IL-8 staining with biochemical recurrence was evaluated by univariate and multivariate models.
Results
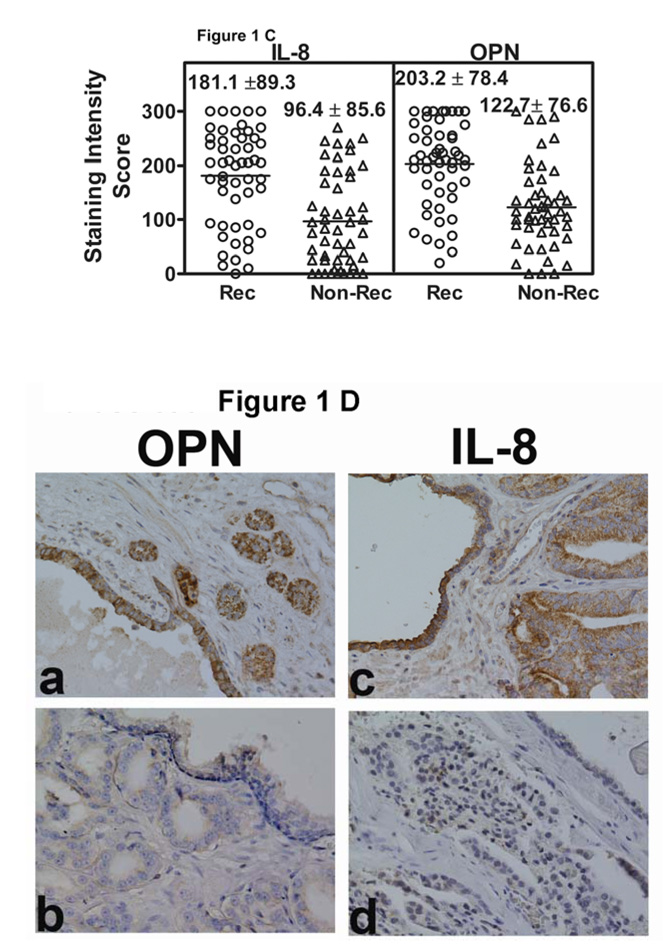
In tumor cells, OPN and IL-8 staining was higher in the recurred group (203.2 ± 78.4; 181.1 ± 89.3) than in the non-recurred group (122.7 ± 76.6; 96.4 ± 85.6; p < 0.001). Higher OPN and IL-8 staining was also observed in benign areas adjacent to tumor in the recurred group, than in non-recurred group. In univariate analysis, except age, all pre- and post-operative parameters and OPN and IL-8 staining scores significantly associated with biochemical recurrence (P < 0.05). In multivariate analysis, margin status and OPN staining independently associated with biochemical recurrence within 72 months. OPN, either alone or with IL-8 and seminal vesicle invasion was a significant parameter in predicting biochemical recurrence within 24 months. OPN and IL-8 staining predicted recurrence with high sensitivity (75.5% 73.6%) and specificity (76%, 70.6%).
Conclusion
In prostatectomy specimens, OPN expression is independently associated with biochemical recurrence. Both OPN and IL-8 may be predictors of early disease progression.
Keywords: chemokines, Inflammation, immunohistochemistry, PSA, seminal vesicle invasion, cancer recurrence, prognostic biomarkers
INTRODUCTION
The number of organ-confined prostate cancer (CaP) cases has significantly increased due to the widespread use of PSA. Despite careful selection of patients, a substantial percentage of patients with localized CaP will experience disease recurrence after undergoing radical prostatectomy or radiotherapy [1, 2]. Although existing parameters such as Gleason sum or preoperative PSA provide some prognostic information, it is a challenge estimating prognosis in patients with CaP considering two-thirds will have a Gleason sum of 5–7 and serum PSA levels of 4–10 ng/dl. Therefore, there is a need for more accurate prognostic markers to identify the biologic potential of the tumor.
Osteopontin (OPN) is a glycosylated phosphoprotein comprising about 2% of the noncollageneous proteins of the bone [3]. It is involved in osteoblastic differentiation and bone formation, as well as, anchorage of osteoclasts to bone and reabsorption of bone [4]. OPN is over expressed in a variety of cancers and is involved in invasion and metastasis [5]. High levels of OPN expression are associated with a poor prognosis in breast cancer patients [6]. High serum-OPN levels have been reported in patients with metastatic CaP [7]. A recent study causally linked high OPN expression with CaP cell proliferation and metastasis [8]. Another study showed that increased OPN expression correlates with Gleason sum and decreased survival in CaP patients [9]. OPN is also an inflammatory chemokine and has been linked to inflammatory atrophy of large colon [10].
A causal link between chronic or recurrent inflammation has been suggested for genesis and progression of CaP. IL-8, also known as the CXC ligand-8 is a member of the CXC chemokine family and is a pro-inflammatory cytokine. IL-8 is a common chemotactic factor regulating pathologic angiogenesis, tumor growth, and metastasis. For example, high level of IL-8 is associated with CaP invasion and metastasis, and inhibition of IL-8 production in experimental CaP models decreases metastatic potential [11]. We have recently shown that IL-8 expression in androgen dependent/responsive CaP cells induces androgen independence and increased survival when exposed to chemotherapeutic drugs. [12]. A study involving a small number of patients has linked high serum level of IL-8 to Gleason sum and pathologic stage of CaP [13]. Furthermore, an increased serum IL-8 level was linked to CaP bone metastasis [14]. IL-8 gene polymorphism that correlates with elevated IL-8 expression may also be associated with increased risk for development of CaP [15, 16]. However, the prognostic potential of IL-8 to predict biochemical recurrence in patients with clinically localized CaP has not been evaluated.
IL-8 and OPN have been co-expressed in a variety of tumors and also increased IL-8 levels lead to increased OPN expression in some benign conditions, suggesting deregulation of IL-8 and OPN during disease progression [17]. However, such IL-8 mediated increase of OPN expression has yet not been reported in tumors. In this study, we investigated OPN and IL-8 expression in archival radical prostatectomy specimens from 103 CaP patients with a mean follow-up of 96.3 months. Our results show that both OPN and IL-8 expression is elevated in CaP cells and associates with biochemical recurrence.
MATERIALS AND METHODS
Specimens and study patients
We analyzed 103 CaP specimens obtained from patients who underwent a radical prostatectomy with bilateral pelvic lymphadenectomy between 1992 and 2001. This study was conducted under a protocol approved by the University of Miami’s Institutional Review Board. During 1992–2001, a total of 911 patients underwent radical prostatectomy, out of which 695 did not receive neoadjuvant androgen deprivation therapy. A minimum 5-year follow-up was available on 381 patients. The pathology resource selected 117 blocks for the study based on the availability of well preserved tissue blocks containing tumor and the reevaluation of H&E slides for pathologic parameters. Due to staining artifacts (i.e., poor fixation, not enough tumor in the specimen, and sloughing off tissues) 14 of the 117 specimens could not be evaluated for IL-8 and OPN staining. The minimum available follow-up on study patients (n = 103) was 72 months. The majority of the patients (n = 99) had no metastasis to the pelvic lymph nodes. Of the 103 patients, 47 had biochemical or clinical recurrence before 72 months and 56 patients were free of biochemical recurrence. We used the Kaplan-Meier method to estimate the distribution function of the times to recurrence and obtained the mean, and median time to recur (mean: 36.3 months; median: 21.15 months, 95% confidence interval (CI): 14.75 – 45.74 months). The mean, and median time of total follow-up was also computed by the Kaplan-Meier method (mean: 96.3 months; median: 91.9 months, 95% CI: 84.53 – 99.54; range for total follow-up 71.96 – 145.77 months).
Biochemical recurrence was defined as a PSA level > 0.4 ng/ml in 2 successive measurements following radical prostatectomy, in which case the first date of elevated PSA was considered as the date of recurrence. This is a clinical definition of biochemical recurrence and treatment decisions are based on the rising PSA levels and PSA velocity (how fast the PSA rises in successive measurements). Thus, the data was censored as those who experienced rise in PSA following radical prostatectomy within 72 months versus those who did not. Those patients who experienced rise in PSA beyond 72 months (6 patients) were considered as non-recurred at 72 months. The patient characteristics with respect to age, preoperative PSA, preoperative clinical stage, and tumor (i.e. Gleason sum, stage, margin, extra-prostatic extension (EPE), seminal vesicle invasion (SVI) and lymph node status) are shown in Table 1.
Table 1. Pre- and postoperative parameters of the study patients.
The information on the post-operative variables (Gleason sum, margin status, EPE, SVI and lymph node status) was assessed by pathologic examination of the surgical specimens immediately after surgery, and therefore, these parameters are considered as baseline covariates.
| Progression | Age (yrs) | PSA (ng/ml) | Clinical Stage | Gleason sum | 1EPE | Margin | 2SVI | Lymph node |
|---|---|---|---|---|---|---|---|---|
| Biochemical | Mean: | Mean: | T1c: 23 | 5 = 1 | (+) = 32 | (+) = 40 | (+) = 16 | (+) = 4 |
| recurrence* | 63.8± 5.9 | 11.5± 8.8 | (43.3%) | (1.9%) | (60.4%) | (75.5%) | (32%) | (7.6%) |
| (n = 53) | Median: | Median: | ||||||
| 65.5 | 8.9 | T2a: 10 | 6 = 4 | (−) = 21 | (−) = 13 | (−) = 37 | (−) = 49 | |
| Range: | Range: | (18.9%) | 7.5%) | (39.6%) | (26%) | (69.8%) | (92.5% | |
| 51 – 73 | 1.4 – 41.3 | |||||||
| T2b: 20 | 7 = 20 | |||||||
| (37.7%) | (37.7%) | |||||||
| 8 = 17 | ||||||||
| (32.1%) | ||||||||
| 9 = 11 | ||||||||
| (20.8%) | ||||||||
| No | Mean: | Mean: 6.6 | T1c: 32 | 4 = 1 | (+) = 7 | (+) = 14 | (+) = 2 | (+) = 0 |
| biochemical | 61.7± 7.1 | ± 3.0 | (64%) | (2%) | (14%) | (28%) | (4%) | (0%) |
| or clinical | Median: | Median: | ||||||
| recurrence | 63 | 6.2 | T2a: 10 | 5 = 4 | (−) = 43 | (−) = 36 | (−) = 48 | (−) = 50 |
| (n = 50) | Range: | Range: | (20%) | (8%) | (86%) | (72%) | (56%) | (100%0 |
| 41 – 75 | 0.5 – 15.5 | ≥T2b: 8 | ||||||
| (16%) | 6 = 12 | |||||||
| (24%) | ||||||||
| 7 = 29 | ||||||||
| (58%) | ||||||||
| 8 = 3 | ||||||||
| (6%) | ||||||||
| 9 = 1 | ||||||||
| (2%) | ||||||||
Out of the 53 patients who experienced biochemical recurrence, 47 recurred within 72 months and 29 recurred within 24 months.
EPE = extra-prostatic extension of tumor.
SVI = seminal vesicle invasion.
Three of the patients without recurrence had an unspecified T2 clinical stage.
Immunohistochemistry
For all specimens, paraffin embedded blocks, containing CaP tissues, representing the major Gleason score, were selected from an archival repository. These specimens were processed for embedding by standard histology procedures involving formalin fixation and paraffin embedding and evaluated for standard pathology (i.e., determination of Gleason sum, margin, etc). Thus, no special tissue processing procedures were undertaken for this study. We sectioned specimens for 10 slides from each block. Two slides, one each stained for OPN and IL-8 staining were scored. The remaining slides were available for the optimization of OPN and IL-8 antibody concentration for staining, determination of non-specific staining, and repeating the staining in case of any discrepancies. The specimen slides were deparafinized, rehydrated and treated with an antigen retrieval solution (Dako Laboratories (USA) Carpinteria, CA). The slides were incubated with either anti-OPN or anti-IL-8 antibodies (affinity purified IgG; Sigma-Aldrich, St Louis, MO and R&D Systems, Minneapolis, MN, respectively) at 4° C for 16 hours. The concentration of antibodies used for OPN and IL-8 staining were 0.25 µ/ml and 2 µ/ml, respectively. The slides were developed using the Dako LASB kit (DakoCytomation, Carpinteria, CA) and 3,3′-diaminobenzidine staining. The slides were counterstained with hematoxylin and mounted, as described before [18]. To determine the reproducibility of staining, 25 slides were restained twice and 10 slides were restained three times and scored independently in a blinded fashion by both readers. For both readers, the differences in the staining scores obtained by restaining the slides twice or three times were within 10% of the initial staining score.
Slide grading
Two readers independently evaluated all slides in a blinded fashion (i.e., the readers were blinded to whether the slides were from the recurred or the non-recurred group during the staining procedure and also when evaluating the slides). OPN and IL-8 staining of tumor cells in each slide was graded for intensity (0 to 3+) and percentage of the specimen stained with a particular intensity. A final score was then determined by multiplying the intensity score and the percentage of the specimen. For example, if a specimen exhibited a staining distribution of 50% 2+ and 50% 1+, then the final score is 150 (i.e., 2 × 50 + 1× 50). Therefore, the weighted scores ranged between 0 and 300. Of the 103 stained slides, there was a discrepancy in 15 slides. These discrepancies were resolved by both readers reexamining those slides simultaneously. In addition, 20% (21 slides) of the slides were also evaluated using the IP Image Analysis software and these results were comparable with the readers’ scores.
Statistical Analysis
Based on the availability, in this study, 117 patients/blocks were selected and 103 were ultimately included in the analysis. Among these 103 patients, 47 had biochemical recurrence within 72 months following prostatectomy and 56 patients were recurrence free at 72 months or more. Therefore, based on the way the specimens were collected for analysis, the study design is that of a cohort study. To determine the correlation of each pre- and post-operative parameters and IL-8 and OPN staining scores (analyzed as continuous variables) with biochemical recurrence, we performed logistic regression single parameter analysis. Since this is a cohort study, we performed the Cox proportional hazard analysis on the whole model, which included all pre- and post-operative parameters listed in Table 1, and the staining intensity scores of IL-8 and OPN to determine the parameters that jointly predict biochemical recurrence. In addition, we performed the Cox analysis using a parsimonious subset of parameters that included the intensity scores of IL-8 and OPN and those parameters (i.e., Gleason sum categorized as < 8 or ≥8 and margin status) which were found to be significant in the Cox analysis that contained only the pre- and post-operative parameters described in Table 1.
We also performed the Cox whole model analysis to determine the predictors of early progression. For this analysis, patients who had biochemical recurrence were categorized as those who recurred before 24 months (n = 29) and those who recurred after 24 months (n = 24). Cox proportional hazard analysis was performed using the whole model, i.e., all pre- and post-operative parameters plus the intensity scores of IL-8 and OPN. Cox proportional analysis using the parsimonious model was not performed as none of the pre- and post-operative parameters reached statistical significance when included by themselves in the absence of IL-8 or OPN staining inferences. As described for the Cox analysis using the entire cohort, for determining the early predictors of progression, Gleason sum was included in the model either as a continuous variable, or as a stratified variable (< 8 or ≥ 8) and the intensity scores of OPN and IL-8 were included as continuous variables.
Receiver operating curves were generated to determine the association between staining scores (i.e., for IL-8 or OPN) and biochemical recurrence within 72 months. Cut off values selected by a statistical program (JMP®6 Software from SAS) were used for defining high- or low-grade expression of OPN (cut-off 160) and IL-8 (cut-off 138). The program selects the cut-off limit that yields the highest Sensitivity – (1-Specificity) value. A staining score above the cut-off value was considered as a true positive if the patient had biochemical recurrence and the score lower than the cut-off value was considered as a true negative if the patient had no biochemical recurrence. The sensitivity and specificity for OPN and IL-8 staining inferences were calculated as described before [19]. Monte-Carlo cross-validation was performed to obtain the mean ± SD and 95% CI for the sensitivity and specificity for OPN and IL-8 staining scores.
Statistical analyses were carried out using the JMP® Software Program (version 6.0; SAS Institute, Cary, NC).
RESULTS
Localization of OPN and IL-8 in CaP tissues
Since CaP is a slow disease to progress, a longer follow-up is necessary to make clinically meaningful predictions regarding biochemical recurrence. Therefore, in this study we examined the prognostic potential of OPN and IL-8, in archival radical prostatectomy specimens from patients with CaP on whom there was a minimum 72 month follow-up. It is noteworthy that the pre- and post-operative parameters of the patients selected for the study (n = 103) were not significantly different from the total number of patients on whom there was six year follow-up (n = 381) or those who were lost to follow-up before six years (n = 314). The parameters compared were age, mean PSA, Gleason sum, (P > 0.05; Mann-Whitney test in each case) and margin status, EPE, seminal vesicle invasion (P > 0.05; chi-square test, in each case). A trend-based chi-square analysis for clinical stage (P > 0.05, DF = 1, chi-square 1.532) also showed no difference between the group of patients on whom there was six year follow-up and those who were lost to follow-up before six years. Therefore, the study patients (n = 103) were most likely not different from those who were not included in the study.
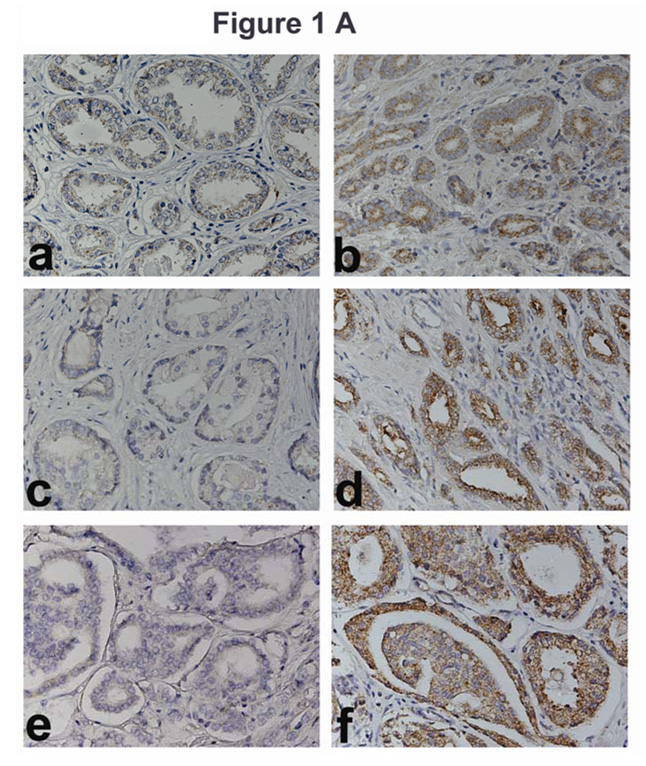
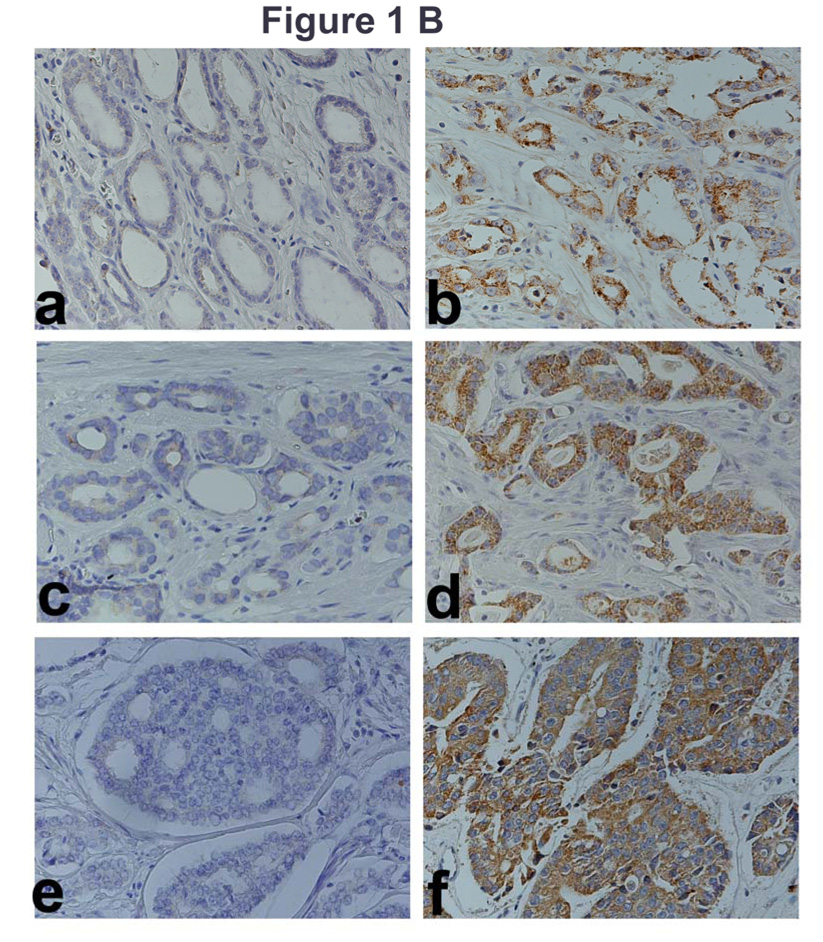
As shown in Figure 1 A, very little OPN staining is observed in specimens from patients with Gleason sum 6, 7, or 8, CaP, who did not have biochemical recurrence (panels a, c, and e). However, high-grade staining is observed in CaP specimens from patients who had biochemical recurrence (panels b, d and f). Figure 1 B (panels a, c and e) shows low-grade IL-8 staining in specimens from patients with Gleason sum 6, 7 or 8 CaP, who did not have biochemical recurrence. Contrarily, high-grade staining is observed in CaP specimens from patients who had biochemical recurrence (Fig 1 B, panels, b, d, f).
Figure 1. Localization of OPN and IL-8 in CaP tissues.
A and B: OPN (A) and IL-8 (B) localization: OPN and IL-8 were localized in CaP specimens from non-recurred patients (panels a, c, e) and recurred patients (panels b, d, f). Panels a, b: Gleason Sum 6; panels c, d: Gleason sum 7 and panels e, f: Gleason sum 8. Original magnification: 400⨯. C: OPN and IL-8 intensity scores. Scatter diagram of OPN and IL-8 staining scores for patients who either had or did not have biochemical recurrence. The mean ± SD scores for OPN (a) and IL-8 (b) staining intensity are indicated. Each CaP specimen could receive a minimum and maximum possible score of 0 and 300, respectively. D: Localization of OPN and IL8 in normal-benign prostate glands near tumor. OPN and IL-8 expression was examined in normal-benign prostate glands, which are adjacent to tumor. Panels a and c: Normal-benign glands near the tumor from a recurred patient. Panels b and d: Normal-benign glands adjacent to tumor from a non-recurred patient.
Figure 1 C shows the IL-8 and OPN staining intensity scores for both the recurred and non-recurred patients. The differences in the mean intensity scores among recurred and non-recurred groups for OPN and IL-8 staining were statistically significant (P < 0.001; DF = 1; unpaired t-test).
We also compared the staining intensity in areas adjacent to the tumors, for both IL-8 and OPN. As shown in Figure 1 D, for both OPN and IL-8, the normal-benign glands adjacent to the tumor cells show high-grade staining, if the specimen was obtained from a patient who recurred (panels a, c). However, the normal-benign glands adjacent to tumor cells do not stain for OPN or IL-8, if the specimen was obtained from a patient who did not recur (panels b, d). In the normal and benign glands, the intensity scores (mean ± SD) for OPN and IL-8 staining in the recurred group (169.1 ± 89.9 and 157.5 ± 91.6 respectively) were 2.8 and 6.0-fold higher than those in the non-recurred group (57.5 ± 65 and 27.2 ± 50.7). Logistic regression analysis showed that the differences in the intensity scores of IL-8 and OPN staining in normal specimens from patients who had biochemical recurrence and those who did not are statistically significant (IL-8: chi-square: 11.8; P < 0.001; odds ratio: 1.01 (95% CI: 1.00 – 1.01; OPN: chi-square: 10.0; P = 0.002; odds ratio: 1.02 (95% CI: 1.01 – 1.03)). In addition, the analyses also showed that the staining intensity in the normal glands correlated with staining intensity in tumor cells for both IL-8 (Spearman r = 0.853; 95% CI: 0.724 0.924; p = < 0.001) and OPN (Spearman r = 0.862; 95% CI: 0.734 0.931; p = < 0.001). This suggests that OPN and IL-8 expression in the benign glands associates with biochemical recurrence.
Association of pre- and post-operative parameters and OPN/IL-8 staining with biochemical recurrence
Univariate analysis
Since in this study all patients had minimum 72 month follow-up, we categorized the patients as those who recurred before 72 months and those who did not recur but had minimum 72 month follow-up. We used the logistic regression analysis to determine the association of each of the preoperative (i.e., age, PSA, and clinical stage) and postoperative (i.e., Gleason sum, margin, EPE, SVI and lymph node status) parameters, as well as staining inferences of OPN and IL-8, with biochemical recurrence. As shown in Table 2, except age, all other pre- and post-operative parameters, as well as OPN and IL-8 staining inferences, were significant in predicting biochemical recurrence.
Table 2. Univariate Analysis of pre- and post-operative parameters and IHC staining inferences.
Logistic regression single parameter analysis was used to determine the association of pre-operative (age, pre-operative PSA, and clinical stage) and post-operative (Gleason sum, margin +/−, EPE +/−, SV invasion +/− and lymph node +/−) parameters and OPN and IL-8 staining inferences with biochemical recurrence.
| Parameter | Chi-Square | P-value | Odds Ratio | 95% CI |
|---|---|---|---|---|
| Gleason Sum | 15.06 | <0.001* | 2.72 | 1.71 – 4.74 |
| Gleason ≥ 7 | 10.09 | 0.001* | 1.92 | 1.368 – 2.697 |
| Gleason ≥ 8 | 24.69 | < 0.001* | 5.22 | 2.056 – 13.263 |
| Age | 2.61 | 0.11 | 0.98 | 0.89 – 1.01 |
| Pre-op PSA | 8.27 | < 0.004 | 1.13** | 1.05 – 1.25 |
| Stage | 11.5 | < 0.001* | 2.95 | 1.318 – 6.599 |
| EPE | 24.14 | < 0.001* | 3.78 | 1.89 – 7.532 |
| Positive Surgical Margin | 24.0 | < 0.001* | 2.85 | 1.766 – 4.611 |
| SVI | 12.529 | < 0.001* | 5.13 | 1.371 – 19.186 |
| Lymph node status | 3.926 | 0.0475* | 9.18 | |
| OPN | 13.43 | <0.001* | 1.01** | 1.00 – 1.01 |
| IL-8 | 11.91 | <0.001* | 1.01** | 1.00 – 1.01 |
Statistically significant.
Change in odds ratio per unit change in the parameter.
Multivariate analyses
To determine the smallest number of variables that can independently associate with biochemical recurrence within 72 months, we performed multivariate analysis using the Cox proportional hazard model. Initially, we performed the Cox analysis using only the pre- and post-operative parameters. As shown in Table 3, in the whole model, when Gleason sum was included as a continuous variable, only the margin status reached statistical significance. However, when Gleason sum was included as a categorized variable (< 8 and ≥ 8) both margin status and Gleason sum reached statistical significance. When Gleason sum was categorized as < 7 and ≥ 7, it did not reach statistical significance. We then determined the statistical significance of the staining scores of IL-8 and/or OPN in the multivariate whole model which included all pre- and post-operative parameters along with the staining scores. In the parsimonious model, we included margin status and Gleason sum (categorized as < 8 or ≥ 8) and the staining scores, to determine whether IL-8 or OPN staining scores reach statistical significance beyond the margin status and Gleason sum. As shown in Table 3, IL-8 staining score did not reach statistical significance either in the whole model or in the parsimonious model. However, OPN staining score along with margin status reached statistical significance in the whole mode, regardless of whether Gleason sum was included as a continuous or as a categorized variable (Table, 3). In the parsimonious model, all three parameters (i.e., margin, Gleason sum categorized and OPN) were found to be statistically significant (Table 3). When both OPN and IL-8 staining scores were included in the whole model, once again margin status and OPN staining score reached statistical significance regardless of whether Gleason sum was categorized or not. Categorized Gleason sum, along with margin status and OPN staining score reached statistical significance in the parsimonious model. It is noteworthy that lymph node status did not reach statistical significance in any of the models. The obvious explanation for this is that there were only 4 patients in the entire cohort who had positive lymph node status and all of these patients experienced biochemical recurrence; 3 in < 72 months.
Table 3. Multivariate analyses of pre- and post-operative parameters and IHC staining inferences.
Cox proportional hazard analysis was performed by including: 1. only the pre-operative (i.e., age, PSA, and clinical stage) and post-operative (i.e., Gleason sum, EPE, margin +/−, and SV invasion, lymph node status) parameters; 2. Pre-and post-operative parameters and IL-8 staining score (IL-8 whole model); 3. Margin, Gleason sum and IL-8 staining score (IL-8 parsimonious model); 4. Pre- and post operative parameters and OPN staining score (OPN whole model); 3. Margin, Gleason sum and OPN staining score (OPN parsimonious model); 5. Pre- and post-operative parameters and OPN and IL-8 staining scores included as individual parameters (OPN and IL-8 whole model); 6. Margin, Gleason sum and OPN and IL-8 staining scores (OPN and IL-8 parsimonious model). The whole model analyses were also performed by including the Gleason sum as a categorical variable (Gleason sum < 8 or ≥ 8). The significant parameters (P < 0.05) selected by the model are shown. In all of the analyses, IL-8, OPN or IL-8 and OPN staining scores were included as continuous variables.
| Parameter | Chi-Square | P-value | Risk Ratio | 95% CI |
|---|---|---|---|---|
| No IL-8 and/or OPN included (Whole model) | ||||
| Margin | 6.21 | 0.013 | 1.59 | 1.10 – 2.34 |
| No IL-8 and/or OPN included (Whole model, Gleason categorized) | ||||
| Margin | 6.09 | 0.014 | 1.58 | 1.10 – 2.33 |
| Gleason categorized | 5.06 | 0.025 | 1.51 | 1.06 – 2.18 |
| IL-8 (Whole model; Gleason continuous) | ||||
| Margin | 4.6 | 0.032 | 1.51 | 1.04 – 2.25 |
| IL-8 (Whole Model; Gleason categorized) | ||||
| Margin | 4.75 | 0.029 | 1.52 | 1.04 – 2.28 |
| Gleason Categorized | 3.88 | 0.049 | 1.46 | 1.00 – 2.13 |
| IL-8 (Parsimonious model; Gleason categorized) | ||||
| Margin | 10.93 | < 0.001 | 1.74 | 1.25 – 2.51 |
| Gleason Categorized | 10.84 | 0.001 | 1.7 | 1.24 – 2.34 |
| OPN (Whole model; Gleason continuous) | ||||
| Margin | 5.75 | 0.017 | 1.55 | 1.08 – 2.29 |
| OPN | 7.88 | 0.005 | 1.00* | 1.00 – 1.01 |
| OPN (Whole model; Gleason categorized) | ||||
| Margin | 5.83 | 0.016 | 1.55 | 1.08 – 2.29 |
| OPN | 6.75 | 0.009 | 1.00* | 1.00 – 1.01 |
| OPN (Parsimonious model; Gleason categorized) | ||||
| Margin | 13.51 | < 0.001 | 1.78 | 1.29 – 2.54 |
| Gleason categorized | 6.13 | 0.013 | 1.47 | 1.08 – 2.02 |
| OPN | 9.19 | 0.002 | 1.01* | 1.00 – 1.01 |
| OPN and IL-8 (Whole model; Gleason continuous) | ||||
| Margin | 5.16 | 0.023 | 1.52 | 1.06 – 2.25 |
| OPN | 7.36 | 0.007 | 1.01* | 1.00 – 1.01 |
| OPN and IL-8 (Whole model; Gleason categorized) | ||||
| Margin | 5.27 | 0.022 | 1.53 | 1.07 – 2.26 |
| OPN | 6.46 | 0.011 | 1.01* | 1.00 – 1.01 |
| OPN and IL-8 (Parsimonious model; Gleason categorized) | ||||
| Margin | 12.16 | < 0.001 | 1.81 | 1.27 – 2.54 |
| OPN | 8.5 | 0.004 | 1.01* | 1.00 – 1.01 |
| Gleason ≥ 8 | 4.75 | 0.029 | 1.44 | 1.04– 2.00 |
Change in risk ratio per unit change in OPN staining score.
Sensitivity, specificity, accuracy of OPN and IL-8 expression
To test whether there was any association between IL-8/OPN levels and biochemical recurrence, receiver operating characteristic (ROC) curves were generated for OPN and IL-8 staining scores. For IL-8, the area under the ROC curve was 0.7458 (chi-square, 17.19; P < 0.001) and for OPN, it was 0.767 (chi-square, 18.3; P < 0.001). To increase the observed degree of association, cut-off values were obtained from the ROC curves for IL-8 (138) and OPN (160), respectively. These cut-off values were then used for calculating sensitivity and specificity of both markers to predict biochemical recurrence. As shown in Table 4, at 72 months OPN staining has 75.5% sensitivity and 76% specificity to predict biochemical recurrence. The sensitivity (73.6%) and specificity (70.6%) for IL-8 staining are slightly lower than that for OPN staining. Although, the sensitivity and specificity of OPN or IL-8 staining to predict biochemical recurrence within 72 months, will require independent confirmation, the cross-validation results presented in Table 4, strengthen the results somewhat.
Table 4. Determination of sensitivity and specificity of OPN and IL-8 staining inferences for predicting biochemical recurrence.
The sensitivity and specificity for OPN and IL-8 staining were determined at 72 month follow-up using the cut-off limits determined from the ROC curves and then cross-valideated.
| Parameter | Value (%) | Cross-validation Value (%) |
|---|---|---|
| IL-8 | ||
| Sensitivity | 73.6 | 66.9 ± 16.2; 95% CI: 63.0 – 70.9 |
| Specificity | 70.6 | 68.1 ± 17.1; 95% CI: 63.9 – 72.2 |
| OPN | ||
| Sensitivity | 75.5 | 68.4 ± 13.8; 66.5 ± 70.3 |
| Specificity | 76 | 76.8 ± 12.1; 75.1 – 78.5 |
We also determined the sensitivity and specificity of combined OPN and IL-8 staining inferences, by considering one or both marker positive as a positive inference for the combined marker (either true positive or false positive). The combined OPN+IL-8 marker had 95% sensitivity and 65% specificity to predict biochemical recurrence at 72 months. Monte-Carlo cross-validation analysis showed 86 ± 8.5% (95% CI: 84.4% -88.2%) sensitivity and 60.5 ± 6.5 (95% CI: 59% - 61.9%) specificity for the combined marker.
Predictors of early progression
It has been suggested that biochemical recurrence within 1 to 2 years indicates systemic disease, whereas biochemical recurrence beyond 24 months suggests local recurrence [20]. The patients who had biochemical recurrence (n = 53) were categorized as those who recurred before 24 months (n = 29) and those who did not recur before 24 months (n = 24). Cox proportional hazard model, which included only the pre- and post operative parameters, no single parameter reached statistical significance, regardless of whether Gleason sum was categorized (< 8 and ≥ 8) or included as a continuous variable (Table 5). In the Cox model which included IL-8 staining score and all of the pre-and post-operative parameters, no parameter reached statistical significance regardless of whether Gleason sum is included as a continuous or categorical variable. Contrarily, OPN staining score reached statistical significance in the whole model regardless of whether Gleason sum was categorized or included as a continuous variable (Table 5). When both OPN and IL-8 staining scores were included in the Cox model (as separate variables) OPN together with IL-8 and SVI reached statistical significance (Table 5).
Table 5. Multivariate analyses of pre- and post-operative parameters and IL-8 and OPN staining scores to predict biochemical recurrence within 24 months.
Cox proportional hazard analyses were performed to identify those parameters that associated with PSA biochemical recurrence within 24 months. The analyses were carried out as follows: 1. only the pre-operative and post-operative parameters; 2. Pre-and post-operative parameters and IL-8 staining score (IL-8 whole model); 3. Pre- and post-operative parameters and OPN staining score (OPN whole model); 4. Pre- and post-operative parameters and OPN and IL-8 staining scores, included as individual parameters (OPN and IL-8 whole model). The analysis for each of the above four categories was also performed by including Gleason sum as a categorical variable (Gleason sum ≥ 8). The significant parameters (P < 0.05) selected by the model are shown. In all of the analyses, IL-8, OPN or IL-8 and OPN staining scores were included as continuous variables.
| Parameter | Chi-square | P value | Risk Ratio | 95% CI |
|---|---|---|---|---|
|
No IL-8 and/or OPN (Whole model; Gleason continuous or categorized) No pre- or post-operative parameter reached significance | ||||
|
IL-8 (Whole model; Gleason continuous or categorized) No parameter reached significance | ||||
| OPN (Whole model; Gleason continuous) | ||||
| OPN | 4.32 | 0.038 | 1.01* | 1.00 – 1.01 |
| OPN (Whole model; Gleason categorized) | ||||
| OPN | 4.16 | 0.041 | 1.01* | 1.00 – 1.01 |
| IL-8 and OPN (Whole model; Gleason continuous) | ||||
| OPN | 7.13 | 0.008 | 1.01* | 1.00 – 1.02 |
| IL-8 | 5.63 | 0.018 | 1.01* | 1.00 – 1.01 |
| SVI | 4.64 | 0.031 | 1.74 | 1.05 – 2.86 |
| IL-8 and OPN (Whole model; Gleason categorized) | ||||
| OPN | 6.59 | 0.010 | 1.01* | 1.00 – 1.01 |
| IL-8 | 5.63 | 0.018 | 1.01* | 1.00 – 1.01 |
| SVI | 5.15 | 0.023 | 1.78 | 1.08 – 2.88 |
Increase in risk per unit increase in the staining score.
DISCUSSION
The majority of the newly diagnosed CaP patients have clinically organ-confined disease [22]. Although, nomograms based on pre- and post-operative parameters offer some information about patients’ prognosis, limited knowledge about which CaP is likely to progress, as well as, when it will recur severely impedes individualized selection of therapy and subsequent prediction of outcome [23]. Recent studies have shown that chemokines, cytokines and other proteins that are associated with inflammatory processes may function in promoting tumor invasion and metastasis [24–26]. Furthermore, some of these molecules, such as cyclooxygease-2 have shown prognostic potential for predicting CaP progression [18]. In this study, with a minimum 6 year on all patients, we demonstrate that the expression of OPN, in radical prostatectomy specimens is independently associated with biochemical recurrence. Furthermore, OPN and LI-8 may be early predictors of disease progression.
In prostate tissues, OPN levels have been shown to be elevated in carcinoma when compared to normal and benign prostatic hyperplasia tissues [9]. Consistent with these findings, we found that benign prostate specimens stained for OPN with low intensity. Furthermore, we observed that OPN staining correlated with Gleason sum (P < 0.001; logistic regression analysis; unpublished results. This may explain why when OPN staining is included in the multivariate model, Gleason sum did not reach statistical significance in predicting biochemical recurrence even when included as a categorized (< 8, ≥ 8). Although the over expression of OPN has been previously shown to correlate with poor survival, our study is the first to report that high OPN staining may indicate biochemical recurrence in the future. OPN was found to be independently associated with biochemical recurrence within 72 months. The high sensitivity and specificity (∼ 76%) of OPN staining to predict biochemical recurrence also suggest that it may be a clinically useful marker for predicting biochemical recurrence. The observation that high intensity staining of normal glands surrounding tumor cells for both IL-8 and OPN proteins is an indication of the possible role of the paracrine induction of pro-inflammatory factors in the development of reactive stroma, which in turn, promotes tumor progression [27].
The expression of IL-8 mRNA has been shown to correlate with Gleason sum and pathologic stage [13], but whether it independently associates with biochemical recurrence has not been evaluated. In our study, IL-8 expression correlated with Gleason sum (P = 0.019; logistic regression analysis) but not with clinical stage (p = 0.8; unpublished results). Furthermore, IL-8 staining also does not independently associate with biochemical recurrence within 72 months.
It is noteworthy that among the group of CaP patients who recurred, OPN was able to distinguish between those who recurred before 24 months and those recurring after 24 months in the multivariate model. Although, IL-8 staining score when included by itself in the multivariate model was not an early predictor of biochemical recurrence, when included in the model along with the OPN staining, it did contribute to recognizing those patients who will have early biochemical recurrence, and hence may be at risk for systemic disease. This result is consistent with the role of OPN and IL-8 in cancer metastasis and progression.
Taken together our study shows that OPN in radical prostatectomy specimens is independently associated with CaP progression i.e., biochemical recurrence, and when combined with margin status, can stratify patients into different risk categories for developing biochemical recurrence within six years. In addition, OPN either alone or together with IL-8 and SVI may be able to stratify patients at risk for early disease progression.
CONCLUSION
OPN and IL-8 expression is higher in radical prostatectomy specimens from patients who experience biochemical recurrence within 72 months. OPN and to a lesser extent IL-8 expression are independently associated with biochemical recurrence in prostate cancer patients and may have potential to predict early disease progression.
Acknowledgments
Grant support: NIH/NCI 2R01-CA061038 (BLL); R01 CA 123063-01 (VBL); Department of Urology and Sylvester Comprehensive Cancer Center, University of Miami
Abbreviations used
- CI
confidence interval
- IL-8
Interleukin-8
- OPN
osteopontin
- PSA
prostate specific antigen
REFERENCES
- 1.Amling CL. Biochemical recurrence after localized treatment. Urol Clin North Am. 2006;33:147–159. doi: 10.1016/j.ucl.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Routh JC, Leibovich BC. Adenocarcinoma of the prostate: epidemiological trends, screening, diagnosis, and surgical management of localized disease. Mayo Clin Proc. 2005;80:899–907. doi: 10.4065/80.7.899. [DOI] [PubMed] [Google Scholar]
- 3.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 4.Ravallese EM. Osteopontin: a bridge between bone and the immune system. J Clin Invest. 2003;112:147–149. doi: 10.1172/JCI19190. Erratum in: J Clin Invest 2003;112:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Rudland PS, Platt-Higgins A, El-Tanani M, et al. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62:3417–3427. [PubMed] [Google Scholar]
- 7.Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7:4060–4066. [PubMed] [Google Scholar]
- 8.Khodavirdi AC, Song Z, Yang S, et al. Increased expression of osteopontin contributes to the progression of prostate cancer. Cancer Res. 2006;66:883–888. doi: 10.1158/0008-5472.CAN-05-2816. [DOI] [PubMed] [Google Scholar]
- 9.Forootan SS, Foster CS, Aachi VR, et al. Prognostic significance of osteopontin expression in human prostate cancer. Int J Cancer. 2006;118:2255–2261. doi: 10.1002/ijc.21619. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Ananthula S, Milhorn DM, Krishnaswamy G, Singh K. Osteopontin: anovel inflammatory mediator of cardiovascular disease. Front Biosci. 2007;12:214–221. doi: 10.2741/2059. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Uehara H, Karashima T, McCarty M, Shih N, Fidler IJ. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3:33–42. doi: 10.1038/sj.neo.7900124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araki S, Omori Y, Lyn D, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–6862. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 13.Uehara H, Troncoso P, Johnston D, et al. Expression of interleukin-8 gene in radical prostatectomy specimens is associated with advanced pathologic stage. Prostate. 2005;15(64):40–49. doi: 10.1002/pros.20223. [DOI] [PubMed] [Google Scholar]
- 14.Lehrer S, Diamond EJ, Mamkine B, Stone NN, Stock RG. Serum interleukin-8 is elevated in men with prostate cancer and bone metastases. Technol Cancer Res Treat. 2004;3:411. doi: 10.1177/153303460400300501. [DOI] [PubMed] [Google Scholar]
- 15.Michaud DS, Daugherty SE, Berndt SI, et al. Genetic polymorphisms of interleukin-1B (IL-1B), IL-6, IL-8, and IL-10 and risk of prostate cancer. Cancer Res. 2006;66:4525–4530. doi: 10.1158/0008-5472.CAN-05-3987. [DOI] [PubMed] [Google Scholar]
- 16.Yang HP, Woodson K, Taylor PR, et al. Genetic variation in interleukin 8 and its receptor genes and its influence on the risk and prognosis of prostate cancer among Finnish men in a large cancer prevention trial. Eur J Cancer Prev. 2006;15:249–253. doi: 10.1097/01.cej.0000199504.07947.e7. [DOI] [PubMed] [Google Scholar]
- 17.Attur MG, Dave MN, Clancy RM, Patel IR, Abramson SB, Amin AR. Functional genomic analysis in arthritis-affected cartilage: yin-yang regulation of inflammatory mediators by alpha 5 beta 1 and alpha V beta 3 integrins. J Immunol. 2000;164:2684–2691. doi: 10.4049/jimmunol.164.5.2684. [DOI] [PubMed] [Google Scholar]
- 18.Cohen BL, Gomez P, Omori Y, et al. Cyclooxygenase-2 (COX-2) expression is an independent predictor of prostate cancer recurrence. Int J Cancer. 2006;119:1082–1087. doi: 10.1002/ijc.21749. [DOI] [PubMed] [Google Scholar]
- 19.Lokeshwar VB, Habuchi T, Grossman HB, et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005;66(Suppl 1):35–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 20.Canto EI, Shariat SF, Slawin KM. Biochemical staging of prostate cancer. Urol Clin North Am. 2003;30:263–277. doi: 10.1016/s0094-0143(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method) Br Med J. 1998;317:1572. doi: 10.1136/bmj.317.7172.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braeckman J, Michielsen D. Prognostic factors in prostate cancer. Recent Results Cancer Res. 2007;175:25–32. doi: 10.1007/978-3-540-40901-4_3. [DOI] [PubMed] [Google Scholar]
- 23.Amling CL. Biochemical recurrence after localized treatment. Urol Clin North Am. 2006;33:147–159. doi: 10.1016/j.ucl.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Schalken JA, Bergh A, Bono A, et al. Molecular prostate cancer pathology: current issues and achievements. Scand J Urol Nephrol Suppl. 2005;216:82–93. doi: 10.1080/03008880510030950. [DOI] [PubMed] [Google Scholar]
- 25.Flaig TW, Nordeen SK, Lucia MS, Harrison GS, Glode LM. Conference report and review: current status of biomarkers potentially associated with prostate cancer outcomes. J Urol. 2007;177:1229–1237. doi: 10.1016/j.juro.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–555. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 27.Rowley DR. Stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17:411–419. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]